ABSTRACT
Pancreatic cancer is among the most lethal malignant neoplasms, and few patients with pancreatic cancer benefit from immunotherapy. We retrospectively analyzed advanced pancreatic cancer patients who received PD-1 inhibitor-based combination therapies during 2019–2021 in our institution. The clinical characteristics and peripheral blood inflammatory markers (neutrophil-to-lymphocyte ratio [NLR], platelet-to-lymphocyte ratio [PLR], lymphocyte-to-monocyte ratio [LMR], and lactate dehydrogenase [LDH]) were collected at baseline. Chi-squared and Fisher’s exact tests were used to evaluate relationships between the above parameters and tumor response. Cox regression analyses were employed to assess the effects of baseline factors on patients’ survival and immune-related adverse events (irAEs). Overall, 67 patients who received at least two cycles of PD-1 inhibitor were considered evaluable. A lower NLR was independent predictor for objective response rate (38.1% vs. 15.2%, P = .037) and disease control rate (81.0% vs. 52.2%, P = .032). In our study population, patients with lower LDH had superior progression-free survival (PFS) and overall survival(OS) (mPFS, 5.4 vs. 2.8 months, P < .001; mOS, 13.3 vs. 3.6 months, P < .001). Liver metastasis was verified to be a negative prognostic factor for PFS (2.4 vs. 7.8 months, P < .001) and OS (5.7 vs. 18.0 months, P < .001). The most common irAEs were hypothyroidism (13.4%) and rash (10.5%). Our study demonstrated that the pretreatment inflammatory markers were independent predictors for tumor response, and the baseline LDH level and liver metastasis were potential prognostic markers of survival in patients with pancreatic cancer treated with PD-1 inhibitors.
KEYWORDS: Pancreatic cancer, PD-1 inhibitors, inflammatory markers, liver metastasis, biomarkers
Introduction
Pancreatic cancer is among the most lethal malignant neoplasms worldwide, with a 5-year overall survival rate of less than 10%.1 The incidence of pancreatic cancer and associated deaths are increasing worldwide, and it is projected to become the second leading cause of cancer-related deaths in the US before 2030.2 Currently, radical surgical resection is the only potentially curative treatment for pancreatic cancer; however, fewer than 20% of patients are resectable at initial presentation. Furthermore, most patients (50%–55%) present with metastatic disease at diagnosis, for which chemotherapy and radiotherapy remain the dominant therapeutic regimens. Therefore, it is critical to explore novel treatment modalities for pancreatic cancer.
In recent years, immune checkpoint inhibitors (ICIs) have achieved excellent results in the treatment of various tumors, especially in patients with programmed cell death-ligand 1 (PD-L1) positive, microsatellite instability-high/deficient mismatch repair (MSI-H/dMMR), or tumor mutational burden-high (TMB-H). Unfortunately, only limited clinical activity of ICIs was observed in patients with pancreatic cancer, which is likely due to the profoundly suppressive tumor immune microenvironment.3 Le and colleagues4 found that patients with advanced mismatch repair deficiency (dMMR) cancers were responsive to anti-PD-1 therapy. Similar results were further confirmed by the phase II KEYNOTE-158 study, which reported that the objective response rate (ORR) of pancreatic cancer cohort was 18.2% and median progression-free survival (mPFS) and median overall survival (mOS) of 2.1 and 4.0 months, respectively.5 Additionally, patients with TMB-H (≥10 mutations/Mb) achieved a higher ORR compared to those in the tumor mutational burden-low (TMB-L) group (29% vs. 5%) across ten different tumor types.6 According to the National Comprehensive Cancer Network (NCCN) guidelines, pembrolizumab (an anti-PD-1 receptor antibody) was recommended for the subset of patients with MSI-H/dMMR or TMB-H.7 Only a minority of patients with pancreatic cancer meet these conditions, and the detection of these biomarkers is extremely expensive. Hence, it would be desirable to identify other biomarkers that are convenient to use and can accurately predict the efficacy of immunotherapy.
Inflammation is known to be closely related to tumor development and progression.8 The regulation of the tumor microenvironment (TME) largely relies on the infiltration of inflammatory cells and the release of pro-inflammatory molecules, which are closely involved in tumor proliferation, differentiation, and metastasis.9 Anti-inflammatory agents, such as non-steroidal anti-inflammatory drugs, can effectively treat cancer and reduce distant metastasis. Similar positive results were also reported in different cancers with cytokine or chemokine-targeted therapy.10 Recently, inflammatory response factors, such as prognostic nutritional index (PNI), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), and lactate dehydrogenase (LDH) were found to be associated with tumor response and patients’ prognosis. In a retrospective study of peripheral blood markers predictive of the outcome in non-small cell lung cancer (NSCLC), it was found that NLR < 5, LDH < 240 U/L, or PNI ≥ 45 was favorably associated with better outcomes.11 In another retrospective analysis of inflammatory markers that could predict survival in patients with advanced gastric and colorectal cancers receiving anti-PD-1 therapy, monocyte-to-lymphocyte ratio (MLR) > 0.31, NLR > 5, and PLR > 135 were found to be poor prognostic factors.12 Moreover, Waninger et al reported that liver metastases and elevated LDH levels appear to be important prognostic indicators of PFS and OS in patients with metastatic cutaneous melanoma receiving PD-1 inhibitor therapy.13 However, the potential predictors for pancreatic cancer have not been fully clarified.
In this study, we retrospectively analyzed patients with advanced pancreatic cancer who underwent anti-PD-1 therapy in our institution and further explored the potential biomarkers that can predict the efficacy and prognosis of immunotherapy.
Materials and methods
Patients
This was a single-center, retrospective analysis of patients with advanced pancreatic cancer who were treated with ICI-based combination approaches from February 2019 to November 2021 at the Comprehensive Cancer Center of Nanjing Drum Tower Hospital. Only patients who agreed to the study and provided signed consent were included. Further inclusion criteria were as follows: (1) histological or cytological diagnosis of pancreatic cancer, at a locally advanced or metastatic stage, (2) an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2 points, (3) received anti-PD-1 therapy at least twice and had a post-baseline computed tomography scan, (4) peripheral blood cell count before the initiation of anti-PD-1 inhibitor was determined, (5) and acquired survival status. Patients with any of the following criteria were excluded: bone marrow deficiency, defined as platelets <100 × 109/L and/or white blood cells <3.5 × 109/L; abnormal liver functions, defined as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels greater than 5× the upper limit of normal; and renal dysfunction, defined as a creatinine clearance of less than 30 mL/min. Patients who suffered from any severe concomitant disease were also excluded. Flow diagram of the study population is shown in Figure 1.
Figure 1.
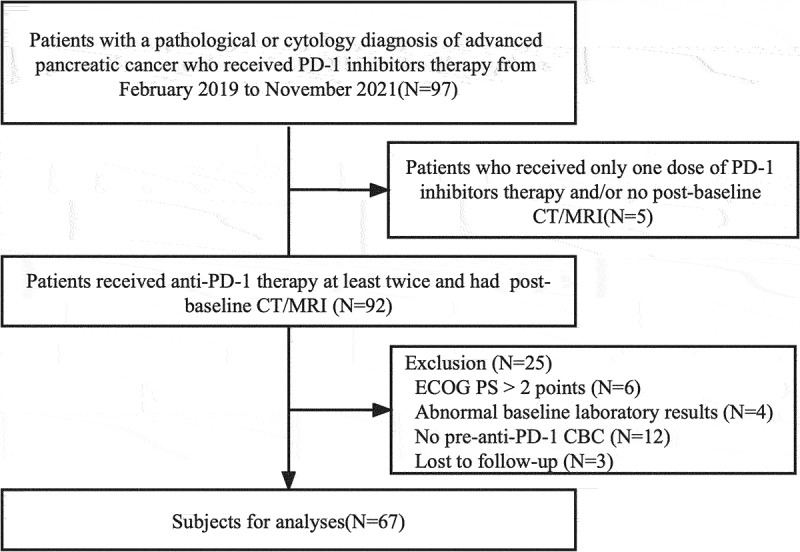
Flow diagram of the study population.
Abbreviations: CT, Computed tomography; MRI, magnetic resonance imaging; ECOG PS, Eastern Cooperative Oncology Group performance status; CBC, complete blood count.
Treatment
All patients were treated with PD-1 inhibitors from different producers according to the instructions, such as Toripalimab (3 mg/kg every 2 weeks), Sintilimab (200 mg every 3 weeks), and Pembrolizumab (2 mg/kg every 3 weeks), and in combination with other treatment schemes, including chemotherapy, radiotherapy and targeted therapy. Patients received immunotherapy until disease progression, the occurrence of unacceptable toxicity, and/or the patient’s decision to discontinue.
Assessment
Computed tomography (CT) and/or magnetic resonance imaging (MRI) were conducted at baseline and every two cycles during the treatment. Tumor responses were evaluated according to the response evaluation criteria in solid tumors (RECIST) version 1.1, in which, we assessed the PFS, OS, ORR [defined as the sum of the portion of complete response (CR) and partial response (PR)], and disease control rate [DCR; defined as the sum of CR, PR, and stable disease (SD)]. Additionally, the clinical characteristics and peripheral blood data of NLR, PLR, LMR, and LDH were collected at baseline. Meanwhile, every enrollment underwent histological next-generation sequencing (NGS) analysis. TMB was estimated by counting the coding somatic mutations, including SNVs and Indels, per megabase of the sequence examined in each patient. The MSI status was inferred based on the MANTIS score, and microsatellite regions were manually reviewed in Integrative Genomics Viewer for confirmation. PD-L1 expression was interpreted as a combined positive score (CPS), which was defined as the number of PD-L1-positive cells (including tumor cells, lymphocytes, and macrophages) divided by the total number of tumor cells, and then multiplied by 100. The safety was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. All patients were followed up until death or the last follow-up (15th January 2022).
Statistical analysis
The cutoff values for PLR and LMR were 135 and 2, respectively, in accordance with previous literature.12,14 The optimal cutoff values for NLR and LDH were 2 and 265, respectively, and were determined using R Foundation (version 4.0.1) and SPSS software (version 26.0). The Chi-squared test and Fisher’s exact test were used to evaluate relationships between the inflammatory markers and tumor response. Survival curves were calculated using the Kaplan – Meier method and compared using an unadjusted log-rank test. The hazard ratio (HR) and 95%CI were tested using the Cox proportional hazard model. Univariate and multivariate regression analyses were used to estimate the relevant factors for efficacy and safety. P < .05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
The baseline characteristics of the enrolled patients are summarized in Table 1. From February 2019 to November 2021, 67 patients with locally advanced or metastatic pancreatic cancer received at least two cycles of anti-PD-1 therapy and had a post-baseline CT scan in our institution (Figure 1). In total, the median age of the study population was 61.4 years (range 33–84 years), and 44 (65.7%) patients were male. Most participants (n = 47, 70.1%) had an ECOG PS of 0–1. Of the enrolled patients, 46 (68.7%) were diagnosed with metastatic pancreatic cancer, and 36 patients (53.7%) showed liver metastasis. There were 54 patients who showed a higher carbohydrate antigen 19–9 (CA19–9) level before treatment. Additionally, 4 patients were TMB-H, only 1 patient was MSI-H, and 3 showed PD-L1-positive tumors with CPS≥1. A total of 25 patients (37.3%) received PD-1 inhibitors as the first-line treatment and 26 patients (38.8%) underwent radiotherapy during the treatment. The selection of systematic treatment for advanced pancreatic cancer is shown in the Supplementary Table S1.
Table 1.
The baseline characteristics of patients.
| Characteristics | Total (N = 67) N (%) |
|---|---|
| Age (years) | |
| Median (range) | 61.4 (33–84) |
| <65 | 44 (65.7) |
| ≥65 | 23 (34.3) |
| Sex | |
| Male | 44 (65.7) |
| Female | 23 (34.3) |
| ECOG PS | |
| 0–1 | 47 (70.1) |
| 2 | 20 (29.9) |
| Tumor stage | |
| Locally advanced | 21 (31.3) |
| Metastatic | 46 (68.7) |
| Treatment line | |
| First | 25 (37.3) |
| ≥Second | 42 (62.7) |
| Baseline CA19–9 (U/ml) | |
| ≤37 | 13 (19.4) |
| >37 | 54 (80.6) |
| TMB (muts/Mb) | |
| <10 | 63 (94.0) |
| ≥10 | 4 (6.0) |
| MSI status | |
| MSI-H | 1 (1.5) |
| MSS | 66 (98.5) |
| PD-L1 expression | |
| CPS≥1 | 3 (4.5) |
| CPS<1 | 64 (95.5) |
| Liver metastasis | |
| Yes | 36 (53.7) |
| No | 31 (46.3) |
| With radiotherapy | |
| Yes | 26 (38.8) |
| No | 41 (61.2) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; CA19–9, carbohydrate antigen 19–9; TMB, tumor mutational burden; MSI, microsatellite instability; MSI-H, microsatellite instability-High; MSS, microsatellite stable; PD-L1, programmed death-ligand 1; CPS, combined positive score.
Tumor response
At the last follow-up (15 January 2022), the median follow-up time was 13.5 months (range 1.7–29.0 months, interquartile range 10.1–19.0 months). Of the 67 patients, 2 (3.0%) had CR, 13 (19.4%) had confirmed PR, and 26 (38.8%) were evaluated as SD. The ORR and DCR were 22.4% and 61.2%, respectively (Table 2). Furthermore, analysis of the association between the inflammatory markers in peripheral blood and treatment response revealed that patients with lower NLR (NLR≤2) achieved higher ORR (38.1% vs. 15.2%, P = .037) and DCR (81.0% vs. 52.2%, P = .032) compared to the group. Additionally, a higher DCR was observed in patients with PLR≤135 (vs. PLR>135, P = .021), LMR>2 (vs. LMR≤2, P = .038), and LDH≤265 (vs. LDH>265, P = .001); however, the ORR in these groups was not statistically significant (Table 2).
Table 2.
Statistical associations between baseline NLR, PLR, LMR, LDH and tumor response.
| Total (N = 67) N (%) | NLR N(%) |
PLR N (%) |
LMR N (%) |
LDH N (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤2 | >2 | P-value | ≤135 | >135 | P-value | ≤2 | >2 | P-value | ≤265 | >265 | P-value | ||
| Best overall response | |||||||||||||
| CR | 2(3.0) | 2(100) | 0(0) | 2(100) | 0(0) | 0(0) | 2(100) | 2(100) | 0(0) | ||||
| PR | 13(19.4) | 6(46.2) | 7(53.8) | 9(69.2) | 4(30.8) | 0(0) | 13(100) | 11(84.6) | 2(15.4) | ||||
| SD | 26(38.8) | 9(34.6) | 17(65.4) | 15(57.7) | 11(42.3) | 3(11.5) | 23(88.5) | 24(92.3) | 2(7.7) | ||||
| PD | 26(38.8) | 4(15.4) | 22(84.6) | 9(34.6) | 17(65.4) | 7(26.9) | 19(73.1) | 14(53.8) | 12(46.2) | ||||
| DCR(CR+PR+SD) | 22.4 | 17(81.0) | 24(52.2) | .032 | 26(74.3) | 15(46.9) | .021 | 3(30) | 38(66.7) | .038 | 37(72.5) | 4(25.0) | .001 |
| ORR(CR+PR) | 61.2 | 8(38.1) | 7(15.2) | .037 | 11(31.4) | 4(12.5) | .063 | 0(0) | 15(26.3) | .101 | 13(25.5) | 2(12.5) | .492 |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DCR,disease control rate; ORR,objective response rate; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; LDH, lactate dehydrogenase.
P values in bold indicated statistically significant differences (P < .05).
Survival
As shown in Supplementary Figure S1, the median PFS and OS were 4.4 months (95%CI 3.3–6.4 months) and 9.7 months (95%CI 6.6–16.6 months), respectively. According to the univariate and multivariate regression analyses, liver metastasis and baseline LDH levels were found to be negative prognostic factors for patients with advanced pancreatic cancer treated with anti-PD-1 therapy (Table 3). Patients with LDH-L (LDH≤265) had superior PFS and OS than those with LDH-H (LDH>265) (mPFS 5.4 vs. 2.8 months, HR 0.36, 95%CI 0.20–0.67, P < .001; mOS 13.3 vs. 3.6 months, HR 0.16, 95%CI 0.08–0.31, P < .001) (Figure 2a,b). Patients suffering from liver metastasis were verified to have worse PFS and OS compared to those without liver metastasis (mPFS 2.4 vs. 7.8 months, HR 4.48, 95%CI 2.43–8.27, P < .001; mOS 5.7 vs. 18.0 months, HR 4.15, 95%CI 2.07–8.33, P < .001) (Figure 2c,d). Subsequently, we divided all patients into three groups, namely Group A: with LDH-H and liver metastasis; Group B: LDH-H without liver metastasis or with liver metastasis but LDH-L; and Group C: LDH-L and without liver metastasis. The data showed that the PFS and OS for patients in Group C were greater than those for patients in Groups B and A (mPFS, 1.8 vs. 3.5 vs. 9.3 months, P < .001; mOS, 3.3 vs. 8.5 vs. 18.0 months, P < .001) (Figure 3). Furthermore, patients treated with radiotherapy were found to be significantly related to a longer PFS (HR 0.367, 95%CI 0.207–0.650, P < .001) and OS (HR 0.406, 95%CI 0.202–0.814, P = .009) by univariate Cox regression analysis, but there was no statistical significance in multivariate analysis (Table 3).
Table 3.
Uni- and multivariate Cox regression analyses of PFS and OS.
| Factors | Category | PFS |
OS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Treatment line | First | 0.440 (0.254–0.763) | .001 | 1.598 (0.527–4.850) | .408 | 0.410 (0.205–0.821) | .003 | 1.309 (0.764–0.570) | .526 |
| TMB(muts/Mb) | ≥10 | 0.490 (0.15–1.573) | .220 | 1.27e-0。8(0-Inf) | .035 | ||||
| PD-L1 | CPS≥1 | 3.066(0.423–22.22) | .268 | 1.433 (0.196–10.49) | .723 | ||||
| NLR | ≤2 | 0.616(0.357–1.064) | .079 | 0.492 (0.239–1.014) | .050 | ||||
| PLR | ≤135 | 0.390(0.226–0.673) | <.001 | 0.898 (0.411–1.963) | .787 | 0.454 (0.242–0.850) | .012 | ||
| LMR | ≤2 | 1.988(0.996–3.968) | .05 | 2.401 (1.138–5.066) | .018 | ||||
| LDH | ≤265 | 0.364(0.200–0.665) | <.001 | 0.280 (0.133–0.588) | .001 | 0.157 (0.079–0.312) | <.001 | 0.128 (0.059–0.2780) | <.001 |
| Liver metastasis | Yes | 4.483(2.429–8.275) | <.001 | 5.591 (1.71–18.285) | .004 | 4.151 (2.068–8.333) | <.001 | 5.011 (1.901–13.210) | .001 |
| With radiotherapy | Yes | 0.367(0.207–0.650) | <.001 | 0.560 (0.211–1.485) | .243 | 0.406 (0.202–0.814) | .009 | 0.961 (0.375–2.464) | .934 |
Abbreviations: PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; TMB, tumor mutational burden; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; LDH, lactate dehydrogenase.
P values in bold indicated statistically significant differences in multivariate analysis (P < .05).
Figure 2.
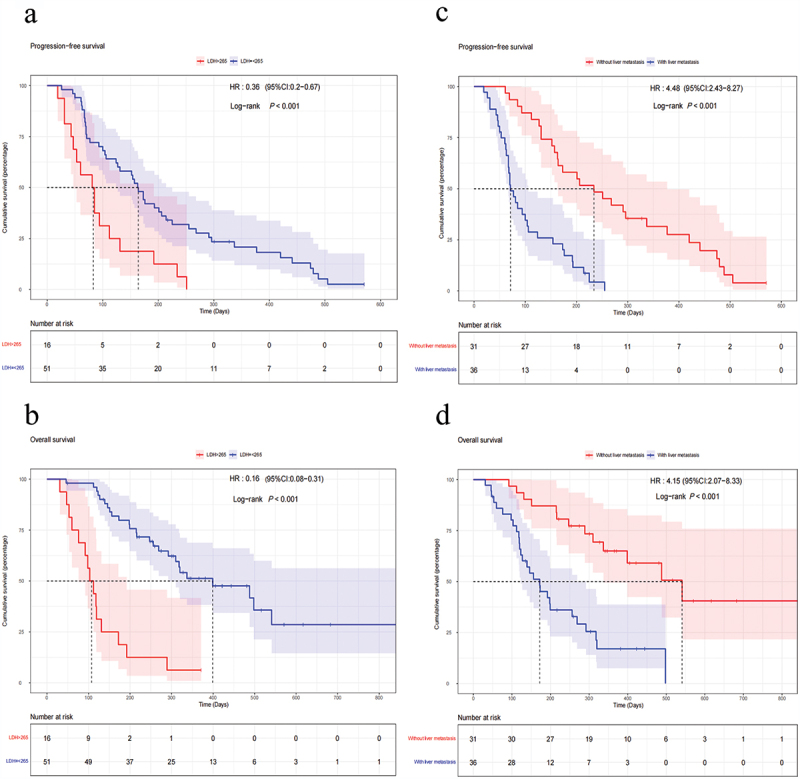
Kaplan–Meier analysis of survival and biomarkers. PFS based on LDH level (a) and liver metastasis (c). OS based on LDH level (b) and liver metastasis (d).
Abbreviations: PFS, progression-free survival; OS, overall survival; LDH, lactate dehydrogenase; HR, hazard ratio; CI, confidence interval.
Figure 3.
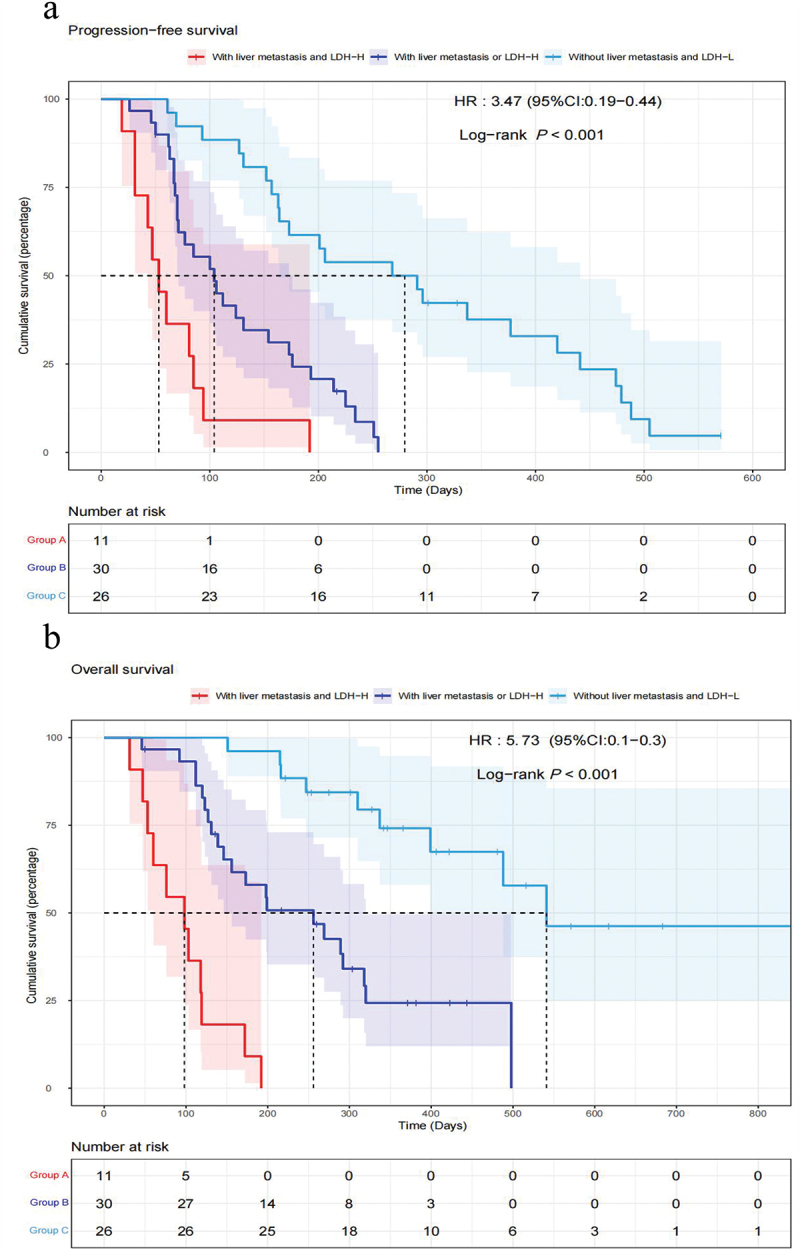
Kaplan–Meier PFS curves (a) and OS curves (b) of the multifactor model according to LDH levels and liver metastasis.
Abbreviations: Group A: patients with LDH-H and liver metastasis; Group B: patients with LDH-H without liver metastasis or with liver metastasis but LDH-L; and Group C: patients with LDH-L and without liver metastasis. PFS, progression-free survival; OS, overall survival; LDH, lactate dehydrogenase; HR, hazard ratio; CI, confidence interval.
Safety
Seventeen patients (25.4%) showed irAEs, with the common irAEs being hypothyroidism (n = 9, 13.4%) and rash (n = 7, 10.5%). Additionally, 2 patients (3.0%) experienced severe immune rash, and no patient died during the treatment (Table 4). Although not significantly different, the PFS for patients with irAEs tended to be longer than for those without irAEs (5.8 vs. 4.1 months, P = .83, Supplementary Figure S2). Additionally, the OS between these two groups was comparable (9.0 vs. 9.7 months, P = .55). The multivariate Cox regression analysis indicated that peripheral blood inflammatory markers were not independent predictors for the onset of irAEs (Supplementary Figure S3).
Table 4.
Incidence of adverse events.
| Grade |
|||
|---|---|---|---|
| 1–2 | 3 | ≥4 | |
| N (%) | N (%) | N (%) | |
| TRAEs | |||
| Leukopenia | 37(55.2) | 9(13.4) | 0(0) |
| Thrombocytopenia | 31(46.3) | 7(10.4) | 1(1.5) |
| Increased ALT or AST | 19(28.4) | 3(4.5) | 0(0) |
| Fatigue | 20(29.9) | 4(6.0) | 0(0) |
| Nausea or emesis | 10(14.9) | 1(1.5) | 0(0) |
| Colitisor Diarrhea | 17(25.4) | 6(9.0) | 0(0) |
| irAEs | |||
| Rash | 2(3.0) | 3(4.5) | 2(3.0) |
| Pneumonitis | 0(0) | 1(1.5) | 0(0) |
| Hypothyroidism | 9(13.4) | 0(0) | 0(0) |
| Hyperthyroidism | 4(6.0) | 0(0) | 0(0) |
| Myositis | 0(0) | 2(3.0) | 1(1.5) |
| Hepatitis | 0(0) | 2(3.0) | 0(0) |
| Others | 15(22.4) | 0(0) | 0(0) |
Abbreviations: TRAEs, treatment-related adverse events; irAEs, immune-related adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
Chemotherapy remains the mainstream treatment for pancreatic cancer. Our previous study has shown that a combination of nab-paclitaxel, gemcitabine, and hypofractionated tomotherapy with simultaneous integrated boost can improve the local control rate and PFS; however, the OS remains unsatisfactory.15 This prompted us to further explore novel treatment regimens for patients with pancreatic cancer.
Although immunotherapy has achieved remarkable results in many malignant tumors, patients with pancreatic cancer benefit less from ICI monotherapy. Recent studies have reported that compared to checkpoint blockade monotherapy, the combination of anti-PD-1 antibody and chemotherapy, targeted therapy, or other immunotherapeutic agents have yielded promising results.16,17 In a phase II clinical trial, after initial treatment based on 5-FU or gemcitabine for patients with advanced pancreatic cancer, the median OS of durvalumab monotherapy or durvalumab plus tremelimumab was 3.6 and 3.1 months, respectively.18 A phase II clinical trial was designed to evaluate the efficacy and safety of the CXCR4 antagonist BL-8040 combined with pembrolizumab and chemotherapy for second-line therapy of metastatic pancreatic cancer. The overall response rate was 32%, and the median duration of response was 7.8 months.19 The most common biomarkers for predicting immunotherapy efficacy in clinical practice consist of PD-L1 expression, TMB level, and MSI status.20 In our study, we checked these biomarkers by the NGS-based method and found 3 patients to be PD-L1 positive (CPS≥1), 4 patients showing high TMB (≥10 mutations/Mb), and only 1 patient (1.5%) with MSI-H; this latter finding was in agreement with a previous report of ~1%–2% patients showing MSI-H status.21 Considering the lower prevalence of MSI-H in our study population, we did not test its relationship with tumor response. Furthermore, we failed to verify if the PD-L1 expression level or TMB status affects the responses to ICIs. Therefore, studies using a larger sample size to further evaluate the predictive roles of biomarkers on immunotherapy efficacy in a real-world setting are warranted.
Considering the significant roles of inflammation in cancer initiation, promotion, and progression, we conducted a retrospective analysis to evaluate the association between peripheral blood inflammatory markers and clinical response for patients with advanced pancreatic cancer receiving PD-1 inhibitor therapy. Interestingly, we found that the baseline NLR level was able to predict tumor response. Patients with NLR≤2 achieved higher ORR and DCR, which is consistent with a previous report.22 This result is reasonable because neutrophils can suppress lymphocytes and natural killer cells’ immune activity by producing chemokines and cytokines. As an index of systemic inflammation, lower NLR levels represent relative lymphocyte advantage and a favorable inflammatory microenvironment.23 We analyzed the relationship between inflammatory markers and survival, and found that pre-treatment LDH levels appeared to be inversely associated with PFS and OS, which might be attributable to the ability of LDH to induce cancer cell proliferation, promote tumor angiogenesis, inhibit natural killer cytolytic activity, and regulate myeloid-derived suppressor cells.24,25 LDH has also been proved to be a negative prognostic biomarker for melanoma,26 lung cancer,27 and urothelial carcinoma28 treated with immunotherapy.
The liver is the most common metastatic organ for advanced pancreatic cancer. Consistent with this, in our study population, 36 patients (53.7%) showed liver metastasis. For patients with liver metastasis, neither systemic treatments (chemotherapy, immunotherapy, and targeted therapy), nor local-regional therapies, including radiofrequency ablation, transcatheter arterial chemoembolization, and transcatheter arterial radioembolization, can give encouraging results.29 Recently, Hashimoto et al30 found that insulin-like growth factor inhibitors could alter the suppressive TME, reduce the activation of hepatic stellate cells, and thereby inhibit pancreatic cancer liver metastases both in vitro and in vivo. Increasingly, it has become evident that liver involvement is a poor prognostic factor in breast cancer31 and colorectal cancer.32 By univariate and multivariate Cox regression analyses, we found that liver metastasis was associated with poor survival of patients with pancreatic cancer receiving PD-1 inhibitor therapy; these patients exhibited a shorter median PFS and OS of 2.4 and 5.7 months, respectively, compared with those (7.8 and 18.0 months, respectively) in patients without liver metastasis. This finding is biologically reasonable considering the following. Liver metastasis has been shown to siphon activated CD8+ T cells from systemic circulation and subsequently induce cell apoptosis following their interaction with monocyte-derived macrophages via the Fas-FasL signaling pathway. This tremendously reduces peripheral antigen-specific T-cell numbers and diminishes the efficacy of immunotherapy in patients and preclinical models.33
More recently, the phase II CheckPAC trial34 showed that patients with refractory metastatic pancreatic cancer could also benefit from treatment with nivolumab, ipilimumab, and stereotactic body radiotherapy. The synergistic mechanisms of radiotherapy combined with immunotherapy include the reshaping of the TME and induction of ‘immunogenic cell death.’ Radiotherapy causes immune activation via MHC class I upregulation, increasing the visibility of tumor cells to the host immune system, and the up-regulation of PD-L1 via activation of the cGAS-STING (cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon gene) pathway.35–38 Sharabi et al. reported that stereotactic body radiotherapy can upregulate antigen cross-presentation by tumor-specific antigen – MHC complexes and increase tumor T cell infiltration, and thus enhance anti-tumor immune response.39 In our study, 38.8% (26/67) of the patients underwent immunotherapy and radiotherapy concomitantly, and this combination regimen was found to be significantly related to a better prognosis by univariate Cox regression analysis, while there was no statistical significance in multivariate analysis. As radiotherapy was commonly employed for patients with local recurrence or distant metastasis in our study population, we did not distinguish the enrollments by the treatment line or other characteristics. This could have led to a failure to identify survival benefits for those patients treated with a combination of immunotherapy and radiotherapy.
Compared to traditional chemotherapies, adverse events of immunotherapy are different. In this study, the most common irAE was hypothyroidism (13.4%), and 4 patients experienced severe adverse events. We further analyzed the relationship between predictive factors and irAEs, but no correlation was found between them. Therefore, there is a need to further explore novel factors to evaluate potential adverse events before immunotherapy.
Our study has several limitations. Firstly, the major limitation of this study is the prognostic parameters can only be applied to a population after they have started treatment, and the study population only included pancreatic cancer patients who received at least twice anti-PD-1 therapy. Secondly, this was a retrospective study with a small sample size and lacked control cohorts. Thirdly, there was a certain heterogeneity in our cohort of patients, including patients with locally advanced and metastatic pancreatic cancer, as well as patients who received anti-PD-1 therapy as the first-line and follow-up line therapy; the PFS and OS also varied widely among the patients.
In conclusion, the pretreatment peripheral blood marker NLR might correlate with tumor response in patients with advanced pancreatic cancer receiving anti-PD-1 therapy, and the baseline LDH level and liver metastasis are potential factors that can predict survival benefits from immunotherapy. Our findings are beneficial for patient selection before immunotherapy, and further studies with larger sample sizes in a randomized, controlled setting are needed to validate our results.
Supplementary Material
Funding Statement
The study was supported by the National Natural Science Foundation of China (82072926), the Special Fund of Health Science and Technology Development of Nanjing (YKK20080), and the Beijing Medical Award Foundation (YXJL-2020-0236-0050).
Disclosure statement
No potential conflict of interest was reported by the authors.
Ethical approval and ethical standards
The study was performed in accordance with the Declaration of Helsinki.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2178791
References
- 1.Park W, Chawla A, O’reilly EM.. Pancreatic cancer: a review. JAMA. 2021;326:851–9. doi: 10.1001/jama.2021.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahib L, Wehner MR, Matrisian LM, Nead KT.. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. 2021;4:e214708. doi: 10.1001/jamanetworkopen.2021.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bear AS, Vonderheide RH, O’hara MH. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell. 2020;38:788–802. doi: 10.1016/j.ccell.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord J-P, Geva R, Gottfried M, Penel N, Hansen AR, et al. Efficacy of Pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:38. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 7.Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN Clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2021;19(4):439–57. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 9.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dougan M, Luoma AM, Dougan SK, Wucherpfennig KW. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell. 2021;184:1575–88. doi: 10.1016/j.cell.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, Qian X, Li Y. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother. 2020;69:1813–22. doi: 10.1007/s00262-020-02585-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan X, Wang D, Zhang W, Liu J, Liu C, Li Q, Ma Z, Li H, Guan X, Bai Y, et al. Inflammatory markers predict survival in patients with advanced gastric and colorectal cancers receiving Anti–PD-1 therapy. Front Cell Dev Biol. 2021;9:638312. doi: 10.3389/fcell.2021.638312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waninger JJ, Ma VT, Journey S, Skvarce J, Chopra Z, Tezel A, Bryant AK, Mayo C, Sun Y, Sankar K, et al. Validation of the American Joint Committee on cancer eighth edition staging of patients with metastatic cutaneous melanoma treated with immune checkpoint inhibitors. JAMA Netw Open. 2021;4:e210980. doi: 10.1001/jamanetworkopen.2021.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimono J, Izumiyama K, Ito S, Tsutsmi Y, Kondo T, Kakinoki Y, Teshima T. Lymphocyte-monocyte ratio (LMR) can predict bendamustine therapeutic efficacy in low-grade B-cell lymphoma. Int J Lab Hematol. 2020;42:431–38. doi: 10.1111/ijlh.13216. [DOI] [PubMed] [Google Scholar]
- 15.Shi Z, Yang J, Kong W, Qiu X, Lu C, Liu J, Liu B, Du J. Use of Nab-Paclitaxel plus gemcitabine followed by hypofractionated tomotherapy with simultaneous integrated boost in patients with locally advanced pancreatic cancer. Front Oncol. 2022;12:782730. doi: 10.3389/fonc.2022.782730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiss KA, Mick R, Teitelbaum U, O’hara M, Schneider C, Massa R, Karasic T, Tondon R, Onyiah C, Gosselin MK, et al. Niraparib plus nivolumab or niraparib plus ipilimumab in patients with platinum-sensitive advanced pancreatic cancer: a randomised, phase 1b/2 trial. Lancet Oncol. 2022;23:1009–20. doi: 10.1016/S1470-2045(22)00369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X, Cao Y, Liu W, Ju X, Zhao X, Jiang L, Ye Y, Jin G, Zhang H. Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: an open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2021;22:1093–102. doi: 10.1016/S1470-2045(21)00286-2. [DOI] [PubMed] [Google Scholar]
- 18.O’reilly EM, Oh D-Y, Dhani N, Renouf DJ, Lee MA, Sun W, Fisher G, Hezel A, Chang S-C, Vlahovic G, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1431–38. doi: 10.1001/jamaoncol.2019.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bockorny B, Semenisty V, Macarulla T, Borazanci E, Wolpin BM, Stemmer SM, Golan T, Geva R, Borad MJ, Pedersen KS, et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med. 2020;26:878–85. doi: 10.1038/s41591-020-0880-x. [DOI] [PubMed] [Google Scholar]
- 20.Majidpoor J, Mortezaee K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin Immunol. 2021;226:108707. doi: 10.1016/j.clim.2021.108707. [DOI] [PubMed] [Google Scholar]
- 21.Luchini C, Brosens LAA, Wood LD, Chatterjee D, Shin JI, Sciammarella C, Fiadone G, Malleo G, Salvia R, Kryklyva V, et al. Comprehensive characterisation of pancreatic ductal adenocarcinoma with microsatellite instability: histology, molecular pathology and clinical implications. Gut. 2021;70:148–56. doi: 10.1136/gutjnl-2020-320726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valero C, Lee M, Hoen D, Weiss K, Kelly DW, Adusumilli PS, Paik PK, Plitas G, Ladanyi M, Postow MA, et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun. 2021;12:729. doi: 10.1038/s41467-021-20935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–16. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 24.Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017;19:353–63. doi: 10.3233/CBM-160336. [DOI] [PubMed] [Google Scholar]
- 25.Van Wilpe S, Koornstra R, Den Brok M, De Groot JW, Blank C, De Vries J, Gerritsen W, Mehra N. Lactate dehydrogenase: a marker of diminished antitumor immunity. Oncoimmunology. 2020;9:1731942. doi: 10.1080/2162402X.2020.1731942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ny L, Jespersen H, Karlsson J, Alsén S, Filges S, All-Eriksson C, Andersson B, Carneiro A, Helgadottir H, Levin M, et al. The PEMDAC phase 2 study of pembrolizumab and entinostat in patients with metastatic uveal melanoma. Nat Commun. 2021;12:5155. doi: 10.1038/s41467-021-25332-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L, Audigier-Valette C, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non–small cell lung cancer. JAMA Oncol. 2018;4:351–57. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fornarini G, Rebuzzi SE, Banna GL, Calabrò F, Scandurra G, De Giorgi U, Masini C, Baldessari C, Naglieri E, Caserta C, et al. Immune-inflammatory biomarkers as prognostic factors for immunotherapy in pretreated advanced urinary tract cancer patients: an analysis of the Italian SAUL cohort. ESMO Open. 2021;6:100118. doi: 10.1016/j.esmoop.2021.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren SQ, Chen Q, Yuan CH. Treatment strategies of pancreatic neuroendocrine neoplasms with liver metastases. Zhonghua Wai Ke Za Zhi. 2020;58:499–504. doi: 10.3760/cma.j.cn112139-20200310-00205. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto M, Konda JD, Perrino S, Celia Fernandez M, Lowy AM, Brodt P. Targeting the IGF-axis potentiates immunotherapy for pancreatic ductal adenocarcinoma liver metastases by altering the immunosuppressive microenvironment. Mol Cancer Ther. 2021;20:2469–82. doi: 10.1158/1535-7163.MCT-20-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien PA. Liver metastases from breast cancer: long-term survival after curative resection. Surgery. 2000;127:383–89. doi: 10.1067/msy.2000.103883. [DOI] [PubMed] [Google Scholar]
- 32.Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases – a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. doi: 10.1186/s12885-017-3925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–64. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen IM, Johansen JS, Theile S, Hjaltelin JX, Novitski SI, Brunak S, Hasselby JP, Willemoe GL, Lorentzen T, Madsen K, et al. Randomized phase II study of Nivolumab with or without Ipilimumab combined with stereotactic body radiotherapy for refractory metastatic pancreatic cancer (CheckPAC). J Clin Oncol. 2022;40:3180–89. doi: 10.1200/JCO.21.02511. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. 2020;21:120–34. doi: 10.1038/s41590-019-0561-4. [DOI] [PubMed] [Google Scholar]
- 36.S-S D, Chen G-W, Yang P, Chen Y-X, Hu Y, Zhao Q-Q, Zhang Y, Liu R, Zheng D-X, Zhou J, et al. Radiation therapy promotes hepatocellular carcinoma immune cloaking via PD-L1 upregulation induced by cGAS-STING activation. Int J Radiat Oncol Biol Phys. 2022;112:1243–55. doi: 10.1016/j.ijrobp.2021.12.162. [DOI] [PubMed] [Google Scholar]
- 37.Arina A, Gutiontov SI, Weichselbaum RR. Radiotherapy and immunotherapy for cancer: from “Systemic” to “Multisite”. Clin Cancer Res. 2020;26:2777–82. doi: 10.1158/1078-0432.CCR-19-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–77. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic radiation therapy augments antigen-specific PD-1–mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3:345–55. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


