Abstract
DR3 (Ws1, Apo3, LARD, TRAMP, TNFSFR12) is a member of the death domain-containing tumor necrosis factor receptor (TNFR) superfamily, members of which mediate a variety of developmental events including the regulation of cell proliferation, differentiation, and apoptosis. We have investigated the in vivo role(s) of DR3 by generating mice congenitally deficient in the expression of the DR3 gene. We show that negative selection and anti-CD3-induced apoptosis are significantly impaired in DR3-null mice. In contrast, both superantigen-induced negative selection and positive selection are normal. The pre-T-cell receptor-mediated checkpoint, which is dependent on TNFR signaling, is also unaffected in DR3-deficient mice. These data reveal a nonredundant in vivo role for this TNF receptor family member in the removal of self-reactive T cells in the thymus.
The tumor necrosis factor receptor (TNFR) superfamily comprise a growing family of type I membrane bound glycoproteins which interact with the TNF family of soluble mediators and type II transmembrane proteins. At least 23 TNFR superfamily members and 17 known ligands have been identified in mammals (reviewed in references 3, 35, and 44). These receptors trigger pleiotropic responses, ranging from apoptosis and differentiation to proliferation, and have been implicated in immune regulation, host defense and lymphoid organ development.
Members of the TNFR family are characterized by the presence of varying numbers (three to six) of cysteine-rich repeats in their cytoplasmic domains (52). TNFRs are subdivided based on the presence or absence of a 70- to 80-amino-acid region of homology in the cytoplasmic region called the death domain, through which these receptors trigger apoptosis (20, 48). DR3 (also called Ws1, Apo3, TRAMP, LARD, TR3, and TNFRSF12) is one of six death domain-containing TNFR family members (the others are TNFR1, CD95/FAS, DR4, DR5, and DR6) and is the one most closely related to TNFR1. Studies on the TNFR1 crystal structure suggest that ligand binding or receptor overexpression results in receptor trimerization and recruitment of trimeric intracellular signaling molecules (4, 36). DR3, like TNFR1, recruits TNFR1-associated death domain protein (TRADD) and Fas-associated death domain-containing protein (FADD) (5, 6, 11, 12, 24) as downstream effectors of apoptosis. These, in turn, interact with caspase 8 (FLICE/MACH) (7, 31), and a cascade of interleukin-1β-converting enzyme-like cysteine proteases which trigger cell death (13, 17, 27). DR3 also recruits TRAF2 via TRADD (18, 24, 29, 39) and thus activates the transcription factor, NF-κB, that induces the transcription of a number of immune genes (19). In this respect, DR3 (like TNFR1) is capable of inducing both apoptosis and expression of survival/activation genes and is likely to have multiple functions depending on the context of its expression.
DR3 was first reported as the only death domain-containing TNFR family member with lymphoid organ-restricted expression (11, 24). More recent studies have, however, shown DR3 expression to be less restricted, though not as ubiquitous expression of as its other death domain-containing TNFR relatives (47, 51). DR3 expression patterns may be further complicated by the presence of at least 13 human (24, 42, 53) and 3 mouse (51) splice variants. DR3 splicing may be developmentally regulated since human peripheral blood leukocytes have been shown to express full-length DR3 mRNA only following activation (42). While the ligand for DR3 has been reported as TWEAK (30, 10), it has also been shown that TWEAK can bind and signal in a DR3-negative cell line via a TNF/TNFR1-related mechanism (40). Thus, the problems with identifying a single ligand together with the complex expression of DR3 have contributed to the lack of functional data for this gene. To date, the only ex vivo indication, albeit indirect, that DR3 has an apoptotic role stems from the detection of translocations affecting the DR3 gene in several neuroblastoma cell lines (16).
To determine the in vivo function(s) of DR3, we generated mice that are deficient in the expression of its murine homologue. DR3-deficient mice show no defects in organ development (lymphoid or otherwise), lymphoid proliferation, or apoptosis triggered by glucocorticoids or DNA-damaging agents. There is also no defect in the progression of double-negative (DN) thymocytes through the pre-T-cell receptor (TCR)-mediated checkpoint, a developmental transition at which TNFR signaling has been shown to be important (33). However, an impairment of negative selection, as well as anti-CD3-mediated cell death of thymocytes, is observed in DR3-deficient mice, suggesting a nonredundant role in TCR-induced apoptosis that establishes central tolerance in vivo.
MATERIALS AND METHODS
Generation of DR3−/− mice.
A human cDNA corresponding to the full-length DR3 gene was used as a probe to isolate clones from an EMBL3A phage library of 129/Sv mouse strain genomic DNA (51). A 6-kb SmaI-MfeI fragment covering the whole of the coding region of the DR3 gene was replaced with a cassette containing an internal ribosome entry site (IRES), lacZ poly(A), and neomycin resistance (neo) gene flanked by loxP sequences (a gift from Andrew Smith, University of Edinburgh, Edinburgh, United Kingdom). The DR3 gene targeting construct was linearized and transfected into GK129 embryonic stem (ES) cells by electroporation. ES cell clones were selected in G418-containing medium on a monolayer of mitotically inactivated STO feeder cells. Clones were picked and screened for homologous recombinants using the probes shown in Fig. 1A. ES clones with correct 5′ and 3′ recombinations were microinjected into C57BL/6 blastocysts and introduced into pseudopregnant C57BL/6 mice. Male chimeric offspring were bred to obtain germ line mutant mice which were screened either by Southern blotting or by PCR.
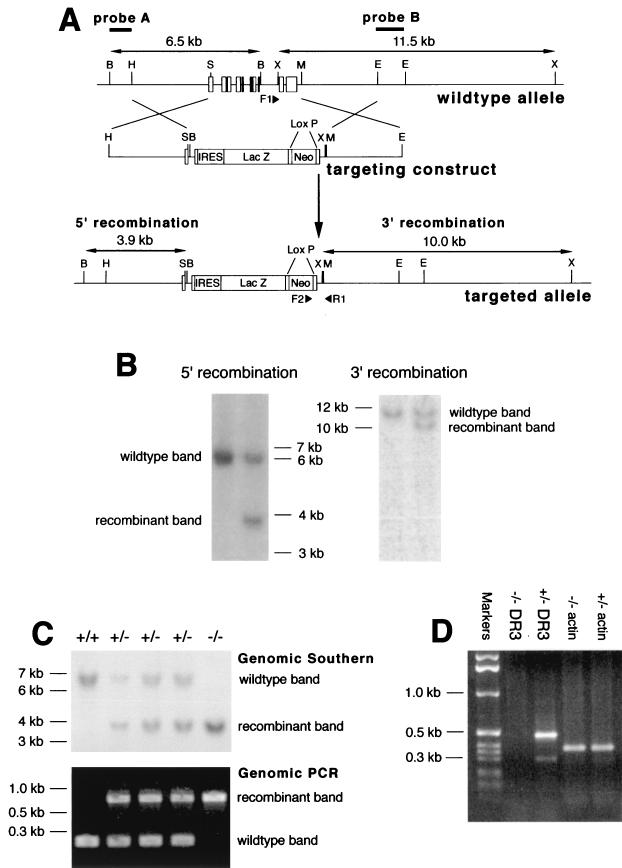
FIG. 1.
Generation and screening of DR3−/− mice. (A) Map of the coding region of the DR3 gene (top), the targeting construct (middle), and the targeted locus (bottom). Restriction enzyme sites are indicated by single letters; B, BamHI; E, EcoRI; H, HindIII; M, MfeI; S, SmaI; X, XbaI. The positions of the DR3 exons are shown. An IRES/lacZ/loxP/neo vector was used to replace the whole coding region of the DR3 gene. The 5′ arm was generated using a 3-kb HindIII-SmaI fragment, while the 3′ arm consisted of a 3-kb MfeI-EcoRI fragment. (B) Southern blot screening for homologous recombination in ES cell clones. Genomic DNA was isolated from transfected ES cell clones, digested with either BamHI or XbaI, and screened with probe A (left) for 5′ recombination or probe B (right) for 3′ recombination, respectively. Only ES cell clones with correct recombination at both ends were used for injections. Sizes of fragments are indicated. (C) Southern blot analysis and genomic PCR of representative mouse tail DNA. Genomic DNA was isolated from mouse tails, screened for correct 5′ recombination by Southern blotting using a BamHI digest and probe A, and screened for correct 3′ recombination by genomic PCR using the three primers (filled triangles) F1, F2 (in the neo gene), and R1. Sizes of fragments, recombinant and wild-type bands are indicated. (D) Reverse transcription-PCR analysis of thymus cDNA. cDNA prepared from total thymus RNA was subjected to PCR analysis using specific primers corresponding to sequences within the DR3 death domain exon. Actin primers were used a positive control. Sizes of fragments are indicated.
Maintenance and breeding of mouse strains.
All mice were kept under barrier conditions at the Imperial Cancer Research Fund animal unit. H-Y TCR transgenic mice were kindly provided by H. von Boehmer (22). Rag 1−/− mice were acquired from the Jackson Laboratory. For H-Y TCR transgenic studies, DR3−/− × H-Y TCR mice were obtained by crossing DR3−/− mice with DR3+/− H-Y TCR heterozygote transgenics, thus deriving DR3+/− and DR3−/− mice with and without the H-Y TCR transgene. All mice analyzed were between 6 and 14 weeks of age. For analysis of each age group (6, 10, and 14 weeks), mice were sacrificed within one day of their weekly age.
Flow cytometric analysis.
Fluorescence-activated cell sorting (FACS) analysis was performed on a FACScan (Becton Dickinson) using CellQuest software. Single-cell suspensions were prepared from freshly isolated bone marrow, lymph nodes (inguinal), spleen, and thymus. All samples were gated on standard forward scatter versus-side scatter gates. Thymocytes were stained using combinations of the antibodies from Becton Dickinson (anti-CD4-fluorescein isothiocyanate [FITC], anti-CD4-phycoerythrin [PE], anti-CD8-FITC, anti-CD8-biotin, anti-CD30, anti-CD44-PE, anti-CD25-biotin, anti-Vβ8-FITC [F23.1], anti-CD3-FITC [2C11], anti-αβTCR-FITC, anti-γδTCR-FITC, anti-B220-FITC, and anti-5-bromo-2′-deoxyuridine [BrdU]) or Caltag Laboratories (anti-CD8-Tricolor, streptavidin-Tricolor, anti-neutrophil-FITC, and anti-monocyte-FITC) or kindly donated by P. Kieselow and H. von Boehmer for H-Y transgenic analysis (T3.70 and T3.70-FITC specific for Vα3 [49]). BrdU and unconjugated T3.70 or CD30 were visualized using anti-mouse immunoglobulin G (IgG)-FITC (Dako).
Lymphocyte proliferation assays.
In vivo BrdU incorporation assays were carried out as described elsewhere (56). In brief, 1 mg of BrdU (Sigma) was injected twice intraperitoneally, 30 min apart. Mice were then sacrificed after 6 h, thymuses were extracted, and single-cell-suspension thymocytes were isolated. BrdU incorporation was visualized using a standard BrdU-labeling protocol (28) with an anti-BrdU antibody (Becton Dickinson) and anti-mouse Ig-FITC secondary (Dako) and analyzed on a FACScan (Becton Dickinson).
For in vitro [3H]thymidine uptake assays, 2 × 105 lymphocytes, isolated as described above, were aliquoted into 96-well flat-bottomed plates and stimulated with anti-CD3 (2C11), anti-CD3, and anti-CD28 (37.51; Pharmingen), 1 ng of phorbol myristate acetate (PMA) and ionomycin (Sigma) per ml, or 5 μg of concanavalin A (Sigma) per ml. For antibody stimulations, plates were previously coated with the monoclonal antibodies (MAbs) overnight at 4°C at 10 μg/ml in phosphate-buffered saline (PBS) and washed five times with PBS before use. Cultures were performed in RPMI medium supplemented with 10% heat-inactivated fetal calf serum and 50 μM β-mercaptoethanol. Stimulations were carried out in triplicate and incubated for 72 h before pulsing for 18 to 24 h with [3H]thymidine (1 mCi/well; Dupont NEN, Boston, Mass.). Cells were then harvested onto fiberglass filter mats (Pharmacia, Uppsala, Sweden) and washed with a cell harvester (Skatron). Beta emission was counted on a Betaplate counter (Pharmacia). Results are presented as a stimulation index, calculated as the geometric mean of stimulated cultures/geometric mean of control cultures with medium only ± 1 standard deviation. No significant differences were observed between the medium-only controls of DR3+/− and DR3−/− lymphocytes.
Apoptosis assays.
For anti-CD3-induced apoptosis assays, experiments were carried out as described elsewhere (46). In brief, 96-well flat-bottomed plates were coated with anti-CD3 MAb 2C11 (2, 10, or 50 μg/ml) or anti-CD3 and anti-CD28 MAb (each at 10 μg/ml) in PBS, overnight at 4°C, or with rat Ig as a control. Plates were washed three times with PBS before use. Thymocytes were extracted, resuspended in single-cell suspension in Dulbecco's modified Eagle's medium (Gibco BRL) supplemented with 10% heat-inactivated fetal calf serum, and aliquoted into plates at 5 × 105 cells/well. For PMA (Sigma) stimulation, a final concentration of 1 ng/nl was used. Samples were then incubated at 37°C for the indicated times before analysis for apoptosing cells using annexin V-FITC (Pharmingen) and propidium iodide staining to gate out dead cells. All samples were performed in triplicate. Values for percent anti-CD3-induced apoptosis were calculated using the following formula: (% annexin V+ cells after anti-CD3 − % annexin V+ cells in rat Ig controls)/(100 − % annexin V+ cells in rat Ig controls) × 100. For other apoptosis assays, thymocytes were cultured in vitro in standard RPMI medium supplemented with 10% heat-inactivated fetal calf serum and 50 μM β-mercaptoethanol, and apoptosis was induced by stimulation with the following reagents: cycloheximide (30 μg/ml) and anti-Fas antibody (1 μg/ml); dexamethasone (2 μM); and etoposide (50 μM). Samples were taken at 4, 8, 12, 24, and 48 h poststimulation. Apoptosis was visualized using both annexin V-FITC staining, or pre-G1 peak DNA staining with propidium iodide.
RESULTS
Generation of DR3−/− mice.
The strategy used to inactivate the DR3 gene in ES cells is shown in Fig. 1A. We chose to disrupt exon 1 and replace all of the coding exons of the gene to exclude the possibility of any expression of DR3 splice variants. The entire coding region of the DR3 gene, from exon 1 (56 bp upstream of the ATG initiation codon) to 297 bp downstream of the polyadenylation site, was replaced with a construct containing an IRES, β-galactosidase (lacZ) gene, and neo gene flanked by laxP sites. ES cell clones with correct 5′ and 3′ recombinations were detected by screening using probes outside the recombination arms of the targeting vector. 5′ recombination was detected by the reduction of a 6.5-kb wild-type BamHI fragment to 3.9 kb and 3′ recombination by the reduction of an 11.5-kb wild-type XbaI band to 10 kb (Fig. 1A and B). The homologous recombination frequency was 4.2%. Four clones were chosen for injection into blastocysts. Mice heterozygous for the targeted allele, as detected by screening for 5′ recombination in genomic Southern blots of tail DNA (Fig. 1C), were generated from two ES cell clones with correct 5′ and 3′ integrations of the targeting construct.
Breeding of heterozygous (DR3+/−) mice generated homozygote DR3-null (DR3−/−) mice (Fig. 1C) in normal Mendelian and male/female ratios (data not shown). Due to the targeting strategy (deletion of the entire coding region of the DR3 gene), the mutation must clearly be null. In agreement with this, no transcripts were detected by PCR analysis using any combination of primers specific for the DR3 coding sequence. A representative reverse transcription-PCR demonstrating this using primers encoding sequences within the DR3 death domain is shown in Fig. 1D.
Normal development of major organs, peripheral lymphoid organs, and lymphocyte subsets in DR3−/− mice.
The pattern of expression of mouse DR3 in the brain, heart, and kidney as well as lymphoid organs (51) prompted us to investigate whether there were any general developmental defects in DR3−/− mice. Histological analysis of all major organs (including brain, heart, and kidney) revealed no abnormalities (data not shown). In particular, the peripheral lymphoid organs such as thymus, spleen, lymph nodes, and Peyer's patches all showed normal development. Primary B-cell follicles and follicular dendritic cell networks were present in the peripheral lymphoid organs from DR3−/− mice (data not shown), unlike their TNFR1−/− counterparts (25, 32, 37).
A more detailed analysis of the B- and T-lymphocyte populations in peripheral lymphoid organs from DR3-deficient mice was carried out by FACS analysis using a battery of antibodies that define the various lymphoid populations. No significant differences were observed between DR3−/− mice and their heterozygous or wild-type littermates (data not shown). There were also no significant differences in the weight and size of spleens or lymph nodes at any age examined (data not shown).
Increased thymus size but normal turnover in DR3−/− mice.
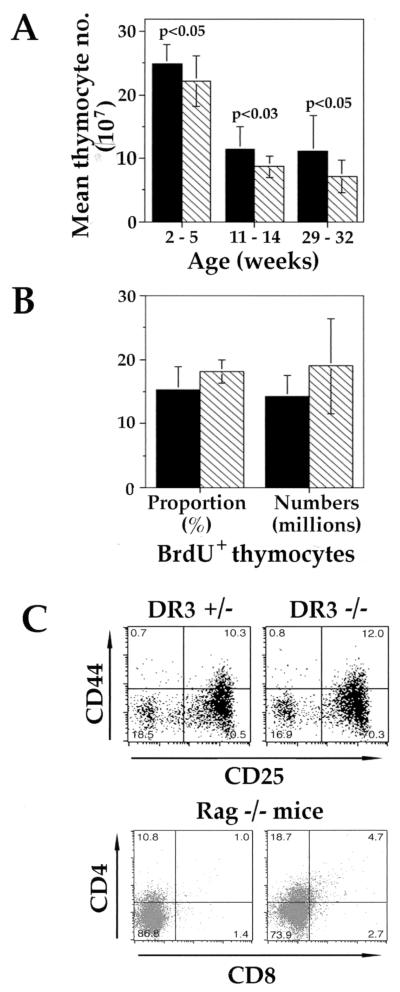
TNFR family members have been shown to be important regulators of developmental processes. The expression of DR3 in developing thymocytes suggested that DR3 may be a regulator of thymocyte development. To determine whether DR3 is required for normal thymocyte development, thymocyte subpopulations were analyzed by flow cytometry. There were no significant differences in the four major thymocyte populations defined by the expression of the CD4 and CD8 markers. However, a small but significant increase in the average total number of thymocytes derived from DR3-deficient mice, compared to heterozygous littermates, was observed (Fig. 2A). At 2 to 5 weeks of age, DR3−/− thymuses were generally about 10% larger than their heterozygote counterparts, and by 29 to 32 weeks this difference had increased to about 30%. A larger study (data not shown), covering mice aged from 26 to 233 days, showed these differences to be significant using a different statistical analysis (Mann-Whitney U test; U = 5,965, P < 0.05). No difference in thymocyte number was observed between wild-type and heterozygous mice (data not shown). This increase in thymus size appeared not to be a consequence of an increase in thymocyte proliferation and/or turnover, since neither the proportion nor the numbers of thymocytes which incorporated BrdU differed significantly between DR3−/− and DR3+/− mice (Fig. 2B).
FIG. 2.
Analysis of thymocyte development in DR3+/− and DR3−/− mice. (A) Thymus size in DR3−/− mice. Total thymocyte numbers were collected for mice at the ages indicated (DR3+/− and DR3−/−, n ≥ 11 and n ≥ 10, respectively, for each age group), and means were generated. DR3−/− thymuses were generally larger than their DR3−/− counterparts, irrespective of age. Significance using t tests is indicated. (B) Turnover and proliferation in DR3−/− thymus. The proportion (left) and number (right) of BrdU+ thymocytes were estimated as described in Materials and Methods. Filled bars, DR3−/−; hatched bars, DR3+/−. (C) Early thymocyte development in DR3−/− mice. Upper panels depict representative FACS plots showing expression of CD44 and CD25 in early thymocyte subsets from DR3+/− (left) and DR3−/− (right) mice. Thymocytes were extracted and depleted of cells expressing CD4 and CD8 using specific antibodies and comple- ment. The remaining thymocytes were stained with anti-CD44-PE, anti-CD25-biotin with streptavidin-Tricolor and anti-CD4-FITC, anti-CD8-FITC, anti-B220-FITC, γδTCR-FITC, and αβTCR-FITC. DN thymocytes were analyzed by gating on the CD4−, CD8−, B220−, αβTCR−, and γδTCR− populations. Lower panels depict representative FACS plots showing CD4 and CD8 thymocyte subsets in Rag-1-deficient DR3+/− (left) and DR3−/− (right) mice.
Early thymocyte development is unaffected by the absence of DR3.
The small but significant increase in total thymocyte numbers in DR3-deficient mice was consistent with a regulatory role of DR3 at one or more stages of thymocyte development. There are two major checkpoints at which thymocyte development is controlled. The DN-to-double-positive (DP) thymocyte transition selects immature thymocytes with in-frame TCRβ rearrangements and is regulated by the pre-TCR. The DP-to-single-positive (SP) thymocyte transition selects major histocompatibility complex (MHC)-restricted, nonautoreactive thymocytes and is regulated by the mature αβTCR. To investigate whether DR3 plays an essential role in early thymocyte development, we initially analyzed DN thymocyte subsets, as defined by the absence of CD4, CD8, αβTCR, γδTCR, and B220 surface markers and the differential expression of CD44 and CD25, by flow cytometry. As shown in Fig. 2C (upper panels), this analysis revealed no consistent differences in the relative proportions of DN populations in DR3−/− compared to DR3+/− mice.
Analysis of transgenic mice expressing dominant negative FADD (DN-FADD)/MORT1 (which binds to TNFR death domains) has implicated a role for death receptor signaling in early thymocyte development at the pre-TCR checkpoint (33). This study showed that the DN-FADD transgene bypassed the requirement for pre-TCR signaling, promoting the survival and differentiation, but not the proliferation, of CD25+ CD44− thymocytes in Rag-deficient mice. These results have suggested a model in which dual signaling, via the pre-TCR and death receptors, triggers the development of early pre-T cells, whereas death receptor signaling in thymocytes that lack a pre-TCR induces apoptosis. To test whether FADD recruitment to DR3 is essential for the regulation of the pre-TCR-mediated checkpoint, Rag1 × DR3 doubly deficient mice were analyzed. As shown in Fig. 2C (lower panels), thymocyte development was completely arrested at the CD25+ CD44− DN stage in Rag−/− × DR3−/− mice, demonstrating that DR3 is not essential for FADD-mediated deletion of DN thymocytes that fail to make in-frame rearrangements at the TCRβ locus.
Negative selection is impaired in DR3−/−, H-Y transgenic mice.
The DP-to-SP thymocyte transition is a checkpoint at which a developmental decision is made between cell death and survival. Thymocytes expressing αβTCRs that have no detectable affinity for ligand, or which are autoreactive, undergo apoptosis, whereas cells expressing αβTCRs with intermediate affinities receive survival signals and generate the pool of mature SP thymocytes and αβT cells. To investigate whether DR3 is essential for efficient negative selection of thymocytes, the H-Y TCR transgenic mouse model was used (22). The H-Y TCR recognizes a male-specific antigen in the context of H-2b In male mice, thymocytes expressing the transgenic TCR are deleted, whereas in female mice, transgene-expressing cells undergo positive selection, resulting in an increased proportion of CD8 SP cells.
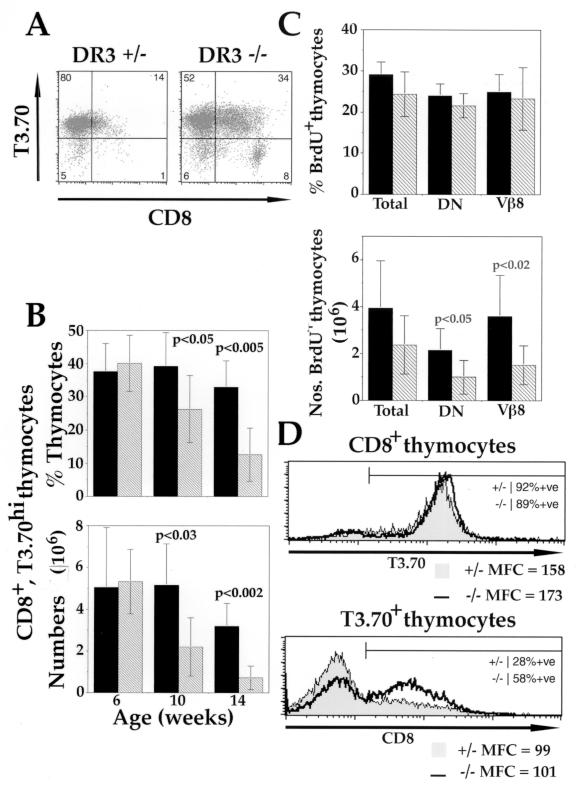
In male H-Y TCR H-2b transgenic mice, CD8+ TCR-expressing cells are efficiently deleted by negative selection. To determine whether this deletion was dependent on the expression of DR3, the proportion of CD8+ TCR-expressing thymocytes from DR3-deficient, H-Y TCR male mice was analyzed by flow cytometry using MAb T3.70, which recognizes the Vα3 chain of the H-Y TCR. This analysis was carried out using male mice 6, 10, and 14 weeks of age. As shown in Fig. 3A and B, and as reported previously (22), an age-dependent deletion of transgenic TCR-positive, CD8+ thymocytes was observed in male mice. At 14 weeks of age, the total numbers and proportion of H-Y TCR CD8 SP thymocytes were barely detectable. A striking increase in the numbers and proportions of CD8+ T3.70+ thymocytes, was observed in DR3-deficient H-Y TCR transgenic male mice compared to DR3+/− littermates. A significant difference was apparent in 10-week-old male mice and, by 14 weeks of age there was at least a fourfold difference in the numbers of transgenic TCR-positive CD8+ SP cells in DR3-deficient male mice.
FIG. 3.
Negative selection in male DR3−/− × H-Y transgenic mice. (A) Representative FACS plots showing expression of CD8 and T3.70 in 14-week-old male H-Y transgenic mice. Thymocytes were extracted and stained with anti-CD4-PE, anti-CD8-Tricolor, and anti-T3.70-FITC. DR3−/− × H-Y mice (right) showed an increase in the proportions of CD8+ T3.70+ thymocytes compared to their DR3+/− × H-Y littermates. (B) Proportions and numbers of CD8+ T3.70+ thymocytes in H-Y TCR transgenic DR3−/− and DR3+/− mice with age. Male DR3−/− × H-Y mice showed higher proportions (top) and numbers (bottom) of CD8+ T3.70+ thymocytes with age compared to their DR3+/− × H-Y littermates. Differences were statistically significant using parametric (for numbers) or nonparametric (for percentages) t tests from 10 weeks of age and are indicated on the graphs. Means were calculated from n ≥ 7 mice. Standard deviations of the means are indicated by bars. Filled bars, DR3−/−; hatched bars, DR3+/−. (C) BrdU uptake of male DR3−/− × H-Y transgenic mice, determined as described in Materials and Methods. The mean from n ≥ 5 mice for each thymocyte population is shown. All mice were 10 weeks of age (±1 day). Proportions (top) of BrdU+ thymocytes did not differ between H-Y transgenic, DR3−/− and DR3+/− mice, but numbers (bottom) of BrdU+ thymocytes were increased in DR3−/− × H-Y mice compared to their DR3+/− littermates at 10 weeks of age. Increased numbers of total BrdU+ cells were due to increases in the BrdU+ DN and transgenic Vβ8+ thymocyte populations. Filled bars, DR3−/−; hatched bars, DR3+/−. (D) CD8 and T3.70 expression on DR3−/− × H-Y transgenic thymocytes. Thymocytes were extracted from 10-week-old mice and stained with anti-CD4-PE, anti-CD8-Tricolor, and anti-T3.70-FITC. Thymocytes were either gated for CD8 expression and their T3.70 expression (top) or gated for high T3.70 expression and their CD8 expression shown (bottom). Representative overlay histograms are displayed and labeled with the proportion of positive cells and the level of each surface marker, as measured by their mean fluorescence channel (MFC). Male DR3−/− × H-Y mice (thick line) showed an increase in the proportion of T3.70hi thymocytes expressing CD8 compared to their DR3+/− × H-Y (shaded) littermates but no difference in the level of CD8 expression (lower panel). There was no significant difference in the proportion of CD8+ thymocytes expressing T3.70 or the level of T370 expression on CD8+ thymocytes compared with male DR3−/− and DR3+/− H-Y transgenic mice.
The observed increase in the numbers of CD8+ T3.70+ thymocytes could be a consequence of either an increase in proliferation and/or a decrease in apoptosis of this subset. To distinguish between these possibilities, the turnover of thymocytes in male DR3−/− H-Y and DR3+/− H-Y TCR transgenic littermates was investigated by examining their BrdU incorporation. Mice 10 weeks age were chosen, as no significant differences in CD8+ T3.70+ thymocyte numbers or thymus size were observed at 6 weeks (Fig. 3B and data not shown), while at 14 weeks around half the DR3+/− H-Y mice had virtually undetectable thymuses. The proportion of thymocytes incorporating BrdU into DNA did not differ in the DR3+/− or DR3−/− background, but as expected from the increase in thymus size, there was a significant increase in the numbers of BrdU+ thymocytes in DR3−/− H-Y male mice (Fig. 3C). Significantly, the numbers of transgenic Vβ8+ thymocytes were increased and accounted for the rise in observed BrdU uptake (Fig. 3C).
One means by which thymocytes escape negative selection in male H-Y transgenic mice is by down-regulation of the CD8 coreceptor, allowing a small proportion of T3.70+ transgenic thymocytes to migrate to the periphery (22). Thus, CD8lo T3.70+ T cells can be detected in the peripheral lymphoid organs, though these cells remain unresponsive to H-Y antigen due to undefined peripheral tolerance mechanisms. Increased down-regulation of the CD8 coreceptor may therefore be one possible mechanism for the defect in negative selection that is observed in DR3−/− H-Y TCR transgenic mice. However, as shown in Fig. 3A and D, there was an increased proportion of CD8+ T3.70+ thymocytes in male DR3−/− mice compared to their heterozygous littermates, demonstrating that coreceptor down-regulation is not the major mechanism of escape from negative selection in these mice. A reduction in the surface level of the H-Y TCR may also enable thymocytes to escape negative selection. However, as shown in Fig. 3D, surface H-Y TCR levels were equivalent in DR3−/− and DR3+/− mice.
Defective negative selection in male H-Y TCR transgenic DR3−/− mice might be expected to result in an equivalent increase in the CD8+ T3.70+ T-cell load in the peripheral lymphoid organs. To test this possibility, the number of transgenic peripheral T cells was determined. As no differences in the development of different lymph nodes were observed in DR3−/− mice (data not shown), inguinal lymph nodes from H-Y TCR transgenic mice were used as a representative lymph node. While no differences were detected in inguinal lymph nodes, a significant increase (∼40%) in the numbers of CD8+ T3.70+ T cells was observed in the spleens of DR3−/− mice.
A previous analysis has revealed that mice deficient in the expression of CD30, a TNFR family member that lacks a death domain, exhibit defective negative selection (2). Since CD30 maps to the same chromosomal location as DR3 (8), it is possible that the observed negative selection defect in DR3-null mice is a consequence of an indirect effect of the gene targeting strategy on CD30 gene expression. To rule out this possibility, CD30 expression on thymocytes from DR3−/− mice was assayed by flow cytometry. There were no differences in the overall proportions of CD30-expressing thymocytes between DR3−/− thymocytes and their heterozygous or wild-type littermates. However, a more detailed analysis identified a CD4lo CD8lo CD30+ population that was significantly reduced in DR3−/− mice compared to their DR3+/− counterparts (data not shown). The reduction in this thymocyte subset is age dependent, but CD4lo CD8lo CD30+ cells were generally lower in both proportion and number in DR3−/− mice. There were no significant differences in this subset between DR3+/− and DR3+/+ mice (data not shown).
Endogenous superantigen-mediated deletion of T cells is unaffected in DR3-deficient mice.
T cells expressing TCRs that recognize minor lymphocyte-stimulating determinants encoded by endogenous superantigens in the mouse genome together with MHC class II molecules are deleted. Minor lymphocyte-stimulating determinants expressed on the 129 × C57BL/6 background of DR3-deficient mice result in the deletion of several Vβ-expressing CD4+ T cells including Vβ5 (1). To determine whether endogenous superantigen-driven deletion is defective in DR3-null mice, lymph node T cells, splenic T cells, and SP thymocytes were analyzed using antibodies to specific Vβ families. As shown in Table 1, Vβ5-bearing CD4+ T cells were efficiently deleted in DR3-null mice, whereas the proportion of nondeleting Vβ8 cells was unaffected. These data show that DR3 is not essential for superantigen-mediated deletion.
TABLE 1.
MMTV superantigen deletion of CD4 and CD8 SP cells
| SP cell type | Thymus
|
Spleen
|
Lymph node
|
|||
|---|---|---|---|---|---|---|
| Vβ5 | Vβ8 | Vβ5 | Vβ8 | Vβ5 | Vβ8 | |
| CD4 | ||||||
| DR3+/+ | 3.0 ± 0.4 | 15.0 ± 1.3 | 2.7 ± 0.6 | 17.5 ± 1.8 | 2.2 ± 0.3 | 19.0 ± 0.8 |
| DR3−/− | 3.2 ± 0.9 | 13.7 ± 1.3 | 2.5 ± 0.3 | 17.3 ± 1.5 | 2.2 ± 0.1 | 18.5 ± 0.2 |
| CD8 | ||||||
| DR3+/+ | 10.7 ± 0.9 | 9.9 ± 1.3 | 12.4 ± 0.6 | 17.9 ± 1.2 | 13.3 ± 0.8 | 16.0 ± 1.5 |
| DR3−/− | 11.0 ± 4.1 | 7.4 ± 1.1 | 14.8 ± 2.0 | 19.7 ± 3.7 | 13.0 ± 0.3 | 16.6 ± 1.7 |
Anti-CD3-induced, but not non-antigen receptor-mediated, apoptosis is impaired in DR3-deficient mice.
Immature DP thymocytes and T cells are susceptible to an array of cell death-inducing stimuli, including anti-CD3 cross-linking, glucocorticoid treatment, Fas ligation, and DNA-damaging agents. To assess whether these forms of apoptosis are also dependent on DR3 expression, thymocytes and peripheral T cells were subjected to treatment with anti-CD3 cross-linking, the steroid dexamethasone, the DNA-damaging agent etoposide, and the protein synthesis inhibitor cycloheximide in conjunction with an anti-Fas signal.
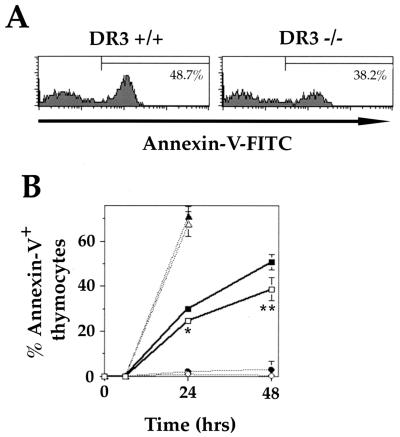
Using propidium iodide to exclude all dead cells and annexin V surface labeling, the proportion of live thymocytes undergoing early stages of apoptosis was measured at different time points up to 48 h after stimulation. Cross-linking of surface CD3 on DR3−/− thymocytes resulted in significantly reduced levels of apoptosis compared to DR3+/− thymocytes at lower concentrations of anti-CD3 MAb (2 μg/ml [Fig. 4A and B] and 10 μg/ml [data not shown]) but not higher concentrations (50 μg/ml) of coating anti-CD3 MAb (data not shown). The reduction in the proportion of apoptosing cells induced by anti-CD3 treatment was observed at 12 h (data not shown) 24 and 48 h (Fig. 4B) and ranged from 15 to 45%. In contrast, the levels of apoptosis of thymocytes induced by PMA or anti-CD3 and anti-CD28 (Fig. 4B) were indistinguishable between DR3+/− and DR3−/− thymocytes.
FIG. 4.
Apoptosis of thymocytes following CD3 ligation. (A) Representative FACS histograms showing reduced proportions of annexin V-positive thymocytes from DR3−/− mice compared to DR3+/+ age- and sex-matched controls after CD3 cross-linking. A stimulation of 2 μg/ml was used, and thymocytes were stained after 48 h. Dead cells were gated out using propidium iodide. (B) Thymocyte suspensions were stimulated for up to 48 h with plate bound anti-CD3 or rat Ig as a negative control. Percent apoptosis was measured as the proportion of propidium iodide-negative thymocytes, which were positive for annexin V. Anti-CD3 treatment at 2 μg/ml resulted in an increase of 50.7% in apoptosing thymocytes above rat Ig controls by 48 h in DR3+/− thymocytes (filled squares) compared to 38.7% in DR3−/− thymocytes (open squares). There were no differences in apoptosis of DR3−/− (open) and DR3+/+ (filled) thymocytes following stimulation with PMA (triangles) or anti-CD3 and anti-CD28 (circles). Results are the means of 3 DR3−/− and DR3+/− littermates, with duplicate cultures performed on each mouse. Standard deviations are shown as bars. ∗ and ∗∗ represent P < 0.0003 and P < 0.004, respectively, using t tests assuming unequal variance.
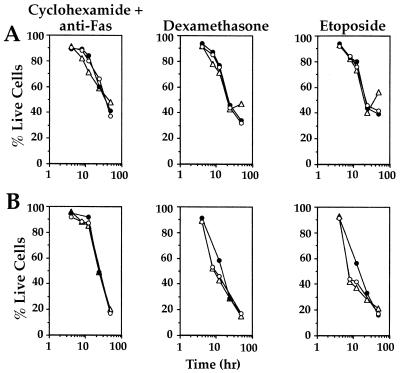
In contrast, neither DR3−/− thymocytes nor lymphocytes extracted from inguinal lymph nodes were resistant to any of the other apoptosis-inducing agents used. There were no differences in the rate of apoptosis over 48 h following treatment with these agents compared to lymphocytes from DR3+/+ and DR3+/− mice (Fig. 5).
FIG. 5.
Apoptosis of thymocytes and lymphocytes after exposure to various apoptosis inducing agents. Assays were carried out over 48 h with apoptosis measured using annexin V-FITC staining or sub-G1 peak following staining with propidium iodide as described in Materials and Methods. There were no differences in the induction of apoptosis using cycloheximide and anti-Fas, dexamethasone, or etoposide on thymocytes (A) or lymphocytes (B) derived from inguinal lymph nodes from DR3+/+ (open triangles), DR3+/− (closed circles), or DR3−/− (open circles) mice.
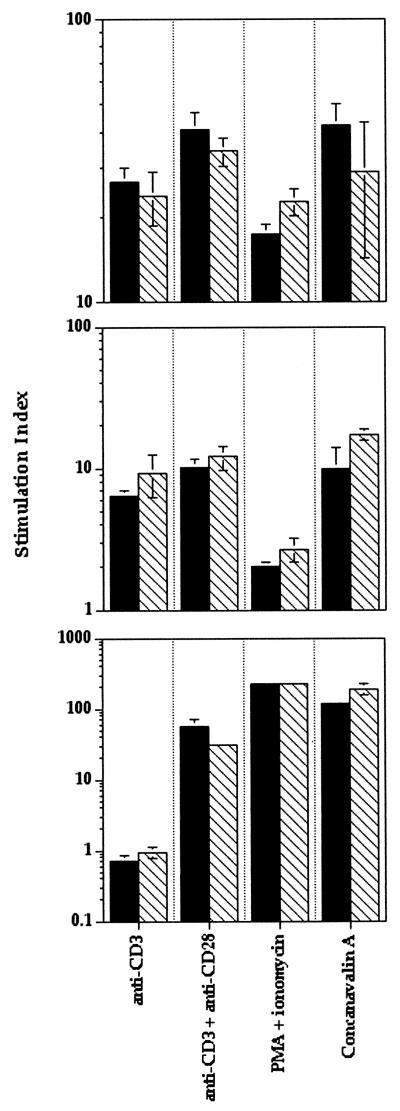
We extended these investigations by performing proliferation assays using a variety of polyclonal stimulators. Previous studies have shown that lymphocytes from transgenic mice expressing a dominant negative form of FADD, a downstream signaling molecule of DR3, have impaired proliferative ability (34, 56). Therefore, the ability of DR3-deficient thymocytes and T cells to respond to a variety of proliferative stimuli was assessed. DR3−/− lymph node cells, splenocytes, and thymocytes exhibited no impairments in proliferation to anti-CD3, anti-CD3, and anti-CD28, concanavalin A, or PMA plus ionomycin compared to heterozygote or wild-type littermates (Fig. 6).
FIG. 6.
Proliferation of lymphocytes, thymocytes, and splenocytes to various stimuli Assays were carried out over 96 h with proliferation measured by [3H]thymidine uptake over the final 18 to 24 h as described in Materials and Methods. There were no significant differences in thymidine uptake of lymphocytes (top), splenocytes (middle), or thymocytes (bottom) between DR3+/− and DR3−/− cells using anti-CD3, anti-CD3, and anti-CD28, PMA and ionomycin, or concanavalin A as stimuli. Data are presented as a stimulation index over medium-only controls, which were not significantly different between DR3+/− and DR3−/− cells. Filled bars, DR3−/−; hatched bars, DR3+/+.
Positive selection is unimpaired in DR3−/− mice.
The αβTCR is first expressed at the DP stage, resulting in a population of αβTCRlo and CD3lo thymocytes which then undergo positive selection to generate the αβTCRhi and CD3hi SP subsets. Positively selected thymocytes also transiently up-regulate the expression of CD69. Analysis of CD3 and CD69 expression on thymocytes from DR3-deficient mice showed no significant difference in the proportions of CD3hi or CD69-positive cells between DR3-null mice and their heterozygous littermates, suggesting that DR3 is not absolutely required for positive selection (data not shown). This conclusion was consistent with a flow cytometric analysis of DR3-deficient female mice expressing the H-Y TCR. No difference was observed in the numbers or proportions of H-Y TCR-expressing CD8 SP cells between DR3-expressing or -deficient thymocytes (Table 2). The H-Y TCR is H-2Kb restricted and therefore selects on both C57BL/6 and 129 mouse strain backgrounds. Thus, the mixed strain nature of the knockouts should not affect the efficiency of positive or negative selection. Taken together, these results demonstrate that DR3 is not required for positive selection during thymocyte development.
TABLE 2.
Positive selection in female H-Y transgenic DR3−/− and DR3+/− mice
| Organ (cell type) | Age (wk) | No. of cells (106 ± SD)a
|
|
|---|---|---|---|
| KO (n) | HET (n) | ||
| Thymus (all thymocytes) | 6 | 129 ± 29 (7) | 134 ± 42 (10) |
| 10 | 95 ± 29 (7) | 98 ± 31 (12) | |
| 14 | 74 ± 49 (7) | 75 ± 24 (6) | |
| Thymus (CD8+ T370+) | 6 | 33.2 ± 12.1 (7) | 51.8 ± 30.3 (10) |
| 10 | 25.4 ± 7.3 (7) | 31.1 ± 13.3 (12) | |
| 14 | 24.4 ± 10.0 (7) | 22.2 ± 6.5 (6) | |
| Spleen (CD8+ T370+) | 6 | 2.9 ± 0.4 (7) | 3.3 ± 2.1 (10) |
| 10 | 4.7 ± 4.0 (7) | 4.8 ± 2.1 (12) | |
| 14 | 2.7 ± 1.6 (7) | 3.2 ± 1.7 (6) | |
| Inguinal LN (CD8+ T370+) | 6 | 0.38 ± 0.21 (7) | 0.68 ± 0.56 (10) |
| 10 | 0.39 ± 0.16 (7) | 0.66 ± 0.35 (12) | |
| 14 | 0.73 ± 0.52 (7) | 0.63 ± 0.34 (6) | |
In all cases, the difference between KO and HET values was nonsignificant.
DISCUSSION
DR3 belongs to a family of receptors that plays an important role in regulating cell survival and proliferation. These processes are tightly regulated during T-cell development. Thus, signals originating from the pre-TCR mediate the survival and proliferation of pre-T cells that have undergone in-frame TCRβ gene rearrangements. The αβTCR controls the survival of DP thymocytes, selecting those cells expressing TCR with intermediate affinity for MHC ligand and inducing programmed cell death of high-affinity, autoreactive T cells. DP thymocytes that fail to express an αβTCR also undergo apoptosis. The expression of DR3 in developing thymocytes prompted the hypothesis that it is a key regulator of life/death decisions during thymocyte development. This notion was tested by generating and analyzing mice deficient in DR3 expression.
TNFR family members containing death domains (TNFR1, Fas, DR3, DR4, DR5, and DR6) transduce death signals by interaction of their death domains with FADD, a death domain-containing cytoplasmic protein. FADD then recruits other cytoplasmic effectors such as caspase 8, TRADD, TRAF2, and RIP that activate the apoptotic machinery (41). Studies of transgenic mice expressing DN-FADD in developing thymocytes have given rise to the hypothesis that stimulation of death domain-containing TNFRs on early pre-T cells induces cell death via FADD only in the absence of pre-TCR-derived signals (33). In the presence of pre-TCR-derived signals, the TNFR signals proliferation. The present study demonstrates that DR3, which is expressed on early and late pre-T cells, is not essential for the transduction of apoptosis or proliferation signals at the pre-TCR-mediated developmental checkpoint. It may, however, participate in the regulation of β-selected thymocytes in concert with other death receptors, such as DR5, Fas, and TNFR1. An analysis of mice deficient in the expression of multiple death receptors would resolve this question.
The DP-to-SP thymocyte transition is associated with extensive cell death. The pathway(s) that, in concert with signals derived from high-affinity TCRs, induce apoptosis in DP thymocytes have not been defined. TNFRs have, however, been implicated in negative selection in the thymus. Thus, a role for Fas in negative selection at high doses of antigen has been proposed (23). Also, negative selection has been shown to be either enhanced following CD30 overexpression (9) or partially impaired in mice deficient in the expression of CD30, a non-death domain-containing member of the TNFR superfamily (2). We show here that DR3 is also important for negative selection, which is impaired in the absence of this receptor. Thus, the induction of apoptosis in DP thymocytes by anti-CD3, an agonist that is presumed to mimic a high-affinity TCR interaction, is impaired at least at low concentrations of anti-CD3 (2 and 10 μg/ml) in DR3-null mice. Interestingly, this effect is abrogated by high anti-CD3 concentrations, suggesting a greater contribution of DR3 to apoptosis when the signaling strength is not maximal. Also, the deletion of H-Y TCR transgenic thymocytes in male mice is partially inhibited, an effect which is manifested most clearly in older mice. Although the numbers and proportions of H-Y TCR transgenic thymocytes were indistinguishable between DR3−/− and DR3+/− backgrounds in 6-week-old male mice, a significant inhibition of negative selection occurred in DR3-null mice at 10 weeks, at 14 weeks, when H-Y TCR-expressing thymocytes were virtually undetectable in most male mice expressing DR3, transgene-positive cells were easily detectable in all DR3-null mice analyzed. However, substantial negative selection had clearly occurred in male DR3-null mice, since at 14 weeks, male thymuses contained only about 1/100 the numbers of cells found in the thymuses of female transgenic mice.
The notion that death domain-containing TNFRs play a role in negative selection is in apparent contradiction to studies of mice expressing DN-FADD in thymocytes. In one such study, negative selection was unaffected by DN-FADD, although in this study negative selection was assayed in 6-week-old H-Y TCR male mice (50). At this age, we observed no difference in negative selection in the presence or absence of DR3. In an independent study, the deletion of autoreactive thymocytes was enhanced in H-Y TCR+ DN-FADD+ male thymocytes (34). These studies suggest that FADD signaling does not lead exclusively to cell death in thymocytes. This, in turn, suggests that FADD-independent pathways from death domain-containing TNFRs activate caspases in negative selection. Adapter molecules such as RIP, RAIDD, and Daxx have been shown to interact with the CD95 death domain, thereby activating a caspase cascade leading to apoptosis (15, 55). It is possible that these cytoplasmic signaling molecules also transduce DR3-mediated signals in thymocytes. A recent study has, however, failed to support a role for caspase activity in negative selection. In this study, mice expressing an inhibitor (p35) of caspase activity in thymocytes showed unimpaired negative selection (14). However, an independent study, using an identical strategy, revealed a reduction of negative selection upon inhibition of caspase activity (21). Therefore, the role of caspases in thymocyte negative selection remains unresolved.
The observation that DR3-null mice exhibit a partial impairment of negative selection can be interpreted within a model in which several different surface molecules act in concert to control the removal of autoreactive thymocytes (23). According to this model, disruption of a single surface receptor or signal transduction pathway would not be expected to result in a complete block in negative selection, a prediction that is observed in practice. The age dependency of the negative selection defect in DR3-null mice in H-Y TCR-transgenic mice may be due to developmental regulation of the ligand(s) for DR3 or, alternatively, to the developmental stage at which deletion occurs in the H-Y TCR model which is likely to be at the DN stage or in the transition from DN to DP thymocytes. Thus, the relative importance of a particular deletional mechanism may vary with age or with the stage of thymocyte development at which deletion of a particular TCR occurs.
Despite the impairment of negative selection in DR3-null mice, there were no signs of autoimmunity in these mice as indicated by the presence of DNA autoantibodies, nor was development of autoimmunity significantly accelerated on an lpr (Fas mutant) background (unpublished data). Furthermore, several other forms of apoptosis were unaffected. There was no difference in the rate of spontaneous cell death of thymocytes in culture or of the rate of apoptosis of thymocytes or T cells after treatment with glucocorticoids, DNA-damaging agents, or anti-Fas in the presence of protein synthesis inhibitors. Moreover, no difference in endogenous superantigen-mediated deletion of T cells was detected. This observation is consistent with the analysis of other transgenic models with defects in negative selection. For example, CD30-null mice (2) and mice lacking expression of the helix-loop-helix inhibitor protein Id3 (38) have impaired negative selection, but T cells specific for endogenous superantigens are efficiently deleted. Conversely, the antiapoptotic protein Bcl-2 inhibits multiple forms of apoptosis but not endogenous superantigen-induced negative selection in thymocytes (43), although bcl-2 transgenic mice have impaired negative selection when tested on a TCR transgenic background (45, 54). Taken together with the present study, these data suggest that there are qualitatively or quantitatively distinct cell death programs that operate at different stages of T-cell development to ensure effective central tolerance.
ACKNOWLEDGMENTS
We thank J. Williamson for chromosome counting of ES cell clones, I. Rosewell and his group for microinjections and chimeric mouse breeding, P. Hagger and colleagues for breeding and maintenance of the DR3−/− mouse colony, and P. Kieselow and H. von Boehmer for the kind gift of MAb T3.70.
This work was supported by the Imperial Cancer Research Fund. Eddie Wang was the recipient of a fellowship from the Beit Memorial Foundation for Medical Research, Anette Thern is a fellow of the Swedish Cancer Society, and Angela Denzel was the recipient of a Glaxo-Wellcome-BBSRC CASE Studentship.
REFERENCES
- 1.Acha-Orbea H. Superantigens and tolerance. In: Bell J I, Owen M I, Simpson E, editors. T cell receptors. Oxford, United Kingdom: Oxford University Press; 1995. pp. 224–265. [Google Scholar]
- 2.Amakawa R, Hakem A, Kundig T M, Matsuyama T, Simard J J L, Timms E, Wakeham A, Mittruecker H-W, Griesser H, Takimoto H, Schmits R, Shahinian A, Ohashi P S, Penninger J M, Mak T W. Impaired negative selection of T cells in Hodgkin's disease antigen CD30-deficient mice. Cell. 1996;84:551–562. doi: 10.1016/s0092-8674(00)81031-4. [DOI] [PubMed] [Google Scholar]
- 3.Baker S J, Reddy E P. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998;17:3261–3270. doi: 10.1038/sj.onc.1202568. [DOI] [PubMed] [Google Scholar]
- 4.Banner D W, D'Arey A, Janes W, Gentz R, Schoenfeld H J, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kD TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 5.Bodmer J-L, Burns K, Schneider P, Hoffman K, Steiner V, Thome M, Bornand T, Hahne M, Schroter M, Becker K, Wilson A, French L E, Browning J L, Robson Macdonald H, Tschopp J. TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas (Apo-1/CD95) Immunity. 1997;6:79–88. doi: 10.1016/s1074-7613(00)80244-7. [DOI] [PubMed] [Google Scholar]
- 6.Boldin M P, Verfolomeev E E, Pancer C, Mett I L, Carmonis J H, Wallach D. A novel protein that interacts with the death domain of Fas/Apo1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7787. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 7.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 8.Bowen M A, Lee R K, Miraglilotta G, Nam S Y, Podack E R. Structure and expression of murine CD30 and its role in cytokine production. J Immunol. 1996;156:442–449. [PubMed] [Google Scholar]
- 9.Chiarle R, Podda A, Prolla G, Podack E R, Thorbecke G J, Inghirami G. CD30 overexpression enhances negative selection in the thymus and mediates programmed cell death via a Bcl-2 sensitive pathway. J Immunol. 1999;163:194–205. [PubMed] [Google Scholar]
- 10.Chicheportiche Y, Bourdon P R, Xu H, Hsu Y M, Scoyy H, Hession C, Garcia I, Browning J L. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 11.Chinnaiyan A M, O'Rourke K, Yu G L, Lyons R H, Garg M, Duan D R, Xing L, Gentz R, Ni J, Dixit V M. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274:990–992. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 12.Chinnaiyan A M, O'Rourke K, Tewari M, Dixit V M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 13.Dixit V M. The cell-death machine. Curr Biol. 1996;6:555–562. doi: 10.1016/s0960-9822(02)00541-9. [DOI] [PubMed] [Google Scholar]
- 14.Doerfler P, Forbush K A, Perlmutter R M. Caspase enzyme activity is not essential for apoptosis during thymocyte development. J Immunol. 2000;164:4071–4079. doi: 10.4049/jimmunol.164.8.4071. [DOI] [PubMed] [Google Scholar]
- 15.Duan H, Dixit V M. RAIDD is a new “death” adaptor molecule. Nature. 1997;385:86–89. doi: 10.1038/385086a0. [DOI] [PubMed] [Google Scholar]
- 16.Grenet J, Valentine V, Kitson J, Li H, Farrow S N, Kidd V J. Duplication of the DR3 gene on human chromosome 1p36 and its deletion in human neuroblastoma. Genomics. 1998;49:385–393. doi: 10.1006/geno.1998.5300. [DOI] [PubMed] [Google Scholar]
- 17.Henkart P A. ICE family proteases: mediators of all apoptotic cell death? Immunity. 1996;4:195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- 18.Hsu H, Shu S B, Pan M G, Goeddel D V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 19.Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- 20.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 21.Izquierdo M, Grandien A, Criado L M, Robles S, Leonardo E, Albar J P, de-Buitrago G G, Martinez A C. Blocked negative selection of developing T cells in mice expressing the baculovirus p35 caspase inhibitor. EMBO J. 1999;18:156–166. doi: 10.1093/emboj/18.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kieselow P, Bluthmann H, Staerz U D, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 23.Kishimoto H, Sprent J. Several different cell surface molecules control negative selection of medullary thymocytes. J Exp Med. 1999;190:65–73. doi: 10.1084/jem.190.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitson J, Raven T, Jiang Y P, Goeddel D V, Giles K M, Pun K T, Grinham C J, Brown R, Farrow S N. A death domain-containing receptor that mediates apoptosis. Nature. 1996;384:372–375. doi: 10.1038/384372a0. [DOI] [PubMed] [Google Scholar]
- 25.Koni P A, Flavell R A. A role for tumor necrosis factor receptor type 1 in gut-associated lymphoid tissue development: genetic evidence of synergism with lymphotoxin β. J Exp Med. 1998;187:1977–1983. doi: 10.1084/jem.187.12.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koni P A, Sacca R, Lawton P, Browning J L, Ruddle N H, Flavell R A. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin β-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S. ICE-like proteases in apoptosis. Trends Biochem Sci. 1996;20:198–202. doi: 10.1016/s0968-0004(00)89007-6. [DOI] [PubMed] [Google Scholar]
- 28.Lucas B, Vasseur F, Penit C. Normal sequence of phenotypic transitions in one cohort of 5-bromo-2′-deoxyuridine-pulse labeled thymocytes. J Immunol. 1997;151:4574–4582. [PubMed] [Google Scholar]
- 29.Marsters S A, Sheridan J P, Donahue C J, Pitti R M, Gray C L, Goddard A D, Bauer K D, Ashkenazi A. Apo-3, a new member of the tumor necrosis factor receptor family, contains a death domain and activates apoptosis and NF-κB. Curr Biol. 1996;6:1669–1676. doi: 10.1016/s0960-9822(02)70791-4. [DOI] [PubMed] [Google Scholar]
- 30.Marsters S A, Sheridan J P, Pitti R M, Brush J, Goddard A, Ashkenazi A. Identification of a ligand for the death-domain-containing receptor Apo3. Curr Biol. 1998;8:525–528. doi: 10.1016/s0960-9822(98)70204-0. [DOI] [PubMed] [Google Scholar]
- 31.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95(Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 32.Neumann B A, Luz K, Pfeffer K, Holzmann B. Defective Peyer's patch organogenesis in mice lacking the 55-kD receptor for tumor necrosis factor. J Exp Med. 1996;184:259–264. doi: 10.1084/jem.184.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton K, Harris A W, Strasser A. FADD/MORT1 regulates the pre-TCR checkpoint and can function as a tumor suppressor gene. EMBO J. 2000;19:931–941. doi: 10.1093/emboj/19.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newton K, Harris A W, Bath M L, Smith K C G, Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orlinick J R, Chao M V. TNF-related ligands and their receptors. Cell Signal. 1998;10:543–551. doi: 10.1016/s0898-6568(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 36.Park Y C, Burkitt V, Villa A R, Tong L, Wu H. Structural basis for self-association and receptor recognition of human TRAF2. Nature. 1999;398:533–538. doi: 10.1038/19110. [DOI] [PubMed] [Google Scholar]
- 37.Pasparakis M, Alexopoulou L, Grell M, Pfizenmaier K, Bleuthmann H, Kollias G. Peyer's patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc Natl Acad Sci USA. 1997;94:6319–6323. doi: 10.1073/pnas.94.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivera R R, Johns C P, Quan J, Johnson R S, Murre C. Thymocyte selection is regulated by the helix-loop-helix inhibitor protein, Id3. Immunity. 2000;12:17–26. doi: 10.1016/s1074-7613(00)80155-7. [DOI] [PubMed] [Google Scholar]
- 39.Roth M, Wong S, Henzel W, Goeddel D V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 40.Schneider P, Schwenzer R, Haas E, Muhlenbeck F, Schubert G, Scheurich P, Tschopp J, Wajant H. TWEAK can induce cell death via endogenous TNF and TNF receptor 1. Eur J Immunol. 1999;29:1785–1792. doi: 10.1002/(SICI)1521-4141(199906)29:06<1785::AID-IMMU1785>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 41.Screaton G, Xu X-N. T cell life and death signalling via TNF-receptor family members. Curr Opin Immunol. 2000;12:316–322. doi: 10.1016/s0952-7915(00)00093-5. [DOI] [PubMed] [Google Scholar]
- 42.Screaton G R, Xu X-N, Olsen A L, Cowper A E, Tan R, McMichael A J, Bell J I. LARD, a new lymphoid-specific death domain containing receptor regulated by alternative pre-mRNA splicing. Proc Natl Acad Sci USA. 1997;94:4615–4619. doi: 10.1073/pnas.94.9.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sentman C L, Shutter J R, Hockenbery D, Kanagawa O, Korsmeyer S J. Bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 44.Smith C A, Farrah T, Goodwin R G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation and death. Cell. 1994;76:958–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 45.Strasser A, Harris A W, von Boehmer H, Cory S. Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene. Proc Natl Acad Sci USA. 1994;91:1376–1380. doi: 10.1073/pnas.91.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takayama E, Kina T, Katsura Y, Tadakuma T. Enhancement of activation-induced cell death by fibronectin in murine CD4+ CD8+ thymocytes. Immunology. 1998;95:553–558. doi: 10.1046/j.1365-2567.1998.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan K B, Harrop J, Reddy M, Young P, Terrett J, Emery J, Moore G, Truneh A. Characterization of a novel TNF-like ligand and recently described TNF ligand and TNF receptor superfamily genes and their constitutive and inducible expression in hematopoetic and non-hematopoietic cells. Gene. 1997;204:35–46. doi: 10.1016/s0378-1119(97)00509-x. [DOI] [PubMed] [Google Scholar]
- 48.Tartaglia L A, Ayers T M, Wong G H W, Goeddel D V. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 49.Teh H S, Kisielow P, Scott B, Kishi H, Uematsu Y, Bluthmann H, von Boehmer H. Thymic major histocompatibility complex antigens and the αβ T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988;335:229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- 50.Walsh C M, Wen B G, Chinnaiyan A M, O'Rourke K, Dixit V M, Hedrick S M. A role for FADD in T cell activation and development. Immunity. 1998;8:439–449. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 51.Wang, E. C., J. Kitson, A. Thern, J. Williamson, S. N. Farrow, and M. J. Owen. Genomic structure, expression and chromosome mapping of the murine homologue for the WSL-1 (DR3, Apo3, TRAMP, LARD, TR3, TNFRSF12) gene. Immunogenetics, in press. [DOI] [PubMed]
- 52.Ware C F, Vanarsdale S, Vanarsdale T L. Apoptosis mediated by the TNF-related cytokine and receptor families. J Cell Biochem. 1996;60:47–55. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C47::AID-JCB8%3E3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 53.Warzocha K, Ribeiro P, Charlot C, Renard N, Coiffier B, Salles G. A new death receptor 3 isoform: expression in human lymphoid cell lines and non-Hodgkin's lymphomas. Biochem Biophys Res Commun. 1998;242:376–379. doi: 10.1006/bbrc.1997.7948. [DOI] [PubMed] [Google Scholar]
- 54.Williams O, Norton T, Halligey M, Kioussis D, Brady H J M. The action of Bax and bcl-2 on T cell selection. J Exp Med. 1998;188:1125–1133. doi: 10.1084/jem.188.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X, Khosravi-far R, Chang H Y, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zornig M, Huber A-O, Evan G. p53-dependent impairment of T-cell proliferation in FADD dominant-negative transgenic mice. Curr Biol. 1998;8:467–470. doi: 10.1016/s0960-9822(98)70182-4. [DOI] [PubMed] [Google Scholar]