Abstract
Background/Aim: The inflammatory response plays an important role in the activation and progression of many inflammation-related diseases. Cannabis sativa and Morinda citrifolia have long been used in folk medicine to treat inflammation. Cannabidiol is the most abundant non-psychoactive phytocannabinoid in C. sativa and exhibits anti-inflammatory activity. The objective of this study was to examine the anti-inflammatory effect of cannabidiol in combination with M. citrifolia and compare its effects with those of cannabidiol alone.
Materials and Methods: RAW264 cells stimulated with lipopolysaccharide (200 ng/ml) were treated with cannabidiol (0-10 μM), M. citrifolia seed extract (0-100 μg/ml), or a combination of both for 8 or 24 h. Following the treatments, nitric oxide production in the activated RAW264 cells and the expression of inducible nitric oxide synthase were assessed.
Results: Our results showed that combination of cannabidiol (2.5 μM) and M. citrifolia seed extract (100 μg/ml) exhibited more efficient inhibition of nitric oxide production than cannabidiol treatment alone in lipopolysaccharide-stimulated RAW264 cells. The combination treatment also reduced the expression of inducible nitric oxide synthase.
Conclusion: These results suggest that the anti-inflammatory effect of combined treatment with cannabidiol and M. citrifolia seed extract causes a reduction in the expression of inflammatory mediators.
Keywords: Cannabidiol, Morinda citrifolia, nitric oxide, RAW264 cells, anti-inflammatory
Inflammation occurs in response to injury and plays an important role in removing harmful stimuli (1). It is related to the progression of diseases, including pancreatitis, and cardiovascular and lung inflammatory diseases (2). The inhibition of the inflammatory response has become a therapeutic strategy for the treatment of these diseases.
Macrophages are important for the pathological process of inflammation. RAW264 cells, a murine macrophage-like cell line, stimulated by lipopolysaccharide (LPS), have frequently been used to investigate macrophage activation in in vitro models (3). Activated macrophages produce pro-inflammatory mediators such as interleukin-6 and nitric oxide (NO) (4). Traditional medicine continues to use medicinal plants as a substitute for modern medicines (5). It has been considered that plant-derived natural products or extracts include immunomodulators (6,7).
Cannabis sativa L., commonly known as marijuana, and Morinda citrifolia L., known as noni, have been used as folk remedies by many cultures for centuries. The anti-inflammatory and antioxidant effects of natural products derived from plants have been extensively studied (8,9). C. sativa plant contains more than 500 natural compounds and over 100 phytocannabinoids (10). Cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC) are the two most abundant cannabinoids present in cannabis. Although THC exhibits anti-inflammatory effects, the application of THC in clinical therapy has been limited mainly because of its psychotropic effects. In contrast, CBD exerts anti-inflammatory and antioxidant effects without causing any psychotropic effects (11).
M. citrifolia has been used in the treatment of many diseases, including diabetes, cancer, and hypertension (12-14). M. citrifolia seed extract (MCS-ext) has been reported to have human leukocyte elastase-inhibitory, tyrosinase-inhibitory, antioxidant, and anti-inflammatory properties (15,16).
In this study, we evaluated the anti-inflammatory activity of combination treatment with CBD and MCS-ext, in comparison with CBD alone, in lipopolysaccharide (LPS)-stimulated RAW264 cells.
Materials and Methods
Cell culture experiment. RAW264 cell line was obtained from RIKEN BRC (Ibaraki, Japan). This cell line was cultured in Eagle’s minimum essential medium with L-glutamine and phenol red supplemented with 10% fetal bovine serum (Biowest, Nuaille, France), streptomycin (100 μg/ml), penicillin (100 U/ml), and 1% minimum essential medium with non-essential amino acids (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The experiments were performed using a modified version of previously described protocols (15,17). Briefly, RAW264 cells were plated in Dulbecco’s modified Eagle’s medium (high glucose) without L-glutamine and phenol red containing 0.5% fetal bovine serum, 2 mM L-glutamine (FUJIFILM Wako Pure Chemical Corporation), streptomycin (100 μg/ml), and penicillin (100 U/ml) and cultured overnight at 37˚C in a humidified atmosphere with 5% CO2. MCS-ext (Oryza Oil & Fat Chemical Co., Ltd., Aichi, Japan) was added to the medium 0.5 h before CBD (2.5 μM) (Cayman Chemical, Ann Arbor, MI, USA) and LPS from Escherichia coli O111 (200 ng/ml) (FUJIFILM Wako Pure Chemical Corporation) addition. The final concentration of MCS-ext in samples incubated with LPS was 0-100 μg/ml. After 24 h treatment with the mixture, NO accumulation in the cell culture medium was measured using the Griess method. The absorbance at 550 nm was measured with a microplate reader. The cell viability was assessed by Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan). The absorbance was measured at 450 nm using a microplate reader. The final concentration of dimethyl sulfoxide was adjusted to be less than 0.15%.
RNA isolation and real-time reverse transcriptase–polymerase chain reaction. MCS-ext (100 μg/ml) was added to the medium 0.5 h before CBD (2.5 μM) and LPS (200 ng/ml) addition and the cells were incubated with the mixture for 8 h. The RNA from the cells was then isolated using Isogen II (Nippon Gene Co., Ltd., Tokyo, Japan). The RNA was reverse-transcribed into cDNA with FastGene Scriptase II cDNA Synthesis (NIPPON Genetics Co., Ltd.). Real-time reverse transcriptase–polymerase chain reaction was performed in a Thermal Cycler Dice Real-Time System (Takara Bio Inc., Shiga, Japan) with EvaGreen (Biotium, Inc., Fremont, CA, USA) and Taq DNA Polymerase (New England BioLabs, Ipswich, MA, USA). The primers were as followed: Mouse β-actin (Actb): sense and antisense, 5’-CGG TTC CGA TGC CCT GAG GCT CTT-3’ and 5’-CGT CAC ACT TCA TGA TGG AAT TGA-3’; mouse inducible nitric oxide synthase (Nos2) sense and antisense, 5’-TGG AGC CAG TTG TGG ATT GTC-3’ and 5’-GGT CGT AAT GTC CAG GAA GTA G-3’. The 2−ΔΔCt method was used to normalize the relative mRNA expression levels to that of Actb.
Statistical analysis. Analysis of variance followed by Dunnett’s multiple comparison test was carried out. The significance of differences was analyzed using Student’s t-test. In all cases, statistical significance was accepted at p<0.05.
Results
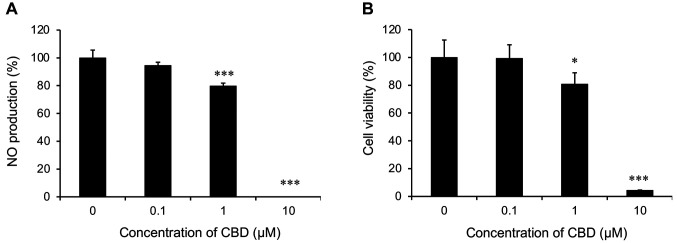
Effect of CBD on NO production. Firstly, we examined whether CBD treatment inhibited NO production in activated (LPS-stimulated) RAW264 cells. RAW264 cells were treated with CBD and LPS for 24 h, and NO production was measured using the Griess reagent system. CBD suppressed NO production at concentrations ranging from 1 to 10 μM and dose-dependently reduced LPS-induced NO production (Figure 1A). We also examined whether CBD treatment of LPS-stimulated RAW264 cells induced cytotoxic effects. Treatment with CBD reduced the viability of LPS-stimulated RAW264 cells as its concentration increased (Figure 1B). Therefore, the inhibitory effect of CBD on NO production may be related to cell viability. These results suggest that it is not appropriate to simply increase CBD content to expect the inhibitory effect on NO production.
Figure 1. Effect of cannabidiol (CBD) on nitric oxide production and cell viability of lipopolysaccharide (LPS)-stimulated RAW 264 cells. Cells were incubated with the indicated concentrations of CBD and 200 ng/ml LPS for 24 h. The final concentration of the samples incubated with LPS is indicated. A: The level of nitric oxide (NO) in the culture medium was measured using the Griess reaction. B: Cell viability was measured using a Cell Counting Kit-8. Data are presented as the mean±standard deviation (n=3-4). Significantly different at: *p<0.05 and ***p<0.001 compared to samples without CBD.
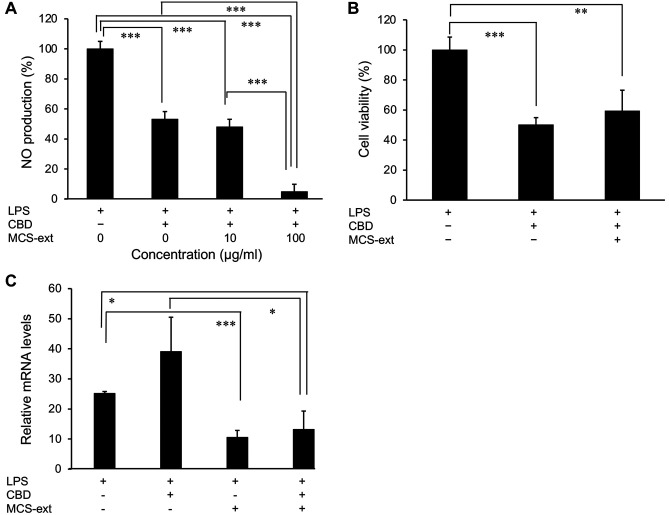
Effect of CBD in combination with MCS-ext on NO production. To examine the anti-inflammatory effect of CBD in combination with natural product extracts, we used M. citrifolia seed extract (MCS-ext). Various natural extracts have been reported to exert anti-inflammatory effects. We have previously reported the anti-inflammatory effect of MCS-ext in LPS-stimulated RAW264 cells in vitro (15). Noni juice has also been reported to exhibit anti-inflammatory and antioxidative effects and reduce carrageenan-induced paw edema (18) and lung inflammation sensitized by ovalbumin (19) in animal models of inflammation. MCS-ext (0-100 μg/ml) was added to the medium and cells were incubated for 0.5 h. We next added LPS and CBD to the medium and NO production was measured after 24 h. We found that the combination of CBD and MCS-ext led to a greater reduction than CBD alone (Figure 2A). However, the cell viability did not decrease when LPS- and CBD-stimulated RAW264 cells were treated with 100 μg/ml MCS-ext (Figure 2B). These results indicated that the addition of MCS-ext beforehand can be expected to have a higher inhibitory effect on NO production than the addition of CBD alone, without an increase in cytotoxicity.
Figure 2. Inhibitory effect of combination of Morinda citrifolia seed extract (MCS-ext) and cannabidiol (CBD) in lipopolysaccharide (LPS)- stimulated RAW 264 cells. MCS-ext was added to cells 0.5 h before incubation with cannabidiol (CBD) (2.5 μM) and lipopolysaccharides (LPS) (200 ng/ml). The final concentration of MCS-ext in samples incubated with LPS is indicated. A: The level of nitric oxide (NO) in the culture medium was measured using the Griess reaction. B: Cell viability was measured using Cell Counting Kit-8 assay. C: Total RNA was isolated using Isogen II after LPS stimulation. Inducible nitric oxide synthase (Nos2) messenger RNA (mRNA) expression level was analyzed by real-time reverse transcriptase-polymerase chain reaction. Relative mRNA levels were determined using 2−ΔΔCt method and normalized to Actb expression. The relative mRNA level of the nontreated samples was set to 1. Data are presented as the mean±SD (n=3-4). Significantly different at: *p<0.05, **p<0.01, and ***p<0.001 as determined by Student’s t-test except for comparisons with CBD alone and MCS-ext alone.
Additionally, we examined whether combination treatment with CBD and MCS-ext modulates the expression of inducible nitric oxide synthase, and whether this is related to NO production in LPS-stimulated RAW264 cells. Activated macrophages produce pro-inflammatory mediators, such as inducible nitric oxide synthase, which synthesizes NO using L-arginine as a substrate (4,20). MCS-ext (100 μg/ml) was added to cells 0.5 h before incubation with CBD and LPS for 8 h. The expression level of Nos2 messenger RNA in LPS-induced cells was enhanced compared to that of non-treated cells. However, MCS-ext alone and the combination of CBD and MCS-ext inhibited expression of Nos2 mRNA of LPS-stimulated RAW264 cells (Figure 2C). CBD treatment alone caused no significant change in Nos2 mRNA expression in LPS-stimulated.
Discussion
Recently, foods, drinks and creams containing CBD have become widely available around the world. CBD is used for its relaxant, antioxidant, and anti-inflammatory effects. In this study, we focused on the anti-inflammatory effects and suggested the potential significance of a combination of CBD with other materials. Our results demonstrate that CBD and MCS-ext appear to exhibit an anti-inflammatory effect in LPS-stimulated RAW264 cells.
Previous studies have found that CBD induced apoptosis of HL-60 cells, human myeloblastic leukemia cells (at 1-8 μg/ml or 3.226 μM) (21), primary human monocytic cells (at 4-16 μM) (22,23), and human and murine leukemia cells (at 1.25-10 μM) (24). In our study, CBD reduced cell viability at 1-10 μM (Figure 1B). These results indicate that a high concentration of CBD alone potentially has not only an anti-inflammatory effect but also causes cell damage.
To maintain the anti-inflammatory effect of CBD with low cytotoxicity, we evaluated the anti-inflammatory effect of MCS-ext in combination with a low concentration of CBD in LPS-stimulated RAW264 cells. The combination of CBD and MCS-ext (100 μg/ml) inhibited NO production in LPS-stimulated RAW264 cells more than CBD alone (Figure 2A). The combination of CBD and MCS-ext did not reduce cell viability compared to treatment with CBD alone (Figure 2B). However, the combination of CBD and MCS-ext treatment significantly reduced NO production compared to CBD treatment. Thus, the significant anti-inflammatory effect in LPS-stimulated RAW264 cells treated with the combination of CBD and MCS-ext is unrelated to the reduction in cell viability. MCS-ext alone and in combination with CBD suppressed Nos2 expression in LPS-stimulated RAW264 cells (Figure 2C). The detailed mechanism of inhibition of NO production caused by the combination of MCS-ext and CBD is unclear. Inducible nitric oxide synthase is known to be regulated by nuclear factor-ĸB (NF-ĸB) and becomes active after degradation of the inhibitory protein, I-ĸB (25,26). It has been reported that compounds from Hawaiian noni fruit juice suppress NO production through the inhibition of the IKKα/β, I-ĸBα, and NF-ĸB p65 signaling pathways in LPS-stimulated macrophages (27). Therefore, MCS-ext might reduce NO production through the inhibition of the NF-ĸB signaling pathway. CBD treatment did not lead to a significant decrease in inducible nitric oxide synthase expression in LPS-stimulated macrophages compared to dimethyl sulfoxide treatment (28). CBD has potential antioxidant activity because cation free radicals have several resonance structures in which unpaired electrons are mainly distributed on the ether and alkyl moieties, as well as the benzene ring (29). The decrease in NO may be related to the scavenging capacity of CBD. In the present study, we measured only NO production as a pro-inflammatory mediator to examine the anti-inflammatory effect of the combination of CBD and MCS-ext in LPS-stimulated RAW264 cells. Activated macrophages secret various cytokines such as tumor necrosis factor, and interleukins 1 and 6 (30). It is important to further investigate the inhibitory effects of CBD and MCS-ext combination on other pro-inflammatory mediators in LPS-stimulated macrophages. Future studies are necessary to elucidate the molecular mechanism underlying the anti-inflammatory effects of CBD and MCS-ext combination treatment in LPS-stimulated macrophages. Furthermore, in general, natural products, including M. citrifolia L. may be affected by several environmental and genetic factors including the area and variety cultivated. Because our studies performed only one set of MCS-ext samples in this study, further studies are required. Finally, the combination of CBD and MCS-ext might be a new, potential anti-inflammatory treatment option against inflammation-related disorders.
Conflicts of Interest
The Authors have no conflicts of interest to declare.
Authors’ Contributions
Conceptualization and supervision, T.T.; Methodology, T.T., M.K., Y.H., N.T., A.U., F.I., T.Y. and Y.I.; Formal analysis, T.T. and M.K.; writing—original draft preparation, T.T.; writing—review and editing, T.T., M.K., Y.H., N.T., A.U., F.I., T.Y. and Y.I. All Authors have read and agreed to the published version of the article.
References
- 1.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147(2):227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakanishi-Matsui M, Yano S, Matsumoto N, Futai M. Lipopolysaccharide induces multinuclear cell from RAW264.7 line with increased phagocytosis activity. Biochem Biophys Res Commun. 2012;425(2):144–149. doi: 10.1016/j.bbrc.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 4.Guo Q, Bi D, Wu M, Yu B, Hu L, Liu C, Gu L, Zhu H, Lei A, Xu X, Wang J. Immune activation of murine RAW264.7 macrophages by sonicated and alkalized paramylon from Euglena gracilis. BMC Microbiol. 2020;20(1):171. doi: 10.1186/s12866-020-01782-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinheiro MM, Fernandes SB, Fingolo CE, Boylan F, Fernandes PD. Anti-inflammatory activity of ethanol extract and fractions from Couroupita guianensis Aublet leaves. J Ethnopharmacol. 2013;146(1):324–330. doi: 10.1016/j.jep.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 6.Jantan I, Ahmad W, Bukhari SN. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front Plant Sci. 2015;6:655. doi: 10.3389/fpls.2015.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kure C, Timmer J, Stough C. The immunomodulatory effects of plant extracts and plant secondary metabolites on chronic neuroinflammation and cognitive aging: a mechanistic and empirical review. Front Pharmacol. 2017;8:117. doi: 10.3389/fphar.2017.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautam R, Jachak SM. Recent developments in anti-inflammatory natural products. Med Res Rev. 2009;29(5):767–820. doi: 10.1002/med.20156. [DOI] [PubMed] [Google Scholar]
- 9.Abourashed EA. Bioavailability of plant-derived antioxidants. Antioxidants (Basel) 2013;2(4):309–325. doi: 10.3390/antiox2040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33(12):1357–1392. doi: 10.1039/c6np00074f. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa K, Mishima K, Fujiwara M. Therapeutic potential of non-psychotropic cannabidiol in ischemic stroke. Pharmaceuticals (Basel) 2010;3(7):2197–2212. doi: 10.3390/ph3072197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potterat O, Hamburger M. Morinda citrifolia (Noni) fruit—phytochemistry, pharmacology, safety. Planta Med. 2007;73(3):191–199. doi: 10.1055/s-2007-967115. [DOI] [PubMed] [Google Scholar]
- 13.Samoylenko V, Zhao J, Dunbar DC, Khan IA, Rushing JW, Muhammad I. New constituents from noni (Morinda citrifolia) fruit juice. J Agric Food Chem. 2006;54(17):6398–6402. doi: 10.1021/jf060672u. [DOI] [PubMed] [Google Scholar]
- 14.Wang MY, West BJ, Jensen CJ, Nowicki D, Su C, Palu AK, Anderson G. Morinda citrifolia (Noni): a literature review and recent advances in Noni research. Acta Pharmacol Sin. 2002;23(12):1127–1141. [PubMed] [Google Scholar]
- 15.Tanikawa T, Kitamura M, Hayashi Y, Tomida N, Uwaya A, Isami F, Inoue Y. Anti-inflammatory effects of Morinda citrifolia extract against lipopolysaccharide-induced inflammation in RAW264 cells. Medicines (Basel) 2021;8(8):43. doi: 10.3390/medicines8080043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuda M, Murata K, Fukuhama A, Naruto S, Fujita T, Uwaya A, Isami F, Matsuda H. Inhibitory effects of constituents of Morinda citrifolia seeds on elastase and tyrosinase. J Nat Med. 2009;63(3):267–273. doi: 10.1007/s11418-009-0328-6. [DOI] [PubMed] [Google Scholar]
- 17.Tanikawa T, Kitamura M, Hayashi Y, Yokogawa T, Inoue Y. Curcumae Longae Rhizoma and Saussureae Radix inhibit nitric oxide production and cannabinoid receptor 2 down-regulation. In Vivo. 2022;36(1):227–232. doi: 10.21873/invivo.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dussossoy E, Brat P, Bony E, Boudard F, Poucheret P, Mertz C, Giaimis J, Michel A. Characterization, anti-oxidative and anti-inflammatory effects of Costa Rican noni juice (Morinda citrifolia L.) J Ethnopharmacol. 2011;133(1):108–115. doi: 10.1016/j.jep.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 19.Dussossoy E, Bichon F, Bony E, Portet K, Brat P, Vaillant F, Michel A, Poucheret P. Pulmonary anti-inflammatory effects and spasmolytic properties of Costa Rican noni juice (Morinda citrifolia L.) J Ethnopharmacol. 2016;192:264–272. doi: 10.1016/j.jep.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 20.Pekarova M, Lojek A, Martiskova H, Vasicek O, Bino L, Klinke A, Lau D, Kuchta R, Kadlec J, Vrba R, Kubala L. New role for L-arginine in regulation of inducible nitric-oxide-synthase-derived superoxide anion production in raw 264.7 macrophages. ScientificWorldJournal. 2011;11:2443–2457. doi: 10.1100/2011/321979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols JM, Kaplan BLF. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res. 2020;5(1):12–31. doi: 10.1089/can.2018.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu HY, Chang AC, Wang CC, Kuo FH, Lee CY, Liu DZ, Jan TR. Cannabidiol induced a contrasting pro-apoptotic effect between freshly isolated and precultured human monocytes. Toxicol Appl Pharmacol. 2010;246(3):141–147. doi: 10.1016/j.taap.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Wu HY, Huang CH, Lin YH, Wang CC, Jan TR. Cannabidiol induced apoptosis in human monocytes through mitochondrial permeability transition pore-mediated ROS production. Free Radic Biol Med. 2018;124:311–318. doi: 10.1016/j.freeradbiomed.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 24.McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS, Nagarkatti M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol. 2006;70(3):897–908. doi: 10.1124/mol.106.023937. [DOI] [PubMed] [Google Scholar]
- 25.Kolyada AY, Savikovsky N, Madias NE. Transcriptional regulation of the human iNOS gene in vascular-smooth-muscle cells and macrophages: evidence for tissue specificity. Biochem Biophys Res Commun. 1996;220(3):600–605. doi: 10.1006/bbrc.1996.0449. [DOI] [PubMed] [Google Scholar]
- 26.Morris KR, Lutz RD, Choi HS, Kamitani T, Chmura K, Chan ED. Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide. Infect Immun. 2003;71(3):1442–1452. doi: 10.1128/IAI.71.3.1442-1452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee D, Yu JS, Huang P, Qader M, Manavalan A, Wu X, Kim JC, Pang C, Cao S, Kang KS, Kim KH. Identification of anti-inflammatory compounds from Hawaiian Noni (Morinda citrifolia L.) fruit juice. Molecules. 2020;25(21):4968. doi: 10.3390/molecules25214968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajan TS, Giacoppo S, Iori R, De Nicola GR, Grassi G, Pollastro F, Bramanti P, Mazzon E. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia. 2016;112:104–115. doi: 10.1016/j.fitote.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Borges RS, Batista J Jr, Viana RB, Baetas AC, Orestes E, Andrade MA, Honório KM, da Silva AB. Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules. 2013;18(10):12663–12674. doi: 10.3390/molecules181012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]