Abstract
Purpose of Review
Cardiovascular disease (CVD) and cancer are the first and second most common causes of death within the USA. It is well established that a diagnosis of cancer increases risk and predisposes the patient to CVD, and vice versa. Despite these associations, cancer is not yet incorporated into current CVD risk calculators, necessitating additional CV risk markers for improved stratification in this at-risk population. In this review, we consider the utility of breast arterial calcification (BAC), coronary artery calcification (CAC), clonal hematopoiesis of indeterminate potential (CHIP), and cancer and cancer treatment in CVD risk assessment.
Recent Findings
There is evidence supporting the use of BAC, CAC, CHIP, and cancer and cancer treatment for improved CV risk stratification in patients with cancer and those who are being screened for cancer. BAC has been shown to predict CAC, coronary atherosclerotic plaque on coronary CTA, coronary artery stenosis on coronary angiography, and CVD events and accordingly enhances CVD risk stratification beyond the atherosclerotic CVD (ASCVD) risk pooled cohort equation. Additionally, CAC visualized on CT utilized for lung cancer screening, radiation planning, and cancer staging is predictive of coronary artery disease (CAD). Furthermore, CHIP can also be utilized in risk stratification, as the presence of CHIP carries a 40% increase in CV risk independent of traditional CV risk factors. Finally, cancer and many oncologic therapies confer a lifelong increased risk of CVD.
Summary
We propose an emerging set of tools to be incorporated into the routine continuum of CVD risk assessment in individuals who have been treated for cancer or who are being screened for cancer development. In this review, we discuss BAC, CAC, CHIP, and cancer and cancer treatment as emerging risk markers in cardiovascular health assessment. Their effectiveness in predicting and influencing the burden of CVD will be discussed, along with suggestions on their incorporation into preventive cardio-oncology practice. Future research will focus on short- and long-term CVD outcomes in these populations.
Keywords: Prevention, Cardio-oncology, Risk, Cancer, Breast arterial calcification, Coronary artery calcification, Clonal hematopoiesis of indeterminate potential
Introduction
CVD and cancer are the first and second most common causes of death within the USA accounting for 1.1 million deaths per year and approximately $400 billion in annual medical costs [1]. While CVD and cancer are historically siloed within separate subspecialty disciplines, these conditions have many common modifiable risk factors, are acquired through similar pathophysiologic mechanisms, and often develop in similar patient populations. Recently, collaborative efforts supporting a joint approach to treating patients with both CV and oncologic disease have yielded a novel paradigm: cardio-oncology. This distinct field is dedicated to characterizing and improving outcomes for this unique population.
This integrated subspecialty evolved from a need to provide long-term CV care to cancer patients [2]. Therapeutic advancements in the treatment of malignancies, including the development of monoclonal antibodies, checkpoint inhibitors, and targeted therapeutics, have enormously improved oncologic patient oncologic outcomes and survival. Despite the significant successes in oncology, one in ten cancer survivors will die of CVD, with their greatest risk within the first year of diagnosis. Furthermore, their overall risk for CVD is 2–6 times higher than in patients without cancer, a consequence of treatment-related toxicities and prolonged survival [3]. Improved cancer survivorship, therefore, propels the need for further investigation and risk assessment of treatment-related cardiotoxicities in addition to prevention and optimization of CVD prior to and during treatment.
Preventive cardio-oncology aims to mitigate CV side effects of cancer therapies by optimizing CV function and risk factors prior, throughout, and after oncologic therapy [2, 4]. A cancer diagnosis should prompt immediate CV risk assessment and early involvement of a multidisciplinary team. Periodic screening, surveillance, shared-decision making, and evidence-based therapies are current tools used to identify and treat cancer patients with cardiotoxicities. Another way the field may advance this mission is through the utilization of next generation risk markers which aid in CVD risk stratification in patients with and without malignancy. Here, we discuss these emerging risk markers, which include BAC, CAC, CHIP, and abstractly, cancer and cancer treatment, as well as discuss their effectiveness in predicting and influencing the burden of CVD in cancer patients. While many of these tools have shown strong associations to CVD, their incorporation into preventive measures in cardio-oncology is so far limited. This paper will describe their importance in the prevention and management of CVD and suggest how the field of preventive cardio-oncology can bridge this gap.
Breast Arterial Calcification
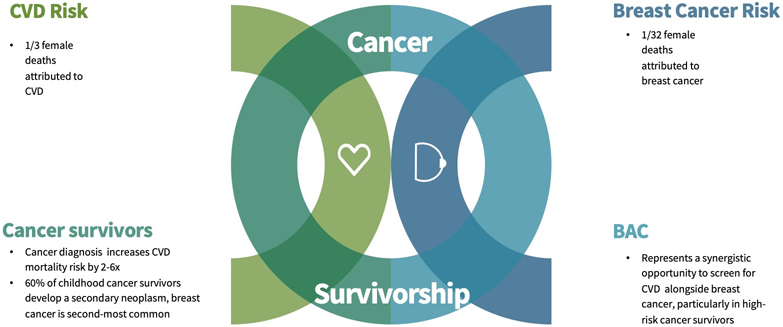
BAC, defined as medial calcification within the arteries of the breast, is displayed as tubular and radio-opaque parallel tracks on both screening and diagnostic mammograms [5•]. Previously considered an incidental finding, these calcifications are associated with established CVD risk factors and more importantly increased risk of CAD and CV outcomes [6-11]. Despite these associations, BAC is not routinely documented on screening mammography [12], nor is BAC on mammography used to initiate, expedite, or intensify CVD risk assessment in at-risk women. Mammograms, currently utilized annually by more than 65% of women over the age of 40 [6], therefore provide what Handy et al. describe as synergistic opportunities in screening for both CVD and breast cancer (Fig. 1A) [5•]. This is of great benefit to the general female population and among those with heightened risk, specifically childhood cancer survivors and breast cancer patients.
Fig. 1.
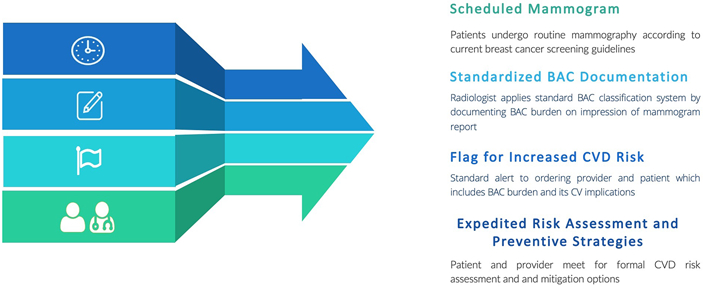
Synergistic screening in preventive cardio-oncology. A Rational for combining breast cancer and CVD screening with mammography [3, 13, 28, 31]. B Purposed workflow for standardized breast arterial calcification (BAC) reporting and follow-up CVD risk assessment. Figure components are from Infografia
While much emphasis is placed on breast cancer prevention, the 2014 Center for Disease Control attributes 1 in 32 female deaths to breast cancer and 1 in 3 female deaths to CVD, making CVD the leading cause of morbidity and mortality for women in the USA [13]. Despite these statistics, the need for improved risk stratification in women is immense. Currently, CVD risk assessments fall short in accurately stratifying CVD risk in female populations [14, 15]. Existing algorithms both underestimate the presence and burden of atherosclerotic disease in women [16]. Many women who experience CV events actually have a 10-year estimated atherosclerotic cardiovascular disease (ASCVD) risk of < 7.5% and would therefore have been classified as “low risk” [17]. Appropriate risk modifiers are therefore needed to more adequately risk classify women. In this setting, BAC has the potential for use as a risk modifier, specifically in women [6-11].
BAC is reported to be found on 13% [7, 18] of all screening mammograms and increases with patient age, with up to 50% of 80-year-old women demonstrating calcifications on imaging [18]. In addition to age, BAC also correlates with diabetes mellitus, hypertension, chronic kidney disease, parity, and a history of CAD [6, 19]. Additionally, women with BAC had higher levels of serum triglycerides, homocysteine, C-reactive protein, and an elevated LDL-C/HDL-C ratio, all of which are serum markers for CV risk [20, 21].
BAC not only is correlated to CVD risk markers but also CV outcomes. In one cohort study, BAC presence resulted in a 1.3 and 1.5 increased risk of CAD and heart failure, respectively [22]. Another showed an odds ratio (OR) of 3.5 (95% confidence interval (CI) 2.28–5.50) for CVD in women who had BAC on the mammograph [22, 23]. Furthermore, the burden of arterial calcification on mammography appears to further stratify as a series of case-cohort studies demonstrates that “severe” quantification on imaging resulted in a threefold increase in CVD when traditional CV risk factors were accounted for [22]. In a large-scale retrospective cohort study, the hazard ratio (HR) for overall mortality in patients with BAC was 1.3 (95% CI 1.06–1.58) and up to 1.74 ( 95% CI 1.19–2.56) in diabetic women with BAC [24]. In regard to other imaging, BAC has been shown to predict CAC [6-10], coronary atherosclerotic plaque on coronary CTA [6], and coronary artery stenosis on coronary angiography [25]. BAC presence increases the likelihood of CVD events [11, 22], and enhances CVD risk stratification beyond the atherosclerotic CVD (ASCVD) risk pooled cohort Eq. (8). In women referred for coronary angiography for high suspicion of CAD, BAC showed a correlation with the presence of coronary artery stenosis [26]. Used as a risk enhancer, BAC can be applied similarly to CAC on a CT scan, which is used to reclassify individuals with ASCVD risk estimation between 5 and 20% [27].
The application of BAC is favored in women with comorbidities, specifically a history of cancer and cardiotoxic treatments, which increase CVD risk but are not incorporated into current risk calculators. While CVD and CV mortality trends in cancer patients are multifactorial and complex, overall, a cancer diagnosis increases the risk of CVD mortality 2–6 times compared to the general public [3, 28]. Generally, this risk is the highest within the first year of a diagnosis with what Hermann describes as the acute phase, where toxic therapies and tumor burden come to a dangerous intersection. A chronic phase follows, which extends into survivorship and requires continued clinical surveillance. Currently, the 4 million breast cancer survivors in the USA are more likely to die from CVD than reoccurrence of their malignancy [1, 29]. Mammography, through the assessment and reporting of BAC, may improve preventive strategies in this under-stratified population.
The application of BAC in this context goes beyond adult cancer patients as 1 in 10 survivors of childhood cancer will develop CVD [30]. Beyond CVD risk, 60% of female adult survivors of childhood cancer will develop a secondary neoplasm, with breast cancer being the most frequent after nonmelanoma skin cancer [31]. Emerging data recommends annual breast cancer screening via imaging in survivors of childhood cancer between the age of 25 and 30 years, nearly a decade before general guidelines [32]. The benefits of mammography are multifactorial in this population as adult survivors of childhood cancer carry a greater risk of both breast cancer and CVD than the general population.
The evidence supports routine, standardized reporting of BAC, given its association with CVD risk independent of typical risk markers. Increased awareness of BAC on mammography can facilitate the creation of a more robust and personalized risk prediction tool for women. Furthermore, 96% of patients in one study indicated a preference to be informed of BAC presence [33], highlighting the opportunity for earlier patient-provider dialogue about CVD and expedited referral to preventive cardiology or cardio-oncology clinics for aggressive modification of cardiac risk factors. As no universal interpretation, quantification, and clinical application of BAC exist, the next step to a large-scale application is the creation of guidelines and algorithms for incorporating BAC into the current risk prediction paradigm. We suggest this standardized classification of BAC be similar to the Coronary Artery Disease-Reporting and Data System (CAD-RADS) used for CCTA reporting. This would also involve widespread education about BAC and its implications to radiology, cardiology, and primary care providers as well as the development of BAC clinical management programs (Fig. 1B). With emerging systems in place, BAC can be prospectively followed to assess significant improvements in CVD morbidity and mortality in female patients, specifically cancer patients and survivors. Until then, BAC presence should prompt a thorough assessment of CV risk so preventive therapies and screening can be appropriately implemented. In conclusion, mammograms, through the detection of BAC, can potentially simultaneously improve outcomes in both breast cancer and CVD in female populations, making it an emerging risk marker in preventative cardio-oncology.
Coronary Artery Calcification
CAC score is a measurement of coronary artery calcium used as a marker of overall coronary artery plaque burden, and therefore by extension, CV risk [34, 35]. Unlike coronary angiography, which directly measures CAD, CAC is a proxy for this measurement that still provides prognostic value and guidance of primary prevention of CAD [36-38]. CAC is typically assessed using non-contrast, cardiac-gated computed tomography (CT) and is reported as an absolute number or “score” to quantify the burden of CAC. This score is compared to others with similar characteristics and reported as a percentile, allowing for stratification of risk and calculation of a hazard ratio for a CV event [35]. The clinical relevance of CAC is numerous. First, the absolute burden of CAC is used to guide prevention measures. CAC is a Class IIA recommendation in the 2019 ACC/AHA prevention guidelines for adults at intermediate risk for atherosclerotic CVD [39]. Taken together with lifestyle and genetic risk factors, this score can be used to further stratify risk and individualize prevention strategies for CAD [39]. Taken one step further, a CAC = 0 can be used to “downgrade” ASCVD risk, with sensitivity as high as 98% and specificity up to 40% [39, 40]. This high sensitivity was demonstrated in the MESA (Multi-Ethnic Study of Atherosclerosis) trial, with CAC = 0 illustrated as the strongest negative risk factor for ASCVD in middle-aged adults [41]. Most recently, a study from the CAC Consortium, comprised of 66,636 scans on asymptomatic patients without CVD, demonstrated that across traditional risk factors, CAC was the most consistent predictor of long-term, all-cause mortality with the greatest proportion of deaths from CVD [5•, 42].
While formal assessment of CAC is performed using cardiac-gated CT imaging, evidence of CAC can be seen on other imaging modalities [35, 43, 44]. Currently, low-dose CT screening of the chest is recommended by the US Preventive Services Task in patients between the age of 55 and 80 years with a 30 pack-year smoking history. Though not EKG (electrocardiogram)-gated, CAC is present on these CT scans, with increasing CAC scores from these images associated with increased risk of CV events and death [45]. Given that cigarette smoking is a major risk factor in the development of both CVD and malignancy, low-dose CT images provide yet another synergistic opportunity in screening and prevention of both CVD and cancer in high-risk populations [5•].
In addition to screening CT scans, incidental CAC is found on CT scans used for cancer staging and radiotherapy planning [36]. A recent retrospective analysis of non-contrast CT scans in breast cancer patients undergoing radiation identified CAC > 0 in over half of their patients [46]. Of those with CAC > 0, one-third then qualified for statin as their risk was reclassified beyond their ASCVD 10-year risk calculation [46]. This highlights the importance of identifying subclinical CVD in an already at-risk population. Currently underway, the BRAGASTON Study seeks to deliver cost-effective quantification of CAC on chest CTs being utilized for radiation planning, providing further high-quality data to specialists on the burden of CAD prior to treatment [47]. Additionally, baseline CAC data may be helpful, especially since those with higher CVD risk at baseline may be at greater risk for the development of premature clinical CAD following radiation therapy (RT) involving the heart in the treatment field [48•]. For this noted to have baseline CAC greater than 0, coronary artery dose volumes could potentially be adjusted preemptively [48•].
Finally, CAC assessment may be utilized in survivorship, specifically in patients who have undergone RT. In one study, CAC was found almost exclusively in patients who had baseline CV risk factors, suggesting potentially expedited atherosclerosis after undergoing RT, particularly in this population [48•]. Higher radiation exposure strongly correlated with increased CAC on CT even after controlling for typical cardiac risk markers, especially noted in the specific coronary arteries that were irradiated [48•]. This was noted at a median of 32 months after radiation therapy. Accordingly, we suggest obtaining a CAC scan in those not already known to have clinical or subclinical CAD—within 2 to 10 years after the conclusion of RT. Timing should be based on risk factors such as age and concurrent chemotherapy exposure, similar to expert consensus recommendations from the Society for Cardiovascular Angiography and Intervention’s recommendations for cardiac computed tomography angiography (CCTA) after RT [49]. Further surveillance with a CAC scan, often in addition to EKG and echocardiogram, at 5 to 10 years after an initial CAC scan or CCTA may be warranted in specific high-risk populations such as those who are > 60 years of age, have more than one CV risk factor, or known CAD [49, 50].
Resources should be allocated to increase awareness, reporting, and application of CAC, when found on both CT scans, utilized for cancer screening and therapy planning (Fig. 2). In cancer survivors, CAC CT has utility as a component of CVD screening protocols. CAC should be regarded as an emerging risk marker in the field of cardio-oncology because, like BAC, CAC can be used as a risk enhancer to more precisely risk-stratify patients. Standardized training of radiology, oncology, and cardiology specialists to facilitate interpretation and reporting of CAC on these CT scans is needed. From this, prospective analysis and outcomes should be measured, further supporting the effort of CAC documentation.
Fig. 2.
CAC on CT in preventive cardio-oncology. Figure components are from Microsoft Powerpoint and Infografia and not of actual patients
Clonal Hematopoiesis of Indeterminate Potential
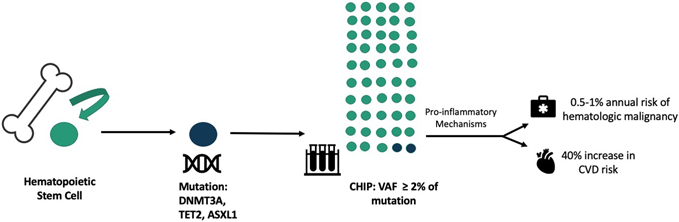
CHIP is a hematologic disorder characterized by a clonal hematopoietic stem cell expansion driven by an acquired mutation in a gene that causes blood cancer [51]. CHIP was first identified by analyzing genome sequences of large numbers of healthy individuals to identify premalignant changes in the blood that increase the risk for blood cancer. Surprisingly, a large fraction of individuals had somatic mutations (i.e., mutations acquired after birth) in the same small set of genes including DNMT3A, TET2, ASXL1, and JAK2. These mutations caused a growth advantage over other hematopoietic stem cells, leading to clonal expansion (Fig. 3A). CHIP was subsequently defined as the presence of one of these mutations that cause blood cancer in the absence of hematologic abnormalities [51, 52].
Fig. 3.
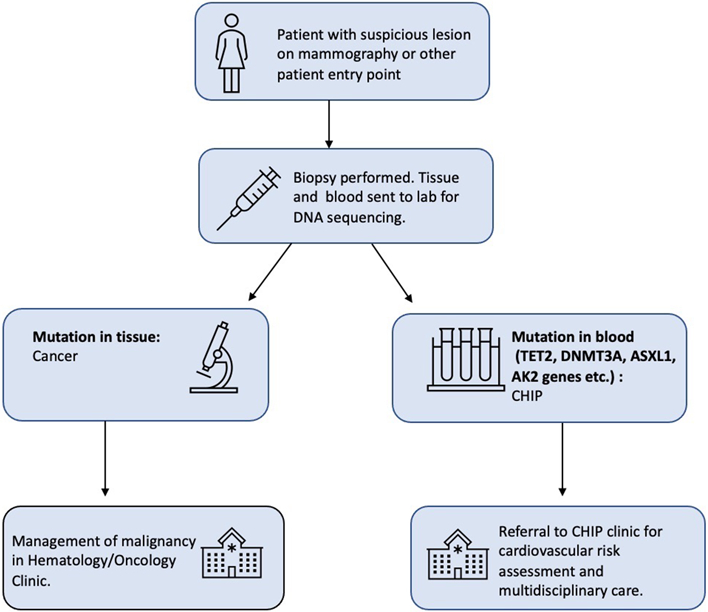
CHIP in preventive cardio-oncology. A CHIP pathophysiology [51, 65]. B Example of CHIP workflow. Figure components are from Infografia
Intriguingly, progression from CHIP to overt neoplasia occurs at a modest rate of 0.5–1% annually [51]. However, individuals with CHIP have a significantly increased risk of mortality due to CVD, with a 40% increase in CV risk independent of traditional CV risk factors (53–55). As such, CHIP has not only been of great interest to the oncology community but has emerged as a potent, next generation CV risk marker.
The association of CHIP with CVD appears to be driven by multiple pro-inflammatory mechanisms caused by the underlying somatic mutation that drives the CHIP clonal expansion. [53-55]. This expansion of peripheral leukocytes causes inflammation by irritating the vessel endothelium [55]. While the most common CHIP-related mutations, DNMT3A and TET2, drive this proinflammatory cycle through cytokine expression and subsequent cardiac dysfunction, the JAK2 mutation accelerates atherosclerotic plaque formation and confers a 12-fold increased risk of CAD compared to a twofold increased risk with DNMT3A and TET2 mutations [53, 54, 56, 57, 58•, 59, 60]. Less common CHIP mutations in PPM1D and TP53 are of special interest to the preventive cardio-oncologist because they are more prevalent in patients exposed to cytotoxic cancer therapeutic agents [61, 62]. For example, TP53-mediated therapy-related clonal hematopoiesis has been found to play a role in doxorubicin-induced cardiomyopathy [63]. Data, therefore, suggests the need for further investigation of chemotherapy’s role in CHIP and CVD outcomes.
Blood sequencing remains the sole method to identify CHIP, and as a result, individuals with CHIP are usually identified incidentally to other clinical workups typically in hematology/oncology (Fig. 3B). The most common clinical scenario is the identification of CHIP in patients with a known solid malignancy undergoing molecular genetic profiling. In this process, a patient’s tumor biopsy is sequenced and compared to non-cancerous control tissue—most often a sample of the patient’s blood. The goal of this testing is to identify mutations in the tumor sample. However, this can also result in the discovery of a mutation in the blood sample used as control tissue, identifying an individual as having CHIP (Fig. 1). Another common scenario that can reveal individuals with CHIP occurs when a clinician orders genetic sequence testing for a patient presenting with blood count abnormalities of unknown cause. Finally, CHIP is occasionally found in those who undergo direct-to-consumer or “elective” genetic sequencing [60, 64, 65].
As DNA sequencing is more broadly applied, detection of CHIP requires a range of clinical management strategies. Most commonly, individuals with CHIP are first referred to as hematology. The hematologic management of CHIP typically involves periodic monitoring, unique to each patient. A similar approach is used in the management of myelodysplastic syndrome (MDS), which is associated with an up to 10% annual risk of progression to blood cancer [66]. Although evidence-based guidelines do not exist, the consensus is to obtain routine lab work, including complete blood count and C-reactive protein and monitor blood counts every 3–6 months [65]. The hematologic considerations of CHIP must, of course, be thoroughly discussed with the patient. However, hematologists frequently refer patients to preventive cardiology or cardio-oncology clinic as CHIP confers an even greater CV risk than hematologic malignancy risk.
In the absence of evidence-based guidelines for the CV management of CHIP patients, the management of this population relies on a preventive cardiology evaluation, similar to the general population that includes a thorough history, utilization of labs, and application of available imaging, perhaps BAC or CAC. In short, the focus remains on targeting modifiable CVD risk factors such as cholesterol, blood pressure, diabetes, regular physical exercise, a heart-healthy diet, and smoking cessation [65]. In regard to pharmacologic therapies, CHIP may be a risk factor used to support the initiation of a lipid-lowering agent for cholesterol management. In patients with CHIP and diabetes, there may be a benefit in the initiation of either a GLP-1 agonist or SGLT-2 blocker, which are glucose-lowering agents shown to lower CV risk in select individuals [65, 67]. At this time, aspirin use is not recommended in CHIP patients without ischemic history, as recent data suggests an association of CHIP with intracerebral hemorrhage [68]. However, individuals with CHIP who harbor JAK2 V617F mutations are at an especially increased risk of thrombotic events and aspirin prophylaxis remains an essential pharmacologic therapy [69].
Laboratory investigations point to potential future management strategies for CHIP that will require rigorous evaluation. Studies have shown that inflammatory biomarkers, including CRP and interleukin 6-B (IL-6B), are elevated in patients within the general population who have significant atherosclerosis, independent of cholesterol levels [70]. Furthermore, CHIP’s previously referenced loss of the TET2 allele is associated with increased expression of IL-1B, another inflammatory marker [56]. Although not yet standard of care, the use of biologics and immune modulators targeting markers of inflammation show promise as treatment strategies for CHIP and CVD. Canakinumab, a monoclonal antibody, has shown potential through the inhibition of Interleukin-1B, which is the cytokine driving the IL-6B cytokine inflammatory pathway. In the CANTOS study, patients with high inflammatory markers and a history of myocardial infarction who received canakinumab at a threshold dose were found to have a significant decrease in both subsequent CV events and inflammatory marker levels, compared to patients who received a placebo drug [70]. These findings suggest a potential therapeutic role for canakinumab in the CHIP population.
The intricate relationship and subsequent management among CHIP, cardiovascular disease, and hematologic malignancy remain extremely complex and therefore requires a multidisciplinary approach that includes skilled clinicians from multiple specialties. As a result, CHIP clinics have emerged. Multidisciplinary providers in these clinics frequently include hematology, oncology, and cardiology. Given that CHIP is most frequently an incidental finding identified in patients undergoing sequencing for their solid tumor malignancy, cardio-oncologists have unique expertise in this patient population and therefore benefit CHIP clinics. The advantages that CHIP clinics provide to patients are numerous and include ease of access and recruitment into clinical trials designed to both reduce the risk of progression to neoplasia and decrease the risk of CV complications [69]. In summary, CHIP is a next generation risk marker in the field of cardio-oncology given the increased risk of both malignancy and CVD it confers. CHIP clinics are a key innovation in furthering our understanding of the meaningful application of CHIP in both CVD and cancer. They afford us the opportunity to follow patients with CHIP while also allowing for the development of formal and evidence-based guidelines so that we may best manage individuals with CHIP.
Cancer and Cancer Treatment as a Risk Marker
As new cancer therapies are discovered and implemented, cancer survivorship in patients has led to an increased risk of CVD. While the increased prevalence of CVD in these patients is often attributed to cardiotoxic therapies, the connection is proving to be multifaceted. Existing cardiac risk factors coupled with the inflammatory processes that drive malignancy have shown to also be significant players, as these make patients more susceptible to CVD and catalyze CV events in what Jones et al. describe as the multihit hypothesis [71]. This theory depicts CVD in cancer patients as a series of concomitant events that leave patients vulnerable to reduced cardiovascular reserves and the development of CVD and CV events. With CVD as the leading cause of death in cancer survivors, a current or previous cancer diagnosis should be viewed and utilized by the cardio-oncology team as a novel risk marker and risk equivalent when personalizing a preventive plan.
Before therapies are initiated, cancer patients, in general, are already more likely to have preexisting risk factors for CVD, as compared to their counterparts. Risk factor examples, most of which are modifiable, include tobacco use, obesity, diabetes mellitus, hyperlipidemia, sedentary lifestyle, and diets high in saturated fat [5•]. In a large prospective observational study, patients without CVD or cancer who had a 10-year ASCVD risk of 20% compared to a 10-year ASCVD risk of 5% were more likely to develop cancer [72]. In the same observational study, patients who experienced a new cardiac event had a sevenfold increased risk for cancer than those who had not developed CVD [72]. Beyond risk factors, CVD predisposes patients to cancer.
These shared preexisting conditions promote increased inflammation, a mechanism central to the pathophysiology of both CVD and cancer. As mentioned in the CHIP section of this manuscript, inflammation is the driving force of atherosclerotic progression and therefore, CVD. Hypertension, smoking, hyperlipidemia, and insulin resistance contribute to this process as they allow for the expression of adhesion molecules on endothelial cells, which promote leukocyte attachment, recruitment of proinflammatory cytokines, and cause disruption within vessel walls [73]. Inflammation is also fundamental to carcinogenesis and tumor growth through similar mechanisms [74]. This can be easily demonstrated by infections that are known to cause cancer through induction of a chronic inflammatory environment (Helicobacter pylori in stomach cancer, Epstein–Barr virus in lymphoma, human papillomavirus in cervical and oropharyngeal cancers). Furthermore, tumors harness inflammatory pathways to further grow, transform, and enlist immune cells via chemosignaling [74]. Oxidative stress, caused by smoking, diabetes mellitus, hypertension, and obesity, also causes inflammation and is another mechanism significant to the development of both CVD and cancer [74]. The overlap of preexisting conditions with critical biologic mechanisms demonstrates the intricate connection between CVD and cancer.
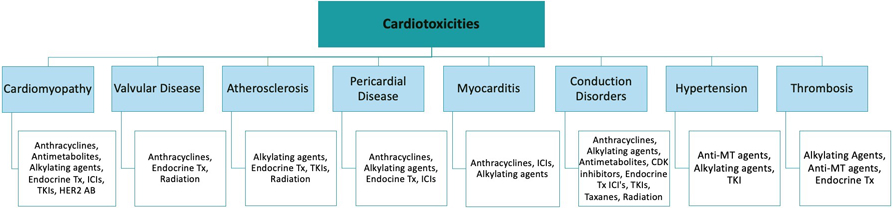
Adjuvant therapies for cancer treatments deliver additional cardiovascular insults, providing the next “hit” in the progression of atherosclerosis and CVD. Specific cancer therapies have been associated with an array of early and delayed cardiotoxicities varying from LV dysfunction to overt heart failure, hypertension, arrhythmias, myocardial ischemia, valvular disease, thromboembolic disease, pulmonary hypertension, and pericarditis (Fig. 4A) [1]. For the purpose of this manuscript, the atherogenic therapies will be highlighted as they relate most closely to CVD. HER2-targeted therapies, vascular endothelial growth factor (VEGF) inhibitors, combination RAF and MEK inhibitor treatment, and androgen deprivation therapies (ADT) have been shown to cause treatment-related hypertension, a well-established risk factor for heart disease. VEGF inhibitors, BCR-ABL tyrosine kinase inhibitors (TKIs), proteasome inhibitors (PIs) and immunomodulatory drugs (IMIDs), ADT, and immune checkpoint inhibitors have shown treatment-related increases in atherosclerosis and/or cardiac events [75]. Endocrine therapy, which plays an important role in the treatment of patients with breast cancer expressing estrogen (ER) or progesterone receptors (ER), is hypothesized to increase the risk of CVD through estrogen depletion [1]. For brain cancer survivors, the risk for CVD is strongly increased due to dyslipidemia, central obesity, and elevated systolic blood pressure, particularly for those with growth hormone deficiency [76]. Panhypopituitarism is also associated with a cluster of CVD risk factors, such as hormonal deficiencies, altered lipoprotein metabolism, hypertension, and obesity [77]. Comorbid conditions also have the ability to influence the degree of cardiac toxicity patients experience. For treatments such as anthracyclines, age, prior cardiac dysfunction, coronary disease, hypertension, and obesity are all known risk factors for increased likelihood of cardiotoxicity [78]. Cancer drugs targeting growth factors, such as anti-epidermal growth factor receptors, present compounded cardiotoxicities when accounting for additional risk factors such as obesity and diabetes mellitus [74].
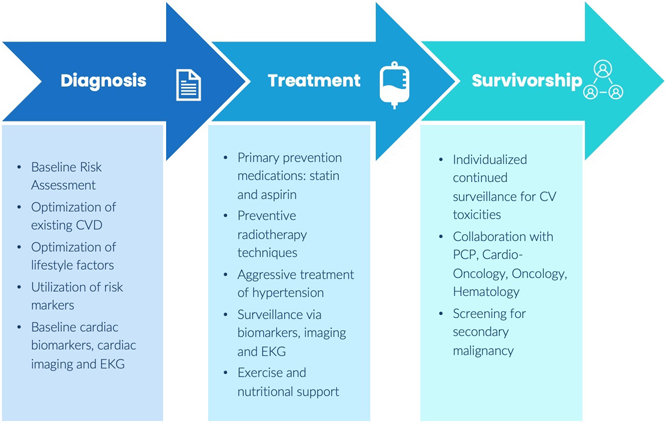
Fig. 4.
Considerations in preventive cardio-oncology. A Cancer treatment cardiotoxicities. B Preventive cardio-oncology: continuum of care in cancer patients. Figure components are from Infografia
Of additional clinical importance for many patients is radiation-induced heart disease (RIHD). In large blood vessels, radiotherapy (XRT) causes inflammation and oxidative damage and leads to lipid peroxidation and the formation of foam cells that initiate the atherosclerotic process in the presence of high cholesterol [1]. While the incidence of RIHD is still being studied, it has been well established that mediastinal XRT for the treatment of cancer (breast cancer, lymphoma, lung cancer) is associated with an increased risk of CVD [79]. The disease appears to be more severe in those treated with XRT at a younger age (< 50), with standing CV risk factors, lack of radiation protection shielding of the heart, high cumulative doses of RT (> 300 Gy or > 2 Gy/day), tumor location in close proximity to the heart, XRT to the anterior chest, or concomitant chemotherapy [80]. Combined, these increase risk of CV events and death. In one study with women treated with RT for breast cancer, rates of major coronary events increase linearly with the mean dose to heart by 7.4% per gray [80, 81]. This increase was similar in women with and without cardiac risk factors at the time of radiotherapy (80).
The summation of baseline CVD risk factors, multiple inflammatory mechanisms of malignancy, and the cardiotoxic treatments result in a dangerous triad for cancer patients; therefore, a diagnosis of cancer should be viewed and applied as an emerging risk marker for CVD. It is our recommendation that malignancy acts as a risk-enhancing factor when stratifying CV risk and follow-up. Such stratification and monitoring should be applied before, during, and after treatment (Fig. 4B). Special attention must be given to this patient population by primary care physicians, cardiologists, and cardio-oncologists before, throughout, and after cancer treatments in order to maximize the eradication of cancer while minimizing CVD outcomes.
Conclusion
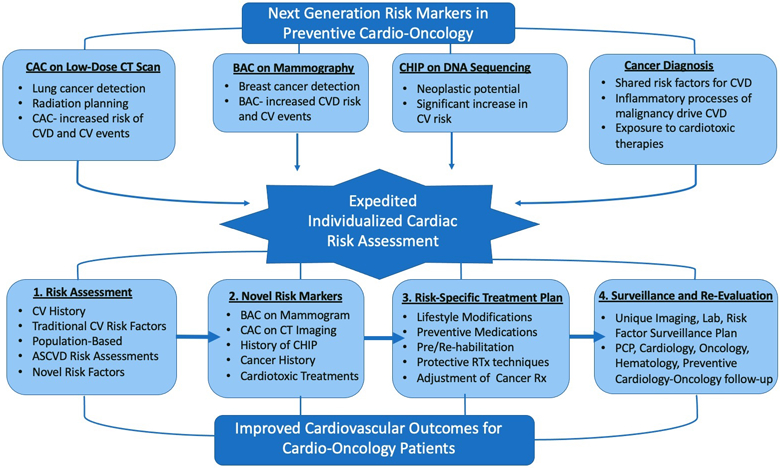
CVD and cancer remain the most common causes of morbidity and mortality within the USA. Therapeutic advancements have resulted in an aging population who have a complex overlap of risk factors for and disease burden from both CVD and cancer. For this reason, the intersection of cardiac and oncologic prevention, screening, and management protocols, with the inclusion of these next generation risk markers, may provide efficient and synergistic analysis of CVD and cancer disease or risk factor burden unique to each patient (Fig. 5, Table 1). Collaborative multidisciplinary preventive cardio-oncology teams well versed in the application and limitations of such tools will be instrumental for preemptive and timely guidance through management protocols. These teams will be well equipped to appropriately implement these next generation risk markers in the risk assessment of individuals with or at risk for cancer and CVD. The goal will be to further customize preventive therapies and surveillance. While the associations of BAC, CAC, CHIP, and cancer with CVD and CV events have been established, there remains no universal guideline on how to appropriately incorporate these in preventive cardio-oncology. Therefore, we suggest that BAC, CAC, CHIP, and the presence of cancer be additive to established CVD risks and intensify concerns about a patient’s risk of CVD during cancer screening and surveillance, and before, during, and after cancer treatments.
Fig. 5.
Graphic abstract. Next generation risk markers in preventive cardio-oncology
Table 1.
Next generation risk markers in preventive cardio-oncology
| Why risk marker | How/when/by whom | Implications | |
|---|---|---|---|
| BAC on mammography | BAC is associated with CVD risk factors, CVD events, and increased all-cause mortality | How: screening and diagnostic mammograms When: per breast cancer screening guidelines By whom: reported by radiologists. Applied via PCP, cardio-oncology, cardiology |
Expedited CVD risk assessment and preventions Breast cancer screening |
| CAC on CT scan | CAC scores on CT scans for lung cancer, radiation planning, and cancer staging are associated with CV events and death | How: cardiac-gated and non-cardiac-gated CT scans When: per lung cancer screening guidelines, cancer staging, and radiation planning By whom: reported by radiologists and radiooncologists. Applied via PCP, cardio-oncology, and cardiology |
Expedited CVD risk assessment and preventions Screening for lung CA, staging of cancer, and radiation planning |
| CHIP | Confers increased risk of hematologic malignancy and even a great risk of CVD | How: blood sequencing When: known solid malignancy, genetic sequencing in hematologic abnormality, or direct-to-consumer sequencing By whom: reported by geneticist, pathologist, or oncologist. Applied by oncology, cardiology, and cardio-oncology |
Expedited CVD risk assessment and preventions Surveillance for hematologic neoplasia |
| Cancer and cancer treatment | Cancer and CVD have shared baseline risk factors and are driven through similar inflammatory processes. Cardiotoxic therapies also contribute to CVD | How: Cancer diagnosis should prompt immediate CVD risk assessment with continued reevaluation When: diagnosis, treatment, and survivorship By whom: continued CVD surveillance by PCP, cardiology, and cardio-oncology |
Aggressive CVD risk assessment, prevention, and treatment |
Funding
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Numbers UL1TR001436 and KL2TR001438. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors of this paper.
Conflict of Interest Rachel Goodman reports the following: owning stock in Pfizer and Viatris; having investments in mutual funds that may or may not have stock in healthcare-related companies; she is a class B nonvoting member in a family LLC that may or may not have stock or mutual fund investments in healthcare-related companies, but she has no authority to buy or sell stocks, have no decision making authority of any kind, and have no knowledge of any investments made or sold in this family LLC. James MacLeod is co-president of the Medical College of Wisconsin Student Surgical Society. Alexander Bick reports the following: consulting fees from TenSixteen Bio to him. He also owns TenSixteen Bio stock or stock options. The other authors declare that they have no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137(8):e30–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellinger AM, Arteaga CL, Force T, Humphreys BD, Demetri GD, Druker BJ, et al. Cardio-oncology: how new targeted cancer therapies and precision medicine can inform cardiovascular discovery. Circulation. 2015;132(23):2248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown S-A. Preventive cardio-oncology: the time has come. Frontiers in Cardiovascular Medicine. 2020;6:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. •. Handy CE, Quispe R, Pinto X, Blaha MJ, Blumenthal RS, Michos ED, et al. Synergistic opportunities in the interplay between cancer screening and cardiovascular disease risk assessment: together we are stronger. Circulation. 2018;138(7):727–34. Describes overlap in screening for CVD and malignancy.
- 6.Bui QM, Daniels LB. A review of the role of breast arterial calcification for cardiovascular risk stratification in women. Circulation. 2019;139(8):1094–101. [DOI] [PubMed] [Google Scholar]
- 7.Maas AHEM, van der Schouw YT, Atsma F, Beijerinck D, Deurenberg JJM, Willem PTM, et al. Breast arterial calcifications are correlated with subsequent development of coronary artery calcifications, but their aetiology is predominantly different. Eur J Radiol. 2007;63(3):396–400. [DOI] [PubMed] [Google Scholar]
- 8.Yoon YE, Kim KM, Han JS, Kang S-H, Chun EJ, Ahn S, et al. Prediction of subclinical coronary artery disease with breast arterial calcification and low bone mass in asymptomatic women: registry for the women health cohort for the BBC study. JACC Cardiovascular Imaging. 2019;12(7 Part 1):1202–11. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura ME, Maksimik C, Martinez MW, Weiss M, Newcomb J, Harris K, et al. Breast artery calcium noted on screening mammography is predictive of high risk coronary calcium in asymptomatic women: a case control study. Vasa. 2013;42(6):429–33. [DOI] [PubMed] [Google Scholar]
- 10.Margolies L, Salvatore M, Hecht HS, Kotkin S, Yip R, Baber U, et al. Digital mammography and screening for coronary artery disease. JACC Cardiovascular Imaging. 2016;9(4):350–60. [DOI] [PubMed] [Google Scholar]
- 11.Karm D, Marks DS, Wein M, Kong AL. Benign arterial calcification on screening mammogram: a marker for coronary artery disease? J Womens Health. 2015;24(10):795–800. [DOI] [PubMed] [Google Scholar]
- 12.Datta T, Pena I, Inciardi A, Kappler A, Brem RF, Mazhari R, et al. Relationship of breast artery calcification to coronary artery stenosis: is mammography the new screening test for coronary artery stenosis in women? J Am Coll Cardiol. 2018;71(11 Supplement):A1874. [Google Scholar]
- 13.Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: final data for 2014. 2016. [PubMed] [Google Scholar]
- 14.Wenger NK. Female-friendly focus: 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease. Clin Cardiol. 2019;42(8):706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michos ED, Nasir K, Braunstein JB, Rumberger JA, Budoff MJ, Post WS, et al. Framingham risk equation underestimates subclinical atherosclerosis risk in asymptomatic women. Atherosclerosis. 2006;184(1):201–6. [DOI] [PubMed] [Google Scholar]
- 16.Nasir K, McEvoy JW. Recognizing breast arterial calcification as atherosclerotic CVD risk equivalent: from evidence to action. JACC Cardiovasc Imaging. 2016;9(4):361–3. [DOI] [PubMed] [Google Scholar]
- 17.Polonsky TS, Greenland P. Breast arterial calcification: expanding the reach of cardiovascular prevention. Circulation. 2017;135(6):499–501. [DOI] [PubMed] [Google Scholar]
- 18.Hendriks EJE. Medial arterial calcification: novel insights into its determinants and cardiovascular implications. 2017. [Google Scholar]
- 19.Lee SC, Phillips M, Bellinge J, Stone J, Wylie E, Schultz C. Is breast arterial calcification associated with coronary artery disease?—a systematic review and meta-analysis. PloS one. 2020;15(7):e0236598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Noord PA, Beijerinck D, Kemmeren JM, Van der Graaf Y. Mammograms may convey more than breast cancer risk: breast arterial calcification and arterio-sclerotic related diseases in women of the DOM cohort. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP). 1996;5(6):483–7. [PubMed] [Google Scholar]
- 21.Pidal D, Vidal MTS, Rodríguez JC, Corte MD, Pravia P, Guinea O, et al. Relationship between arterial vascular calcifications seen on screening mammograms and biochemical markers of endothelial injury. Eur J Radiol. 2009;69(1):87–92. [DOI] [PubMed] [Google Scholar]
- 22.Hendriks EJE, de Jong PA, van der Graaf Y, Willem PTM, van der Schouw YT, Beulens JWJ. Breast arterial calcifications: a systematic review and meta-analysis of their determinants and their association with cardiovascular events. Atherosclerosis. 2015;239(1):11–20. [DOI] [PubMed] [Google Scholar]
- 23.Schnatz PF, Marakovits KA, O’Sullivan DM. The association of breast arterial calcification and coronary heart disease. Obstet Gynecol. 2011;117(2):233–41. [DOI] [PubMed] [Google Scholar]
- 24.Kemmeren JM, van Noord PAH, Beijerinck D, Fracheboud J, Banga J-D, van der Graaf Y. Arterial calcification found on breast cancer screening mammograms and cardiovascular mortality in women: the DOM project. Am J Epidemiol. 1998;147(4):333–41. [DOI] [PubMed] [Google Scholar]
- 25.Pecchi A, Rossi R, Coppi F, Ligabue G, Modena MG, Romagnoli R. Association of breast arterial calcifications detected by mammography and coronary artery calcifications quantified by multislice CT in a population of post-menopausal women. Radiol Med (Torino). 2003;106(4):305–12. [PubMed] [Google Scholar]
- 26.Hendriks EJE, Beulens JWJ, Mali W, Beijerinck D, Van Der Graaf Y, de Jong PA, et al. Breast arterial calcifications and their association with incident cardiovascular disease and diabetes: the Prospect-EPIC cohort. J Am Coll Cardiol. 2015;65(8):859–60. [DOI] [PubMed] [Google Scholar]
- 27.Tota-Maharaj R, Blaha MJ, Blankstein R, Silverman MG, Eng J, Shaw LJ, et al. , editors. Association of coronary artery calcium and coronary heart disease events in young and elderly participants in the multi-ethnic study of atherosclerosis: a secondary analysis of a prospective, population-based cohort 2014: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrmann J From trends to transformation: where cardio-oncology is to make a difference. European heart journal. 2019. [DOI] [PubMed] [Google Scholar]
- 29.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turcotte LM, Whitton J, Friedman DL, Hammond S, Armstrong GT, Leisenring WM, et al. Subsequent neoplasms in the fifth and sixth decades of life in the Childhood Cancer Survivor Study cohort. American Society of Clinical Oncology; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh JM, Lowry KP, Schechter CB, Diller LR, Alagoz O, Armstrong GT, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation: a comparative modeling study. Ann Intern Med. 2020;173(5):331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolies LR, Yip R, Hwang E, Oudsema RH, Subramaniam VR, Hecht H, et al. Breast arterial calcification in the mammogram report: the patient perspective. Am J Roentgenol. 2019;212(1):209–14. [DOI] [PubMed] [Google Scholar]
- 34.Peng AW, Mirbolouk M, Orimoloye OA, Osei AD, Dardari Z, Dzaye O, et al. Long-term all-cause and cause-specific mortality in asymptomatic patients with CAC ≥1,000: results from the CAC Consortium. JACC Cardiovasc Imaging. 2020;13(1 Pt 1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging. 2015;8(5):579–96. [DOI] [PubMed] [Google Scholar]
- 36.Hecht H, Blaha MJ, Berman DS, Nasir K, Budoff M, Leipsic J, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2017;11(2):157–68. [DOI] [PubMed] [Google Scholar]
- 37.Hausleiter J, Meyer T, Hermann F, Hadamitzky M, Krebs M, Gerber TC, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500–7. [DOI] [PubMed] [Google Scholar]
- 38.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaha MJ, Blankstein R, Nasir K. Coronary artery calcium scores of zero and establishing the concept of negative risk factors. J Am Coll Cardiol. 2019;74(1):12–4. [DOI] [PubMed] [Google Scholar]
- 40.Blaha MJ, Blumenthal RS, Budoff MJ, Nasir K. Understanding the utility of zero coronary calcium as a prognostic test: a Bayesian approach. Circulation Cardiovascular Quality and Outcomes. 2011;4(2):253–6. [DOI] [PubMed] [Google Scholar]
- 41.Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grandhi GR, Mirbolouk M, Dardari ZA, Al-Mallah MH, Rumberger JA, Shaw LJ, et al. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD mortality: the CAC Consortium. Cardiovascular Imaging. 2020;13(5):1175–86. [DOI] [PubMed] [Google Scholar]
- 43.Dunleavy MP, Guha A, Cardona A, Fortuna C, Daoud EG, Raman SV, et al. Prevalence of coronary artery calcification on pre-atrial fibrillation ablation CT pulmonary venograms and its impact on selection for statin therapy. J Clin Med. 2020;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dekker M, Waissi F, Bank IEM, Lessmann N, Išgum I, Velthuis BK, et al. Automated calcium scores collected during myocardial perfusion imaging improve identification of obstructive coronary artery disease. Int J Cardiol Heart Vasc. 2020;26:100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie X, Zhao Y, de Bock GH, de Jong PA, Mali WP, Oudkerk M, et al. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: systematic review and meta-analysis. Circulation Cardiovascular Imaging. 2013;6(4):514–21. [DOI] [PubMed] [Google Scholar]
- 46.Amin NP, Kim SM, Lasio G, Zhou J, Romar L, Shipman K, et al. Incidental coronary artery calcium on breast radiation therapy planning scans identifies patients for cardiac preventive therapy. Am J Clin Oncol. 2020;43(11):826–31. [DOI] [PubMed] [Google Scholar]
- 47.Emaus MJ, Išgum I, van Velzen SGM, van den Bongard HJGD, Gernaat SAM, Lessmann N, et al. Bragatston study protocol: a multicentre cohort study on automated quantification of cardiovascular calcifications on radiotherapy planning CT scans for cardiovascular risk prediction in patients with breast cancer. BMJ open. 2019;9(7):e028752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. •. Milgrom SA, Varghese B, Gladish GW, Choi AD, Dong W, Patel ZS, et al. Coronary artery dose-volume parameters predict risk of calcification after radiation therapy. Journal of Cardiovascular Imaging. 2019;27(4):268–79. Demonstrates that radiation exposure is associated with CAC when controlling for major cardiac risk factors.
- 49.Iliescu CA, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, et al. SCAI Expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of india, and sociedad Latino Americana de Cardiologıa intervencionista). Catheter Cardiovasc Interv. 2016;87(5):E202–23. [DOI] [PubMed] [Google Scholar]
- 50.Puckett LL, Saba SG, Henry S, Rosen S, Rooney E, Filosa SL, et al. Cardiotoxicity screening of long-term, breast cancer survivors—the CAROLE (Cardiac-Related Oncologic Late Effects) study. Cancer Med. 2021;10(15):5051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506(7488):328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibson CJ, Steensma DP. New insights from studies of clonal hematopoiesis. Clin Cancer Res. 2018;24(19):4633–42. [DOI] [PubMed] [Google Scholar]
- 56.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355(6327):842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K. CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res. 2018;123(3):335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. •. Wang W, Liu W, Fidler T, Wang Y, Tang Y, Woods B, et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2. Circ Res. 2018;123(11):e35–47. Describes the pathogenesis of Jak2 positve clonal hematoposesis driven atheroscleosis.
- 59.Libby P, Molinaro R, Sellar RS, Ebert BL. Jak-ing up the plaque’s lipid core…and even more. Circ Res. 2018;123(11):1180–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sidlow R, Lin AE, Gupta D, Bolton KL, Steensma DP, Levine RL, et al. The clinical challenge of clonal hematopoiesis, a newly recognized cardiovascular risk factor. JAMA Cardiol. 2020. [DOI] [PubMed] [Google Scholar]
- 61.Swisher EM, Harrell MI, Norquist BM, Walsh T, Brady M, Lee M, et al. Somatic mosaic mutations in PPM1D and TP53 in the blood of women with ovarian carcinoma. JAMA Oncol. 2016;2(3):370–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21(3):374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sano S, Wang Y, Ogawa H, Horitani K, Sano M, Polizio AH, et al. TP53-mediated therapy-related clonal hematopoiesis contributes to doxorubicin-induced cardiomyopathy by augmenting a neutrophil-mediated cytotoxic response. JCI insight. 2021;6(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coombs CC, Gillis NK, Tan X, Berg JS, Ball M, Balasis ME, et al. Identification of clonal hematopoiesis mutations in solid tumor patients undergoing unpaired next-generation sequencing assays. Clin Cancer Res. 2018;24(23):5918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Libby P, Sidlow R, Lin AE, Gupta D, Jones LW, Moslehi J, et al. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74(4):567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 67.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139(25):e1082–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raber I, McCarthy CP, Vaduganathan M, Bhatt DL, Wood DA, Cleland JGF, et al. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet. 2019;393(10186):2155–67. [DOI] [PubMed] [Google Scholar]
- 69.Bolton KL, Zehir A, Ptashkin RN, Patel M, Gupta D, Sidlow R, et al. The clinical management of clonal hematopoiesis: creation of a clonal hematopoiesis clinic. Hematol Oncol Clin North Am. 2020;34(2):357–67. [DOI] [PubMed] [Google Scholar]
- 70.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. [DOI] [PubMed] [Google Scholar]
- 71.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50(15):1435–41. [DOI] [PubMed] [Google Scholar]
- 72.Lau E, Paniagua SM, Liu E, Jovani M, Li S, Takvorian K, et al. The association of cardiovascular disease and future cancer. American Heart Association; Philadelphia, Pennsylvania: 2019. [Google Scholar]
- 73.Masoudkabir F, Sarrafzadegan N, Gotay C, Ignaszewski A, Krahn AD, Davis MK, et al. Cardiovascular disease and cancer: evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis. 2017;263:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. European Journal of Heart Failure. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heikens J, Ubbink MC, van der Pal HP, Bakker PJ, Fliers E, Smilde TJ, et al. Long term survivors of childhood brain cancer have an increased risk for cardiovascular disease. Cancer. 2000;88(9):2116–21. [PubMed] [Google Scholar]
- 77.Rosén T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990;336(8710):285–8. [DOI] [PubMed] [Google Scholar]
- 78.Lipshultz SE, Cochran TR, Franco VI, Miller TL. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol. 2013;10(12):697. [DOI] [PubMed] [Google Scholar]
- 79.Desai MY, Jellis CL, Kotecha R, Johnston DR, Griffin BP. Radiation-associated cardiac disease: a practical approach to diagnosis and management. JACC Cardiovascular Imaging. 2018;11(8):1132–49. [DOI] [PubMed] [Google Scholar]
- 80.Desai MY, Windecker S, Lancellotti P, Bax JJ, Griffin BP, Cahlon O, et al. Prevention, diagnosis, and management of radiation-associated cardiac disease: JACC scientific expert panel. J Am Coll Cardiol. 2019;74(7):905–27. [DOI] [PubMed] [Google Scholar]
- 81.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98. [DOI] [PubMed] [Google Scholar]