ABSTRACT
Switching a vaccine for another on a pediatric national immunization program is often done for the betterment of society. However, if poorly implemented, switching vaccines could result in suboptimal transitions with negative effects. A systematic review was conducted to evaluate the existing knowledge from identifiable documents on implementation challenges of pediatric vaccine switches and the real-world impact of those challenges. Thirty-three studies met the inclusion criteria. We synthesized three themes: vaccine availability, vaccination program deployment, and vaccine acceptability. Switching pediatric vaccines can pose unforeseen challenges to health-care systems worldwide and additional resources are often required to overcome those challenges. Yet, the magnitude of the impact, especially economic and societal, was frequently under-researched with variability in reporting. Therefore, an efficient vaccine switch requires a thorough consideration of the added benefits of replacing the existing vaccine, preparation, planning, additional resource allocation, implementation timing, public–private partnerships, outreach campaigns, and surveillance for program evaluation.
KEYWORDS: Pediatric, child, adolescent, vaccine, immunization program, switch, systematic review
Introduction
A vaccine switch occurs when a vaccine for a particular indication is substituted for another vaccine covering the same indication but has different product attributes, such as antigenic coverage, formulation, and number of doses per container. With the introduction of newer, more expensive pediatric vaccines, the efficient use of resources is an important consideration prior to the introduction in a national immunization program (NIP). Various factors contribute to the wide adoption of vaccine switching globally, and decisions are frequently taken at the national or sub-national level (e.g., regional or health-care facility) to meet specific local needs. The rationale for switching vaccines on NIPs may include improving protection against infectious diseases,1–3 optimizing budget spending,4–6 increasing supply chain efficiency,7 and addressing vaccine shortages.8,9
A vaccine switch has the potential to have both positive and negative impacts on health systems.10 While a vaccine switch is frequently carried out in an effort to enhance the health-care system, it also involves a variety of activities, resources, considerations, and implementation challenges. Given the complexities of vaccine switching, even a well-resourced national or sub-national delivery system may encounter unexpected barriers during program deployment. Such implementation challenges can arise in the supply chain, logistical management, training of health-care professionals, surveillance, and monitoring, as well as the evaluation of new vaccines for safety, efficacy, and quality.3,11 Inadequately planned vaccine rollout initiatives can disrupt routine immunization schedules while posing the risk of increasing the burden of major communicable diseases on society.12,13 Therefore, healthcare and policy decision-makers should leverage existing knowledge to identify potential implementation challenges in a pediatric vaccine switch.
One of the most well-known pediatric vaccine switches is the globally synchronized switch of the poliovirus vaccine with an aim to achieve a polio-free world.14 This global campaign switched the trivalent oral poliovirus vaccine (tOPV) to bivalent OPV (bOPV) by removing type-2 poliovirus from the new vaccine formulation. As type-2 poliovirus has been declared to be eradicated, this vaccine switch responds to the changing disease burden and lowers the risk of vaccine-derived poliovirus (VDPV) outbreak.14 Another example includes the pneumococcal conjugate vaccine (PCV) for children, which has been largely effective in reducing pneumococcal disease associated with serotypes included in PCV formulations, including the heptavalent (PCV7, no longer licensed), the 10-valent (PCV10), and the 13-valent (PCV13).15 PCVs have been switched on NIPs multiple times in both directions; broader coverage for improving protection against more disease-causing serotypes (PCV7 to PCV13)16 and reducing serotype coverage to save on vaccination costs (PCV13 to PCV10).5,6 In many of these decisions, PCV programs may be undervalued, which has been demonstrated by many economic evaluations of PCVs globally.17,18 However, vaccine switches may also introduce implementation challenges from unaccounted resources consumed during vaccine transition activities and suboptimal transitions.19 Implementation challenges could adversely affect vaccine access, produce negative public health impacts, and are important considerations for optimal resource allocation;12 yet these are rarely included in current economic evaluations of pediatric vaccines.18
Several published studies describe and evaluate the processes and impact of vaccine switches for pediatric infectious diseases.1–8 However, no study summarizes the evidence and challenges associated with implementing a pediatric vaccine switch. Understanding the real-world implementation challenges and cost drivers associated with switching a vaccine can help inform policy decisions of impacts, obstacles, or inefficiencies that might emerge. Hence, we aim to perform a systematic literature review to summarize the global knowledge of implementation challenges and real-world impacts of pediatric vaccine switches based on existing documents identified through our best attempt for a comprehensive search.
Materials and methods
We conducted a systematic literature review to identify published articles describing real-world implementation challenges of pediatric vaccine switches and the impact of those challenges. This systematic review was conducted following the approaches of the Cochrane handbook for systematic reviews of interventions.20 General considerations of vaccine switches, fundamental and basic processes required for considering the introduction of any new vaccines, and expected or modeled implementation challenges were not captured in this review.
The protocol of this systematic review was registered with PROSPERO (CRD42022331134).21 This study reported following the 2020 Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (Table S1 in Supplementary Material).22
Search strategy and selection process
Four electronic databases, including PubMed, Embase, CENTRAL, and LILACS, were searched for articles published from database inception to April 30, 2022. The search term used was (Child* OR Infant* OR Pediatric* OR Paediatric*) AND (Vaccines[MeSH] OR Vaccin*[tiab] OR Immunis*[tiab] OR Immuniz*[tiab] OR Inoculat*[tiab]) AND Switch*[tiab] that was adapted to match searching strategy of each database (Table S2 in the Supplementary material). No language restriction was applied. Identified articles were imported to EndNote, and duplicates were removed. We also perform gray literature search in the following data sources: OpenGrey, EBSCO Open Dissertations, World Health Organization (WHO) website, WHO European Region (EURO) website, WHO Eastern Mediterranean Region (EMRO) website, WHO South-East Asia Region (SEAR) website, WHO Pan American Health Organization (PAHO), and WHO African Region (AFRO) website.
Two reviewers (CP and WK) independently screened titles and abstracts of identified articles for relevance and selected articles after examining the full text of the potentially eligible articles. Any discrepancies in article selection between the two reviewers were resolved through discussion with the third reviewer (NC).
Eligibility criteria
Eligible articles must describe the real-world implementation challenges of switching any pediatric vaccine and/or the impact of the challenges, e.g., clinical or economic impact in any country. Eligible articles could include but are not limited to commentaries, letters, news, correspondences, review articles, original articles, policy analyses, reports, models, or economic evaluations.
Data extraction
Data extraction was performed independently by two reviewers (CP and WK). Discrepancies were resolved with consensus among the reviewers (CP, WK, and NC). A data extraction sheet was developed and pilot-tested on five randomly selected articles and then refined until finalization.
The following data were extracted from eligible articles: the name of the first author, year of publication, country/region, title, type of article, study aim, study design, data collection approach, type of vaccine, type of vaccine switch, setting level, reasons for vaccine switch, implementation challenges, and impact of implementation challenges.
Data synthesis
Following the data extraction, we utilized thematic synthesis to classify the identified implementation challenges and impact of a vaccine switch into themes and sub-themes based on the extracted data. One reviewer (WK) constructed an initial coding framework to categorize the extracted data based on processes or structures affected or related to implementing a pediatric vaccine switch. Themes and subthemes were developed by discovering, interpreting, and reporting patterns and clusters of meaning within the extracted data. Themes and subthemes were refined until thematic saturation was reached when no more themes and sub-themes were identified. The synthesized themes and sub-themes were refined and finalized upon discussion with the other reviewers (CP and NC). The impact of implementation challenges includes explicit impact described in the articles and implicit impact that the reviewers synthesized based on the extracted data.
We conducted a subgroup analysis to determine whether the implementation challenges differed between low- and middle-income countries (LMICs) and high-income countries (HICs) by excluding studies that focused on the regional or global level. The identified countries were classified according to the World Bank’s income levels.23
Quality assessment
Eligible articles were assessed for risk of bias and/or reporting bias. We employed external tools for evaluating the quality of different types of articles and study designs, including the Scale for the Assessment of Narrative Review Articles (SANRA) for narrative review articles,24 The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement25 and Risk Of Bias In Non-randomized Studies – of Exposure (ROBINS-E) for observational studies,26 and A Consensus-Based Checklist for Reporting of Survey Studies (CROSS) for survey studies.27
Two reviewers independently performed the quality assessment of the eligible articles (CP and WK). Any disagreements during the quality assessment were resolved in consensus upon discussion with the third reviewer (NC).
Results
We identified 1,674 articles through searches in four electronic databases and 1,191 articles from the gray literature search. We included 33 studies that met the eligibility criteria as shown in Figure 1.28–59 We provided reasons for exclusion after assessing full-text articles in Table S3 in the Supplementary material. The included studies comprised narrative reviews (n = 25),28–49–57,58 observational studies (n = 4),51–54 survey studies (n = 3),50,55,56 and news (n = 1).59 The study characteristics of the included articles are summarized in Table 1.
Figure 1.
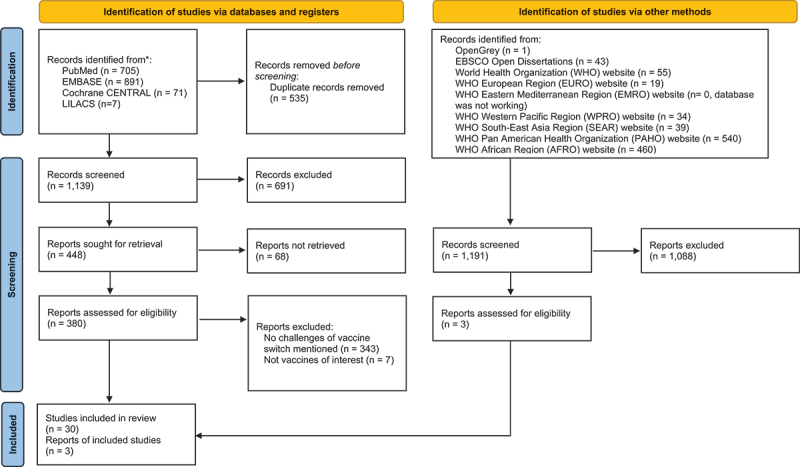
PRISMA flow diagram showing selection process of included studies.
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/.
Table 1.
Characteristics of the included studies.
| Author, year | Country/Region | Income Level | Study Design/Article type | Vaccine of Interest | Type of Switch | Year of Switch |
|---|---|---|---|---|---|---|
| Bahl, 201725 | South-East Asia Region | Middle to High | Narrative review | Polio | Switch of serotype coverage (tOPV to bOPV) | 2016 |
| Califano, 201626 | Argentina | High | Narrative review | Polio | Switch of serotype coverage (tOPV to bOPV) | 2016 |
| Fahmy, 201727 | Eastern Mediterranean Region | Low to High | Narrative review | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
2016 |
| Freed, 200652 | US | High | Survey study | Combination vaccine (diphtheria, tetanus, acellular pertussis, hepatitis B, IPV) | Switch from multiple vaccinations to a combination vaccine (Pentavalent vaccine) | 2002 |
| Gamage, 201828 | Sri Lanka | Lower Middle | Narrative review | Polio | Switch of route of administration (OPV to IPV and IM to ID) | 2016 |
| Garg, 201829 | Southeast Asia Region | Middle to High | Narrative review | Polio | Switch of serotype coverage (tOPV to bOPV) | 2016 |
| Garon, 201730 | Global | NA | Narrative review | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
2016 |
| Garon, 201631 | Global | NA | Narrative review | Polio | Switch of serotype coverage (tOPV to bOPV) | 2016 |
| Gurung, 201732 | Western Pacific Region | Middle to High | Narrative review | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
2016 |
| Hampton, 201633 | Global | NA | Narrative review | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
2016 |
| Hampton, 201734 | Global | NA | Narrative review | Polio | Switch of serotype coverage (tOPV to bOPV) | NA |
| Horn, 202135 | Global | NA | Narrative review | PCV | Switch of serotype coverage (PCV7 to PCV10 or PCV13, PCV10 to PCV13, and PCV13 to PCV10) | 2009 |
| Icardi, 201836 | Global (Nigeria, Pakistan and Afghanistan were mentioned as examples) | NA | Narrative review | Polio | Switch of serotype coverage (tOPV to bOPV) | 2016 |
| Jog, 201637 | India | Lower Middle | Narrative review | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
2016 |
| John, 201338 | India | Lower Middle | Narrative review | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
NA |
| Kaucley, 202047 | Benin | Lower Middle | Survey study | PCV | Switch of number of doses per vial (single-dose vial to multi-dose vial) | 2011 |
| Kolasa, 20048 | US | High | Observational study | Polio DTP |
Switch of route of administration (OPV to IPV) and additional injection of DTaP) |
1997 |
| Menning, 201739 | Global | NA | Narrative review | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
2016 |
| Nafi, 201940 | Global | NA | Narrative review | Polio | Switch of route of administration (OPV to IPV) | NA |
| Orenstein, 201541 | Global | NA | Narrative review | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
NA |
| Pedreira, 201742 | Region of Americas | Middle to High | Narrative review | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
2015–2016 |
| Pervaiz, 201743 | Pakistan | Lower Middle | Narrative review | Polio | Switch of route of administration (OPV to IPV and IM to ID) | 2016 |
| Ramirez Gonzalez, 201744 | Global | NA | Narrative review | Polio PCV as an example |
Switch of serotype coverage (tOPV to bOPV) | 2016 |
| Snelling, 201545 | Global | NA | Narrative review | Pertussis | Switch of types of vaccines (whole cell to acellular) | NA |
| Soeters, 201949 | Burkina Faso | Low | Observational study | PCV | Switch of vaccination schedule (3 + 0 to 2 + 1) | NA |
| Suarez, 201650 | Peru | Upper Middle | Observational study | PCV | Switch of vaccination schedule (3, 5, and 12 months to 2, 4, and 12 months) Switch of serotype coverage (PCV7 to PCV10) |
2010 and 2011 |
| Tevi-Benissan, 201746 | African Region | Low to High | Narrative review | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
2016 |
| Thacker, 20163 | Global (Mainly Discussed on Pakistan, Afghanistan, India, and Nigeria) | Low to Lower Middle | Narrative review | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
2016 |
| Usuf, 201453 | Gambia | Low | Survey study | Combination vaccine and PCV | Switch from multiple vaccinations to a combination vaccine (Pentavalent vaccine) and introduction of PCV13 | 2009 |
| Wahjuhono, 201451 | Indonesia | Lower Middle | Prospective cohort study | Polio | Switch of route of administration (OPV to IPV) | 2007 |
| World Health Organization, Regional Office the Europe, 201654 | European Region | Low to High | News | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
2016 |
| Pan American Health Organization, 201755 | Region of Americas | Middle to High | Narrative review | Polio | Switch of route of administration (OPV to IPV) Switch of serotype coverage (tOPV to bOPV) |
2016 |
| Fine, 199956 | Not specified | NA | Narrative review | BCG | Switch of strains (switch to a more reactogenic strain vaccines) | NA |
Abbreviations: 2 + 1, 2 primary doses with a booster dose; 3 + 0, 3 primary doses without a booster dose; BCG, Bacillus Calmette-Guérin; bOPV, Bivalent Oral Poliovirus vaccine; DTaP, Diphtheria-Tetanus-Pertussis; ID, Intradermal; IM, Intramuscular; IPV, Inactivated Poliovirus vaccine; NA, Not Available; OPV, Oral Poliovirus vaccine; PCV, Pneumococcal Conjugate vaccine; tOPV, Trivalent Oral Poliovirus vaccine.
Of 33 included studies, types of vaccine switches are switch of serotype coverage and route of administration (n = 12),30–35,36–40–42–44,45–48,49–57–59 switch of serotype coverage only (n = 8),3–28,29–32–34–37–39 switch of route of administration only (n = 6),31,33,43,46,51,54 switch from multiple vaccinations to a combination vaccine (n = 2),55,56 switch of number of doses per vial (n = 1),50 switch of type of vaccine (n = 1),47 switch of vaccine schedule only (n = 1),52 switch of vaccine schedule and serotype coverage (n = 1),53 and switch to of vaccine strains.58 Vaccines of interest include poliovirus vaccines (n = 21),28–37–39–42–46,48,49,51,54,57,59 PCV (n = 6),38,40,41,50,52,53 combination vaccines (n = 2),55,56 pertussis vaccines (n = 1),47 and Bacillus Calmette – Guérin (BCG) vaccine.58 Real-world implementation challenges were described at the global level (n = 12),3–33,34–36–44–47–49 regional level (n = 6),28,30,32,35,45,48,57,59 and country level (n = 13).29–31–40,41-46–50-56–58
We identified three themes with seven sub-themes reflecting the real-world implementation challenges of vaccine switch, as shown in Figure 2. The themes and sub-themes are as follows:
Challenges regarding vaccine availability; 1.1) vaccine supply and 1.2) vaccine logistics
Challenges regarding vaccination program deployment; 2.1) training of health-care professionals, 2.2) infrastructure and resources, and 2.3) management of pre-switched vaccines
Challenges regarding vaccine acceptability; 3.1) parental acceptability and 3.2) health-care professional acceptability.
Figure 2.
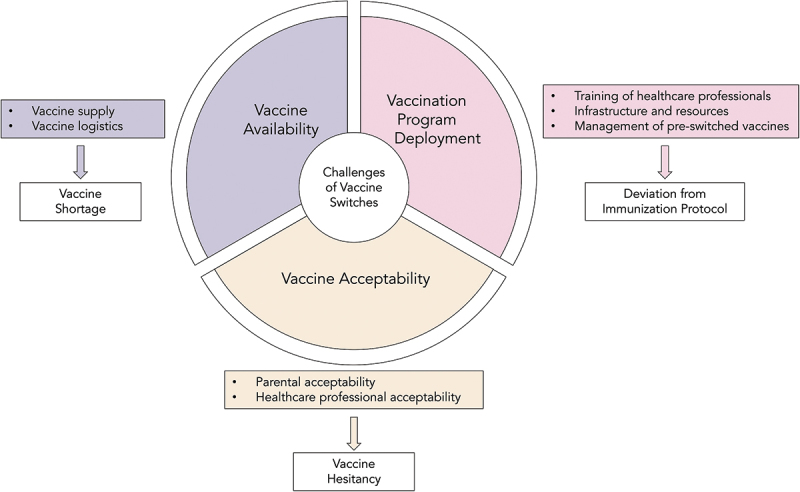
Themes and sub-themes of real-world implementation challenges of pediatric vaccine switches.
We found that the impacts of the challenges were not comprehensively captured and adequately explained in the included articles. We summarize the implementation challenges of vaccine switches and the impact of these challenges by themes and sub-themes in Table 2.
Table 2.
Implementation challenges and the impact of pediatric vaccine switches by themes.
| Vaccine implementation challenge | Country/region facing implementation challenge | Type of vaccine switches (Type of vaccine) |
Description of implementation challenge | Resource and costs caused by implementation challenge |
|---|---|---|---|---|
| 1. Vaccine availability | ||||
| 1.1 Vaccine supply | ||||
| Ensuring sufficient production scale-up |
|
|||
| 1.2 Vaccine logistics | ||||
| Ensuring the availability of new vaccines at the point of use |
|
|
||
| Ensuring sufficient cold chain requirements and storage management |
|
|
||
| 2. Vaccination program deployment | ||||
| 2.1 Training of healthcare professionals | ||||
| Training and Supervision of Healthcare Professionals |
|
|||
| 2.2 Infrastructure and resources | ||||
| Monitoring the effectiveness and safety of vaccine deployment |
|
|
||
| Ensuring sufficient human resources |
|
|
|
|
| 2.3 Management of pre-switched vaccines | ||||
| Ensuring withdrawal of pre-switched vaccines |
|
|
||
| 3. Vaccine acceptability | ||||
| 3.1 Parental acceptability | ||||
| Concern about the switch among parents and communication with parents about the switch |
|
|
||
| Healthcare professional acceptability | ||||
| Healthcare professional reluctance in providing new vaccines to children |
|
|||
*Implicit impact of the challenges of vaccine switch based on the included articles and authors’ speculation. Abbreviations: 2+1; 2 primary doses with a booster dose; 3+0; 3 primary doses without a booster dose; BCG, Bacillus Calmette-Guérin; bOPV, Bivalent Oral Poliovirus vaccine; DTaP, Diphtheria-Tetanus-Pertussis; ID, Intradermal; IM, Intramuscular; IPV, Inactivated Poliovirus vaccine; OPV, Oral Poliovirus vaccine; PCV, Pneumococcal Conjugate vaccine; tOPV, Trivalent Oral Poliovirus vaccine.
Vaccine availability
Vaccine availability is crucial. To ensure that new vaccines are readily available and easily accessible at the points of use, countries have faced various difficulties securing sufficient vaccine supply and efficient vaccine logistics.
Vaccine supply
Challenges in inadequate vaccine supply due to inefficient production scale-up were mentioned in 12 articles, all of which focused on the switching from OPV to inactivated poliovirus vaccine (IPV).30–31-33–42-45–46-57 These studies discussed the switch from tOPV to bOPV in response to the changing poliovirus disease burden. In addition to the switch of the serotype coverage of the vaccine, a single dose of the poliovirus vaccine schedule with IPV was introduced to replace three doses of OPV, thereby reducing the risk of VDPV outbreak.14
Following the vaccine switch, global demand for IPV increased substantially. At the same time, manufacturers were not able to scale up their productions to meet the heightened global demand by the time the synchronized global switch started. This manufacturing-related challenge led to the global shortage of IPV. This challenge was described in studies from several geographical regions, including the Eastern Mediterranean Region,30 the West Pacific Region,35 Region of Americas,45,57 Sri Lanka,31 India,40,41 Pakistan,46 and on a global scale.33,34,37,42 To this end, countries had to delay the IPV introduction into their immunization programs.2,33,37 Countries already introduced IPV faced shortages and had to use intradermal fractional IPV instead.31,46,57 Impact of this challenge in vaccine supply included insufficient access to vaccines, which resulted in children missing scheduled vaccinations. No other impacts were reported.
Vaccine logistics
An efficient logistics system is key to delivering pediatric vaccines to the point of use. Ineffective vaccine logistics led to deviation from immunization protocol due to the unavailability of vaccines. Challenges related to logistics systems were mentioned in studies from several locations, including Peru,53 Gambia,56 Eastern Mediterranean Region,30 South-East Asia Region,28 Region of Americas,57 and a global study.44
Switches in vaccine product characteristics, including the switch from multiple vaccinations to a combination vaccine,54 the switch of the route of administration,30,44,57 and the switch of serotype coverage,30,44,57 created the challenge of ensuring sufficient cold chain requirements and storage management. Some types of vaccine switches required additional space and equipment to contain the products, while others necessitated using different equipment. For example, OPV retains its potency over a long period when stored in a freezer (−20°C and below), while IPV is freeze-sensitive and unable to be stored in a freezer.60,61
This logistical challenge with the vaccine delivery systems is one of the possible factors contributing to missed vaccination opportunities due to vaccine unavailability at the point of use and deviations from the recommended immunization protocol.53 Additional supply chain resources and human resources were further demanded to overcome this barrier. According to a study from the Gambia, the switch from multiple vaccinations to a combination vaccine (pentavalent vaccine) occurred concurrently with the introduction of PCV13 to the NIP. Costs of additional cold chain investment collected from six health administrative regions in 2009 were $US 373,000 to the switch to the pentavalent vaccine. In addition, incremental transportation costs for solely the switch to combination vaccine were $US 7,251.56
Vaccination program deployment
To effectively deploy immunization programs for a new vaccine, there are challenges in training health-care personnel, preparing appropriate infrastructure and resources, and managing pre-switched vaccines.
Training of healthcare professionals
Training and supervision of health-care professionals was a challenge in almost all types of vaccine switches mentioned in the included studies. These were switch of the route of administration (poliovirus vaccines: OPV to IPV,30,49,51,57 and from intramuscular full-dose to intradermal fractional dose of IPV),31,46 switch of serotype coverage (poliovirus vaccines: tOPV to bOPV),30,37,49 switch of the number of doses per vial (PCV: single-dose vial to multi-dose vial),50 and switch of vaccination schedule of PCV (3 primary doses without a booster (3+0) to 2 primary doses with a booster dose (2+1),52 and 3, 5, and 12 months to 2, 4, and 12 months).53
During the global shortage of IPV, countries that had already introduced IPV to their NIPs were disrupted by unexpected vaccine supply constraints. Subsequently, the World Health Organization (WHO) recommended using the fractional dose of intradermal IPV, one-fifth of the standard intramuscular dose, to conserve limited IPV vaccines and address the supply shortage. Yet, the deviation from the routine vaccination practice also brought a challenge in the additional training and supervision of health-care providers as the supply shortage related to the switch altered the administration of products as well as the dose of vaccines. This situation was reported in Sri Lanka and the Sindh Province of Pakistan.31,46
The challenge of health-care professional training was also mentioned with PCV for the switch from a single-dose vial to a multi-dose vial of PCV13 in Benin,50 and the switch of PCV vaccine schedule in Burkina Faso and Peru.52,53 In Benin, the training for new activities related to the switch was limited to central and intermediate-level health-care professionals who were not at the point of service, while the operational personnel at the point of immunization service only resorted to using training materials and peer-to-peer training.50 In contrast, the switch of the vaccination schedule for PCV in Burkina Faso was anticipated to require intensive communication efforts to immunization staff to deliver a proper vaccination following the immunization protocol.52 This led to challenges in ensuring adequate training of health-care professionals to appropriately deploy a vaccination program and adhere to the immunization protocol of the new vaccines.
The impact of inefficient training of health-care professionals included deviation and non-adherence to the immunization protocol. It was also anticipated that non-adherence to the immunization protocol could result in the unintentional use of the pre-switched vaccines.37 Aside from the clinical concerns, training of health-care professionals and ensuring adherence to the vaccine switch protocol entailed monetary and societal costs to a country, including human resources, worktime used for training and monitoring, as well as resources needed for preparing training materials.
Infrastructure and resources
Infrastructure and resources for monitoring the effectiveness and safety of vaccine deployment was a challenge in various locations ranging from a global scale,3,38,43 to Eastern Mediterranean Region,30 South-East Asia Region,32 African Region,48 Region of Americas,57 European Region,59 and Burkina Faso.52 This challenge was reported in several types of switches, including the switch of the route of administration in poliovirus vaccines (OPV to IPV),30,43,57 switch of serotype coverage of poliovirus vaccines (tOPV to bOPV),3,30,32,48,59 switch of PCVs (PCV7 to PCV10 or PCV13, PCV10 to PCV13, and PCV13 to PCV10),38 switch of vaccination schedule of PCVs (3+0 to 2+1),52 and switch of strains of BCG (switch to more reactogenic strains).58
Challenges regarding monitoring the effectiveness and safety of vaccine deployment usually involved system preparedness. For instance, during the globally synchronized switch of poliovirus vaccines, countries were expected to complete the vaccine switch into their NIPs in a similar time frame. However, in the real world, countries had varying levels of system readiness due to variations in infrastructure and resources, presenting challenges to implementing the program simultaneously.3,30,32,48 Furthermore, an inadequate reliable information and reporting system led to difficulties in safety monitoring and stock management.28–30,31–50–57–59 In studies focusing on the switch from tOPV to bOPV, monitoring and controlling transmission of the withdrawn serotype and vaccine-derived poliovirus was essential to ensure a successful switch.3,32,48 This was especially relevant in countries that required improvements in sanitation, where OPV would result in more benefits due to passive environmental immunization to those who are not vaccinated because the vaccine virus replicates in the intestine before being excreted to the environment.62 Therefore, IPV alone was anticipated to have insufficient protective action.43
In the review, the monitoring of vaccine switches was reported to be further complicated by war and international conflicts. For example, in countries in the Eastern Mediterranean Region, such as Libya, it was difficult to monitor and evaluate the vaccine switching implementation due to the impact of internal civil disorders.30 Meanwhile, a vaccine that had multiple switches of serotype coverage and/or vaccination schedule, like PCV, also made it challenging to evaluate the full public health impact of the switch. These challenges include estimating the exact number of vaccinated individuals, as well as the impact of alternative dosing schedules, indirect effects, PCV use in adults, and the potential impact of PCVs on nonspecific disease outcomes.38,52
Furthermore, following the implication of a switch, it is essential to monitor vaccination coverage to evaluate and maintain the effectiveness of the immunization program. Declines in vaccination coverage were reported in the European and American regions.57,59 Moreover, an influx of refugees and migrants in the European region was of concern. Hence, there was an attempt to ensure accessibility to the immunization service for those populations.57
Human resources are the key element in every step of vaccination, from the logistics/distribution process, the vaccination at the point of service, to communicating with parents. One study mentioned an understaffing issue during the globally synchronized switch from tOPV to bOPV in Argentina.29 Challenges in system readiness in terms of infrastructure and resources influenced inefficient deployment, monitoring, and evaluation of the vaccine switches. Consequently, countries found it difficult to access the effectiveness, safety, and impact of the vaccine switches they implemented.
Management of the pre-switched vaccine
Managing the pre-switched vaccine, including withdrawal and disposal, presents a challenge in implementing the vaccine switch. Incomplete withdrawal of pre-switched vaccines may result in unintended use of the pre-switched vaccines, causing deviation from the recommended vaccination schedule. In the case of switching poliovirus vaccines from tOPV to bOPV, incomplete withdrawal of tOPV increases the risk of VDPV outbreak.14 This challenge was found at the global level,3,49 regional level (South-East Asia Region),28 and country level (Argentina).29 All four studies highlighting the sub-theme discuss the challenge of managing pre-switched vaccines during poliovirus vaccine switches.3,28,29,49 In Argentina, for instance, several health-care facilities still had tOPVs stockpiled after the switch, leading to inappropriate handling and administration of the pre-switched vaccines.29
Overcoming this implementation challenge necessitates proper coordination with health-care professionals, human resources, and other stakeholders to monitor the withdrawal processes. For example, in 2010, the US expanded the serotype coverage of PCVs by switching from PCV7 to PCV13. During the switch, manufacturers attempted to ensure the withdrawal of the PCV7 and availability of the PCV13 by buying back the product and directing thousands of its representatives to monitor stock levels in health-care facilities.3
Vaccine acceptability
New and unfamiliar vaccines brought doubt to both parents and health-care professionals.39,42,47,51,52,54,55,58 Doubtful parents were hesitant to have their children receive the new vaccine, which ultimately led to vaccine rejection.39,42 Additionally, doubtful health-care professionals were reluctant to provide new vaccines to children.47 Thus, vaccine acceptability is one of the challenges determining the success of a vaccine switch.
Parental acceptability
Vaccine acceptability challenges among parents were found in several types of pediatric vaccine switches: switch of serotype coverage of poliovirus vaccines (tOPV to bOPV) with an additional injection of IPV to the immunization program, switch of the route of administration (from OPV to IPV),51,54,57 the switch of PCV vaccination schedule (3+0 to 2+1),52 switch of types of vaccines (whole cell to acellular pertussis),47 switch strain of BCG (switch to a more reactogenic strain).58
Among those switches, psychological barriers were discussed the most. Additional injections in the same visit caused parents’ concern and reluctance in allowing their children to receive vaccines,42,51,57 which led to a delay in vaccination in some cases.54 On the other hand, fundamental and religious oppositions in some areas caused an unsuccessful switch of poliovirus vaccines.39
Healthcare professional acceptability
The issue of new vaccine acceptability among parents and health-care professionals resulted in health-care professionals’ reluctance to provide the new vaccine to children. This challenge was discussed in studies concerning the switch from multiple vaccinations to a combination vaccine (pentavalent vaccine) in the US,55 the switch of the route of administration from OPV to IPV in Yogyakarta, Indonesia,54 and the switch of strains of BCG.58
In the switch to a combination vaccine, the reluctance happened because of clinical concerns regarding the potential for extra and unnecessary doses due to combination vaccines. This resulted in the use of the pre-switched vaccine instead.55 Meanwhile, the switch of the route of administration of poliovirus vaccines from OPV to IPV contributed to an additional injection during a visit, leading to health-care professionals’ reluctance to provide a new vaccine to children.54 The reluctance was not reported to result in vaccine hesitancy, as the delay in IPV adoption was not observed after the switch in Indonesia.54 For the switch of strains of BCG, a need to support peripheral staff in order to communicate with parents was mentioned as a lesson learned. However, it was not mentioned to cause a negative impact.58
Comparison of real-world implementation challenges of vaccine switches across different income levels
We performed a sub-group analysis to investigate the difference in challenges of vaccine switch between HICs and LMICs. Only 14 studies describing real-world implementation challenges of vaccine switches in specific countries were included in this sub-group analysis.29–31–39–41–46–49–56
Three studies were from HICs. The switches in these studies were the switch of tOPV to bOPV along with the introduction of IPV in Argentina, the switch of OPV to IPV along with an introduction of DTaP in the US, and the switch from multiple injections to a pentavalent combination vaccine in the US.29,51,55 These studies described implementation challenges related to an understaffing issue and incomplete withdrawal of tOPV in Argentina. In contrast, in the US, the challenges were the reluctance to comply with the new vaccination protocol among parents and providers due to the additional switch.29,51,55
In 11 studies from LMICs, the challenges varied.31–39–41–46–49,50-52–54-56 The most frequently discussed challenges in this group of studies are IPV vaccine shortage, insufficient vaccine-related equipment and transportation capacity, vaccine stock management and ensuring the withdrawal of pre-switched vaccines, and training of health-care professionals. The rest involved adherence to the switch, monitoring and evaluation, and providers’ reluctance to administer the additional injection.31–39–41–46–49,50-52–54-56
Quality assessment
A quality assessment of the included articles was presented in Tables S4–S7 in the Online Supplement Documents. The reporting quality of the included articles varied. For the included narrative review articles assessed by SANRA, the average score was 9.12 out of 12. The description of the literature search was the lowest rated item, while the appropriate presentation of data, justification of the article’s importance for readership, and referencing were the highest rated items. Evaluation of the included survey and observational studies found that the methods were underreported. The risk of bias assessment of four observational studies showed one study with a low risk of bias,52 one with some concerns,54 and two studies with a high risk of bias.51,53
Discussion
To our knowledge, this is the first systematic review that summarizes the implementation challenges of switching pediatric vaccines and the consequences of the challenges. In all included studies, most decisions to switch pediatric vaccines were reported to be for the betterment of society. Although we have identified several important implementation challenges from switching pediatric vaccines, the impacts of these challenges were not often reported, comprehensively captured, or fully discussed in the existing literature. Each phase of a vaccine switch (decision-making, system preparation, facility preparation, personnel preparation, implementation of switching, and monitoring and evaluation) bears cost considerations and resource demands (Figure 3). However, these expenditures and resources, especially those required for planning and preparing a vaccine switch, have not been sufficiently quantified in the included articles of this review. Additionally, we did not review examples of the guidance given before switches to assess how well it met these needs, as it is beyond the scope of our study.
Figure 3.
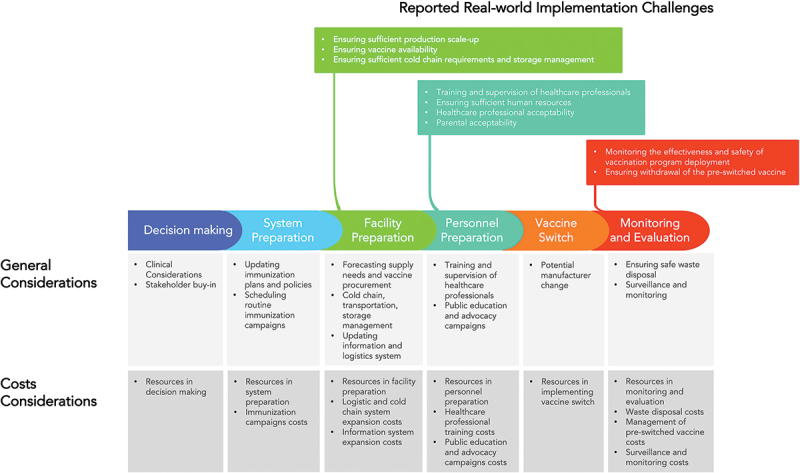
General considerations and real-world implementation challenges and impact of pediatric vaccine switches.
Vaccine availability has received significant global attention, with vaccine supply and logistics being two of the most frequently reported implementation challenges in our review. A well-documented supply challenge involved a global shortage of IPV due to manufacturer delays in production scale-up and inaccurate global demand forecasts.30–31-33–42-45–46-57 Consequently, many birth cohorts worldwide faced IPV unavailability. Countries either delayed IPV introduction or experienced national stockouts, creating an increased risk of polio disease. Many children required catch-up vaccination or received additional shots due to fractional dosing schedules.2,13,31,33,37,46 Therefore, manufacturers must have adequate capacity to rapidly expand vaccine production and reliably produce quantities sufficient to fulfill demand by the time of the switch, or else vaccine access and other potential consequences may arise. In our review, manufacturer-related implementation challenges were rarely reported beyond delays in production scale-up. Switching manufacturers, in addition to switching vaccines, may cause added complications from changes in supply forecasts, product characteristics, packaging, delivery, and management. Moreover, supplier contracts should be managed appropriately to minimize waste of the pre-switched vaccine when being replaced and to guide efficient distribution and timely patient access to the newly switched vaccines via established distribution networks. Upon new vaccine recommendations, decisions on whether to destroy pre-switched vaccines should be strategically made depending on the nature of the disease and vaccines. In the case of the switch from tOPV to bOPV, it is essential to ensure that tOPV is no longer available at the point of use because using the pre-switch vaccine increases the risk of VDPV. In some other cases, pre-switched vaccines do not always have to be destroyed. These pre-switched vaccines will require proper management and allocation, but they can still be used in accordance with the switch guidance or recommendation.
As for vaccine logistics challenges, studies frequently reported on inefficient in-country distribution systems, limited vaccine storage capacity, and the unavailability of vaccines at the point of use. Most of the studies facing such challenges were reported in LMICs.28,53,56 To this, vaccine deployment challenges were found to be connected to the readiness of personnel, resources, and infrastructure.3,30,32,48,57
Additionally, inequitable access to vaccines was reported among refugees, immigrants, and under-reached populations.59 This reflects inequity in healthcare access, which would not be restricted to only immunization and should be of concern in all health-care services.
We found fewer publications on implementation challenges in HICs compared with LMICs. In LMICs, system readiness was cited more frequently as a challenge.31–39–41–46–49,50-52–54-56 LMICs and hard-to-reach geographical locations/populations in HICs tend to have less well-resourced vaccination delivery systems. In contrast, the use of new pediatric vaccines was met with reluctance from parents and health-care professionals in HICs, particularly in relation to schedule changes.29,51,55 Nonetheless, all countries faced common challenges in ensuring the withdrawal of the old vaccine and timely transition to the new vaccine. Countries of all income levels also faced challenges in effectively coordinating roles among government entities and other stakeholders in the vaccine delivery system. As a result, poor coordination can lead to confusion and duplicated efforts, further presenting a barrier to switching pediatric vaccines for countries or regions.
Many general implementation challenges were not reported in the included studies. For example, program evaluation challenges were rarely mentioned. Closely monitoring supply, delivery, safety, vaccine effectiveness, and epidemiologic conditions after implementing a switch is critical. This monitoring effort, however, would demand extra resources for complex vaccination schedule switches or multiple switches in fast succession, which may heighten challenges and complicate program evaluation. In our review, replacing tOPV with bOPV during the global synchronization switch and replacing full dose IPV with fractional dose IPV to cope with IPV shortage required global funding, technical assistance, and other resources.27 However, program evaluation challenges after bOPV and IPV were not well documented in the included studies. Another example identified in our review was the multiple switches of pediatric PCVs, which have different schedules (i.e., 3 + 1, 2 + 1, and 3 + 0).52 Each schedule has different timings for priming doses and booster dose administration. Challenges may occur in the next decade given the different infant PCV formulations that are anticipated for licensure and occurring in quick succession.38 If multiple vaccines with the same indication are anticipated in short intervals, horizon scanning to detect near- and long-term availability of vaccines or simultaneous review of vaccines should be performed to help with prioritization, resource allocation, implementation, and future program evaluations.
Vaccine acceptability is another implementation challenge worth discussing. In this review, parents’ and health-care professionals’ acceptability was highlighted in several pediatric vaccine switches. One impact of vaccine switching resulted in vaccine-hesitant parents postponing or refusing vaccination for their children.54,55 Adequate communication and education on vaccine switch is the key to tackling this challenge. For health-care professionals, reluctance involved administering a new vaccine that was perceived to potentially increase risks of adverse events among children due to the use of a combination vaccine, or a vaccine requiring a schedule change compared to previous practice.55 Furthermore, health-care professional communications and behavior can strongly influence parental acceptability and uptake.63 As a result, outreach campaigns and education programs for health-care professionals and parents are essential to deliver accurate safety information to maximize acceptability during the vaccine switch. Vaccine-related misinformation should be appropriately managed to reduce vaccine hesitancy and rejection among the general population and health-care professionals.64
The globally synchronized switch of poliovirus vaccine is a valuable experience given that it is the only switch that occurred worldwide. Therefore, this switch has its uniqueness from other types of national- or subnational-level switches. The switch required consensus among all countries and strong solidarity from governments, international and private organizations. Technical and financial support was provided unprecedently to ensure system readiness for all countries. Even so, financial constraints remained a challenge for countries to properly implement the program.57
Although several challenges regarding this switch have been reported, the global switch has successfully proceeded. This switch does not affect only the type of vaccines used; simultaneously, it enhances systems for each country. This switch would result in increased capacity for routine immunization and future changes to programs.
Limitations
Several limitations of this review deserve discussion. Our gray literature search was based on international organizations but did not include local data sources (e.g., searching websites of the Ministries of Health or national authorities responsible for NIP). Although we believe that the data sources are good proxies, it constrained our ability to identify all implementation challenges of pediatric vaccine switches and their impacts that were not documented. Our study focuses on reported outcomes. Hence, switches that may have been well conducted and did not report their challenges are not included in our study.
Human papillomavirus (HPV) vaccines, for example, are available in three valences (i.e., 2-, 4-, and 9-valent HPV vaccines) with varying immunization schedules.65 Despite the fact that switching HPVs is anticipated to present implementation challenges due to changes in multiple suppliers and immunization schedules, global experience with switching HPVs has gone undocumented in the literature. Moreover, some countries may have attempted to switch vaccines but ultimately decided against it due to budget constraints, insufficient infrastructure, or a lack of resources, which were not captured in this review. In addition, because this information in the eligible articles was not sufficiently reported, variations in the social and economic contexts of each switch could not be incorporated into the data analysis. Finally, the identified existing literature on real-world implementation challenges was limited to a small number of vaccines, in which global experience with poliovirus vaccines and PCVs are discussed extensively in this review. However, the findings in this review could be generalizable to other pediatric vaccines, given that the process for implementing immunization programs is similar across other vaccines.
Conclusion
Switching pediatric vaccines is associated with various types of implementation challenges. Yet, the impacts of these challenges are not comprehensively captured in the literature. Countries aiming to switch vaccines should thoroughly plan a smooth transition to ensure timely access to essential vaccines while considering the overall benefits and burdens of the switch. An efficient vaccine switch requires thorough preparation, planning, resource allocation, implementation timing, public–private partnerships, and constant program evaluation. It is essential that all the aspects of a switch are considered prior to decision-making to provide optimum public health benefits under an appropriate timeline. We emphasize that future research should be conducted to comprehensively capture the underrecognized impact, resources consumed, and costs of implementing a vaccine switch.
Supplementary Material
Acknowledgments
The authors would like to thank Taylor Ann Shufelt, Emma Therese Behan, and Luke Joseph Schwerer for their diligent proofreading of this article.
Funding Statement
This study was funded by Pfizer Inc.
Disclosure statement
Johnna Perdrizet and Xiuyan Li are employees of Pfizer. Other authors reported no potential conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2177459.
References
- 1.Immunization Systems Management Group of the Global Polio Eradication Initiative . Introduction of inactivated poliovirus vaccine and switch from trivalent to bivalent oral poliovirus vaccine—worldwide, 2013–2016. Morb Mort Wkly Rep. 2015;64(25):699–15. [PMC free article] [PubMed] [Google Scholar]
- 2.Pedreira C, Thrush E, Jauregui B.. Systematization of the introduction of IPV and switch from tOPV to bOPV in the Americas. J Infect Dis. 2017;216(suppl_1):S76–s85. doi: 10.1093/infdis/jiw557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramirez Gonzalez A, Farrell M, Menning L, Garon J, Everts H, Hampton LM, Dolan SB, Shendale S, Wanyoike S, Veira CL, et al. Implementing the synchronized global switch from trivalent to bivalent oral polio vaccines—lessons learned from the global perspective. J Infect Dis. 2017;216(suppl_1):S183–92. doi: 10.1093/infdis/jiw626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suwantika AA, Zakiyah N, Kusuma AS, Abdulah R, Postma MJ.. Impact of switch options on the economics of pneumococcal conjugate vaccine (PCV) introduction in Indonesia. Vaccines. 2020;8(2):233. doi: 10.3390/vaccines8020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson M, Wasserman M, Jadavi T, Postma M, Breton MC, Peloquin F, Earnshaw S, McDade C, Sings H, Farkouh R. Clinical and economic impact of a potential switch from 13-valent to 10-valent pneumococcal conjugate infant vaccination in Canada. Infect Dis Ther. 2018;7(3):353–71. doi: 10.1007/s40121-018-0206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wouters I, Desmet S, Van Heirstraeten L, Blaizot S, Verhaegen J, Van Damme P, Malhotra-Kumar S, Theeten H. Follow-up of serotype distribution and antimicrobial susceptibility of streptococcus pneumoniae in child carriage after a PCV13-to-PCV10 vaccine switch in Belgium. Vaccine. 2019;37(8):1080–86. doi: 10.1016/j.vaccine.2018.12.068. [DOI] [PubMed] [Google Scholar]
- 7.Wedlock PT, Cox SN, Bartsch SM, Randall SL, O’shea KJ, Ferguson MC, Siegmund SS, Lee BY. Should countries switch to using five- or ten-dose rotavirus vaccines now that they are available? Vaccine. 2021;39(31):4335–42. doi: 10.1016/j.vaccine.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klar S, Harris T, Wong K, Fediurek J, Deeks SL. Vaccine safety implications of Ontario, Canada’s switch from DTaP-IPV to Tdap-IPV for the pre-school booster. Vaccine. 2014;32(48):6360–63. doi: 10.1016/j.vaccine.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Parmar D, Baruwa EM, Zuber P, Kone S. Impact of wastage on single and multi-dose vaccine vials: implications for introducing pneumococcal vaccines in developing countries. Hum Vaccin. 2010;6(3):270–78. doi: 10.4161/hv.6.3.10397. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . Principles and considerations for adding a vaccine to a national immunization programme. 2014.
- 11.Gordon WS, Jones A, Wecker J. Introducing multiple vaccines in low-and lower-middle-income countries: issues, opportunities and challenges. Health Policy Plan. 2012;27(suppl_2):ii17–26. doi: 10.1093/heapol/czs040. [DOI] [PubMed] [Google Scholar]
- 12.De Oliveira LH, Danovaro-Holliday MC, Matus CR, Andrus JK. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert Rev Vaccines. 2008;7(3):345–53. doi: 10.1586/14760584.7.3.345. [DOI] [PubMed] [Google Scholar]
- 13.Sutter RW, Cochi SL. Inactivated poliovirus vaccine supply shortage: is there light at the end of the tunnel? The Journal of Infectious Diseases. 2019;220(10):1545–46. 10.1093/infdis/jiy739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Global Polio Eradication Initiative . Polio eradication & endgame strategic plan 2013-2018. Geneva, Switzerland; 2013. https://polioeradication.org/who-we-are/strategic-plan-2013-2018/. [Google Scholar]
- 15.Centers for Disease Control and Prevention . Pneumococcal vaccination: what everyone should know [internet]. Centers for Disease Control and Prevention; 2022. Jan 24 [accessed 2022 Apr 25]. https://www.cdc.gov/vaccines/vpd/pneumo/public/index.html.
- 16.Asogwa OA, de Hoog MLA, Bruijning-Verhagen P. Impact of 7-valent versus 10-valent pneumococcal conjugate vaccines on primary care consultations across various age groups in the Netherlands, 5 years after the switch: a time-series analysis. Vaccine. 2022;40(2):334–43. doi: 10.1016/j.vaccine.2021.11.059. [DOI] [PubMed] [Google Scholar]
- 17.Wu DB-C, Chaiyakunapruk N, Chong H-Y, Beutels P. Choosing between 7-, 10-and 13-valent pneumococcal conjugate vaccines in childhood: a review of economic evaluations (2006–2014). Vaccine. 2015;33(14):1633–58. doi: 10.1016/j.vaccine.2015.01.081. [DOI] [PubMed] [Google Scholar]
- 18.Syeed MS, Ghule P, Le L, Veettil SK, Horn EK, Perdrizet J, Wasserman M, Thakkinstian A, Chaiyakunapruk N. Pneumococcal vaccination in children: a systematic review and meta-analysis of cost-effectiveness studies. Value in Health. 2022. doi: 10.1016/j.jval.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Xu J, Xu J, Tan H, Zhang C. Current situation, causes, and countermeasures to NIP vaccine shortages in Guangzhou, China. Human Vaccines Immunother. 2020;16(1):76–79. doi: 10.1080/21645515.2019.1644883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, VA Welch. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane; 2022. [accessed 2022 Apr 30]. www.training.cochrane.org/handbook.
- 21.Chaiyakunapruk N, Patikorn C, Kategaew W. Implementation challenges and impact of pediatric vaccine switches on immunization programs: a systematic literature review. PROSPERO 2022 CRD42022331134; 2022. [accessed 2022 Jul 12]. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022331134.
- 22.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):1–11. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Bank Country and Lending Groups [Internet] . 2022. [accessed 2022 Jul 6]. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 24.Baethge C, Goldbeck-Wood S, Mertens S. Sanra—a scale for the quality assessment of narrative review articles. Res Integr and Peer Rev. 2019;4(1):1–7. doi: 10.1186/s41073-019-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–77. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 26.ROBINS-E Development Group . Risk of bias in non-randomized studies - of exposure (ROBINS-E). Launch version; 2022. [accessed 2022 Jun 1]. https://www.riskofbias.info/welcome/robins-e-tool.
- 27.Sharma A, Minh Duc NT, Luu Lam Thang T, Nam NH, Ng SJ, Abbas KS, Huy NT, Marušić A, Paul CL, Kwok J, et al. A consensus-based checklist for reporting of survey studies (CROSS). J Gen Intern Med. 2021;36(10):3179–87. doi: 10.1007/s11606-021-06737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahl S, Hasman A, Eltayeb AO, James Noble D, Thapa A. The switch from trivalent to bivalent oral poliovirus vaccine in the South-East Asia Region. J Infect Dis. 2017;216(suppl_1):S94–s100. doi: 10.1093/infdis/jiw602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Califano G, Sagradini S, Magariños M, González Capria S, Álvarez M, Aquino A, Hernández L, Zubieta A, Vizzotti C. Experiencia Argentina en la etapa final de erradicación de la polio: cambio de vacuna oral trivalente a bivalente. Rev argent salud publica. 2016;7(28):34–37. [Google Scholar]
- 30.Fahmy K, Hampton LM, Langar H, Patel M, Mir T, Soloman C, Hasman A, Yusuf N, Teleb N. Introduction of inactivated polio vaccine, withdrawal of type 2 oral polio vaccine, and routine immunization strengthening in the Eastern Mediterranean region. J Infect Dis. 2017;216(suppl_1):S86–s93. doi: 10.1093/infdis/jix133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamage D, Ginige S, Palihawadana P. National introduction of fractional-dose inactivated polio vaccine in Sri Lanka following the global “switch”. WHO South East Asia J Public Health. 2018;7(2):79–83. doi: 10.4103/2224-3151.239418. [DOI] [PubMed] [Google Scholar]
- 32.Garg A, Pattamadilok S, Bahl S. Successes and challenges of expansion of environmental poliovirus surveillance in the WHO South-East Asia Region. WHO South East Asia J Public Health. 2018;7(2):122–28. doi: 10.4103/2224-3151.239424. [DOI] [PubMed] [Google Scholar]
- 33.Garon J, Patel M. The polio endgame: rationale behind the change in immunisation. Arch Dis Child. 2017;102(4):362–65. doi: 10.1136/archdischild-2016-311171. [DOI] [PubMed] [Google Scholar]
- 34.Garon J, Seib K, Orenstein WA, Ramirez Gonzalez A, Chang Blanc D, Zaffran M, Patel M. Polio endgame: the global switch from tOPV to bOPV. Expert Rev Vaccines. 2016;15(6):693–708. doi: 10.1586/14760584.2016.1140041. [DOI] [PubMed] [Google Scholar]
- 35.Gurung S, Harris JB, Eltayeb AO, Hampton LM, Diorditsa S, Avagyan T, Schluter WW. Experience with inactivated polio vaccine introduction and the “Switch” from trivalent to bivalent oral polio vaccine in the World Health Organization’s Western Pacific Region. J Infect Dis. 2017;216(suppl_1):S101–s8. doi: 10.1093/infdis/jiw574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hampton LM, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale S, Lewis I, Rubin J, Garon J, Harris J, Hyde T, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine — worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(35):934–38. doi: 10.15585/mmwr.mm6535a3. [DOI] [PubMed] [Google Scholar]
- 37.Hampton LM, du Châtellier GM, Fournier-Caruana J, Ottosen A, Rubin J, Menning L, Farrell M, Shendale S, Patel M. Considerations for the full global withdrawal of oral polio vaccine after eradication of polio. J Infect Dis. 2017;216(suppl_1):S217–s25. doi: 10.1093/infdis/jix105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horn EK, Wasserman MD, Hall-Murray C, Sings HL, Chapman R, Farkouh RA. Public health impact of pneumococcal conjugate vaccination: a review of measurement challenges. Expert Rev Vaccines. 2021;20(10):1291–309. doi: 10.1080/14760584.2021.1971521. [DOI] [PubMed] [Google Scholar]
- 39.Icardi G, Tassinari F. Anti-polio vaccinations in the third millennia. Ann Ig. 2018;30(4 Supple 1):11–15. doi: 10.7416/ai.2018.2228. [DOI] [PubMed] [Google Scholar]
- 40.Jog PP. Goodbye switch and imminent polio victory. Indian Pediatr. 2016;53(4):285–88. doi: 10.1007/s13312-016-0837-9. [DOI] [PubMed] [Google Scholar]
- 41.John TJ, Vashishtha VM. Eradicating poliomyelitis: India’s journey from hyperendemic to polio-free status. Indian J Med Res. 2013;137:881–94. [PMC free article] [PubMed] [Google Scholar]
- 42.Menning L, Garg G, Pokharel D, Thrush E, Farrell M, Kodio FK, Veira CL, Wanyoike S, Malik S, Patel M, et al. Communications, immunization, and polio vaccines: lessons from a global perspective on generating political will, informing decision-making and planning, and engaging local support. J Infect Dis. 2017;216(suppl_1):S24–s32. doi: 10.1093/infdis/jix059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nafi OA, Ramadan B. Sabin vaccine in poliomyelitis eradication: achievements and risks. J Pure Appl Microbiol. 2019;13(1):413–18. doi: 10.22207/JPAM.13.1.45. [DOI] [Google Scholar]
- 44.Orenstein WA. Eradicating polio: how the world’s pediatricians can help stop this crippling illness forever. Pediatrics. 2015;135(1):196–202. doi: 10.1542/peds.2014-3163. [DOI] [PubMed] [Google Scholar]
- 45.Pedreira C, Thrush E, Rey-Benito G, Chévez AE, Jauregui B. The path towards polio eradication over 40 years of the expanded program on immunization in the Americas. Rev panam salud pública. 2017;41:e154. doi: 10.26633/RPSP.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pervaiz A, Mbaeyi C, Baig MA, Burman A, Ahmed JA, Akter S, Jatoi FA, Mahamud A, Asghar RJ, Azam N, et al. Fractional-dose inactivated poliovirus vaccine campaign — Sindh Province, Pakistan, 2016. Morb Mortal Wkly Rep. 2017;66(47):1295–99. doi: 10.15585/mmwr.mm6647a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snelling TL, Waddington CS. Whither pertussis? Expert Rev Anti Infect Ther. 2015;13(2):145–48. doi: 10.1586/14787210.2015.986462. [DOI] [PubMed] [Google Scholar]
- 48.Tevi-Benissan C, Okeibunor J, du Châtellier GM, Assefa A, Biey JN, Cheikh D, Eshetu M, Anya B-P, Dao H, Nasir Y, et al. Introduction of inactivated poliovirus vaccine and trivalent oral polio vaccine/bivalent oral polio vaccine switch in the African Region. J Infect Dis. 2017;216(suppl_1):S66–s75. doi: 10.1093/infdis/jiw616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thacker N, Yewale VN, Pathak A. Global polio eradication,The journey so far. Indian Pediatr. 2016;53:S61–s4. [PubMed] [Google Scholar]
- 50.Kaucley L, Essoh TA, Ilboudo GP, Abdoulaye Alfa D, Dicko M, Houngnihin RA, Satoulou-Maleyo A, Bété FH, Agossadou DC. Decision making process, programmatic and logistic impact of the transition from a single-dose vial to a multi-dose vial of the 13-valent pneumococcal vaccine in Benin. Vaccine. 2020;38(43):6807–13. doi: 10.1016/j.vaccine.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 51.Kolasa MS, Petersen TJ, Brink EW, Bulim ID, Stevenson JM, Rodewald LE. Impact of multiple injections on immunization rates among vulnerable children. Am J Prev Med. 2001;21(4):261–66. doi: 10.1016/S0749-3797(01)00371-3. [DOI] [PubMed] [Google Scholar]
- 52.Soeters HM, Kambiré D, Sawadogo G, Ouédraogo-Traoré R, Bicaba B, Medah I, Sangaré L, Ouédraogo A-S, Ouangraoua S, Yaméogo I, et al. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis, Burkina Faso, 2016–2017. J Infect Dis. 2019;220(220 Suppl 4):S253–s62. doi: 10.1093/infdis/jiz301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suarez V, Michel F, Toscano CM, Bierrenbach AL, Gonzales M, Alencar AP, CR Matus, JK Andrus, de Oliveira LH. Impact of pneumococcal conjugate vaccine in children morbidity and mortality in Peru: time series analyses. Vaccine. 2016;34(39):4738–43. doi: 10.1016/j.vaccine.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 54.Wahjuhono G, Revolusiana J, Widhiastuti D, Sundoro J, Mardani T, WU Ratih, Sutomo R, Safitri I, Sampurno OD, Rana B, et al. Switch from oral to inactivated poliovirus vaccine in Yogyakarta Province, Indonesia: summary of coverage, immunity, and environmental surveillance. J Infect Dis. 2014;210 Suppl 1(suppl_1):S347–52. doi: 10.1093/infdis/jiu060. [DOI] [PubMed] [Google Scholar]
- 55.Freed GL, Cowan AE, Clark SJ, Santoli J, Bradley J. Use of a new combined vaccine in pediatric practices. Pediatrics. 2006;118(2):e251–7. doi: 10.1542/peds.2006-0114. [DOI] [PubMed] [Google Scholar]
- 56.Usuf E, Mackenzie G, Lowe-Jallow Y, Boye B, Atherly D, Suraratdecha C, Griffiths UK. Costs of vaccine delivery in the Gambia before and after, pentavalent and pneumococcal conjugate vaccine introductions. Vaccine. 2014;32(17):1975–81. doi: 10.1016/j.vaccine.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 57.Pan American Health Organization . Lessons learned on IPV introduction and the switch from tOPV to bOPV in the Americas. Washington (DC): PAHO; 2017. [Google Scholar]
- 58.Fine PEM, Carneiro IAM, Milstien JB, Clements CJ, World Health O. Issues relating to the use of BCG in immunization programmes: a discussion document. Geneva: World Health Organization; 1999. [Google Scholar]
- 59.World Health Organization ROfE . European Region must remain on alert for polio copenhagen2016. https://www.who.int/europe/news/item/14-06-2016-european-region-must-remain-on-alert-for-polio.
- 60.Sokhey J, Gupta CK, Sharma B, Singh H. Stability of oral polio vaccine at different temperatures. Vaccine. 1988;6(1):12–13. doi: 10.1016/0264-410X(88)90006-0. [DOI] [PubMed] [Google Scholar]
- 61.White JA, Estrada M, Weldon WC, Chumakov K, Kouiavskaia D, Fournier-Caruana J, Stevens E, Gary HE, Maes EF, Oberste MS, et al. Assessing the potency and immunogenicity of inactivated poliovirus vaccine after exposure to freezing temperatures. Biologicals. 2018;53:30–38. doi: 10.1016/j.biologicals.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Polio Global Eradication Initiative . Oral poliovirus vaccine. https://polioeradication.org/polio-today/polio-prevention/the-vaccines/opv/.
- 63.Opel DJ, Mangione-Smith R, Robinson JD, Heritage J, DeVere V, Salas HS, Zhou C, Taylor JA. The influence of provider communication behaviors on parental vaccine acceptance and visit experience. Am J Public Health. 2015;105(10):1998–2004. doi: 10.2105/AJPH.2014.302425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SK, Sun J, Jang S, Connelly S. Misinformation of COVID-19 vaccines and vaccine hesitancy. Sci Rep. 2022;12(1):1–11. doi: 10.1038/s41598-022-17430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Centers for Disease Control and Prevention . Human papillomavirus (HPV) vaccination: what everyone should know; 2021. [accessed 2021 Nov 16]. https://www.cdc.gov/vaccines/vpd/hpv/public/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


