The current literature provides insufficient data to conclude whether postpartum venous thromboembolism rates differ between those exposed to postpartum pharmacologic prophylaxis and those unexposed.
OBJECTIVE:
To evaluate the effectiveness of pharmacologic venous thromboembolism (VTE) prophylaxis in postpartum patients.
DATA SOURCES:
On February 21, 2022, a literature search was conducted on Embase.com, Ovid-Medline All, Cochrane Library, Scopus, and ClinicalTrials.gov using terms postpartum period AND thromboprophylaxis AND antithrombin medications including heparin and low molecular weight heparin.
METHODS OF STUDY SELECTION:
Studies that evaluated the outcome of VTE among postpartum patients exposed to pharmacologic VTE prophylaxis with or without a comparator group were eligible for inclusion. Studies of patients who received antepartum VTE prophylaxis, studies in which this prophylaxis could not be definitively ruled out, and studies of patients who received therapeutic dosing of anticoagulation for specific medical problems or treatment of VTE were excluded. Titles and abstracts were independently screened by two authors. Relevant full-text articles were retrieved and independently reviewed for inclusion or exclusion by two authors.
TABULATION, INTEGRATION, AND RESULTS:
A total of 944 studies were screened by title and abstract, and 54 full-text studies were retrieved for further evaluation after 890 studies were excluded. Fourteen studies including 11,944 patients were analyzed: eight randomized controlled trials (8,001 patients) and six observational studies (3,943 patients). Among the eight studies with a comparator group, there was no difference in the risk of VTE between patients who were exposed to postpartum pharmacologic VTE prophylaxis and those who were unexposed (pooled relative risk 1.02, 95% CI 0.29–3.51); however, six of eight studies had no events in either the exposed or unexposed group. Among the six studies without a comparator group, the pooled proportion of postpartum VTE events was 0.00, likely due to five of six studies having no events.
CONCLUSION:
The current literature provided an insufficient sample size to conclude whether postpartum VTE rates differ between those exposed to postpartum pharmacologic prophylaxis and those unexposed, given the rarity of VTE events.
SYSTEMATIC REVIEW REGISTRATION:
PROSPERO, CRD42022323841.
Venous thromboembolism (VTE), which includes deep vein thrombosis and pulmonary embolism, is responsible for 9–30% of pregnancy-related mortality in high resource countries and remains a significant, increasing cause of severe maternal morbidity.1–4 Although rising rates of comorbidities known to be risk factors for VTE (such as obesity and older age at the time of delivery) have contributed to this problem, pregnancy itself is associated with both physiologic and anatomic changes that increase the risk for VTE approximately fivefold compared with the nonpregnant state due to hypercoagulability and venous stasis.5–7 Approximately 50% of all VTE events occur in the early postpartum period and represents a window of time that patients remain at significant risk for adverse outcomes.8–10 Specifically, the hypercoagulable state favoring thrombosis does not resolve to prepregnancy physiology until approximately 6–8 weeks postpartum.11
In light of the need for effective strategies for VTE prevention specific to postpartum patients, many professional obstetric societies and governing bodies have put forth recommendations addressing the role of postpartum pharmacologic VTE prophylaxis (ie, anticoagulation).5,12–16 Yet, these guidelines lack consensus in regard to the specifics of thromboprophylaxis, due to the heterogeneity of both obstetric and nonobstetric literature used to develop them.16 As a result the efficacy of thromboprophylaxis is unclear. The objective of this study was to conduct a systematic review and meta-analysis to estimate the magnitude to which pharmacologic prophylaxis affects the risk of VTE among postpartum patients.
SOURCES
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines and was registered in PROSPERO International Prospective Register of Systematic Reviews (CRD42022323841) before beginning review and data abstraction.17 The published literature was searched by a medical librarian (A.H.) for the terms postpartum period AND thromboprophylaxis AND antithrombin medications including heparin and low molecular weight heparin. These search terms were created using a combination of controlled vocabulary terms and keywords, executed in Embase.com, Ovid-Medline All, Cochrane Library, Scopus, and Clinicaltrials.gov from database inception (Appendix 1, available online at http://links.lww.com/AOG/D74). Results were limited to English-language through the use of database-supplied filters. All database searches were completed on February 21, 2022. Because all data were de-identified and available in the public domain, this study was exempt from Institutional Review Board approval. After duplicates were removed, two of the authors (M.C.O. and M.R.) independently screened the titles and abstracts of remaining publications for fulfillment of inclusion, and exclusion criteria and relevance to the present study. Additional publications were identified by reviewing the bibliographies of selected studies.
STUDY SELECTION
The PICOT method (population, intervention, comparison, outcome, time) was used to develop the study question and inclusion criteria, which included studies of postpartum patients’ status after vaginal or cesarean delivery who received pharmacologic VTE prophylaxis (either with or without an unexposed comparator group) with the outcome of VTE.18 Study designs that compared postpartum patients exposed to heparin (unfractionated or low molecular weight) with an unexposed group (either placebo or no intervention) were eligible for inclusion. Studies were also eligible for inclusion if their design compared two or more groups that were exposed to pharmacologic thromboprophylaxis (for instance, two groups both exposed to a low molecular weight heparin but for different durations of prophylaxis) with or without the presence of an unexposed group. Exclusion criteria were studies that included patients who received antepartum pharmacologic prophylaxis or studies in which this exposure type could not be definitively ruled out, and patients who received therapeutic dosing of anticoagulation for specific medical problems or treatment of VTE. We excluded case reports, case series, review articles, abstracts without a corresponding full-text article, and full-text articles not published in a peer-reviewed journal.
The primary outcome of this study was postpartum VTE. Titles and abstracts were independently screened by two of the authors (M.C.O. and M.R.). Full-text articles were then retrieved if they were deemed relevant or relevance was queried. Full-text articles were again independently reviewed by two of the authors (M.C.O. and M.R.) against inclusion and exclusion criteria. Discrepancies regarding the decision to include or exclude a study were resolved by consultation with the senior author (A.I.F.) as needed. Data abstraction into a standardized form was performed by two of the authors (M.C.O. and M.R.). A single attempt was made to contact authors by email if there was insufficient information to complete the data abstraction.
In addition to information on the primary outcome, additional data points that were abstracted included: study inclusion and exclusion criteria; type and dose of anticoagulant studied; duration of anticoagulant exposure; presence or absence of an unexposed or other comparator group; duration of surveillance for postpartum VTE; descriptor of how VTE events were identified; and participant characteristics. Missing or unclear information was clearly denoted as “not measured” or “unknown.”
Although published quality scoring systems for both randomized and nonrandomized studies of health care interventions exist (eg, the Downs and Black19 checklist), such checklists still involve subjectivity and there is a lack of consensus as to a cut-point that discriminates between high-quality and low-quality studies. On reviewing these checklists, it was felt that they did not adequately discriminate study quality for the purposes of our systematic review and meta-analysis. Thus, we assessed study quality and risk for pertinent forms of bias based on the presence or absence of five characteristics most likely to influence study validity: 1) documented anticoagulant type and dose, 2) documented duration of anticoagulation exposure, 3) presence of an unexposed comparator group, 4) documented VTE surveillance duration, and 5) the process of identifying and documenting postpartum VTE events.20,21 The presence of criteria 1 and 2 were considered to reflect reduced risk for information bias. The presence of criterion 3 was considered to reflect a reduced risk for selection bias. The presence of criteria 4 and 5 were considered to reflect a reduced risk for misclassification bias and detection bias. The quality of each study was assessed independently by two study authors (M.C.O. and M.R.) with any discrepancies adjudicated by the senior author (A.I.F.). Overall study quality was determined to be high if all five criteria were met.
Data were abstracted from each study and, for studies with an unexposed comparator group, combined using the Der-Simonian-Laird random-effects model to account for both within- and between-study variances. Zero cells were adjusted for with a continuity correction of 0.5. The Peto odds ratio (OR) method also was performed given the rarity of events.22,23 Pooled relative risk (RR) with 95% CIs were calculated for the primary outcome among studies with an unexposed comparator group. If a study included two or more different groups exposed to pharmacologic thromboprophylaxis (eg, two different anticoagulant types, and one unexposed comparator group), all exposed patients were combined and compared with the single unexposed comparator group. The proportion of VTE events and 95% CI was calculated among studies with no unexposed comparator group, for only those exposed to anticoagulation from studies with an unexposed or control group, and as a pooled proportion. Similarly, the pooled proportion of VTE events was calculated among all unexposed or control groups. Forest plots were used to graphically represent the data.
Heterogeneity was explored using Higgins' I2.24 Given the low statistical power of tests of heterogeneity, heterogeneity was further classified as small (I2<25%), moderate (I2 25–50%), or large (I2>50%). The presence of moderate-to-large heterogeneity was further explored by stratification by study quality. Publication bias was assessed graphically using contrast-enhanced funnel plots, and small-study bias was assessed using the Harbord test.25 Data were analyzed using STATA 16.0.
RESULTS
A flow diagram of study identification for the meta-analysis is shown in Figure 1. The initial literature search yielded 1,570 citations. Of those, 626 citations were duplicates and removed, leaving 944 studies to be screened by title and abstract.26 After titles and abstracts were reviewed for relevance and screened against inclusion and exclusion criteria, 890 studies were excluded, and 54 full-text studies were retrieved for further evaluation. Review of bibliographies of selected papers against study criteria resulted in an additional six studies for consideration. Studies were subsequently excluded for the following reasons: abstract only (n=22); no full text available (n=4); did not include primary outcome of interest (n=6); data set was duplicated from another study (n=2); study included antenatal exposure to anticoagulation (n=3); inability to extract exposure data (n=8); and full-text not available in English (n=1).
Fig. 1. Flowchart of study selection methodology.
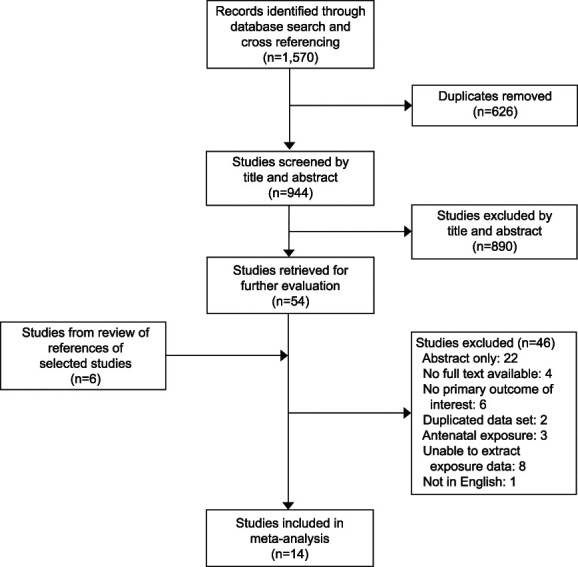
Oakes. Review of Postpartum Thromboprophylaxis. Obstet Gynecol 2023.
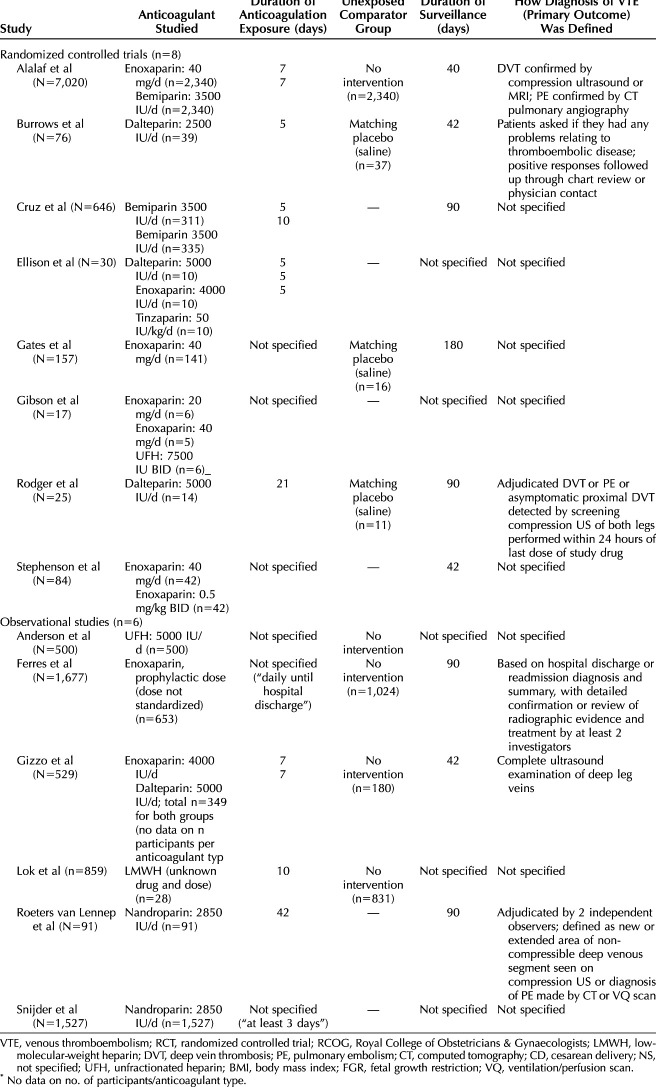
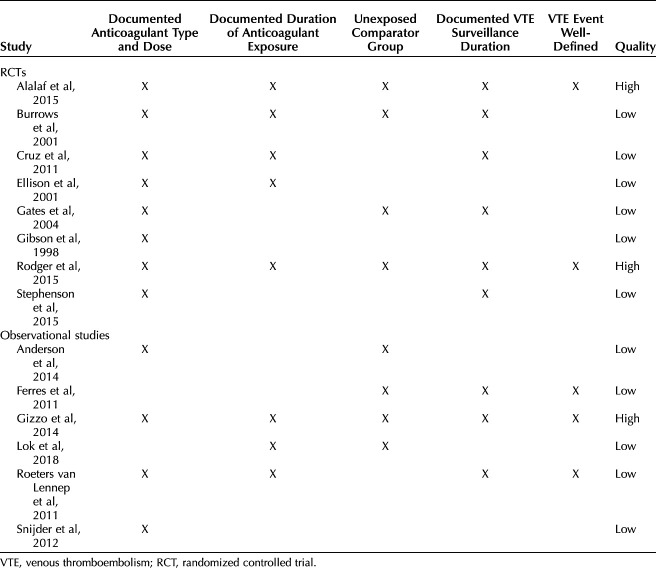
Fourteen studies including 11,944 patients were analyzed: eight randomized controlled trials ([RCTs] 8,001 patients) and six observational studies (3,943 patients).27–40 Study characteristics are shown in Table 1, including each study's year of publication, county of origin, inclusion and exclusion criteria, characteristics of prophylactic anticoagulant studied, definition of primary outcome (VTE), duration of surveillance, and whether an unexposed comparator group was included. The results of the methodologic quality assessment are shown in Table 2. Based on evaluation in five categories, three studies were deemed high-quality, and 11 studies were deemed low quality. Low-quality studies were at higher risk for misclassification bias with unspecified surveillance durations and ambiguous or unclear definitions of a VTE event; they were also more likely to have lower quality data with unclear details regarding anticoagulation dosing and duration of exposure.
Table 1.
Characteristics of Studies Included in Final Meta-Analysis
Table 2.
Quality Assessment of Included Studies
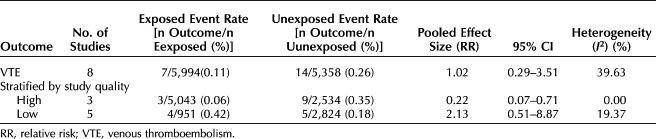
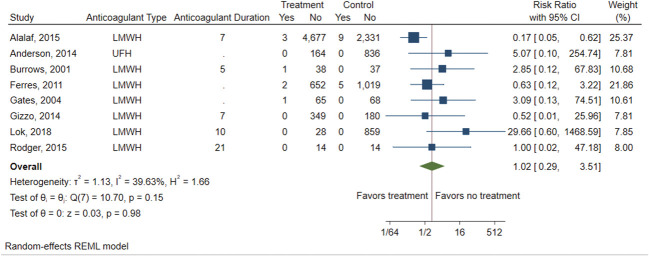
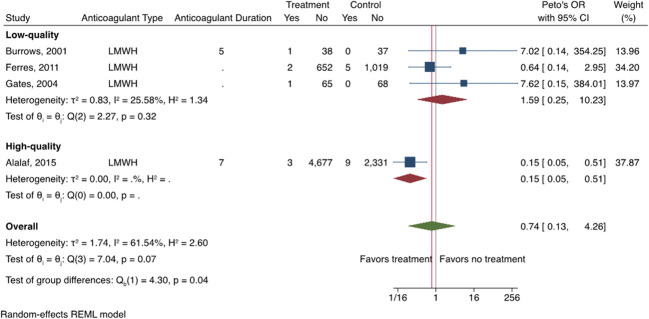
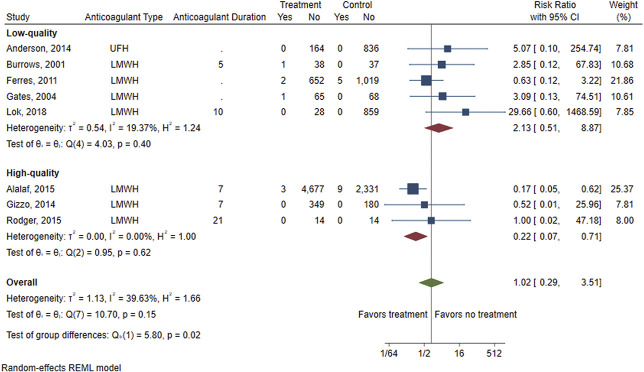
There were eight studies (four RCTs, four observational studies) that compared the risk of VTE between those exposed to postpartum pharmacologic prophylaxis and an unexposed comparator group (either placebo or no intervention).27,28,31,33,35–38 Table 3 shows VTE rates in the exposed and unexposed groups, pooled RR and 95% CI, and heterogeneity of the primary outcome of VTE, as well as results of the stratified analysis. Overall, there was no difference in the risk of VTE between patients who were exposed to postpartum pharmacologic VTE prophylaxis and those who were unexposed (eight studies including six studies with imputed values for primary outcome due to no VTE events, pooled RR 1.02, 95% CI 0.29–3.51) (Fig. 2). There was also no difference in risk of VTE when using the Peto OR method to account for the rarity of VTE events (pooled RR 0.74, 95% CI 0.13–4.26) (Fig. 3). A moderate amount of heterogeneity was noted (I2=39.63%), which was explored further with an analysis stratified by study quality (Fig. 4). Evaluation of high-quality studies (three studies, including two with imputed values for primary outcome due to no VTE events) revealed a significantly decreased RR of postpartum VTE for those exposed to pharmacologic VTE prophylaxis compared with those unexposed (RR 0.22, 95% CI 0.07–0.71) and no heterogeneity among studies (I2=0.00%). Low-quality studies (five studies, including four with imputed values for primary outcome due to no VTE events) did not demonstrate a significant difference in risk for postpartum VTE between exposed and unexposed groups (RR 2.13, 95% CI 0.51–8.87; I2=19.37%). Findings were similar using the Peto OR method (Appendix 2, available online at http://links.lww.com/AOG/D74).
Table 3.
Rates and Pooled Estimates for Primary Outcome and Analysis Stratified by Study Quality
Fig. 2. Forest plot showing the risk of venous thromboembolism (VTE) for those exposed to postpartum pharmacologic VTE prophylaxis vs those unexposed. LMWH, low-molecular-weight heparin; UFH, unfractionated heparin; REML, restricted maximum likelihood.
Oakes. Review of Postpartum Thromboprophylaxis. Obstet Gynecol 2023.
Fig. 3. Forest plot showing the risk of venous thromboembolism (VTE) for those exposed to postpartum pharmacologic VTE prophylaxis vs those unexposed, using Peto's odds ratio (OR) method. LMWH, low-molecular-weight heparin; REML, restricted maximum likelihood.
Oakes. Review of Postpartum Thromboprophylaxis. Obstet Gynecol 2023.
Fig. 4. Forest plot showing the risk of venous thromboembolism (VTE) for those exposed to postpartum pharmacologic VTE prophylaxis vs those unexposed, stratified by study quality. UFH, unfractionated heparin; LMWH, low-molecular-weight heparin; REML, restricted maximum likelihood.
Oakes. Review of Postpartum Thromboprophylaxis. Obstet Gynecol 2023.
Visual inspection of a contrast-enhanced funnel plot of studies with an unexposed comparator group demonstrated evidence of publication bias. Specifically, there was a lack of studies with nonsignificant results favorable to the intervention (postpartum pharmacologic prophylaxis) (Fig. 5). The Harbord test was significant (P=.01), suggesting the presence of a small study effect.
Fig. 5. Contour-enhanced funnel plot showing the risk of venous thromboembolism (VTE) for those exposed to postpartum pharmacologic VTE prophylaxis vs those unexposed.
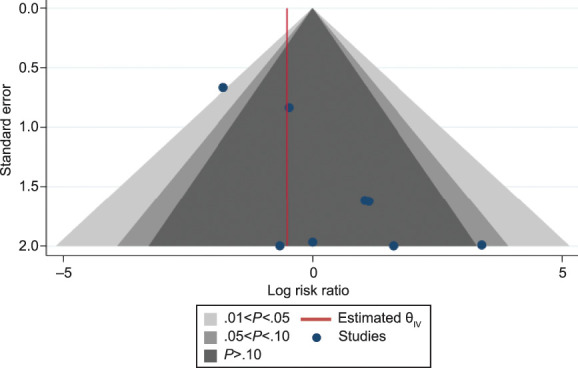
Oakes. Review of Postpartum Thromboprophylaxis. Obstet Gynecol 2023.
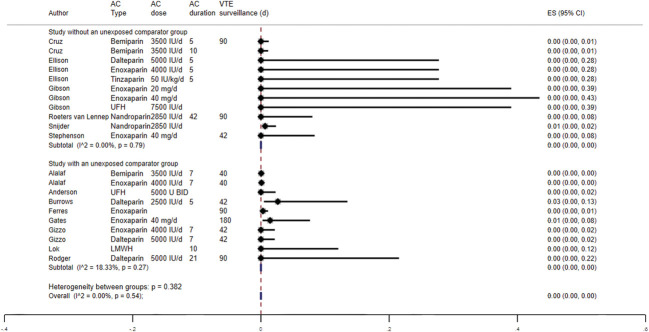
The effect of postpartum pharmacologic VTE prophylaxis was assessed among studies that did not have an unexposed comparator group (n=6, four RCTs and two observational studies).29,30,32,34,39,40 The pooled proportion of postpartum VTE events was 0.00 (5/6 studies with imputed values for primary outcome due to no VTE events) with no significant heterogeneity (I2=0.00%), likely due to the extremely small number of events across studies. When including the exposed groups from studies with an unexposed comparator group (n=8; total 14 studies, including five with imputed values for primary outcome due to no VTE events), there was no difference in the overall pooled proportion of VTE events (0.00%) (Fig. 6).27,28,31,35–38 The pooled proportion of VTE events among those that did not receive anticoagulation from studies with an unexposed comparator group was 0.00% (eight studies, including four with imputed values for primary outcome due to no VTE events) (Fig. 7).
Fig. 6. Forest plot showing the proportion of venous thromboembolism (VTE) for those exposed to postpartum pharmacologic VTE prophylaxis. AC, anticoagulation; ES, effect size; UFH, unfractionated heparin; LMWH, low-molecular-weight heparin.
Oakes. Review of Postpartum Thromboprophylaxis. Obstet Gynecol 2023.
Fig. 7. Forest plot showing the proportion of venous thromboembolism (VTE) for those unexposed to postpartum pharmacologic VTE prophylaxis. ES, effect size.
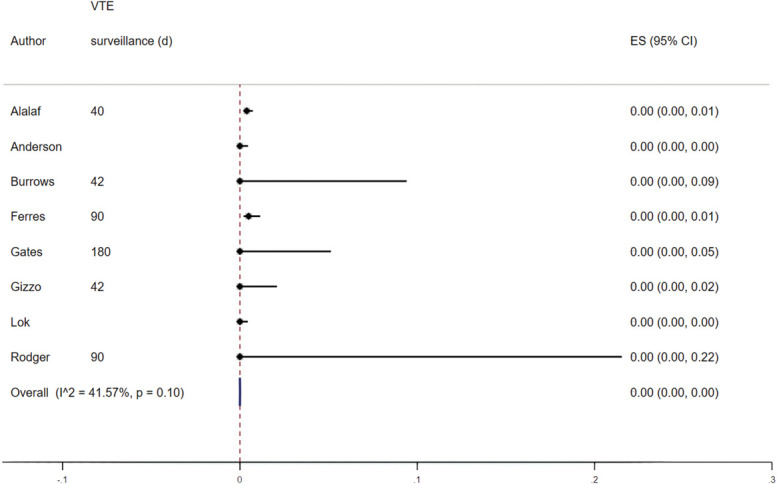
Oakes. Review of Postpartum Thromboprophylaxis. Obstet Gynecol 2023.
DISCUSSION
Analysis of 16 studies published in the peer-reviewed literature that met inclusion criteria (N=11,944) for this meta-analysis found the risk of postpartum VTE did not differ between those exposed to thromboprophylaxis and unexposed groups. However, this study illustrates that, although universal postpartum VTE thromboprophylaxis is a recommended practice by some professional societies, not only is the rate of postpartum VTE exceedingly low, but the limited high-quality evidence surrounding its efficacy limits the ability of this study to definitively make conclusions regarding its efficacy.
It is also important to note the high level of heterogeneity in the available studies included in our analysis. The two most important sources of heterogeneity were in patient selection and study population and definition or diagnosis of the primary outcome of VTE. The specific risk factors that defined populations as “at risk” for postpartum VTE and, therefore, those in need of pharmacologic prophylactic anticoagulation, varied greatly. A majority of studies in this analysis included only patients who underwent cesarean delivery (11/14, 78.6%), which is the most common surgery performed in the United States, with a rate that continues to rise.28–32,34–38,40 In a review of published evidence-based guidelines on VTE prevention in pregnant and postpartum patients, almost half of the guidelines (4/9) recommended initiation of thromboprophylaxis for people who undergo cesarean delivery with the presence of an additional risk factor (eg, obesity).16,31,35,37 Although it was not within the scope of this study to evaluate specific practice guidelines, our findings allude to a need for further studies to explore the effect of postpartum VTE prophylaxis, particularly in high-risk groups.
The diagnostic criteria for VTE in the studies included in this meta-analysis ranged from patient-reported symptoms to objective screening of all patients with compression ultrasonography. Further, 8 of 14 (57%) included studies did not provide details in regard to how VTE was diagnosed (including 5/8 [62.5%] of RCTs). The heterogeneity in method of VTE diagnosis and duration of screening introduces a great degree of selection bias, making the applicability of the findings challenging. The analysis presented here highlights that future studies should clearly state postpartum risk factors for VTE and identify the optimal drug, dose, and duration for VTE prevention. These studies must also include detailed VTE surveillance protocols and methods for identifying VTE events. Additionally, the duration of surveillance should span at least 6 weeks after delivery when the hypercoagulable state associated with pregnancy resolves.11
Among the strengths of this study are the rigorous, transparent data-collection methods and analysis, including an extensive literature search across five databases, performed by two reviewers with the assistance of a medical librarian with a Master of Library and Information Science. Three contemporary systematic review and meta-analyses concluded that either postpartum VTE prophylaxis did not reduce the risk of VTE events or there were insufficient data to make conclusions regarding the efficacy of postpartum VTE prophylaxis.41–43 However, the present meta-analysis is unique in that it includes both patients who underwent vaginal and cesarean delivery and excludes studies that evaluated the effect of pharmacologic agents other than unfractionated and low-molecular-weight heparin used to prevent thrombosis (such as aspirin, warfarin, and hydroxyethyl starch). Further, the present meta-analysis includes both observational studies and RCTs.
Several limitations to this systematic review must be considered. Four of the eight comparative studies had zero events in both the exposed and unexposed groups, and an additional two of eight comparative studies had zero events in the unexposed group resulting in a need for imputation of the primary outcome to perform a meta-analysis. Despite the inclusion of both observational and randomized studies, our study was underpowered to detect a difference in the primary outcome between those exposed and unexposed to postpartum pharmacologic VTE prophylaxis, given the limited number of available patients and rarity of the primary outcome. Only two comparative studies included had VTE events in the unexposed group, and the rate of VTE events (0.96/1,000 births) was lower than estimated in the literature (1.72–1.86/1,000 births). However, the exclusion of studies that had patients with an elevated VTE risk independent of pregnancy (such as known thrombophilia) may account for some degree of this discrepancy. Studies that did not have an unexposed comparator group (such as those that compared two different durations of prophylactic anticoagulation without a control or untreated group) were included, given that these studies still contributed to estimating the proportion of VTE events among those exposed to prophylactic anticoagulation. However, comparing the pooled rate of VTE among all patients who received prophylaxis in the included studies (n=14) to patients who did not receive prophylaxis from studies that had an unexposed comparator group (n=8) arguably introduces a higher risk of selection bias. Finally, given the heterogeneity in reporting, we were unable to comment on important secondary and safety outcomes, such as postpartum bleeding and wound complications related to anticoagulation.
In conclusion, results of this systematic review and meta-analysis suggest that, although high-quality studies may signal a benefit of postpartum pharmacologic VTE prophylaxis, this evidence is extremely limited, and, currently, the existing literature is insufficient to conclude whether postpartum pharmacologic prophylaxis affects VTE risk. The effect of postpartum prophylactic anticoagulation needs to be further clarified by larger, well-conducted studies with particular attention to strict methods of identifying postpartum VTE events and appropriate surveillance duration to identify the appropriate postpartum population for whom this preventative strategy may be appropriate.
Footnotes
Financial Disclosure The authors did not report any potential conflicts of interest.
Dr. Oakes is supported by UL1 TR002345 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D75.
Figure.
No available caption
REFERENCES
- 1.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol 2017;130:366–73. doi: 10.1097/AOG.0000000000002114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol 2012;120:1029–36. doi: 10.1097/aog.0b013e31826d60c5 [DOI] [PubMed] [Google Scholar]
- 3.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. The Lancet 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9 [DOI] [PubMed] [Google Scholar]
- 4.Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving mothers' lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The eighth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom [published erratum appears in BJOG 2015;122:e1]. BJOG 2011;118(suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x [DOI] [PubMed] [Google Scholar]
- 5.Thromboembolism in pregnancy. ACOG Practice Bulletin No. 196. American College of Obstetricians and Gynecologists. Obstet Gynecol 2018;132:e1–e17. doi: 10.1097/AOG.0000000000002706 [DOI] [PubMed] [Google Scholar]
- 6.Ghaji N, Boulet SL, Tepper N, Hooper WC. Trends in venous thromboembolism among pregnancy-related hospitalizations, United States, 1994-2009. Am J Obstet Gynecol 2013;209:433.e1–8. doi: 10.1016/j.ajog.2013.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pabinger IGH, Grafenhofer H. Thrombosis during pregnancy: risk factors, diagnosis and treatment. Pathophysiology Haemost Thromb 2002;32:322–4. doi: 10.1159/000073590 [DOI] [PubMed] [Google Scholar]
- 8.Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ, 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med 2005;143:697–706. doi: 10.7326/0003-4819-143-10-200511150-00006 [DOI] [PubMed] [Google Scholar]
- 9.James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol 2006;194:1311–5. doi: 10.1016/j.ajog.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 10.James AH, Tapson VF, Goldhaber SZ. Thrombosis during pregnancy and the postpartum period. Am J Obstet Gynecol 2005;193:216–9. doi: 10.1016/j.ajog.2004.11.037 [DOI] [PubMed] [Google Scholar]
- 11.Saha P, Stott D, Atalla R. Haemostatic changes in the puerperium '6 weeks postpartum' (HIP Study) - implication for maternal thromboembolism. BJOG: Int J Obstet Gynaecol 2009;116:1602–12. doi: 10.1111/j.1471-0528.2009.02295.x [DOI] [PubMed] [Google Scholar]
- 12.D'Alton ME, Friedman AM, Smiley RM, Montgomery DM, Paidas MJ, D’Oria R, et al. National Partnership for Maternal Safety: consensus bundle on venous thromboembolism [published erratum appears in Obstet Gynecol 2019;133:1288]. Obstet Gynecol 2016;128:688–98. doi: 10.1097/AOG.0000000000001579 [DOI] [PubMed] [Google Scholar]
- 13.Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. Green-top Guideline No. 37a. RCOG; 2015. [Google Scholar]
- 14.Hameed AB, Friedman A, Peterson N, Morton CH, Montgomery D. Improving health care response to maternal venous thromboembolism. (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). CMQCC; 2017. [Google Scholar]
- 15.Bates SM, Greer IA, Pabinger I, Sofaer S, Hirsh J. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians evidence-based clinical practice guidelines (9th edition). Chest 2008;133:844–86S. doi: 10.1378/chest.08-0761 [DOI] [PubMed] [Google Scholar]
- 16.Okoroh EM, Azonobi IC, Grosse SD, Grant AM, Atrash HK, James AH. Prevention of venous thromboembolism in pregnancy: a review of guidelines, 2000-2011. J Women's Health 2012;21:611–5. doi: 10.1089/jwh.2012.3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 18.Brian Haynes R. Forming research questions. J Clin Epidemiol 2006;59:881–6. doi: 10.1016/j.jclinepi.2006.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. doi: 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JAC, Hernán MA, McAleenan A, Reeves BC, Higgins JPT. Assessing risk of bias in a non-randomized study. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions version 6.3. John Wiley & Sons; 2022. [Google Scholar]
- 21.Higgins JPT, Savovic J, Page MJ, Elbers RG, Sterne JAC. Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions version 6.3. John Wiley & Sons; 2022. [Google Scholar]
- 22.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985;27:335–71. doi: 10.1016/s0033-0620(85)80003-7 [DOI] [PubMed] [Google Scholar]
- 23.Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions version 6.3. John Wiley & Sons; 2022. [Google Scholar]
- 24.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 25.Harbord RM, Egger M, Sterne JAC. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443–57. doi: 10.1002/sim.2380 [DOI] [PubMed] [Google Scholar]
- 26.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016;104:240–3. doi: 10.3163/1536-5050.104.3.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alalaf SK, Jawad RK, Muhammad PR, Ali MS, Al Tawil NG. Bemiparin versus enoxaparin as thromboprophylaxis following vaginal and abdominal deliveries: a prospective clinical trial. BMC Pregnancy Childbirth 2015;15:72. doi: 10.1186/s12884-015-0515-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burrows RF, Gan ET, Gallus AS, Wallace EM, Burrows EA. A randomised double-blind placebo controlled trial of low molecular weight heparin as prophylaxis in preventing venous thrombolic events after caesarean section: a pilot study. BJOG 2001;108:835–9. doi: 10.1111/j.1471-0528.2001.00198.x [DOI] [PubMed] [Google Scholar]
- 29.Cruz M, Fernández-Alonso AM, Rodríguez I, Garrigosa l, Caño A, Carretero P, et al. Postcesarean thromboprophylaxis with two different regimens of bemiparin. Obstet Gynecol Int 2011;2011:1–6. doi: 10.1155/2011/548327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson AJ, Ellison J, Conkie JA, McCall F, Walker ID, Greer IA. Thromboprophylaxis following caesarean section. Thromb Haemost 2001;86:1374–8. doi: 10.1055/s-0037-1616738 [DOI] [PubMed] [Google Scholar]
- 31.Gates S, Brocklehurst P, Ayers S, Bowler U. Thromboprophylaxis and pregnancy: two randomized controlled pilot trials that used low-molecular-weight heparin. Am J Obstet Gynecol 2004;191:1296–303. doi: 10.1016/j.ajog.2004.03.039 [DOI] [PubMed] [Google Scholar]
- 32.Gibson JL, Ekevall K, Walker I, Greer IA. Puerperal thromboprophylaxis: comparison of the anti-Xa activity of enoxaparin and unfractionated heparin. BJOG: Int J Obstet Gynaecol 1998;105:795–7. doi: 10.1111/j.1471-0528.1998.tb10212.x [DOI] [PubMed] [Google Scholar]
- 33.Rodger MA, Phillips P, Kahn SR, Bates S, McDonald S, Khurana R, et al. Low molecular weight heparin to prevent postpartum venous thromboembolism: a pilot study to assess the feasibility of a randomized, open-label trial. Thromb Res 2016;142:17–20. doi: 10.1016/j.thromres.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 34.Stephenson ML, Serra AE, Neeper JM, Caballero DC, McNulty J. 26: comparing anti-Xa levels in women with body mass index ≥35 post cesarean delivery undergoing enoxaparin thromboprophylaxis with weight-based dosing twice daily versus fixed dose 40 mg daily: a randomized, controlled trial [abstract]. Am J Obstet Gynecol 2015;212:S18. doi: 10.1016/j.ajog.2014.10.072 [DOI] [Google Scholar]
- 35.Anderson SB, Lin SN, Reiss J, Skupski D, Grunebaum A. Peripartum thromboprophylaxis before and after implementation of a uniform heparin protocol. J Perinatal Med 2014;42:219–23. doi: 10.1515/jpm-2013-0165 [DOI] [PubMed] [Google Scholar]
- 36.Ferres MA, Olivarez SA, Trinh V, Davidson C, Sangi-Haghpeykar H, Aagaard-Tillery KM. Rate of wound complications with enoxaparin use among women at high risk for postpartum thrombosis. Obstet Gynecol 2011;117:119–24. doi: 10.1097/AOG.0b013e3182029180 [DOI] [PubMed] [Google Scholar]
- 37.Gizzo S, Noventa M, Anis O, Saccardi C, Zambon A, Di Gangi S, et al. Pharmacological anti-thrombotic prophylaxis after elective caesarean delivery in thrombophilia unscreened women: should maternal age have a role in decision making? J Perinatal Med 2014;42:339–47. doi: 10.1515/jpm-2013-0160 [DOI] [PubMed] [Google Scholar]
- 38.Lok WY, Kong CW, To WWK. A local risk score model for venous thromboembolism prophylaxis for caesarean section in Chinese women and comparison with international guidelines. Taiwanese J Obstet Gynecol 2019;58:520–5. doi: 10.1016/j.tjog.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 39.Roeters van Lennep JE, Meijer E, Klumper FJ, Middeldorp JM, Bloemenkamp KW, Middeldorp S. Prophylaxis with low-dose low-molecular-weight heparin during pregnancy and postpartum: is it effective? J Thromb Haemost 2011;9:473–80. doi: 10.1111/j.1538-7836.2011.04186.x [DOI] [PubMed] [Google Scholar]
- 40.Snijder CA, Cornette JMW, Hop WCJ, Kruip MJHA, Duvekot JJ. Thrombophylaxis and bleeding complications after cesarean section. Acta Obstetricia Gynecologica Scand 2012;91:560–5. doi: 10.1111/j.1600-0412.2012.01351.x [DOI] [PubMed] [Google Scholar]
- 41.Yang R, Zhao X, Yang Y, Huang X, Li H, Su L. The efficacy and safety of pharmacologic thromboprophylaxis following caesarean section: a systematic review and meta-analysis. PLoS One 2018;13:e0208725. doi: 10.1371/journal.pone.0208725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bain E, Wilson A, Tooher R, Gates S, Davis LJ, Middleton P. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. The Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No.: CD001689. doi: 10.1002/14651858.CD001689.pub3 [DOI] [PubMed] [Google Scholar]
- 43.Middleton P, Shepherd E, Gomersall JC. Venous thromboembolism prophylaxis for women at risk during pregnancy and the early postnatal period. The Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No.: CD001689. doi: 10.1002/14651858.CD001689.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]