Abstract
The CNS critically relies on the formation and proper function of its vasculature during development, adult homeostasis and disease. Angiogenesis — the formation of new blood vessels — is highly active during brain development, enters almost complete quiescence in the healthy adult brain and is reactivated in vascular-dependent brain pathologies such as brain vascular malformations and brain tumours. Despite major advances in the understanding of the cellular and molecular mechanisms driving angiogenesis in peripheral tissues, developmental signalling pathways orchestrating angiogenic processes in the healthy and the diseased CNS remain incompletely understood. Molecular signalling pathways of the ‘neurovascular link’ defining common mechanisms of nerve and vessel wiring have emerged as crucial regulators of peripheral vascular growth, but their relevance for angiogenesis in brain development and disease remains largely unexplored. Here we review the current knowledge of general and CNS-specific mechanisms of angiogenesis during brain development and in brain vascular malformations and brain tumours, including how key molecular signalling pathways are reactivated in vascular-dependent diseases. We also discuss how these topics can be studied in the single-cell multi-omics era.
Subject terms: CNS cancer, Sequencing, Blood-brain barrier, Neuro-vascular interactions, Cerebrovascular disorders
The CNS critically relies on an extensive and complex vasculature to function properly. In this Review, Wälchli and colleagues examine the general and CNS-specific mechanisms that underlie angiogenesis in brain development, brain vascular malformations and brain tumours.
Introduction
The human brain constitutes only 2% of body mass but receives 20% of cardiac output and consumes 20% of the body’s total oxygen and glucose, underlining the crucial importance of the CNS vasculature for a properly functioning brain1,2. Accordingly, the human brain vasculature is composed of an extensive and complex network of blood vessels, with a total length of 400 miles and including up to 100 billion capillaries2. The brain vascular network is established during embryonic and postnatal development via vasculogenesis (de novo formation of blood vessels) and sprouting angiogenesis (formation of new blood vessels from pre-existing ones), driven by various pro-angiogenic and anti-angiogenic factors3.
The endothelium of the brain vasculature displays specific properties that distinguish blood vessels in the CNS from those outside the CNS4. The most characteristic feature of the brain endothelium is the presence of a functional blood–brain barrier (BBB) — the highly selective semipermeable border between the vascular lumen of capillaries and the CNS parenchyma — established during embryonic and postnatal development by extrinsic cues provided by the perivascular microenvironment3,5 and intrinsic endothelial cell (EC) regulation mediated by homeobox transcription factors6. Blood vessels in the brain are embedded in an anatomical or structural unit termed the ‘perivascular niche’ (PVN), which describes a microenvironment that, in addition to ECs, includes perivascular cells (PVCs), such as astrocytes, pericytes, perivascular fibroblasts, neurons, stem cells, microglia and vascular smooth muscle cells (vSMCs)3,7–9. Together, ECs and PVCs in the PVN form the neurovascular unit (NVU)9–11, which is the functional correlate of the structural PVN9–11. Cellular and molecular interactions between ECs and PVCs in the NVU contribute to regulation of CNS angiogenesis9–11.
Developmental vascular growth in the CNS involves general angiogenic mechanisms (that is, mechanisms involved in angiogenesis inside and outside the CNS9) and CNS-specific angiogenic mechanisms. The NVU becomes deregulated in vascular-dependent brain pathologies such as brain tumours and brain vascular malformations, in which angiogenic signalling pathways become activated and lead to the formation of leaky, tortuous and dysfunctional neovessels via various modes of neovascularization9,12,13. These angiogenic pathways are, at least in part, reactivated signalling cascades regulating vascularization and the NVU and PVN during brain development9,12,13, but how these molecular mechanisms are involved in the initiation and progression of vascular-dependent brain pathologies remains poorly understood.
In this Review, we provide an overview of our current understanding of neovascularization in the developing, healthy adult and pathological brain (Fig. 1). Moreover, we describe recent insights into the human brain vasculature at the single-cell level, emphasizing the expanding knowledge of cerebrovascular cell type heterogeneity and the reactivation of developmental angiogenic signalling pathways in ECs of vascular-dependent brain pathologies. We review recent evidence regarding reactivated developmental signalling pathways in disease, focusing on molecules involved in angiogenesis and the neurovascular link (NVL), defined as the shared molecular mechanisms regulating both the vascular system and the nervous system9,14–17 (Fig. 2). We describe the involvement of these signalling cues in glial brain tumours and brain arteriovenous malformations (AVMs), two typical vascular-dependent CNS pathologies, with special focus on the distinction between CNS-specific cues and general molecular cues. Finally, we discuss several outstanding questions and emphasize how novel technologies used in the field of single-cell multi-omics may influence our understanding of brain vascular biology.
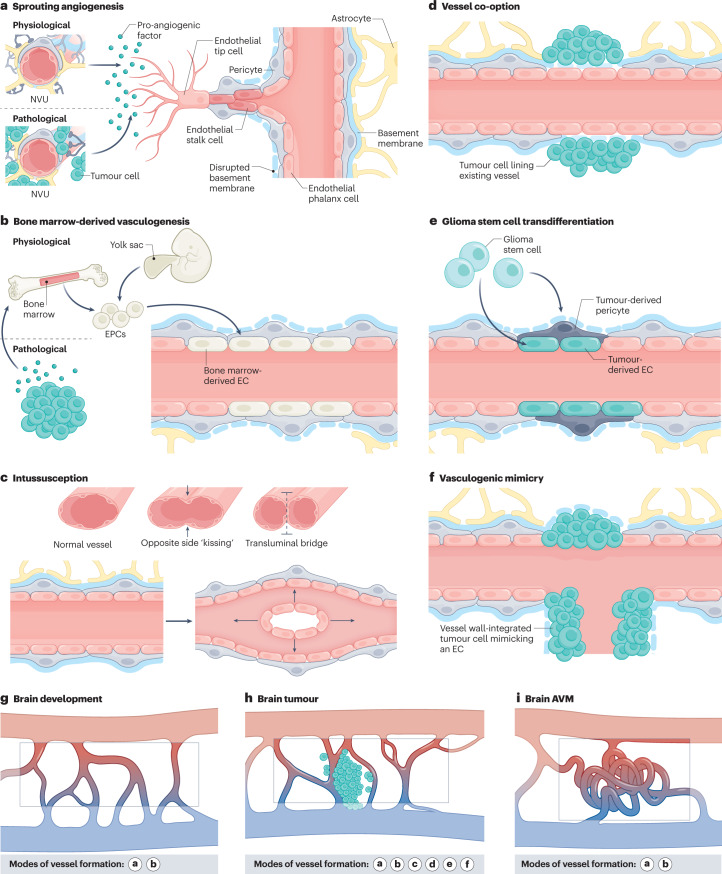
Fig. 1. Modes of vessel formation during brain development, in brain tumours and in brain AVMs.
Vascularization during brain development, in brain tumours and in brain arteriovenous malformations (AVMs) can occur via different modes of neovascularization. a, Neovascularization is possible via the formation of new blood vessels from pre-existing ones in response to pro-angiogenic signalling molecules secreted by components of the neurovascular unit (NVU) (defined as physiological sprouting angiogenesis) or by tumour cells (defined as pathological sprouting angiogenesis). For simplicity, the NVU (in physiological conditions) and tumour cells (in pathological conditions) are illustrated as sources of pro-angiogenic molecules for this mode of neovascularization. Note that the secretion of pro-angiogenic molecules is not limited to these sources but can also occur from brain vascular malformations and other vascular-dependent brain pathologies as well as from components of the extracellular matrix. New vessel sprouts are guided by specialized endothelial tip cells extending multiple filopodial protrusions sensing and reacting to pro-angiogenic, anti-angiogenic and hypoxia-related cues in the microenvironment. At the back of the leading tip cell, proliferating endothelial stalk cells elongate the growing blood vessel and initiate the formation of a functional lumen. Phalanx cells are the most quiescent of the endothelial cell (EC) subtypes, extend few filopodia and migrate and divide poorly in response to VEGF. Endothelial phalanx cells line vessels once the new vessel branches have been consolidated. b, Physiological vasculogenesis is defined as the de novo generation of blood vessels from either yolk sac-derived endothelial progenitor cells (EPCs) or bone marrow-derived EPCs, depending on the developmental time point. Pathological vasculogenesis occurs upon secretion of pro-angiogenic molecules by tumour cells that activate bone marrow to produce EPCs. Both indirect paracrine secretion of pro-angiogenic growth factors and direct luminal incorporation into sprouting nascent vessels contribute to vasculogenesis. Note that the secretion of pro-angiogenic molecules is not limited to these sources but can also occur from brain vascular malformations and other vascular-dependent brain pathologies as well as from components of the extracellular matrix. c, The splitting of existing blood vessels — vascular intussusception — allows the reorganization of existing cells without a corresponding increase in EC number. During this process, the opposite capillary walls invaginate into the vessel lumen in consecutive steps with the formation of a transluminal bridge of pericytes, myofibroblasts and extracellular matrix. d–f, Pathological conditions such as tumours or regenerative processes can exhibit the aforementioned modes of vessel formation and three additional ones, namely vessel co-option, glioma stem cell to EC transdifferentiation or glioma stem cell to pericyte transdifferentiation, and vasculogenic mimicry. Vessel co-option occurs when tumour cells co-opt existing vessels in response to angiopoietin 2 (ANG2) expression gradients (part d). In glioma stem cell transdifferentiation, glioma stem-like cells differentiate into either tumour-derived ECs or tumour-derived pericytes, induced predominantly by the TGFβ and NOTCH1 pathways in hypoxic conditions (part e). In vasculogenic mimicry, tumour cells (instead of ECs) are incorporated into the inner vessel wall, forming functional vessel-like structures and thereby mimicking ECs (part f). g–i, Modes of vessel formation involved in angiogenesis during brain development (part g), in brain tumours (part h) and in brain AVMs (part i).
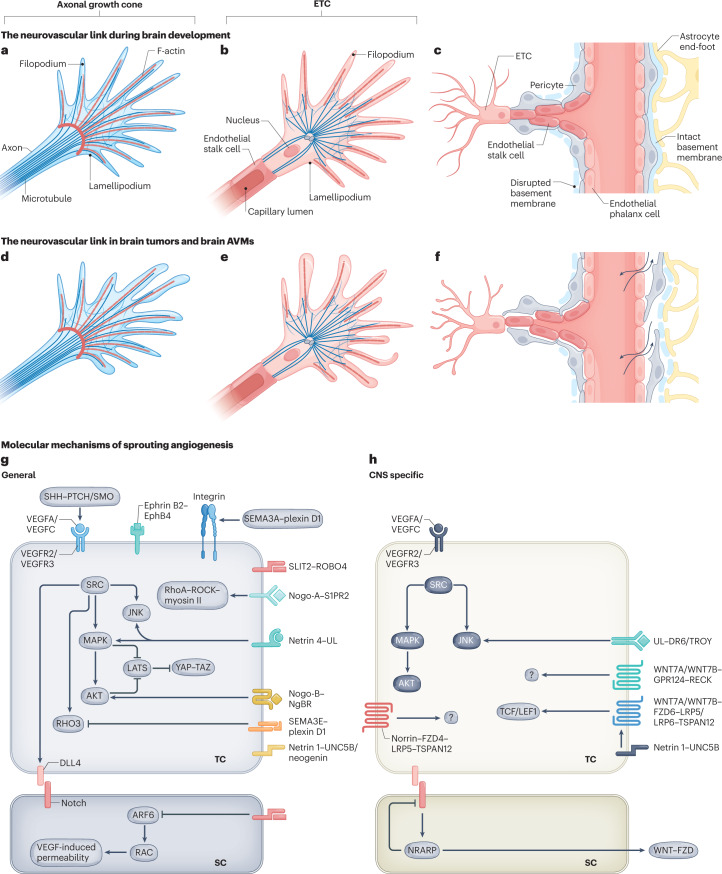
Fig. 2. Neurovascular link molecules affecting endothelial tip cell sprouting during vascular brain development, in brain tumours and in brain AVMs.
a, The axonal growth cone at the leading edge of a growing axon is a specialized, subcellular ‘hand-like’ structure at the tip of an extending neuron. In the axonal growth cone, lamellipodia and filopodia sense and integrate attractive and repulsive guidance cues in the local tissue microenvironment, thereby guiding the extending axon to its target. The central domain of an axonal growth cone is rich in microtubules, whereas the peripheral domain predominantly contains filopodia (composed of F-actin bundles) and lamellipodia (composed of an actin meshwork). Some microtubules extend into the peripheral domain and rarely into filopodia. b, The endothelial tip cell (ETC) is a specialized vascular endothelial cell type at the tip of the newly forming blood vessel, followed by proliferating endothelial stalk cells. Similarly to axonal growth cones, ETCs are specialized, ‘hand-like’ structures at the forefront of growing blood vessels that sense environmental cues using lamellipodia and ‘finger-like’ filopodia, thereby guiding the growing blood vessels to their respective targets. Endothelial phalanx cells comprise a third, mostly silent vascular endothelial cell type, lining the border of functional, established blood vessels (not shown). ETCs use actin-based lamellipodia and filopodia sensing attractive and repulsive guidance cues in the local tissue microenvironment to reach their target. Microtubules have not been detected in filopodia so far. c, A newly forming blood vessel sprout including a migrating ETC extending multiple filopodia, followed by proliferating endothelial stalk cells creating a newly formed capillary lumen, and quiescent endothelial phalanx cells lining an established vascular blood vessel. Pericytes, astrocytes and the basement membrane are also depicted. d–f, Schematic illustrations showing the characteristics of the axonal growth cone (part d), ETC (part e) and vessel sprouting (part f) in pathological conditions. Newly formed vessels often show a disrupted basement membrane, vascular leakage and a reduced pericyte coverage (part f). g,h, Molecularly, sprouting angiogenesis into the CNS is regulated by neurovascular link molecules that act in a non-CNS-specific way (part g), such as VEGFA–VEGFR2, SEMA3A/SEMA3E–plexin D1, ephrin B2–EphB4 and SLIT2–ROBO4, or a CNS-specific manner (part h), such as WNT7A/WNT7B–GPR124–FZD6–RECK and DR6–TROY. Of note, the VEGFA/VEGFC–VEGFR2/VEGFR3 and netrin 1–UNC5B signalling axes are shown in part h because even though they represent non-CNS-specific mechanisms, multiple CNS-specific mechanisms interact with these pathways downstream. AVM, arteriovenous malformation; SC, stalk cell; TC, tip cell; UL, unknown ligand.
Modes of neovascularization
The neovascularization of organs and tissues can occur via different mechanisms (Fig. 1). During physiological development, such vascularization may involve the formation of new blood vessels from pre-existing ones, defined as sprouting angiogenesis (by far the best-described mode)9,12,15,18 (Fig. 1a), the de novo generation of blood vessels from mesodermal angioblasts or haemangioblasts (which differentiate into endothelial progenitor cells (EPCs) and subsequently into ECs) in a process called ‘vasculogenesis’19 (Fig. 1b), and/or the splitting of existing blood vessels, named ‘intussusception’12 (Fig. 1c). Three additional pathological modes of neovascularization may occur in glial brain tumours and in tissues undergoing regenerative processes (for example, following ischaemic stroke): vascular co-option, in which tumour cells co-opt blood vessels to grow along pre-existing healthy blood vessels (Fig. 1d), glioma (or glioblastoma) stem cell (GSC)-to-EC transdifferentiation or GSC-to-pericyte transdifferentiation20–22 (Fig. 1e) and vasculogenic (or vascular) mimicry, in which tumour cells integrate into the blood vessel wall, mimicking ECs12 (Fig. 1f). Whereas sprouting angiogenesis and vasculogenesis are primary contributors to neovascularization during brain development and in brain AVMs (Fig. 1g,i), all six modes of vessel formation have been described in brain tumours23–26 (Fig. 1h), as discussed later herein.
Sprouting angiogenesis
On a cellular level, sprouting vessels are guided by specialized ECs that extend multiple filopodia, the endothelial tip cells (ETCs)9,12,18. Behind the leading ETC, proliferating endothelial stalk cells are responsible for the elongation of blood vessels and the formation of a functional lumen3,9,12,15,18 (Fig. 1a). Subsequently, sprouting vessels anastomose and establish a three-dimensional, perfused and fully functional vascular network9,18 (Fig. 1a,g). Quiescent endothelial phalanx cells line the newly formed lumenized vessels and can be reactivated by pro-angiogenic stimuli3,12,18. Sprouting angiogenesis and ETCs, stalk cells and phalanx cells are regulated by pro-angiogenic and anti-angiogenic molecules, the balance between them being thought to determine the angiogenic response3,12,18,27 (Supplementary Table 1). Findings of recent studies have complemented this traditional view on sprouting and ETCs by suggesting a key role of venous ECs as the primary subtype of ECs — which proliferate and migrate against the flow to acquire the ETC position — that are responsible for sprouting angiogenesis and expanding vascular networks28.
On a molecular level, the VEGF–VEGFR–DLL4–Jagged–Notch signalling cascade is a key regulator of sprouting angiogenesis in both CNS tissues and non-CNS tissues and is thought to be the central pattern generator underlying ETC, stalk cell and phalanx cell differentiation3,9,29,30 in development and disease. The most important Notch ligands — DLL4 and Jagged 1 — have opposing roles in vessel formation, with DLL4 being anti-angiogenic and Jagged 1 being pro-angiogenic31. Interestingly, ETC and stalk cell specification is dynamically regulated by a feedback loop between the VEGF–VEGFR pathway and the DLL4–Jagged 1–Notch pathway32. Competition for the tip cell position occurs when activated ECs — expressing VEGFR1, VEGFR2, VEGFR3 and neuropilin 1 (NRP1) — upregulate DLL4 on their membrane, giving these ECs an advantage for the tip cell position29,32,33. DLL4 on ETCs activates Notch signalling in adjacent stalk cells, thereby downregulating VEGFR2, VEGFR3 and NRP1, upregulating VEGFR1 and restricting the ability of stalk cells to acquire the tip cell position30,34 and limiting tip cell numbers35. In contrast to DLL4, Jagged–Notch signalling drives tip cell selection and sprouting angiogenesis by antagonizing DLL4–Notch signalling31. MPDZ and the transcription factor ERG are key regulators of endothelial Notch–DLL4–Jagged 1 signalling36, underlining the dynamic nature of EC specification into ETCs, stalk cells and phalanx cells.
We previously described the regulatory effects of NVL molecules on peripheral and CNS angiogenesis during development, including their modes of action as either general cues or CNS-specific cues for vascular growth and their emerging molecular interactions with the VEGF–VEGFR–DLL4–Jagged–Notch pathway, and we do not comprehensively revisit this topic here9.
Vasculogenesis and intussusception
During embryonic development, vasculogenesis gives rise to the heart and the primitive vascular plexus. The vascular system is generated from precursor cells (angioblasts or haemangioblasts), and its establishment occurs in parallel with haematopoiesis (the formation of blood cells)37 (Fig. 1b). Angioblasts and blood cells constitute blood islets, which then fuse and give rise to a honeycomb-shaped primitive vascular plexus before the onset of heartbeats37. Once blood circulation has been established, primary vascular plexuses are remodelled into hierarchical networks with arteriovenous distinction37 (Fig. 1g). Subsequently, PVCs, including vSMCs (in the case of arteries and veins) and pericytes (in the case of capillaries), are recruited and stabilize the vascular network37,38. Molecularly, fibroblast growth factors (FGFs) induce the formation of angioblasts, whereas VEGFA plays key roles in the differentiation and chemotaxis of angioblasts and EPCs37.
Intussusceptive angiogenesis is defined as the invagination of the capillary wall into the lumen to split a single vessel in two39,40 (Fig. 1c). This mode of neovascularization was first observed during the development of peripheral organs41–44 and was subsequently characterized in CNS tissue45,46 and in several cancers, including glioblastoma47. Transcapillary intraluminal tissue pillars arise by invagination of the capillary wall into the vessel lumen in four consecutive steps40. First, a contact zone is established between two opposing capillary walls40. Second, reorganization of EC junctions and perforation of the vessel bilayer allows growth factors and cells to penetrate the lumen40. Third, an interstitial pillar core forms between the two new vessels at the contact zone and is filled with pericytes and myofibroblasts40. Finally, the pillars increase in diameter40 (Fig. 1c). Interestingly, intussusceptive angiogenesis allows reorganization of existing cells without the need for an increase in EC number, which is especially important during distinct stages of embryonic development in which the growth rate surpasses the cellular resources40. The molecular basis of vascular intussusception remains unknown.
ECs and PVCs in the NVU and BBB
Newly formed sprouting vessels are initially fragile and become stabilized by the recruitment of PVCs (such as pericytes, vSMCs and astrocytes)9,12, which is important for the establishment of functional, perfused blood vessels integrated into a three-dimensional vascular network3,9,48,49 (Fig. 3). Accordingly, ECs invading the CNS closely interact with PVCs of the surrounding parenchyma, thereby forming a functional NVU9,15,50,51 (Fig. 3a–d). As initially postulated in 1981, the CNS parenchyma provides instructive signals regulating EC sprouting into the CNS and induction of CNS-specific properties in ECs5,52. These structural and functional EC–PVC interactions result in the specific properties of CNS blood vessels, most importantly the establishment of the BBB53 (Fig. 3c,d), which is already established during embryonic development54,55 in a process regulated by extrinsic cues provided by the local CNS microenvironment5,9,52,56–58. Tight junction-specific proteins, such as CLDN5 and OCLN, are present at the BBB interface directly after blood vessels invade the brain at the embryonic stage and achieve functionality to meet barrier functions (which go beyond the presence or absence of passive permeability) according to the particular stage of brain development during the early postnatal period54,57–60. This highly regulated physical permeability barrier can become leaky in CNS pathologies such as brain tumours, brain vascular malformations, ischaemic stroke and some neurodevelopmental and neurodegenerative disorders4,60–63 (Fig. 4).
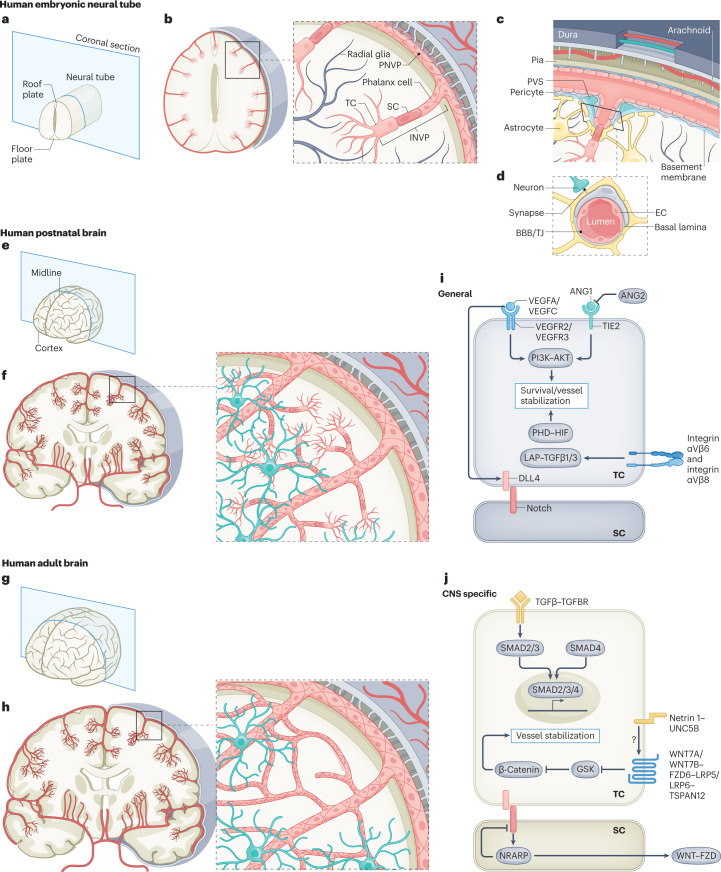
Fig. 3. Structural and molecular mechanisms of angiogenesis at the embryonic, postnatal and adult stages of vascular brain development.
a, A human neural tube at the embryonic stage with the roof and floorplate illustrated on the coronal cutting plane. b, Sprouting angiogenesis into the neural tube during embryogenesis. The perineural vascular plexus (PNVP) is formed by vasculogenesis from mesodermal-derived angioblasts at around 7 weeks of gestational age in humans (embryonic day 8.5 (E8.5) in mice). Subsequently, at around 8 weeks of gestational age in humans (E9.5 in mice), angiogenic sprouts of the intraneural vascular plexus (INVP) are formed along radial glia via sprouting angiogenesis using endothelial tip cell (ETC) filopodia, invading the CNS parenchyma and migrating towards the ventricle, where pro-angiogenic and anti-angiogenic factors such as VEGFA and WNT proteins are produced. At the forefront of these angiogenic sprouts, ETCs guide the CNS-invading blood vessels using ETC filopodia. c, The anatomical organization of the meningeal layers, including dura, arachnoid and pia mater with intradural lymphatic vessels (blue) and blood vessels (red). An angiogenic vascular sprout emanating from the extraparenchymal PNVP composed of ETCs, endothelial stalk cells and endothelial phalanx cells invading the intraparenchymal INVP is shown. A perivascular space (PVS) surrounds the base of the vascular sprout. d, The neurovascular unit (NVU) for established blood vessels that is composed of a variety of cell types, including endothelial cells (ECs), pericytes, astrocytes and neurons. ECs and pericytes are ensheathed by a common basal lamina, the endothelial basement membrane. The blood–brain barrier (BBB) is composed of microvascular ECs that are mutually connected via complex tight junctions (TJs), thereby regulating or inhibiting paracellular diffusion of water-soluble molecules. ECs regulate the transport of molecules between the blood and the brain parenchyma via the expression of influx and efflux transporters. e, A coronal section of a human brain during postnatal development. f, At the postnatal stage, sprouting angiogenesis is the main mode of neovascularization, and vascular sprouting occurs in all directions throughout cortical layers 1–6. Endothelial sprouts invading the CNS parenchyma from week 8 of gestational age (E9.5 in mice) onwards grow along radial glia fibres towards the ventricle. g,h, In the healthy adult brain, the vasculature is almost quiescent, with only very few ECs proliferating. i,j, Molecularly, numerous pathways have been implicated in EC quiescence, survival and maintained inhibition of paracellular permeability, and the molecular cues can be either non-CNS specific or CNS specific. The TGFβ–TGFβR signalling axis is shown here because even though it is a non-CNS-specific mechanism of angiogenesis, it interacts downstream with the CNS-specific WNT7A/WNT7B–GPR124–FZD6–RECK pathway. ANG1, angiopoietin 1; ANG2, angiopoietin 2; SC, stalk cell; TC, tip cell.
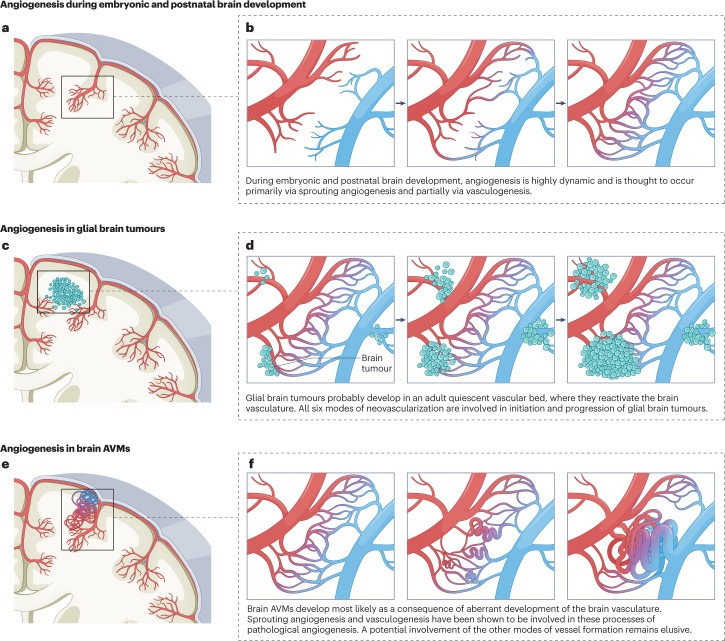
Fig. 4. Angiogenesis during brain development, in glial brain tumours and in brain AVMs.
a,b, Angiogenesis during embryonic and postnatal brain development is initiated by bone marrow-derived de novo vasculogenesis followed by sprouting angiogenesis with the formation and elongation of new vessel sprouts from pre-existing vessels. Newly formed vessels fuse with other vascular sprouts in a process called ‘anastomosis’, thereby forming a healthy capillary bed within a three-dimensional network of perfused, functional vasculature. c,d, Glial brain tumours develop in a vascular bed where they reactivate the surrounding quiescent brain vasculature but also form their own blood vessels within the tumour mass. All six modes of neovascularization are active in glial brain tumours. e,f, Brain arteriovenous malformations (AVMs) develop as a consequence of aberrant vascular development of a healthy capillary bed in which the initial formation of arteriovenous shunts leads to further progression towards brain AVMs. Sprouting angiogenesis and bone marrow-derived vasculogenesis (in the AVM nidus) play an important role during the initiation and progression of brain AVMs.
NVL molecules
Both the vascular system and the nervous system require coordinated guidance of their cellular and subcellular elements9,15,61. At the cellular level, axonal growth cones and ETCs exhibit similar lamellipodia and filopodia9,12,16,18,64 (Fig. 2a–c). At the subcellular level, axonal growth cones consist of a central domain containing microtubules and a peripheral domain composed of an actin meshwork (in lamellipodia) and F-actin bundles (in filopodia)9. Fan-like filopodial protrusions sense stimulatory and inhibitory guidance signals in the microenvironment and steer both the growing axon65,66 and the developing, newly forming blood vessels12,16,18,64,67 (Fig. 2a,b). F-actin structures have been found in ETC filopodia68, but the cytoskeletal organization of tip cells is less well described than that of axonal growth cones, mainly owing to technical limitations and the lack of specific ETC markers. Suggested tip cell markers — such as ESM1, APLN, RAMP3 and CLDN5 — that have emerged from microarray analysis and single-cell RNA sequencing (scRNA-seq) studies69–76 await full validation.
At the molecular level, numerous cues have been discovered that guide both ETCs and axonal growth cones9,12,15,16 (Fig. 2g,h). These cues include the four canonical axon guidance molecule families — netrins, semaphorins, ephrins and Slit proteins9,12,14–16,77 — and other axon guidance molecules, such as WNT proteins, SHH, bone morphogenetic protein (BMP), Nogo-A and Nogo-B, exert similar repulsive and attractive effects on neuronal growth cones78 and ETCs9,14,15,79,80 (Supplementary Table 2). In addition to these neural cues guiding blood vessels, classic angiogenic factors such as VEGFA and FGF2 and their receptors, endothelin 3, artemin and the receptor complex RET–GFRα3 can direct neuronal development and axonal growth during brain development9,14,15,79 (Fig. 2g). The NVL relies on direct cellular interactions between vascular cells and neural cells. For instance, sensory neurons and Schwann cells in the peripheral nervous system provide a template for the patterning of arteries but not veins during skin development, whereas neuronal release of VEGF induces arterial differentiation81. In the CNS, retinal ganglion cells and astrocytes provide a physical template for sprouting ECs while releasing pro-angiogenic and anti-angiogenic factors such as VEGFA, semaphorins and Nogo-A. Conversely, vessel-derived cues such as artemin and endothelin 3 guide growing axons in the retina82,83. Accordingly, ablation of radial glia84, oligodendrocyte precursor cells85 or astroglia86 results in a severe reduction in developmental angiogenesis14. Many of the NVL molecules interact with key downstream angiogenic signalling axes, most notably the VEGF–DLL4–Jagged 1–Notch and YAP–TAZ pathways9.
Angiogenesis and brain development
Embryonic CNS angiogenesis
Cellular mechanisms during embryonic brain development
During brain development in mice at embryonic day 8.5 (E8.5), a perineural vascular plexus (PNVP) (non-CNS tissue of mesodermal origin) forms around the neuroectodermal-derived neural tube via vasculogenesis (Fig. 3a–d and Supplementary Table 1), in which VEGFA derived from the neural tube interacts with VEGFR2 expressed on PNVP angioblasts9,50. This PNVP will later be transformed into arteries and veins of the pia and the arachnoid mater (leptomeninges) ensheathing the CNS tissue87. At E9.5, vessel sprouts from the PNVP invade the CNS parenchyma and form the intraneural vascular plexus (INVP) via sprouting angiogenesis9,54,64,88 (Fig. 3a,b). These perforating vessels of the INVP follow a radial course towards the ventricles. Once they are inside the ventricular zone, they branch in a circumferential fashion parallel to the ependyma, giving rise to a periventricular vascular plexus89 (Fig. 3a,b). Only after this lateral branching at the periventricular level do lateral branches from the INVP sprout at several levels throughout the cortical layers89.
In humans, the pial capillary anastomotic plexus is considered the functional and structural analogue of the PNVP in embryonic mice90. The pial capillary anastomotic plexus is a meningeal layer of extracerebral or non-CNS origin and is the source of all perforating vessels entering the cerebral cortex during later embryonic and postnatal stages67,90. The pial capillary anastomotic plexus is already detectable in 6-week-old human embryos and is separated from the underlying cortical tissue by the brain’s external glial limiting membrane90. Subsequently, pial capillaries perforate the external glial limiting membrane and grow into the cerebral cortex (comparable to the formation of the INVP in mice) from the eighth week of gestation onwards90. Whereas the CNS is, after vasculogenic formation of the PNVP, predominantly vascularized by sprouting angiogenesis27, vascularization of non-CNS tissues mainly relies on vasculogenesis91,92, for reasons that remain elusive.
General molecular mechanisms during embryonic brain development
Various general developmental pathways are active in both the CNS tissue and peripheral tissue, including the following: VEGFA–VEGFR–DLL4–Jagged 1–Notch signalling for appropriate vessel sprouting, patterning and vascular remodelling34,50,93,94 (see earlier herein for a description of this signalling pathway); YAP and TAZ as essential co-transcriptional activators of the Hippo pathway in ECs95; angiopoietins and their receptors TIE1 and TIE2 as modulators of vessel stability96–98; the classic axon guidance ligand–receptor pairs SLIT2–ROBO4 (refs. 99–101), SEMA3E–plexin D1 (ref. 102), netrin 4–UNC5B103 and ephrin B2–EphB4 (ref. 104); and the non-classic axon guidance cues, namely integrin αVβ8-activated TGFβ signalling105, WNT78, BMP78 and SHH78,79 (Supplementary Table 2). Although many of these pathways are active and important in CNS angiogenesis, they were first discovered in peripheral tissues, acting through a general (non-CNS-specific) molecular mode of action.
YAP and TAZ are transcriptional co-activators regulating the Hippo pathway and have crucial roles in organogenesis and embryonic vascular brain development in a non-CNS-specific manner. The VEGF and YAP–TAZ signalling pathways converge: VEGF stimulates Rho family members, thereby altering cytoskeletal dynamics, contributing to the activation of YAP–TAZ signalling106. YAP and TAZ, in turn, upregulate the gene expression of Rho family members, providing actin cytoskeletal rearrangements needed for ETC migration and stalk cell proliferation during embryonic and postnatal vascular brain development95,106.
Angiopoietin 1 (ANG1) and ANG2 bind to the tyrosine kinases TIE1 and TIE2 and directly act on ECs by modulating cell–cell and cell–extracellular matrix (ECM) communication and promoting or inhibiting angiogenesis, which is of crucial importance before E13.5 (refs. 107,108). ANG1 and ANG2 often have complementary roles in the development of a healthy vasculature; they modulate vessel stability and can be either pro-angiogenic or anti-angiogenic depending on the context96,98,108.
Classic axon guidance cue signalling, such as SLIT-dependent activation of the EC-specific receptor ROBO4 inhibits endothelial hyperpermeability induced by pro-angiogenic factors and enhances vascular stability99. ROBO4-mediated SLIT2-dependent suppression of cellular permeability occurs through inhibition of the small GTPases ARF6 and RAC109. In vivo, inhibition of ARF6 resembles ROBO4 activation by reducing pathological angiogenesis and vessel leakage in retinal hyperpermeability models during vascular development inside and outside the CNS99,101,110. The effects of ROBO4 silencing on human brain microvascular EC proliferation, migration and tube formation remain controversial101,110.
SEMA3A is a secreted protein mediating anti-angiogenesis via the NRP1 and plexin A–plexin D1 receptor complex111. The exact role of SEMA3A during developmental CNS angiogenesis is unknown, given the absence of a vascular phenotype in Sema3a−/− embryos112 and in NRP1sema mice113, which express a mutated variant of NRP1 that lacks the SEMA-binding domain. At E10, SEMA3A is expressed in vascular ECs in the spinal cord and dorsal aorta111. Interestingly, at E12.5, SEMA3A expression is stronger on ETCs than on stalk cells during INVP sprouting into the brain parenchyma and retina, indicating that its expressed on actively sprouting endothelium71,114. In zebrafish, Sema3A–plexin D1 signalling negatively regulates angiogenesis through modulation of soluble Flt1 expression115, illustrating the role of Sema3A–plexin D1 during embryonic brain vascularization in a non-CNS-specific manner.
SEMA3E–plexin D1 signalling negatively regulates angiogenesis inside and outside the CNS via interaction with the VEGF–DLL4–Jagged–Notch pathway. Plexin D1 can be detected in mouse embryos as early as E9.5 (refs. 102,116) as well as postnatally (postnatal day 2 to postnatal day 6) in the mouse retina102,116, where plexin D1 is expressed in ETCs and stalk cells but is absent in mature vessels, indicating that it has a role during developmental sprouting angiogenesis117. SEMA3E–plexin D1 signalling leads to downstream activation of the small GTPase RhoJ, with subsequent VEGF-induced DLL4 expression in retinal ETCs in vivo118 and in human umbilical vein ECs in vitro, contributing to the ETC and stalk cell selection in both the CNS vasculature and the non-CNS vasculature117. Whether SEMA3A–plexin D1 signalling or SEMA3E–plexin D1 signalling regulates PNVP and INVP formation during embryonic human CNS development remains to be explored.
Netrin 1 and netrin 4 are anti-angiogenic factors that act through binding to UNC5B (in the case of netrin 1) or to neogenin with recruitment of UNC5B (in the case of netrin 4) in peripheral tissues and the CNS in a general (non-CNS-specific) manner119–121. Netrin 1 and netrin 4 and their receptors act as repulsive or attractive cues, partially via regulation of VEGF signalling119, starting during embryonic developmental angiogenesis inside and outside the CNS119,120.
Last, the Eph family of receptor tyrosine kinases interacts with membrane-bound ligands called ‘ephrins’122. Ephrin B2, being the sole transmembrane ligand for EphB4, is specifically expressed in arterial angioblasts starting at around E9 (ref. 123). EC and perivascular mesenchymal cell123 interactions lead to activation of the ephrin B2–EphB4 axis, providing attractive and repulsive guidance cues for EphB-expressing cells in angiogenesis as well as regulation of migratory and invasive cellular functions in a non-CNS-specific way122,123.
Non-classic axon guidance cues such as the five members of the αV integrin subfamily (αVβ1, αVβ3, αVβ5, αVβ6 and αVβ8) are expressed by many different cell types, notably by neurons and ECs of the brain (acting as NVL molecules) but also in other organs and tissues, and bind to RGD peptide motifs present on many shared ECM ligands, most importantly to latent TGFβ proteins124. The αV integrin is of particular interest in genetic studies in mice as it is an important regulator of embryonic cerebrovascular morphogenesis (although the actions of αV integrin are not exclusively CNS specific)125,126. Integrin αVβ8 activates ventral–dorsal TGFβ gradients in the brain, inhibiting EC sprouting and stabilizing blood vessels via downstream TGFβ1–TGFBR2–ALK5–SMAD3 signalling105,125,127,128. Ablation of αV integrin-coding or β8 integrin-coding genes in embryonic brain ECs causes pathological vascular phenotypes, including EC hyperproliferation and intracerebral haemorrhages105,127. In mice, knocking out either of the genes encoding the TGFβ signalling co-receptors — that is, ALK1 (encoded by Acvrl1, also known as Alk1) and endoglin (ENG; encoded by Eng) — causes embryonic lethality at E11.5 (refs. 129,130).
Several axon guidance molecules, including the WNT proteins, SHH and BMP, guide both axonal growth cones78 and ETCs according to the concept of the NVL79 (Fig. 2). The specific effects of NVL molecules on ETC guidance, with the exception of the CNS-specific WNT ligands WNT7A and WNT7B (which are discussed later), are less clear than their roles in axon guidance15.
CNS-specific molecular mechanisms during embryonic brain development
CNS-specific molecular cues that are active in developmental angiogenesis include WNT7A and WNT7B, GPR124 and its co-receptor RECK131–137 with suggested upstream involvement of netrin 1–UNC5B138,139, DR6 and TROY50,140, the norrin–FZD4–LRP5–TSPAN12 complex141–143 and the recently discovered brain EC-specific WNT regulator PPIL4 (ref. 144) (Supplementary Table 2). Even though absolute CNS specificity is nearly impossible to prove, most of the CNS-specific molecular mechanisms that regulate the vasculature were shown to be absent in a number of peripheral tissues.
Endothelial β-catenin signalling is crucial for the establishment and maintenance of a functional BBB during embryonic and postnatal brain development145,146. To activate the β-catenin pathway in a CNS-specific manner, the ligands WNT7A and WNT7B and/or norrin with its co-activator TSPAN12 (in retinal angiogenesis) is produced by glial cells or neurons to activate the co-receptors LRP5 and LRP6 on ECs146. Mutations in the genes encoding β-catenin, norrin, FZD4, LRP5, LRP6 and TSPAN12 can cause inherited defects in retinal vascularization, whereas targeted mutations in the genes encoding WNT7A and WNT7B cause defects in both retinal and brain angiogenesis143. The binding of WNT7A and WNT7B to two membrane proteins expressed on CNS ECs —GPR124 and RECK — specifically enhances intracellular β-catenin signalling and is crucial for proper vessel ingression into the CNS parenchyma and the formation of CNS-specific properties of the INVP131,133–136,147,148. Interestingly, in regions where the barrier function of the BBB is physiologically reduced to monitor serum osmolarity and electrolyte balance — most notably the microvasculature of the circumventricular organs, the choroid plexus and the choriocapillaris and ciliary bodies in the eye — EC WNT–β-catenin signalling is kept at low rates, resulting in strict maintenance of this high-permeability state149,150.
Recent studies showed that EC-specific deletion of the gene encoding the non-CNS-specific receptor UNC5B in mice induces loss of BBB integrity, characterized by reduced CLDN5 levels and increased expression of the permeability protein PLVAP138,139. UNC5B-bound netrin 1 interacts with the CNS-specific WNT7A and WNT7B co-receptor LRP6, leading to downstream activation of the WNT–β-catenin pathway inside but not outside the CNS (for example, there are no effects on the vasculature in the lungs, heart and kidneys). This signalling might be an important CNS-specific downstream mechanism regulating BBB integrity138.
Embryonically, mutations in Gpr124 (also known as Adgra2) or Reck severely impair CNS angiogenesis and barriergenesis133,136,148. Endothelial-specific Gpr124 deletion causes embryonic lethality in mice from E15.5 onwards owing to angiogenic defects in the forebrain and neural tube, whereas Gpr124 overexpression produces CNS-specific hyperproliferative vascular malformations135. This forebrain (but not midbrain or hindbrain) localization pattern suggests that GPR124 mediates EC migration towards regional guidance cues in the embryonic CNS135. Endothelial β-catenin signalling promotes sprouting angiogenesis, ETC formation and VEGFR expression during postnatal brain and retinal vascular development151. Increased β-catenin levels also lead to upregulation of DR6 and TROY, which are required for vascular and BBB development and maintenance in a CNS-specific manner in zebrafish and mice140. ppil4−/− zebrafish exhibited a brain EC-specific phenotype, including necrosis in the dorsal midbrain and embryonic lethality 2 days after fertilization144. Interestingly, PPIL4 exerts brain EC-specific modes of action via a downstream effect on WNT signalling cascades144. Finally, the formation of arteriovenous connections during CNS development is partially mediated by the receptor–ligand pair Cxcr4–Cxcl12b in the CNS but not in the trunk of zebrafish embryos, suggesting it has a CNS-specific nature152.
Postnatal CNS angiogenesis
Cellular angiogenic mechanisms during postnatal brain development
Sprouting angiogenesis continues postnatally and further remodels and expands the CNS vascular network3,9,153 (Fig. 3e,f). Whereas sprouting angiogenesis and ETCs advance in a radial manner during embryonic development88, postnatally, ETCs spread in all directions of the various cortical layers, mostly emanating from the main vessel branches established during brain embryogenesis3,153,154 (Fig. 3e,f).
General angiogenic molecular mechanisms during postnatal brain development
Much less is known about the molecular regulation of brain angiogenesis and vascular patterning postnatally than in the embryonic stage. Many molecules and molecular pathways are probably active during both developmental stages, including the VEGFA–VEGFR–DLL4–Jagged 1–Notch pathway, YAP–TAZ, integrin αVβ8, SEMA3A and SEMA3E, and ephrin B2–EphB4 (ref. 50) (Supplementary Table 2). We identified Nogo-A as a major negative regulator of sprouting angiogenesis, ETCs and vascular network formation in the postnatal brain17, whereas its role during embryonic vascular brain development remains unclear. The vascular receptor for the Nogo-A isoform Nogo-B, NgBR155, regulates both embryonic and postnatal brain angiogenesis9,156–159. NgBR knockdown in zebrafish models stopped Nogo-B-stimulated EC migration and reduced VEGF-induced phosphorylation of AKT and EC morphogenesis in a general (non-CNS-specific) manner9,156.
CNS-specific angiogenic molecular mechanisms during postnatal brain development
Similarly to observations made during the embryonic stage, postnatal deletion of Gpr124, Reck or Ndp (which encodes norrin) compromises angiogenesis and BBB integrity in a CNS-specific manner133,136. Whereas most of the CNS-specific mechanisms regulating vascular brain development at the embryonic stage also regulate postnatal brain angiogenesis and barriergenesis, little is known about the molecular mechanisms that regulate CNS vascular development solely at the postnatal stage (Supplementary Table 2).
Summary
In conclusion, during both embryonic and postnatal brain development, sprouting angiogenesis is highly active and vascular sprouts led by ETC filopodia invade the CNS tissue to establish a functional vascular network. Molecular pathways regulating developmental brain angiogenesis in a general or CNS-specific way are increasingly being discovered, but our knowledge of these molecular processes and their interactions with the VEGF–VEGFR–DLL4–Jagged–Notch pathway and the Hippo–YAP–TAZ pathway remains incomplete9,50 (Supplementary Table 2). In the adult human brain vasculature, most of the aforementioned developmental pathways are downregulated, keeping the vasculature in a quiescent homeostatic state9,61,160,161 (Fig. 3g–j).
Angiogenesis in brain tumours
In contrast to the healthy adult quiescent vasculature, brain tumours are characterized by aberrant angiogenesis and alterations to the BBB61,162, to CNS specificity and to arteriovenous specification of ECs24, but to what extent developmental signalling axes are reactivated in brain tumours remains poorly understood. Here we focus on intra-axial glial brain tumours, which are a classic example of highly angiogenic brain tumours characterized by the crucial role of their vasculature and aberrant capillary beds in disease initiation and progression163–166.
Glial brain tumours
Vascular proliferation is an important pathological hallmark of glioblastomas (high-grade gliomas), which have one of the most extensive vascular systems among all solid tumours and vascular proliferation is an important pathological hallmark164–166. However, targeting glioma vascularization using an anti-VEGF therapy167, a combined anti-FGF–anti-VEGF therapy168 or other approaches has resulted in disappointing results166,169–171, probably owing to an incomplete understanding of the cellular and molecular mechanisms regulating angiogenesis and the NVU and PVN in glial brain tumours.
Modes of neovascularization
In glial brain tumours, all six mechanisms of neovascularization have been characterized23–26,172 (Figs. 1, 4c,d and 5 and Supplementary Table 1).
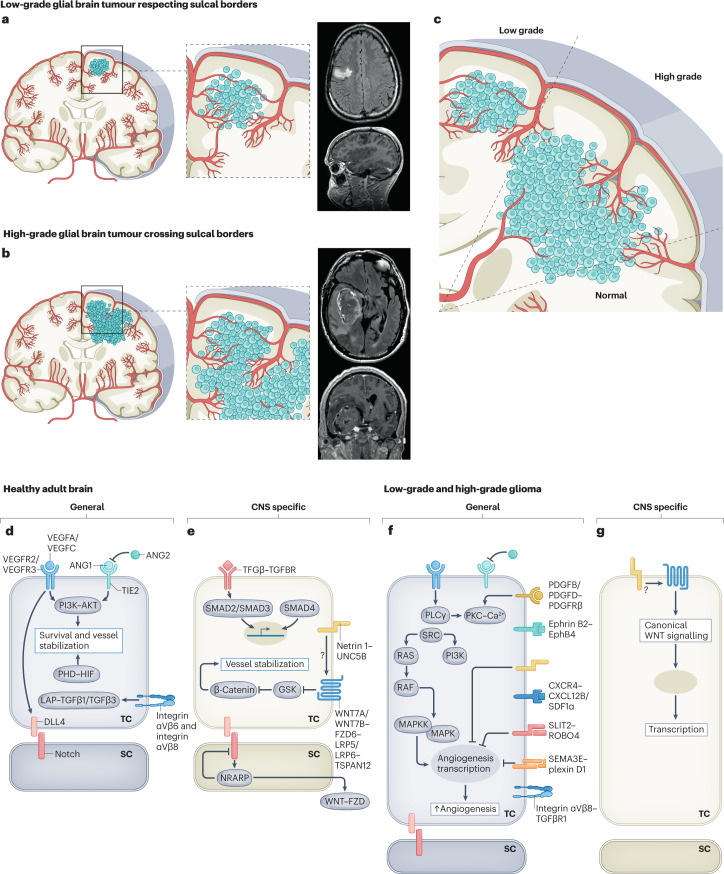
Fig. 5. Molecular mechanisms regulating the vasculature during initiation and progression of glial brain tumours.
Figure illustrating the hypothetical concept postulating the gyral confinement and respect of sulcal borders during progression from low-grade glial brain tumours to high grade glial brain tumours based on radiological observations and the concept of sprouting angiogenesis and recruitment of blood vessels from the adjacent brain parenchyma. a–c, Cross sections of the adult human brain in the coronal plane showing pathological angiogenesis in glial brain tumours. Illustrations and T1-weighted coronal and sagittal MRI scans with gadolinium show that low-grade gliomas are often confined to one gyrus, thereby respecting sulcal borders (parts a,c). Illustrations and T1-weighted coronal and sagittal MRI scans with gadolinium show that invasive high-grade gliomas do often not respect gyral confinement and cross sulcal borders (parts b,c). d,e, Molecularly, numerous signalling pathways have been implicated in the adult healthy brain, regulating endothelial cell quiescence, survival and maintained inhibition of paracellular permeability. Molecular cues can be either non-CNS specific (part d) or CNS specific (part e). These signalling pathways are thought to be of importance during both embryonic and postnatal vascular brain development, as well as to contribute to the maintenance of the quiescent healthy adult brain vasculature. f,g, Molecularly, different non-CNS-specific and CNS-specific angiogenic molecular mechanisms have been implicated in glioma initiation and progression, and they include the reactivation of developmentally active ligand–receptor pairs. ANG1, angiopoietin 1; ANG2, angiopoietin 2; SC, stalk cell; TC, tip cell. Images in parts a,b courtesy of P. Nicholson.
Vascular co-option
Chronologically, the first mode of neovascularization in glial tumours is vascular co-option, involving the organization of tumour cells into perivascular cuffs around microvessels of the surrounding healthy brain tissue to form an early, initially well vascularized tumour mass25 (Figs. 1d and 4c,d and Supplementary Table 1). This process mostly occurs in highly vascularized tissues but may also occur in malignancies both inside and outside the CNS, including liver cancer173, lung tumours174, breast-to-brain metastases175 and glial brain tumours176, as well as in tumour recurrence and metastatic growth following administration of anti-angiogenic therapies in glioblastoma13,176 (Figs. 1d,h and 4c,d and Supplementary Table 1).
At the cellular level, cytoplasmic extensions of glioblastoma cells termed ‘flectopodia’ modify the normal contractile activity of pericytes surrounding pre-existing vessels, resulting in co-option of these blood vessels, thereby illustrating cellular interactions within the tumour NVU and PVN177. Molecularly, inhibition of the small GTPase CDC42, a principal regulator of cell polarity and actin cytoskeletal organization, impairs vessel co-option, thereby favouring an innate immune response against the tumour177. Co-opted vessels do not undergo sprouting angiogenesis as a direct next step but first regress via disruption of EC interactions and proteolysis of the basement membrane and ECM, mediated by expression of ANG2 (ref. 178) (Supplementary Table 1). ANG2 is expressed by ECs in co-opted vessels at an early stage and appears to counter the constitutive expression of ANG1 in healthy tissues. ANG2 is upregulated through HIF1α-dependent mechanisms and contributes to the formation of the leaky, tortuous and dysfunctional vessel characteristics of glioblastoma179. Other molecular players in vascular co-option include bradykinin, EGFRvIII180, MDGI181 and ephrin B2 (ref. 182). Ultimately, the remaining tumour is rescued by sprouting angiogenesis at the tumour borders25,39,182 (discussed later). To date, no CNS-specific mechanisms regulating vascular co-option in glial tumours have been identified.
Sprouting angiogenesis
Glioma-associated sprouting angiogenesis begins after ANG1-mediated and ANG2-mediated breakdown of existing, co-opted vessels. In the presence of ANG2, VEGF promotes EC migration and proliferation and stimulates sprouting of pre-existing blood vessels29. Under hypoxic conditions characterized by high HIF1α expression, VEGF ligands and receptors are upregulated and VEGFA binds VEGFR2 and VEGFR3, resulting in MAPK (ERK)-dependent upregulation of VEGF signalling in gliomas64. DLL4 inhibition leads to non-productive angiogenesis with aberrantly high ETC and filopodia numbers and suppression of tumour growth in glioma models, whereas prolonged complete inhibition of DLL4 resulted in highly vascular tumours with a haemangioblastoma phenotype, illustrating this carefully balanced mechanism183 (Figs. 1a,h, 4c,d and 5f,g and Supplementary Tables 1 and 2). Stabilization of the newly formed capillaries requires interactions between ECs, PVCs and ECM components184–187. For instance, during vessel lumen formation, pericytes are recruited towards the newly formed vessels in response to platelet-derived growth factor (PDGF) and matrix metalloproteinase upregulation in activated glioma ECs to stabilize the vascular sprout53,185,187,188.
Bone marrow-derived vasculogenesis
Vasculogenesis is important in tumour biology, and involves the differentiation of three types of circulating bone marrow-derived cells: most importantly, EPCs and pericyte progenitor cells25, and the less well characterized CD45+ vascular modulatory cells189 (Figs. 1b,h and 4c,d and Supplementary Table 1). Multiple studies showed that impaired recruitment of EPCs interferes with tumour progression in human gliomas190,191. EPCs, defined by the expression of progenitor markers (CD34 and CD133) and EC markers (CD31 and VEGFR2) regulate angiogenesis-mediated tumour progression indirectly via paracrine secretion of pro-angiogenic growth factors192 and by direct luminal incorporation into nascent sprouting vessels81,193.
In a transgenic mouse model of liver carcinogenesis, CCR2+ and CCR5+ EPCs were incorporated into the tumour vasculature191. Glioblastoma recruits CXCR4+ EPCs in the process of bone marrow-derived vasculogenesis through activity of HIF1α and its target SDF1α194. Bone marrow-derived vasculogenesis is important in glioblastoma resistance to initial chemoradiotherapy and pharmacological VEGF inhibition195, and clinical trials targeting inhibition of the SDF–CXCR4–CXCR7 axis combined with anti-VEGF therapy in glioblastoma are ongoing196. Clinically, the number of EPCs in peripheral blood of patients correlates with glioblastoma blood vessel density and angiogenic activity and might serve as a biomarker for the identification of patients who may benefit from anti-angiogenic therapy197. The contribution of pericyte progenitor cells to pathological glioblastoma angiogenesis is a matter of debate, given that the pericyte progenitor cell population varies dramatically depending on the stage of disease and that glioblastoma shows a relatively low pericyte coverage of 10–20% (with substantial interpatient variability), compared with 67% in mammary carcinomas and 65% in colon carcinomas198.
Molecularly, EPC migration and proliferation are regulated by VEGFA–VEGFR2–VEGFR3–MAPK signalling, with VEGFR2 and VEGFR3 being expressed on EPCs199, whereas EPC homing is regulated by key angiogenic chemokines (CXCL1, CXCL7, CXCL12 and CCL2), their respective receptors (CXCR2, CXCR4 and CCR2) and the TGFβ–SDF1α–CXCL12 axis200. CNS-specific molecular mechanisms involved in vasculogenesis remain to be discovered.
Intussusception
Intussusceptive angiogenesis has been characterized in several cancers39, including glioblastoma47. Nico et al. detected a number of connections of intraluminal tissue folds with the opposite vessel walls (corresponding to a key step in the process of intussusception (Fig. 1c)), thereby suggesting the existence of this mode of neovascularization in human glioblastoma47. The relevance of intussusception to human brain development and brain disease remains unknown, as do its underlying molecular mechanisms and whether it displays a CNS-specific or general mode of action.
Glioma stem cell to EC and glioma stem cell to pericyte transdifferentiation
Located in the glioblastoma PVN, GSCs are closely associated with microvascular ECs, and studies have proposed that soluble factors secreted by ECs — including VEGFA201, IL-8 (ref. 202), SHH203 and CD9 (ref. 204) — and adhesive connections between ECs and GSCs control the fate and survival of GSCs, thereby affecting the aggressiveness of glioblastoma (Figs. 1e,h and 4c,d and Supplementary Table 1). A subpopulation of glioblastoma-derived ECs harbours the same somatic mutations (for example, mutation in the gene encoding EGFRvIII and chromosome 7 amplification) as GSCs, indicating that a notable portion of the vascular endothelium has a neoplastic origin and GSCs can transdifferentiate into functional ECs, thereby contributing to tumour vascularization20,21,205. Recently, the P4HA1–COL6A1 axis was identified as a modulator of GSC-to-EC transdifferentiation206. Additional candidate modulators of this process include ETV2, a master regulator of EC development, and the transcription regulator TWIST1, and their expression positively correlates with malignancy grade207,208.
Mechanistically, treatment with the chemotherapeutic drug temozolomide increases the expression of GSC-specific markers in glioblastoma ECs and induces the transdifferentiation of GSCs to glioblastoma ECs, thus identifying chemotherapeutic stress as a driver of this mode of neovascularization209. Ionizing radiation has also been shown to initiate GSC-to-EC transdifferentiation through the previously described TIE2 pathway210,211. Interestingly, GSCs can also give rise to tumour pericytes supporting vessel function and tumourigenesis22. In vivo cell lineage tracing in a glioblastoma xenograft model demonstrated that GSCs generate the majority of glioblastoma pericytes (predominantly via TGFβ signalling) and revealed that selective cell arrest of GSC-derived pericytes led to vessel wall disruption in vivo22. Transdifferentiation of GSCs to pericytes along with stem cell plasticity and angiogenic properties of GSCs are regulated predominantly by the NOTCH1 pathway in hypoxic conditions212. The observation that GSC-derived pericytes bear tumour-specific genetic alterations distinguishing them molecularly from normal pericytes (for example, mutations in the gene encoding EGFRvIII, chromosome 7 amplification, or PTEN or chromosome 10 deletion) provides possibilities to specifically target these tumour-derived pericytes22.
Clinically, pericyte coverage of tumour vasculature inversely correlates with response to chemotherapy and survival in individuals with glioblastoma, suggesting that pericytes with a neoplastic origin in glioblastoma may regulate the brain tumour barrier, which impacts the efficiency of drug delivery213. Tumour vascular endothelium and GSC-derived pericytes have been suggested as novel targets for anti-angiogenic therapy165,166,214. Cancer stem cell to EC or pericyte transdifferentiation is a non-CNS-specific process that has been described in non-CNS tumours215.
Vasculogenic mimicry
‘Vasculogenic mimicry’ (VM) refers to the ability of tumour cells to form functional vessel-like networks216,217 (Figs. 1f,h and 4c,d). Tumour cells lining these erythrocyte-containing ‘vascular’ channels, which are devoid of ECs, continue to express tumour cell markers. First identified in melanomas216, this mode of neovascularization has been reported in various cancers inside and outside the CNS218–220 and in glial brain tumours221.
Molecularly, hypoxia promotes VM through expression of VE-cadherin (also known as CD144) on tumour ECs and tumour cells222. In glioblastoma, tumour cells lining the vasculature display an undifferentiated embryonic-like biological and molecular phenotype, suggesting the involvement of GSCs and reactivation of neurodevelopmental signalling programmes223. Several molecules and ligand–receptor pairs associated with anaplastic properties of these GSCs are associated with VM formation, including TGFβ, Nodal, EphE2 and VE-cadherin224. The incidence of VM was markedly higher in high-grade gliomas than in lower-grade gliomas225. Overall survival was notably lower and microvascular density was higher in people with VM-positive high-grade gliomas than in individuals with VM-negative high-grade gliomas, indicating a notable contribution of VM channels to glioma blood supply225. IGFBP2 (ref. 226), leptin receptor ObR227, the RNA-binding protein ZRANB2 (ref. 228) and several specific long non-coding RNAs229 and microRNAs230 stimulate VM, whereas histone deacetylase inhibitors impair the process of VM in human glioblastoma231. CNS-specific mechanisms of VM have not been discovered to date.
Developmental pathways in glial tumours
General molecular mechanisms reactivated in glial brain tumour angiogenesis
Typical examples of developmentally active general mechanisms that are reactivated in pathological glial brain tumorigenesis include VEGF–VEGFR, DLL4–Jagged–Notch, YAP–TAZ, PDGF–PDGFR, SLIT2–ROBO4, semaphorin–plexin, semaphorin–neuropilin, ANG2–TIE1, ANG2–TIE2 and ephrin B2–EphB4 signalling (Fig. 5f and Supplementary Table 2). An increase in VEGFA expression has been associated with an increase in glioma malignancy and poor prognosis232. A frequent hallmark of glioma-associated angiogenesis is the activation of the developmentally active RTK signalling pathways233, most commonly caused by amplifications of, mutations in or overexpression of EGFR in GSCs and ECs or pericytes234, contributing to sprouting angiogenesis and stem cell to EC transdifferentiation or stem cell to pericyte transdifferentiation233. Mutations in EGFR, in particular mutations encoding the EGFRvIII variant, lead to ligand-independent and constitutive activation of the EGFR signalling pathway235. This prolonged activation leads to tumour progression and stimulation of angiogenesis via secretion of proteases, which degrade the ECM and enable ECs to proliferate in the surrounding matrix via upregulation of unidentified pro-angiogenic molecules235.
The Notch pathway is linked to several glioblastoma-specific responses to hypoxia, angiogenesis and tumour growth183,236,237. Combined targeting of EGFR signalling and Notch signalling results in decreased cell viability and EC sprouting compared with use of either of the monotherapies, supporting an important role of Notch–EGFR signalling crosstalk in glioblastoma238. However, inhibition of both the EGFR signalling pathway and the Notch signalling pathway is not sufficient to fully stop EC sprouting in human glioblastoma cell cultures, despite almost complete inhibition of VEGF secretion upon combined treatment, suggesting that VEGF-independent pro-angiogenic factors contribute to sprouting angiogenesis238. Indeed, VEGF-independent YAP–TAZ upregulation was observed in glioblastoma on both glial tumour cells and tumour-associated ECs, and this correlated with malignancy grade95,239.
PDGFs, which have several critical roles in physiological embryonic development, are also known to have an important role in sprouting angiogenesis in human glial brain tumours240,241. Five different PDGF isoforms (PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC and PDGF-DD) activate cellular responses through two different receptors (PDGFRα and PDGFRβ; the latter is mainly involved in tumour ECs)242. PDGF-mediated endothelial-to-mesenchymal transition induces EC resistance to anti-angiogenic therapies that target VEGF pathways by downregulating VEGFR2 expression in ECs that were isolated from human glioblastoma samples241.
Among the reactivated general molecular mechanisms regulating glial brain tumour vasculature, signalling by the classic axon guidance cue ephrin B2–EphB4 regulates ETC guidance in brain tumour angiogenesis, and ephrin B2–EphB4 expression is associated with accelerated glioma progression and a worse clinical prognosis in patients with glioblastoma123,243. Sawamiphak et al. found a reduction of tumour volume of up to 25% in an intracranial glioma model in ephrin B2-deficient mice104. Furthermore, ephrin B2 activation in ETC filopodia regulates VEGFR2 internalization, which is required for downstream signalling and VEGF-induced tip cell filopodial extension and sprouting angiogenesis104. Additionally, in a glioblastoma EphB4 overexpression model, reactivation of this developmentally active ephrin B2–EphB4 receptor–ligand pair in glial brain tumours and subsequent overexpression of EphB4 leads to a stabilization of pericyte–EC interactions, intact pericyte coverage and cellular proliferation, all hallmarks of anti-angiogenic therapy-resistant tumour vessels244.
Active during physiological embryonic and postnatal vascular development, TIE1-bound ANG2 and TIE2-bound ANG2 were also detected in tumour cells and ECs in high-grade gliomas (they are present at negligible levels in low-grade gliomas)245,246. Reactivation of TIE receptor signalling during ectopic overexpression of ANG2 in glioblastoma accelerates tumour progression and compromises the benefits of anti-VEGFR treatment in murine glioblastoma models247. Dual inhibition of ANG2 and VEGF receptors normalizes tumour vasculature and prolongs survival in glioblastoma models247.
SLIT2–ROBO4 signalling constitutes another classic axon guidance cue regulating vascular development99,248. ROBO4 is markedly downregulated in ECs cultured in glioma-conditioned medium, and binding of SLIT2 to ROBO4 suppresses glioma-induced EC proliferation, migration and tube formation in vitro by inhibiting VEGFR signalling249.
Among the five members of the αV integrin subfamily, αVβ8 — expressed in neurons, ECs and PVCs — is of particular interest as an important regulator of angiogenesis in the developing brain125,126. In mosaic mouse models of astrocytoma, xenografts and cell culture systems of human glioblastoma, αVβ8 integrin-activated TGFβ proteins suppress pathological angiogenesis and differentially regulate glioblastoma (vessel) growth via autocrine activation of TGFβ signalling pathways250.
Other classic axon guidance cues such as netrin 1 and semaphorins (for example, SEMA3D, SEMA3E, SEMA3F and SEMA4D) play important roles in glioblastoma tumorigenesis and progression by affecting infiltration patterns and the aggressiveness of GSCs251–253.
Recently, we identified nucleolin, a neurodevelopmental regulator of angiogenesis in the human fetal brain vasculature, as a reactivated, positive regulator of sprouting angiogenesis in glioblastoma254. In our own scRNA-seq dataset, we have identified various reactivated fetal signalling pathways in human low-grade and high-grade glioma or glioblastoma with a general (non-CNS-specific) mode of action, including, cell–ECM interaction-related and cell–cell interaction-related signalling pathways, as well as WNT, BRAF, Notch, VEGF–VEGFR1 and VEGF–VEGFR2, IL-8–CXCR1, PI3K–AKT, PDGF–PDGFR, Hedgehog, angiopoietin–TIE1, angiopoietin–TIE2, ephrin and integrin signalling cascades163.
CNS-specific molecular mechanisms reactivated in glial brain tumour angiogenesis
Only a few studies have been published to date relating to the CNS-specific regulation of angiogenesis in primary glial brain tumours12,132 (Fig. 5g and Supplementary Table 2). WNT7A/WNT7B–β-catenin signalling, regulating embryonic and postnatal developmental angiogenesis in a CNS-specific manner via the co-activator GPR124, also regulates pathological angiogenesis in mouse models of glioblastoma and ischaemic stroke132,145,146. Mice in which Gpr124 was conditionally knocked out in ECs (Gpr124-CKO mice) exhibited decreased vessel density and increased loss of CNS microvascular integrity, measured by BBB leakage, compared with heterozygous control animals in both the model of stroke255 and the model of glioblastoma132. To investigate whether GPR124 functions via downstream WNT–β-catenin signalling to regulate BBB function, primary cultured brain ECs from adult Gpr124-CKO mice and the Gpr124-heterozygous control group were transduced with Wnt7b-expressing adenovirus. Upregulation of WNT7B signalling resulted in increased BBB integrity in glioblastoma by positively regulating tight junction proteins, pericyte coverage and cell–ECM interactions in the ECs from adult global Gpr124-heterozygous mice but not in those from Gpr124-CKO mice132, indicating a crucial role for WNT7A/WNT7B–GPR124–RECK–FZD–LRP signalling in brain tumour BBB integrity and identifying this molecular signalling pathway as a possible therapeutic CNS-specific target in glioblastoma132,256. More recently, engineered WNT7A ligands were shown to enable BBB repair in mouse models of stroke and glioblastoma by selectively binding the WNT7A/WNT7B-specific GPR124–RECK co-receptor complex, thereby acting as BBB-specific WNT activators to induce WNT signalling257. It remains to be determined whether WNT–GPR124 signalling also affects pathological vascularization in non-CNS tumours or whether this signalling axis keeps its developmental CNS specificity in vascular-dependent CNS pathologies such as brain AVMs.
Other regulators of developmental brain angiogenesis such as norrin, DR6 and TROY have been reported to have effects in brain tumours such as medulloblastoma (mainly on neuronal migration, not on angiogenesis)258,259, but their potential regulatory roles in angiogenesis in glial brain tumours and other non-CNS tumours remain to be investigated. Similarly, in light of the recently identified CNS-specific UNC5B–netrin 1-mediated interaction with LRP6 (ref. 138), it would be interesting to see whether intravenous injection of netrin 1 could increase WNT–β-catenin signalling in the BBB and repair CNS endothelial barrier breakdown in glial brain tumours.
From the findings taken together, reactivation of the VEGF–VEGFR–DLL4–Jagged–Notch signalling axis, along with the YAP–TAZ pathway, is of crucial importance in the initiation and progression of angiogenesis in glial brain tumours. Many of the discussed classic axon guidance cues of the NVL are reactivated in glial brain tumours in a general way. Besides possible involvement of netrin 1 and semaphorins in glioblastoma vascularization252, the role of additional classic and non-classic axon guidance cues and CNS-specific cues in this process remains to be explored.
Molecular mechanisms in glial brain tumour vasculature at the single-cell level
scRNA-seq is a powerful approach to study brain tumour (including low-grade and high-grade glioma) biology260–264. Single-cell techniques enable the study of genetic heterogeneity265,266, developmental cellular lineages and hierarchies, and stem cell programmes261,262,264,267, as well as the investigation of the various cell types in the tumour microenvironment266. Until recently, however, single-cell sequencing had not been applied to the study of the glioma vasculature. Xie and colleagues used scRNA-seq to study freshly isolated ECs from human glioblastoma tissues, gaining molecular insight into the heterogeneity of the human BBB and the pathological neovascularization in glioblastoma265. They identified distinct EC clusters that represent different states of angiogenesis and EC activation and impairment of the BBB in both the tumour centre and the tumour periphery, thereby highlighting the importance of different regions within the tumour with regard to the tumour vasculature.
To address the molecular heterogeneity of brain ECs (and PVCs) across development and disease, we recently created the first large-scale single-cell molecular atlas of the developing fetal, healthy adult and diseased human brain vasculature, focusing on brain vascular malformations and brain tumours, including AVMs and low-grade and high-grade gliomas163. We performed scRNA-seq on approximately 600,000 freshly isolated ECs and PVCs from 47 fetuses and adult patients163. This unprecedented insight into EC and PVC heterogeneity and functional specialization of the human brain vasculature in development, health and disease at the single-cell level revealed alterations in arteriovenous differentiation and CNS-specific properties, upregulation of major histocompatibility complex class II molecules and a central role for ECs in the brain NVU in pathological ECs across different brain diseases, including brain tumours and brain vascular malformations. Notably, we observed a marked increase in the angiogenic capillary EC cluster in glioblastoma (and lung cancer brain metastases) and to a lesser extent in lower-grade gliomas as compared with the adult control brain, indicative of the angiogenic nature of lower-grade and especially high-grade brain tumours. Moreover, these findings unravelled the top differentially regulated pathways (belonging to five major groups, namely angiogenesis-related pathways, development and NVL molecules, cell–cell and cell–ECM interactions, immune-related processes and metabolism) in both fetal and pathological brain ECs as compared with healthy adult brain ECs. Most interestingly, more than half of the differentially regulated pathways in pathological brain ECs also showed differential regulation in fetal brain ECs163. This observation was also made in both low-grade and high-grade gliomas, with the reactivated pathways belonging to the five canonical groups listed above.
In summary, these results showed that, in the human brain, pathological ECs share common hallmarks across various diseases, including brain tumours and brain vascular malformations. Comparison of fetal and pathological ECs also suggested that signalling pathways regulating vascular growth during fetal brain development are silenced in adulthood and subsequently activated again in the vasculature of brain tumours and brain vascular malformations, thereby highlighting the potential importance of developmental pathways in various vascular-dependent brain pathologies. Notably, the observed similarities between fetal and pathological brain ECs at the level of active signalling pathways (for example, reactivated developmental pathways versus persistence of a less differentiated cell type) as well as their functional importance are currently incompletely understood and warrant further investigation.
A developmental look at glial brain tumours
From surgical and neuroradiological observations, glial brain tumours are frequently confined to specific brain regions (Fig. 5), as illustrated by gliomas largely having a gyral or subgyral locatation268,269. Low-grade gliomas (from which many high-grade gliomas arise) are typically confined to a gyrus while respecting pial borders, rarely crossing sulci268,270 (Fig. 5a,c), but the cellular and molecular mechanisms underlying these observations are unknown. In light of compartment-specific embryonic vascular development6,56, it is intriguing to speculate that the restriction of the brain tumour extension within defined gyri might, at least partially, be due to its territorial vascular supply. Interestingly, upon malignant transformation of a low-grade glioma to a high-grade glioma, the tumour mass often spreads on a radial axis, crossing sulci and extending to adjacent gyri270 (Fig. 5b,c).
Strikingly, this brain tumour extension or progression looks comparable to the axis of brain AVM growth towards the ventricle, with infiltration along white matter tracts, such as the corpus callosum and subgyral short association fibres270,271 (Fig. 6). As long as glial tumours are localized within gyri and respect the sulcal borders, their blood supply is thought to be provided by neovessels forming via sprouting angiogenesis from pre-existing arteries running within the sulci271. High-grade gliomas crossing these borders may find ways to break those boundaries and recruit neovessels from adjacent sulci or gyri (for example, via CNS-specific and/or general reactivated NVL molecules or endothelial metabolism cues) via sprouting angiogenesis and other modes of vessel formation (Fig. 1), but this intriguing hypothesis needs further testing.
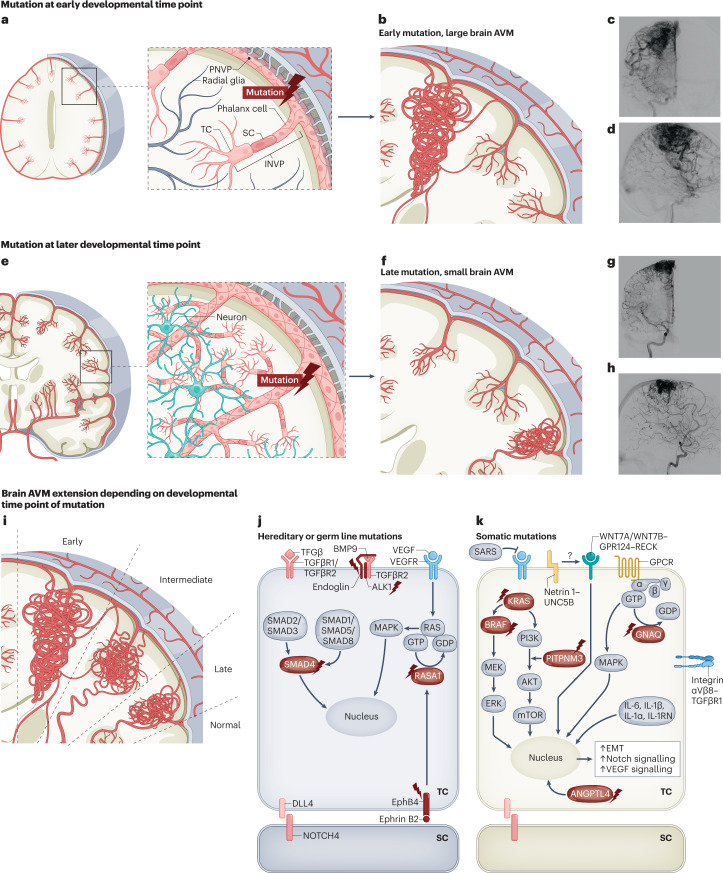
Fig. 6. Molecular mechanisms regulating the vasculature during initiation and progression of brain AVMs.
Figure illustrating the hypothesis stating that the timing of mutation influences the size and location of the arteriovenous malformation (AVM). a,b, Cross section of the human brain in the coronal plane illustrating that mutations occurring in progenitor endothelial cells (ECs) at an early developmental time point will ‘trace’ the future developmental territory of their daughter cells, resulting in a large lesion spreading along a radial axis from the pial cortical surface to the ventricles. c,d, Anterior–posterior (c) and lateral (d) digital subtraction angiography of the right intracarotid artery showing a large AVM. e,f, Cross section of the human brain in the coronal plane illustrating that mutations at later developmental time point result in smaller lesions restricted to a local vascular territory. Note that these smaller AVMs are located around the pial, sulcal and cortical areas or alternatively in the ventricular, ependymal and subependymal zones (that is, choroidal AVMs) but do not occur isolated midway in the white matter without reaching either the cortical surface or the ventricular surface. g,h, Anterior–posterior and lateral digital subtraction angiography of the right intracarotid artery showing a smaller AVM. i, AVM extension as result of early, intermediate and late time points of mutation. j,k, Various molecular pathways have been implicated in AVM initiation and progression. The mutations shown belong to either hereditary or germ line mutations (part j) or somatic mutations in genes in the endothelial tip and stalk cells (part k). The proteins encoded by mutated genes are indicated with a flash symbol. Additional molecules and ligand–receptor pairs involved in regulating the vasculature during initiation and progression of brain AVMs can be found in Supplementary Table 2. BMP9, bone morphogenetic protein 9; EMT, endothelial-to-mesenchymal transition; GPCR, G protein-coupled receptor; INVP, intraneural vascular plexus; SARS, seryl-tRNA synthetase 1; SC, stalk cell; TC, tip cell. Images in parts c,d,g,h courtesy of P. Nicholson.
Angiogenesis in brain AVMs
Brain vascular malformations are characterized by abnormal blood vessel growth and altered maturation of the vessel wall61,162. Here, owing to space limitations, we focus on brain AVMs, which are one of the most commonly encountered brain vascular malformations and are a leading cause of haemorrhage in children and young adults272. Brain AVMs are characterized by aberrant angiogenesis and a malformed capillary bed, thereby representing an exemplar pathology to understand brain vascular biology across arteriovenous zonation273 (Figs. 4e,f and 6). For in-depth discussions of other types of brain vascular malformations, we refer readers to review articles on cerebral cavernous malformations274–276, vein of Galen malformations277 and dural arteriovenous fistulas278.
Brain AVMs
High-pressure arterial blood from feeding arteries shunts directly into the low-pressure outflow veins, rendering brain AVMs prone to rupture273. Regarding their potential developmental origin, brain AVMs so far not been detected in utero (via either ultrasound or MRI techniques). As the same detection methods are capable of detecting similarly sized vein of Galen vascular malformations in utero279, brain AVMs might not develop during embryonic or fetal stages of development. Moreover, the existence of more than ten case reports of de novo formation of brain AVMs in children (for example, they are not present on initial postnatal imaging after trauma but are present on subsequent postnatal imaging280) suggests a postnatal rather than a fetal or embryonic origin.
During normal vascular (brain) development, arteries and veins follow a parallel and countercurrent course without direct communication273. They are separated by capillary networks in the respective tissues, and premature arteriovenous connections are prevented by specific developmentally active molecular control systems (involving, for example, COUP transcription factor 2, NRP2, VEGFR3–FLT4 and EphB4 (refs. 273,281,282)). CNS and peripheral AVMs are thought to occur as a consequence of a failure in these control systems273. Whereas the molecular basis of this aberrant arteriovenous separation leading to AVM formation is unclear, genetic AVM syndromes have provided insight into some crucial signalling pathways that govern arteriovenous patterning273,283–285.
Hereditary brain and peripheral AVMs
Hereditary haemorrhagic telangiectasia
Hereditary haemorrhagic telangiectasia (HHT), or Osler–Weber–Rendu syndrome, is an autosomal dominant disorder characterized by germ line mutations in genes encoding components of the TGFβ signalling pathway27,273,286. As TGFβ is required in embryonic and postnatal development for the establishment and remodelling of the INVP via molecular regulation of EC proliferation, migration and differentiation as well as of pericyte and vSMC recruitment to newly formed blood vessels, it can be considered an important developmentally active signalling cascade that is reactivated in AVMs14,27,124 (Supplementary Table 2).
Mutations in ENG, encoding a TGFβ co-receptor that potentiates TGFβ signalling27,50, cause HHT type 1 (refs. 287,288) (Fig. 6j). Eng−/− mice die at E11.5 owing to defective (both CNS and non-CNS) vascular development, caused by a lack of functional vSMCs and arrested vascular remodelling130. Thus, ENG is required for both CNS and peripheral vasculogenesis and angiogenesis289. Mutations in ALK1, encoding a type 1 TGFβ receptor that stimulates kinase activity290, cause HHT type 2 (ref. 288) (Fig. 6j). Alk1−/− mice die at E11.5 owing to comparable non-CNS-specific vascular defects such as AVMs in the intra-embryonic aortic endothelium, decreased vSMC coverage and disrupted arterial identity129,291. Mutations in SMAD4, encoding a downstream effector of TGFβ signalling290, lead to the combined syndrome of HHT and juvenile polyposis292 (Fig. 6j).
In addition, BMP9 and BMP10, which are important for vascular brain and retinal development293 and vessel normalization in breast cancer294, bind ALK1 with high affinity and induce downstream SMAD signalling, and their genes are mutated in a vascular anomaly syndrome that has phenotypic overlap with HHT295–297 (Fig. 6j). Increasing evidence shows that the BMP9–TGFBR–ENG–ALK1 signalling axis is a developmental (and non-CNS-specific) angiogenic pathway crucially involved in the formation of hereditary AVM syndromes298 (Fig. 6j). Whereas ENG and ALK1 are involved in sprouting angiogenesis during development in a non-CNS-specific manner299, BMP9 and BMP10 are critical for postnatal retinal vascular remodelling and embryonic vascular development inside and outside the CNS293,300.
Differences between mouse models of brain AVMs in adult mice versus developing mice might be due to the dynamic vessel remodelling and highly angiogenic character of the vascular bed during development versus the relatively stable and quiescent nature of the vasculature at the adult stage. Accordingly, in adult mice, regional or tissue-specific CKO of Eng or Alk1 produced AVMs in the lung, brain and gastrointestinal tract but only if angiogenesis was simultaneously stimulated by VEGF301–303. This ‘second hit’ theory304 postulates that a genetic predisposition (the first hit) in combination with an angiogenic trigger (for example, a repetitive injury; the second hit) leads to the reactivation of several developmental angiogenic pathways (for example, the TGFβ pathway)303. As HHT-related mutations involve loss of function in TGFβ pathway-linked genes in ECs but AVMs occur in only certain organs affected by these mutations, it may be that TGFβ haploinsufficiency is not sufficient to initiate a brain AVM in adulthood and requires another somatic mutation (a ‘second hit’) affecting the TGFβ pathway.
Accordingly, whereas in the adult mouse, with a stable or quiescent brain vasculature, this second hit is required to initiate brain AVM formation, in the developing (embryonic or postnatal) mouse, with a dynamic or active brain vasculature, brain AVM formation can occur without a second hit303. In about 15% of patients with clinical features of HHT, no mutations in genes encoding components of the TGFβ pathway are found and the origin of the malformation is unknown305.
Capillary malformation–AVM syndrome
Another hereditary genetic syndrome is capillary malformation–AVM syndrome type 1, caused by heterozygous germ line mutations in RASA1, encoding the cytoplasmic protein RasGAP, a negative regulator of the RAS–MAPK signalling pathway crucial for growth regulation and EC and PVC proliferation in various tissues306–309. RasGAP inactivates RAS by hydrolysing GTP to GDP, thereby negatively regulating the RAS–MAPK signal transduction pathway, with a loss of RasGAP activity resulting in the excessive activation of RAS and downstream signalling pathways295,307,309,310 (Fig. 6j). Mechanistically, RasGAP acts downstream of the endothelial receptor EphB4, a marker of venous endothelial identity and a regulator of developmental and brain tumour angiogenesis, by promoting venous differentiation311. Accordingly, RASA1 mutations result in dysregulation of arteriovenous patterning (with a shift from venous to arterial differentiation) and formation of AVMs inside and outside the CNS310,312. Germ line mutations in EPHB4 have been identified in CM–AVMs that are negative for RASA1 mutations and are therefore categorized as capillary malformation–AVM type 2 (ref. 313).
Sporadic brain and peripheral AVMs
Somatic mutations are increasingly being reported in studies investigating the genetic basis of sporadic (brain) vascular malformations314–317. Many of these mutations are common non-coding single-nucleotide polymorphisms. For example, non-CNS-specific venous malformations are associated with somatic mutations in PIK3CA and TIE2 (refs. 315,316), lymphatic malformations are associated with mutations in PIK3CA318, Sturge–Weber syndrome, capillary malformations and congenital haemangiomas are linked to GNAQ mutations319,320, verrucous venous malformations are linked to MAP3K3 mutations321, extracranial AVMs are associated with MAP2K1 mutations322 and brain AVMs were recently associated with activating somatic mutations in KRAS162,323–325. Other groups studying brain AVMs have reported single-nucleotide polymorphisms located in ALK1 (refs. 326,327), ENG328, IL1B329, ITGB8 (ref. 330), ANGPTL4 (ref. 331), GPR124 (ref. 332), VEGFA333, MMP3 (ref. 334) and MMP9 (ref. 317) (Fig. 6k; see Supplementary Table 2 for additional candidate genes for brain AVM initiation and progression).
Sturge–Weber syndrome is caused by non-hereditary somatic mutations in the protein GNAQ, characterized by port wine stains on the face and leptomeningeal angiomatosis with brain vascular malformations, indicating an underlying general/non-CNS-specific molecular mechanism319. Mutations in GNAQ decrease GTPase activity and increase signalling of associated G proteins, leading to increased MAPK activity319,335 (Fig. 6k and Supplementary Table 2). It remains to be investigated whether genetic risk factors in the context of hereditary AVM syndromes render individuals more susceptible to developing sporadic AVMs.
A key future step in the improvement of the clinical management of brain AVMs would be the development of novel anti-angiogenic therapies336,337, for instance targeting the pathways downstream of KRAS mutations with MEK inhibitors (which are already approved for the treatment of brain tumours338,339) or other targets emanating from single-cell studies75,163,265. For explorations of the future clinical and pharmacological treatment of brain AVMs, we refer readers to recent reviews on this topic336,337.
Developmental pathways in brain AVMs
General molecular mechanisms reactivated in brain AVMs
Interestingly, most of the mutations associated with vascular malformations characterized so far are linked to the RAS–RAF–MAPK and PI3K–PTEN–AKT–mTOR pathways, both of which have pivotal roles in physiological (CNS and non-CNS) vascular development (Fig. 6j,k and Supplementary Table 2). In particular, high-flow AVMs are associated with the latter, as most brain and spinal AVMs have mutations in KRAS162,323–325, whereas low-flow vascular malformations are often associated with activating mutations affecting the PI3K pathway314,316. These observations strongly suggest that the RAS–RAF–MAPK pathway is a central signalling node for the development of AVMs in the brain and spinal cord as well as in non-CNS organs. It remains, however, unclear whether and how the BMP9–TGFβ–SMAD pathway involved in HHT-related AVMs (but also somatic mutations, for example, found in ITGB8) and genes affecting the RAS–RAF–MAPK pathway overlap or interact during normal brain vascular development and (CNS and non-CNS) AVM initiation and progression.
Currently, the downstream effector signalling pathways that are required for AVM development are not well characterized in humans but they are hypothesized to be crucial regulators of arteriovenous specification and zonation273. Several AVM mouse models have elucidated underlying molecular mechanisms driving brain AVM initiation and progression340. In particular, manipulation of the developmentally active DLL4–Jagged–Notch pathway resulted in the development of (CNS and non-CNS) vascular malformations in mice340. Whereas genetic ablation of both Notch1 and Notch4 resulted in embryonic lethality, haploinsufficiency of Dll4 induced AVM-like brain (and non-CNS, including dorsal aorta and cardinal veins) lesions at the embryonic stage that were characterized by the lack of a capillary bed between feeding arteries and draining veins341.
At the postnatal stage, endothelial-specific inducible postnatal expression of constitutively active NOTCH4 induced brain AVMs in mice342, which resulted from the increase in length and calibre, and not the absence, of brain capillaries343. Strikingly, these AVMs were reversible upon normalization of NOTCH4 expression342. vSMCs and ECs in human brain AVMs exhibited upregulated DLL4–Jagged–NOTCH1 signalling compared with healthy cerebral vessels344, indicating that NOTCH1 signalling contributes to the development of human brain AVMs. In arteriovenous differentiation of ECs during development, NOTCH1 and NOTCH4 are major determinants of arterial fate choice, associated with expression of the arterial markers ephrin B2, CXCR4 and connexin 40 (ref. 345). Lack of Notch signalling results in a default phenotype characterized by venous markers such as COUP transcription factor 2, NRP2 and VEGFR3 and the receptor EphB4 (refs. 281,282). Furthermore, activating mutations in RAS–RAF–MAPK pathway genes would result in constitutively active and VEGF-independent activation of the Notch pathway. Indeed, expression of mutant active KRAS in ECs results in overexpression of the Notch pathway and angiogenic cascades downstream of VEGF162 along with endothelial-to-mesenchymal transition. At a cellular level, mutant KRAS induced a migratory phenotype of brain (and peripheral) ECs, loss of tight junctions and disorganization of cytoskeletal actin with intact proliferation162.
A better understanding of the signalling downstream of the RAS–RAF–MAPK and PI3K–PTEN–AKT–mTOR pathways during normal vascular development in CNS and non-CNS tissues and in AVMs may help to develop a more comprehensive picture of arteriovenous morphogenesis. A recent study addressed endothelial aberrancy in brain AVMs at the single-cell level, linking the transcriptional state of ECs isolated from human brain AVMs to a dysregulation of arteriovenous zonation, evidenced by a strong enrichment of arterial and venous transcriptional identity but not of capillary or venule transcriptional identity75. That study further found an upregulation of PLVAP (a marker of fenestrated endothelium75) and the pro-angiogenic protein PGF in the AVM nidus75.
Along those lines, in our own scRNA-seq dataset, we found upregulated PLVAP predominantly in angiogenic capillary ECs of brain AVMs as well as reactivated fetal signalling pathways in human AVMs with a general (non-CNS-specific) mode of action, involving the integrin, TGFβ, angiopoietin–TIE, epithelial-to-mesenchymal transition-related, inflammatory-related and IL4-mediated signalling cascades163.
CNS-specific molecular mechanisms in brain AVMs
Most of the molecules involved in vascular brain development that are reactivated in brain AVMs act via a general (non-CNS-specific) mechanism of action (Fig. 6j,k and Supplementary Table 2). Interestingly, somatic mutations in the gene encoding the CNS-specific angiogenesis regulator GPR124 were identified in human brain AVMs. This finding, however, could not be substantiated in a replication cohort or meta-analysis of individuals with brain AVMs332.
Molecular mechanisms in brain AVM vasculature at the single-cell level
scRNA-seq allows the study of the biology of brain vascular malformations (including brain AVMs) at the single-cell level75,346,347, yielding insights into EC and PVC heterogeneity, their interactions in the blood vessel microenvironment, the intermediate cell types that arise during blood and lymphatic vessel development, and cell type-specific responses to disease347.
Recently, Winkler and colleagues presented a human cerebrovascular cell atlas that compared isolated cells from the adult human brain with cells isolated from resected human brain AVM tissue75. They uncovered a previously unknown heterogeneity in PVCs, revealed transcriptional variation within SMCs and perivascular fibroblasts, and identified SMC-like cells known as fibromyocytes75. In addition to a loss of physiological arteriovenous zonation, which is characteristic of brain AVM pathology, they reported a distinct transcriptomic state in a subset or cluster of ECs relating to heightened angiogenic potential and immunogenicity, indicating that this subset of ECs may originate from the AVM nidus75.
In our molecular single-cell atlas, we found an increase in the number of venous EC clusters in brain AVMs and cavernomas compared with adult control brain tissue163, suggesting an involvement of venous ECs in the pathophysiology of brain vascular malformations, as reported for cavernomas in mice346. Similarly to the situation observed in glial brain tumours, we identified alteration of arteriovenous differentiation and CNS-specific properties, upregulation of major histocompatibility complex class II molecules and reactivated developmental pathways in brain AVMs (although these were less numerous than those in brain tumours) belonging to the aforementioned five major groups of pathways163, indicating some common mechanisms across brain tumours and brain vascular malformations163.
Although shared signalling pathways seem to regulate vascular growth in brain pathologies (including brain tumours and brain vascular malformations) and in the fetal brain, it remains to be clarified whether the pathways observed in brain pathologies are reactivated developmental pathways or rather reflect the persistence (for example, the presence since development) of a less differentiated cell type (or even a combination of these two). Moreover, the functional relevance of these developmental pathways in vascular-dependent brain pathologies is not clear, and further studies will be needed to elucidate their translational potential in terms of developing therapies targeting the vasculature in brain tumours and brain vascular malformations. Single-cell atlases such as those discussed above will inform such endeavours347.
A developmental look at brain AVMs
On the basis of neuroradiological and surgical observations, most brain AVMs occupy a defined segment of the brain’s vascular tree and do not grow after diagnosis273,348,349. It is currently thought that AVMs develop during early postnatal life, at highly active developmental stages, as mentioned earlier herein (Fig. 6a–i). Postnatal development of brain AVMs is supported by the lack of cases reported in utero (which indicates an embryonic AVM development). However, this does not exclude the possibility that somatic mutations and brain AVM initiation occur during embryonic development but remain undetectable until later stages of postnatal life. KRAS mutations seem restricted to the endothelium in brain AVMs, suggesting that somatic mutations occurring in progenitor ECs will ‘trace’ the future developmental territory (for example, the vascular network field) of their daughter ECs. Accordingly, large brain AVMs would result from somatic mutations occurring early in development (spanning larger vascular territories) (Fig. 6a–d), whereas small AVMs may reflect later mutations spanning a restricted vascular territory (Fig. 6e–h). Strikingly, many brain AVMs spread preferentially along a radial axis extending from the ventricles to the pial cortical surface. When small, they can be constrained in and around the pial, sulcal and cortical areas or alternatively in the ventricular, ependymal and subependymal zones (for example, choroidal AVMs), but they do not occur isolated midway in the white matter without reaching either the cortical surface or the ventricular surface (Fig. 6a–i). These observations prompt a comparison with the radial ventriculocortical axis of the radial glia and cortical neuron migration as well as of sprouting angiogenesis during embryonic and postnatal brain vascular development and maturation (Fig. 3). Could somatic mutations in EC progenitors actually be genetic tracers of the migrating and dividing EC progenitors recruited in sprouting angiogenesis and could brain AVMs, consequently, be an aberrant, dysmorphic and oversized capillary network occupying a developmentally defined vascular zone? This tempting but speculative hypothesis may clarify the temporo-spatial organization of sprouting angiogenesis in the developing CNS vascular network and developmental morphogenesis of brain AVMs.
Perspectives and conclusion
Several outstanding questions exist regarding the cellular and molecular mechanisms and the EC and PVC heterogeneity that underlie the brain vasculature during brain development, in the adult healthy brain and in vascular-dependent CNS pathologies and the shared angiogenic pathways between brain development and pathologies. First, how do CNS-specific and general cues interact molecularly to govern CNS angiogenesis during embryological and postnatal brain development and in vascular-dependent CNS pathologies? The CNS-specific cues that are known to regulate developmental angiogenesis show striking region specificity (for example, between the hindbrain and the forebrain)133,135,136,148. Moreover, brain region-specific intrinsic transcription factors were shown to govern embryonic brain angiogenesis in a spatially regulated manner6. These are interesting observations that lead to the question of whether region-specific vascular growth might be linked to region-specific brain function during development and in disease. Furthermore, both glial brain tumours and brain AVMs are most often confined to specific brain regions, but whether CNS-specific and region-specific regulators6 of angiogenesis participate in the molecular mechanisms underlying these observations, suggestive of another link between the developmental brain vasculature and the pathological brain vasculature, remains unknown.
All currently known molecules and signalling pathways underlying hereditary AVM syndromes and sporadic brain AVMs characterized by somatic mutations are non-CNS-specific regulators of angiogenesis162,287,288,323,325,328 (although the CNS-specific signalling receptor GPR124 is expressed in brain AVM ECs332, a functional role for it in brain AVM has not been established to date). The lack of CNS specificity in this signalling is in line with the fact that multiple organs are affected by AVMs in these syndromes. Regarding sporadic AVMs, KRAS and BRAF mutations in ECs cause brain and spinal cord AVMs (peripheral AVMs were not reported)162,323,325, whereas RAS and MAPK variants cause sporadic brain AVMs and skin vascular malformations350, indicating specificity for neuroectodermal-derived tissues. Given the highly specialized vasculature of the CNS9 and the observed alteration of the CNS-specific gene profile in pathological brain ECs (for example, pathological brain ECs partially acquiring a gene profile that is characteristic of peripheral or non-CNS ECs)163, we think that the role of CNS-specific and general regulators of angiogenesis in brain tumours, brain AVMs and other CNS pathologies warrants further investigation. For instance, conducting single-cell multi-omics studies of the vasculature in different compartments of the developing brain as well as of brain region-confined pathologies (for example, brain tumours in defined gyri, for instance superior temporal lobe glioblastoma351) will be an important step forward to elucidate these very exciting concepts.
The second question is how different or comparable are the mechanisms governing angiogenesis during brain development, in brain tumours and in brain AVMs, and how can this be addressed by single-cell analyses in the multi-omics era? Currently, it remains incompletely understood to what extent developmental signalling pathways reactivated in pathologies differ from those active during (brain) development. Regulatory effects of neurodevelopmental programmes in glioblastoma cells267 as well as oncofetal reprogramming of ECs in hepatocellular carcinoma have been reported in single-cell studies352, but the relevance of fetal pathways in the pathological brain vasculature has not been described so far. Therefore, direct comparison between ECs derived from developing (fetal or embryonic or postnatal) brains, ECs derived from healthy adult control brains and ECs derived from vascular-dependent CNS pathologies at single-cell resolution is of crucial importance.
Recently, the power of single-cell analyses enabled us to unravel key signalling pathways in brain ECs active during development that were reactivated in brain tumour and brain vascular malformation ECs163. Our finding that more than half of all regulated pathways in pathological ECs are of developmental origin confirm a paradigm in which signalling axes driving vascular growth during fetal human brain development are silenced in the adult human control brain and (re)activated across various human brain pathologies, including various brain tumours and brain vascular malformations163. The crucial importance of developmental pathways in vascular-dependent brain pathologies and the suggested functional plasticity of ECs353,354 across developmental and disease states will need to be functionally validated using emerging novel techniques such as single-cell genomics355, spatial transcriptomics (for example, Slide-seq356 or other spatial transcriptomics techniques357) and single-cell proteomics (for example, imaging mass cytometry358, single-cell cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq)359, and single-cell western blotting360). These techniques provide exciting novel avenues allowing direct measurement of RNA and protein expression in isolated human brain ECs (and PVCs of the NVU), thereby providing insights into the molecular and genetic basis while retaining spatial information of both developmental and pathological CNS angiogenesis using an unbiased approach. These cellular and molecular insights at single-cell precision leading to the identification of novel molecular angiogenic signalling cascades then need to be studied using in vivo models of angiogenesis for both CNS (brain, spinal cord and retina) and non-CNS tissues/organs using xenograft models and other strategies361–363.
The third crucial question for future studies is can single-cell multi-omics techniques be used to further clarify the role of inflammatory or immune-related processes in pathological angiogenesis beyond what is currently known (for example, inflammation-induced pro-angiogenic effects on the vasculature in brain tumours and brain vascular malformations364–366)? Notably, recent single-cell studies have further emphasized additional roles of inflammatory processes in pathological ECs across various brain diseases, including brain AVMs75,163, brain tumours163 and neurodegenerative diseases367–369. Interestingly, pathological ECs show upregulation of inflammatory or immune-related pathways and of major histocompatibility complex class II molecules in brain tumours (including glial brain tumours) and brain vascular malformations (including brain AVMs)75,163 as well as elevated levels of immune cell–EC interactions in brain tumours (including glial brain tumours) and brain vascular malformations (including brain AVMs)163 as well as in Alzheimer disease and Huntington disease367,368. These very interesting observations warrant further investigation at both the single-cell level and the functional level, with potentially crucial implications for both basic biological understanding and translational settings.
The fourth question is how important are NVL-related developmental pathways for pathological brain angiogenesis, and are they of a CNS-specific nature or a general nature? We anticipate that addressing the role of NVL molecules in ECs and PVCs within the developing and the pathological CNS (for example, through leveraging single-cell multi-omics techniques163,370–372) will provide important insights that have to be characterized at multiple organizational levels.
At the molecular level, NVL-related pathways are of crucial importance during vascular brain development, and many are reactivated in vascular brain pathologies, as evidenced by our recent single-cell atlas9,12,14,79,373. As the cellular and molecular interaction and bilateral crosstalk between neuronal and vascular tissue are especially tight in the CNS9,14,374, we reason that NVL molecules (being of a general or a CNS-specific nature) are of crucial importance in the healthy and the diseased brain and need to be studied in more detail in the future.
Regarding the layered organization of the human brain375, neurovascular interactions might be fundamentally different in distinct CNS compartments, with predominantly neuron-to-EC interactions in CNS grey matter and mainly oligodendrocyte-to-EC interactions in the CNS white matter, both involving classic and non-classic axon guidance cues9.
Members of the classic axonal guidance and NVL molecule families such as netrins, semaphorins, ephrins and Slit proteins, and their receptors, as well as non-classic axon guidance and NVL molecules such as WNT proteins, SHH and BMPs are implicated in arteriovenous differentiation14,15,163,201,374,376. NVL molecules interact with the Notch pathway9, and interactions between NVL molecules and Notch are important in arteriovenous differentiation9,14,201. Most notably, NOTCH1 and NOTCH4 are major drivers of arterial fate, associated with expression of the arterial markers ephrin B2, CXCR4 and connexin 40 (ref. 345). Lack of Notch signalling results in upregulation of venous markers such as COUP transcription factor 2, NRP2, VEGFR3 and the receptor EphB4 (refs. 75,281,282). It remains to be explored how NVL molecules contribute to these phenomena, and analyses of NVL molecules and pathways in distinct arteriovenous compartments at the single-cell level75,163,367,368 comprise a promising approach.
The final question is as follows: given the current focus on sprouting angiogenesis, how important are other modes of vessel formation during brain development, in brain tumours and in brain AVMs, how do they differ between distinct vascular beds inside and outside the CNS and how are they regulated at the molecular level? Modes of vessel formation other than sprouting angiogenesis probably have roles in both brain development and vascular-dependent brain pathologies24. Vasculogenesis is important during PNVP formation, whereas the INVP is predominantly vascularized by sprouting angiogenesis27, but the involvement of other modes of vessel formation during these developmental stages remains to be determined. In tumours located inside and outside the CNS, GSC transdifferentiation into tumour ECs20,21,215 or tumour pericytes22 directly involves PVCs of the NVU. Similarly, vascular co-option and mimicry in liver cancer exemplifies PVC–EC interactions outside the CNS173. The molecular mechanisms underlying vascular co-option, vascular mimicry and vascular intussusception remain largely unexplored. A more thorough investigation of the influence of PVCs and cellular interactions within the NVU in the setting of these additional modes of neovascularization using single-cell sequencing (for example, scRNA-seq377 and CITE-seq359) and imaging (for example, fluorescence light sheet microscopy and high-throughput microscopy378) techniques is key for future progress in developmental and pathological settings.
In conclusion, it has become increasingly evident that the remarkable cellular heterogeneity and molecular heterogeneity of the human brain vasculature within and between individuals across development and disease as well as its specific characteristics, such as CNS specificity and arteriovenous zonation, require thorough characterization at the single-cell level. Furthermore, a clearer mechanistic understanding of the silencing of developmentally active angiogenic processes in the healthy adult brain and subsequent reactivation in disease at the single-cell level will be crucial for the development of future therapies aimed at targeting vascular pathology. We anticipate that the constantly evolving multi-omics approaches (including scDNA-seq, single-cell assay for transposase-accessible chromatin with sequencing (scATAC-seq), imaging cytometry by time of flight and spatial transcriptomics) will enable various long-standing questions in the field of neurovascular biology to be answered and will continue to increase our knowledge of the human brain vasculature in development, adulthood and disease.
Supplementary information
Acknowledgements
The authors thank N. Chu Ji for help with the illustrations, P. Nicholson for providing neuroradiological images of the brain AVMs and brain tumours, J. Fish, M. Ghobrial, H. Zhong and F. Farnhammer for discussion regarding the scRNA-seq data and A. Thomson for help with English proofreading. T.W. was supported by the OPO Foundation, Swiss Cancer Research (KFS-3880-02-2016-R and KFS-4758-02-2019-R), the Stiftung zur Krebsbekampfung, the Kurt und Senta Herrmann Foundation, Forschungskredit of the University of Zurich, the Zurich Cancer League, the Theodor und Ida Herzog Egli Foundation, the Novartis Foundation for Medical-Biological Research and the HOPE Foundation.
Glossary
- Blood–brain barrier
(BBB). A physiological barrier formed by the brain endothelium to regulate trafficking of most compounds from the blood to the brain.
- Brain arteriovenous malformations
High-flow low-resistance vascular malformations characterized by a loss of vascular organization, a network of tortuous, dysplastic vascular channels (termed ‘nidus’) in between one or multiple feeding arteries and one or multiple draining veins in lieu of a normal intervening capillary network.
- Brain vascular malformations
Malformations characterized by abnormal blood vessel growth and altered maturation of the vessel wall, including brain arteriovenous malformations, cerebral cavernous malformations, developmental venous anomalies, dural and pial arteriovenous fistulas, capillary telangiectasias, vein of Galen malformations and carotid-cavernous fistulae.
- Glial brain tumours
Primary brain tumours originating from neuroglial stem or progenitor cells, accounting for almost 30% of all primary brain tumours and for 80% of all malignant primary brain tumours.
- Glioma (or glioblastoma) stem cell
(GSC). A subpopulation of tumour cells with stem cell-like properties that contribute to tumour initiation, progression and resistance to anticancer therapies.
- Neurovascular link
(NVL). The similar appearance and coordinated guidance of the cellular and subcellular elements of both the vascular system and the nervous system.
- Neurovascular unit
(NVU). The functional unit of the complex crosstalk between endothelial cells and perivascular cells in the perivascular niche.
- Perivascular niche
(PVN). The microenvironment around a blood vessel; it includes endothelial cells and perivascular cells such as astrocytes, pericytes, neurons, stem cells, microglia and vascular smooth muscle cells.
- Reactivated developmental signalling pathways
Molecular signalling cues and pathways that are active during embryonic and/or postnatal vascular brain development, are silenced in the adult healthy brain vasculature and might be reactivated in vascular-dependent CNS diseases, including brain tumours and brain vascular malformations.
- Single-nucleotide polymorphisms
A somatic mutation characterized by a single nucleotide change in the DNA sequence that can modulate biological mechanisms. Somatic mutations do not occur in the germ line but occur in a postzygotic progenitor or differentiated cell and are well described in both CNS and non-CNS cancer development.
Author contributions
T.W. had the idea for the Review, wrote the manuscript with J.B., designed the figures and, with J.B., created the figures. T.W. and J.B. researched data for the article, provided substantial contributions to discussion of its content, and reviewed and edited the manuscript before submission. P.C., G.Z., P.P.M., K.D.B. and I.R. provided substantial contributions to discussion of the article’s content and reviewed and edited the manuscript before submission. I.R. also helped write the article.
Peer review
Peer review information
Nature Reviews Neuroscience thanks S. Liebner; J. Siegenthaler; R. Wang, who co-reviewed with S. Yuan; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41583-023-00684-y.
References
- 1.Mink JW, Blumenschine RJ, Adams DB. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am. J. Physiol. 1981;241:R203–R212. doi: 10.1152/ajpregu.1981.241.3.R203. [DOI] [PubMed] [Google Scholar]
- 2.Zlokovic BV, Apuzzo ML. Strategies to circumvent vascular barriers of the central nervous system. Neurosurgery. 1998;43:877–878. doi: 10.1097/00006123-199810000-00089. [DOI] [PubMed] [Google Scholar]
- 3.Wälchli T, et al. Quantitative assessment of angiogenesis, perfused blood vessels and endothelial tip cells in the postnatal mouse brain. Nat. Protoc. 2015;10:53–74. doi: 10.1038/nprot.2015.002. [DOI] [PubMed] [Google Scholar]
- 4.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail–chick transplantation chimeras. Dev. Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- 6.Vasudevan A, Long JE, Crandall JE, Rubenstein JL, Bhide PG. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat. Neurosci. 2008;11:429–439. doi: 10.1038/nn2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komsany, A. & Pezzella, F. in Tumor Vascularization (eds Ribatti, D. & Pezzella, F.) 113–127 (Academic Press, 2020).
- 8.Ghajar CM, et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wälchli T, et al. Wiring the vascular network with neural cues: a CNS perspective. Neuron. 2015;87:271–296. doi: 10.1016/j.neuron.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Muoio V, Persson PB, Sendeski MM. The neurovascular unit – concept review. Acta Physiol. 2014;210:790–798. doi: 10.1111/apha.12250. [DOI] [PubMed] [Google Scholar]
- 11.Eichmann A, Thomas JL. Molecular parallels between neural and vascular development. Cold Spring Harb. Perspect. Med. 2013;3:a006551. doi: 10.1101/cshperspect.a006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paredes I, Himmels P, Ruiz de Almodovar C. Neurovascular communication during CNS development. Dev. Cell. 2018;45:10–32. doi: 10.1016/j.devcel.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 17.Wälchli T, et al. Nogo-A is a negative regulator of CNS angiogenesis. Proc. Natl Acad. Sci. USA. 2013;110:E1943. doi: 10.1073/pnas.1216203110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson JE, 3rd, Kelley RW, Patterson C. Mechanisms of endothelial differentiation in embryonic vasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2005;25:2246–2254. doi: 10.1161/01.ATV.0000183609.55154.44. [DOI] [PubMed] [Google Scholar]
- 20.Ricci-Vitiani L, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 21.Wang R, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 22.Cheng L, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer. 2020;20:26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain RK, Carmeliet P. SnapShot: tumor angiogenesis. Cell. 2012;149:1408–1408.e1401. doi: 10.1016/j.cell.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Hardee ME, Zagzag D. Mechanisms of glioma-associated neovascularization. Am. J. Pathol. 2012;181:1126–1141. doi: 10.1016/j.ajpath.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boire A, Brastianos PK, Garzia L, Valiente M. Brain metastasis. Nat. Rev. Cancer. 2020;20:4–11. doi: 10.1038/s41568-019-0220-y. [DOI] [PubMed] [Google Scholar]
- 27.Vallon M, Chang J, Zhang H, Kuo CJ. Developmental and pathological angiogenesis in the central nervous system. Cell. Mol. Life Sci. 2014;71:3489–3506. doi: 10.1007/s00018-014-1625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HW, et al. Role of venous endothelial cells in developmental and pathologic angiogenesis. Circulation. 2021;144:1308–1322. doi: 10.1161/CIRCULATIONAHA.121.054071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellstrom M, Phng LK, Gerhardt H. VEGF and Notch signaling: the yin and yang of angiogenic sprouting. Cell Adh. Migr. 2007;1:133–136. doi: 10.4161/cam.1.3.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 2013;3:a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue Y, et al. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum. Mol. Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- 32.Jakobsson L, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 33.Bentley K, et al. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat. Cell Biol. 2014;16:309–321. doi: 10.1038/ncb2926. [DOI] [PubMed] [Google Scholar]
- 34.Pitulescu ME, et al. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat. Cell Biol. 2017;19:915–927. doi: 10.1038/ncb3555. [DOI] [PubMed] [Google Scholar]
- 35.Hellstrom M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 36.Shah AV, et al. The endothelial transcription factor ERG mediates angiopoietin-1-dependent control of Notch signalling and vascular stability. Nat. Commun. 2017;8:16002. doi: 10.1038/ncomms16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 38.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali Z, et al. Intussusceptive vascular remodeling precedes pathological neovascularization. Arterioscler. Thromb. Vasc. Biol. 2019;39:1402–1418. doi: 10.1161/ATVBAHA.118.312190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Djonov V, Baum O, Burri PH. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003;314:107–117. doi: 10.1007/s00441-003-0784-3. [DOI] [PubMed] [Google Scholar]
- 41.Patan S, Alvarez MJ, Schittny JC, Burri PH. Intussusceptive microvascular growth: a common alternative to capillary sprouting. Arch. Histol. Cytol. 1992;55:65–75. doi: 10.1679/aohc.55.Suppl_65. [DOI] [PubMed] [Google Scholar]
- 42.Patan S, Haenni B, Burri PH. Evidence for intussusceptive capillary growth in the chicken chorio-allantoic membrane (CAM) Anat. Embryol. 1993;187:121–130. doi: 10.1007/BF00171743. [DOI] [PubMed] [Google Scholar]
- 43.Makanya AN, Stauffer D, Ribatti D, Burri PH, Djonov V. Microvascular growth, development, and remodeling in the embryonic avian kidney: the interplay between sprouting and intussusceptive angiogenic mechanisms. Microsc. Res. Tech. 2005;66:275–288. doi: 10.1002/jemt.20169. [DOI] [PubMed] [Google Scholar]
- 44.Gargett CE, Rogers PA. Human endometrial angiogenesis. Reproduction. 2001;121:181–186. doi: 10.1530/rep.0.1210181. [DOI] [PubMed] [Google Scholar]
- 45.Djonov V, Schmid M, Tschanz SA, Burri PH. Intussusceptive angiogenesis: its role in embryonic vascular network formation. Circ. Res. 2000;86:286–292. doi: 10.1161/01.RES.86.3.286. [DOI] [PubMed] [Google Scholar]
- 46.Zhang ZG, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Nico B, et al. Intussusceptive microvascular growth in human glioma. Clin. Exp. Med. 2010;10:93–98. doi: 10.1007/s10238-009-0076-7. [DOI] [PubMed] [Google Scholar]
- 48.Ornelas S, et al. Three-dimensional ultrastructure of the brain pericyte-endothelial interface. J. Cereb. Blood Flow. Metab. 2021;41:2185–2200. doi: 10.1177/0271678X211012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartmann DA, et al. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci. 2021;24:633–645. doi: 10.1038/s41593-020-00793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mancuso MR, Kuhnert F, Kuo CJ. Developmental angiogenesis of the central nervous system. Lymphat. Res. Biol. 2008;6:173–180. doi: 10.1089/lrb.2008.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu. Rev. Neurosci. 2010;33:379–408. doi: 10.1146/annurev-neuro-060909-152829. [DOI] [PubMed] [Google Scholar]
- 53.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansson PA, et al. Blood-CSF barrier function in the rat embryo. Eur. J. Neurosci. 2006;24:65–76. doi: 10.1111/j.1460-9568.2006.04904.x. [DOI] [PubMed] [Google Scholar]
- 56.Saunders A, et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell. 2018;174:1015–1030.e1016. doi: 10.1016/j.cell.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saunders NR, et al. The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Front. Neurosci. 2014;8:404–404. doi: 10.3389/fnins.2014.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front. Pharmacol. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ek CJ, Dziegielewska KM, Stolp H, Saunders NR. Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica) J. Comp. Neurol. 2006;496:13–26. doi: 10.1002/cne.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Storkebaum E, Quaegebeur A, Vikkula M, Carmeliet P. Cerebrovascular disorders: molecular insights and therapeutic opportunities. Nat. Neurosci. 2011;14:1390–1397. doi: 10.1038/nn.2947. [DOI] [PubMed] [Google Scholar]
- 62.Segarra M, Aburto MR, Acker-Palmer A. Blood-brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 2021 doi: 10.1016/j.tins.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Munji RN, et al. Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood-brain barrier dysfunction module. Nat. Neurosci. 2019;22:1892–1902. doi: 10.1038/s41593-019-0497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerhardt H, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 66.Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marin-Padilla M. Early vascularization of the embryonic cerebral cortex: Golgi and electron microscopic studies. J. Comp. Neurol. 1985;241:237–249. doi: 10.1002/cne.902410210. [DOI] [PubMed] [Google Scholar]
- 68.Phng LK, Stanchi F, Gerhardt H. Filopodia are dispensable for endothelial tip cell guidance. Development. 2013;140:4031–4040. doi: 10.1242/dev.097352. [DOI] [PubMed] [Google Scholar]
- 69.del Toro R, et al. Identification and functional analysis of endothelial tip cell-enriched genes. Blood. 2010;116:4025–4033. doi: 10.1182/blood-2010-02-270819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao Q, et al. Single-cell transcriptome analyses reveal endothelial cell heterogeneity in tumors and changes following antiangiogenic treatment. Cancer Res. 2018;78:2370–2382. doi: 10.1158/0008-5472.CAN-17-2728. [DOI] [PubMed] [Google Scholar]
- 71.Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood. 2010;115:5102–5110. doi: 10.1182/blood-2009-07-230284. [DOI] [PubMed] [Google Scholar]
- 72.Rocha SF, et al. Esm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circ. Res. 2014;115:581–590. doi: 10.1161/CIRCRESAHA.115.304718. [DOI] [PubMed] [Google Scholar]
- 73.Goveia J, et al. An integrated gene expression landscape profiling approach to identify lung tumor endothelial cell heterogeneity and angiogenic candidates. Cancer Cell. 2020;37:21–36.e13. doi: 10.1016/j.ccell.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 74.Kalucka J, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180:764–779.e720. doi: 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 75.Winkler EA, et al. A single-cell atlas of the normal and malformed human brain vasculature. Science. 2022;375:eabi7377. doi: 10.1126/science.abi7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vanlandewijck M, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 77.Segarra M, Aburto MR, Hefendehl J, Acker-Palmer A. Neurovascular interactions in the nervous system. Annu. Rev. Cell Dev. Biol. 2019;35:615–635. doi: 10.1146/annurev-cellbio-100818-125142. [DOI] [PubMed] [Google Scholar]
- 78.Charron F, Tessier-Lavigne M. The Hedgehog, TGF-beta/BMP and Wnt families of morphogens in axon guidance. Adv. Exp. Med. Biol. 2007;621:116–133. doi: 10.1007/978-0-387-76715-4_9. [DOI] [PubMed] [Google Scholar]
- 79.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 80.Walchli T, et al. Nogo-A regulates vascular network architecture in the postnatal brain. J. Cereb. Blood Flow. Metab. 2017;37:614–631. doi: 10.1177/0271678X16675182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li W, et al. Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin. Dev. Cell. 2013;24:359–371. doi: 10.1016/j.devcel.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Honma Y, et al. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35:267–282. doi: 10.1016/S0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- 83.Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452:759–763. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma S, Kwon HJ, Johng H, Zang K, Huang Z. Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling. PLoS Biol. 2013;11:e1001469. doi: 10.1371/journal.pbio.1001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Minocha S, et al. NG2 glia are required for vessel network formation during embryonic development. eLife. 2015;4:e09102. doi: 10.7554/eLife.09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma S, Kwon HJ, Huang Z. A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS ONE. 2012;7:e48001. doi: 10.1371/journal.pone.0048001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coelho-Santos V, Shih AY. Postnatal development of cerebrovascular structure and the neurogliovascular unit. Wiley Interdiscip. Rev. Dev. Biol. 2020;9:e363. doi: 10.1002/wdev.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fantin A, Vieira JM, Plein A, Maden CH, Ruhrberg C. The embryonic mouse hindbrain as a qualitative and quantitative model for studying the molecular and cellular mechanisms of angiogenesis. Nat. Protoc. 2013;8:418–429. doi: 10.1038/nprot.2013.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Puelles L, et al. Patterned vascularization of embryonic mouse forebrain, and neuromeric topology of major human subarachnoidal arterial branches: a prosomeric mapping. Front. Neuroanat. 2019;13:59. doi: 10.3389/fnana.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marín-Padilla M. The human brain intracerebral microvascular system: development and structure. Front. Neuroanat. 2012 doi: 10.3389/fnana.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pereda J, Sulz L, San Martin S, Godoy-Guzman C. The human lung during the embryonic period: vasculogenesis and primitive erythroblasts circulation. J. Anat. 2013;222:487–494. doi: 10.1111/joa.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 93.Benedito R, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 94.Leslie JD, et al. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- 95.Wang X, et al. YAP/TAZ Orchestrate VEGF signaling during developmental angiogenesis. Dev. Cell. 2017;42:462–478.e467. doi: 10.1016/j.devcel.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 96.Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA. Angiopoietin-2 plays an important role in retinal angiogenesis. J. Cell Physiol. 2002;192:182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- 97.Sato TN, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 98.Suri C, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/S0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 99.Jones CA, et al. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat. Cell Biol. 2009;11:1325–1331. doi: 10.1038/ncb1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bedell VM, et al. roundabout4 is essential for angiogenesis in vivo. Proc. Natl Acad. Sci. USA. 2005;102:6373–6378. doi: 10.1073/pnas.0408318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tong M, Jun T, Nie Y, Hao J, Fan D. The role of the Slit/Robo signaling pathway. J. Cancer. 2019;10:2694–2705. doi: 10.7150/jca.31877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gu C, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 103.Lejmi E, et al. Netrin-4 promotes mural cell adhesion and recruitment to endothelial cells. Vasc. Cell. 2014;6:1. doi: 10.1186/2045-824X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sawamiphak S, et al. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 105.McCarty JH, et al. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 106.Elaimy AL, Mercurio AM. Convergence of VEGF and YAP/TAZ signaling: implications for angiogenesis and cancer biology. Sci. Signal. 2018 doi: 10.1126/scisignal.aau1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jeansson M, et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J. Clin. Invest. 2011;121:2278–2289. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y, Kontos CD, Annex BH, Popel AS. Angiopoietin-Tie signaling pathway in endothelial cells: a computational model. iScience. 2019;20:497–511. doi: 10.1016/j.isci.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park KW, et al. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev. Biol. 2003;261:251–267. doi: 10.1016/S0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 110.Dai C, Gong Q, Cheng Y, Su G. Regulatory mechanisms of Robo4 and their effects on angiogenesis. Biosci. Rep. 2019 doi: 10.1042/bsr20190513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Serini G, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 112.Vieira JM, Schwarz Q, Ruhrberg C. Selective requirements for NRP1 ligands during neurovascular patterning. Development. 2007;134:1833–1843. doi: 10.1242/dev.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gu C, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell. 2003;5:45–57. doi: 10.1016/S1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fantin A, et al. NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood. 2013;121:2352–2362. doi: 10.1182/blood-2012-05-424713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zygmunt T, et al. Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Dev. Cell. 2011;21:301–314. doi: 10.1016/j.devcel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van der Zwaag B, et al. PLEXIN-D1, a novel plexin family member, is expressed in vascular endothelium and the central nervous system during mouse embryogenesis. Dev. Dyn. 2002;225:336–343. doi: 10.1002/dvdy.10159. [DOI] [PubMed] [Google Scholar]
- 117.Fukushima Y, et al. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J. Clin. Invest. 2011;121:1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev. 2011;25:1399–1411. doi: 10.1101/gad.2042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lejmi E, et al. Netrin-4 inhibits angiogenesis via binding to neogenin and recruitment of Unc5B. Proc. Natl Acad. Sci. USA. 2008;105:12491–12496. doi: 10.1073/pnas.0804008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Larrivee B, et al. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21:2433–2447. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lambert E, Coissieux M-M, Laudet V, Mehlen P. Netrin-4 acts as a pro-angiogenic factor during zebrafish development. J. Biol. Chem. 2012;287:3987–3999. doi: 10.1074/jbc.M111.289371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pasquale EB. The Eph family of receptors. Curr. Opin. Cell Biol. 1997;9:608–615. doi: 10.1016/S0955-0674(97)80113-5. [DOI] [PubMed] [Google Scholar]
- 123.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat. Rev. Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y, Yang X. The roles of TGF-β signaling in cerebrovascular diseases. Front. Cell Dev. Biol. 2020;8:567682. doi: 10.3389/fcell.2020.567682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McCarty JH, et al. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol. Cell. Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu J, et al. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arnold TD, et al. Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking αVβ8-TGFβ signaling in the brain. Development. 2014;141:4489–4499. doi: 10.1242/dev.107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hirota S, et al. Neuropilin 1 balances β8 integrin-activated TGFβ signaling to control sprouting angiogenesis in the brain. Development. 2015;142:4363–4373. doi: 10.1242/dev.113746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oh SP, et al. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc. Natl Acad. Sci. USA. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li DY, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 131.Anderson KD, et al. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc. Natl Acad. Sci. USA. 2011;108:2807–2812. doi: 10.1073/pnas.1019761108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chang J, et al. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat. Med. 2017;23:450–460. doi: 10.1038/nm.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cho C, Smallwood PM, Nathans J. Reck and Gpr124 are essential receptor cofactors for Wnt7a/Wnt7b-specific signaling in mammalian CNS angiogenesis and blood-brain barrier regulation. Neuron. 2017;95:1056–1073.e1055. doi: 10.1016/j.neuron.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cullen M, et al. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc. Natl Acad. Sci. USA. 2011;108:5759–5764. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuhnert F, et al. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou Y, Nathans J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical Wnt signaling. Dev. Cell. 2014;31:248–256. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chang TH, Hsieh FL, Smallwood PM, Gabelli SB, Nathans J. Structure of the RECK CC domain, an evolutionary anomaly. Proc. Natl Acad. Sci. USA. 2020;117:15104–15111. doi: 10.1073/pnas.2006332117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boyé K, et al. Endothelial Unc5B controls blood-brain barrier integrity. Nat. Commun. 2022;13:1169. doi: 10.1038/s41467-022-28785-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huyghe A, et al. Netrin-1 promotes naive pluripotency through Neo1 and Unc5b co-regulation of Wnt and MAPK signalling. Nat. Cell Biol. 2020;22:389–400. doi: 10.1038/s41556-020-0483-2. [DOI] [PubMed] [Google Scholar]
- 140.Tam SJ, et al. Death receptors DR6 and TROY regulate brain vascular development. Dev. Cell. 2012;22:403–417. doi: 10.1016/j.devcel.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 141.Ye X, Wang Y, Nathans J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol. Med. 2010;16:417–425. doi: 10.1016/j.molmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Y, et al. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Z, Liu CH, Huang S, Chen J. Wnt signaling in vascular eye diseases. Prog. Retin. Eye Res. 2019;70:110–133. doi: 10.1016/j.preteyeres.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barak T, et al. PPIL4 is essential for brain angiogenesis and implicated in intracranial aneurysms in humans. Nat. Med. 2021;27:2165–2175. doi: 10.1038/s41591-021-01572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stenman JM, et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 146.Daneman R, et al. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl Acad. Sci. USA. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Posokhova E, et al. GPR124 functions as a WNT7-specific coactivator of canonical beta-catenin signaling. Cell Rep. 2015;10:123–130. doi: 10.1016/j.celrep.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cho C, Wang Y, Smallwood PM, Williams J, Nathans J. Molecular determinants in Frizzled, Reck, and Wnt7a for ligand-specific signaling in neurovascular development. Elife. 2019 doi: 10.7554/eLife.47300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Y, et al. Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. eLife. 2019;8:e43257. doi: 10.7554/eLife.43257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Benz F, et al. Low wnt/β-catenin signaling determines leaky vessels in the subfornical organ and affects water homeostasis in mice. eLife. 2019;8:e43818. doi: 10.7554/eLife.43818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Martowicz A, et al. Endothelial beta-catenin signaling supports postnatal brain and retinal angiogenesis by promoting sprouting, tip cell formation, and VEGFR (vascular endothelial growth factor receptor) 2 expression. Arterioscler. Thromb. Vasc. Biol. 2019 doi: 10.1161/atvbaha.119.312749. [DOI] [PubMed] [Google Scholar]
- 152.Fujita M, et al. Assembly and patterning of the vascular network of the vertebrate hindbrain. Development. 2011;138:1705–1715. doi: 10.1242/dev.058776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Coelho-Santos V, Berthiaume A-A, Ornelas S, Stuhlmann H, Shih AY. Imaging the construction of capillary networks in the neonatal mouse brain. Proc. Natl Acad. Sci. USA. 2021;118:e2100866118. doi: 10.1073/pnas.2100866118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wälchli T, et al. Hierarchical imaging and computational analysis of three-dimensional vascular network architecture in the entire postnatal and adult mouse brain. Nat. Protoc. 2021;16:4564–4610. doi: 10.1038/s41596-021-00587-1. [DOI] [PubMed] [Google Scholar]
- 155.Miao RQ, et al. Identification of a receptor necessary for Nogo-B stimulated chemotaxis and morphogenesis of endothelial cells. Proc. Natl Acad. Sci. USA. 2006;103:10997–11002. doi: 10.1073/pnas.0602427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhao B, et al. Nogo-B receptor is essential for angiogenesis in zebrafish via Akt pathway. Blood. 2010;116:5423–5433. doi: 10.1182/blood-2010-02-271577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schwab ME. Functions of Nogo proteins and their receptors in the nervous system. Nat. Rev. Neurosci. 2010;11:799–811. doi: 10.1038/nrn2936. [DOI] [PubMed] [Google Scholar]
- 158.Rana U, et al. Nogo-B receptor deficiency causes cerebral vasculature defects during embryonic development in mice. Dev. Biol. 2016;410:190–201. doi: 10.1016/j.ydbio.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park EJ, Grabinska KA, Guan Z, Sessa WC. NgBR is essential for endothelial cell glycosylation and vascular development. EMBO Rep. 2016;17:167–177. doi: 10.15252/embr.201540789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rohlenova K, Veys K, Miranda-Santos I, De Bock K, Carmeliet P. Endothelial cell metabolism in health and disease. Trends Cell Biol. 2018;28:224–236. doi: 10.1016/j.tcb.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 161.Li X, Sun X, Carmeliet P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metab. 2019;30:414–433. doi: 10.1016/j.cmet.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 162.Nikolaev SI, et al. Somatic activating KRAS mutations in arteriovenous malformations of the brain. N. Engl. J. Med. 2018;378:250–261. doi: 10.1056/NEJMoa1709449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wälchli T, et al. Molecular atlas of the human brain vasculature at the single-cell level. bioRxiv. 2021 doi: 10.1101/2021.10.18.464715. [DOI] [Google Scholar]
- 164.Weller M, et al. Glioma. Nat. Rev. Dis. Prim. 2015;1:15017. doi: 10.1038/nrdp.2015.17. [DOI] [PubMed] [Google Scholar]
- 165.Boyd NH, et al. Glioma stem cells and their roles within the hypoxic tumor microenvironment. Theranostics. 2021;11:665–683. doi: 10.7150/thno.41692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wirsching HG, Roth P, Weller M. A vasculature-centric approach to developing novel treatment options for glioblastoma. Expert. Opin. Ther. Targets. 2021 doi: 10.1080/14728222.2021.1881062. [DOI] [PubMed] [Google Scholar]
- 167.Diaz RJ, et al. The role of bevacizumab in the treatment of glioblastoma. J. Neurooncol. 2017;133:455–467. doi: 10.1007/s11060-017-2477-x. [DOI] [PubMed] [Google Scholar]
- 168.Thanasupawat T, et al. Dovitinib enhances temozolomide efficacy in glioblastoma cells. Mol. Oncol. 2017;11:1078–1098. doi: 10.1002/1878-0261.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sharma M, et al. Phase II study of dovitinib in recurrent glioblastoma. J. Neurooncol. 2019;144:359–368. doi: 10.1007/s11060-019-03236-6. [DOI] [PubMed] [Google Scholar]
- 170.Stupp R, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–1108. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 171.Balana C, et al. Sunitinib administered prior to radiotherapy in patients with non-resectable glioblastoma: results of a phase II study. Target. Oncol. 2014;9:321–329. doi: 10.1007/s11523-014-0305-1. [DOI] [PubMed] [Google Scholar]
- 172.Mosteiro A, et al. The vascular microenvironment in glioblastoma: a comprehensive review. Biomedicines. 2022 doi: 10.3390/biomedicines10061285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Frentzas S, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat. Med. 2016;22:1294–1302. doi: 10.1038/nm.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bridgeman VL, et al. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J. Pathol. 2017;241:362–374. doi: 10.1002/path.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Budde MD, Gold E, Jordan EK, Smith-Brown M, Frank JA. Phase contrast MRI is an early marker of micrometastatic breast cancer development in the rat brain. NMR Biomed. 2012;25:726–736. doi: 10.1002/nbm.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Donnem T, et al. Vessel co-option in primary human tumors and metastases: an obstacle to effective anti-angiogenic treatment? Cancer Med. 2013;2:427–436. doi: 10.1002/cam4.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Caspani EM, Crossley PH, Redondo-Garcia C, Martinez S. Glioblastoma: a pathogenic crosstalk between tumor cells and pericytes. PLoS ONE. 2014;9:e101402. doi: 10.1371/journal.pone.0101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Holash J, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 179.Simon MP, Tournaire R, Pouyssegur J. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1. J. Cell Physiol. 2008;217:809–818. doi: 10.1002/jcp.21558. [DOI] [PubMed] [Google Scholar]
- 180.Lindberg OR, et al. GBM heterogeneity as a function of variable epidermal growth factor receptor variant III activity. Oncotarget. 2016;7:79101–79116. doi: 10.18632/oncotarget.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Le Joncour V, et al. Vulnerability of invasive glioblastoma cells to lysosomal membrane destabilization. EMBO Mol. Med. 2019;11:e9034. doi: 10.15252/emmm.201809034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Seano G, Jain RK. Vessel co-option in glioblastoma: emerging insights and opportunities. Angiogenesis. 2020;23:9–16. doi: 10.1007/s10456-019-09691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Noguera-Troise I, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 184.Davis GE, Norden PR, Bowers SL. Molecular control of capillary morphogenesis and maturation by recognition and remodeling of the extracellular matrix: functional roles of endothelial cells and pericytes in health and disease. Connect. Tissue Res. 2015;56:392–402. doi: 10.3109/03008207.2015.1066781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chantrain CF, et al. Mechanisms of pericyte recruitment in tumour angiogenesis: a new role for metalloproteinases. Eur. J. Cancer. 2006;42:310–318. doi: 10.1016/j.ejca.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 186.Sattiraju A, Mintz A. Pericytes in glioblastomas: multifaceted role within tumor microenvironments and potential for therapeutic interventions. Adv. Exp. Med. Biol. 2019;1147:65–91. doi: 10.1007/978-3-030-16908-4_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bruna A, et al. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 188.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 189.Grunewald M, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 190.Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 191.Spring H, Schuler T, Arnold B, Hammerling GJ, Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proc. Natl Acad. Sci. USA. 2005;102:18111–18116. doi: 10.1073/pnas.0507158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rajantie I, et al. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gao D, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 194.Patel JR, McCandless EE, Dorsey D, Klein RS. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc. Natl Acad. Sci. USA. 2010;107:11062. doi: 10.1073/pnas.1006301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kioi M, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Urbantat RM, Vajkoczy P, Brandenburg S. Advances in chemokine signaling pathways as therapeutic targets in glioblastoma. Cancers. 2021 doi: 10.3390/cancers13122983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Burrell K, Singh S, Jalali S, Hill RP, Zadeh G. VEGF regulates region-specific localization of perivascular bone marrow-derived cells in glioblastoma. Cancer Res. 2014;74:3727–3739. doi: 10.1158/0008-5472.CAN-13-3119. [DOI] [PubMed] [Google Scholar]
- 198.Eberhard A, et al. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–1393. [PubMed] [Google Scholar]
- 199.Young PP, Hofling AA, Sands MS. VEGF increases engraftment of bone marrow-derived endothelial progenitor cells (EPCs) into vasculature of newborn murine recipients. Proc. Natl Acad. Sci. USA. 2002;99:11951–11956. doi: 10.1073/pnas.182215799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tabatabai G, Frank B, Möhle R, Weller M, Wick W. Irradiation and hypoxia promote homing of haematopoietic progenitor cells towards gliomas by TGF-beta-dependent HIF-1alpha-mediated induction of CXCL12. Brain. 2006;129:2426–2435. doi: 10.1093/brain/awl173. [DOI] [PubMed] [Google Scholar]
- 201.Hjelmeland AB, Lathia JD, Sathornsumetee S, Rich JN. Twisted tango: brain tumor neurovascular interactions. Nat. Neurosci. 2011;14:1375–1381. doi: 10.1038/nn.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Infanger DW, et al. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res. 2013;73:7079–7089. doi: 10.1158/0008-5472.CAN-13-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yan GN, et al. Endothelial cells promote stem-like phenotype of glioma cells through activating the Hedgehog pathway. J. Pathol. 2014;234:11–22. doi: 10.1002/path.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li D, et al. Glioma-associated human endothelial cell-derived extracellular vesicles specifically promote the tumourigenicity of glioma stem cells via CD9. Oncogene. 2019 doi: 10.1038/s41388-019-0903-6. [DOI] [PubMed] [Google Scholar]
- 205.Emlet DR, et al. Targeting a glioblastoma cancer stem-cell population defined by EGF receptor variant III. Cancer Res. 2014;74:1238–1249. doi: 10.1158/0008-5472.CAN-13-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Han X, et al. P4HA1 Regulates CD31 via COL6A1 in the transition of glioblastoma stem-like cells to tumor endothelioid cells. Front. Oncol. 2022;12:836511. doi: 10.3389/fonc.2022.836511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhao C, et al. ETV2 mediates endothelial transdifferentiation of glioblastoma. Signal. Transduct. Target. Ther. 2018;3:4. doi: 10.1038/s41392-018-0007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chen HF, et al. Twist1 induces endothelial differentiation of tumour cells through the Jagged1-KLF4 axis. Nat. Commun. 2014;5:4697. doi: 10.1038/ncomms5697. [DOI] [PubMed] [Google Scholar]
- 209.Baisiwala S, et al. Chemotherapeutic stress induces transdifferentiation of glioblastoma cells to endothelial cells and promotes vascular mimicry. Stem Cell Int. 2019;2019:6107456. doi: 10.1155/2019/6107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.De Pascalis I, et al. Endothelial trans-differentiation in glioblastoma recurring after radiotherapy. Mod. Pathol. 2018;31:1361–1366. doi: 10.1038/s41379-018-0046-2. [DOI] [PubMed] [Google Scholar]
- 211.Deshors P, et al. Ionizing radiation induces endothelial transdifferentiation of glioblastoma stem-like cells through the Tie2 signaling pathway. Cell Death Dis. 2019;10:816. doi: 10.1038/s41419-019-2055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Guichet P-O, et al. Notch1 stimulation induces a vascularization switch with pericyte-like cell differentiation of glioblastoma stem cells. Stem Cell. 2015;33:21–34. doi: 10.1002/stem.1767. [DOI] [PubMed] [Google Scholar]
- 213.Zhou W, et al. Targeting glioma stem cell-derived pericytes disrupts the blood-tumor barrier and improves chemotherapeutic efficacy. Cell stem Cell. 2017;21:591–603.e594. doi: 10.1016/j.stem.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Soda Y, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc. Natl Acad. Sci. USA. 2011;108:4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shangguan W, et al. Endothelium originated from colorectal cancer stem cells constitute cancer blood vessels. Cancer Sci. 2017;108:1357–1367. doi: 10.1111/cas.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Maniotis AJ, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J. Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.El Hallani S, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133:973–982. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jue C, et al. Vasculogenic mimicry in hepatocellular carcinoma contributes to portal vein invasion. Oncotarget. 2016;7:77987–77997. doi: 10.18632/oncotarget.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Williamson SC, et al. Vasculogenic mimicry in small cell lung cancer. Nat. Commun. 2016;7:13322. doi: 10.1038/ncomms13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Baeten CI, Hillen F, Pauwels P, de Bruine AP, Baeten CG. Prognostic role of vasculogenic mimicry in colorectal cancer. Dis. Colon. Rectum. 2009;52:2028–2035. doi: 10.1007/DCR.0b013e3181beb4ff. [DOI] [PubMed] [Google Scholar]
- 221.Ge H, Luo H. Overview of advances in vasculogenic mimicry-a potential target for tumor therapy. Cancer Manag. Res. 2018;10:2429–2437. doi: 10.2147/CMAR.S164675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Le Bras A, et al. HIF-2alpha specifically activates the VE-cadherin promoter independently of hypoxia and in synergy with Ets-1 through two essential ETS-binding sites. Oncogene. 2007;26:7480–7489. doi: 10.1038/sj.onc.1210566. [DOI] [PubMed] [Google Scholar]
- 223.Yao X, et al. Vascular endothelial growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic mimicry formation, neovascularization and tumor initiation by glioma stem-like cells. PLoS ONE. 2013;8:e57188. doi: 10.1371/journal.pone.0057188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mao JM, Liu J, Guo G, Mao XG, Li CX. Glioblastoma vasculogenic mimicry: signaling pathways progression and potential anti-angiogenesis targets. Biomark. Res. 2015;3:8. doi: 10.1186/s40364-015-0034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liu XM, et al. Clinical significance of vasculogenic mimicry in human gliomas. J. Neurooncol. 2011;105:173–179. doi: 10.1007/s11060-011-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liu Y, et al. IGFBP2 promotes vasculogenic mimicry formation via regulating CD144 and MMP2 expression in glioma. Oncogene. 2019;38:1815–1831. doi: 10.1038/s41388-018-0525-4. [DOI] [PubMed] [Google Scholar]
- 227.Han G, et al. Overexpression of leptin receptor in human glioblastoma: correlation with vasculogenic mimicry and poor prognosis. Oncotarget. 2017;8:58163–58171. doi: 10.18632/oncotarget.17344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li X, et al. ZRANB2/SNHG20/FOXK1 Axis regulates vasculogenic mimicry formation in glioma. J. Exp. Clin. Cancer Res. 2019;38:68. doi: 10.1186/s13046-019-1073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Guo J, et al. Long non-coding RNA LINC00339 stimulates glioma vasculogenic mimicry formation by regulating the miR-539-5p/TWIST1/MMPs Axis. Mol. Ther. Nucleic Acids. 2018;10:170–186. doi: 10.1016/j.omtn.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Li G, et al. miR141 inhibits glioma vasculogenic mimicry by controlling EphA2 expression. Mol. Med. Rep. 2018;18:1395–1404. doi: 10.3892/mmr.2018.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Treps L, Faure S, Clere N. Vasculogenic mimicry, a complex and devious process favoring tumorigenesis-interest in making it a therapeutic target. Pharmacol. Ther. 2021 doi: 10.1016/j.pharmthera.2021.107805. [DOI] [PubMed] [Google Scholar]
- 232.Chen W, et al. Overexpression of vascular endothelial growth factor indicates poor outcomes of glioma: a systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2015;8:8709–8719. [PMC free article] [PubMed] [Google Scholar]
- 233.Tilak M, Holborn J, New LA, Lalonde J, Jones N. Receptor tyrosine kinase signaling and targeting in glioblastoma multiforme. Int. J. Mol. Sci. 2021 doi: 10.3390/ijms22041831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat. Res. 1992;276:299–306. doi: 10.1016/0165-1110(92)90016-3. [DOI] [PubMed] [Google Scholar]
- 235.Nishikawa R, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc. Natl Acad. Sci. USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl Acad. Sci. USA. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ridgway J, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 238.Staberg M, et al. Combined EGFR- and notch inhibition display additive inhibitory effect on glioblastoma cell viability and glioblastoma-induced endothelial cell sprouting in vitro. Cancer Cell Int. 2016;16:34. doi: 10.1186/s12935-016-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Xu C, et al. TAZ Expression on endothelial cells is closely related to blood vascular density and VEGFR2 expression in astrocytomas. J. Neuropathol. Exp. Neurol. 2019;78:172–180. doi: 10.1093/jnen/nly122. [DOI] [PubMed] [Google Scholar]
- 240.Cantanhede IG, de Oliveira JRM. PDGF Family expression in glioblastoma multiforme: data compilation from ivy glioblastoma atlas project database. Sci. Rep. 2017;7:15271. doi: 10.1038/s41598-017-15045-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liu T, et al. PDGF-mediated mesenchymal transformation renders endothelial resistance to anti-VEGF treatment in glioblastoma. Nat. Commun. 2018;9:3439. doi: 10.1038/s41467-018-05982-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Williams LT. Signal transduction by the platelet-derived growth factor receptor. Science. 1989;243:1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- 243.Tu Y, et al. Expression of EphrinB2 and EphB4 in glioma tissues correlated to the progression of glioma and the prognosis of glioblastoma patients. Clin. Transl. Oncol. 2012;14:214–220. doi: 10.1007/s12094-012-0786-2. [DOI] [PubMed] [Google Scholar]
- 244.Uhl C, et al. EphB4 mediates resistance to antiangiogenic therapy in experimental glioma. Angiogenesis. 2018;21:873–881. doi: 10.1007/s10456-018-9633-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Audero E, et al. Expression of angiopoietin-1 in human glioblastomas regulates tumor-induced angiogenesis: in vivo and in vitro studies. Arterioscler. Thromb. Vasc. Biol. 2001;21:536–541. doi: 10.1161/01.ATV.21.4.536. [DOI] [PubMed] [Google Scholar]
- 246.Park JS, et al. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell. 2016;30:953–967. doi: 10.1016/j.ccell.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 247.Chae SS, et al. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clin. Cancer Res. 2010;16:3618–3627. doi: 10.1158/1078-0432.CCR-09-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jones CA, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat. Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cai H, et al. Roundabout4 suppresses glioma-induced endothelial cell proliferation, migration and tube formation in vitro by inhibiting VEGR2-mediated PI3K/AKT and FAK signaling pathways. Cell Physiol. Biochem. 2015;35:1689–1705. doi: 10.1159/000373982. [DOI] [PubMed] [Google Scholar]
- 250.Tchaicha JH, Mobley AK, Hossain MG, Aldape KD, McCarty JH. A mosaic mouse model of astrocytoma identifies alphavbeta8 integrin as a negative regulator of tumor angiogenesis. Oncogene. 2010;29:4460–4472. doi: 10.1038/onc.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 252.Vásquez X, Sánchez-Gómez P, Palma V. Netrin-1 in Glioblastoma neovascularization: the new partner in crime? Int. J. Mol. Sci. 2021 doi: 10.3390/ijms22158248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Angelucci C, Lama G, Sica G. Multifaceted functional role of semaphorins in Glioblastoma. Int. J. Mol. Sci. 2019;20:2144. doi: 10.3390/ijms20092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Schwab M, et al. Nucleolin is expressed in human fetal brain development and reactivated in human glial brain tumors regulating angiogenesis and vascular metabolism. bioRxiv. 2020 doi: 10.1101/2020.10.14.337824. [DOI] [Google Scholar]
- 255.Ta S, et al. Variants of WNT7A and GPR124 are associated with hemorrhagic transformation following intravenous thrombolysis in ischemic stroke. CNS Neurosci. Ther. 2021;27:71–81. doi: 10.1111/cns.13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Reis M, et al. Endothelial Wnt/β-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J. Exp. Med. 2012;209:1611–1627. doi: 10.1084/jem.20111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Martin M, et al. Engineered Wnt ligands enable blood-brain barrier repair in neurological disorders. Science. 2022;375:eabm4459. doi: 10.1126/science.abm4459. [DOI] [PubMed] [Google Scholar]
- 258.Bassett EA, et al. Norrin/Frizzled4 signalling in the preneoplastic niche blocks medulloblastoma initiation. eLife. 2016;5:e16764. doi: 10.7554/eLife.16764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Liu X, et al. TROY interacts with RKIP to promote glioma development. Oncogene. 2019;38:1544–1559. doi: 10.1038/s41388-018-0503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Griveau A, et al. A glial signature and Wnt7 signaling regulate glioma-vascular interactions and tumor microenvironment. Cancer Cell. 2018;33:874–889.e877. doi: 10.1016/j.ccell.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Neftel C, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178:835–849.e821. doi: 10.1016/j.cell.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Suvà ML, et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell. 2014;157:580–594. doi: 10.1016/j.cell.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Suvà ML, Tirosh I. The glioma stem cell model in the era of single-cell genomics. Cancer Cell. 2020;37:630–636. doi: 10.1016/j.ccell.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 264.Tirosh I, et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature. 2016;539:309–313. doi: 10.1038/nature20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Xie Y, et al. Key molecular alterations in endothelial cells in human glioblastoma uncovered through single-cell RNA sequencing. JCI Insight. 2021 doi: 10.1172/jci.insight.150861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tirosh I, Suvà ML. Dissecting human gliomas by single-cell RNA sequencing. Neuro Oncol. 2018;20:37–43. doi: 10.1093/neuonc/nox126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Couturier CP, et al. Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nat. Commun. 2020;11:3406. doi: 10.1038/s41467-020-17186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Toms SA, Ferson DZ, Sawaya R. Basic surgical techniques in the resection of malignant gliomas. J. Neurooncol. 1999;42:215–226. doi: 10.1023/A:1006121817861. [DOI] [PubMed] [Google Scholar]
- 269.Akeret K, et al. Anatomical phenotyping and staging of brain tumours. Brain. 2021 doi: 10.1093/brain/awab352. [DOI] [PubMed] [Google Scholar]
- 270.Salazar OM, Rubin P. The spread of glioblastoma multiforme as a determining factor in the radiation treated volume. Int. J. Radiat. Oncol. Biol. Phys. 1976;1:627–637. doi: 10.1016/0360-3016(76)90144-9. [DOI] [PubMed] [Google Scholar]
- 271.Burger PC, Heinz ER, Shibata T, Kleihues P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J. Neurosurg. 1988;68:698–704. doi: 10.3171/jns.1988.68.5.0698. [DOI] [PubMed] [Google Scholar]
- 272.Rutledge WC, Ko NU, Lawton MT, Kim H. Hemorrhage rates and risk factors in the natural history course of brain arteriovenous malformations. Transl. stroke Res. 2014;5:538–542. doi: 10.1007/s12975-014-0351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lawton MT, et al. Brain arteriovenous malformations. Nat. Rev. Dis. Prim. 2015;1:15008. doi: 10.1038/nrdp.2015.8. [DOI] [PubMed] [Google Scholar]
- 274.Rigamonti D, et al. Cerebral cavernous malformations. N. Engl. J. Med. 1988;319:343–347. doi: 10.1056/NEJM198808113190605. [DOI] [PubMed] [Google Scholar]
- 275.Choquet H, Pawlikowska L, Lawton MT, Kim H. Genetics of cerebral cavernous malformations: current status and future prospects. J. Neurosurg. Sci. 2015;59:211–220. [PMC free article] [PubMed] [Google Scholar]
- 276.Malinverno M, et al. Endothelial cell clonal expansion in the development of cerebral cavernous malformations. Nat. Commun. 2019;10:2761. doi: 10.1038/s41467-019-10707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Duran D, et al. Human genetics and molecular mechanisms of vein of Galen malformation. J. Neurosurg. Pediatr. 2018;21:367–374. doi: 10.3171/2017.9.PEDS17365. [DOI] [PubMed] [Google Scholar]
- 278.Elhammady MS, Ambekar S, Heros RC. Epidemiology, clinical presentation, diagnostic evaluation, and prognosis of cerebral dural arteriovenous fistulas. Handb. Clin. Neurol. 2017;143:99–105. doi: 10.1016/B978-0-444-63640-9.00009-6. [DOI] [PubMed] [Google Scholar]
- 279.Yuval Y, et al. Prenatal diagnosis of vein of Galen aneurysmal malformation: report of two cases with proposal for prognostic indices. Prenat. Diagn. 1997;17:972–977. doi: 10.1002/(SICI)1097-0223(199710)17:10<972::AID-PD167>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 280.Neil JA, Li D, Stiefel MF, Hu YC. Symptomatic de novo arteriovenous malformation in an adult: case report and review of the literature. Surg. Neurol. Int. 2014;5:148. doi: 10.4103/2152-7806.142796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.You LR, et al. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 282.Mack JJ, Iruela-Arispe ML. NOTCH regulation of the endothelial cell phenotype. Curr. Opin. Hematol. 2018;25:212–218. doi: 10.1097/MOH.0000000000000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Leblanc GG, Golanov E, Awad IA, Young WL. Biology of vascular malformations of the brain. Stroke. 2009;40:e694–e702. doi: 10.1161/STROKEAHA.109.563692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Whitehead KJ, Smith MC, Li DY. Arteriovenous malformations and other vascular malformation syndromes. Cold Spring Harb. Perspect. Med. 2013;3:a006635. doi: 10.1101/cshperspect.a006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Winkler EA, et al. Defective vascular signaling & prospective therapeutic targets in brain arteriovenous malformations. Neurochem. Int. 2019;126:126–138. doi: 10.1016/j.neuint.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 286.ten Dijke P, Arthur HM. Extracellular control of TGFβ signalling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 2007;8:857. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 287.McAllister KA, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 288.Johnson DW, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat. Genet. 1996;13:189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 289.Zhu W, Ma L, Zhang R, Su H. The roles of endoglin gene in cerebrovascular diseases. Neuroimmunol. Neuroinflamm. 2017;4:199–210. doi: 10.20517/2347-8659.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- 291.Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat. Genet. 2000;26:328–331. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- 292.Govani FS, Shovlin CL. Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur. J. Hum. Genet. 2009;17:860. doi: 10.1038/ejhg.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ricard N, et al. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119:6162. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ouarne M, et al. BMP9, but not BMP10, acts as a quiescence factor on tumor growth, vessel normalization and metastasis in a mouse model of breast cancer. J. Exp. Clin. Cancer Res. 2018;37:209. doi: 10.1186/s13046-018-0885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wang K, et al. Perturbations of BMP/TGF-β and VEGF/VEGFR signalling pathways in non-syndromic sporadic brain arteriovenous malformations (BAVM) J. Med. Genet. 2018;55:675–684. doi: 10.1136/jmedgenet-2017-105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Crist AM, Lee AR, Patel NR, Westhoff DE, Meadows SM. Vascular deficiency of Smad4 causes arteriovenous malformations: a mouse model of hereditary hemorrhagic telangiectasia. Angiogenesis. 2018;21:363–380. doi: 10.1007/s10456-018-9602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tual-Chalot S, Oh P, Arthur H. Mouse models of hereditary haemorrhagic telangiectasia: recent advances and future challenges. Front. Genet. 2015 doi: 10.3389/fgene.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Saito T, et al. Structural basis of the human endoglin-BMP9 interaction: insights into BMP signaling and HHT1. Cell Rep. 2017;19:1917–1928. doi: 10.1016/j.celrep.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Roman BL, Hinck AP. ALK1 signaling in development and disease: new paradigms. Cell. Mol. Life Sci. 2017;74:4539–4560. doi: 10.1007/s00018-017-2636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Suzuki Y, et al. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J. Cell Sci. 2010;123:1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 301.Park SO, et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J. Clin. Invest. 2009;119:3487–3496. doi: 10.1172/JCI39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Garrido-Martin EM, et al. Common and distinctive pathogenetic features of arteriovenous malformations in hereditary hemorrhagic telangiectasia 1 and hereditary hemorrhagic telangiectasia 2 animal models–brief report. Arterioscler. Thromb. Vasc. Biol. 2014;34:2232–2236. doi: 10.1161/ATVBAHA.114.303984. [DOI] [PubMed] [Google Scholar]
- 303.Bernabeu C, Bayrak-Toydemir P, McDonald J, Letarte M. Potential second-hits in hereditary hemorrhagic telangiectasia. J. Clin. Med. 2020 doi: 10.3390/jcm9113571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Knudson AG. Cancer genetics through a personal retrospectroscope. Genes Chromosomes Cancer. 2003;38:288–291. doi: 10.1002/gcc.10254. [DOI] [PubMed] [Google Scholar]
- 305.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109:1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 306.Boon LM, Mulliken JB, Vikkula M. RASA1: variable phenotype with capillary and arteriovenous malformations. Curr. Opin. Genet. Dev. 2005;15:265–269. doi: 10.1016/j.gde.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 307.Moteki Y, Akagawa H, Niimi Y, Okada Y, Kawamata T. Novel RASA1 mutations in Japanese pedigrees with capillary malformation-arteriovenous malformation. Brain Dev. 2019 doi: 10.1016/j.braindev.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 308.Revencu N, et al. RASA1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Hum. Mutat. 2013;34:1632–1641. doi: 10.1002/humu.22431. [DOI] [PubMed] [Google Scholar]
- 309.Revencu N, et al. RASA1 mosaic mutations in patients with capillary malformation-arteriovenous malformation. J. Med. Genet. 2019 doi: 10.1136/jmedgenet-2019-106024. [DOI] [PubMed] [Google Scholar]
- 310.Zeng X, et al. EphrinB2-EphB4-RASA1 signaling in human cerebrovascular development and disease. Trends Mol. Med. 2019;25:265–286. doi: 10.1016/j.molmed.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Bai J, Wang YJ, Liu L, Zhao YL. Ephrin B2 and EphB4 selectively mark arterial and venous vessels in cerebral arteriovenous malformation. J. Int. Med. Res. 2014;42:405–415. doi: 10.1177/0300060513478091. [DOI] [PubMed] [Google Scholar]
- 312.Kawasaki J, et al. RASA1 functions in EPHB4 signaling pathway to suppress endothelial mTORC1 activity. J. Clin. Invest. 2014;124:2774–2784. doi: 10.1172/JCI67084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Amyere M, et al. Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation. 2017;136:1037–1048. doi: 10.1161/CIRCULATIONAHA.116.026886. [DOI] [PubMed] [Google Scholar]
- 314.Ren AA, et al. PIK3CA and CCM mutations fuel cavernomas through a cancer-like mechanism. Nature. 2021;594:271–276. doi: 10.1038/s41586-021-03562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Limaye N, et al. Somatic activating PIK3CA mutations cause venous malformation. Am. J. Hum. Genet. 2015;97:914–921. doi: 10.1016/j.ajhg.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Limaye N, et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat. Genet. 2009;41:118–124. doi: 10.1038/ng.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sun B, et al. The rs9509 polymorphism of MMP-9 is associated with risk of hemorrhage in brain arteriovenous malformations. J. Clin. Neurosci. 2012;19:1287–1290. doi: 10.1016/j.jocn.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 318.Luks VL, et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J. Pediatr. 2015;166:1048–1054.e1041-1045. doi: 10.1016/j.jpeds.2014.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Shirley MD, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N. Engl. J. Med. 2013;368:1971–1979. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Couto JA, et al. Endothelial cells from capillary malformations are enriched for somatic GNAQ mutations. Plast. Reconstr. Surg. 2016;137:77e–82e. doi: 10.1097/PRS.0000000000001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Couto JavierA, et al. A somatic MAP3K3 mutation is associated with verrucous venous malformation. Am. J. Hum. Genet. 2015;96:480–486. doi: 10.1016/j.ajhg.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Couto JA, et al. Somatic MAP2K1 mutations are associated with extracranial arteriovenous malformation. Am. J. Hum. Genet. 2017;100:546–554. doi: 10.1016/j.ajhg.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Hong T, et al. High prevalence of KRAS/BRAF somatic mutations in brain and spinal cord arteriovenous malformations. Brain. 2019;142:23–34. doi: 10.1093/brain/awy307. [DOI] [PubMed] [Google Scholar]
- 324.Li QF, Decker-Rockefeller B, Bajaj A, Pumiglia K. Activation of Ras in the vascular endothelium induces brain vascular malformations and hemorrhagic stroke. Cell Rep. 2018;24:2869–2882. doi: 10.1016/j.celrep.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 325.Oka M, et al. KRAS G12D or G12V mutation in human brain arteriovenous malformations. World Neurosurg. 2019;126:e1365–e1373. doi: 10.1016/j.wneu.2019.03.105. [DOI] [PubMed] [Google Scholar]
- 326.Chen Y, et al. Interleukin-6 involvement in brain arteriovenous malformations. Ann. Neurol. 2006;59:72–80. doi: 10.1002/ana.20697. [DOI] [PubMed] [Google Scholar]
- 327.Simon M, et al. Association of a polymorphism of the ACVRL1 gene with sporadic arteriovenous malformations of the central nervous system. J. Neurosurg. 2006;104:945–949. doi: 10.3171/jns.2006.104.6.945. [DOI] [PubMed] [Google Scholar]
- 328.Pawlikowska L, et al. Polymorphisms in transforming growth factor-beta-related genes ALK1 and ENG are associated with sporadic brain arteriovenous malformations. Stroke. 2005;36:2278–2280. doi: 10.1161/01.STR.0000182253.91167.fa. [DOI] [PubMed] [Google Scholar]
- 329.Kim H, et al. Common variants in interleukin-1-beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc. Dis. 2009;27:176–182. doi: 10.1159/000185609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Su H, et al. Reduced expression of integrin alphavbeta8 is associated with brain arteriovenous malformation pathogenesis. Am. J. Pathol. 2010;176:1018–1027. doi: 10.2353/ajpath.2010.090453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Mikhak B, et al. Angiopoietin-like 4 (ANGPTL4) gene polymorphisms and risk of brain arteriovenous malformations. Cerebrovasc. Dis. 2011;31:338–345. doi: 10.1159/000322601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Weinsheimer S, et al. G protein-coupled receptor 124 (GPR124) gene polymorphisms and risk of brain arteriovenous malformation. Transl. Stroke Res. 2012;3:418–427. doi: 10.1007/s12975-012-0202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Chen H, et al. Polymorphisms of the vascular endothelial growth factor A gene and susceptibility to sporadic brain arteriovenous malformation in a Chinese population. J. Clin. Neurosci. 2011;18:549–553. doi: 10.1016/j.jocn.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 334.Zhao Y, et al. The rs522616 polymorphism in the matrix metalloproteinase-3 (MMP-3) gene is associated with sporadic brain arteriovenous malformation in a Chinese population. J. Clin. Neurosci. 2010;17:1568–1572. doi: 10.1016/j.jocn.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 335.Van Raamsdonk CD, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2008;457:599. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pan P, et al. Review of treatment and therapeutic targets in brain arteriovenous malformation. J. Cereb. Blood Flow. Metab. 2021;41:3141–3156. doi: 10.1177/0271678X211026771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Scherschinski L, et al. Genetics and emerging therapies for brain arteriovenous malformations. World Neurosurg. 2022;159:327–337. doi: 10.1016/j.wneu.2021.10.127. [DOI] [PubMed] [Google Scholar]
- 338.Wen PY, et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2022;23:53–64. doi: 10.1016/S1470-2045(21)00578-7. [DOI] [PubMed] [Google Scholar]
- 339.Selvasaravanan KD, et al. The limitations of targeting MEK signalling in Glioblastoma therapy. Sci. Rep. 2020;10:7401. doi: 10.1038/s41598-020-64289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Carlson TR, et al. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc. Natl Acad. Sci. USA. 2005;102:9884–9889. doi: 10.1073/pnas.0504391102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Krebs LT, et al. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Murphy PA, et al. Constitutively active Notch4 receptor elicits brain arteriovenous malformations through enlargement of capillary-like vessels. Proc. Natl Acad. Sci. USA. 2014;111:18007–18012. doi: 10.1073/pnas.1415316111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Murphy PA, et al. Notch4 normalization reduces blood vessel size in arteriovenous malformations. Sci. Transl. Med. 2012;4:117ra118. doi: 10.1126/scitranslmed.3002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.ZhuGe Q, et al. Notch-1 signalling is activated in brain arteriovenous malformations in humans. Brain. 2009;132:3231–3241. doi: 10.1093/brain/awp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Sivarapatna A, et al. Arterial specification of endothelial cells derived from human induced pluripotent stem cells in a biomimetic flow bioreactor. Biomaterials. 2015;53:621–633. doi: 10.1016/j.biomaterials.2015.02.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Orsenigo F, et al. Mapping endothelial-cell diversity in cerebral cavernous malformations at single-cell resolution. Elife. 2020 doi: 10.7554/eLife.61413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Chavkin NW, Hirschi KK. Single cell analysis in vascular biology. Front. Cardiovasc. Med. 2020 doi: 10.3389/fcvm.2020.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Morgan MK, Davidson AS, Assaad NNA, Stoodley MA. Critical review of brain AVM surgery, surgical results and natural history in 2017. Acta Neurochir. 2017;159:1457–1478. doi: 10.1007/s00701-017-3217-x. [DOI] [PubMed] [Google Scholar]
- 349.William LY, et al. Arteriovenous malformation. J. Neurosurg. 2007;106:731–732. doi: 10.3171/jns.2007.106.4.731. [DOI] [PubMed] [Google Scholar]
- 350.Al-Olabi L, et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J. Clin. Invest. 2018;128:1496–1508. doi: 10.1172/JCI98589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Gould J. Breaking down the epidemiology of brain cancer. Nature. 2018;561:S40–s41. doi: 10.1038/d41586-018-06704-7. [DOI] [PubMed] [Google Scholar]
- 352.Sharma A, et al. Onco-fetal reprogramming of endothelial cells drives immunosuppressive macrophages in hepatocellular carcinoma. Cell. 2020;183:377–394.e321. doi: 10.1016/j.cell.2020.08.040. [DOI] [PubMed] [Google Scholar]
- 353.Guo F-H, et al. Single-cell transcriptome analysis reveals embryonic endothelial heterogeneity at spatiotemporal level and multifunctions of microRNA-126 in mice. Arterioscler. Thromb. Vasc. Biol. 2022;42:326–342. doi: 10.1161/ATVBAHA.121.317093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Wälchli T, Farnhammer F, Fish JE. MicroRNA-based regulation of embryonic endothelial cell heterogeneity at single-cell resolution. Arterioscler. Thromb. Vasc. Biol. 2022;42:343–347. doi: 10.1161/ATVBAHA.122.317400. [DOI] [PubMed] [Google Scholar]
- 355.Haque A, Engel J, Teichmann SA, Lonnberg T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 2017;9:75. doi: 10.1186/s13073-017-0467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Rodriques SG, et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science. 2019;363:1463–1467. doi: 10.1126/science.aaw1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Ståhl PL, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353:78. doi: 10.1126/science.aaf2403. [DOI] [PubMed] [Google Scholar]
- 358.Ali HR, et al. Imaging mass cytometry and multiplatform genomics define the phenogenomic landscape of breast cancer. Nat. Cancer. 2020;1:163–175. doi: 10.1038/s43018-020-0026-6. [DOI] [PubMed] [Google Scholar]
- 359.Stoeckius M, et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods. 2017;14:865–868. doi: 10.1038/nmeth.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Hughes AJ, et al. Single-cell western blotting. Nat. Methods. 2014;11:749–755. doi: 10.1038/nmeth.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Eklund L, Bry M, Alitalo K. Mouse models for studying angiogenesis and lymphangiogenesis in cancer. Mol. Oncol. 2013;7:259–282. doi: 10.1016/j.molonc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Papagiannaki C, et al. Development of an angiogenesis animal model featuring brain arteriovenous malformation histological characteristics. J. NeuroInterventional Surg. 2017;9:204. doi: 10.1136/neurintsurg-2015-012173. [DOI] [PubMed] [Google Scholar]
- 363.Tsukada Y, et al. An in vivo model allowing continuous observation of human vascular formation in the same animal over time. Sci. Rep. 2021;11:745. doi: 10.1038/s41598-020-80497-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Zhu H, et al. Inflammation-mediated angiogenesis in Ischemic stroke. Front. Cell. Neurosci. 2021 doi: 10.3389/fncel.2021.652647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ha ET, et al. Chronic inflammation drives glioma growth: cellular and molecular factors responsible for an immunosuppressive microenvironment. Neuroimmunol. Neuroinflamm. 2014;1:66–76. doi: 10.4103/2347-8659.139717. [DOI] [Google Scholar]
- 366.Murat A, et al. Modulation of angiogenic and inflammatory response in glioblastoma by hypoxia. PLoS ONE. 2009;4:e5947. doi: 10.1371/journal.pone.0005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Garcia FJ, et al. Single-cell dissection of the human brain vasculature. Nature. 2022;603:893–899. doi: 10.1038/s41586-022-04521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Yang AC, et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature. 2022;603:885–892. doi: 10.1038/s41586-021-04369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Gerrits E, et al. Neurovascular dysfunction in GRN-associated frontotemporal dementia identified by single-nucleus RNA sequencing of human cerebral cortex. Nat. Neurosci. 2022;25:1034–1048. doi: 10.1038/s41593-022-01124-3. [DOI] [PubMed] [Google Scholar]
- 370.Ghobrial M, et al. The human brain vasculature shows a distinct expression pattern of SARS-CoV-2 entry factors. bioRxiv. 2020 doi: 10.1101/2020.10.10.334664. [DOI] [Google Scholar]
- 371.Yang AC, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Hodge RD, et al. Conserved cell types with divergent features in human versus mouse cortex. Nature. 2019;573:61–68. doi: 10.1038/s41586-019-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Rosińska S, Gavard J. Tumor vessels fuel the fire in glioblastoma. Int. J. Mol. Sci. 2021 doi: 10.3390/ijms22126514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Segura I, De Smet F, Hohensinner PJ, Ruiz de Almodovar C, Carmeliet P. The neurovascular link in health and disease: an update. Trends Mol. Med. 2009;15:439–451. doi: 10.1016/j.molmed.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 375.Amunts K, Zilles K. Architectonic mapping of the human brain beyond brodmann. Neuron. 2015;88:1086–1107. doi: 10.1016/j.neuron.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 376.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 377.Eberwine J, Sul J-Y, Bartfai T, Kim J. The promise of single-cell sequencing. Nat. Methods. 2014;11:25–27. doi: 10.1038/nmeth.2769. [DOI] [PubMed] [Google Scholar]
- 378.Hasle N, et al. High-throughput, microscope-based sorting to dissect cellular heterogeneity. Mol. Syst. Biol. 2020;16:e9442. doi: 10.15252/msb.20209442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.