Abstract
Background and Objectives
With the increasing use of visually evoked potentials (VEPs) as quantitative outcome parameters for myelin in clinical trials, an in-depth understanding of longitudinal VEP latency changes and their prognostic potential for subsequent neuronal loss will be required. In this longitudinal multicenter study, we evaluated the association and prognostic potential of VEP latency for retinal neurodegeneration, measured by optical coherence tomography (OCT), in relapsing-remitting MS (RRMS).
Methods
We included 293 eyes of 147 patients with RRMS (age [years, median ± SD] 36 ± 10, male sex 35%, F/U [years, median {IQR} 2.1 {1.5–3.9}]): 41 eyes had a history of optic neuritis (ON) ≥6 months before baseline (CHRONIC-ON), and 252 eyes had no history of ON (CHRONIC-NON). P100 latency (VEP), macular combined ganglion cell and inner plexiform layer volume (GCIPL), and peripapillary retinal nerve fiber layer thickness (pRNFL) (OCT) were quantified.
Results
P100 latency change over the first year predicted subsequent GCIPL loss (36 months) across the entire chronic cohort (p = 0.001) and in (and driven by) the CHRONIC-NON subset (p = 0.019) but not in the CHRONIC-ON subset (p = 0.680). P100 latency and pRNFL were correlated at baseline (CHRONIC-NON p = 0.004, CHRONIC-ON p < 0.001), but change in P100 latency and pRNFL were not correlated. P100 latency did not differ longitudinally between protocols or centers.
Discussion
VEP in non-ON eyes seems to be a promising marker of demyelination in RRMS and of potential prognostic value for subsequent retinal ganglion cell loss. This study also provides evidence that VEP may be a useful and reliable biomarker for multicenter studies.
Multiple sclerosis (MS) is an autoimmune inflammatory disease of the CNS characterized by inflammation, demyelination, and neurodegeneration.1 Most approved disease-modifying treatments (DMTs) for MS are targeting the inflammatory disease component with the goal of decelerating or stopping disease progression.2 Clinical trials, which led to the approval of these DMTs, have used structural outcome parameters both for inflammatory disease activity such as new CNS lesions and for neurodegeneration such as brain atrophy.3,4 New regenerative treatment strategies for MS are imminent, which are aiming to not only repair myelin but also, by doing so, prevent neuroaxonal damage and reduce permanent disability.5-7 Studies on potential remyelinating agents showed promising results.5,6,8,9 Yet, evidence that remyelination protects axons in humans is still needed in spite of good experimental evidence.10-12
No gold standard has been established for the noninvasive imaging of myelin within the CNS.13-16 Myelin is a critical determinant of conduction speed in the CNS.17-20 Noninvasive evoked potentials measure conduction speed across large fiber tract within the CNS. During the first trials for remyelinating agents, pattern reversal visual evoked potentials (VEPs) have detected a response to therapy, although these trials were not long enough to show consequences for following neuroaxonal loss and disability accrual.5,6,21-23 Pattern reversal VEPs have been established as an interim standard for remyelination trials. Their benefits further include the well-defined conduction pathway from the retina to the occipital cortex and published standards for clinical trial application by the International Society for Clinical Electrophysiology of Vision (ISCEV).24,25
Historically, VEPs were mainly used as a diagnostic instrument for optic neuritis (ON), a typical manifestation of relapsing-remitting MS (RRMS).26-28 For diagnostic purposes, it was also known that VEP latencies are delayed in non-ON eyes of patients with MS likely due to subclinical demyelination,29 but the application of longitudinal VEP measurements in clinical practice has been uncommon.29-32 With the increasing use of VEPs as primary and secondary outcome parameters in clinical trials for remyelination, we need to understand their prognostic potential for neuroaxonal loss and resultant permanent disability. In line with our current understanding of MS pathology, demyelination accelerates neurodegeneration, which subsequently leads to disability accrual. It remains unclear whether VEP latency changes can adequately reflect part of this process and whether they have prognostic potential for subsequent neurodegeneration—especially in the chronic stage. Hence, the aim of this study was to evaluate the association and prognostic potential of VEP latency for retinal neurodegeneration (measured by optical coherence tomography [OCT]) in patients with chronic RRMS.
Methods
Participants
We included patients in a chronic disease stage, defined as no history of ON within the last 6 months before baseline or during follow-up (F/U), from Charité-Universitätsmedizin Berlin, Universitätsklinikum Düsseldorf, Universitätsklinikum Münster (Germany), and University of California San Francisco (UCSF, United States). Inclusion criteria were (1) a diagnosis of RRMS according to the 2017 criteria,22 (2) age 18–65 years at baseline, and (3) longitudinal VEP measurements with ≥2 visits and a F/U of ≥1 year. ON episodes were defined clinically. We excluded patients with comorbidities potentially influencing afferent visual system measurements (e.g., glaucoma, congenital or developmental visual disorders, and other significant neurologic diseases that can affect the visual system) and/or who have been treated with clemastine. At each visit, patients underwent neurologic and comprehensive visual system and eye examination. Their disability was scored according to the expanded disability status scale under supervision of an experienced MS neurologist.33 High-contrast and low-contrast visual acuity were acquired for descriptive purposes only by center-specific protocols and are reported as logarithm of the minimum angle of resolution to ensure normal distribution. Data are reported according to STROBE reporting guidelines.34
Standard Protocol Approvals, Registrations, and Patient Consents
Data were acquired in several separate local studies and registries, which were approved by the local ethics committees (ethics committee of the Heinrich-Heine-University Düsseldorf 5794R and 4389R, Münster 2014-545-f-A, Berlin EA1/163/12 and EA1/182/10) and performed in line with the applicable German and US laws and the current version of the Declaration of Helsinki. All patients gave written informed consent before study inclusion.
Visual Evoked Potentials
Centers used different VEP devices and protocols, but the same device was used for longitudinal measurements at each center. All protocols were in accordance with the ISCEV24 and used monocular full-field stimulation with pattern reversal black/white checkerboards (check size: 41′ for Universitätsklinikum Münster and Düsseldorf29; 60′ for Charité-Universitätsmedizin Berlin and UCSF). Scalp recordings were made with gold disc electrodes placed over the left, right, and mid-occipital lobe referenced to a mid-frontal electrode placed 12 cm to the nasion. VEP recordings were repeated ≥2 per eye averaging ≥100 responses. VEP measurements with a P100 latency measurement >200 ms or not measurable VEP latency (conduction block) were excluded. P100 latency was the primary investigated predictor. VEP and OCT reading were performed on the discretion of each center (Charité-Universitätsmedizin Berlin: C. Bereuter, F.C. Oertel, H.G. Zimmermann, and S. Motamedi; Universitätsklinikum Düsseldorf: N. Dimitriou, P. Albrecht; Universitätsklinikum Münster: J. Krämer; UCSF: A.J. Green, A. Keihani, F.C. Oertel, S. Condor-Montes). VEP thresholds were defined in accordance with each center's healthy reference population (Charité-Universitätsmedizin Berlin: 117 ms; Universitätsklinikum Düsseldorf: 120 ms; Universitätsklinikum Münster: 120 ms; UCSF: 118 ms).
Optical Coherence Tomography
OCT acquisition, quality control, and reading were performed by experienced technicians. OCT data were acquired with Spectralis SD-OCT devices (Heidelberg Engineering, Heidelberg, Germany) and reported according to APOSTEL recommendations.35,36 Image quality was assessed by OSCAR-IB criteria; scans with insufficient image quality were excluded.37 Semiautomatic intraretinal layer segmentation was performed using the software provided by the OCT manufacturer (Eye Explorer; Heidelberg Engineering). We quantified markers of retinal neuroaxonal content: The combined macular ganglion cell and inner plexiform layer thickness (GCIPL) were measured using a 3.45-mm diameter cylinder around the fovea from a macular volume scan. The peripapillary retinal nerve fiber layer thickness (pRNFL) was measured using 12° ring scans around the optic nerve head with activated eye tracker.
Statistical Testing
All parameters are described as mean ± SD, if not stated otherwise. Subset analyses were performed where necessary based on eye-specific ON history. Cross-sectional and longitudinal group differences and correlations were tested by linear mixed-effect models accounting for within-subject intereye correlations as a random intercept and controlling for age, sex, and center as fixed effect (if necessary). Statistical significance was established at p < 0.05. Analyses were performed with R 4.1.0. Figures were created using R and Adobe Illustrator.
Data Availability
All anonymized data are available from the corresponding author on reasonable request with limitations only subject to patients' privacy.
Results
Cohort Description
Two hundred ninety-three eyes of 147 patients with chronic RRMS (time since onset [months, median {IQR}]: 46 [12–90]) were included (Charité-Universitätsmedizin Berlin N = 41, UCSF N = 27, Universitätsklinikum Düsseldorf N = 71, Universitätsklinikum Münster N = 8). Forty-one eyes had a history of ON ≥6 months before baseline (CHRONIC-ON, time since ON [months, median {IQR}]: 23 [12–51], see abovementioned exclusion criteria), and 252 eyes had no history of ON (CHRONIC-NON) (Table 1).
Table 1.
Cohort Description
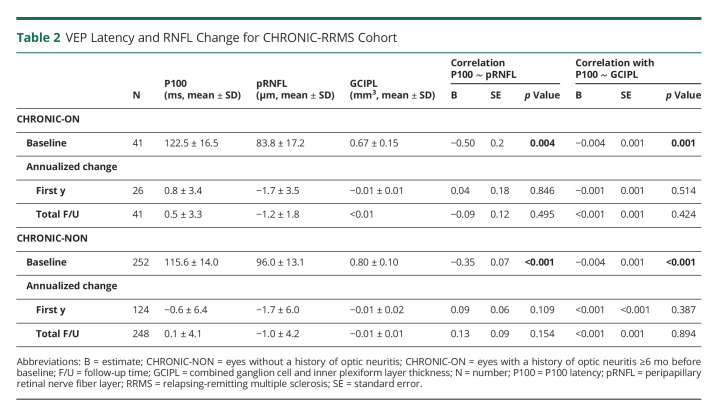
VEP in Chronic RRMS
At baseline, CHRONIC-ON had reduced pRNFL ([μm], B = −14.2, standard error [SE] = 1.8, p < 0.001), GCIPL ([mm3], B = −0.14, SE = 0.02, p < 0.001), and prolonged P100 latency ([ms], B = 8.9, SE = 2.1, p < 0.001) compared with CHRONIC-NON. The amount of neuroaxonal damage (pRNFL and GCIPL) was associated with P100 latency for CHRONIC-ON and CHRONIC-NON (Table 2).
Table 2.
VEP Latency and RNFL Change for CHRONIC-RRMS Cohort
To investigate the hypothesis that a change in P100 latency would be associated with subsequent retinal neurodegeneration, we tested neuroaxonal loss in CHRONIC patients dichotomized by median P100 latency change within the rounded first year (above vs below the median of 1.5 ms). Participants with P100 latency prolongation ≥1.5 ms during their (rounded) first year of F/U had stronger subsequent GCIPL thinning during the full subsequent F/U (starting at rounded year 1) (B = 0.21, SE = 0.06, p = 0.001) and the first 36 months of F/U (B = 0.34, SE = 0.12, p = 0.001). This effect was not evident in the analysis limited to the CHRONIC-ON subset (median first-year change 1.0 ms; full F/U: B = 0.67, SE = 0.28, p = 0.13; 36 months: B = 1.08, SE = 1.09, p = 0.68) but remained for the CHRONIC-NON subset (median first-year change: 1.5 ms; full F/U: B = 0.16, SE = 0.06, p = 0.016; 36 months: B = 0.30, SE = 0.12, p = 0.019) (Figure 1). In contrast to the prognostic potential of first-year VEP change, the neuroaxonal loss in patients dichotomized by the traditional device-specific VEP threshold at baseline did not differ longitudinally (eTable 1, links.lww.com/NXI/A809).
Figure 1. Plots of Longitudinal Change in GCIPL Change.
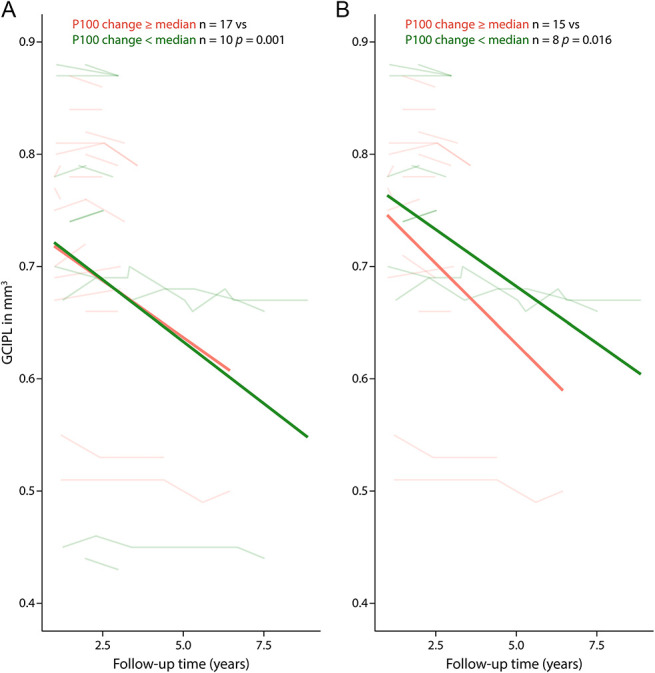
For eyes with high (salmon) and low (green) P100 latency change within the first year of F/U for (A) all CHRONIC eyes and (B) CHRONIC-NON. Transparent lines for eye-based values and thicker line for fitted linear mixed model. Plotted for full F/U starting at year 1. CHRONIC-NON = eyes without a history of optic neuritis; CHRONIC = all eyes of the chronic data set; F/U = follow-up; GCIPL = combined ganglion cell and inner plexiform layer.
Next, we excluded the possibility that the change in P100 latency and retinal neurodegeneration was contemporaneous: During F/U in CHRONIC-ON and CHRONIC-NON, pRNFL and GCIPL thinned over time, whereas P100 latency did not change significantly (Figure 2, CHRONIC-ON pRNFL: B = −0.04, SE = 0.01, p < 0.001; GCIPL: B < −0.001, SE < 0.001, p < 0.001; CHRONIC-NON: pRNFL: B = −0.05, SE = 0.01, p < 0.001; GCIPL: B < 0.001, SE < 0.001, p < 0.001) The longitudinal changes in P100 latency, pRNFL, and GCIPL did not differ between CHRONIC-ON and CHRONIC-NON. In addition, the rate of change in neuroaxonal parameters (pRNFL and GCIPL) and P100 latency were not correlated for the first year or for the total F/U time. VEP latency did differ cross-sectionally but not longitudinally between centers using protocols with 41′ vs 60′ check sizes—and no differences were found between centers using protocols with the same check size (eTable2, links.lww.com/NXI/A809).
Figure 2. Plots of Longitudinal Change in (A) pRNFL, (B) GCIPL, and (C) P100 Latency.
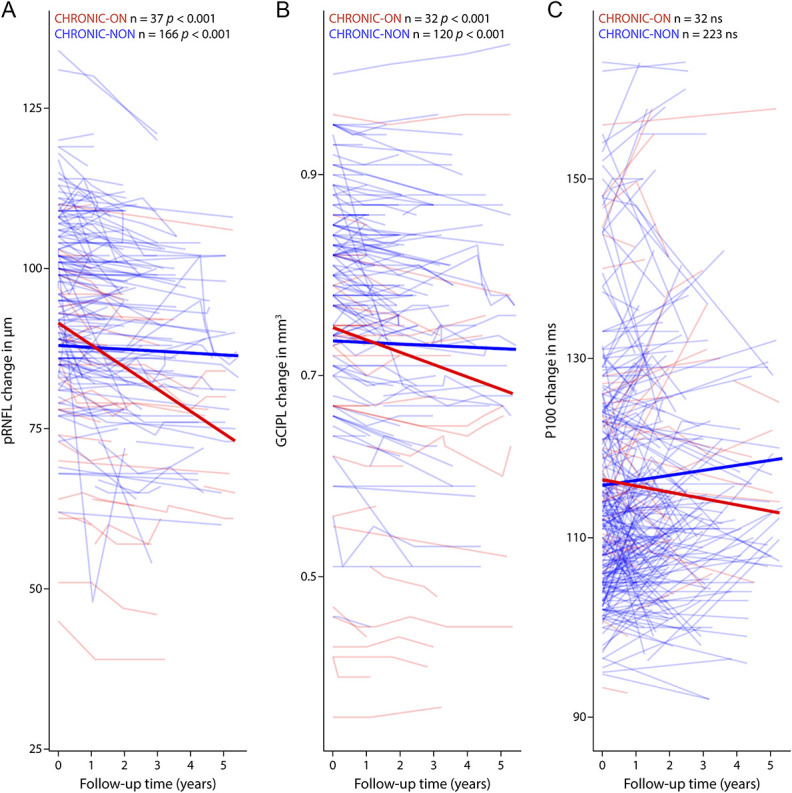
For CHRONIC-ON (red) and CHRONIC-NON (blue) with transparent lines for eye-based values and thicker line for fitted linear-mixed model. Plotted until F/U year 5. CHRONIC-NON = eyes without a history of optic neuritis; CHRONIC-ON = eyes with a history of optic neuritis; F/U = follow-up; GCIPL = combined ganglion cell and inner plexiform layer; P100 = P100 latency; pRNFL = peripapillary retinal nerve fiber layer.
Discussion
This study provides evidence that increasing VEP latency delay is predictive of subsequent neuroaxonal loss in the anterior visual pathway particularly in the setting of chronic demyelination (i.e., non-ON eyes of patients with preexisting RRMS). Of importance, this suggests that chronic demyelination may be a major driver of neuroaxonal loss and related permanent disability and may help us understand an important component of the biological basis behind disease progression independent of relapse activity.38
Our results indicate that P100 latency change can serve as a prognostic tool for retinal ganglion cell loss in patients with RRMS. This is important because it documents that chronic neurodegeneration in a specific pathway is determined by prior demyelination in that pathway. This observation may help us understand why immunotherapies capable of preventing all—or nearly all—new gadolinium-enhancing lesions, and relapses are still only partially effective at preventing disability accumulation. Furthermore, we already know that GCIPL in non-ON eyes predicts medium to long-term disability.39 Therefore, the findings here extend prior limited evidence,20 suggesting that VEP latency change may have predictive capacity for longer-term general disease progression.
On the contrary, VEP baseline values or change rates were not found to be predictive for GCIPL loss after ON—with several potential explanations. In the subacute stage, neuroaxonal loss might occur partially independent of prior demyelination, and neuroaxonal loss might be hard to differentiate from the reduction of axoedema. Moreover, it is important to note that axons that do not conduct (either because they are lost, disrupted, or have conduction failure or block) cannot contribute to the VEP. In a related fashion, more severe inflammation with axon loss as a bystander phenomenon may lead to decoupling of the relationship between axonal loss and myelin injury—especially because disrupted axons do not contribute to the VEP latency—and may be associated with apparent latency improvement (i.e., better myelinated axons are the only ones left behind). In addition, with active inflammation, concurrent remyelination and demyelination may reduce VEPs capacity to document the component of myelin loss that is permanent or persistent. Last, demyelination and subsequent neuroaxonal loss might be temporally more closely associated so that the impact of demyelination on axonal loss cannot be resolved at the time scale in which measurements were performed. All these processes could potentially happen in parallel in ON eyes leading to a dissociation of GCIPL and VEP measurements.
The predictivity of eyes without any history of ON did not differ from non-ON eyes from patients with a history of contralateral ON long term. Although our sample was too small to completely exclude all effects on non-ON eyes, the predictive value of VEP latency in non-ON eyes seems promising. Similar results between centers and OCT protocols also encourage the use of VEP in large multicenter clinical trials.
The involvement of multiple centers in this analysis likely contributes to a higher variability and heterogeneity of data and time points, but this makes these results applicable to routine clinical practice. As in this study, standardized equipment and protocols will be necessary for a clinical application of VEP as a prognostic marker in the future. The lack of consistency between the sites regarding data on visual performance and clinical disability limited our ability to draw conclusions regarding VEP's capacity to predict clinical function. Furthermore, the understanding of the full capacity of VEP latency as an outcome parameter in ON eyes will require a larger systematic study with multiple prespecified time points for assessment after ON or a larger clinical trial for remyelinating agents that consider ON as a stratification variable.
Prior small studies investigated the value of evoked potentials as potential predictors of long-term disability.20,40 With the burgeoning development of remyelinating treatments, VEP and other evoked potentials should be investigated and understood in depth before being applied in clinical trials so as to avoid frustrating and potentially costly trial failures. The data here demonstrate that VEPs in non-ON eyes provide a promising marker for demyelination in chronic RRMS with prognostic value for subsequent retinal ganglion cell loss.
Acknowledgment
The authors thank Daisy Perez and Denise Bolivar for their excellent technical support.
Glossary
- CHRONIC-NON
eyes without a history of optic neuritis
- CHRONIC-ON
eyes with a history of optic neuritis
- DMTs
disease-modifying treatments
- F/U
follow-up
- GCIPL
ganglion cell and inner plexiform layer thickness
- ISCEV
International Society for Clinical Electrophysiology of Vision
- MS
multiple sclerosis
- OCT
optical coherence tomography
- ON
optic neuritis
- pRNFL
peripapillary retinal nerve fiber layer thickness
- RRMS
relapsing-remitting MS
- SE
standard error
- VEPs
visual evoked potentials
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
F.C. Oertel reports grants from National Multiple Sclerosis Society, grants from American Academy of Neurology, grants from Deutsche Gesellschaft für Neurologie, all outside the submitted work. J. Krämer received honoraria for lecturing for Biogen, Novartis, Mylan, Teva, Roche, Sanofi, and Genzyme and financial research support from Sanofi, Genzyme, Roche, and American Therapeutics. S. Motamedi has nothing to disclose. A. Keihani has nothing to disclose. H. Zimmermann reports grants from Novartis and personal fees from Bayer Healthcare, all outside the submitted work. N.G. Dimitriou has nothing to disclose. S. Condor-Montes has nothing to disclose. C. Bereuter has nothing to disclose. C. Cordano has nothing to disclose. A.C. Abdelhak has nothing to disclose. A. Trip has nothing to disclose. O. Aktas reports honoraria for lectures and support for attending meetings by Alexion, Bayer, Biogen, Novartis, Roche, and Sanofi, all outside the submitted work. S.G. Meuth has nothing to disclose. H. Wiendl reports personal fees from Biogen, Genzyme, Merck Serono, Novartis, Roche Pharma AG, Sanofi-Aventis, UCB, Alexion, Biologix, Cognomed, F. Hoffmann-La Roche Ltd, Gemeinnützige Hertie-Stiftung, TEVA, WebMD Global, Actelion, IGES, Johnson & Johnson, and Swiss Multiple Sclerosis Society, grants from German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, European Union, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, and GlaxoSmithKline, all outside the submitted work. K. Ruprecht reports grants from Novartis Pharma, Merck Serono, German Ministry of Education and Research, European Union (821,283-2), Stiftung Charité, and Arthur Arnstein Foundation and other from Guthy Jackson Charitable Foundation, all outside the submitted work. J. Bellmann-Strobl reports personal fees and nonfinancial support from Sanofi-Aventis GmbH, personal fees and nonfinancial support from Roche Pharma AG, personal fees and nonfinancial support from Bayer AG, and personal fees from Merck Serono GmbH, al outside the submitted work. F. Paul reports research support from Bayer, Novartis, Biogen, Teva, Sanofi-Aventis/Genzyme, Alexion, and Merck Serono and research support from the German Research Council, Werth Stiftung of the City of Cologne, German Ministry of Education and Research, Arthur Arnstein Stiftung Berlin, EU FP7 Framework Program, Guthy-Jackson Charitable Foundation, and NMSS. He also reports consulting fees as an associate editor for Neurology, Neuroimmunology & Neuroinflammation and as an academic editor for PloS ONE and consultant fees for Sanofi Genzyme, Biogen, MedImmune, Shire, and Alexion. He also reports speaker honoraria from Bayer, Novartis, Biogen, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, and Shire. He is advisory board member for Novartis and MedImmune Scientific and holds stocks of Nocturne GmbH—all outside the submitted work. A. Petzold reports personal fees from Novartis, Heidelberg Engineering, and Zeiss, reports grants from Novartis, outside the submitted work; and is part of the steering committee of the OCTiMS study that is sponsored by Novartis and the Angio-OCT steering committee, which is sponsored by Zeiss. He does not receive compensation for these activities. AS reports compensation for consulting services and speaker honoraria from Merck-Serono, Biogen-Idec, Sanofi, Novartis, Roche, Janssen, and Alexion. A.U. Brandt reports grants from Deutsche Forschungsgemeinschaft, during the conduct of the study; shares from Nocturne GmbH, and shares from Motognosis GmbH, outside the submitted work; In addition, A.U. Brandt has multiple patents regarding retinal image analysis technology owned by Charite and UCI and licensed to Nocturne GmbH. A.J. Green reports other from Bionure, grants, personal fees, and other from Inception Sciences, grants from Sherak Foundation, personal fees and other from Pipeline Pharmaceuticals, grants from Hilton Foundation, grants from Adelson Foundation, grants from National MS Society, personal fees from JAMA Neurology, personal fees and other from Mediimmune/Viela, outside the submitted work; In addition, A.J. Green has a patent small molecule drug for remyelination pending and has worked on testing off-label compounds for remyelination. Go to Neurology.org/NN for full disclosures.
References
- 1.Thompson AJ, Banwell BL, Barkhof F, et al. . Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi. 10.1016/s1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 2.Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med. 2020;133(12):1380-1390.e2. doi. 10.1016/j.amjmed.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol. 2006;5(2):158-170. doi. 10.1016/s1474-4422(06)70349-0. [DOI] [PubMed] [Google Scholar]
- 4.Giovannoni G, Bermel R, Phillips T, Rudick R. A brief history of NEDA. Mult Scler Relat Disord. 2018;20:228-230. doi. 10.1016/j.msard.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Green AJ, Gelfand JM, Cree BA, et al. . Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481-2489. doi. 10.1016/s0140-6736(17)32346-2. [DOI] [PubMed] [Google Scholar]
- 6.Wooliscroft L, Altowaijri G, Hildebrand A, et al. . Phase I randomized trial of liothyronine for remyelination in multiple sclerosis: a dose-ranging study with assessment of reliability of visual outcomes. Mult Scler Relat Disord. 2020;41:102015. doi. 10.1016/j.msard.2020.102015. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich M, Helling N, Hilla A, et al. . Early alpha-lipoic acid therapy protects from degeneration of the inner retinal layers and vision loss in an experimental autoimmune encephalomyelitis-optic neuritis model. J Neuroinflammation. 2018;15(1):71. doi. 10.1186/s12974-018-1111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadavid D, Balcer L, Galetta S, et al. . Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16(3):189-199. doi. 10.1016/s1474-4422(16)30377-5. [DOI] [PubMed] [Google Scholar]
- 9.Bove RM, Green AJ. Remyelinating pharmacotherapies in multiple sclerosis. Neurotherapeutics. 2017;14(4):894-904. doi. 10.1007/s13311-017-0577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mei F, Lehmann-Horn K, Shen Y-AA, et al. . Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. eLife. 2016;5:e18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y, Morrison BM, Li Y, et al. . Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443-448. doi. 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fünfschilling U, Supplie LM, Mahad D, et al. . Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485(7399):517-521. doi. 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laule C, Vavasour IM, Kolind SH, et al. . Magnetic resonance imaging of myelin. Neurotherapeutics. 2007;4(3):460-484. doi. 10.1016/j.nurt.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heath F, Hurley SA, Johansen-Berg H, Sampaio-Baptista C. Advances in noninvasive myelin imaging. Dev Neurobiol. 2018;78(2):136-151. doi. 10.1002/dneu.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silbermann E, Wooliscroft L, Bourdette D. Using the anterior visual system to assess neuroprotection and remyelination in multiple sclerosis trials. Curr Neurol Neurosci Rep. 2018;18(8):49. doi. 10.1007/s11910-018-0858-y. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Hyun J-W, Lee J, et al. . So you want to image myelin using MRI: an overview and practical guide for myelin water imaging. J Magn Reson Imaging. 2021;53(2):360-373. doi. 10.1002/jmri.27059. [DOI] [PubMed] [Google Scholar]
- 17.Heidari M, Radcliff AB, McLellan GJ, et al. . Evoked potentials as a biomarker of remyelination. Proc Natl Acad Sci U S A. 2019;116(52):27074-27083. doi. 10.1073/pnas.1906358116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlaeger R, Hardmeier M, D'Souza M, et al. . Monitoring multiple sclerosis by multimodal evoked potentials: numerically versus ordinally scaled scoring systems. Clin Neurophysiol. 2016;127(3):1864-1871. doi. 10.1016/j.clinph.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Schlaeger R, D'Souza M, Schindler C, Grize L, Kappos L, Fuhr P. Prediction of MS disability by multimodal evoked potentials: investigation during relapse or in the relapse-free interval? Clin Neurophysiol. 2014;125(9):1889-1892. doi. 10.1016/j.clinph.2013.12.117. [DOI] [PubMed] [Google Scholar]
- 20.Schlaeger R, D'Souza M, Schindler C, Grize L, Kappos L, Fuhr P. Combined evoked potentials as markers and predictors of disability in early multiple sclerosis. Clin Neurophysiol. 2012;123(2):406-410. doi. 10.1016/j.clinph.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Brown JWL, Cunniffe NG, Prados F, et al. . Safety and efficacy of bexarotene in patients with relapsing-remitting multiple sclerosis (CCMR One): a randomised, double-blind, placebo-controlled, parallel-group, phase 2a study. Lancet Neurol. 2021;20(9):709-720. doi. 10.1016/s1474-4422(21)00179-4. [DOI] [PubMed] [Google Scholar]
- 22.Henderson APD, Altmann DR, Trip AS, et al. . A serial study of retinal changes following optic neuritis with sample size estimates for acute neuroprotection trials. Brain. 2010;133(9):2592-2602. doi. 10.1093/brain/awq146. [DOI] [PubMed] [Google Scholar]
- 23.Henderson APD, Altmann DR, Trip SA, et al. . Early factors associated with axonal loss after optic neuritis. Ann Neurol. 2011;70(6):955-963. doi. 10.1002/ana.22554. [DOI] [PubMed] [Google Scholar]
- 24.Odom JV, Bach M, Brigell M, et al. . ISCEV standard for clinical visual evoked potentials: (2016 update). Doc Ophthalmol. 2016;133(1):1-9. doi. 10.1007/s10633-016-9553-y. [DOI] [PubMed] [Google Scholar]
- 25.Oertel FC, Zimmermann HG, Brandt AU, Paul F. Novel uses of retinal imaging with optical coherence tomography in multiple sclerosis. Expert Rev Neurother. 2019;19(1):31-43. [DOI] [PubMed] [Google Scholar]
- 26.Andorrà M, Alba-Arbalat S, Camos-Carreras A, et al. . Using acute optic neuritis trials to assess neuroprotective and remyelinating therapies in multiple sclerosis. JAMA Neurol. 2020;77(2):234-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costello F. Evaluating the use of optical coherence tomography in optic neuritis. Mult Scler Int. 2011;2011:148394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costello F, Pan YI, Yeh EA, Hodge W, Burton JM, Kardon R. The temporal evolution of structural and functional measures after acute optic neuritis. J Neurol Neurosurg Psychiatry. 2015;86(12):1369-1373. doi. 10.1136/jnnp-2014-309704. [DOI] [PubMed] [Google Scholar]
- 29.Brusa A, Jones SJ, Plant GT. Long-term remyelination after optic neuritis: a 2-year visual evoked potential and psychophysical serial study. Brain. 2001;124(3):468-479. doi. 10.1093/brain/124.3.468. [DOI] [PubMed] [Google Scholar]
- 30.Janáky M, Jánossy Á, Horváth G, Benedek G, Braunitzer G. VEP and PERG in patients with multiple sclerosis, with and without a history of optic neuritis. Doc Ophthalmol. 2017;134(3):185-193. doi. 10.1007/s10633-017-9589-7. [DOI] [PubMed] [Google Scholar]
- 31.Berman S, Backner Y, Krupnik R, et al. . Conduction delays in the visual pathways of progressive multiple sclerosis patients covary with brain structure. Neuroimage. 2020;221:117204. [DOI] [PubMed] [Google Scholar]
- 32.Backner Y, Kuchling J, Massarwa S, et al. . Anatomical wiring and functional networking changes in the visual system following optic neuritis. JAMA Neurol. 2018;75(3):287-295. doi. 10.1001/jamaneurol.2017.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. doi. 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 34.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. doi. 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. . The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303-2309. doi. 10.1212/wnl.0000000000002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aytulun A, Cruz-Herranz A, Aktas O, et al. . The APOSTEL 2.0 recommendations for reporting quantitative optical coherence tomography studieson behalf of the American Academy of Neurology. Neurology. 2021;97(2):68-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tewarie P, Balk L, Costello F, et al. . The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823. doi. 10.1371/journal.pone.0034823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kappos L, Wolinsky JS, Giovannoni G, et al. . Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132-1140. doi. 10.1001/jamaneurol.2020.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmermann HG, Knier B, Oberwahrenbrock T, et al. . Association of retinal ganglion cell layer thickness with future disease activity in patients with clinically isolated syndrome. JAMA Neurol. 2018;75(9):1071. doi. 10.1001/jamaneurol.2018.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlaeger R, D'Souza M, Schindler C, et al. . Prediction of long-term disability in multiple sclerosis. Mult Scler. 2012;18(1):31-38. doi. 10.1177/1352458511416836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All anonymized data are available from the corresponding author on reasonable request with limitations only subject to patients' privacy.