Abstract
Background/Aim: To monitor adverse events rapidly and accurately during combination chemotherapy, we established an innovative medication instruction sheet (MIS) including cytarabine and idarubicin induction therapy. However, it is unclear whether this MIS allows for the accurate prediction of adverse events and their onset timing in a clinically significant manner. We therefore evaluated the clinical usefulness of our MIS for monitoring adverse events.
Patients and Methods: Patients who received cytarabine and idarubicin induction therapy for acute myeloid leukemia (AML) at the Department of Hematology, Kyushu University Hospital between January 2013 and February 2022 were included. The real-world clinical data were compared to the MIS to determine the accuracy of the MIS for predicting the onset and duration of adverse events in patients with AML during induction chemotherapy.
Results: Thirty-nine patients with AML were included in this study. Overall, 294 adverse events were noted, all of which were predicted items in the MIS. Among the 192 non-hematological adverse events, 131 (68.2%) occurred during a similar period as that listed in the MIS, whereas among the 102 hematological adverse events, 98 (96.1%) appeared earlier than expected. For the non-hematological events, the onset and duration of elevated aspartate aminotransferase levels and nausea/vomiting coincided well with those listed in the MIS, whereas the predictive accuracy for rashes was the lowest.
Conclusion: Hematological toxicity was not predicted because of the bone marrow failure associated with AML. Our MIS was useful for rapidly monitoring non-hematological adverse events in patients with AML receiving cytarabine and idarubicin induction therapy.
Keywords: Chemotherapy, medication instruction sheet, adverse events, acute myeloid leukemia, cytarabine and idarubicin induction therapy
Acute myeloid leukemia (AML) is the most common and lethal form of acute leukemia in adults. Curative therapy for AML requires induction therapy followed by curative-intent post-induction therapy, often including allogeneic hematopoietic stem cell transplantation (1). The most common induction therapy for AML is cytarabine administered continuously for 7 days in combination with an anthracycline for the first 3 days, commonly referred to as “7+3” (2-4).
Outcomes for patients with AML have improved in recent years owing to advances in therapeutic agents and steady improvements in supportive care (5,6). Ensuring optimal supportive care is therefore essential for improving clinical outcomes.
Fatal adverse events including sepsis, bleeding, febrile neutropenia, acute cardiac toxicity, and late-onset cardiac failure have been reported in Japanese patients receiving induction therapy with cytarabine and an anthracycline (4). However, the adverse events associated with subjective symptoms that significantly reduce patients’ quality of life have been reported less frequently. In addition, information about the onset and timing of adverse events during induction therapy with cytarabine and an anthracycline is currently limited.
Clinical pharmacists are responsible for ensuring safe and effective chemotherapy and allaying patients’ anxiety about the incidence of adverse events. They achieve this by reviewing prescription orders based on the chemotherapy regimen, providing safe and effective supportive care medicine, and offering extensive explanations regarding the onset, symptom severity, and duration of expected adverse events (7-13).
Therefore, to monitor for adverse events quickly and accurately during combination chemotherapy, we previously established an innovative medication instruction sheet (MIS) for monitoring for adverse events that are anticipated to occur during combination chemotherapy. The MIS covers 300 chemotherapy regimens, including cytarabine and idarubicin induction therapy (7).
This MIS allows for the chemotherapy treatment schedule and the type, onset, and duration of adverse events to be easily visualized and rapidly recognized. To predict the type, onset, and duration of adverse events, the MIS was created with reference to package inserts, manufacturers’ brochures, and previous literature (14-16).
However, the accuracy of the MIS for predicting adverse events associated with induction chemotherapy in the clinical setting has not been determined. Therefore, in this study, we evaluated the clinical usefulness of the MIS for patients with AML receiving cytarabine and idarubicin induction therapy.
Patients and Methods
Assessment of the MIS. Patients received cytarabine and idarubicin induction therapy for AML at the Department of Hematology, Kyushu University Hospital from January 2013 to February 2022 were included in the present study. Hematologists, nurses, and pharmacists monitored for adverse events using the MIS for cytarabine and idarubicin induction therapy and verified the prescription of supportive care medicines when any sign of potential adverse events appeared. Documented adverse events were graded according to the Common Toxicity Criteria, version 5.0 (National Cancer Institute, Bethesda, MD, USA).
In the present study, we evaluated the clinical usefulness of our MIS by comparing adverse events and their onset in the clinical setting with that indicated by the MIS.
Cytarabine and idarubicin induction therapy. Patients received 24-hour infusions of cytarabine (100 mg/m2) for 7 days starting on day 1 and 30-minute infusions of idarubicin (12 mg/m2) for 3 days starting on day 1.
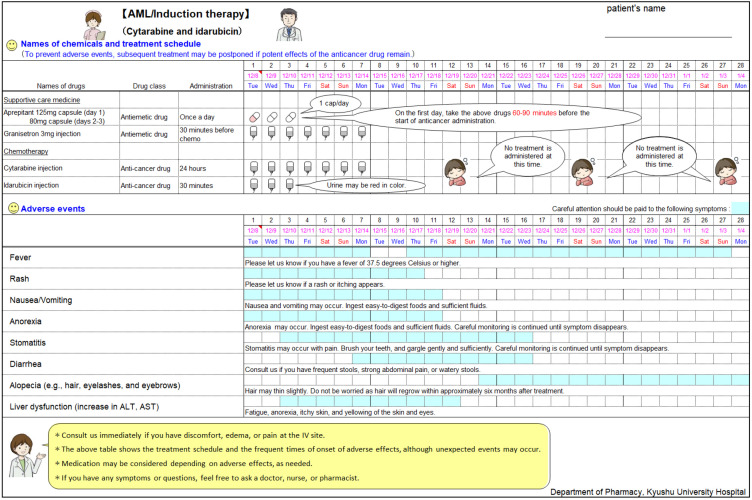
Preparation of the MIS. Using Microsoft Excel® 2010 and later Microsoft Windows platform versions, we created an MIS template consisting of two sections: a treatment schedule section for chemotherapeutic agents and supportive care medicine and an anticipated adverse event section (Figure 1). The MIS lists the prophylactic and supportive care medicines and includes a brief description for clarity. The illustrations included in the MIS were originally drawn by pharmacists at the Department of Pharmacy, Kyushu University Hospital. Adverse events with an incidence rate >10% were listed in the MIS as essential for medical professionals to monitor. The onset timing and duration of adverse events were marked in color to allow for the prompt recognition of adverse events that required careful attention. Hematological toxicities were excluded from the MIS because bone marrow failure, such as decreased hemoglobin levels (95%), thrombocytopenia (87%), and leukopenia (36%) are associated with AML and appear before the induction of chemotherapy and thus would likely complicate the monitoring of chemotherapy-associated hematological adverse events. Our MIS was completed after approval from the hematologists at our Department of Hematology.
Figure 1. The medication instruction sheet (MIS) for cytarabine and idarubicin induction therapy. This sheet was used to monitor for adverse events and verify the chemotherapy and supportive care medicine treatment schedule for patients with acute myeloid leukemia. The upper section indicates the treatment schedule for the chemotherapeutic agents and supportive care medicine, while the lower section represents predicted adverse events, their onset, and duration. A brief description for each adverse event is also included. AST: Aspartate aminotransferase; ALT: alanine aminotransferase.
Data analysis. The onset and duration of each adverse event were compared between the clinical data and the MIS. The predictive accuracy of the MIS was defined as the concordance rate of the onset timing and duration of each adverse event in the clinical setting with that predicted by the MIS.
The data are shown as the predictive accuracy rate and 95% confidence interval (CI) for the proportion of the population, as reported by Rumsey (17). The accuracy rate was statistically compared for each adverse event of any grade and for the different adverse event grades using the Kruskal-Wallis test followed by Scheffe’s test. Other non-parametric analyses, such as the chi-square test, were used to compare two groups. Data were analyzed using JMP Pro® 16.2 (SAS Institute, Cary, NC, USA), and a p-value <0.05 was considered statistically significant.
Ethics and consent. All study protocols were approved by the Medical Ethics Review Committee of Kyushu University Graduate School and Faculty of Medicine (approval No. 22151-00) and the Doshisha Women’s College of Liberal Arts (approval No. 2022-19). All procedures were performed in accordance with the ethical standards of the Kyushu University Graduate School and Faculty of Medicine, the Institutional Medical Ethics Review Committee at Doshisha Women’s College of Liberal Arts, and the 1964 Declaration of Helsinki and its later amendments. Given the retrospective nature of this study, the need for informed consent was waived in accordance with the standards of the Kyushu University Graduate School and Faculty of Medicine and Doshisha Women’s College of Liberal Arts of Medicine Institutional Medical Ethics Review Committee.
Results
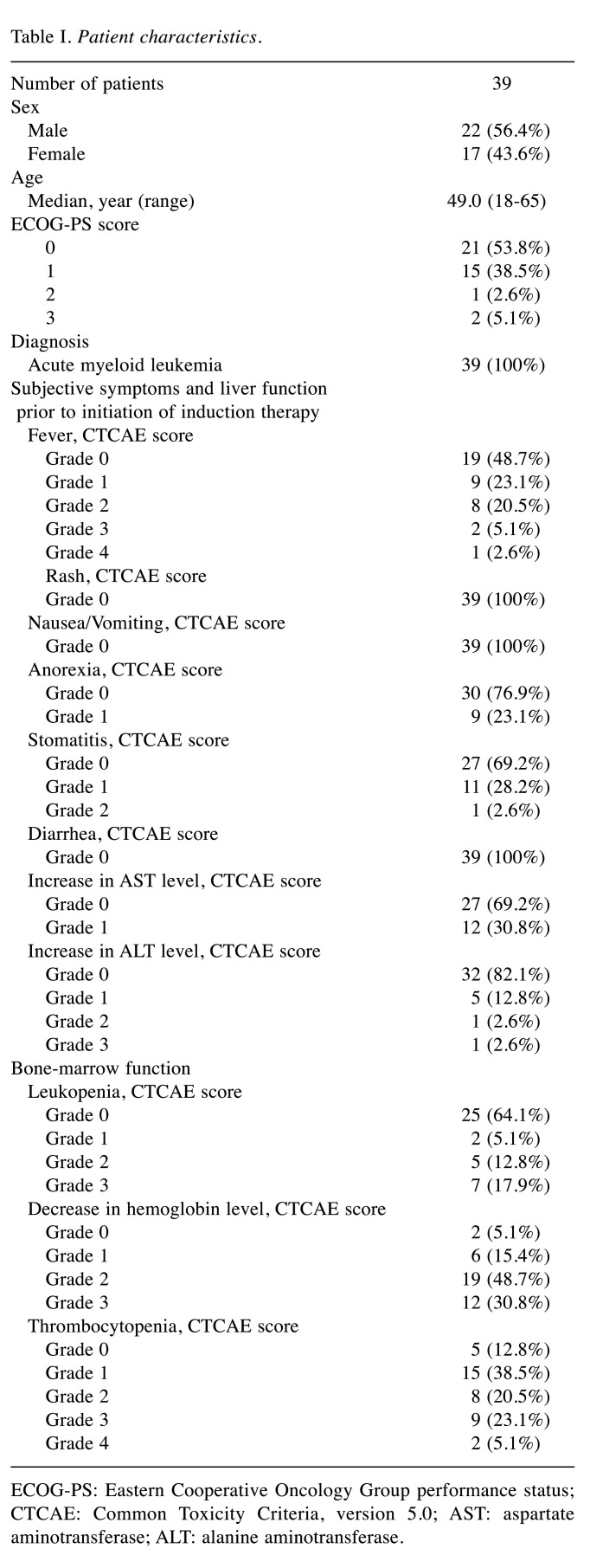
Baseline clinical characteristics. Of the 41 potentially eligible patients retrieved from the medical records, 39 were included in the present study. One patient was excluded due to a cerebellar hemorrhage on day 19 and the other patient was excluded due to septic shock on day 23 after the start of cytarabine and idarubicin induction therapy. Table I presents the baseline clinical characteristics of the 39 patients. The median age of the patients was 49 years (range=18-65 years). Most patients presented with a good performance status (0-1). Before the start of cytarabine and idarubicin induction therapy, 20 patients (51.3%) had fevers, 9 (23.1%) had anorexia, 12 (30.8%) had stomatitis, and 19 (24.4%) had abnormal liver function test levels, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Table I. Patient characteristics.
ECOG-PS: Eastern Cooperative Oncology Group performance status; CTCAE: Common Toxicity Criteria, version 5.0; AST: aspartate aminotransferase; ALT: alanine aminotransferase.
Additionally, most of the patients showed a reduction in bone marrow function before chemotherapy: 37 (94.9%) had decreased hemoglobin levels, 34 (87.2%) had thrombocytopenia, and 14 patients (35.9%) had leukopenia.
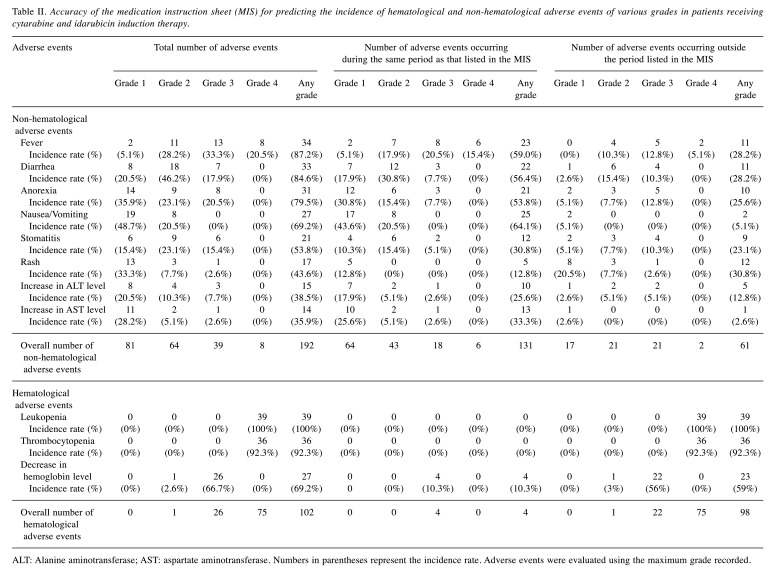
Clinical usefulness of the MIS for predicting adverse events, their onset timing, and duration. As shown in Table II, 294 adverse events in total were recorded among the 39 included patients. Among the 192 non-hematological adverse events, there were 34 incidences of fever (incidence rate, 87.2%), 33 incidences of diarrhea (84.6%), 31 incidences of anorexia (79.4%), 27 incidences of nausea/vomiting (69.2%), 21 incidences of stomatitis (53.8%), 17 incidences of rash (43.6%), 15 incidences of increased ALT levels (38.5%), and 14 incidences of increased AST levels (35.9%). Among the 102 hematological adverse events, 39 incidences of leukopenia (100%), 36 incidences of thrombocytopenia (92.3%), and 27 incidences of decreased hemoglobin levels (69.2%) were recorded.
Table II. Accuracy of the medication instruction sheet (MIS) for predicting the incidence of hematological and non-hematological adverse events of various grades in patients receiving cytarabine and idarubicin induction therapy.
ALT: Alanine aminotransferase; AST: aspartate aminotransferase. Numbers in parentheses represent the incidence rate. Adverse events were evaluated using the maximum grade recorded.
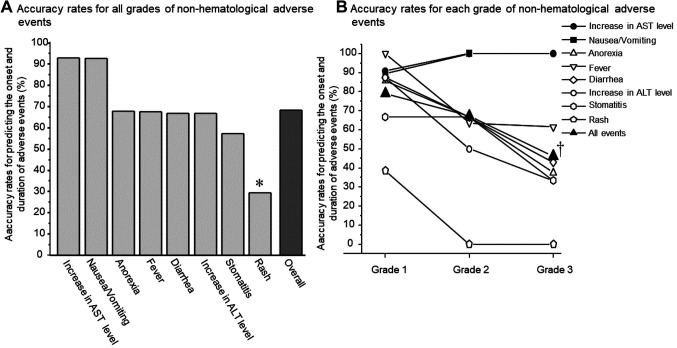
It is noteworthy that all of these adverse events were predictable items described in the MIS. Among the 192 non-hematological adverse events, 131 occurred during the same period as that anticipated by the MIS, resulting in a 68.2% (95%CI=61.1-74.7) prediction accuracy. As shown in Figure 2A, the accuracy rate of the MIS for predicting the occurrence and duration of adverse events was highest for increased AST levels (92.9%, 95%CI=63.9-99.99) and nausea and vomiting (92.6%, 95%CI=75.7-99.1). The MIS had a moderate accuracy rate for anorexia (67.7%, 95%CI=48.6-83.3), fever (67.6%, 95%CI=49.3-82.9), diarrhea (66.7%, 95%CI=48.2-82.0), increased ALT levels (66.7%, 95%CI=38.4-88.2), and stomatitis (57.1%, 95%CI=34.0-78.2), whereas the accuracy rate for rashes was the lowest (29.4%, 95%CI=10.3-56.2) among the various non-hematological adverse events. Among the 12 incidences of rash that appeared outside the duration range described in the MIS, the onset was delayed in five cases, whereas the symptoms persisted beyond the predicted recovery day in other seven cases. Interestingly, the accuracy rate for predicting the onset and duration of rashes tended to be higher, though not significantly, in patients without pre-existing leukopenia than in those with pre-existing leukopenia (11.1%, 95%CI=0.3-48.2 versus 50.0%, 95%CI=15.7-84.3, p=0.221 using the chi-square test). Moreover, the incidence rate and grade of the rash tended to be lower in patients without pre-existing leukopenia than in those with pre-existing leukopenia (incidence rate, 32.0% versus 64.3%, p=0.107 using the chi-square test; average rash grade, 0.4 versus 0.9, p=0.192 using the Kruskal-Wallis test; data not shown).
Figure 2. Comparison of the accuracy rates for predicting the onset and duration of various non-hematological adverse events. The accuracy rate was compared for each adverse event of any grade (A) and for the different adverse event grades (B). In (A), the accuracy rate for rashes was significantly lower than that for increased AST levels and nausea/vomiting. AST: Αspartate aminotransferase; ALT: alanine aminotransferase. *p<0.05 using the Kruskal-Wallis test followed by Scheffe’s test. In (B), significant differences in the accuracy rate were found according to the grade of the total adverse events. †p<0.01 vs. Grade 1 using the Kruskal-Wallis test followed by Scheffe’s test. However, the accuracy rate was not significantly different among the grades of individual adverse events.
The accuracy rate for the total adverse events decreased as the grade of the adverse events increased (p<0.01 using the Kruskal-Wallis test); however, the accuracy rate was not significantly different among the different grades of individual adverse events (Figure 2B).
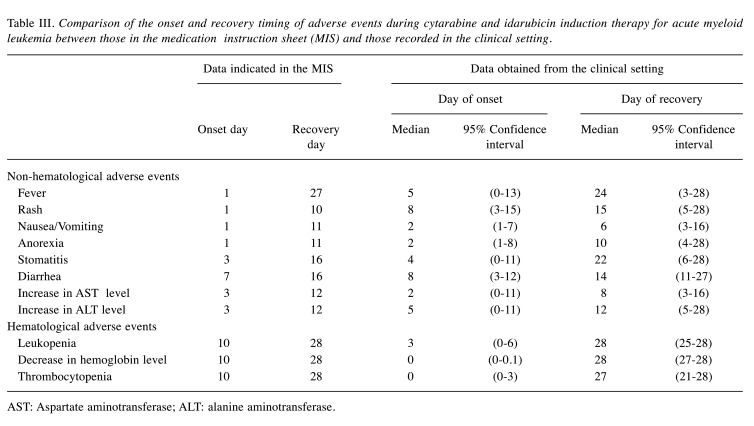
Among the 102 hematological events, however, 98 appeared much earlier than expected, indicating an accuracy rate of only 3.9% (95%CI=1.1-9.7) (Table II). Table III shows a comparison of the onset and recovery timing for hematological and non-hematological adverse events between the MIS and clinical data. The median day of onset for the non-hematological adverse events observed in the clinical setting was generally later than that described in the MIS. However, hematological adverse events appeared earlier (day 0 to day 3) in the clinical setting than in the MIS (each, day 10). On the other hand, the timing of recovery was generally comparable between the MIS and clinical setting, although the incidences of rash (MIS, 10 days versus clinical data, 15 days) and stomatitis (MIS, 16 days versus clinical data, 22 days) persisted beyond the predicted recovery day.
Table III. Comparison of the onset and recovery timing of adverse events during cytarabine and idarubicin induction therapy for acute myeloid leukemia between those in the medication instruction sheet (MIS) and those recorded in the clinical setting.
AST: Aspartate aminotransferase; ALT: alanine aminotransferase.
Discussion
In the present study, we evaluated the accuracy of our newly developed MIS for predicting adverse events and their onset and duration in patients with AML receiving cytarabine and idarubicin induction therapy. Various adverse events occurred, among which, the predominant hematological adverse events were leukopenia (100%) and thrombocytopenia (92.3%), and the predominant non-hematological events were fever (87.2%), diarrhea (84.6%), and anorexia (79.4%). Notably, the adverse events observed in the present study were all predicted items described in the MIS.
However, our MIS could not be used to predict the onset of decreased hemoglobin levels, leukopenia, or thrombocytopenia. This is likely due to the patients’ bone marrow failure at induction therapy (decreased hemoglobin levels, 94.9%; thrombocytopenia, 87.2%; and leukopenia, 35.9%). Such disease-based bone marrow failure is caused by a massive increase in leukemia cells, which inhibits the production of normal blood cells and platelets. Most patients with AML have pancytopenia, weakness, fatigue, infections, and other hemorrhagic findings as a result of a reduction in the capacity of stem cells to differentiate into mature cells due to the clonal proliferation of leukemia cells (2,18). Mucosal bleeding, ulcerations, and petechiae are the most commonly observed oral manifestations of leukemia (19). Therefore, monitoring chemotherapy-associated myelosuppression is complicated by the preexistence of disease-specific bone marrow failure. Myelosuppression was thus excluded from our MIS, and our evaluation of the accuracy of the MIS was restricted to the onset and duration of non-hematological adverse events.
In the present study, 192 non-hematological adverse events (any grade) were observed, among which 131 occurred with a similar onset and duration as that predicted by the MIS, indicating an accuracy rate of 68.2% (95%CI=61.1-74.7). This value is generally consistent with that reported previously for patients with Non-Hodgkin’s lymphoma treated with rituximab, etoposide, methylprednisolone, cisplatin, and cytarabine therapy (61%) (15) and rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy (71%) (16).
Among the various non-hematological adverse events, the onset and duration of increased AST levels (accuracy rate, 92.9%) and nausea/vomiting (92.6%) were highly predictable using our MIS. However, our MIS had the lowest accuracy rate for predicting the incidence of rash (29.4%). The median onset of rash (day 8) was much later than that predicted by the MIS (day 1) and the duration was longer than predicted (15 days versus 10 days). When patients were divided into those with pre-existing leukopenia and those without pre-existing leukopenia, the accuracy rate for predicting the onset and duration of rashes tended to be higher in the group without pre-existing leukopenia (50.0% versus 11.1%). In addition, the incidence rate and grade of the rash tended to be lower in the group without pre-existing leukopenia than in the group with pre-existing leukopenia (incidence, 32.0% versus 64.3%; average grade, 0.4 versus 0.9). Yemisen et al. reported that skin lesions such as maculopapular eruption and drug-induced eruption are frequent adverse events that occur in patients with hematological malignancies, including AML (20). They also showed that the most common cause of such skin lesions is infections associated with neutropenia (20). Therefore, it seems likely that the enhancement of the incidence rate and the degree of severity of rash observed in patients with pre-existed leukopenia is due to the increased susceptibility to infection associated with immunosuppression. Taken together, it is suggested that monitoring for rashes earlier may be necessary for patients with pre-existing leukopenia.
Overall, our MIS had good accuracy for predicting the onset and duration of non-hematological adverse events in patients with AML undergoing cytarabine and idarubicin induction therapy. As serious adverse events associated with chemotherapy can lead to a decline in a patient’s quality of life and/or death, patients are understandably anxious about when and which adverse events may occur. Patients with AML undergoing intensive chemotherapy may additionally be anxious about treatment efficacy.
In this regard, our MIS appears to be useful for monitoring and managing adverse events in patients with AML receiving cytarabine and idarubicin induction chemotherapy. In addition, the MIS was prepared in the form of a clinical pathway, making it visually comprehensible to not only cancer patients but also to healthcare professionals, including pharmacists (7,13). Thus, this MIS could enable healthcare professionals to identify adverse events in an accurate and timely manner regardless of whether they have sufficient practical experience.
Limitations
Our MIS could not be used to accurately monitor hematological adverse events because of the presence of bone marrow failure associated with AML. In addition, this study included a small number of patients from a single institution, and the data were analyzed retrospectively. A larger multicenter study is needed to further confirm the accuracy of our MIS.
Conclusion
We compared our MIS data with the clinical data of patients with AML receiving cytarabine and idarubicin induction therapy. A total of 192 non-hematological adverse events (any grade) were observed, all of which were predicted by our MIS. Among them, the onset and duration of 131 of the events were accurately predicted by the MIS, indicating an accuracy rate of 68.2% (95%CI=61.1-74.7). However, evidence of myelosuppression, such as decreased hemoglobin levels, leukopenia, and thrombocytopenia, occurred much earlier than expected. These findings suggest that our MIS is useful for rapidly monitoring adverse events in patients with AML receiving cytarabine and idarubicin induction therapy.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Mayako Uchida: Conceptualization, methodology, data collection, formal analysis, and writing – original draft preparation. Erika Mochizuki, Shigeru Ishida, Nana Ozawa, Hiroko Yonemitsu, Hideki Ochiai, and Hanae Nakamura: Data collection. Takehiro Kawashiri, Koji Kato, and Nobuaki Egashira: Writing – review and editing. Koichi Akashi and Ichiro Ieiri: Writing – review and editing, supervision.
Acknowledgements
The Authors are grateful to Professor Ryozo Oishi, Professor Emeritus at Kyushu University (Fukuoka, Japan); Professor Yoshinori Itoh, Professor Emeritus at Gifu University (Gifu, Japan); and Dr. Hiroaki Ikesue at the Department of Pharmacy, Kobe City Medical Center General Hospital (Kobe, Japan).
References
- 1.Zeidan AM, Podoltsev NA, Wang X, Zhang C, Bewersdorf JP, Shallis RM, Huntington SF, Neparidze N, Giri S, Gore SD, Davidoff AJ, Ma X, Wang R. Patterns of care and clinical outcomes with cytarabine-anthracycline induction chemotherapy for AML patients in the United States. Blood Adv. 2020;4(8):1615–1623. doi: 10.1182/bloodadvances.2020001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, Ebert BL, Fenaux P, Godley LA, Hasserjian RP, Larson RA, Levine RL, Miyazaki Y, Niederwieser D, Ossenkoppele G, Röllig C, Sierra J, Stein EM, Tallman MS, Tien HF, Wang J, Wierzbowska A, Löwenberg B. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–1377. doi: 10.1182/blood2022016867. [DOI] [PubMed] [Google Scholar]
- 3.NCCN.org: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Acute Myeloid Leukemia, National Comprehensive Cancer Network, Version 2.2022 - June 14, 2022. Available at: https://www.nccn.org/guidelines/guidelinesdetail?category=1&id=1411. [Last accessed on January 18, 2023]
- 4.Ohtake S, Miyawaki S, Fujita H, Kiyoi H, Shinagawa K, Usui N, Okumura H, Miyamura K, Nakaseko C, Miyazaki Y, Fujieda A, Nagai T, Yamane T, Taniwaki M, Takahashi M, Yagasaki F, Kimura Y, Asou N, Sakamaki H, Handa H, Honda S, Ohnishi K, Naoe T, Ohno R. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSG AML201 Study. Blood. 2011;117(8):2358–2365. doi: 10.1182/blood-2010-03-273243. [DOI] [PubMed] [Google Scholar]
- 5.Reville PK, Kadia TM. Maintenance therapy in AML. Front Oncol. 2021;10:619085. doi: 10.3389/fonc.2020.619085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S, Cho BS, Kim HJ. New agents in acute myeloid leukemia (AML) Blood Res. 2020;55(S1):S14–S18. doi: 10.5045/br.2020.S003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchida M, Nakamura T, Watanabe H, Kato K, Miyamoto T, Akashi K, Masuda S. Usefulness of medication instruction sheets for sharing information on cancer chemotherapy within the health care team. Pharmazie. 2019;74(9):566–569. doi: 10.1691/ph.2019.9467. [DOI] [PubMed] [Google Scholar]
- 8.Patel H, Gurumurthy P. Implementation and evaluation of medicine and therapeutic information service by clinical pharmacists in oncology care setting. J Oncol Pharm Pract. 2019;25(1):60–67. doi: 10.1177/1078155217727141. [DOI] [PubMed] [Google Scholar]
- 9.Boşnak AS, Birand N, Diker Ö, Abdi A, Başgut B. The role of the pharmacist in the multidisciplinary approach to the prevention and resolution of drug-related problems in cancer chemotherapy. J Oncol Pharm Pract. 2019;25(6):1312–1320. doi: 10.1177/1078155218786048. [DOI] [PubMed] [Google Scholar]
- 10.Fornasier G, Taborelli M, Francescon S, Polesel J, Aliberti M, De Paoli P, Baldo P. Targeted therapies and adverse drug reactions in oncology: the role of clinical pharmacist in pharmacovigilance. Int J Clin Pharm. 2018;40(4):795–802. doi: 10.1007/s11096-018-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aziz MT, Rehman TU, Qureshi S, Andleeb S. Effects of multidisciplinary teams and an integrated follow-up electronic system on clinical pharmacist interventions in a cancer hospital. Int J Clin Pharm. 2017;39(6):1175–1184. doi: 10.1007/s11096-017-0530-7. [DOI] [PubMed] [Google Scholar]
- 12.Holle LM, Harris CS, Chan A, Fahrenbruch RJ, Labdi BA, Mohs JE, Norris LB, Perkins J, Vela CM. Pharmacists’ roles in oncology pharmacy services: Results of a global survey. J Oncol Pharm Pract. 2017;23(3):185–194. doi: 10.1177/1078155216629827. [DOI] [PubMed] [Google Scholar]
- 13.Ikesue H, Ishida M, Uchida M, Harada M, Haro T, Mishima K, Itoh Y, Kotsubo K, Yoshikawa M, Oishi R. Monitoring for potential adverse drug reactions in patients receiving chemotherapy. Am J Health Syst Pharm. 2004;61(22):2366,2368–2366,2369. doi: 10.1093/ajhp/61.22.2366. [DOI] [PubMed] [Google Scholar]
- 14.Makieda D, Hisaeda S, Kinoshita H, Uchida M, Ikesue H, Mishima K, Watanabe H, Sueyasu M, Miyamoto T, Egashira N, Oishi R. Investigation of adverse drug reactions in bortezomib therapy for relapsed multiple myeloma. Iryo Yakugaku (Japanese Journal of Pharmaceutical Health Care and Sciences) 2021;36(4):270–276. doi: 10.5649/jjphcs.36.270. [DOI] [Google Scholar]
- 15.Uchida M, Murata S, Morikawa H, Yonemitsu H, Ishida S, Suetsugu K, Tsuji T, Watanabe H, Kawashiri T, Kato K, Hosohata K, Miyamoto T, Egashira N, Nakamura T, Akashi K, Ieiri I. Usefulness of medication guidance sheets for patients with non-Hodgkin’s lymphoma receiving ESHAP±R therapy. Anticancer Res. 2022;42(4):2053–2060. doi: 10.21873/anticanres.15686. [DOI] [PubMed] [Google Scholar]
- 16.Uchida M, Kawai R, Hisamitsu R, Mai S, Ishida S, Watanabe H, Kawashiri T, Kato K, Hosohata K, Miyamoto T, Egashira N, Nakamura T, Akashi K, Ieiri I. Evaluation of medication instruction sheets for patients undergoing R-CHOP therapy in non-Hodgkin’s lymphoma. In Vivo. 2022;36(3):1461–1467. doi: 10.21873/invivo.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rumsey DJ. How to determine the confidence interval for a population proportion. Available at: https://www.dummies.com/education/math/statistics/how-to-determine-the-confidenceinterval-for-a-population-proportion/ [Last accessed on January 11, 2021]
- 18.Rowe JM. Perspectives on current survival and new developments in AML. Best Pract Res Clin Haematol. 2021;34(1):101248. doi: 10.1016/j.beha.2021.101248. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Fantasia JE, Kaplan R. Oral manifestations of acute myelomonocytic leukemia: a case report and review of the classification of leukemias. J Periodontol. 2002;73(6):664–668. doi: 10.1902/jop.2002.73.6.664. [DOI] [PubMed] [Google Scholar]
- 20.Yemisen M, Vatan A, Balkan I, Salihoglu A, Elverdi T, Eskazan E, Mete B, Ar MC, Ongoren S, Baslar Z. 466 Skin lesions in febrile neutropenic patients. Open Forum Infectious Diseases, Oxford University Press. 2014:pp. S174–S175. doi: 10.1093/ofid/ofu052.332. [DOI] [Google Scholar]