Abstract
Introduction:
Reward deficits negatively impact recovery from substance use disorder (SUD). LETS ACT, a behavioral activation treatment targeting substance-free reward, has demonstrated effectiveness in reducing post treatment substance use. There remains room for modifications to extend recovery gains, and LETS ACT remains largely untested in outpatient treatment. We tested the effect of LETS ACT when delivered alongside intensive outpatient SUD treatment, with and without a smartphone app designed to extend access to treatment content outside of clinician-administered sessions.
Methods:
In this three-arm randomized controlled trial (N=206; 54% White, 67% male), all participants received intensive outpatient SUD treatment as usual (TAU) and either LETS ACT (n=56), smartphone-enhanced LETS ACT (n=65), or assessments only (n=61). Substance use days and substance related problems were assessed through 12 months posttreatment.
Results:
Generalized estimating equations indicated a significant condition*time interaction for substance use days; Days of substance use significantly declined from pretreatment until 1-month for TAU, 3-months for LETS ACT-SE, and 6-months for LETS ACT. Decreases in substance-related problems were maintained across all conditions through 12 months.
Conclusions:
Adding LETS ACT to intensive outpatient treatment resulted in significant decreases in substance use through 6 months posttreatment, yet these gains were not sustained through 12 months posttreatment. A smartphone app did not facilitate superior treatment outcomes. Future studies should consider factors impacting treatment efficacy in outpatient settings and the utility of providing more than six sessions of behavioral activation.
Keywords: Substance Use Disorder, Smartphone App, mHealth, Behavioral Activation, Treatment
1. Introduction
One of the many challenges individuals entering treatment for substance use disorder (SUD) face during recovery is the depletion of reward sensitivity and substance-free forms of reinforcement. These deficits stem from the effect of SUD on the availability of, and ability to experience pleasure from, substance-free rewards (Acuff et al., 2019; Baskin-Sommers & Foti, 2015; Koob & Volkow, 2010). Recovery efforts are therefore difficult to sustain due to the heightened reward value and availability of substance use.
The Life Enhancement Treatment for Substance Use (LETS ACT) is a behavioral activation (BA) treatment that aims to increase the availability of substance-free rewards and the frequency of daily substance-free positive reinforcement. LETS ACT delivered in small groups to low-income adults with an SUD enrolled in publicly funded residential treatment improves treatment retention (Magidson et al., 2011) and reduces the likelihood of returning to substance use and related problems up to one year posttreatment (Daughters et al., 2018) compared to control conditions. BA has also demonstrated effectiveness in treating smoking (MacPherson et al., 2010) and hazardous alcohol use and related problems among college students (Reynolds et al., 2011). A systematic review concluded that BA has a significant effect on substance use and problems (Fazzino et al., 2019).
Despite growing empirical support for LETS ACT, there remains room for improvement and expansion. A substantial proportion of patients who receive abstinence-focused treatment supplemented with LETS ACT return to substance use, suggesting a need for modifications that would support sustained treatment gains (Daughters et al., 2018). Given the importance of treatment engagement and homework completion in cognitive-behavioral therapy broadly (e.g., Decker et al., 2016), and of daily activity planning and completion in BA in particular, targeting engagement in treatment skills outside of sessions represents a promising next step. Smartphone apps have the potential to bolster skill building and treatment engagement via theoretically based guidance, prompts, and reminders and in the context of SUD treatment have proven to be accessible, acceptable, and an effective means of behavior change (Carreiro et al., 2020; Dahne & Lejuez, 2015; Fowler et al., 2016; Meshesha et al., 2020; Tofighi et al., 2019). To this end, we designed a LETS ACT smartphone app to increase access to BA during and after the conclusion of the clinician-administered treatment sessions (Paquette et al., 2021).
In addition to the importance of increasing accessibility in low resource settings, most adults with an SUD receive outpatient treatment services, with the proportion receiving residential or inpatient treatment declining yearly over the past decade (SAMHSA, 2021). It is therefore important to test the effect of LETS ACT when administered to adults enrolled in publicly funded outpatient treatment. Compared to residential settings, a possible advantage of outpatient treatment is patient access to daily out-of-session engagement in value-based activities within their natural environment, a key mechanism of BA. Evidence from a pilot trial of LETS ACT delivered in an individual outpatient format to adults with co-occurring depression and SUD in the United Kingdom indicates superior effects on depression and substance use compared to treatment as usual up to three months posttreatment (Pott et al., 2022).
The aims of this study were to test the effect of LETS ACT when administered in conjunction with a publicly funded outpatient SUD treatment program, and whether a smartphone app improves recovery outcomes. It was predicted that patients who received the smartphone-enhanced condition (LETS ACT-SE) would outperform standard LETS ACT, and that both conditions would outperform those who only received the outpatient treatment as usual (TAU), on the number of substance use days and related problems up to one year posttreatment.
2. Material and Methods
2.1. Study Design
This study was a single-site three-arm clinical trial (Technology Enhanced Behavioral Activation Treatment for Substance Use, NCT#: 02707887) conducted at an intensive outpatient SUD treatment center in Raleigh, NC. All participants (N=206) received treatment as usual (TAU). Participants were randomized to LETS ACT (n=77), LETS ACT-SE (n=68), or TAU with assessments only (n=61). Assessments were conducted by trained research assistants and occurred at pre- and posttreatment and at 1, 3, 6, and 12-month posttreatment follow-ups. All study procedures received Institutional Review Board approval.
2.2. Sample recruitment and retention
The research team recruited patients who were currently enrolled in an intensive outpatient SUD treatment program between February of 2016 and April of 2019; recruitment ended when the target sample size had been reached. Target sample size was determined using an empirical power analysis to ensure the ability to detect clinically meaningful differences in our outcomes between conditions over time. Those who were interested in participating were assessed for eligibility, provided informed consent, and completed the pre-treatment assessment. Randomization occurred at the group level and was conducted by an off-site consultant using computerized urn randomization procedures (Stout et al., 1994), with 3 conditions. Participants were recruited in groups (N=64) and unaware of condition assignments. Study exclusion criteria were: (1) aged >65 or <18, (2) < fifth grade English reading level (i.e., score <42 on the Wide Range Achievement Test – Word Reading Subtest (Jastak & Wilkinson, 1984), (3) current psychotic symptoms (measured by the Mini-International Neuropsychiatric Interview 7.0 (MINI; Sheehan et al., 1998), (4) completion of > four weeks of TAU, and (5) inability to provide written informed consent to participate. Participants completed posttreatment and follow-up assessments at the outpatient treatment facility or a public location with adequate privacy (e.g., public library). Follow-up assessments occurred between February of 2016 and June of 2020. Study flow from recruitment to analysis is reported in Figure 1. Follow-up rates at each assessment ranged from 75.38% to 95.08%, excluding individuals who were withdrawn from the study due to nonattendance following the pretreatment assessment and therefore not followed subsequently. In total, 83.98% randomized participants attended at least one posttreatment or follow-up assessment and 64.83% of those retained beyond the pretreatment assessment attended all six assessments.
Figure 1.
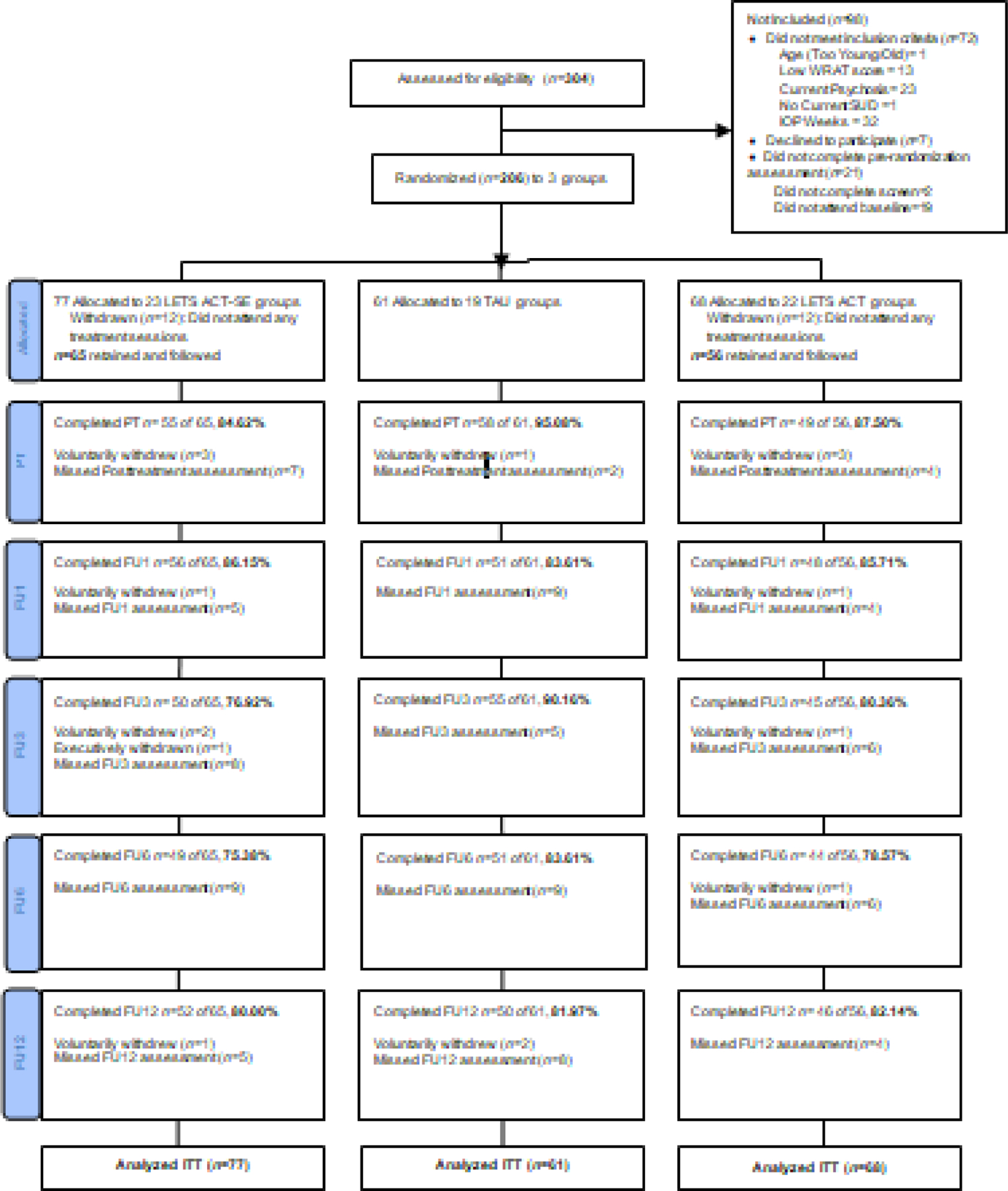
CONSORT Diagram
= Life Enhancement Treatment for Substance Use, Smartphone-Enhanced Condition; TAU = Treatment as Usual; LETS ACT = Life Enhancement Treatment for Substance Use; PT = Posttreatment; FU1 = 1 month follow-up; FU3 = 3 month follow-up; FU6 = 6 month follow-up; FU12 = 12 month follow-up; ITT = Intent to treat
2.3. Intervention
2.3.1. Treatment as Usual (TAU)
All study participants were enrolled in an abstinence-based SUD intensive outpatient program (IOP) which is based on the Matrix Model of Intensive Outpatient Treatment (Rawson et al., 1995). Main components of the program include 12 weeks of group therapy for three hours per day, three days per week, weekly individual case management appointments, and up to two optional individual counseling sessions per week. Group sessions do not have a set curriculum, but typically include individual check-ins, psychoeducation, and time to verbally process and share. Abstinence from substance use is required and patients are aware that a positive urine drug screen administered by the treatment program may result in dismissal.
2.3.2. LETS ACT
The Life Enhancement Treatment for Substance Use (LETS ACT; Daughters et al., 2018) is a group-based brief BA treatment for substance use. Six one-hour sessions were provided in small groups of six or fewer participants twice weekly over three weeks. Sessions began with discussion of the treatment rationale, which is a functional analysis describing a cycle of negative mood, urges, and maladaptive behaviors (e.g., substance use). Participants learn that the goal of treatment is to break this cycle by engaging in healthy, rewarding behaviors that generate a sense of enjoyment and/or accomplishment, which in turn helps reduce the likelihood of experiencing urges to use substances or engage in other maladaptive behaviors in response to difficult emotions. Following the treatment rationale, participants record daily activities and rate them on enjoyment and importance in order to identify patterns of inactivation and opportunities to increase positive reinforcement. Next, emphasis shifts to identifying value-based activities within a variety of life areas (e.g., education and work, emotional health, hobbies and recreation, relationships). In later sessions, the focus shifts to planning and implementing value-based activities in a daily plan, problem-solving challenges to adherence, and posttreatment planning (see Daughters et al., 2016 for a detailed description of session content).
2.3.3. Smartphone-Enhanced LETS ACT
The smartphone app was developed as an adjunct to LETS ACT; the development, design, feasibility, and use of the app have been described previously in a manuscript available via open access (Paquette et al., 2021). In the Smartphone-Enhanced condition (LETS ACT-SE), participants were provided with Apple iPhone 6 smartphones with the installed LETS ACT app at the second treatment session. Participants were introduced to each app component during the sessions, with a quick therapist-led tutorial followed by in-session practice. Participants were asked to use the LETS ACT app to complete treatment homework. Primary features and functions of the app included the life areas, values, and activities (LAVA) library, plan ahead, daily plan, weekly progress, and emergency button, accessible via icons on the home screen. The LAVA library stores user-generated value-based activities. The plan ahead feature allows the app user to schedule value-based activities for specific days and times. The daily plan feature lists planned activities for the coming week, which app users can mark as complete by checking a box. Weekly progress allows the app user to view their proportion of completed activities. The emergency button is available for high-risk situations and lists user-generated activity or healthy coping behavior options. Participants were permitted to keep the smartphones until the three-month posttreatment follow-up appointment, at which time they returned the phones to the research team. Phone plans (which included calls, texting, and wireless data) were set up and paid for by the research study. The majority of participants (37/54, 69%) self-reported use of the LETS ACT app until one month posttreatment, with this proportion decreasing significantly (to 20/54, 37%) by 3 months posttreatment (see Paquette et al., 2021 for more detailed data on LETS ACT app usage and acceptability).
2.3.4. Treatment Attendance and Fidelity
Participants attended an average of 4 of the 6 BA sessions (range=1–6, SD=1.83). A two-tailed t-test indicated that there were no significant differences in number of BA sessions attended between the LETS ACT (mean=4.29) and LETS ACT-SE (mean=3.75) conditions (t(119)=1.61, p=.11).
Study therapists included clinical psychology doctoral students and postdoctoral fellows. Therapist training included didactics, observation, and role-plays and each therapist was trained to administer both conditions. Treatment manuals were followed in both conditions to ensure standardization of treatment and consistency of delivery (Bellg et al., 2004). Therapists (n=9) provided treatment to a total of 45 groups (LETS ACT n=23; LETS ACT-SE n=22), with each therapist averaging 5.0±7.4 groups (range=1–13) and a total of 3.2±1.3 participants (range=3–42). Study therapists were not involved in any research procedures for their groups. All therapy sessions were audiotaped, and clinical supervision was provided weekly for both conditions. Adherence forms were completed for each session and 20% of session audiotapes (n=50) were randomly selected and rated by a trained independent rater. The mean percent adherence for each component within each session indicate a high level of adherence (LETS ACT=93.8±4.7, LETS ACT-SE=95.8±4.4), with no significant differences between BA conditions (t(47)=1.55, p=.13).
2.4. Measures and Outcome Variables
Participants self-reported sociodemographic information including age, race/ethnicity, sex, education level, and income. Trained interviewers administered the MINI 7.0 (Sheehan et al., 1998) at pretreatment to assess for mood, anxiety, and SUD diagnoses using diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Interrater reliability on the MINI was determined via assessment of 10% of audio-recorded baseline clinical interviews by independent, secondary raters blind to original score and diagnosis. The reliability statistic across diagnostic categories was 0.95.
2.4.1. Outcome Measures
The primary outcome representing frequency of substance use was self-reported substance use days, defined as the number of days of any alcohol or illicit substance use within each assessment period for substances for which participants met SUD diagnosis at pretreatment, self-reported using the clinician administered Timeline Followback (TLFB; Sobell et al., 1979). The TLFB is a gold-standard self-report measure of substance use that has high agreement with biological measures (Hjorthoj et al., 2012). The pretreatment time period consisted of the 30 days prior to beginning the IOP program. Subsequent timepoints consisted of the days between each assessment (e.g., pretreatment assessment date to posttreatment assessment date).
Substance-related problems were assessed using the Short Inventory of Problems-Alcohol and Drugs (SIP-AD; Blanchard et al., 2003), a 15-item measure with excellent reliability (α=.95; Kiluk et al., 2013). Individual item responses range from 0–4 (never to daily/almost daily during the past month), with a total score range of 0–60 that reflects adverse consequences from substance use across physical, inter-personal, intra-personal, impulse control and social responsibility domains. Higher scores reflect a greater frequency of adverse consequences attributed to drug and alcohol use. Internal reliability in the current study was α=.98. Given the 1-month time period assessed by the SIP-AD, this measure was administered at pretreatment in reference to the 30-days prior to IOP, and all follow-up assessments except posttreatment because the pretreatment to posttreatment assessment time window was only 3 weeks.
2.5. Statistical Analyses
2.5.1. Covariate Selection
Two covariates were selected a priori based on hypothesized relevance to the outcomes of interest and were included in all analyses. These included the number of days in the IOP program at the pretreatment assessment and the number of full days participants spent in a restricted environment (e.g., hospital, jail, inpatient treatment center) within each assessment period. Chi-square analyses and one-way ANOVAs were used to test group equivalence between the three treatment conditions across demographic variables (age, gender, race/ethnicity, education level, income), DSM-5 diagnoses (see Table 1 for list), and pretreatment substance use (frequency of use in the 30 days before treatment and number of days since last use of any substance). Categories represented in <5% of the sample were excluded from these analyses and are summarized in Supplementary Table 1. To examine if DSM-5 diagnoses were associated with the primary outcomes, omnibus tests were conducted with main effects of these variables included as fixed effects. For each primary outcome (i.e., substance use frequency and substance-related problems), one omnibus test was conducted with all SUD diagnoses coded by severity level (0=no diagnosis, 1=mild, 2=moderate, 3=severe) as well as a variable representing the total number of SUD diagnoses for which the participant met criteria at pretreatment. A second omnibus test included all DSM-5 psychiatric diagnoses.
Table 1.
Pre-treatment sample characteristics
| Total Sample | LETS ACT- | LETS ACT | TAU | |
|---|---|---|---|---|
| (n=206) | SE (n=77) | (n=68) | (n=61) | |
| Age, mean (SD) | 40.29 (11.06) | 41.62 (10.56) | 39.40 (11.63) | 39.61 (11.03) |
| Race/Ethnicity, # (%) | ||||
| White/Caucasian | 111 (53.9) | 45 (58.4) | 40 (58.8) | 26 (42.6) |
| Black/African American | 70 (34.0) | 26 (33.8) | 20 (29.4) | 24 (39.3) |
| Hispanic/Latino | 1 (.5) | 1 (1.3) | 0 (0.0) | 0 (0.0) |
| Native American/American Indian | 4 (1.9) | 1 (1.3) | 1 (1.5) | 2 (3.3) |
| Other Ethnicity | 3 (1.5) | 0 (0.0) | 1 (1.5) | 2 (3.3) |
| Multi-racial/Multi-ethnic | 17 (8.3) | 4 (5.2) | 6 (8.8) | 7 (11.5) |
| Sex, # (%) | ||||
| Female | 69 (33.5) | 29 (37.7) | 25 (36.8) | 15 (24.6) |
| Male | 137 (66.5) | 48 (62.3) | 43 (63.2) | 46 (75.4) |
| Years of Education, mean (SD) | 12.42 (2.5) | 12.27 (2.7) | 13.0 (2.04) | 12.03 (2.68) |
| Monthly Income $, mean (SD) | 301.61 (483.73) | 289.11 (526.17) | 268.86 (393.42) | 353.92 (520.21) |
| Days with any substance use in 30 days prior to IOP, mean (SD) | 7.19 (8.72) | 7.10 (8.49) | 7.01 (8.69) | 7.51 (9.18) |
| Days since last use of any substance, mean (SD) | 58.23 (92.61) | 47.32 (98.05) | 66.78 (108.02) | 51.33 (59.17) |
| Days in IOP, mean (SD) | 19.84 (12.71) | 15.71 (9.09) | 23.44 (16.63) | 21.02 (10.04) |
| DSM-5 Disorders, # (%) | ||||
| Substance Use | ||||
| Alcohol Use Disorder | 135 (65.5) | 53 (68.8) | 43 (63.2) | 39 (63.9) |
| Stimulant Use Disorder | 53 (25.7) | 18 (23.4) | 20 (29.4) | 15 (24.6) |
| Cocaine Use Disorder | 127 (62.0) | 47 (61.0) | 40 (58.8) | 40 (66.7) |
| Opioid Use Disorder | 98 (47.6) | 37 (48.1) | 32 (47.1) | 29 (47.5) |
| Hallucinogen Use Disorder | 19 (9.2) | 6 (7.8) | 7 (10.3) | 6 (9.8) |
| Cannabis Use Disorder | 81 (39.3) | 24 (31.2) | 34 (50.0) | 23 (37.7) |
| Tranquilizer Use Disorder | 53 (25.7) | 14 (18.2) | 26 (38.2) | 13 (21.3) |
| Other Substance Use Disorder | 21 (10.2) | 7 (9.1) | 6 (8.8) | 8 (13.1) |
| More than one SUD Diagnosis | 158 (76.7) | 59 (76.6) | 53 (77.9) | 46 (75.4) |
| Non-Substance Use | ||||
| Antisocial Personality Disorder | 83 (40.3) | 34 (44.2) | 27 (39.7) | 22 (36.1) |
| Borderline Personality Disorder | 38 (18.6) | 13 (16.9) | 12 (18.2) | 13 (21.3) |
| Major Depressive Disorder | 114 (58.2) | 39 (52.0) | 40 (63.5) | 35 (60.3) |
| Panic Disorder | 33 (16.0) | 12 (15.8) | 14 (20.9) | 7 (11.7) |
| Agoraphobia Disorder | 17 (8.3) | 6 (7.8) | 8 (11.8) | 3 (4.9) |
| Social Anxiety Disorder | 38 (18.4) | 13 (16.9) | 13 (19.1) | 12 (19.7) |
| Posttraumatic Stress Disorder | 27 (13.1) | 6 (7.9) | 16 (23.5) | 5 (8.2) |
| Generalized Anxiety Disorder | 26 (12.6) | 7 (9.2) | 15 (22.1) | 4 (6.6) |
| Bipolar I Disorder | 35 (17.2) | 7 (9.1) | 15 (22.7) | 13 (21.7) |
| Bipolar II Disorder | 24 (11.7) | 15 (19.5) | 6 (8.8) | 3 (4.9) |
| Co-occurring Disorders (>1 diagnosis) | 118 (57.3) | 39 (50.6) | 46 (67.6) | 33 (54.1) |
Note. LETS ACT = Life Enhancement Treatment for Substance Use; LETS ACT-SE = Life Enhancement Treatment for Substance Use, Smartphone-Enhanced Condition; TAU = Treatment as usual; SD = Standard deviation; DSM-5 = Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; SUD = Substance use disorder
2.5.2. Analytic approach
Data were analyzed using SPSS version 26, using an intent-to-treat framework. Generalized Estimating Equations (GEE) tested treatment condition effects on substance use days and substance-related problems from pretreatment to the 12-month follow-up. This analytic approach was selected due to the nested nature of the data (i.e., time nested in person) and the suitability of GEE to non-normal data distributions, including zero-inflated “count” data, such as the substance use days outcome (Ballinger, 2004). Additionally, GEE is an optimal analysis method with large numbers of clusters (e.g., over 40), and with many clusters of a small size (Teerenstra et al., 2010), making it well suited to the structure of the current data (specifically, there were 206 subjects with up to six observations, i.e., time points, per subject). The outcome variable for substance use days (number of days with any substance use within the assessment period) was restricted to substances for which each participant met criteria for an SUD at pretreatment. A variable representing the total days within each time period was included as the trials variable, since the number of days between assessment points varied across time points and between participants. This approach achieves a similar goal but is statistically preferable to specifying the outcome as percentage of days used within the assessment period. A binomial probability distribution was selected for the analysis of substance use days, while a Poisson distribution was selected for the analysis of substance-related problems. Participant was included as a random effect in both analyses.
Missing data for both outcome analyses was determined to be missing completely at random (MCAR) using Little’s MCAR tests (all p-values >.17). Multiple imputation was performed for missing outcome variables and covariates at all time points using linear regression with the monotone method. Five datasets were imputed and analyses were run within each dataset. Pooled parameter estimates were obtained using the multiply imputed data and did not differ in direction or significance compared to the analyses without multiple imputation. Thus, the original analyses without imputation are presented here.
3. Results
3.1. Study Retention, Treatment Attendance, and Sample Characteristics
Detailed pretreatment diagnostic and substance use information is displayed in Table 1. The most prevalent diagnoses in the sample were major depressive disorder (58.2%) and alcohol use disorder (65.5%). The sample also displayed a high rate of comorbidity, with 57.3% of the sample meeting criteria for two or more DSM-5 non-substance use disorder diagnoses and 76.7% of the sample meeting criteria for two or more DSM-5 substance use disorder diagnoses. Pretreatment substance use was characterized by days of substance use in the 30 days prior to entering IOP (mean=7.29, SD=8.72) and days since last use of any substance (mean=58.23, SD=92.61).
There were no between group differences in sociodemographic characteristics or pretreatment substance use (frequency of use in the 30 days prior to IOP or days since last use of any substance). There were significant differences in the number of days in IOP at the pretreatment assessment (F(2)=7.49, p<.001), such that individuals in the LETS ACT-SE condition had significantly fewer days in IOP at pretreatment compared to the LETS ACT (p<.001) and TAU (p=.034) conditions. As noted above, this variable was included as a covariate in all analyses based on a priori selection. Significant pretreatment differences between condition were observed for tranquilizer use (χ2(2, N=206)=8.48, p=.01), posttraumatic stress (χ2(2, N=205)=9.55, p=.01) and generalized anxiety (χ2(2, N=205)=8.29, p=.02) disorders. Omnibus tests are reported in Supplementary Tables 2 through 5. No SUD diagnoses were significant predictors of substance use days, while alcohol, cocaine, and opioid use disorders were significant predictors of substance-related problems. No psychiatric diagnoses were significant predictors of substance use days or substance-related problems.
3.2. Substance Use Days
Across conditions in the full sample, participants reported an average of 16.24% substance days at pretreatment, dropping to 5.66% at posttreatment and then rising steadily to 18.28% by the 12-month follow-up (Figure 2). Among those reporting substance use at all timepoints, there was an average of use reported on 24.09%, 15.48%, and 32.51% of days at pretreatment, posttreatment and 12-month follow-up, respectively. Results from the GEE analysis testing substance use days in BA conditions vs. TAU from pretreatment (30 days prior to IOP) to 12 months posttreatment are reported in Tables 2 and 3. On average, there was a significant effect of time (p<.001). Condition was not a significant predictor in the model (p=.82), but the condition by time interaction was significant (p=.02). Post-hoc analyses comparing the probability of substance use on any one day across time points (Table 4 and Figure 3) indicated that participants in all conditions significantly decreased substance use days from pretreatment to posttreatment. Days of use remained significantly lower than pretreatment until 1 month for TAU, 3 months for LETS ACT-SE, and 6 months for LETS ACT. For the TAU group, rates of use at 3 months were not significantly different from pretreatment, days of use at the 6-month follow-up were significantly fewer than at pretreatment. Across conditions, the probability of substance use on any one day was not significantly different at 12 months posttreatment compared to pretreatment. As previously noted, the primary analysis of substance use days restricted the outcome to substances for which participants met criteria for an SUD at pretreatment. Analyses were also tested when defining substance use days for all substances. This analysis produced similar results, although the condition by time interaction was no longer significant (Supplementary Tables 6-8).
Figure 2.
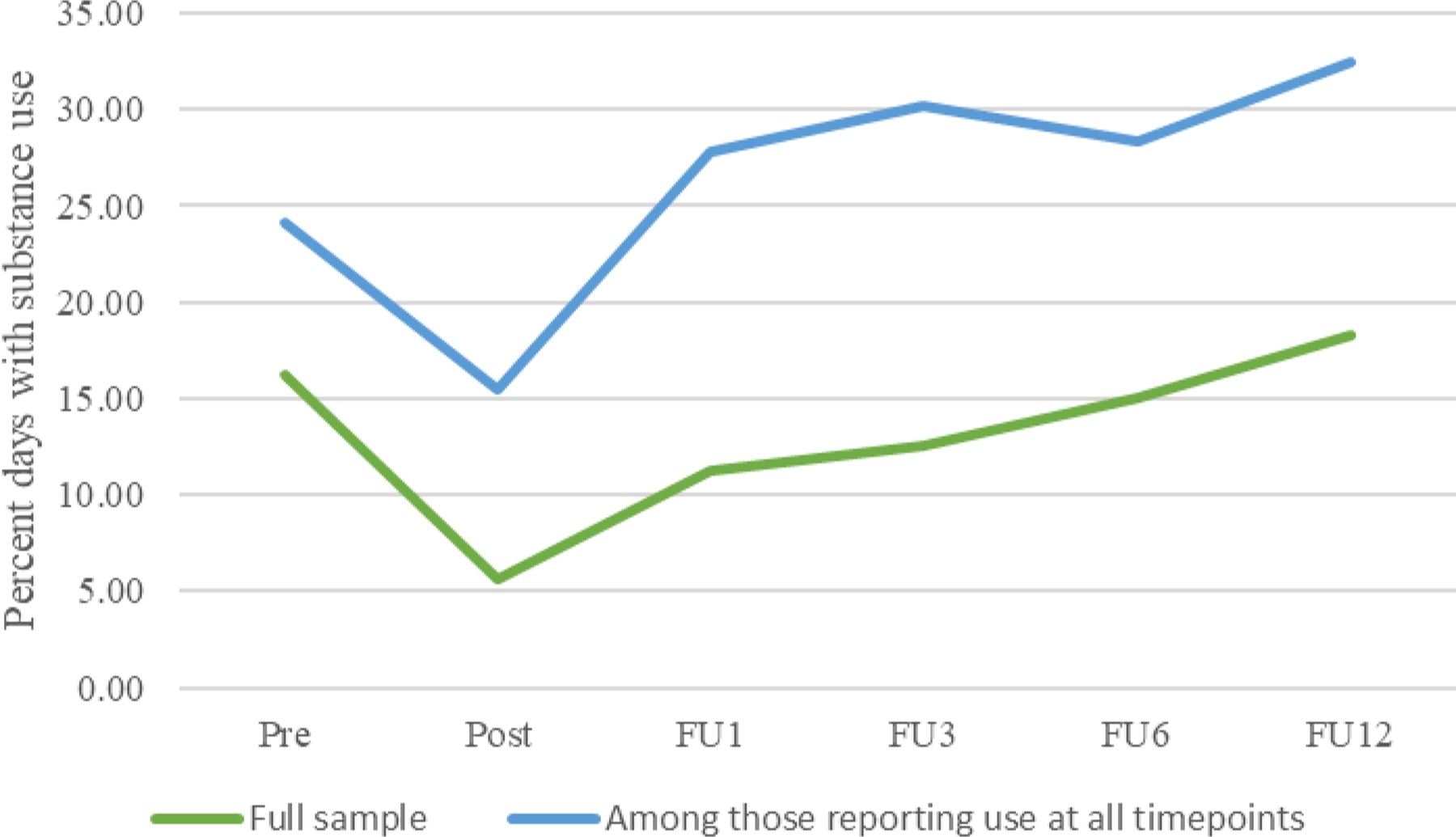
Percent days with substance use over time, across conditions
Table 2.
Generalized estimating equations predicting substance use days from pretreatment to 12 months post-treatment: Tests of model effects
| Source | Wald Chi-Square | df | p |
|---|---|---|---|
| (Intercept) | 244.06 | 1 | <.001 |
| Condition | 0.39 | 2 | .82 |
| Timepoint | 86.35 | 5 | <.001 |
| Days in IOP | 0.29 | 1 | .59 |
| Days in restricted environment | 37.02 | 1 | <.001 |
| Condition*Timepoint | 20.71 | 10 | .02 |
Note. IOP = Intensive Outpatient Treatment; Days in IOP = days in TAU prior to pretreatment assessment.
Table 3.
Generalized estimating equations predicting substance use days: Parameter estimates
| Source | B | SE B | 95% Wald Confidence Interval | Odds Ratio | 95% Confidence Interval for Odds Ratio | p | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||
| (Intercept) | −1.18 | .20 | −1.57 | −.79 | .31 | .21 | .46 | <.001 |
| Condition: LETS ACT-SE | −.17 | .28 | −.71 | .37 | .85 | .49 | 1.45 | .55 |
| Condition: LETS ACT | −.09 | .28 | −.63 | .45 | .91 | .53 | 1.57 | .74 |
| Condition: TAU (Ref.) | 0 | . | . | . | 1 | . | . | . |
| Time: FU12 | −.41 | .39 | −1.18 | .36 | .66 | .31 | 1.43 | .29 |
| Time: FU6 | −.70 | .33 | −1.35 | −.04 | .50 | .26 | .96 | .04 |
| Time: FU3 | −.49 | .33 | −1.14 | .17 | .61 | .32 | 1.18 | .15 |
| Time: FU1 | −1.11 | .35 | −1.80 | −.43 | .33 | .17 | .65 | .001 |
| Time: PT | −1.33 | .37 | −2.05 | −.60 | .27 | .13 | .55 | <.001 |
| Time: Pretreatment (Ref.) | 0 | . | . | . | 1 | . | . | . |
| Days in IOP | .01 | .01 | −.02 | .03 | 1.01 | .98 | 1.03 | .59 |
| Days in restricted environment | −.01 | .002 | −.02 | −.009 | .99 | .98 | .99 | <.001 |
| LETS ACT-SE*FU12 | .20 | .51 | −.81 | 1.21 | 1.22 | .44 | 3.34 | .70 |
| LETS ACT-SE*FU6 | .50 | .46 | −.40 | 1.40 | 1.65 | .67 | 4.05 | .28 |
| LETS ACT-SE*FU3 | −.12 | .48 | −1.07 | .82 | .88 | .35 | 2.26 | .80 |
| LETS ACT-SE*FU1 | .40 | .46 | −.51 | 1.30 | 1.48 | .60 | 3.66 | .39 |
| LETS ACT-SE*PT | −.44 | .55 | −1.52 | .64 | .65 | .22 | 1.90 | .43 |
| LETS ACT-SE*Pretreatment (Ref.) | 0 | . | . | . | 1 | . | . | . |
| LETS ACT*FU12 | .65 | .52 | −.37 | 1.66 | 1.91 | .69 | 5.25 | .21 |
| LETS ACT*FU6 | .16 | .44 | −.71 | 1.02 | 1.17 | .49 | 2.78 | .73 |
| LETS ACT*FU3 | −.50 | .48 | −1.46 | .45 | .60 | .23 | 1.56 | .30 |
| LETS ACT*FU1 | .15 | .53 | −.90 | 1.19 | 1.16 | .41 | 3.27 | .79 |
| LETS ACT*PT | −1.09 | .51 | −2.09 | −.09 | .34 | .12 | .92 | .03 |
| LETS ACT*Pretreatment (Ref.) | 0 | . | . | . | 1 | . | . | . |
Note. LETS ACT = Life Enhancement Treatment for Substance Use; LETS ACT-SE = Life Enhancement Treatment for Substance Use, Smartphone-Enhanced Condition; TAU = Treatment as Usual; FU12 = 12 month follow-up; FU6 = 6 month follow-up; FU3 = 3 month follow-up; FU1 = 1 month follow-up; PT = Posttreatment; IOP = Intensive Outpatient Treatment; Days in IOP = days in TAU prior to pretreatment assessment.
Table 4.
Pairwise comparisons: Probability of substance use
| (I) Condition*Timepoint | Mean Difference (I-J) | Std. Error | df | p | |
|---|---|---|---|---|---|
| TAU (Pretreatment) |
Post | 0.16 | 0.04 | 1 | <.001* |
| FU1 | 0.14 | 0.04 | 1 | <.001* | |
| FU3 | 0.08 | 0.05 | 1 | 0.12 | |
| FU6 | 0.10 | 0.05 | 1 | 0.03* | |
| FU12 | 0.07 | 0.06 | 1 | 0.27 | |
| LETS ACT (Pretreatment) |
Post | 0.20 | 0.03 | 1 | <.001* |
| FU1 | 0.12 | 0.04 | 1 | 0.002* | |
| FU3 | 0.13 | 0.04 | 1 | 0.001* | |
| FU6 | 0.08 | 0.04 | 1 | 0.04* | |
| FU12 | −0.04 | 0.06 | 1 | 0.49 | |
| LETS ACT-SE (Pretreatment) |
Post | 0.16 | 0.03 | 1 | <.001* |
| FU1 | 0.09 | 0.03 | 1 | 0.004* | |
| FU3 | 0.08 | 0.04 | 1 | 0.046* | |
| FU6 | 0.03 | 0.05 | 1 | 0.50 | |
| FU12 | 0.03 | 0.05 | 1 | 0.50 | |
Significant at p<.05 level
Note. TAU = Treatment as Usual; LETS ACT = Life Enhancement Treatment for Substance Use; LETS ACT-SE = Life Enhancement Treatment for Substance Use, Smartphone-Enhanced Condition; Post = Posttreatment; FU1 = 1 month follow-up; FU3 = 3 month follow-up; FU6 = 6 month follow-up; FU12 = 12 month follow-up
Figure 3.
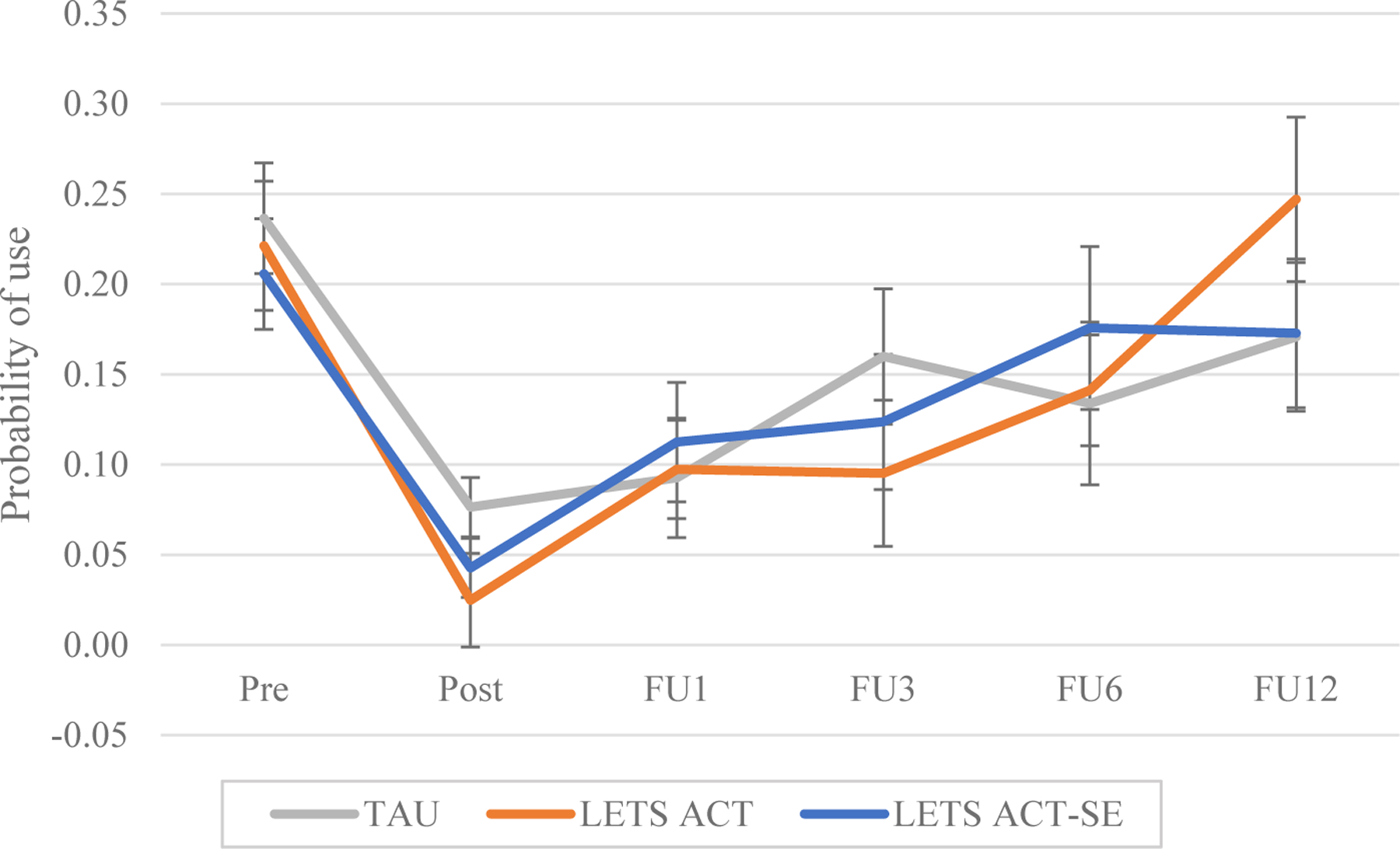
Model-estimated group means for probability of substance use
Note. TAU = Treatment as Usual; LETS ACT = Life Enhancement Treatment for Substance Use; LETS ACT-SE = Life Enhancement Treatment for Substance Use, Smartphone-Enhanced Condition; Pre = Pretreatment; Post = Posttreatment; FU1 = 1 month follow-up; FU3 = 3 month follow-up; FU6 = 6 month follow-up; FU12 = 12 month follow-up
3.3. Substance-Related Problems
Results from the GEE analysis testing changes in substance-related problems from pretreatment to 12 months posttreatment are reported in Tables 5 and 6. The model predicting substance use problems was tested with and without the DSM-5 diagnostic variables that differed significantly between conditions at pretreatment and were significant predictors in omnibus tests. Inclusion of the variables did not change the direction or significance of the results, and thus the model without the DSM-5 covariates was retained (Supplementary Table 9).
Table 5.
Generalized estimating equations examining substance-related problems from pretreatment to 12 months post-treatment: Tests of model effects
| Source | Wald Chi-Square | df | p |
|---|---|---|---|
| (Intercept) | 2517.18 | 1 | <.001 |
| Condition | 2.77 | 2 | .25 |
| Timepoint | 138.54 | 4 | <.001 |
| Days in IOP | 3.58 | 1 | .06 |
| Days in restricted environment | 1.61 | 1 | .21 |
| Condition*Timepoint | 10.36 | 8 | .24 |
Note. IOP = Intensive Outpatient Treatment
Table 6.
Generalized estimating equations examining substance-related problems: Parameter estimates
| Source | B | SE B | 95% Wald Confidence Interval | Odds Ratio | 95% Confidence Interval for Odds Ratio | p | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||
| (Intercept) | 3.28 | .07 | 3.15 | 3.42 | 26.66 | 23.34 | 30.46 | <.001 |
| Condition: LETS ACT-SE | .15 | .08 | −.01 | .31 | 1.16 | .99 | 1.37 | .07 |
| Condition: LETS ACT | .04 | .09 | −.14 | .22 | 1.04 | .87 | 1.25 | .64 |
| Condition: TAU (Ref.) | 0 | . | . | . | 1 | . | . | . |
| Time: FU12 | −.82 | .18 | −1.17 | −.46 | .44 | .31 | .63 | <.001 |
| Time: FU6 | −.71 | .19 | −1.09 | −.34 | .49 | .34 | .71 | <.001 |
| Time: FU3 | −.74 | .18 | −1.08 | −.39 | .48 | .34 | .68 | <.001 |
| Time: FU1 | −1.12 | .20 | −1.52 | −.72 | .33 | .22 | .49 | <.001 |
| Time: Pretreatment (Ref.) | 0 | . | . | . | 1 | . | . | . |
| Days in IOP | .006 | .003 | .000 | .01 | 1.00 | 1.00 | 1.01 | .06 |
| Days in restricted environment | −.001 | .001 | −.003 | .001 | 1.00 | 1.00 | 1.00 | .21 |
| LETS ACT-SE*FU12 | −.05 | .24 | −.52 | .42 | .95 | .60 | 1.52 | .84 |
| LETS ACT-SE*FU6 | −.13 | .25 | −.62 | .36 | .88 | .54 | 1.43 | .60 |
| LETS ACT-SE*FU3 | .04 | .22 | −.40 | .48 | 1.04 | .67 | 1.62 | .86 |
| LETS ACT-SE*FU1 | .35 | .24 | −.13 | .83 | 1.42 | .88 | 2.29 | .15 |
| LETS ACT-SE*Pretreatment (Ref.) | 0 | . | . | . | 1 | . | . | . |
| LETS ACT*FU12 | .09 | .25 | −.39 | .58 | 1.10 | .68 | 1.79 | .70 |
| LETS ACT*FU6 | −.08 | .26 | −.59 | .44 | .93 | .56 | 1.55 | .78 |
| LETS ACT*FU3 | −.46 | .26 | −.97 | .06 | .63 | .38 | 1.06 | .08 |
| LETS ACT*FU1 | .34 | .28 | −.21 | .89 | 1.41 | .81 | 2.43 | .22 |
| LETS ACT*Pretreatment (Ref.) | 0 | . | . | . | 1 | . | . | . |
Note. LETS ACT = Life Enhancement Treatment for Substance Use; LETS ACT-SE = Life Enhancement Treatment for Substance Use, Smartphone-Enhanced Condition; TAU = Treatment as Usual; FU12 = 12 month follow-up; FU6 = 6 month follow-up; FU3 = 3 month follow-up; FU1 = 1 month follow-up; PT = Posttreatment; IOP = Intensive Outpatient Treatment
On average, there was a significant effect of time (p<.001). Condition was not a significant predictor in the model (p=.25), and the condition by time interaction was also nonsignificant (p=.24). Post-hoc analyses comparing substance-related problems across time points (Table 7 and Figure 3) indicated that participants in all conditions significantly decreased substance-related problems from pretreatment to the 1-month follow-up. These reductions were sustained until 12 months posttreatment, such that the model-predicted mean SIP scores at all time points from 1 through 12 months posttreatment were significantly lower compared to pretreatment (all p’s <.001).
Table 7.
Pairwise comparisons: Substance-related problems
| (I) Condition*Timepoint | Mean Difference (I-J) | Std. Error | df | p | |
|---|---|---|---|---|---|
| TAU (Pretreatment) |
Post | 17.92 | 2.31 | 1 | <.001* |
| FU1 | 13.85 | 2.79 | 1 | <.001* | |
| FU3 | 13.54 | 2.98 | 1 | <.001* | |
| FU6 | 14.80 | 2.66 | 1 | <.001* | |
| FU12 | 15.04 | 3.01 | 1 | <.001* | |
| LETS ACT (Pretreatment) |
Post | 19.32 | 2.18 | 1 | <.001* |
| FU1 | 15.11 | 2.71 | 1 | <.001* | |
| FU3 | 14.23 | 2.75 | 1 | <.001* | |
| FU6 | 16.58 | 2.09 | 1 | <.001* | |
| FU12 | 15.48 | 2.32 | 1 | <.001* | |
| LETS ACT-SE (Pretreatment) |
Post | 17.56 | 2.47 | 1 | <.001* |
| FU1 | 17.82 | 2.32 | 1 | <.001* | |
| FU3 | 17.92 | 2.31 | 1 | <.001* | |
| FU6 | 13.85 | 2.79 | 1 | <.001* | |
| FU12 | 13.54 | 2.98 | 1 | <.00* | |
Significant at p<.05 level
Note. TAU = Treatment as Usual; LETS ACT = Life Enhancement Treatment for Substance Use; LETS ACT-SE = Life Enhancement Treatment for Substance Use, Smartphone-Enhanced Condition; Post = Posttreatment; FU1 = 1 month follow-up; FU3 = 3 month follow-up; FU6 = 6 month follow-up; FU12 = 12 month follow-up
4. Discussion
This study tested the effect of LETS ACT delivered in small groups in an outpatient SUD treatment setting and investigated the added benefit of a smartphone-enhanced version on substance use outcomes. Significant reductions in substance-related problems were maintained until 12 months posttreatment for all conditions. In contrast to the sustained change in substance-related problems, substance use days decreased for all groups from pre- to posttreatment but subsequently increased again, such that significant reductions in substance use days were not maintained at 12 months posttreatment for any condition. Individuals in both LETS ACT conditions, but not the TAU condition, were significantly less likely to use substances at 3 months posttreatment compared to pretreatment, and the standard LETS ACT condition maintained significant reductions until 6 months posttreatment. At 6 months posttreatment the TAU condition demonstrated a slight decrease compared to use at 3 months, yet this reduction was not maintained at the 12-month follow-up.
The results of the current study add important and novel information to the current literature on behavioral activation for SUD. First, most research evaluating behavioral activation treatments for SUD has been conducted in residential and inpatient settings despite continuing decline in utilization of these services (SAMHSA, 2021). Therefore, testing the effect of LETS ACT in an outpatient treatment setting that provides low-cost services to individuals meeting diagnostic criteria for multiple SUDs represents a critical step in determining the generalizability of this treatment, as outpatient is rapidly becoming the most common setting of care for adults with SUD (SAMHSA, 2021). Evidence from the current study is promising, as those receiving LETS ACT maintained fewer substance use days through 6 months posttreatment, in comparison to those in TAU whose substance use at 3 months posttreatment was not significantly different than pretreatment. Yet treatment gains were not maintained across any condition by 12 months posttreatment, which is in contrast to expectation and to prior work reporting a significantly greater likelihood of abstinence through 12 months posttreatment among adults receiving LETS ACT in a residential treatment setting (Daughters et al., 2018). Considering similarly short-lived gains observed in a pilot trial of LETS ACT administered in an outpatient SUD treatment setting (Pott et al., 2022), there is a clear need to consider the multitude of factors that may impact LETS ACT treatment efficacy in outpatient versus residential settings.
One specific concern is the degree to which outpatient treatment attendance impacts outcomes in the present study, a consideration less relevant within residential settings. Indeed, treatment attendance is predictive of longevity of favorable outcomes among those receiving SUD treatment (Milward et al., 2014; Pfund et al., 2021). Treatment attendance was variable among individuals randomized to receive LETS ACT (mean=4, range=1–6), raising important questions concerning whether individuals obtained an adequate dose of treatment, and whether specific modules, which may not have been administered to all individuals randomized to LETS ACT by virtue of attendance variability, may represent important mechanisms of change. Relatedly and as hypothesized in previous research (Pott et al., 2022), lack of sustained treatment gains may be due to the limited number of clinician-administered sessions, namely up to six group sessions within three weeks. This format and length of treatment was chosen to fit within the constraints of a low-resourced and time-limited treatment setting, yet likely not adequate to reinforce skills acquisition, given evidence that a minimum of fourteen sessions of cognitive-behavioral therapy are needed to detect a treatment response for depression and anxiety (Robinson 2020). It will be important for future research to determine the adequate dose needed to realize maximum benefit of LETS ACT within outpatient SUD treatment settings.
Integrating additional treatment modalities to incentivize treatment participation, including treatment attendance and activity engagement, may be a promising approach to increase the magnitude and longevity of treatment gains. Several studies demonstrate the benefit of integrating the delivery of additional reinforcers through treatment modalities such as contingency management. Combined contingency management-BA treatment is feasible and well-accepted (Mimiaga et al., 2019), and is associated with greater treatment attendance (Gonzalez et al., 2019) and enhanced abstinence rates (González-Roz et al., 2019; Secades-Villa et al., 2019) among people who smoke cigarettes with depressive symptoms. Further, the potential effect of targeting different treatment components such as reinforcement for abstinence compared to values-based activities with contingency management may be a promising future direction.
The sustained decrease in substance-related problems at all follow-up timepoints across conditions adds to a growing literature indicating that SUD treatment can result in notable positive outcomes even among those who continue using substances or return to use after treatment (Kidorf et al., 2011; Witkiewitz et al., 2020). This underscores the importance of considering a broader range of outcomes in addition to substance use when evaluating the effectiveness of SUD treatment, including reductions in adverse health, social, legal, or economic problems related to substance use. This consideration is especially important considering the movement toward greater acceptance of nonabstinence goals (Paquette et al., 2022; Volkow, 2020) in alignment with harm reduction approaches to SUD treatment. Thus, our assessment of substance-related problems across harm-reduction relevant domains of physical, inter-personal, intra-personal, impulse control and social functioning provides a richer understanding of SUD treatment response than traditionally afforded by abstinence-specific outcome measures. Relatedly, in recognition that not all substance use is problematic, in this study we restricted substance use days to only substances for which participants met criteria for an SUD. Although we did not assess participants’ specific substance use goals, we argue that our approach is preferable to the more common method of assessing frequency of any substance use given that many individuals presenting to SUD treatment, even where treatment is predominantly abstinence-focused, endorse nonabstinence goals (see Paquette et al., 2022 for a summary of this literature). In this study both approaches produced similar outcomes, with rates of use of excluded substances generally low following the pretreatment assessment.
Contrary to study hypotheses, the addition of a smartphone app did not facilitate superior treatment outcomes when compared to standard LETS ACT. Though information regarding feasibility and acceptability of this modality of treatment is reported elsewhere (see Paquette et al., 2021) several challenges specific to app use are important to consider in relation to substance use outcomes. Most notably, and in alignment with trends across smartphone-enhanced interventions for SUD, less than one-third (27%) of participants randomized to the LETS ACT-SE condition continued to use the app during the 3-month posttreatment follow-up period. This attrition was attributed to a variety of reasons, including forgetting to use the app and not having the study-provided smartphone with the app installed on their person outside of treatment sessions (Paquette et al., 2022). While the addition of a smartphone app was intended to enhance treatment engagement outside of LETS ACT treatment sessions, such barriers to consistent app use may explain the negligible effect of this condition on the observed substance use outcomes. Given these findings and other studies demonstrating challenges with sustaining app engagement over time, increasing engagement may be a critical consideration for improving clinical efficacy of smartphone-enhanced treatments. A recent meta-analysis found a greater number of engagement features among mental health smartphone apps is associated with larger clinical effects (Wu et al., 2021). Engagement features can range from capitalizing on social relationships (e.g., promoting social comparison or normative influence of target behaviors) to increasing user-app interactions (e.g., offering reminders, praise, and rewards for target behaviors).
The current results must be interpreted in the context of study limitations. While this study extends generalizability of LETS ACT into outpatient treatment, it is notable that participants in the current study were recruited from an intensive outpatient treatment program serving primarily low-income, high school-educated adults. It will be important to further evaluate LETS ACT efficacy across treatment settings and populations, including within general outpatient treatment settings and among individuals with differing levels of SUD severity (e.g., mild-to-moderate), as well as when delivered in an individual format.
5. Conclusions
This study provides a novel test of LETS ACT, a behavioral activation treatment for SUD, within the context of intensive outpatient treatment. Contrary to our hypotheses, we did not find evidence that LETS ACT was associated with greater decreases in substance use or substance-related problems from pretreatment to 12 months posttreatment compared to intensive outpatient TAU. Study findings did demonstrate that individuals who received LETS ACT as a treatment adjunct continuously maintained significant decreases in posttreatment substance use longer than those who only received TAU, while decreases in posttreatment substance-related problems were maintained similarly across all conditions. Such findings underscore the importance of testing the generalizability of evidence-based treatments for SUD across settings and populations and highlight critical considerations for future treatment development and testing within the context of SUD treatment research.
Supplementary Material
Figure 4.
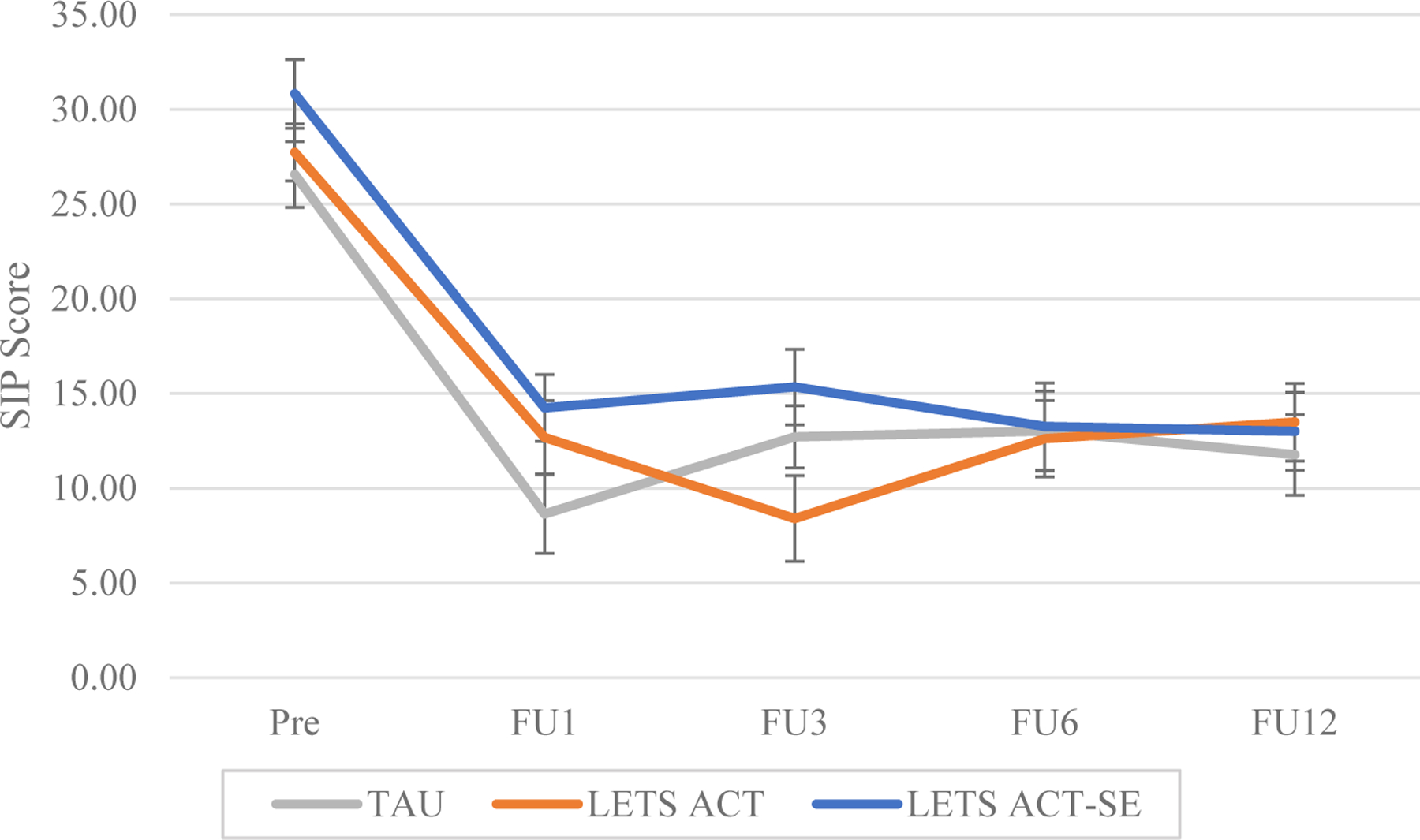
Model-estimated group means for substance-related problems
Note. SIP = Short Inventory of Problems; TAU = Treatment as Usual; LETS ACT = Life Enhancement Treatment for Substance Use; LETS ACT-SE = Life Enhancement Treatment for Substance Use, Smartphone-Enhanced Condition; Pre = Pretreatment; Post = Posttreatment; FU1 = 1 month follow-up; FU3 = 3 month follow-up; FU6 = 6 month follow-up; FU12 = 12 month follow-up
Highlights.
We tested behavioral activation for SUD in intensive outpatient treatment.
All groups decreased substance-related problems through 12 months posttreatment.
Adding a behavioral activation smartphone app did not improve treatment outcomes.
Lengthier behavioral activation treatments may be needed in outpatient settings.
Acknowledgements
The authors wish to thank SouthLight Healthcare for their partnership in this research; Chris Wiesen and the UNC Odum Institute for statistical consultation services; and the incredible research team who made this study happen, including Yun Chen, Deepika Anand, Kimberley Johnson, Sydney Baker, Michael Loeffler, Alexandra Riedel, Dillon Rubalcava and Surabhi Swaminath. We also wish to thank our participants, without whom this research would not be possible.
Role of Funding Source
This research was supported by the National Institute on Drug Abuse, Grant Numbers: R01 DA026424 and 1F31DA049457. The funding source had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: No conflict declared.
Declarations of interest: none
NCT#: 02707887
References
- Acuff SF, Dennhardt AA, Correia CJ, & Murphy JG (2019). Measurement of substance-free reinforcement in addiction: A systematic review. Clinical Psychology Review, 70, 79–90. 10.1016/j.cpr.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger GA (2004). Using generalized estimating equations for longitudinal data analysis. Organizational Research Methods, 7(2), 127–150. 10.1177/1094428104263672 [DOI] [Google Scholar]
- Baskin-Sommers AR & Foti D (2015). Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. International Journal of Psychophysiology, 98(2 pt 2), 227–239. 10.1016/j.ijpsycho.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Ogedegbe G, Orwig D, Ernst D, & Czajkowski S (2004). Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the nih behavior change consortium. Health Psychology, 23(5), 443–451. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- Blanchard KA, Morgenstern J, Morgan TJ, Lobouvie EW, & Bux DA (2003). Assessing consequences of substance use: Psychometric properties of the Inventory of Drug Use Consequences. Psychology of Addictive Behaviors, 17(4), 328–331. 10.1037/0893-164x.17.4.328 [DOI] [PubMed] [Google Scholar]
- Carreiro S, Newcomb M, Leach R, Ostrowski S, Boudreaux ED, & Amante D (2020). Current reporting of usability and impact of mHealth interventions for substance use disorder: A systematic review. Drug and Alcohol Dependence, 215, 108201. 10.1016/j.drugalcdep.2020.108201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahne J, & Lejuez CW (2015). Smartphone and mobile application utilization prior to and following treatment among individuals enrolled in residential substance use treatment. Journal of Substance Abuse Treatment, 58, 95–99. 10.1016/j.jsat.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters S, Magidson J, Anand D, Seitz-Brown CJ, Chen Y, & Baker S (2018). The effect of a behavioral activation treatment for substance use on post-treatment abstinence: A randomized controlled trial. Addiction, 113(3), 535–544. 10.1111/add.14049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker SE, Kiluk BD, Frankforter T, Babuscio T, Nich C, & Carroll KM (2016). Just showing up is not enough: Homework adherence and outcome in cognitive-behavioral therapy for cocaine dependence. Journal of Consulting and Clinical Psychology, 84(10), 907–912. 10.1037/ccp0000126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzino TL, Bjorlie K, & Lejuez CW (2019). A systematic review of reinforcement-based interventions for substance use: Efficacy, mechanisms of action, and moderators of treatment effects. Journal of Substance Abuse Treatment, 104, 83–96. 10.1016/j.jsat.2019.06.016 [DOI] [PubMed] [Google Scholar]
- Fowler LA, Holt SL, & Joshi D (2016). Mobile technology-based interventions for adult users of alcohol: A systematic review of the literature. Addictive behaviors, 62, 25–34. 10.1016/j.addbeh.2016.06.008 [DOI] [PubMed] [Google Scholar]
- González-Roz A, Secades-Villa R, & Alonso-Pérez F (2019). Effects of combining contingency management with behavioral activation for smokers with depression. Addiction Research & Theory, 27(2), 114–121. 10.1080/16066359.2018.1463371 [DOI] [Google Scholar]
- Hjorthoj CR, Hjorthoj AR, & Nordentoft M (2012). Validity of timeline follow-back for self-reported use of cannabis and other illicit substances: Systematic review and meta-analysis. Addictive Behaviors, 37(3), 225–233. 10.1016/j.addbeh.2011.11.025 [DOI] [PubMed] [Google Scholar]
- Jastak S, & Wilkinson G (1984). Wide range achievement test-revised (WRAT-R) The Psychological Corporation. [Google Scholar]
- Kidorf M, King VL, Peirce J, Kolodner K, & Brooner RK (2011). Benefits of concurrent syringe exchange and substance abuse treatment participation. Journal of Substance Abuse Treatment, 40(3), 265–271. 10.1016/j.jsat.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk BD, Dreifuss JA, Weiss RD, Morgenstern J, & Carroll KM (2013). The Short Inventory of Problems – Revised (SIP-R): Psychometric properties within a large, diverse sample of substance use disorder treatment seekers. Psychology of Addictive Behaviors : Journal of the Society of Psychologists in Addictive Behaviors, 27(1), 307–314. 10.1037/a0028445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217–238. 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Tull MT, Matusiewicz AK, Rodman S, Strong DR, Kahler CW, Hopko DR, Zvolensky MJ, Brown RA, & Lejuez C (2010). Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. Journal of Consulting and Clinical Psychology, 78(1), 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magidson JF, Gorka SM, MacPherson L, Hopko DR, Blanco C, Lejuez C, & Daughters SB (2011). Examining the effect of the Life Enhancement Treatment for Substance Use (LETS ACT) on residential substance abuse treatment retention. Addictive Behaviors, 36(6), 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshesha LZ, Soltis KE, Wise EA, Rohsenow DJ, Witkiewitz K, & Murphy JG (2020). Pilot trial investigating a brief behavioral economic intervention as an adjunctive treatment for alcohol use disorder. Journal of Substance Abuse Treatment, 113, 108002. 10.1016/j.jsat.2020.108002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milward J, Lynskey M, & Strang J (2014). Solving the problem of non-attendance in substance abuse services. Drug and Alcohol Review, 33(6), 625–636. 10.1111/dar.12194 [DOI] [PubMed] [Google Scholar]
- Mimiaga MJ, Closson EF, Pantalone DW, Safren SA, & Mitty JA (2019). Applying behavioral activation to sustain and enhance the effects of contingency management for reducing stimulant use among individuals with hiv infection. Psychology, Health & Medicine, 24(3), 374–381. 10.1080/13548506.2018.1515492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette CE, Daughters SB, & Witkiewitz K (2022). Expanding the continuum of substance use disorder treatment: Nonabstinence approaches. Clinical Psychology Review, 91, 102110. 10.1016/j.cpr.2021.102110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette CE, Rubalcava DT, Chen Y, Anand D, & Daughters SB (2021). A mobile app to enhance behavioral activation treatment for substance use disorder: App design, use, and integration into treatment in the context of a randomized controlled trial. JMIR Formative Research, 5(11), e25749. 10.2196/25749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfund RA, Hallgren KA, Maisto SA, Pearson MR, & Witkiewitz K (2021). Dose of psychotherapy and long-term recovery outcomes: An examination of attendance patterns in alcohol use disorder treatment. Journal of Consulting and Clinical Psychology, 89(12), 1026–1034. 10.1037/ccp0000703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott SL, Kellett S, Green S, Daughters S, & Delgadillo J (2022). Behavioral activation for depression delivered by drug and alcohol treatment workers: A pilot randomized controlled trial. Journal of Substance Abuse Treatment, 139, 108769. 10.1016/j.jsat.2022.108769 [DOI] [PubMed] [Google Scholar]
- Rawson RA, Shoptaw SJ, Obert JL, McCann MJ, Hasson AL, Marinelli-Casey PJ, Brethen PR, & Ling W (1995). An intensive outpatient approach for cocaine abuse treatment. Journal of Substance Abuse Treatment, 12(2), 117–127. 10.1016/0740-5472(94)00080-b [DOI] [PubMed] [Google Scholar]
- Reynolds EK, MacPherson L, Tull MT, Baruch DE, & Lejuez C (2011). Integration of the Brief Behavioral Activation Treatment for Depression (BATD) into a college orientation program: Depression and alcohol outcomes. Journal of Counseling Psychology, 58(4), 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secades-Villa R, González-Roz A, Vallejo-Seco G, Weidberg S, García-Pérez Á, & Alonso-Pérez F (2019). Additive effectiveness of contingency management on cognitive behavioural treatment for smokers with depression: Six-month abstinence and depression outcomes. Drug and Alcohol Dependence, 204, 107495. 10.1016/j.drugalcdep.2019.06.003 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, & Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, & Cooper AM (1979). Reliability of alcohol abusers' self-reports of drinking behavior. Behaviour Research and Therapy, 17(2), 157–160. 10.1016/0005-7967(79)90025-1 [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, & Del Boca FK (1994). Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol and Drugs, 12, 70–75. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2021). National Survey of Substance Abuse Treatment Services (N-SSATS): 2016 Data on Substance Abuse Treatment Facilities. [Google Scholar]
- Teerenstra S, Lu B, Preisser JS, van Achterberg T, & Borm GF (2010). Sample size considerations for gee analyses of three-level cluster randomized trials. Biometrics, 66(4), 1230–1237. 10.1111/j.1541-0420.2009.01374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighi B, Leonard N, Greco P, Hadavand A, Acosta MC, & Lee JD (2019). Technology use patterns among patients enrolled in inpatient detoxification treatment. Journal of Addiction Medicine, 13(4). 10.1097/adm.0000000000000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND (2020). Personalizing the treatment of substance use disorders. American Journal of Psychiatry, 177(2), 113–116. 10.1176/appi.ajp.2019.19121284 [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Pearson MR, Wilson AD, Stein ER, Votaw VR, Hallgren KA, Maisto SA, Swan JE, Schwebel FJ, Aldridge A, Zarkin GA, & Tucker JA (2020). Can alcohol use disorder recovery include some heavy drinking? A replication and extension up to 9 years following treatment. Alcoholism: Clinical and Experimental Research, 44(9), 1862–1874. 10.1111/acer.14413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Scult MA, Barnes ED, Betancourt JA, Falk A, & Gunning FM (2021). Smartphone apps for depression and anxiety: A systematic review and meta-analysis of techniques to increase engagement. NPJ Digital Medicine, 4(1). 10.1038/s41746-021-00386-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


