ABSTRACT
Introduction: Freezing Cold Injuries (FCI) have been associated with long-term sequelae including vasospasm. The aims of the pilot study are to explore the research methodology and investigate the tolerability and safety of treatment with Botulinum Toxin-A (BTX-A) in FCI Sequelae.
Methodology: This pilot study tests the logistics, the treatment setting and the follow-up procedure in an early-phase, double-blinded, randomized, controlled trial study-design. The variables in the study were subjective symptoms, peripheral micro-vascularization/rewarming, somatosensory responsiveness, and generic measure of health status.
Results: No major challenges or difficulties were noticed according to the protocol or the study methodology. The monitoring of tolerability and safety of treatment with BTX-A did not reveal any major unwanted and/or adverse reactions among the patients in the pilot study and no challenges occurred during data collection of endpoints. The study revealed an inaccuracy of the 2nd degree FCI diagnosis and uncover a need for relevant and sufficient clinical information for FCI classification.
Conclusions: This pilot study showed the study methodology with minor adjustments is feasible in a future full-scale clinical trial. The recruitment process needs to be more refined to ensure that the eligible study participants are a homogenous group of FCI patients.
KEYWORDS: Frostbite, freezing cold injury, sequelae, botulinum toxin, BTX, military, thermography, quantitative sensory test
Introduction
Throughout history, cold injuries have been a major cause of morbidity during armed conflicts. In 218 BC, crossing the Southern Alps to reach Italy, Hannibal lost nearly half his army of 46,000 soldiers to cold injuries in just 15 days [1]. More recently, cold injury affected the majority of British soldiers fighting in the cold temperate climate of the Falkland Islands [2]. The annual incidence of freezing cold injuries (FCI) among conscripts in the Norwegian Armed Forces during the winter months in cold arctic conditions is about 2.1% [3].
The prognosis and progression of FCI have not previously been studied in large cohorts. Existing reports describe the spectrum of cold injuries as very broad, ranging from minimal tissue damage to major necrosis of the distal limbs with subsequent amputations [4]. In addition to the acute injury, cold injuries are often associated with long-term sequelae, including increased cold hypersensitivity [5–7].
These sequelae are less well studied and may compromise the future operational capability of the soldier and have considerable negative impact on the quality of life. The pathophysiology of these sequelae is still poorly understood, although peripheral neurovascular dysfunction plays an important role [8]. Sequelae of moderate frostbite are associated with an increased tendency to vasospasm [5]. BTX-A has been used to treat vasospasm, not only in FCIs, and has shown long-term symptom relief [9].
A case study has described the off-label use of Botulinum Toxin-A (BTX-A) to treat digital vasospasm to the hands of a Norwegian patient who suffered long-term sequelae, after a winter military exercise. At follow-up, the patient reported less pain, warmer hands and improved sensory function and had no recurrence of his complaints during a follow-up of more than 3 years [10]. Following case histories and off-label treatment experience, there is a need for controlled trials for the proof of concept.
The aims of the study were to test the research methodology, investigate the tolerability and safety, and assess the potential for a future full-scale efficacy study on Botulinum Toxin-A for Freezing Cold Injury.
Material and methods
The patients
The population studied in this trial was recruited from the Norwegian Armed Forces Health Registry (NAFHR) [11]. The NAFHR contains personal, service-related and health-related information for conscripts and Armed Forces personnel, including information and results from military screening examinations and tests [12].
In a quality assurance study, the NAFHR investigated the incidence and severity of frostbite sequelae for the period 01.01.2010–31.12.2014. According to this study, a total of 810 soldiers were registered in the NAFHR with an FCI [3]. This quality assurance study also asked whether the injured soldiers were interested in participating in a clinical trial investigating advanced treatment for frostbite sequelae. The selection of patients for this study is based on the historic data registered in the NAFHR in a previous quality assurance study [3]. In the NAFHR-questionnaire, 96 injured soldiers stated that they had a severe (2nd degree) frostbite injury, were still suffering from an FCI-sequelae, and wanted to be included in a later clinical study (Figure 1).
Figure 1.
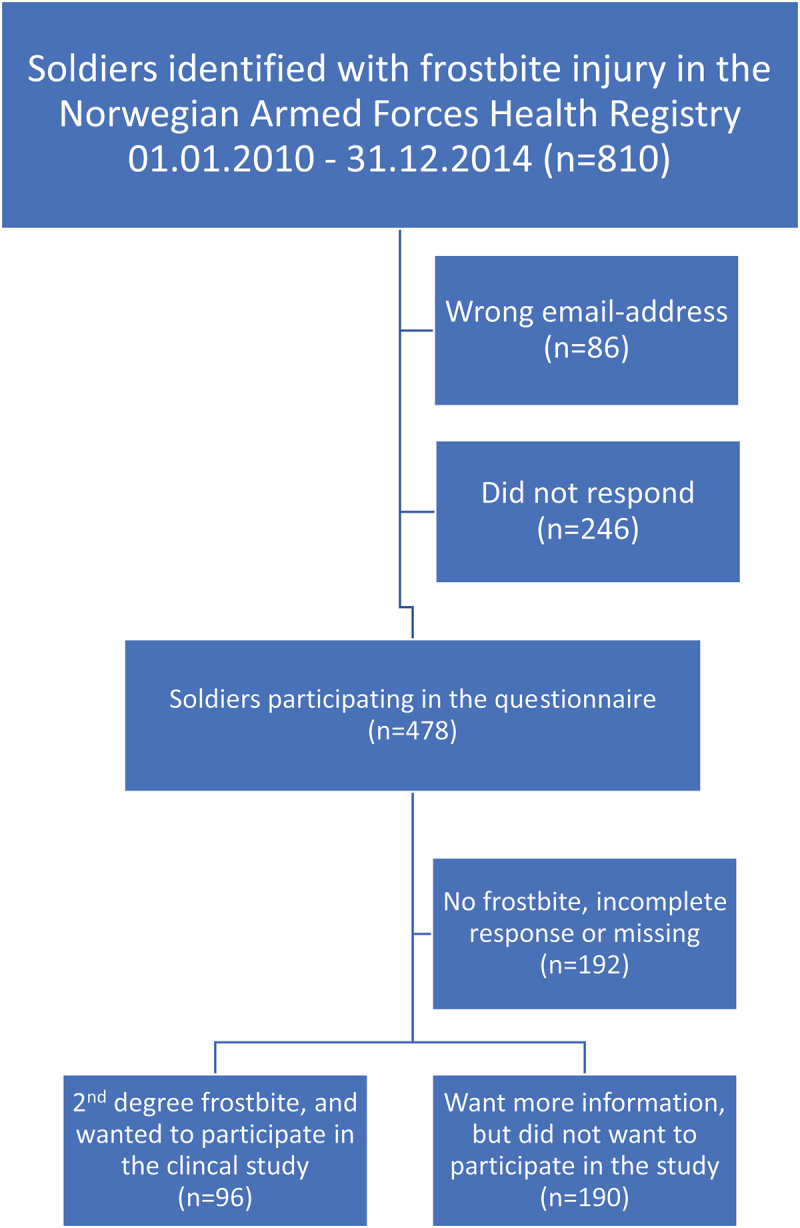
Flow chart for the 96 soldiers with frostbite sequel eligible for the study.
The patients’ statements in the NFHR-questionnaire that they had a 2nd degree including FCI-symptoms, specifically having had blisters with clear/white fluid was used to select a homogenous research cohort. Patients were also asked in the questionnaire whether they had undergone invasive treatment (injections) for FCI or had any contraindications to BTX-A (known hypersensitivity towards BTX-A, use of interactive drugs or drugs that may affect neuromuscular transmission). Any patient with previous invasive treatment and/or contraindication were regarded not eligible for the study. The authors do not know how many who gave such statements in the previous NAFHR quality assurance study.
All genders of all ages were invited to participate in the study. The four subjects in this pilot study (two BTX-A and two placebo) were randomly selected among the remaining 96 eligible injured soldiers. This pilot study was conducted, all the treatments were given, and all observations done from 14.03.2019 onwards.
Study design
This early-phase study used the randomised, double-blinded, placebo-controlled study design. The methodology for the future full-scale study aimed at investigating the efficacy of the intervention where other unspecified contributing factors are levelled out in a thorough and rigid study procedure.
Both the study subjects and all investigators were blinded to the contents of the injection. All participating soldiers got two injections, one at inclusion and one after 6 weeks. The participating soldiers were randomly selected to either the primary treatment group or the secondary treatment group.
The study subjects in the primary treatment group received BTX-A at their first injection at inclusion. The study subjects in the secondary treatment group received placebo as their first injection at inclusion. At 6 weeks follow-up, all study subjects, both in the primary and in the secondary treatment group, received their second injection, and all participating soldiers in both groups received BTX-A (Figure 2).
Figure 2.
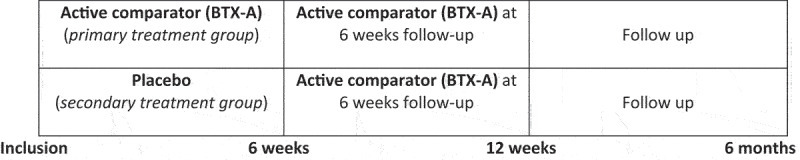
Primary and secondary treatment groups at different timepoints in the study.
At 6 weeks follow-up, the secondary treatment group (initial placebo group) served as a control for the primary treatment group. At a later follow-up at 12 weeks and 6 months, the dose–response effect was observed due to having received one or two injections of BTX-A.
Intervention
The treatments were administered by an experienced frostbite specialist (LdW). The injection of BTX-A was administered near the neurovascular bundle at the A1-A5 pulley at the basis of digit 1–5 in the palm of the hand. The most frequently used dosage was 100 U per hand (concentration 40 U per ml), 8–12 U/site [13].
Data collection
The data collected in the study was implemented in a Case Report Form, embedded in the Research Electronic Data Capture data handling system. Source data (original records or certified copies) were contained and kept under lock by the Research Department at University Hospital of North Norway (UNN). The data collection process was monitored by a Clinical Study Monitor (CSM) at the Research department at UNN. The study subject was assigned to a unique subject number when the subject signed the “Informed Consent Form”.
The study had obtained ethical approval from the Regional Committees for Medical and Health Research Ethics (REK) (ref 2018/1961).
The variables to be piloted in the study were:
subjective symptoms (Patients Subjective Symptom Score (PSSS)),
peripheral micro-vascularization/rewarming (Dynamic Infrared Thermography (DIRT)).
somatosensory responsiveness (Quantitative Sensory Test (QST)).
generic measure of health (EuroQol-5dimensions-3level (EQ-5D-3 L))
Data on these variables were collected at inclusion and during follow-up of the study participants at 6 weeks, 12 weeks, and 6 months.
The PSSS was a self-constructed, piloted, Visual Analogue Scale (VAS) based (1=no symptoms to 10=maximal symptoms) standardised questionnaire assessing the most important subjective symptoms following a primary frostbite including pain, hypersensitivity to cold, numbness of fingers and declined sensitivity of touch. PSSS also evaluated working ability of participants, as working ability tends to be lowered due to frostbite sequelae [5]. The DIRT examined the dynamic regulation of peripheral micro-vascularisation [14] as demonstrated in a case report [10] and in accordance with a Delphi-processed checklist among leading experts in thermography [15]. Examination of the dorsal side of the hands encompassed a pre-cooling phase (T1), a cooling phase with both hands, covered by a thin plastic bag, being immersed in water at 20°C for 1 min (T2), and a 4-min recovery phase (T3, T4, T5 and T6). The QST investigated the nature of the frostbite-related somatosensory abnormalities, monitor their recovery alongside the expected clinical improvement, and assessed the number of abnormal QST parameters using the standard procedure [16–18]. The QST-results from the study were analysed in collaboration with internationally recognised experts of QST at the University of Jena, Germany.
The EQ-5D-3L is a standardised measure of health status developed by the EuroQol-Group [19], regarding 5 dimensions of health (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), expressed at three levels (no problems, some problems, extreme problems) [20].
Statistical analysis
The aim of the study was to test the research methodology. This aim was met without the use of statistical analysis, whereas the personal feedback from patients, clinicians, statisticians and CSM was of major importance. The nature of this early-phase pilot study does not allow any effect-analyses according to the number of four participating soldiers. The separation test [21,22] was used to investigate between-group difference regarding an indication of an effect in a future full-scale effect study [23].
Results
The study was conducted according to the study protocol with four study subjects, two in the primary treatment group and two in the secondary treatment group.
Feasibility of the study protocol
The protocol was based on available scientific knowledge, clinical experience, and collaboration with the research department at UNN. The theoretical content of the protocol was acknowledged as appropriate and sufficient for the implementation of the study.
The protocol underwent high-quality controls according to the Norwegian and European regulation authorities with a particular attention towards adverse events. No major challenges or difficulties were noticed regarding the format or content of the protocol that should have any influence on the planning of the full-scale effect-study.
Methodology
The major challenge in this pilot was the inaccuracy of the FCI diagnosis. The patients were not able to give relevant and sufficient clinical information for classification of their injury, nor was the accuracy of the diagnosis in the Norwegian Armed Forces Health Registry (NAFHR) sufficient to classify patients to the targeted group of 2nd degree FCI.
This led to a concern as to whether two out of four patients probably had ever suffered a 2nd degree FCI, according to information given in the thorough clinical interview at inclusion. A non-freezing cold injury was perhaps just as likely. Nevertheless, all patients selected for the study wanted to be and were included in the pilot, regardless of the uncertainness regarding the clinical symptoms many years ago.
At every study visit, the patient’s medical history, symptoms, and observation of clinical signs were carefully recorded, followed by a clinical examination. The follow-up in the study was on patient subjective symptoms (VAS-scale), changes in rewarming ability (thermography), and the somatosensory nerve function (QST). No challenges occurred during data collection at endpoints at 6 weeks, 12 weeks, and 6 months of follow-up, compared with the results of their own baseline score at inclusion.
The treatment
The BTX-A and placebo (saline water) was prepared in syringes with a randomisation number/study subject number and the indication for use. The syringes contained the same amount of similar appearing fluid and both the investigator and the patient were blinded as to whether the syringe contained BTX-A or placebo. The blinding of the patient and doctor was sufficient and appropriate. No challenges occurred according to the study logistics in preparation of investigational substance and placebo at the hospital pharmacy at UNN.
The BTX-A were injected according to the planned methodology (Figure 3).
Figure 3.
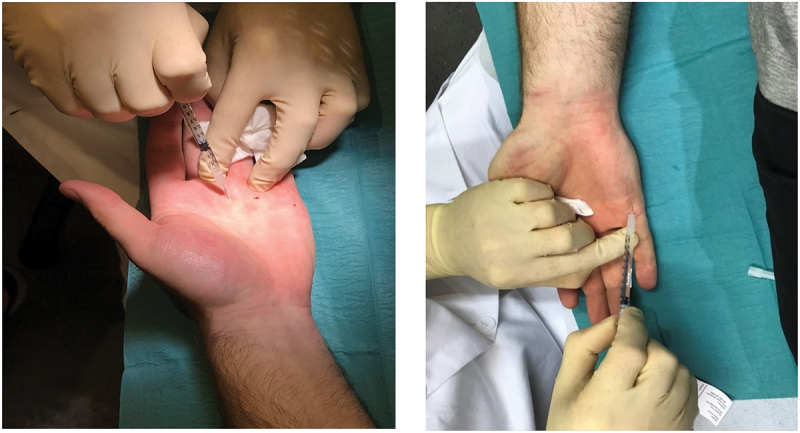
Injection of BTX-A were given near the neurovascular bundle at the A1-A5 pulley at the basis of digit 1–5 in the palm of the hand.
Adverse events
No intercurrent illness, serious adverse events, or unwanted effects were observed that could have had any influence on the judgement of the effect of BTX-A, the patient safety, the ability to deliver treatment, or at the primary study endpoints.
Weakness of muscles adjacent to the injection site was likely related to the spread of BTX-A from the site of injection. The weakness was not severe and resolved spontaneously within 1–2 weeks after injection. One practical aspect we encountered was the local distention of tissue that varied according to the injected volume. Although the procedure took only 4–5 min to execute, the injection of BTX-A was painful for a few minutes.
The injured soldiers
The four study subjects were all male soldiers at average age of 24 years (20–28), average height 189 cm (184–195), average weight 95 kg (84–110), average BMI = 26.6. They were all non-smokers and did not use any chewing or sniffing tobacco. None of the soldiers had tried any medical treatment; however, soldiers 1 and 3 said they had tried alternative treatment without being able to state the modality.
The data
This pilot study was not powered to calculate the effectiveness of BTX-A versus placebo. Nevertheless, all patients reported benefit. The study participants wished to continue treatment after the end of the follow-up. On the other hand, subjects #1 and #2 (placebo and BTX-A, respectively) had worsening PSSS scores at week 6 (after first injections) and worse than baseline scores at 6 months (3 months after their last injection of BTX-A). Being aware that this study does not produce any data on effect from BTX-A, one need also to bear in mind the possibility for an adverse effect to be found in future effect studies.
Self-reported symptoms
Self-reported symptoms and complaints (PSSS) were reported using a Visual Analogue Scale (1–10) where higher PSSS scores mean more symptoms. The sum of four symptoms for the four study subjects in the pilot is shown in Table 1.
Table 1.
Clinical data from the pilot study.
| The patients in the pilot study |
|||||
|---|---|---|---|---|---|
| Measurement | Time | Patient 1 (Placebo) |
Patient 2 (BTX-A) |
Patient 3 (Placebo) |
Patient 4 (BTX-A) |
| Total Patient subjective symptom score (PSSS)a | Inclusion | 22 | 12 | 19 | 27 |
| 6-weeks | 24 | 15 | 14 | 21 | |
| 12-weeks | 12 | 10 | 14 | 8 | |
| 6 months | 30 | 22 | 15 | 14 | |
aPSSS represents the sum of four sensational variables (pain, cold-hypersensitivity, numbness, loss-of-sensitivity of touch).
It is questionable whether PSSS as it currently stands is the best way to measure improvement from treatment with BTX-A. Further, Table 1 shows an example of how subjective data could be displayed, but in a full-scale effect study every single PSSS-symptom must be analysed more thoroughly. There may be relatively small effect sizes, as all subjects were noting improvement.
Rewarming (DIRT)
The rewarming was examined with repeated DIRT at inclusion, 6 weeks, 12-weeks, and 6-months follow-up. Figures 4 and 5 are examples of data presentation in terms of mean hand skin temperature versus time and thermographic images of the dorsal side of the hands encompassed a pre-cooling phase (T1), a cooling phase with hands immersed in water for 1 min (T2), and a four-minute recovery phase (T3, T4, T5 and T6).
Figure 4.
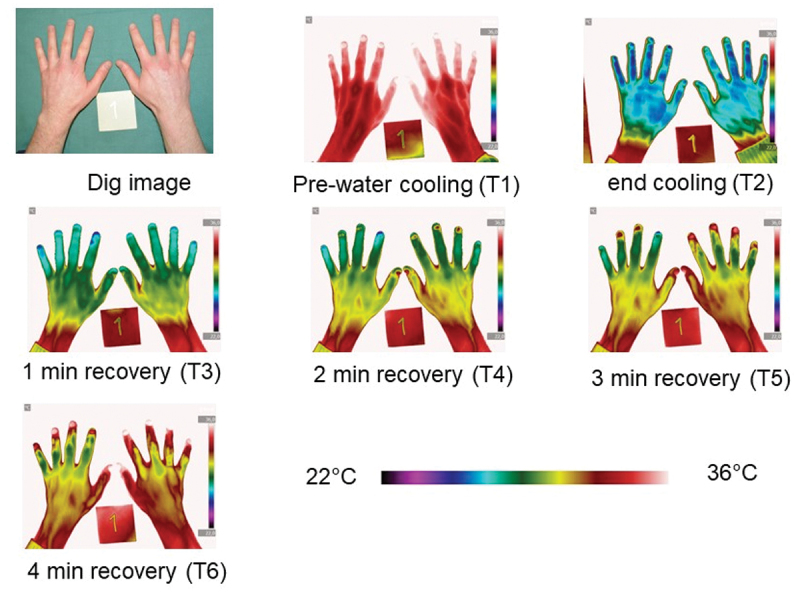
Thermographic images from patient 1 exemplify the rewarming of the dorsal side of the hands at 6 months follow-up encompassed a pre-cooling phase (T1), a cooling phase with hands immersed in water for one minute (T2), and a four-minute recovery phase (T3, T4, T5 and T6) demonstrated by Dynamic Infrared Thermography.
Figure 5.
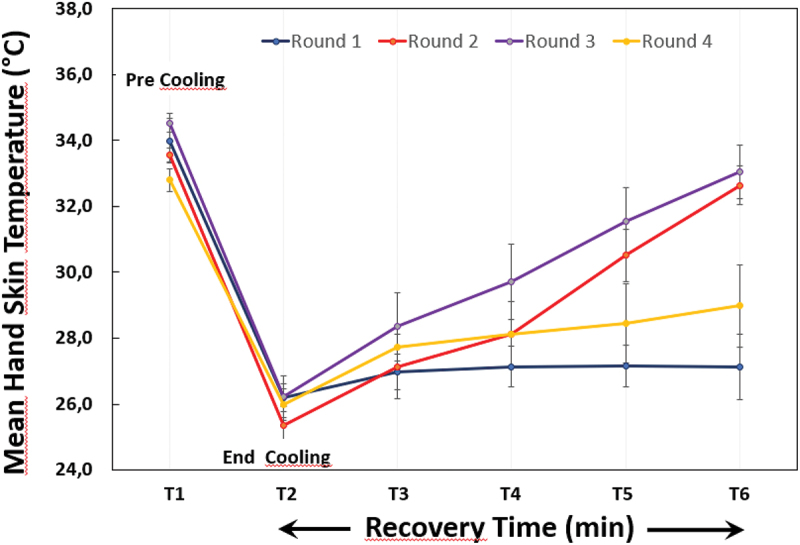
Mean hand skin temperature versus time demonstrated in patient 3 with Dynamic Infrared Thermography (DIRT) at four rounds (inclusion, 6 weeks, 12 weeks, 6 months) encompassed a pre-cooling phase (T1), a cooling phase with hands immersed in water for one minute (T2), and a four-minute recovery phase (T3, T4, T5 and T6).
Somatosensory measurements (QST)
We assessed all 13 somatosensory parameters of the standardised QST protocol [24] for the affected hand and the back as a control area, which is normally not influenced by any frostbite. We found no systematic changes in any parameter in the control area. Additionally, we did not detect any systematic changes in the perception or pain thresholds to mechanic stimulation at the affected hand. However, there were striking abnormalities in all subjects with respect to thermal detection thresholds (see Figure 6 for summary). Interestingly, these changes were reduced in all subjects at the last point of evaluation (Figure 6). Unfortunately, QST-data were not obtained at 6 months follow-up.
Figure 6.
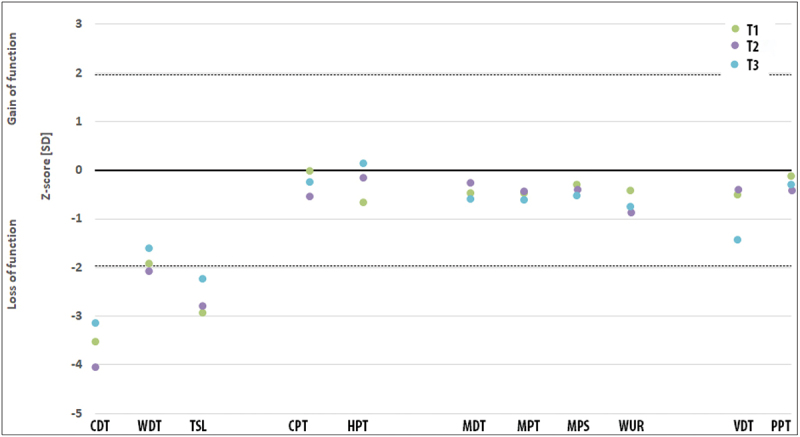
Quantitative sensory testing (QST) at the affected hand for all subjects over time. While the mechanical parameters are all within the confidence interval for age-corrected healthy subjects (dashed line − 95% confidence interval), there are impressive changes for the thermal detection thresholds, especially for the cold detection threshold (CDT). After therapy, these parameters returned in a direction towards normalization. WDT – warm detection threshold, TSL – thermal sensory limen, CPT – cold pain threshold, HPT – heat detection threshold, MDT – mechanical detection threshold, MPT – mechanical pain threshold, MPS – mechanical pain sensitivity, WUR – wind-up ratio, VDT – vibration detection threshold, PPT – pressure pain threshold; T1 – before intervention, T2–6 weeks, T3–12 weeks follow-up. QST-data were not obtained at 6 months follow-up.
Statistical analysis
This study analysed the data with regard to a statistical indication for further research using the separation test [23]. The test uses the standard deviation of the effect estimate (SDE) of the mean difference, then calculated the value of Δ = 1.645*SDE. Further research is indicated if the mean difference exceeds Δ/2 (in the favourable direction), but not if it is lower than Δ/2 (in the unfavourable direction) [21]. In our study, further research is not recommended since the mean difference in score is lower than Δ/2. The results for PSSS in our pilot study are shown in Table 2.
Table 2.
Mean difference of change in mean PSSS between the groups, standard deviation of the difference and threshold (Δ/2) for indication of effect of the between group difference. Further research is not recommended since the difference in score is lower than Δ/2.
| Mean differencea | SDEb | Δ/2 | |
|---|---|---|---|
| Baseline versus 12 weeks | 3.0 | 8.86 | 7.29 |
| Baseline versus 6 months | 3.5 | 12.97 | 10.67 |
aMean difference in score between the intervention group and control (see Table 1).
bSDE, Standard deviation of effect estimates.
Discussion
This study has investigated the research strategy, study-logistics, tolerability, and safety of treatment with BTX-A for frostbite sequelae. The methodology in this pilot study is shown with minor adjustments to be feasible in a future full-scale effect study. Recent literature also recommends BTX-A in FCI treatment [25,26]. However, a major limitation in this study is the questionable diagnosis, and a higher diagnostic precision level may be more feasible. A full-scale study is not warranted by the separation test, at least not without reconsidering the selection process and the study design.
The research methodology
There is need for better diagnostic accuracy and selection of patients for participation in future studies [7]. The recruitment process needs to be more refined in later studies to ensure that the eligible study participants are a homogenous group of patients with a 2nd degree FCI. Recall bias might obscure the accuracy of the recruiting questionnaires. A higher standard for enrolment and, perhaps, reviewing the patients’ medical files before inclusion could limit the recruitment bias in future studies.
Our clinical experience of the pharmacological duration of action of BTX-A is a few weeks, in line with current research [27,28], however the results from the pilot does not add on any evidence to this understanding. The dosage and drug administration in our trial are not outlined in previous research or guidelines but was based on a review on the use of BTX-A treatment of Raynaud’s phenomenon, that were used to decide the injection techniques and botulinum dosages [13].
Additionally, we have used BTX-A as off-label treatment for the last 10 years for different indications, including frostbite sequelae [10]. However, it remains unknown what is the optimal dosing regimen for this condition [5,13,29–31]. Studying the duration of action may help future studies design how frequently and for how long to treat with a specific formulation of BTX. The exact timeline for effect of BTX-A in FCI is still unknown, and further research is needed. Further studies are needed to explore the effect of different dosages and injection site(s).
Temporary muscle weakness is a common and expected pharmacological action of BTX injected into or adjacent to muscle tissue as found in our study. The use of BTX has not previously been associated with serious allergic reactions or anaphylactic shock [13,32]. No serious adverse event was observed in the study or has ever taken place during all years of personal experience (LdW) with BTX-A in off-label treatment.
The QST showed that three parameters were systematically changed in our subjects, i.e. thermal detection thresholds (CDT, WDT, TSL). This is a rather unusual type of change considering the three subtypes of changes in neuropathic pain [33]. However, a previous report on macro-replantation also demonstrated this specific pattern [34]. There may exists a specific pattern of change for frostbite injury. Nevertheless, due to the observed changes in thermal detection thresholds, it is recommended that these parameters be included in a larger study. Interestingly, CDT showed the largest discrepancy and a tendency to normalisation in all subjects, which is plausibly connected to frostbite. If the protocol would allow to include only one parameter, we would suggest including CDT.
It is a limitation of the study that data in the pilot are, due to their nature, are not able to add on to any evidence regarding effect or effectiveness of BTX-A for frostbite sequelae. Further, a full-size clinical trial based on experience in the pilot will need a thorough Statistical Analysis Plan (SAP).
A sample size calculation for a full-scale effectiveness study based on results from this pilot study suffer from uncertainty in both the effect estimate and in the standard deviation caused by low number of participants in the pilot. Nevertheless, the estimated sample size for a two-sample means test comparing BTX-A with Placebo at 12-week observation require n = 276 (n per group = 138).
The separation test applied does not warrant a full-size clinical trial [35]. However, a major concern regarding the separation test is the low number of participating soldiers, and the heterogeneity of the study group. Additionally, in this study, we are comparing the two groups at week 12 and at 6 months where both the intervention group and the placebo group have received the BTX-A injection. We could potentially have detected larger group differences with a “true” intervention/placebo comparison, where the comparing (placebo) group did not receive the BTX-injection at week 6. However, the study design we used was a tradeoff to explore both the intervention-placebo comparison at week 6, and the dose–response relation between the two groups at week 12 and 6 months.
Conclusions
This pilot study showed the study methodology with minor adjustments might be feasible for future research
The selection of FCI-patients’ needs increased attention in future research
BTX-A is in this study was found to have the tolerability and safety for future research
A full-scale study is not warranted by the separation test, at least not without reconsidering the selection process and the study design.
Acknowledgments
The authors are grateful to the Norwegian Armed forces – Joint Medical Forces for the initiative to the study. Much appreciation also goes to Solveig Johansson and Theresa Niesman at the National Research Center for Alternative and Complementary Medicine for the Quantitative Sensory Measurements. The authors wish to thank the Research Unit at UNN for contributing to protocol and handling of the data for the study. We also want to thank the Hospital Pharmacy at UNN for preparing and delivering the Botulinum Toxin and the placebo for the study. We also appreciate all valuable input and discussions with our colleagues at UNN, the Norwegian Armed Forces-Joint medical services, and at UiT - The Arctic University of Norway.
Funding Statement
The publication charges for this article have been funded by a grant from The North Atlantic Treaty Organization (NATO). A grant for the cost of the study was achieved from the Norwegian Armed Forces – Joint Medical Services. No further funding was received.
Disclosure statement
No potential conflict of interest was reported by the authors.
Authors’ contributions
AJN, LdW and JM conceived the study. LdW performed the clinical treatment in the study. JM performed all the Thermographic imaging. TWI conducted the statistical analyses and TWE did the QST analysis. All authors gave input in how to interpret and structure the findings, reviewed subsequent versions. All authors have read and approved the final manuscript.
Availability of data and materials
The raw dataset is not available due to Norwegian military and privacy regulations. Applicants for any data must be prepared to conform to Norwegian privacy regulations.
Ethics approval and trial registration
The study has been approved by the Regional Committee for Medical and Health Research Ethics (REK 2018/1961). Informed consent was obtained from all participants.
EudraCT number 2018-002599-42 (27.06.2018)
References
- [1].Guly H. History of accidental hypothermia. Resuscitation. 2011;82(1):122–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Golden FS, Francis TJ, Gallimore D, et al. Lessons from history: morbidity of cold injury in the Royal Marines during the Falklands Conflict of 1982. Extrem Physiol Med. 2013;2(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Norheim AJ, Borud E. Frostbite in the Norwegian Armed Forces. Tidsskrift for den Norske laegeforening. 2018;138(14). DOI: 10.4045/tidsskr.17.1070 [DOI] [PubMed] [Google Scholar]
- [4].Imray C, Grieve A, Dhillon S, et al. Cold damage to the extremities: frostbite and non-freezing cold injuries. Postgrad Med J. 2009;85(1007):481–488. [DOI] [PubMed] [Google Scholar]
- [5].Ervasti O, Hassi J, Rintamaki H, et al. Sequelae of moderate finger frostbite as assessed by subjective sensations, clinical signs, and thermophysiological responses. Int J Circumpolar Health. 2000;59(2):137–145. [PubMed] [Google Scholar]
- [6].Steinberg T, Norheim AJ, Bjerkan G, et al. Freezing cold injuries among soldiers in the Norwegian Armed Forces - a cross sectional study. Int J Circumpolar Health. in press;2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Norheim AJ, Sulliwan-Kwantes W, Steinberg T, et al., The classification of freezing cold injuries - a NATO research task group position paper. Int J Circumpolar Health. 81: In press. 2022; 10.1080/22423982.2022.2049491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Handford C, Buxton P, Russell K, et al. Frostbite: a practical approach to hospital management. Extrem Physiol Med. 2014;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Van Beek AL, Lim PK, Gear AJ, et al. Management of vasospastic disorders with botulinum toxin a. Plast Reconstr Surg. 2007;119(1):217–226. [DOI] [PubMed] [Google Scholar]
- [10].Norheim AJ, Mercer J, Musial F, et al. A new treatment for frostbite sequelae; Botulinum toxin. Int J Circumpolar Health. 2017;76(1):1273677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Forsvarets helsregister. Available from: https://helsedata.no/no/forvaltere/forsvarsdepartementet/forsvarets-helseregister/
- [12].Forskrift om innsamling og behandling av opplysninger i Forsvarets helseregister. Available from: https://lovdata.no/dokument/SF/forskrift/2005-09-02-1010
- [13].Iorio ML, Masden DL, Higgins JP. Botulinum toxin a treatment of Raynaud’s phenomenon: a review. Semin Arthritis Rheum. 2012;41(4):599–603. [DOI] [PubMed] [Google Scholar]
- [14].Ring EF, Ammer K. Infrared thermal imaging in medicine. Physiol Meas. 2012;33(3):R33–46. [DOI] [PubMed] [Google Scholar]
- [15].Moreira DG, Costello JT, Brito CJ, et al. Thermographic imaging in sports and exercise medicine: a Delphi study and consensus statement on the measurement of human skin temperature. J Therm Biol. 2017;69:155–162. [DOI] [PubMed] [Google Scholar]
- [16].Maier C, Baron R, Tölle T, et al. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150(3):439–450. [DOI] [PubMed] [Google Scholar]
- [17].Magerl W, Krumova EK, Baron R, et al. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151(3):598–605. [DOI] [PubMed] [Google Scholar]
- [18].Pfau DB, Krumova EK, Treede RD, et al. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): reference data for the trunk and application in patients with chronic postherpetic neuralgia. Pain. 2014;155(5):1002–1015. DOI: 10.1016/j.pain.2014.02.004 [DOI] [PubMed] [Google Scholar]
- [19].EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- [20].EQ-5D-3L. Available from: https://euroqol.org/eq-5d-instruments/eq-5d-3l-about/
- [21].Norheim AJ, Fønnebø VM, Lindeland JP, et al. Reflexology for acute rhinosinusitis–results from a blinded, early-phase comparative trial. EXPLORE. 2022;19:36–41. [DOI] [PubMed] [Google Scholar]
- [22].Engen DJ, Wahner-Roedler DL, Nadolny AM, et al. The effect of chair massage on muscular discomfort in cardiac sonographers: a pilot study. BMC Complement Altern Med. 2010;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aickin M. Separation tests for early-phase complementary and alternative medicine comparative trials. Evid-Based Integr Med. 2004;1(4):225–231. [Google Scholar]
- [24].Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. [DOI] [PubMed] [Google Scholar]
- [25].Regli IB, Strapazzon G, Falla M, et al. Long-Term sequelae of frostbite—A scoping review. Int J Environ Res Public Health. 2021;18(18):9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].de Villeneuve Bargemon J-B, Prenaud C. Comment to: frostbite of the extremities–recognition, evaluation and treatment: frostbite and botulinum toxin a. Injury. 2022;53(12):4159. [DOI] [PubMed] [Google Scholar]
- [27].Eleopra R, Rinaldo S, Montecucco C, et al. Clinical duration of action of different botulinum toxin types in humans. Toxicon. 2020;179:84–91. [DOI] [PubMed] [Google Scholar]
- [28].Marvulli R, Ranieri M, Rizzo LB, et al. Clinical onset of action of incobotulinum toxin a preparation. CNS Neurol Disord Drug Targets. 2022;22:916–923. DOI: 10.2174/1871527321666220630154404. [DOI] [PubMed] [Google Scholar]
- [29].Jeynes LC, Gauci CA. Evidence for the use of botulinum toxin in the chronic pain setting—a review of the literature. Pain Pract. 2008;8(4):269–276. [DOI] [PubMed] [Google Scholar]
- [30].Neumeister MW. Botulinum toxin type a in the treatment of Raynaud’s phenomenon. J Hand Surg. 2010;35(12):2085–2092. [DOI] [PubMed] [Google Scholar]
- [31].Rossetto O, Pirazzini M, Montecucco C. Current gaps in basic science knowledge of botulinum neurotoxin biological actions. Toxicon. 2015;107:59–63. [DOI] [PubMed] [Google Scholar]
- [32].Segreto F, Marangi GF, Cerbone V, et al. The role of botulinum toxin a in the treatment of raynaud phenomenon. Ann Plast Surg. 2016;77(3):318–323. [DOI] [PubMed] [Google Scholar]
- [33].Baron R, Maier C, Attal N, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158(2):261–272. DOI: 10.1097/j.pain.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Blume KR, Racz J, Franz M, et al. Quantitative sensory testing after macroreplantation: evidence for a specific somatosensory profile. Pain. 2018;159(7):1289–1296. [DOI] [PubMed] [Google Scholar]
- [35].Aickin M. The importance of early phase research. J Altern Complement Med. 2007;13(4):447–450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw dataset is not available due to Norwegian military and privacy regulations. Applicants for any data must be prepared to conform to Norwegian privacy regulations.


