Abstract
Introduction
The COVID-19 pandemic has significantly impacted the diagnosis of breast cancer (BC). With a large Hispanic/Latinx population, early revocation of mask mandates, and lower vaccination rate than many other states, this study explores the relationship between COVID-19 and the presentation and diagnosis of BC patients in the unique socio-politico-economic context of Central Texas.
Methods
This study is a retrospective review of the Seton Medical Center Austin tumor registry for BC patients from March 1, 2019 to March 2, 2021. We compared demographics, insurance status, clinical and pathologic stage, and time from diagnosis to intervention between “pre-COVID” (March 1, 2019- March 1, 2020) and “post-COVID” (March 2, 2020-March 2, 2021). We utilized descriptive, univariate, and multivariable logistic regression statistics.
Results
There were 781 patients diagnosed with BC, with 113 fewer post-COVID compared to pre-COVID. The proportion of Black patients diagnosed with BC decreased post-COVID compared with pre-COVID (10.1%-4.5%, P = 0.002). When adjusting for other factors, uninsured and underinsured patients had increased odds of presenting with late-stage BC (odds ratio:5.40, P < 0.001). There was also an association between presenting with stage 2 or greater BC and delayed time-to-intervention.
Conclusions
Although fewer women overall were diagnosed with BC post-COVID, the return to baseline diagnoses has yet to be seen. We identified a pandemic-related decrease in BC diagnoses in Black women and increased odds of late-stage cancer among uninsured patients, suggesting a disparate relationship between COVID-19 and health care access and affordability. Outreach and screening efforts should address strategies to engage Black and uninsured patients.
Keywords: Breast cancer, COVID, COVID-19, Disparities, Pandemic
Introduction
The COVID-19 pandemic had a far reaching impact on non-COVID-19 health conditions, with diagnostic and treatment services for cancers of the breast, prostate, lung, and colon being significantly affected.1 A national population-based modeling study out of the United Kingdom found that the pandemic-related delays and management changes for breast cancer would likely cause at least a 7.9% -9.6% increase in the number of deaths due to breast cancer up to 5 y after diagnosis.2
Breast cancer is the most common and second-most deadly cancer in women; screening plays an important role in early detection and intervention.3 An Italian modeling study estimated that lockdowns due to the pandemic would result in 10,000-16,000 missed diagnoses of breast cancer.4 Out of 600 breast care patients surveyed, almost 80% of the respondents reported delays in their breast cancer treatment and care, with younger participants reporting more significant delays than older respondents.5 Screening was stopped or significantly decreased worldwide at the beginning of the pandemic (March 2020-August 2020). Taiwan reported a 60%-90% decrease in screening and screening referrals during the first few months of the pandemic, and due to follow-up diagnostics usually being in a hospital setting those were delayed as well.6
As the pandemic evolved, clinical recommendations regarding breast cancer screening and management changed. Professional societies such as the European Society of Medical Oncology in collaboration with the World Health Organization recommended a priority-based approach when choosing to screen or treat patients during the severe initial phases of the pandemic.7, 8, 9 The American Society of Breast Surgeons and the American College of Radiology developed a joint statement in March 2020 recommending the postponement of all breast screening exams and the discontinuation of nonurgent breast care appointments.10 , 11
There are data suggesting that existing health disparities within breast cancer diagnosis and treatment outcomes were worsened during the pandemic. This has been greatly attributed to the job and insurance loss associated with shelter-in-place mandates and business closures, which disproportionately impact racial-ethnic minorities such as Black and Hispanic women.12 Other barriers contributing to these disparities include language barriers and differential access to care.13 A large study out of Mass General Brigham assessed disparities in cancer screening from November 2019 to August 2020. They found that compared to White women, Asian women were less likely to receive breast cancer screening and that rates of breast cancer screening for Latinx women remained low after the initial pandemic surge.14
The question became whether or not delays in screening and disparities would have an effect on diagnosis, staging, and treatment of breast cancer during the pandemic. The Mayo Clinic Rochester found that the stage at diagnosis, method of detection, histology and tumor markers, and surgical treatment did not differ significantly during the initial pandemic surges compared with pre-COVID. They did find that neoadjuvant endocrine therapy use increased significantly in early-stage hormone receptor-positive disease.15
This study seeks to analyze patterns of diagnosis in women diagnosed with breast cancer before and after the COVID-19 pandemic began, particularly in the unique socio-economic setting of Central Texas that has not been addressed by other studies. Due to the high proportion of Hispanic/Latinx individuals in Central Texas as well as the early revocation of mask mandates and a significantly lower vaccination rate than other states as of the Spring 2021, this study seeks to explore the relationship between COVID-19 and stage distribution, time-to-intervention, and insurance status of patients presenting with breast cancer in the Austin local cancer center. We hypothesized that Hispanic women, Black women, and uninsured or underinsured women, who are known to experience breast cancer disparities would have disparately outcomes in the COVID-19 era. Additionally, it was thought that due to the COVID-19 pandemic, breast cancer stage on presentation and time to diagnosis and first treatment would increase.
Methods
Data source
This is a retrospective review of the Commission on Cancer Accredited Program at Seton Medical Center Austin tumor registry from March 01, 2019 to February 28, 2021. The breast cancer program diagnoses and treats patients referred both internally and externally. Demographic factors assessed included the following: age, sex, race, ethnicity, and insurance payer status.
Approval of the study and waiver of informed consent were obtained from the Institutional Review Board at Dell Medical School at the University of Texas at Austin (STUDY00002055, approved December 21, 2021).
Study population, inclusion criteria, and exclusion criteria
Patients must have been newly diagnosed with breast cancer between March 2019 and March 2021, have completed clinical staging and diagnosis, diagnosed and treated at least partially at Ascension Seton facilities, and have their information entered into the Seton Cancer Registry to be included in this study. Patients were excluded if they had an existing breast cancer diagnosis prior to March 1, 2019 that they were receiving treatment for, or if they were diagnosed after March 2, 2021. Individuals with unknown or incomplete clinical staging in the registry were identified and updated via patient chart review or excluded from analysis if further information was unable to be identified.
For ease of readability, patients were grouped into “pre-COVID” if their diagnosis was made between March 01, 2019 and March 01, 2020, and “post-COVID” if their diagnosis was made between March 02, 2020 and February 28, 2021. Chart review was done using the American Joint Committee on Cancer clinical prognostic staging 8th edition to fill in any missing staging data.16 In the chart review of the unknowns, estrogen-receptor and progesterone-receptor positivity margins were >1%. Human epidermal growth factor positive was characterized by a 3+ on immunohistochemistry. cN0 includes patients with normal lymph nodes on exam or with a negative fine-needle aspiration.
Statistical analyses
Descriptive statistics were used for demographics, clinical characteristics, and outcomes. Categorical variables are reported as N (%). Age is reported as mean ± standard deviation. The time from diagnosis to treatment is reported in median [interquartile range (IQR)] days. Chi-square and Fisher's exact test were utilized to analyze non-continuous variables. Continuous variables with normal distribution were analyzed with unpaired T-test and those with non-normal distributions were analyzed with the Wilcoxon signed-rank test. Multinomial logistic regression was employed to assess for higher-stage breast cancers relative to stage 0. Due to low number of outcomes, clinical stages 3-4 were grouped together to prevent overfitting of the model, as well as grouping Tricare insurance into the “Unknown/Insurance not otherwise specified” category. Significant values within the model were determined using two-tailed Wald tests. All variables included were assessed for collinearity. All statistical tests were two-tailed and a P-value of <0.05 was considered significant. All statistical analyses were performed using R and RStudio.17
Results
Study population
A total of 907 records met inclusion criteria in the study period. A total of 105 individuals were originally found to have unknown or incomplete clinical staging and were updated via chart review. Five of these patients remained with incomplete data and were excluded. The remaining 781 patients were included in the analysis (Fig. 1 , Table 1 ). Of these, 447 (57.2%) were identified in the pre-COVID era, but this decreased to 334 (42.8%) in the post-COVID-19 time frame. Overall, there were 113 (25.3%) fewer patients diagnosed with breast cancer when compared to pre-COVID era.
Fig. 1.
Study population process flow chart.
Table 1.
Patient and tumor characteristics, pre-COVID-19 versus post-COVID-19.
| Factor | Total N (%) | Pre-COVID-19 N (%) | Post-COVID-19 N (%) | P-value |
|---|---|---|---|---|
| Total | 781 | 447 (57.2%) | 334 (42.8%) | |
| Demographics | ||||
| Female | 772 (98.8%) | 443 (99.1%) | 329 (98.5%) | 0.435 |
| Age in y ( ± standard deviation) | 58.8±12.5 | 59.4 | 58.0 | 0.126 |
| Race | ||||
| White | 620 (79.4%) | 342 (76.5%) | 278 (83.2%) | 0.002 |
| Black | 60 (7.7%) | 45 (10.1%) | 15 (4.5%) | |
| Asian | 28 (3.6%) | 11 (2.5%) | 17 (5.1%) | |
| Other | 73 (9.3%) | 49 (11%) | 24 (7.2%) | |
| Ethnicity | ||||
| Hispanic | 176 (22.5%) | 90 (20.1%) | 86 (25.7%) | 0.001 |
| Not Hispanic | 533 (68.2%) | 302 (67.6%) | 231 (69.2%) | |
| Unknown | 72 (9.2%) | 55 (12.3%) | 17 (5.1%) | |
| Insurance | ||||
| Private | 355 (45.5%) | 202 (45.2%) | 153 (45.8%) | 0.102 |
| Medicare | 244 (31.2%) | 144 (32.2%) | 100 (29.9%) | |
| Medicaid | 69 (8.8%) | 38 (8.5%) | 31 (9.3%) | |
| Uninsured | 66 (8.5%) | 34 (7.6%) | 32 (9.6%) | |
| Tricare | 9 (1.2%) | 9 (2.0%) | 0 (0%) | |
| Unknown | 38 (4.9%) | 20 (4.5%) | 18 (5.4%) | |
| Clinical characteristics | ||||
| Clinical stage | ||||
| 0 | 163 (20.9%) | 91 (20.4%) | 72 (21.6%) | 0.646 |
| 1 | 436 (55.8%) | 249 (55.7%) | 187 (56%) | |
| 2 | 103 (13.2%) | 61 (13.6%) | 42 (12.6%) | |
| 3 | 45 (5.8%) | 23 (5.1%) | 22 (6.6%) | |
| 4 | 34 (4.4%) | 23 (5.1%) | 11 (3.3%) | |
| Pathologic stage | ||||
| 0 | 120 (15.4%) | 66 (14.8%) | 54 (16.2%) | 0.008 |
| 1 | 372 (4.8%) | 212 (47.4%) | 160 (47.9%) | |
| 2 | 45 (5.8%) | 37 (8.3%) | 8 (2.4%) | |
| 3 | 12 (1.5%) | 4 (0.9%) | 8 (2.4%) | |
| 4 | 7 (0.9%) | 4 (0.9%) | 3 (0.9%) | |
| Unknown | 225 (28.8%) | 124 (27.7%) | 101 (30.2%) | |
| Treatment characteristic | ||||
| Median days from diagnosis to first treatment [IQR] | 42 [23-56] | 38 [23 – 56] | 39 [23 -58] | 0.605 |
Bold: statistically significant, P < 0.05.
IQR = interquartile range.
Demographics
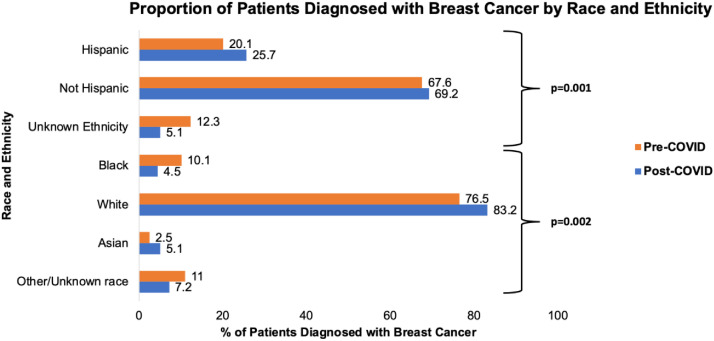
The vast majority of patients identified were female (N = 772, 98.8%). The mean age of breast cancer patients at diagnosis was 58.8 y and was consistent across pre-to-post-COVID time frames (59.4 versus 58.0 y, P = 0.126). There was racial variation identified between pre-COVID and post-COVID (P = 0.002) with an increase in the proportion of White patients (76.5-83.2%), a decrease in Black patients (10.1%-4.5%), but also a decrease in the overall unknowns (11.0 versus 7.2%). Variation was additionally identified among ethnicity (P = 0.001), with an increase in the proportion of Hispanic patients (20.1%-25.7%) and a decrease in unknowns (12.3%-5.1%) over the course of the two time frames (Fig. 2 ). Insurance status was most commonly private (N = 355, 45.5%) with no associations identified from pre-to post-COVID identified (P = 0.102).
Fig. 2.
Proportion of patients diagnosed with breast cancer by race/ethnicity.
Clinical characteristics
Analysis of clinical stage at presentation revealed stage 1 to be the most common (N = 436, 55.8%) and stage 4 to be the least common (N = 34, 4.4%) with no associations in pre-COVID to-post-COVID time frames found (P = 0.646). Histologic analysis showed the majority of cases to be ductal carcinoma (N = 656, 84.0%) and least to be papillary carcinoma (N = 5, 0.6%). There was no difference in the distribution of histologic diagnoses pre-COVID to post-COVID (P = 0.108). The median number of days between breast cancer diagnosis to first treatment was 39 (IQR: 23-56) d without a difference seen between time frames (38 [IQR: 23-56] versus 39 [IQR: 23-58] d, P = 0.605).
Identification of clinical higher-stage breast cancer
Multinomial logistic regression was performed adjusting for pre-COVID versus post-COVID time frames, age, race, ethnicity, insurance status, and time from breast cancer diagnosis to first treatment modality (Table 2 ). COVID-19 time frames did not affect odds of clinical stages 1, 2, or 3/4 breast cancer. Relative to clinical stage 0 breast cancers, there were significant associations identified, including: increased odds of stage 1 breast cancer with older age (relative risk ratio [RRR]: 1.04 [95% confidence interval {CI}: 1.02-1.06] per year increase), increased odds of diagnosing stage 2 breast cancer among those uninsured (RRR: 8.07 [95% CI: 2.68-24.25]), and increased odds of stage 3 or 4 breast cancer among those with Medicaid insurance (RRR: 5.18 [95% CI: 2.04-13.17]) or uninsured patients (RRR: 14.52 [95% CI: 4.57-46.07]). There additionally was an association between presenting with stage 2 or stage 3 or 4 breast cancer and delayed per-day time-to-intervention (RRR: 0.98 [95% CI: 0.98-0.99] and RRR: 0.99 [95% CI: 0.98-1], respectively). There were no associations with race.
Table 2.
Multinomial logistic regression: Relative risk of higher clinical stage breast cancer.
| Factor | Stage 1 |
P value | Stage 2 |
P value | Stage 3 or 4 |
P value |
|---|---|---|---|---|---|---|
| RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | ||||
| Post-COVID-19 | 1 (0.68-1.46) | 0.988 | 0.86 (0.51-1.45) | 0.567 | 0.89 (0.51-1.45) | 0.702 |
| Demographic | ||||||
| Age (per year increase) | 1.04 (1.02-1.06) | 0.001 | 1 (0.97-1.02) | 0.77 | 1 (0.97-1.02) | 0.974 |
| Race | ||||||
| White | Ref. | -- | Ref. | -- | Ref. | -- |
| Black | 0.52 (0.26-1.02) | 0.056 | 0.73 (0.29-1.85) | 0.502 | 0.95 (0.29-1.85) | 0.913 |
| Asian | 0.69 (0.26-1.82) | 0.456 | 0.8 (0.21-3.03) | 0.743 | 0.8 (0.21-3.03) | 0.767 |
| Other/Unknown | 1.99 (0.92-4.29) | 0.08 | 2.5 (0.97-6.44) | 0.059 | 1.1 (0.97-6.44) | 0.862 |
| Ethnicity | ||||||
| Not Hispanic | Ref. | -- | Ref. | -- | Ref. | -- |
| Hispanic | 0.55 (0.33-0.92) | 0.022 | 0.55 (0.27-1.12) | 0.099 | 0.69 (0.27-1.12) | 0.344 |
| Unknown | 0.63 (0.31-1.25) | 0.184 | 0.5 (0.18-1.44) | 0.2 | 1.26 (0.18-1.44) | 0.655 |
| Insurance | ||||||
| Private | Ref. | -- | Ref. | -- | Ref. | -- |
| Medicare | 0.68 (0.38-1.21) | 0.19 | 1.07 (0.47-2.4) | 0.875 | 1.88 (0.47-2.4) | 0.167 |
| Medicaid | 0.7 (0.33-1.49) | 0.357 | 2.12 (0.86-5.22) | 0.103 | 5.18 (0.86-5.22) | 0.001 |
| Uninsured | 2.3 (0.86-6.16) | 0.098 | 8.07 (2.68-24.25) | <0.001 | 14.52 (2.68-24.25) | <0.001 |
| Unknown/Insurance not otherwise specified | 1.2 (0.54-2.66) | 0.653 | 0.83 (0.24-2.88) | 0.775 | 0.78 (0.24-2.88) | 0.765 |
| Treatment characteristic | ||||||
| Time to first intervention (per day increase) | 1 (0.99-1) | 0.156 | 0.98 (0.98-0.99) | 0.001 | 0.99 (0.98-0.99) | 0.018 |
Bold: statistically significant, P < 0.05.
CI = confidence interval; RRR = relative risk ratio.
Discussion
This study assessed patterns in breast cancer diagnosis before and during the first year of the COVID-19 pandemic, especially with regards to incidence, demographics, stage at presentation, and time-to-intervention. Importantly, we were looking to see if the unique socioeconomic and political atmosphere of Central Texas and its population was associated with outcome changes compared to previous reports.14 , 15
In the post-COVID-19 era of our study, 25% fewer women were diagnosed with breast cancer than prior to COVID-19. While breast cancer screening was done less due to safety and patient preference during the major waves of the pandemic, breast cancer surgeries and treatment were not significantly delayed or canceled at this institution. The results of this study are consistent with previous reports demonstrating a decrease in diagnoses during the pandemic. The hypothesis that this decrease is due to decreased screening mammography remains a key question for future studies.15 Over the first year of the pandemic, we observed fewer new diagnoses of breast cancer compared to the prior year, suggesting that cases expected during the pandemic have yet to emerge and still have the potential for later stage at diagnosis. Ongoing efforts to catch up on screening will be the key in preventing further pandemic-related impact on patients diagnosed with breast cancer.
This study demonstrates important implications regarding screening and early detection in the next months and years, especially in our Hispanic, Black, and uninsured populations. Interestingly, while interruptions to screening happened, our data suggest that once patients were diagnosed, there were no significant differences in time to starting treatment. This raises several questions, one of which is whether this cancer center is representative of other health systems in delivering out-patient care to diagnosed cancer patients. This study adds to the growing body of literature analyzing the relationship of COVID-19 and breast cancer diagnosis and treatment in other communities and settings.
Several studies suggest that delay in surgical management of breast cancer is significantly associated with lower survival.18 , 19 Even prior to the effects of COVID-19, wait times between diagnosis and treatment of breast cancer had been increasing, with an interval of <30 d considered not significantly associated with tumor size progression.20 Our data did not find there to be a significant delay in time-to-intervention on univariate analysis associated with the pandemic. However, there was a slight association between presenting with stage 2, stage 3, or stage 4 cancers and delayed per-day time-to-intervention on multinomial analysis. This may reflect the time required for staging studies in later-stage patients prior to initiating treatment.
Regarding socioeconomic and racial factors, this study found that when comparing pre-COVID-19 and post-COVID-19 eras, proportionally fewer Black women (10.1%-4.5%) were diagnosed with breast cancer than their White and Hispanic, and Asian counterparts. Both race and ethnicity demonstrated significant variation between the two eras. While the initial impact of the COVID-19 pandemic did not result in significant staging differences when stratified by race or ethnicity, the disproportionate likelihood of higher stage cancer for underinsured and uninsured patients suggests a negative association of COVID-19 on these populations. An emphasis should be made on improving insurance coverage and screening availability for these populations. Other social factors that could have contributed to this worsened disparity such as transportation, job loss, and language barriers should be investigated in future qualitative studies.
The above findings must be put into the sociocultural context to truly appreciate their significance. The demographic breakdown of Texas compared to the US population at large provides unique challenges in health care delivery, especially during the COVID-19 pandemic. As of March 25, 2021, 65.5% of the US population was fully vaccinated against COVID-19.22 Texas ranked 45th nationally compared to other states in terms of overall percentage of its population being fully vaccinated.21 , 22 Additionally, mask mandates were banned in businesses and schools which may influence patients to avoid public spaces as the only means of protection during surges.23
The relationship between race, ethnicity, and payer status in this study population between pre-COVID-19 and post-COVID-19 eras is reflective of the socioeconomic climate of Texas and Central Texas. The decrease in diagnosis of Black patients post-COVID as well as the significant association with uninsured or underinsured payer status with later-stage presentation could reflect care being sought within a different cancer center in our city. However, this center is responsible for most of the uninsured and underinsured care in Travis County.
Insurance status among Hispanic and Black communities is affected by Texas’ refusal to expand Medicaid, with 10% of African-Americans and 61% of Hispanic/Latinx individuals being uninsured, compared to 14% of African-Americans and 37% of Hispanic/Latinx individuals nationally.24 Additionally, undocumented immigrants who do not have access to health insurance comprise 6% of the population of Texas.24 These findings have been attributed to disproportionate job loss with subsequent loss of insurance in Hispanic and Black populations as well as barriers to health care access including transportation, health literacy, and lower baseline incomes.12 Further research regarding factors influencing these outcome disparities is warranted to better understand and improve breast cancer care in these populations.
The disparities present in breast cancer care were exacerbated in this study by COVID-19, making these minority groups even more at-risk. According to the Center for Disease Control and Prevention data regarding COVID-19 case numbers, the percent of the US population identifying as Hispanic/Latinx is 18%, while in Texas it is 40%.22 However, the percent of COVID-19 cases in the national Hispanic population is 25% while in Texas COVID-19 cases in this group reached 51%.22 The percent of the US population identifying as Black/African American is equivalent to that of Texas (12% and 12.3%, respectively) but the percent of COVID-19 cases in the Black population in the United States is 12%, but in Texas it is 15%.22 Comparably, White Caucasian populations had a lower COVID-19 case percentage compared to their occupied percent of the US and Texas populations. These numbers have significant implications for the prediction and management of health conditions such as breast cancer during and after the COVID-19 pandemic, with Black, uninsured, and underinsured populations being particularly vulnerable to external impact. COVID-19's disproportionate impact on these populations has likely contributed greatly to the breast cancer outcomes and is consistent with this study's findings of decreased breast cancer diagnoses in Hispanic and Black populations post-COVID-19.
Limitations
There are several limitations of this study. Like all nonlongitudinal registry-based studies, there are limited treatment data and follow-up. The method of cancer diagnosis, that is symptom-driven or screening-driven is also not available from the registry data. Assessing the impact of the COVID-19 pandemic on screening methods would be an excellent topic for future studies. This study assessed just one hospital system and was limited to Central Texas. While our demographic data are consistent with that of Texas as a whole, the results are not necessarily generalizable to the state or country at large. It is also possible that certain ethnic or racial groups experienced worse access to care during the time frame of this study independently of the COVID-19 pandemic. However, the worsening from baseline strongly suggests an association with the pandemic.
It is worth noting that while the clinical staging missing from the registry was able to be obtained and completed via chart review, the unknown burden of the pathologic staging was significant (28.8%). The significance of the pathologic staging pre-COVID-19 and post-COVID-19 variation (P = 0.008) is therefore not reliable and was not reported as a key result. It is possible however that the unknown pathologic stage reflects patients receiving neoadjuvant treatment that were awaiting surgery.
As the purpose of this study was to assess disparities in diagnosis and management of breast cancer during COVID, a key variable analyzed was the clinical stage at presentation, or diagnosis. While some treatment data were available from the tumor registry, key contextual information was missing, rendering its exclusion from this study which focuses on initial presentation and diagnosis. However, future studies of the impact of the COVID-19 pandemic on breast cancer treatment would be valuable in regard to providing patient care.
Conclusions
We found 25% fewer patients across all demographics were diagnosed with breast cancer in the first year of the COVID-19 pandemic in Central Texas at a safety-net hospital system, suggesting probable decreases in screening mammography. Black women experienced a disproportionate decrease in COVID-era associated breast cancer diagnoses, but no increase in the risk of higher-stage breast cancers. Uninsured and underinsured patients were more likely to have higher-stage breast cancers. Considering the modeling studies that predict worsening outcomes and mortality over the next 5 y due to COVID-19 disruptions in screening, it is important to proactively identify populations who have had decreases in diagnoses to prevent later stage disease presentations in the upcoming years. Our findings, in a unique sociopolitical and socioeconomic environment not previously described in the literature, highlight the need for future targeted breast cancer screening in Black, underinsured, and uninsured women.
Author Contributions
Lana Schommer contributed to data entry and cleaning and is the manuscript primary author. Matthew Mikulski contributed to data analysis and statistics, methods and is the results author. Boone Goodgame contributed to data acquisition, study design, and is the manuscript editor. Kimberly Brown is the primary investigator and editor.
Disclosure
None declared.
Funding
None.
References
- 1.Chen R.C., Haynes K., Du S., Barron J., Katz A.J. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7:878–884. doi: 10.1001/jamaoncol.2021.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coughlin S.S. In: Breast Cancer Metastasis and Drug Resistance. Vol 1152. Advances in Experimental Medicine and Biology. Ahmad A., editor. Springer International Publishing; Switzerland: 2019. Epidemiology of breast cancer in women; pp. 9–29. [DOI] [PubMed] [Google Scholar]
- 4.Vanni G., Pellicciaro M., Materazzo M., et al. Lockdown of breast cancer screening for COVID-19: possible scenario. Vivo. 2020;34:3047–3053. doi: 10.21873/invivo.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papautsky E.L., Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat. 2020;184:249–254. doi: 10.1007/s10549-020-05828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng S., Yang K., Chan W.P., et al. Impact of the COVID-19 pandemic on a population-based breast cancer screening program. Cancer. 2020;126:5202–5205. doi: 10.1002/cncr.33180. [DOI] [PubMed] [Google Scholar]
- 7.de Azambuja E., Trapani D., Loibl S., et al. ESMO Management and treatment adapted recommendations in the COVID-19 era: breast Cancer. ESMO Open. 2020;5:e000793. doi: 10.1136/esmoopen-2020-000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curigliano G., Cardoso M.J., Poortmans P., et al. Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast. 2020;52:8–16. doi: 10.1016/j.breast.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietz J.R., Moran M.S., Isakoff S.J., et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. the COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020;181:487–497. doi: 10.1007/s10549-020-05644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ASBrS & ACR Joint Statement on Screening Exams COVID-19 resour cent textbar ASBrS. https://www.breastsurgeons.org/management/practice/covid19 Available at:
- 11.Freer P.E. The impact of the COVID-19 pandemic on breast imaging. Radiol Clin North Am. 2021;59:1–11. doi: 10.1016/j.rcl.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman L., Fejerman L., Pal T., et al. Breast cancer disparities through the lens of the COVID-19 pandemic. Curr Breast Cancer Rep. 2021;13:110–112. doi: 10.1007/s12609-021-00419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsapatsaris A., Babagbemi K., Reichman M.B. Barriers to breast cancer screening are worsened amidst COVID-19 pandemic: a review. Clin Imaging. 2022;82:224–227. doi: 10.1016/j.clinimag.2021.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcondes F.O., Cheng D., Warner E.T., Kamran S.C., Haas J.S. The trajectory of racial/ethnic disparities in the use of cancer screening before and during the COVID-19 pandemic: a large U.S. academic center analysis. Prev Med. 2021;151:106640. doi: 10.1016/j.ypmed.2021.106640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tonneson J.E., Hoskin T.L., Day C.N., Durgan D.M., Dilaveri C.A., Boughey J.C. Impact of the COVID-19 pandemic on breast cancer stage at diagnosis, presentation, and patient management. Ann Surg Oncol. 2022;29:2231–2239. doi: 10.1245/s10434-021-11088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giuliano A.E., Connolly J.L., Edge S.B., et al. Breast cancer-major changes in the American joint committee on cancer eighth edition cancer staging manual: updates to the AJCC breast TNM staging system. CA Cancer J Clin. 2017;67:290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 17.The R project for statistical computing. https://www.r-project.org/ Available at:
- 18.Bleicher R.J., Ruth K., Sigurdson E.R., et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2:330–339. doi: 10.1001/jamaoncol.2015.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards M., Westcombe A., Love S., Littlejohns P., Ramirez A. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353:1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.H., Kim Y.S., Han W., et al. Tumor growth rate of invasive breast cancers during wait times for surgery assessed by ultrasonography. Medicine (Baltim) 2016;95:e4874. doi: 10.1097/MD.0000000000004874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harper K.B. Trying to make sense of how Texas ranks on coronavirus vaccinations? Here’s a look behind the numbers. https://www.texastribune.org/2021/04/09/texas-covid-vaccine-rankings/ Available at:
- 22.CDC COVID data tracker. Cent dis control prev. https://covid.cdc.gov/covid-data-tracker Available at:
- 23.Stieg C. Texas and Mississippi ditch mask mandates and other restrictions — here’s how it could affect you, wherever you live. https://www.cnbc.com/2021/03/03/texas-and-mississippi-lifting-mask-mandates-effect-on-ending-pandemic-.html Available at:
- 24.Ojinnaka C.O., Adepoju O.E., Burgess A.V., Woodard L. Factors associated with COVID-related mortality: the case of Texas. J Racial Ethn Health Disparities. 2021;8:1505–1510. doi: 10.1007/s40615-020-00913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]