Abstract
Plant secondary metabolites are bioactive scaffolds that are crucial for plant survival in the environment and to maintain a defense mechanism from predators. These compounds are generally present in plants at a minimal level and interestingly, they are found to have a wide variety of therapeutic values for humans. Several medicinal plants are used for pharmaceutical purposes due to their affordability, fewer adverse effects, and vital role in traditional remedies. Owing to this reason, these plants are exploited at a high range worldwide and therefore many medicinal plants are on the threatened list. There is a need of the hour to tackle this major problem, one effective approach called elicitation can be used to enhance the level of existing and novel plant bioactive compounds using different types of elicitors namely biotic and abiotic. This process can be generally achieved by in vitro and in vivo experiments. The current comprehensive review provides an overview of biotic and abiotic elicitation strategies used in medicinal plants, as well as their effects on secondary metabolites enhancement. Further, this review mainly deals with the enhancement of biomass and biosynthesis of different bioactive compounds by methyl jasmonate (MeJA) and salicylic acid (SA) as elicitors of wide medicinal plants in in vitro by using different cultures. The present review was suggested as a significant groundwork for peers working with medicinal plants by applying elicitation strategies along with advanced biotechnological approaches.
Keywords: Elicitors, Medicinal plants, Salicylic acid, Methyl jasmonate, Bioactive compounds
Introduction
Secondary metabolites (SMs) are bioactive compounds that play a crucial role in plant cross-talks with the environment for adaptation and defense mechanisms but do not have a fundamental role in maintaining the life cycle processes. Higher plants produce a spectrum of SMs from primary metabolites that aid in plant defense against insects, herbivores, pests, phytopathogens, etc. (Jain et al. 2019). Medicinal plants are the primary source of bioactive molecules, with many novel drugs and also components produced directly or indirectly. Based on the WHO reports, approximately 80% of the world’s population relies on traditional medicines notably medicinal plant-based drugs and formulations for their primary healthcare needs (Kumari and Kotecha 2016). These pharmaceutically important chemicals are produced in trace amounts and are dependent on the plant’s developmental and physiological state (Twaij and Hasan 2022). Owing to the higher usage of medicinal plants for curative purposes across the world, increasing market demand leads to over-exploitation of these plants makes them threatened. Due to these reasons, it is critical to identify alternate methods for producing SMs to meet the huge market demand. For the enhanced production of therapeutic metabolites, different biotechnological approaches are used, but elicitation is a mechanistic approach and strong arm for increasing biomass and pharmacologically active phytocompounds production during field cultivation (Halder et al. 2019; Kandoudi et al. 2021). Elicitation is one of the effective biotechnological approaches for inducing novel SMs biosynthesis, and enhancement of biomass as well as the gathering of SMs in in vitro cultures of plants (Wang and Wu, 2013). When plants are exposed to elicitors, signaling molecules specifically bind to the receptors, that urged and they frequently produce a variety of SMs. Elicitors are biotic or abiotic substances that belong to numerous chemical classes and can stimulate or enhance the target SMs biosynthesis (Ghorbanpour et al. 2014). Elicitors also play a pivotal role in plant growth and development as well as in signal transduction. In addition, elicitors signaling mechnisms have a significant impact on bioactivities, mitigation of environmental stress, plant survivability and productivity (Jan et al. 2021). Several phytohormones (Sabagh et al. 2022), transcription factors (TFs) (Zheng et al. 2023) and enzymes (Rajput et al. 2021) associated with various biological pathways, cell compartmentalization and ion exchange play a major role in the development of a signal transduction network. Furthermore, several TFs such as MYB, WRKY, bHLH and AP2/ERF are actively involved in hormonal signaling pathways to enhance the pharamaceutically active phytocompounds in various medicinal plants (Jan et al. 2021; Zheng et al. 2023). During the elicitation process, there are some types of parameters such as concentration, time exposure, elicitor type, and cell culture type that decide the accumulation and enhanced synthesis of metabolite production in plants (Dhiman et al. 2018). Several reports have revealed that the elicitation process increased the biosynthesis of targeted/specific plant-associated SMs in the hairy root and cell suspension cultures, providing a new channel for the pharmaceutical industry to produce a mass of SMs in the future (Srivastava et al. 2017; Singh et al. 2018a, b). To the best of our knowledge, this baseline review sheds the light on potential biotic and abiotic elicitation strategies for medicinal plants and their positive impact on pharmaceutically active and novel SMs production. In addition, this review also aids the scientists and researchers in understanding the novel and important aspects of elicitation to harness the active ingredients of medicinal plants.
Elicitation is a key tool to ameliorate the active metabolites
The defense system of medicinal plants produces chemical substances to combat infections caused by external and internal stresses that can be used to elevate the level of these substances, which are known as SMs. Many biotechnological approaches are considered in in vitro cultures for up gradation or to enhance the production of such essential phytochemicals (Danova and Pistelli 2022; Jakovljevic et al. 2022). The elicitation process is to change the metabolic pathways of medicinal plants to stimulate bioactive compounds through in vitro plant cultures. This method can be used to produce economically viable SMs on a large spectrum level. Elicitors act as a signaling molecule and these signals identified by the plant cellular membrane-bound receptors and activation of the signal transduction pathways and change the expression of regulatory players and resulting in enhanced synthesis and accumulation of phytocompounds (Zhai et al. 2017; Miladinova-Georgieva et al. 2023). Employing in vitro cell culture as a model system, it may also be used to characterize and investigate the impact of various elicitors on plants.
Mechanism of elicitation in plants
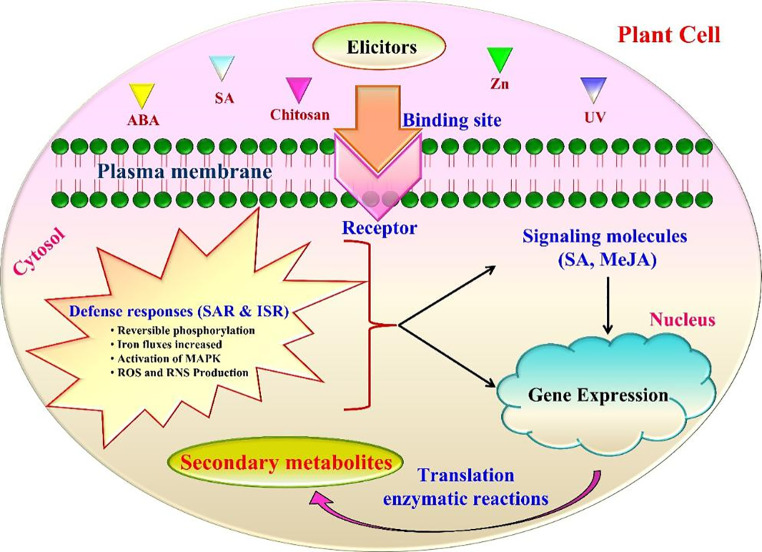
Elicitors are associated with a broad range of different categories of compound scaffolds and biochemical pathways and it is stimulating the enhanced production of novel metabolites in some plants that may be inactive in certain plant species. In contrast, specific elicitors can bind with specific plant membrane receptors. Therefore, this implies that elicitor specificity exists concerning the signal molecules that are triggered by elicitation and the plant has the tendency to recognize certain structurally diverse compounds as signaling molecules (Abdul Malik et al. 2020). Elicitors are recognized by plant cells by interacting with specific receptors on the plasma membranes of plants and these interactions are initiated to generate signals. Those identified signal molecules include chitosan, physical signals, abscisic acid, and systemin. The mechanism of elicitors varies with regard to their origins, specificity in terms of nutrition and concentration, physiochemical environment, and plant development (Meena et al. 2022). The first stage of the elicitor signal transduction cascade is the signal perception, which occurs through a series of events such as the activation of kinases, reactive oxygen species (ROS) burst, ion fluxes NADPH oxidase activation, and cytoplasm acidification followed by nuclear genes activation (Bajwa et al. 2021). Different mechanisms were speculated to describe the biochemical processes that occur in the plant cells when challenged by the elicitor. Apostol et al. (1989) showed that high levels of ROS accumulation in plant tissues occurred through oxidative bursts triggered by elicitor treatment. During the process of elicitation, elicitors can accumulate defense and pathogenesis proteins including endo-polygalacturonases (involved in the pectic oligomers release), chitinases, glycoproteins, and protease inhibitors (Benhamou 1996). A series of events activated by the elicitor is listed as follows:
The plant cell receptor perceives the specific signaling molecule (elicitor), which leads to the activation of complex reactions such as membrane depolarization, reversible phosphorylation of plasma membrane proteins, Cl− and K+ efflux/H+ influx, cytosolic [Ca2+] concentration changes (Gelli et al. 1997), ion fluxes increased (Armero and Tena 2001), NADPH oxidase activation, mitogen-activated protein kinase (MAPK) activation, and ROS and reactive nitrogen species (RNS) production.
Ion influx is a potential event that involves the plant cell physiological processes (Ward et al. 2009). The Ca2+ signals result in conformational changes in a number of ion binding proteins including calcium- dependent protein kinases (CDPKs) calmodulin, calmodulin-like proteins and secondary messengers such as inositol 1,4,5- triphosphate IP3 and diacylglycerol (DAG) (White and Broadley 2003).
Owing to the aforementioned, these binding proteins activates the protein kinase cascades, which leads to the dynamisms in MAPK phosphorylation and it also activate the hormonal signaling pathway messengers such as SA and MeJA.
As a result, activation of transcription and translation of defense-responsive genes/enzymes, which in turn synthetic pathways of SMs production (Ebel and Scheel 1992). The mode of action of the elicitation process is illustrated in Fig. 1.
Fig. 1.
A possible mechanism of the elicitation process. detailed description needed. SAR - Systemic acquired resistance; ISR - induced systemic resistance
Extensive research has been conducted to the mode of action of the potential elicitors in plant biotechnology. Even though in some instances the secondary messengers, receptors, responsive proteins and transduction pathway have been identified, the data is generally inadequate. Furthermore, the unpredictability of this mode of action leads to various metabolic processes. As a result, most research related to the improving impact of elicitation on phytocompounds accumulation in plant tissue cultures was empirical without examining the cellular process at a molecular level.
SMs production by abiotic and biotic elicitors
Plants contain different types of SMs such as phenolics, terpenes or terpenoids, and N-containing compounds. Each of these classes has thousands of SMs (Twaij and Hasan 2022). The presence of certain abiotic and biotic elicitors increases the production of SMs in plants. The origin of elicitors can be classified as abiotic and biotic based on their nature. In abiotic elicitors, there are three types of elicitors categorized as physical, chemical, and hormonal (Wang and Wu 2013). Abiotic elicitors are a group of major elicitors for the biosynthesis and production of novel pharmaceutically active metabolites. So far, there is limited reports are available in the field of abiotic elicitor, but now abiotic elicitation is one of the emerging and effective approaches to enhance the production of bioactive compounds through in vitro culture conditions (Kumari et al. 2020). The impact of abiotic elicitors in plant systems is diverse on cellular processes such as protein synthesis, growth, and development, gene expression, and lipid and carbohydrate metabolisms (Rao and Ravishankar 2002; Hashemi et al. 2021). Recently, various abiotic elicitors were used to modify the cell metabolism of the plant, and these cellular modifications delineated the enhancement of desired phytomolecule production under in vitro conditions (Rodziewicz et al. 2014; Xu et al. 2015) reported that treatment of UV-C along with the combination of Methyl jasmonate (MeJA) and Salicylic acid (SA) had a major impact on the enhancement of stilbene biosynthesis in Vitis vinifera cell suspension cultures. Thus, the combination study, in turn, has shifted the novel paradigm, compared with one elicitor combination application that can effectively enhance the production of active metabolites under in vitro cell cultures. A few examples of abiotic elicitors and phytocompound details are shown in Table 1.
Table 1.
Impact of Abiotic Elicitors on the enhanced synthesis of SMs in Medicinal Plants
| Elicitor | Target metabolite | Plant species | Culture type | References |
|---|---|---|---|---|
| Cadmium (Cd) | Tanshinone | Salvia miltiorrhiza | Cell suspension culture | Zhao et al., 2010 |
| Resveratrol | Vitis vinifera | Cell suspension culture | Cai et al., 2013 | |
| pH | Withanolide A | Withania somnifera | Hairy root culture | Praveen et al. 2012 |
| Gibberellic acid (GA) | Caffeic acid derivatives | Echinacea pupurea | Hairy root culture | Abbasi et al., 2012 |
| Nanosilver | Atropine | Datura metel | Hairy root culture | Shakeran et al., 2015 |
| Copper (Cu) | Bacoside | Bacopa monnieri | Shoot culture | Sharma et al., 2015 |
| Copper sulfate | Bacoside A | Bacopa monnieri | Shoot culture | Sharma et al., 2015 |
| Coniferaldehyde and Methylene dioxycinnamic acid | Lariciresinol, pinoresinol, podophyllotoxin | Linum album | Hairy root culture | Ahmadian Chashmi et al., 2016 |
| Iron oxide (FeNPs) nanoparticles | Hyoscyamine and scopolamine | Hyoscyamus reticulatus | Root culture | Moharrami et al., 2017 |
| Ultraviolet C | Lignans and neolignans | Linum usitatissimum L | Cell suspension culture | Anjum et al., 2017 |
| Drought stress | Cineole | Thymus vulgaris L | Cell suspension culture | Llorens-Molina and Vacas, 2017 |
| Ultraviolet B |
Betacyanins, betaxanthins, and flavonoids |
Alternanthera species | Leaf samples | Klein et al., 2018 |
| Silver nitrate | Atropine | Datura metel | Hairy root culture | Shakeran et al., 2015 |
| Flavonoids, Phenolic acids | Ocimum basilicum | Cell suspension culture | Acıkgoz, 2020 | |
| SA | Bacoside A | Bacopa monnieri | Shoot culture | Sharma et al., 2015 |
| L-3,4-dihydroxyphenylalanine | Hybanthus enneaspermus | Root culture | Sathish et al., 2020 | |
| Bilobalide, ginkgolide A,B,C | Ginkgo biloba | Immobilized cell culture | Sukito and Tachibana, 2016 | |
| Wounding stress | Total phenolic content | Ajuga bracteosa | Root culture | Saeed et al., 2017 |
| Sesquiterpenes | Persicaria minor | Cell suspension culture | Sellapan et al., 2018 | |
| Phenolic compounds | Thevetia peruviana | Cell suspension culture | Mendoza et al., 2018 | |
| Jasmonic acid | Scopolamine | Duboisia genus | Hairy root culture | Singh et al. 2018a, b |
| Rutin, neohesperidin, buddleside | Isatis tinctoria | Hairy root culture | Gai et al., 2019 | |
| Oleanolic acid, glycosides | Calendula officinalis | Hairy root culture | Alsoufi et al., 2019 | |
| Chrysin, wogonin, baicalein | Scutellaria bornmuelleri | Hairy root culture | Gharari et al. 2020a |
Biotic elicitors are derived from plants and microorganisms such as fungal, bacterial, and yeast. Further, they are either of defined composition such as a combination of yeast extract and homogenized fungus, inactivated enzymes, chitosan, curdlan polysaccharides such as Pectin, cellulose, and chitin (Vasconsuelo and Boland 2007). For example, the enhancement of bioactive compounds such as artemisinin, ginsenoside, and anthocyanin is increased to a larger amount with the help of fungal elicitors namely Penicillium oxalicum and Trichoderma harzianum, respectively (Zheng et al. 2016; Biswas et al. 2018). Microalgal elicitors treated suspension cultures of Carthamus tinctorius showed an increased level of red pigment formation (Hanagata et al. 1994). Various biotic elicitors are used for the enhancement of specific pharmaceutically important phytocompounds in medicinal plants. Those elicitors and enhanced compounds are listed in Table 2.
Table 2.
Effect of Biotic Elicitors on therapeutic metabolites production in Plants
| Elicitor | Compounds | Plant species | Culture type | References |
|---|---|---|---|---|
| Sclerotinia sclerotiorum | Lignan | Linum album | Hairy root cultures | Esmaeilzadeh Bahabadi et al., 2014 |
| Sucrose | Leoligin | Leontopodium alpinum | Hairy root cultures | Wawrosch et al., 2014 |
| Penicillium fellutanum | Glycyrrhizic acid |
Taverniera cuneifolia (Roth) Arn. |
Root cultures | Awad et al., 2014 |
| Chitin |
trans-Resveratrol and viniferins |
Vitis vinifera | Cell suspension culture | Taurino et al. 2015 |
| Phenylpropanoids and naphtodianthrones | Hypericum perforatum | Cell suspension culture | Gadzovska-Simic et al., 2015 | |
| Bacillus cereus | Atropine | Datura metel | hairy root cultures | Shakeran et al., 2015 |
| Staphylococcus aureus | Atropine | Datura metel | hairy root cultures | Shakeran et al., 2015 |
| Dextron | Phenylpropanoids and naphtodianthrones | Hypericum perforatum | Shoot, callus, cell suspension cultures | Gadzovska-Simic et al., 2015 |
| Pectin | Phenylpropanoids and naphtodianthrones | Hypericum perforatum | Shoot, callus, cell suspension cultures | Gadzovska-Simic et al., 2015 |
| Aspergillus niger | Oleanolic acid, urosolic acid | Salvia fruticosa | Cell suspension culture | Kummritz et al., 2016 |
| Rhizophagus irregularis | Rosmarinic acid | Ocimum basilicum | Root cultures | Srivastava et al., 2016 |
| Piriformospora indica | Withanolides | Withania somnifera | Hairy root cultures | Ahlawat et al., 2017 |
| Trichoderma harzianum | Ginsenoside and Anthocyanin | Panax sikkimensis | Cell suspension cultures | Biswas et al., 2018 |
| Bacillus subtilis | Ginsenoside | Panax quinquefoliusL | Cell suspension cultures | Biswas et al., 2018 |
| Chitosan | Withaferin | Withania somnifera (L.) | Hairy root culture | Thilip et al., 2019 |
| Coronatine and sorbitol | Artemisinin | Artemisia annua | Hairy root cultures | Salehi et al. 2019a |
| Bacillus subtilis | Diosgenin | Helicteres isora L | Cell suspension cultures | Shaikh et al., 2020 |
| Escherichia coli | Diosgenin | Helicteres isora L | Cell suspension culture | Shaikh et al., 2020 |
| Yeast extract | Flavonoids, phenolic acids | Ocimum basilicum | Cell suspension culture | Acıkgoz, 2020 |
| Alternaria panax | Ginsenoside | Panax ginseng | Cell suspension culture | Hao et al., 2020 |
| Agrobacterium rhizogenus | Paclitaxel | Taxus brevifolia | Hairy root cultures | Jalalipour Parizi et al., 2020 |
All these reports are evidence that any type of abiotic and biotic elicitors given to the plant will undoubtedly have opened up an impact on the SMs production through alterations in various biochemical pathways. There are some cons also noted while using elicitors. Moreover, not all the elicitors affect the SMs production which depends on the concentration and type of biotic elicitors used (Baenas et al. 2014). However, there are no reports available for a combination study of two or more biotic elicitors for phytocompounds enhancement. Developing the combination study of biotic elicitors will pave the way to enhance the synthesis of metabolite production via in vitro conditions and create novel avenues for future researchers.
Among the various types of elicitors, MeJA and SA are the highly reported phytohormone elicitors. Hence, in this review, we have attempted to summarize the effect of MeJA and SA plant hormones for enhancing the production of novel bioactive molecules in medicinal plants through different in vitro cell cultures.
MeJA and SA
In plants, several metabolic activities are mediated by internal signals known as plant hormones. Among the various metabolic processes, stress tolerance is one of the important processes to be able to cause several stresses. In this condition, phytohormones like MeJA, a member of the jasmonate family, modulate important factors of plant physiology (Chen et al. 2006). MeJA was first isolated in 1962 from Jasminum grandiflorum’s essential oil (Demole et al. 1962). MeJA, a volatile methyl ester of jasmonic acid has been identified as a major signaling molecule in abiotic and biotic stresses (Wang et al. 2021). It plays an important role in plants from morphological to molecular functions. Due to its volatile nature as well as the capacity to permeate via biological membranes, MeJA is regarded as a significant phytohormone that can facilitate intra-and inter-communications in plants, regulating defense responses, particularly antioxidant systems. Implementation of exogenous MeJA to plant in vitro cultures has been recognized as a novel method for the enhanced production of SMs, increased activity of antioxidant enzymes, and the expression of genes associated with defense (Murthy et al. 2014; Ho et al. 2020).
SA is a well-known elicitor for inducing systemic acquired resistance and can also elevate the synthesis of SMs in plants. SA is a phenolic compound that has attracted the greatest attention from scientists around the world. The intensive studies on SA in medicinal plants have revealed its role in various developmental and physiological processes, including ethylene production, stomatal movements, photosynthesis, membrane functions, pigment accumulation, enzyme activities, plant growth and development (Ali et al. 2021). Hence, SA and its related precursors are classified as phytohormones (Koo et al. 2020). The significant and functional analog of SA includes salicylic acid, dihydroxy benzoic acid, methyl salicylic acid, and acetylsalicylic acid. They are produced from isocomerate and phenylalanine (Sahu 2013) and are transferred through apoplast, which is aided by pH gradient modulation or SA deprotonation or via cuticle wax in cuticle deficient plants to the mechanism of action for the required performance (Lim et al. 2020).
Regulatory mechanism and biosynthesis of bioactive compounds in plants
MeJA signaling
The primitive stage of MeJA signaling includes a number of metabolic pathways to be activated. In general, the biosynthesis of polypeptides or the formation of free radicals indicates the introduction of biotic and abiotic stress. The activation of cell receptors, and protein molecules as well as over activity of transporters also represent the stressed condition. In response to pathogen attack, oligosaccharide signals were found in symplastic and apoplastic pathways, which indicates the plant is experiencing stess reversal pathway. Moreover, the activation of phospholipases (PLAs) is essential for MeJA biosynthesis and signaling. PLAs produce linoleic acid from the plasma membrane which serves as a precursor for MeJA. Several mutant and transgenic plants have been used to study the PLAs activity (Yan et al. 2013). In MeJA-associated short and long-distance signaling, various plant growth regulators and hormones such as auxin, cytokinin, ethylene and SA play a vital role in the development of complex regulatory network which in turn impact different biochemical processes and SMs accumulation (Chini et al. 2018). On the other hand, various TFs such as WRKY, bZIP, bHLH, NAC, AP2/ERF and MYB are involved in the MeJA signaling process. It has been reported that the bHLH and MYC TF is a master regulator for the MeJA signaling pathway. The MeJA-responsive MYB-TF enhances phenolic acid accumulation in S. miltiorrhiza (Zhou et al. 2021). Moreover, MeJA mediated WRKY TF also plays a crucial role in the production of SMs in medicinal plants. WRKY TF is involved in regulation of SMs biosynthesis such as taxol and artemisinin in Taxus chinensis and A. annua, respectively (Ma et al. 2009; Subramaniyam et al. 2014). MeJA and SA induced TF-WRKY enhances triterpenoid biosynthesis and over-expression of WRKY18 increases salt stress tolerance in Ziziphus jujuba Mill. (Wen et al. 2023; Wang et al. 2017) studied transcriptome analysis of Lycoris aurea seedlings with the treatment of MeJA to determine which molecular mechanisms are responsible for regulating SMs pathway activity in plants. The expression of the plant secondary process highly depends on the different TFs at diverse levels. For instance, TFs participated in MeJA signaling cascades pathways usually regulating the transcription of several genes involved in biosynthesis, to improve the accumulation of bioactive compounds in medicinal plants (Zhou and Memelink 2016). Several TFs have been identified as regulators of SMs biosynthesis in plants, belonging to the families AP2/ERF, MYB, WRKY and bHLH. MeJA signaling activates the MAP kinase cascade, which regulates the calcium channel and interactions with functional molecules. Additionally this signaling pathway interacts with plant growth regulators (PGRs) such as gibberellic acid, SA, abscisic acid, and ethylene to monitor a wide network of various plant physiological and biological processes. Plants are constantly exposed to various environmental stresses. Their stress response is facilitated by a number of bioactive compounds. The exposure of plant cells to diverse stress factors, as well as their primary and secondary defense responses, leads to improved production of phytocompounds, genetic expression, multicomponent signaling, changes in metabolic processes and the involvement of various TFs all this activity is synchronized with both MeJA and SA signals in many ways. There is evidence that plants do regulate TFs to monitor signaling cascades that can reverse the effect of oxidative stress. Research in transgenic plants has revealed the cirical role of various TFs and gene networks in MeJA and SA signaling pathways that are involved in protecting the plants from various stress factors and enhance SMs production (Khare et al. 2020).
SA signaling
SA serves as a mediator in plant development and stress conditions such as cold, drought and salinity, which are partly accomplished through enhanced biosynthesis of SMs. Depending on the elicitor treatment, stress conditions and pathogen attack, SA levels in plants vary. Under stress conditions, SA binds to various metabolic substances and controls the entire molecular signaling mechanisms. SA is strongly involved in the regulation of endoplasmic reticulum (ER) stress, antioxidant mechanism and heavy metal toxicity (Korner et al. 2015; El-Esawi et al. 2017). Exogenous SA treatment can protect the plant against cadmium toxicity; play an important role in plant defense. NPR1 (nonexpresser of pathogenesis-related protein 1) and SA-binding proteins (SABPs) are the major transcriptional regulators of SA signaling pathways (Guo et al. 2019). The ER controls the synthesis the proteins. Disruption of ER homeostasis leads to the production of misfolded proteins, which result in stress-inducing stimuli. SA modulates the redox homeostasis and activate the function of desired TFs. Nowadays, various environmental conditions are leading to a global food shortage. Under this condition, exogenous use of bioactive metabolites like SA may increase the agricultural yield. The accumulation of SA-based derivaties can reduce populations of herbivores. Pretreatment with polyamines may enhance the overall function of SA. Moreover, several TFs function in response to SA signaling. BcWRKY46 and OsWRKY77 effectively respond to SA signaling through defense responses (Wang et al. 2012; Lan et al. 2013). A recent study reported that, SA responsive WRKY TF positively regulates the biosynthesis of taxol in T. chinensis by upregulating the taxol biosynthesis genes expression (Chen et al. 2021). In S. miltiorrhiza, SA stimulated the tanshinone genes expression including SmGGPPS, SmIPPI, SmCPS, SmDXS-II and SmHMGR, consistent with SA induction on enhanced tanshinone production (Hao et al. 2015). In V. vinifera, SA stimulated the production of PAL (phenylalanine ammonia-lyase) mRNA, an essential enzyme in phenylpropanoid metabolism, resulting in a extensive production of phenolic content (Wen et al. 2008; Ma et al. 2017) repoted that li049 an AP2/ERF TF family, serves as a positive regulator for the biosynthesis of SMs called lignan/lignin in Isatis indigotica by the activation of SA signaling pathway. Currently, only a few research on TFs responding to SA have been reported, indicating that more research is required to achieve more results in the future.
Effect of MeJA and SA as elicitors on the biosynthesis of SMs production of potent medicinal plants
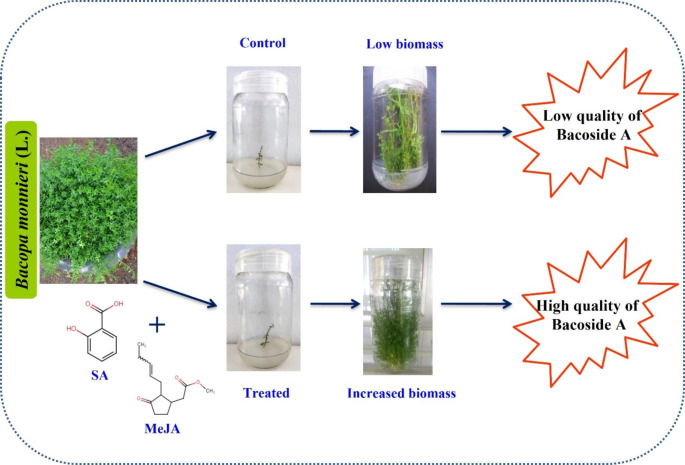
MeJA and SA have been very well-documented chemical elicitors, a response to insect attacks, which deters feeding. MeJA has been hypothesized as an essential signaling elicitation molecule leading to the production of novel and desired phytocompounds through in vitro cell cultures (Zhao et al. 2010). In B. monnieri, the combination of MeJA and SA significantly increased the biomass and bacoside A content in in vitro shoot cultures. An example thematic image was represented in Fig. 2. The bioactive compound bacoside A production was enhanced by using MeJA through in vitro shoot culture of B. monneiri (Sharma et al. 2015; Largia et al. 2015; Kim et al. 2007) reported that the SA and MeJA enhance the asiaticoside content in hairy root cultures of C. asiatica after 3 weeks of elicitation and enhance glycyrrhizin production in Glycyrrhiza glabra after the addition of MeJA and SA. SA and MeJA significantly improve the ginkgolide A, B, C and bilobalide production in the Ginkgo biloba under in vitro conditions in immobilized cell cultures (Sukito and Tachibana 2016). The synergetic effect of MeJA and phenylacetic acid (PAA) were used as elicitors to increase total phenolic and flavonoid content in the root suspension of Ajuga bracteosa. Phytoecdysteroid levels were enhanced by 14 days of MeJA elicitation in A. bracteosa (Saeed et al. 2017). Gadzovska et al. (2013) reported, SA treated suspension culture showed a two-fold increase of hypericin and pseudohyperian production in Hypericum perforatum (L.). MeJA treatment showed low concentrations of exogenous MeJA that could provide a potential protocol for the enhancement of bacoside contents in Bacopa seedlings (Bunjan et al. 2018; Koul and Mallubhotla 2020) reported that SA was used as an elicitor to enhance biomass and bacoside production using in vitro suspension cultures of B. monneiri. Another study was carried out to enhance the bacoside content of B. monnieri through in vitro suspension culture using plant growth regulators such as 6-benzyladenine (BA), kinetin, thidiazuron and chitosan. Among them, thidiazuron-treated samples showed increased bacoside content (Kharde et al. 2018). MeJA, SA, and copper sulfate (CuSO4) were used to enhance the bacoside content in B. monnieri shoot cultures and the results showed, CuSO4 treated shoot cultures exhibited a maximum yield of bacoside content than the other two hormonal elicitors (Sharma et al. 2015). However, scientific experiments through in vivo studies are rare and more contradictory, which indicates more research is undoubtedly urging steps to attain more output in the future (Table 3).
Fig. 2.
Enhancement of biomass through elicitation process in B. monnieri
Table 3.
Effect of MeJA and SA on the enhancement of specific SMs in vitro culture of medicinal plants
| S. No. | Plant species | Elevated Secondary Metabolites | Elicitors | Type of culture | Reference (s) |
|---|---|---|---|---|---|
| 1. | Glycyrrhiza inflata | Glycyrrhizin | MeJA | Hairy root culture | Wongwicha et al. 2011 |
| 2. | Withania somnifera |
Withanolide A, withanone, and withaferin A |
SA | hairy root | Sivanandhan et al. 2013 |
| 3. | Panax ginseng | Ginsenosides | MeJA | Hairy root | Corchete et al. 2013 |
| 4. | Bacopa monnieri | Bacoside A | SA and MeJA | Shoot | Sharma et al. 2015 |
| 5. | Catharanthus roseus | Ajmalicine | MeJA and Cyclodextrin | Cambial meristematic cells | Zhou et al. 2015 |
| 6. | Salvia miltiorrhiza | Tashinones | MeJA | Hairy root | Hao et al. 2015 |
| 7. | Vitis vinifera | Stilbene | SA | cell suspension | Xu et al. 2015 |
| 8. | Gymnema sylvestre | Gymnemic acid | MeJA | Cell suspension | Chodisetti et al. 2015 |
| 9. | Hypericum perforatum | Flavonoid (Hyperin and Quercetin) | MeJA | Cell suspension | Wang et al. 2015 |
| 10. | Ginkgo biloba | Bilobalide, ginkgolide A, ginkgolide B, ginkgolide C | SA and MeJA | immobilized cell cultures | Sukito and Tachibana, 2016 |
| 11. | Ajuga bracteosa | Phytoecydysteroids | MeJA | Hairy root | Saeed et al. 2017 |
| 12. | Ajuga bracteosa | TPC and TFC | MeJA and PAA | Root suspension | Saeed et al. 2017 |
| 13. | Podophyllum hexandrum (ptox) | Podophylloxin | MeJA | Cell | Hazra et al. 2017 |
| 14. | Duboisia genus | Scopolamine | MeJA | Hairy root | Singh et al. 2018a, b |
| 15. | Isatis tinctoria | Epigoitrin, tryptanthrin, indigo, indirubin | SA | hairy root | Gai et al. 2019 |
| 16. | Achillea gypsicola | camphor and phenolic compounds | SA and MeJA | Cell suspension | Acikgoz et al. 2019 |
| 17. | Isatis tinctoria | Rutin, neohesperidin, buddleside | MeJA | Hairy root | Gai et al. 2019 |
| 18. | Bacopa monnieri | Bacoside A | SA | Suspension | Koul and Mallubhotla, 2020 |
| 19. | Hybanthus enneaspermus | L-3,4-dihydroxyphenylalanine | SA | adventitious root | Sathish et al. 2020 |
| 20. | Piper cumanense | 5-hidroximetilfurfural, (Z)-9-octadecenamide and phenol | SA and MeJA | Cell suspension | Rodriguez-Sanchez et al. 2020 |
| 21. | Scutellaria bornmuelleri | Chrysin, baicalein, wogonin | MeJA | hairy root | Gharari et al. 2020a |
| 22. | Vernonia anthelmintica | rhamnetin | SA and MeJA | Cell suspension | Rajan et al. 2020 |
| 23. | Rubia tinctorum | Anthraquinone and phenols | SA | adventitious root cultures | Demirci et al. 2021 |
Conclusion
Medicinal plants are an incredible source for producing pharmaceutically important novel bioactive compounds to reform medicines in the future and also these are all used for industrial and other food materials. Elicitation is one of the effective methods to enhance the synthesis of therapeutic metabolite production in medicinal plants to maintain their resilience and survival. Elicitors can trigger a diverse plant defense mechanism which depends on the type of elicitors encountered. Among the various biotechnological approaches, biotic and abiotic elicitors have gained potential value for the enhanced production of novel bioactive compounds. The current review is a touchstone for the use of MeJA and SA as elicitors in medicinal plants for the enhancement of their phytocompounds using various in vitro culture techniques to meet the market demands of pharmaceuticals. It has been found that medicinal plants are used across the globe to treat diseases such as jaundice, tumor, malaria, depression, cancer, constipation, diabetes, COVID-19, and so on. Because of the presence of bioactive compounds in plants, they have various pharmacological properties. The phytocompounds in the intact plant are limited in quantity, MeJA, and SA are used as elicitors via different in vitro cultures to synthesize the SMs in the desired quantity. However, increasing production via eliciting secondary phytomolecules from different plant cell cultures is a frontier area of research. The main role of elicitors and the amount of enhancement of SMs harvest index of medicinal plants may indicate a future approach for the production of quality and quantity of pharmaceutically active phytocompounds from medicinal plants.
Acknowledgements
RJ acknowledges RUSA 2.0 (Alu/RUSA/Project Fellow – Science-TBRP/2019 dated 07.03.2019), Alagappa University, Karaikudi, Tamil Nadu, India for providing a Research Fellowship in the form of Project Fellow. The authors thankfully acknowledge DST-FIST (Grant No. SR/FST/LSI-639/2015(C)), UGC-SAP (Grant No.F.5 − 1/2018/DRS-II (SAP-II)), and DST-PURSE (Grant No. SR/PURSE Phase 2/38 (G)) for providing instrumentation facilities. The authors also thank RUSA 2.0 [F. 24–51/2014-U, Policy (TN Multi-Gen), Dept of Edn, GOI].
Author contributions
MR and RJ conceived and designed the review. RJ and PM wrote the manuscript. RJ, PM and KK performed the literature search. PM, HS, SHC, J-TC and MR made a critical revision of the review. All authors contributed to the writing-reviewing and editing and approved the final version of the manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbasi BH, Stiles AR, Saxena PK, Liu CZ. Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl Biochem Biotechnol. 2012;168:2057–2066. doi: 10.1007/s12010-012-9917-z. [DOI] [PubMed] [Google Scholar]
- Abdul Malik NA, Kumar IS, Nadarajah K. Elicitor and receptor molecules: orchestrators of plant defense and immunity. Int J Mol Sci. 2020;21(3):963. doi: 10.3390/ijms21030963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acıkgoz MA. Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind Crops Prod. 2020;148:112278. doi: 10.1016/j.indcrop.2020.112278. [DOI] [Google Scholar]
- Acikgoz MA, Kara SM, Aygun AHMET, Ozcan MM, Ay EB. Effects of methyl jasmonate and salicylic acid on the production of camphor and phenolic compounds in cell suspension culture of endemic turkish yarrow (Achillea gypsicola) species. Turkish J Agric Forestry. 2019;43(3):351–359. doi: 10.3906/tar-1809-54. [DOI] [Google Scholar]
- Ahlawat S, Saxena P, Ali A, Khan S, Abdin MZ. Comparative study of withanolide production and the related transcriptional responses of biosynthetic genes in fungi elicited cell suspension culture of Withania somnifera in shake flask and bioreactor. Plant Physiol Biochem. 2017;114:19–28. doi: 10.1016/j.plaphy.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Ahmadian Chashmi N, Sharifi M, Behmanesh M. Lignan enhancement in hairy root cultures of Linum album using coniferaldehyde and methylenedioxycinnamic acid. Prep Biochem Biotechnol. 2016;46:454–460. doi: 10.1080/10826068.2015.1068802. [DOI] [PubMed] [Google Scholar]
- Ali B. Salicylic acid: an efficient elicitor of secondary metabolite production in plants. Biocatal Agric Biotechnol. 2021;31:101884. doi: 10.1016/j.bcab.2020.101884. [DOI] [Google Scholar]
- Alsoufi ASM, Pączkowski C, Szakiel A, Długosz M. Effect of jasmonic acid and chitosan on triterpenoid production in Calendula officinalis hairy root cultures. Phytochem Lett. 2019;31:5–11. doi: 10.1016/j.phytol.2019.02.030. [DOI] [Google Scholar]
- Anjum S, Abbasi BH, Doussot J, Favre-Réguillon A, Hano C. Effects of photoperiod regimes and ultraviolet-C radiations on biosynthesis of industrially important lignans and neolignans in cell cultures of Linum usitatissimum L.(Flax) J Photochem Photobiol B: Biol. 2017;167:216–227. doi: 10.1016/j.jphotobiol.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Apostol L, Heinstein PF. Low PS Rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Plant Physiol. 1989;l90:109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armero J, Tena M. Possible role of plasma membrane H+-ATPase in the elicitation of phytoalexin and related isoflavone root secretion in chickpea (Cicer arietinum L.) seedlings. Plant Sci. 2001;161:791–798. doi: 10.1016/S0168-9452(01)00472-1. [DOI] [Google Scholar]
- Awad V, Kuvalekar A, Harsulkar A. Microbial elicitation in root cultures of Taverniera cuneifolia (Roth) Arn. For elevated glycyrrhizic acid production. Ind Crops Prod. 2014;54:13–16. doi: 10.1016/j.indcrop.2013.12.036. [DOI] [Google Scholar]
- Baenas N, García-Viguera C, Moreno DA. Biotic elicitors effectively increase the glucosinolates content in Brassicaceae sprouts. J Agric Food Chem. 2014;62(8):1881–1889. doi: 10.1021/jf404876z. [DOI] [PubMed] [Google Scholar]
- Bajwa MN, Bibi A, Idrees MZ, Zaman G, Farooq U, Bhatti TT (2021) Elicitation, a mechanistic approach to change the metabolic pathway of plants to produce pharmacological important compounds in in-vitro cell cultures.Glob J. Eng,8
- Benhamou N. Elicitor-induced plant defense pathways. Trends Plant Sci. 1996;1:233–240. doi: 10.1016/S1360-1385(96)86901-0. [DOI] [Google Scholar]
- Biswas T, Pandey SS, Maji D, Gupta V, Kalra A, Singh M, Mathur A, Mathur AK. Enhanced expression of ginsenoside biosynthetic genes and in vitro ginsenoside production in elicited Panax sikkimensis (Ban) cell suspensions. Protoplasma. 2018;255:1147–1160. doi: 10.1007/s00709-018-1219-z. [DOI] [PubMed] [Google Scholar]
- Bunjan W, Sujipuli K, Prasarnpun S. Effect of methyl jasmonate elicitation on biomass, gene expression and saponin accumulation in Bacopa monnieri. Int J Biol Sci. 2018;13(4):369–377. [Google Scholar]
- Cai Z, Kastell A, Speiser C, Smetanska I. Enhanced resveratrol production in Vitis vinifera cell suspension cultures by heavy metals without loss of cell viability. Appl Biochem Biotechnol. 2013;171:330–340. doi: 10.1007/s12010-013-0354-4. [DOI] [PubMed] [Google Scholar]
- Chen H, Jones AD, Howe GA. Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett. 2006;580(11):2540–2546. doi: 10.1016/j.febslet.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang H, Zhang M, Zhang W, Ou Z, Peng Z, Fu C, Zhao C, Yu L (2021) Salicylic acid-responsive factor TcWRKY33 positively regulates taxol biosynthesis in Taxus chinensis in direct and indirect ways.Front. Plant Sci.:1596 [DOI] [PMC free article] [PubMed]
- Chini A, Monte I, Zamarreno AM, Hamberg M, Lassueur S, Reymond P, Weiss S, Stintzi A, Schaller A, Porzel A, Garcia-Mina JM. An OPR3-independent pathway uses 4, 5-didehydrojasmonate for jasmonate synthesis. Nat Chem Biol. 2018;14(2):171–178. doi: 10.1038/nchembio.2540. [DOI] [PubMed] [Google Scholar]
- Chodisetti B, Rao K, Gandi S, Giri A (2015) Gymnemic acid enhancement in the suspension cultures of Gymnema sylvestre by using the signaling molecules—methyl jasmonate and salicylic acid. In vitro Cellular & Developmental Biology-Plant 51:88–92
- Corchete P, Bru R. Proteome alterations monitored by DIGE analysis in Silybum marianum cell cultures elicited with methyl jasmonate and methyl B cyclodextrin. J Proteom. 2013;85:99–108. doi: 10.1016/j.jprot.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Danova K, Pistelli L. Plant tissue culture and secondary metabolites production. Plants. 2022;11(23):3312. doi: 10.3390/plants11233312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci T, Aras Ascı O, Gokturk Baydar N. Influence of salicylic acid and L-phenylalanine on the accumulation of anthraquinone and phenolic compounds in adventitious root cultures of madder (Rubia tinctorum L) Plant Cell Tissue and Organ Culture (PCTOC) 2021;144(2):313–324. doi: 10.1007/s11240-020-01952-w. [DOI] [Google Scholar]
- Demole E, Lederer E, Mercier D. Isolement et détermination de la structure du jasmonate de méthyle, constituant odorant caractéristique de l’essence de jasmin. Helv Chim Acta. 1962;45(2):675–685. doi: 10.1002/hlca.19620450233. [DOI] [Google Scholar]
- Dhiman N, Patial V, Bhattacharya A (2018) The current status and future applications of hairy root cultures. Biotechnological Approaches for Medicinal and Aromatic Plants, Springer, Singapore 87–155
- Ebel DJ, Scheel D (1992) Elicitor recognition and signal transduction. In Genes Involved in Plant Defense T. Boller and F. Meins, (Eds) Vienna: Springer-Verlag 184– 205
- Sabagh EL, Islam A, Hossain MS, Iqbal A, Mubeen MA, Waleed M, Reginato M, Battaglia M, Ahmed M, Rehman S, Arif A. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front agron. 2022;4:4. doi: 10.3389/fagro.2022.765068. [DOI] [Google Scholar]
- El-Esawi MA, Elansary HO, El-Shanhorey NA, Abdel-Hamid AM, Ali HM, Elshikh MS. Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Fron Physiol. 2017;8:716. doi: 10.3389/fphys.2017.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeilzadeh Bahabadi S, Sharifi M, Ahmadian Chashmi N, Murata J, Satake H. Significant enhancement of lignan accumulation in hairy root cultures of Linum album using biotic elicitors. Acta Physiol Plant. 2014;36:3325–3331. doi: 10.1007/s11738-014-1700-z. [DOI] [Google Scholar]
- Gadzovska S, Maury S, Delaunay A, Spasenoski M, Hagege D, Courtois D, Joseph C The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult. 113: 25–39 Gadzovska, Simic S, Tusevski O, Maury S, Delaunay A, Laine E, Joseph C, Hagege D (2015) Polysaccharide elicitors enhance phenylpropanoid and naphtodianthrone production in cell suspension cultures of Hypericum perforatum. Plant Cell Tissue Organ Cult. 122: 649–663. Gai Q-Y, Jiao J, Wang X, Zang Y-P, Niu L-L, Fu Y-J (2013) (2019) Elicitation of Isatis tinctoria L. hairy root cultures by salicylic acid and methyl jasmonate for the enhanced production of pharmacologically active alkaloids and flavonoids. Plant Cell Tissue Organ Cult. 137: 77–86
- Gelli A, Higgins VJ, Blumwald E. Activation of plant plasma membrane Ca2+- vpermeable channels by race-specific fungal elicitors. Plant Physiol. 1997;113:269–279. doi: 10.1104/pp.113.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharari Z, Bagheri K, Danafar H, Sharafi A. Enhanced flavonoid production in hairy root cultures of Scutellaria bornmuelleri by elicitor induced over-expression of MYB7 and FNSП2 genes. Plant Physiol Biochem. 2020;148:35–44. doi: 10.1016/j.plaphy.2020.01.002. [DOI] [PubMed] [Google Scholar]
- Ghorbanpour M, Khavazi K, Hatami M (2014) Chemical compositions and antimicrobial activity of Salvia officinalis L. essential oil under rhizobacteria (Pseudomonas fluorescens and putida) inoculation. European Journal of Soil Biology
- Guo B, Liu C, Liang Y, Li N, Fu Q. Salicylic acid signals plant defence against cadmium toxicity. Int J Mol Sci. 2019;20(12):2960. doi: 10.3390/ijms20122960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder M, Sarkar S, Jha S. Elicitation: a biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng Life Sci. 2019;19(12):880–895. doi: 10.1002/elsc.201900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanagata N, Uehara H, Ito A, Takeuchi T, Karube I. Elicitor for red pigment formation in Carthamus tinctorius cultured cells. J Biotechnol. 1994;34:71–77. doi: 10.1016/0168-1656(94)90167-8. [DOI] [Google Scholar]
- Hao X, Shim M, Cui L, Xu C, Zhang Y, Kai G. Effects of methyl jasmonate and salicylic acid on tanshinone production and biosynthetic gene expression in transgenic Salvia miltiorrhiza hairy roots. Biotechnol Appl Biochem. 2015;62:24–31. doi: 10.1002/bab.1236. [DOI] [PubMed] [Google Scholar]
- Hao YJ, An XL, Sun HD, Piao XC, Gao R, Lian ML. Ginsenoside synthesis of adventitious roots in Panax ginseng is promoted by fungal suspension homogenate of Alternaria panax and regulated by several signaling molecules. Ind Crops Prod. 2020;150:112414. doi: 10.1016/j.indcrop.2020.112414. [DOI] [Google Scholar]
- Hashemi SM, Naghavi MR, Bakhshandeh E, Ghorbani M, Priyanatha C, Zandi P. Effects of abiotic elicitors on expression and accumulation of three candidate benzophenanthridine alkaloids in cultured greater celandine cells. Molecules. 2021;26(5):1395. doi: 10.3390/molecules26051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra S, Bhattacharyya D, Chattopadhyay S (2017) Methyl Jasmonate regulates Podophyllotoxin Accumulation in Podophyllum hexandrum by altering the ROS-Responsive podophyllotoxin pathway gene expression additionally through the Down Regulation of few interfering miRNAs [DOI] [PMC free article] [PubMed]
- Ho TT, Murthy HN, Park SY. Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. Int J Mol Sci. 2020;21(3):716. doi: 10.3390/ijms21030716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain C, Khatana S, Vijayvergia R. Bioactivity of secondary metabolites of various plants: a review. Int J Pharm Sci Res. 2019;10:494–504. [Google Scholar]
- Jakovljevic D, Stankovic M, Warchol M, Skrzype E. Basil (Ocimum L.) cell and organ culture for the secondary metabolites production: a review. Plant Cell Tissue and Organ Culture (PCTOC) 2022;149(1–2):61–79. doi: 10.1007/s11240-022-02286-5. [DOI] [Google Scholar]
- Jalalipour Parizi K, Rahpeyma SA, Pourseyedi S. The novel paclitaxel-producing system: establishment of Corylus avellana L. hairy root culture. Vitr Cell Dev Biol – Plant. 2020;56:290–297. doi: 10.1007/s11627-019-10050-2. [DOI] [Google Scholar]
- Jan R, Asaf S, Numan M, Lubna L, Kim KM. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy. 2021;11:968. doi: 10.3390/agronomy11050968. [DOI] [Google Scholar]
- Kandoudi W, Radácsi P, Gosztola B, Zámborine Németh E. Elicitation of medicinal plants in vivo—Is it a realistic tool? The effect of methyl jasmonate and salicylic acid on lamiaceae species. Horticulturae. 2021;8(1):5. doi: 10.3390/horticulturae8010005. [DOI] [Google Scholar]
- Khare S, Singh NB, Singh A, Hussain I, Niharika KM, Yadav V, Bano C, Yadav RK, Amist N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J Plant Biol. 2020;63:203–216. doi: 10.1007/s12374-020-09245-7. [DOI] [Google Scholar]
- Kharde AV, Kore SV, Khetmalas MB. Elicitation of Bacoside Content using plant growth regulators in cell suspension culture of Bacopa monnieri (L.) Wettst. Plant Tissue Culture and Biotechnology. 2018;28(2):191–199. doi: 10.3329/ptcb.v28i2.39678. [DOI] [Google Scholar]
- Kim OT, Bang KH, Shin YS, Lee MJ, Jung SJ, Hyun DY, Kim YC, Seong NS, Cha SW, Hwang B. Enhanced production of asiaticoside from hairy root cultures of Centella asiatica (L.) Urban elicited by methyl jasmonate. Plant cell rep. 2007;26(11):1941–1949. doi: 10.1007/s00299-007-0400-1. [DOI] [PubMed] [Google Scholar]
- Klein FRS, Reis A, Kleinowski AM, Telles RT, Amarante LD, Peters JA. UV-B radiation as an elicitor of secondary metabolite production in plants of the genus Alternanthera. Acta Bot Brasilica. 2018;32:615–623. doi: 10.1590/0102-33062018abb0120. [DOI] [Google Scholar]
- Korner CJ, Du X, Vollmer ME, Pajerowska-Mukhtar KM. Endoplasmic reticulum stress signaling in plant immunity—at the crossroad of life and death. Int J Mol Sci. 2015;16(11):26582–26598. doi: 10.3390/ijms161125964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo YM, Heo AY, Choi HW. Salicylic acid as a safe plant protector and growth regulator. plant Pathol J. 2020;36(1):1. doi: 10.5423/PPJ.RW.12.2019.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul A, Mallubhotla S. Wettst. Elicitation and enhancement of bacoside production using suspension cultures of Bacopa monnieri (L.) 3. Biotech. 2020;10:1–4. doi: 10.1007/s13205-020-02242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R, Kotecha M. A review on the standardization of herbal medicines. Int J Pharm Sci. 2016;7(2):97–106. [Google Scholar]
- Kumari N, Varghese BA, Khan MA, Jangra S, Kumar A. Abiotic elicitation: a tool for producing bioactive compounds. Plant Arch. 2020;20:2683–2689. [Google Scholar]
- Kummritz S, Louis M, Haas C, Oehmichen F, Gantz S, Delenk H, Steudler S, Bley T, Steingroewer J. Fungal elicitors combined with a sucrose feed significantly enhance triterpene production of a Salvia fruticosa cell suspension. Appl Microbiol Biotechnol. 2016;100:7071–7082. doi: 10.1007/s00253-016-7432-9. [DOI] [PubMed] [Google Scholar]
- Lan A, Huang J, Zhao W, Peng Y, Chen Z, Kang D. A salicylic acid-induced rice (Oryza sativa L.) transcription factor OsWRKY77 is involved in disease resistance of Arabidopsis thaliana. Plant Biol. 2013;15(3):452–461. doi: 10.1111/j.1438-8677.2012.00664.x. [DOI] [PubMed] [Google Scholar]
- Largia MJV, Pothiraj G, Shilpha J, Ramesh M. Methyl jasmonate and salicylic acid synergism enhances bacoside A content in shoot cultures of Bacopa monnieri (L) Plant Cell Tissue Organ Cult. 2015;122(1):9–20. doi: 10.1007/s11240-015-0745-z. [DOI] [Google Scholar]
- Lim GH, Liu H, Yu K, Liu R, Shine MB, Fernandez J, Burch-Smith T, Mobley JK, McLetchie N, Kachroo A, Kachroo P. The plant cuticle regulates apoplastic transport of salicylic acid during systemic acquired resistance. Sci Adv. 2020;6(19):peaaz0478. doi: 10.1126/sciadv.aaz0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Molina JA, Vacas S. Effect of drought stress on essential oil composition of Thymus vulgaris L.(Chemotype 1, 8-cineole) from wild populations of Eastern Iberian Peninsula. J Essent Oil Res. 2017;29:145–155. doi: 10.1080/10412905.2016.1211561. [DOI] [Google Scholar]
- Ma D, Pu G, Lei C, Ma L, Wang H, Guo Y, Chen J, Du Z, Wang H, Li G, Ye H. Isolation and characterization of AaWRKY1, an Artemisia annua transcription factor that regulates the amorpha-4, 11-diene synthase gene, a key gene of artemisinin biosynthesis. Plant Cell Physiol. 2009;50(12):2146–2161. doi: 10.1093/pcp/pcp149. [DOI] [PubMed] [Google Scholar]
- Ma R, Xiao Y, Lv Z, Tan H, Chen R, Li Q, Chen J, Wang Y, Yin J, Zhang L, Chen W. AP2/ERF transcription factor, Ii049, positively regulates lignan biosynthesis in Isatis indigotica through activating salicylic acid signaling and lignan/lignin pathway genes. Front Plant Sci. 2017;8:1361. doi: 10.3389/fpls.2017.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena M, Yadav G, Sonigra P, Nagda A, Mehta T, Swapnil P, Marwal A. Role of elicitors to initiate the induction of systemic resistance in plants to biotic stress. Plant Stress. 2022;5:100103. doi: 10.1016/j.stress.2022.100103. [DOI] [Google Scholar]
- Mendoza D, Cuaspud O, Arias JP, Ruiz O, Arias M (2018) Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnology reports. 19: p.e00273 [DOI] [PMC free article] [PubMed]
- Miladinova-Georgieva K, Geneva M, Stancheva I, Petrova M, Sichanova M, Kirova E. Effects of different elicitors on Micropropagation, Biomass and secondary Metabolite production of Stevia rebaudiana Bertoni—A. Rev Plants. 2023;12(1):153. doi: 10.3390/plants12010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moharrami F, Hosseini B, Sharafi A, Farjaminezhad M (2017) Enhanced production of hyoscyamine and scopolamine from genetically transformed root culture of Hyoscyamus reticulatus L. elicited by iron oxide nanoparticles. In vitro Cellular & Developmental Biology-Plant. 53: 104–111 [DOI] [PMC free article] [PubMed]
- Murthy HN, Lee EJ, Paek KY. Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014;118(1):1–16. doi: 10.1007/s11240-014-0467-7. [DOI] [Google Scholar]
- Praveen N, Murthy HN. Synthesis of withanolide A depends on carbon source and medium pH in hairy root cultures of Withania somnifera. Ind Crops Prod. 2012;35:241–243. doi: 10.1016/j.indcrop.2011.07.009. [DOI] [Google Scholar]
- Rajan M, Feba KS, Chandran V, Shahena S, Mathew L. Enhancement of rhamnetin production in Vernonia anthelmintica (L.) Willd. Cell suspension cultures by eliciting with methyl jasmonate and salicylic acid. Physiol Mol Biol Plants. 2020;26(7):1531–1539. doi: 10.1007/s12298-020-00829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput VD, Singh RK, Verma KK, Sharma L, Quiroz-Figueroa FR, Meena M, Gour VS, Minkina T, Sushkova S, Mandzhieva S. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology. 2021;10(4):267. doi: 10.3390/biology10040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SR, Ravishankar GA. Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv. 2002;20(2):101–153. doi: 10.1016/S0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sanchez LK, Pérez-Bernal JE, Santamaría-Torres MA, Marquínez-Casas X, Cuca-Suarez LE, Prieto-Rodríguez JA, Patino-Ladino OJ. Effect of methyl jasmonate and salicylic acid on the production of metabolites in cell suspensions cultures of Piper cumanense (Piperaceae) Biotechnol Rep. 2020;28:e00559. doi: 10.1016/j.btre.2020.e00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodziewicz P, Swarcewicz B, Chmielewska K, Wojakowska A, Stobiecki M. Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiol Plant. 2014;36(1):1–19. doi: 10.1007/s11738-013-1402-y. [DOI] [Google Scholar]
- Saeed S, Ali H, Khan T, Kayani W, Khan MA. Impacts of methyl jasmonate and phenyl acetic acid on biomass accumulation and antioxidant potential in adventitious roots of Ajuga bracteosa Wall ex Benth., a high valued endangered medicinal plant. Physiol Mol Biol Plants. 2017;23:229–237. doi: 10.1007/s12298-016-0406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu GK (2013) Salicylic acid: Role in plant physiology and stress tolerance. Molecular stress physiology of plants,Springer, India 217–239
- Salehi M, Karimzadeh G, Naghavi MR. Synergistic effect of coronatine and sorbitol on artemisinin production in cell suspension culture of Artemisia annua L. Anamed Plant Cell Tissue Organ Cult. 2019;137:587–597. doi: 10.1007/s11240-019-01593-8. [DOI] [Google Scholar]
- Sathish S, Vasudevan V, Karthik S, Elayaraja D, Pavan G, Ajithan C, Manickavasagam M. Elicitors induced L-Dopa accumulation in adventitious root cultures of Hybanthus enneaspermus (L.) F. Muell Vegetos. 2020;33:304–312. doi: 10.1007/s42535-020-00108-7. [DOI] [Google Scholar]
- Sellapan P, Rohani E, Noor NM. Sesquiterpene production in methyl Jasmonate-induced Persicaria minor cell suspension culture. Sains Malaysiana. 2018;47:3051–3059. doi: 10.17576/jsm-2018-4712-15. [DOI] [Google Scholar]
- Shaikh S, Shriram V, Khare T, Kumar V. Biotic elicitors enhance diosgenin production in Helicteres isora L. suspension cultures via up-regulation of CAS and HMGR genes. Physiol Mol Biol Plants. 2020;26:593–604. doi: 10.1007/s12298-020-00774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeran Z, Keyhanfar M, Asghari G, Ghanadian M. Turkish. Improvement of atropine production by different biotic and abiotic elicitors in hairy root cultures of Datura metel. J Biol. 2015;39:111–118. [Google Scholar]
- Sharma M, Ahuja A, Gupta R, Mallubhotla S. Enhanced bacoside production in shoot cultures of Bacopa monnieri under the influence of abiotic elicitors. Nat Prod Res. 2015;29:745–749. doi: 10.1080/14786419.2014.986657. [DOI] [PubMed] [Google Scholar]
- Singh P, Prasad R, Tewari R, Jaidi M, Kumar S, Rout PK, Rahman L. Silencing of quinolinic acid phosphoribosyl transferase (QPT) gene for enhanced production of scopolamine in hairy root culture of Duboisia leichhardtii. Sci Rep. 2018;8:13939. doi: 10.1038/s41598-018-32396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NR, Rath SK, Behera S, Naik SK, Prasad R, Kumar V (2018b) In vitro secondary metabolite production through fungal elicitation: an approach for sustainability. Fungal Nanobionics: Principles and Applications, Springer, Singapore 215–242
- Sivanandhan G, Dev G, Jeyaraj K, Rajesh M, Arjunan M, Muthuselvam A, Manickavasagam M, Selvaraj M, Ganapathi N. Increased production of withanolide A, withanone, and withaferin A in hairy root cultures of Withania somnifera (L.) Dunal elicited with methyl jasmonate and salicylic acid. Plant Cell Tissue Organ Cult. 2013;114:121–129. doi: 10.1007/s11240-013-0297-z. [DOI] [Google Scholar]
- Srivastava S, Conlan XA, Cahill DM, Adholeya A. Rhizophagus irregularis as an elicitor of rosmarinic acid and antioxidant production by transformed roots of Ocimum basilicum in an in vitro co-culture system. Mycorrhiza. 2016;26:919–930. doi: 10.1007/s00572-016-0721-4. [DOI] [PubMed] [Google Scholar]
- Srivastava V, Mehrotra S, Verma PK (2017) Biotechnological interventions for production of therapeutic secondary metabolites using hairy root cultures of medicinal plants. Current Developments in Biotechnology and Bioengineering: Crop Modification, Nutrition, and Food Production, Elsevier, Netherland 259–282
- Subramaniyam S, Mathiyalagan R, Natarajan S, Kim YJ, Jang MG, Park JH, Yang DC. Transcript expression profiling for adventitious roots of Panax ginseng Meyer. Gene. 2014;546(1):89–96. doi: 10.1016/j.gene.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Sukito A, Tachibana S. Effect of methyl jasmonate and salycilic acid synergism on enhancement of bilobalide and ginkgolide production by immobilized cell cultures of Ginkgo biloba. Bioresour Bioprocess. 2016;3:24. doi: 10.1186/s40643-016-0101-0. [DOI] [Google Scholar]
- Taurino M, Ingrosso I, D’amico L, Domenico SD, Nicoletti I, Corradini D, Santino A, Giovinazzo G (2015) Jasmonates elicit different sets of stilbenes in Vitis vinifera cv. Negramaro cell cultures. SpringerPlus 4: 49 [DOI] [PMC free article] [PubMed]
- Thilip C, Mehaboob VM, Varutharaju K, Faizal K, Raja P, Aslam A, Shajahan A. Elicitation of withaferin-A in hairy root culture of Withania somnifera (L.) Dunal using natural polysaccharides. Biologia. 2019;74:961–968. doi: 10.2478/s11756-019-00236-9. [DOI] [Google Scholar]
- Twaij BM, Hasan MN. Bioactive secondary metabolites from plant sources: types, synthesis, and their therapeutic uses. Int J Plant Biol. 2022;13(1):4–14. doi: 10.3390/ijpb13010003. [DOI] [Google Scholar]
- Vasconsuelo A, Boland R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007;172:861–875. doi: 10.1016/j.plantsci.2007.01.006. [DOI] [Google Scholar]
- Wang JW, Wu JY (2013) Advances in biochemical Engineering/Biotechnology, Springer, Berlin, Heidelberg. Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures.Biotechnology of Hairy Root Systems.55–89 [DOI] [PubMed]
- Wang J, Qian J, Yao L, Lu Y. Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresources and Bioprocessing. 2015;2(1):1–9. doi: 10.1186/s40643-014-0033-5. [DOI] [Google Scholar]
- Wang F, Hou X, Tang J, Wang Z, Wang S, Jiang F, Li Y. A novel cold-inducible gene from pak-choi (Brassica campestris ssp. chinensis), BcWRKY46, enhances the cold, salt and dehydration stress tolerance in transgenic tobacco. Mol Biol Rep. 2012;39:4553–4564. doi: 10.1007/s11033-011-1245-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mostafa S, Zeng W, Jin B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int J Mol Sci. 2021;22(16):8568. doi: 10.3390/ijms22168568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Xu S, Wang N, Xia B, Jiang Y, Wang R (2017) Transcriptome analysis of secondary metabolism pathway, transcription factors, and transporters in response to methyl jasmonate in Lycoris aurea. Front. plant sci. 7:1971 [DOI] [PMC free article] [PubMed]
- Ward JM, Maser P, Schroeder JI. Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol. 2009;71:59–82. doi: 10.1146/annurev.physiol.010908.163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrosch C, Schwaiger S, Stuppner H, Kopp B. Lignan formation in hairy root cultures of Edelweiss (Leontopodium nivale ssp. alpinum (Cass.) Greuter) Fitoterapia. 2014;97:219–223. doi: 10.1016/j.fitote.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen PF, Chen JY, Wan SB, Kong WF, Zhang P, Wang W, Zhan JC, Pan QH, Huang WD. Salicylic acid activates phenylalanine ammonia-lyase in grape berry in response to high temperature stress. Plant Growth Regul. 2008;55:1–10. doi: 10.1007/s10725-007-9250-7. [DOI] [Google Scholar]
- Wen C, Zhang Z, Shi Q, Duan X, Du J, Wu C, Li X. Methyl Jasmonate-and Salicylic Acid-Induced transcription factor ZjWRKY18 regulates Triterpenoid Accumulation and Salt stress tolerance in Jujube. Int J Mol Sci. 2023;24(4):3899. doi: 10.3390/ijms24043899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Broadley MR. Calcium in plants. Ann Botany. 2003;92(4):487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongwicha W, Tanaka H, Shoyama Y, Putalun W. Methyl jasmonate elicitation enhances glycyrrhizin production in Glycyrrhiza inflata hairy roots cultures. Z für Naturforschung C. 2011;66(7–8):423–428. doi: 10.1515/znc-2011-7-815. [DOI] [PubMed] [Google Scholar]
- Xu A, Zhan JC, Huang WD. Effects of ultraviolet C, methyl jasmonate and salicylic acid, alone or in combination, on stilbene biosynthesis in cell suspension cultures of Vitis vinifera L. cv. Cabernet Sauvignon. Plant Cell Tissue Organ Cult. 2015;122:197–211. doi: 10.1007/s11240-015-0761-z. [DOI] [Google Scholar]
- Yan L, Zhai Q, Wei J, Li S, Wang B, Huang T, Du M, Sun J, Kang L, Li CB, Li C. Role of tomato lipoxygenase D in wound-induced jasmonate biosynthesis and plant immunity to insect herbivores. PLoS Genet. 2013;9(12):e1003964. doi: 10.1371/journal.pgen.1003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai X, Jia M, Chen L, Zheng CJ, Rahman K, Han T, Qin LP. The regulatory mechanism of fungal elicitor-induced secondary metabolite biosynthesis in medical plants. Crit Rev Microbiol. 2017;43:238–261. doi: 10.1080/1040841X.2016.1201041. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Zhou LG, Wu JY. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl Microbiol Biotechnol. 2010;87:137–144. doi: 10.1007/s00253-010-2443-4. [DOI] [PubMed] [Google Scholar]
- Zheng LP, Tian H, Yuan YF, Wang JW. The influence of endophytic Penicillium oxalicum B4 on growth and artemisinin biosynthesis of in vitro propagated plantlets of Artemisia annua L. Plant Growth Regul. 2016;80:93–102. doi: 10.1007/s10725-016-0162-2. [DOI] [Google Scholar]
- Zheng H, Fu X, Shao J, Tang Y, Yu M, Li L, Huang L, Tang K (2023) Transcriptional regulatory network of high-value active ingredients in medicinal plants. Trends Plant Sci [DOI] [PubMed]
- Zhou P, Yang J, Zhu J, He S, Zhang W, Yu R, Zi J, Song L, Huang X. Effects of βcyclodextrin and methyl jasmonate on the production of vindoline, catharanthine, and ajmalicine in Catharanthus roseus cambial meristematic cell cultures. Appl Microbiol Biotechnol. 2015;99:7035–7045. doi: 10.1007/s00253-015-6651-9. [DOI] [PubMed] [Google Scholar]
- Zhou W, Shi M, Deng C, Lu S, Huang F, Wang Y, Kai G (2021) The methyl jasmonate-responsive transcription factor SmMYB1 promotes phenolic acid biosynthesis in Salvia miltiorrhiza. Horticulture research, 8 [DOI] [PMC free article] [PubMed]
- Zhou M, Memelink J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol Adv. 2016;34(4):441–449. doi: 10.1016/j.biotechadv.2016.02.004. [DOI] [PubMed] [Google Scholar]