Background:
The inspIRE study (Study for Treatment of Paroxysmal Atrial Fibrillation [PAF] by Pulsed Field Ablation [PFA] System With Irreversible Electroporation [IRE]) evaluated safety and effectiveness of a fully integrated biphasic pulsed field ablation (PFA) system with a variable-loop circular catheter for the treatment of drug-refractory paroxysmal atrial fibrillation.
Methods:
Subjects underwent pulmonary vein (PV) isolation with the PFA system, using at least 12 applications per vein; adenosine/isoproterenol was administered to confirm entrance block. Wave I assessed initial safety, including for esophageal lesions, silent cerebral lesions, and PV stenosis. Wave II (pivotal phase) tested (1) primary safety, incidence of early-onset primary adverse events, and (2) primary effectiveness, confirmed PV isolation with freedom from documented atrial arrhythmia at 12 months. The study design specified an interim analysis to determine early success once 30 subjects reached the 12-month follow-up and all subjects reached 3-month follow-up.
Results:
Across 13 centers in Europe/Canada, 226 subjects were enrolled, met criteria for safety and effectiveness evaluations, and received PFA (Wave I, 40; Wave II, 186). Wave I demonstrated no esophageal thermal lesions or PV stenosis. Among 39 subjects with cerebral magnetic resonance imaging, silent cerebral lesions were detected in 4 of the first 6 subjects, after which workflow enhancements, including a 10-second pause between PFA applications, were implemented; subsequently, only 4 of 33 subjects had silent cerebral lesions. In the Wave II phase, no primary adverse events were reported. Upon declaring early success, 83 subjects reached 12-month follow-up. With 100% entrance block, PV isolation without acute reconnection was achieved in 97.1% of targeted veins. For Wave II, the primary effectiveness end point per Kaplan-Meier at the time of interim analysis was 70.9%; 12-month freedom from symptomatic atrial fibrillation/atrial flutter/atrial tachycardia recurrence and repeat ablation was 78.9% and 92.3%, respectively. Total procedure and transpired PFA times were 70.1±27.7 and 26.7±14.0 minutes, respectively.
Conclusions:
The inspIRE trial confirmed the safety and effectiveness of the novel mapping-integrated PFA system.
Registration:
URL: https://www.clinicaltrials.gov; unique identifier: NCT04524364.
Keywords: atrial fibrillation, catheter ablation, efficacy, electroporation, radiofrequency ablation
What is Known?
Growing evidence on novel pulsed field ablation (PFA) technology shows safety benefits versus conventional radiofrequency ablation in both preclinical and clinical models.
Current publications have limited data on PFA catheter use in conjunction with electroanatomical mapping systems.
What the Study Adds
This study reports on the first clinical experience of paroxysmal atrial fibrillation ablation using a novel fully integrated biphasic PFA system with a variable-loop circular catheter in combination with a multichannel PFA generator and a 3-dimensional mapping system (PFA Platform).
The results confirm the strong safety profile of the PFA system with no reported device-related serious adverse events, esophageal injury, or pulmonary vein stenosis, along with 12-month effectiveness comparable to multicenter experience with radiofrequency ablation technologies.
The study demonstrated practical advantages of utility of a biphasic PFA system integrated with a 3-dimensional mapping system that included the ability to map and track lesion set placement, to perform voltage mapping, and to adopt a low-fluoroscopy workflow.
Pulsed field ablation (PFA) is a novel, largely nonthermal ablative modality that, by virtue of its putative preferential action on myocardial tissue through the process of irreversible electroporation, may replace conventional thermal ablation (eg, radiofrequency, cryothermal, and laser energy) for the ablation of atrial fibrillation (AF). It is postulated that by reducing the potential for damage to neighboring tissues during cardiac ablation, PFA will reduce the risk of rare but serious complications, such as pulmonary vein (PV) stenosis, phrenic nerve palsy, or atrioesophageal fistula, while maintaining effectiveness.1,2 Preclinical studies with PFA have demonstrated both the feasibility of achieving transmural and durable cardiac ablation lesions and a favorable safety profile with minimal effects on the esophagus and phrenic nerve compared with radiofrequency ablation.3–6 The clinical use of PFA in small cohorts of patients with AF has demonstrated successful acute PV isolation (PVI), low rates of arrhythmia recurrence, and minimal safety concerns.1,2,7–10
However, with few PFA devices available, there currently are limited data on the long-term effectiveness of PFA on a multicenter level.8–10 Here, we report results from the first-in-human clinical experience of paroxysmal AF ablation using a novel fully integrated biphasic PFA system with a variable-loop circular catheter (VLCC) in combination with a multichannel PFA generator and a 3-dimensional (3D) mapping system (PFA Platform).
Methods
Study Design and Population
The inspIRE study (Study for Treatment of Paroxysmal Atrial Fibrillation [PAF] by Pulsed Field Ablation [PFA] System With Irreversible Electroporation [IRE]) was a prospective, multicenter, single-arm clinical trial with two phases. Wave I (feasibility phase) of the study assessed initial safety and effectiveness with mandated additional imaging (to assess for esophageal thermal lesions, cerebral embolism, and PV stenosis) in a small cohort of subjects with drug-refractory, symptomatic paroxysmal AF in Europe. The subsequent Wave II (pivotal phase) enrolled a larger cohort of subjects in Europe and Canada: in this phase, primary safety and effectiveness end points were evaluated against prespecified performance goals. Eligible patients were 18 to 75 years of age, had drug-refractory (ie, failed at least 1 Class I–IV antiarrhythmic drug) symptomatic paroxysmal AF, and were candidates for PVI. Exclusion criteria included AF secondary to reversible or noncardiac cause, previous ablation or surgery for AF, anticipation of receiving ablation other than PVI, and persistent AF. The study was approved by national authorities and ethics committees, and all subjects provided written informed consent. All subjects underwent first-time PVI and were followed for 12 months after ablation.
Johnson & Johnson MedTech has an agreement with the Yale Open Data Access Project to serve as the independent review panel for evaluation of requests for clinical study reports and participant-level data from investigators and physicians for scientific research that will advance medical knowledge and public health. Requests for access to the study data can be submitted through the Yale Open Data Access Project site at http://yoda.yale.edu.
Ablation System
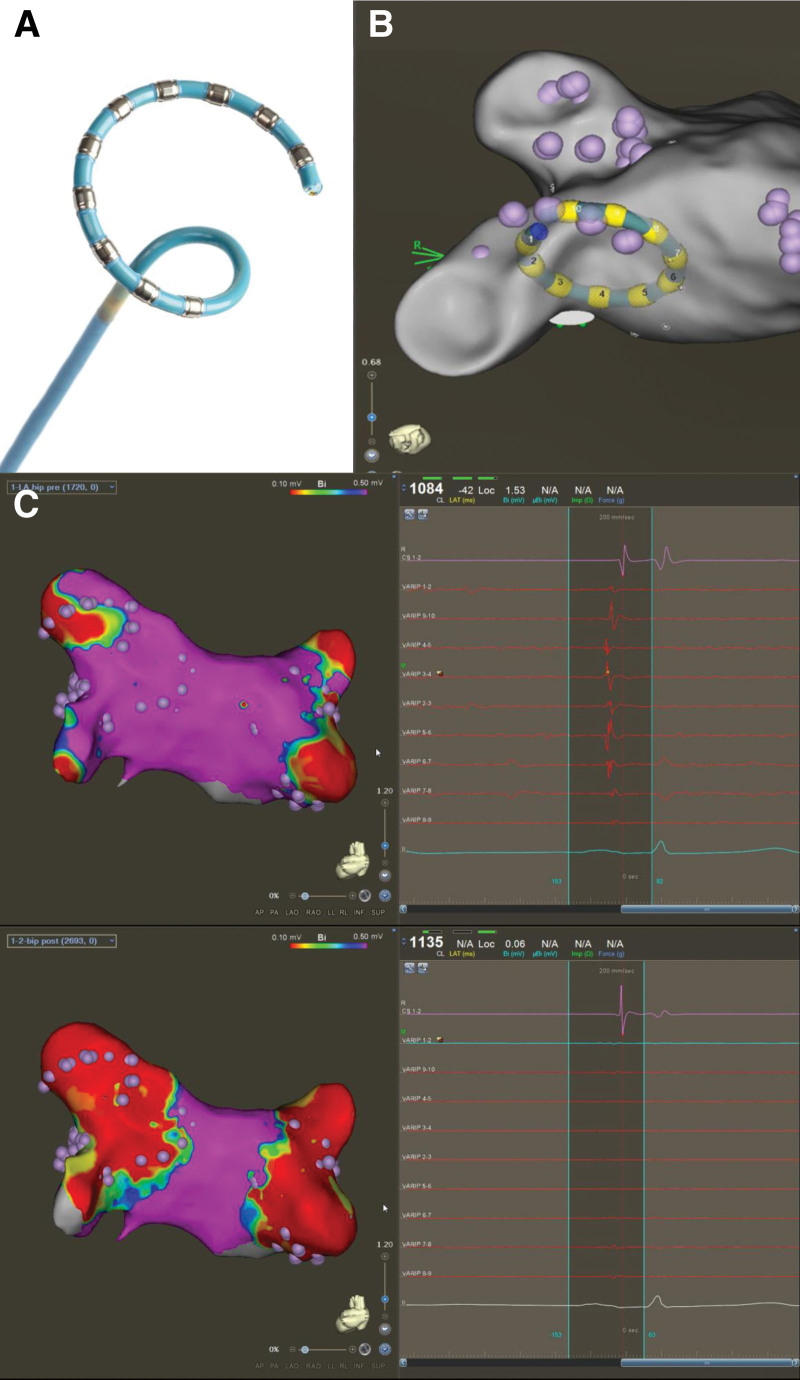
The Multi-Channel PFA Generator (PFA Generator; Biosense Webster, Inc.; Irvine, CA) is compatible with an electroanatomical mapping system (CARTO3 System; Biosense Webster) and incorporates proprietary technology to deliver short-duration, high-voltage bipolar biphasic pulses to a multielectrode ablation catheter (Figure 1A and 1B).5 Each pulse is delivered as a square wave with positive and negative phases. The voltage, pulse length, and number of pulses delivered to an electrode pair have been optimized via bench and preclinical testing; briefly, PFA is applied in a bipolar configuration with an energy of 1800 V. Each pulsed field application includes trains of microsecond-long biphasic pulses in between, for a total application duration of ≈250 ms.11–13 The generator can also be configured to deliver energy to specific electrode pairs and to adjust energy delivery based on clinical need.
Figure 1.
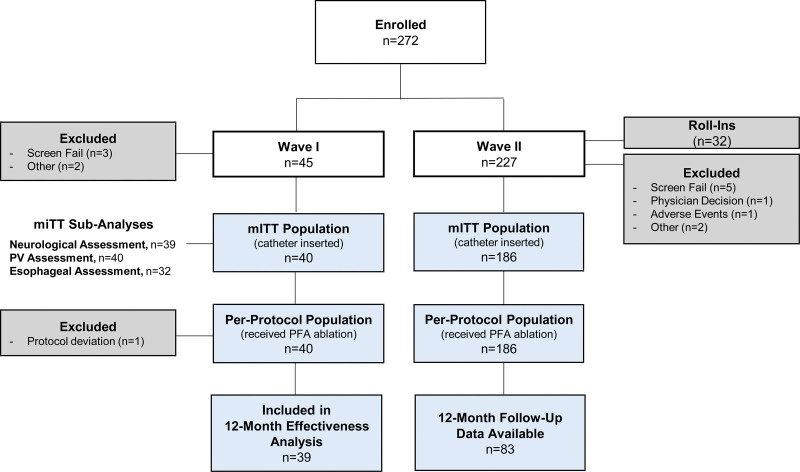
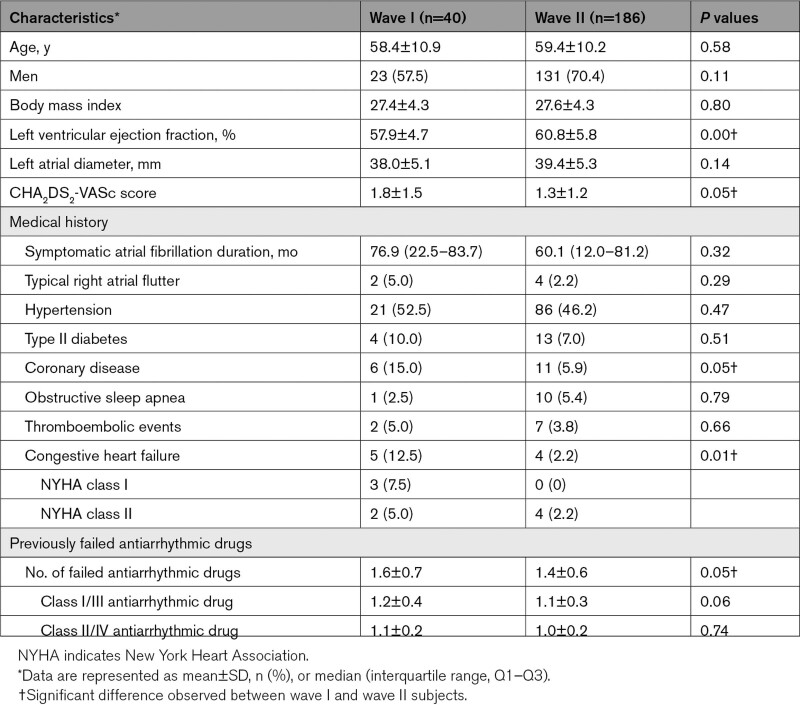
Pre- and postablation voltage maps using the variable-loop circular catheter (VLCC). A, The variable-loop circular catheter (VLCC). B, The VLCC as visualized under the 3-dimensional mapping system. C, Preablation (top) and postablation (bottom) voltage map showing the level of isolation (red) for the pulmonary veins with corresponding electrogram signals in the same subject. Reprinted from Biosense Webster, Inc. with permission. Copyright ©, Biosense Webster, Inc. (A).
The VLCC is a steerable, multielectrode, irrigated (irrigation rate, 4 mL/min) catheter. Attached to the distal end of the 8.5F shaft is a bidirectional circular tip that can be expanded and contracted, as necessary, to fit PVs of different sizes. The 10 platinum/iridium electrode rings are used for visualization, stimulation, recording, and bipolar PFA. All 10 poles of the VLCC are used for ablation, except in the case of electrode overlap, which require the most distal and the most proximal electrodes to be turned off.
Ablation Procedure
Procedures were performed under conscious sedation or general anesthesia. Following transseptal puncture, anatomical mapping of the left atrium was completed using either a diagnostic catheter (LASSO or PENTARAY; Biosense Webster) or the VLCC (Wave II only) with the electroanatomical mapping system, while a voltage map was created per institution standard of care (example of voltage map shown in Figure 1C). For PVI, while not mandated by protocol, workflow recommendations were to use the maximum energy setting to apply at least 12 applications per PV (translating to 48 applications per subject or 36 applications if right/left common veins were present). Acute procedural success was determined by confirmation of entrance block after adenosine/isoproterenol challenge, without a waiting period. Additional index procedural data were collected, including procedure time, transpired PFA time (total time transpiring from the first to last PFA application using the investigational catheter including catheter handling), fluoroscopy time, and study catheter dwell time in the left atrium. Additional learning curve analysis was performed as described in the Supplemental Methods.
Postablation Follow-up
Stringent arrhythmia recurrence assessments were performed via remote arrhythmia monitoring (weekly between months 3 and 5, monthly between months 6 and 12, and following any symptomatic episodes, recorded for a duration of 1 minute) and 24-hour Holter monitoring (at months 3, 6, and 12). ECG monitoring recordings were conducted if part of the standard-of-care assessment at preprocedure, predischarge, and month 1, 3, 6, and 12 follow-up visits. All event tracings and Holter recordings were independently assessed by a core laboratory. For subjects undergoing repeat procedures, the percentage of targeted PVs at the repeat procedure where PV reconnection was observed were documented.
Safety
The primary safety end point was the incidence of early-onset primary adverse events (PAEs) within 7 days of initial ablation, including pericarditis, myocardial infarction, cardiac tamponade/perforation, thromboembolism, stroke or cerebrovascular accident, transient ischemic attack, phrenic nerve paralysis, or major vascular access complication/bleeding, as well as death, PV stenosis, and atrioesophageal fistula that occurred later than 7 days postprocedure. All safety events were adjudicated by an independent clinical end point committee.
Supplementary for the Wave I cohort, endoscopy was performed before discharge and no later than 72 hours after the procedure to evaluate esophageal injury, computed tomography/magnetic resonance angiography was performed at month 3 postablation to assess for the presence and, if present, the severity of PV stenosis. Preablation and postablation neurological evaluation was performed, along with cerebral magnetic resonance imaging to detect silent cerebral lesion (SCL) using either diffusion-weighted or fluid-attenuated inversion recovery imaging sequence. If postprocedure magnetic resonance imaging was abnormal, the patient had a follow-up scan at the next visit with full neurologic assessment, until observations were resolved.
Effectiveness
The primary effectiveness end point was assessed by 12-month freedom from documented asymptomatic or symptomatic atrial arrhythmia episodes (AF, atrial flutter, or atrial tachycardia) of ≥30-s duration based on electrocardiographic data after a 3-month blanking period. Failure to confirm entrance block in all PVs was considered a long-term effectiveness failure as well. Clinical success was assessed by 12-month freedom from documented symptomatic AF/atrial flutter/atrial tachycardia recurrence.
Statistical Methods
Descriptive statistics were computed for demographic characteristics, medical history, and procedural characteristics in both the Wave I and Wave II full analysis set. The 2-sample t test was conducted for continuous variables, and the χ2 or Fisher exact test was applied for categorical variables.
As Wave I of the study was a safety characterization phase, a sample size of 40 subjects was intended to delineate preliminary safety and effectiveness of the investigational system. The Wave II study utilizes an adaptive design of interim sample size analyses at which enrollment may be stopped earlier than the maximum of 330 patients. An interim analysis was to be performed once (1) all subjects in the Wave II main study modified intention-to-treat analyses set reached 3-month follow-up and (2) a minimum of 30 subjects reached 12-month follow-up, upon which early success could be declared once primary safety and effectiveness goals were met.
To claim early success in Wave II, the primary safety analysis was completed using a beta-binomial model with a noninformative uniform prior (1,1) in the modified intention-to-treat analysis population. The safety end point was considered a success if the posterior probability of the PAE rate being <14% was >0.975. For this report, the primary safety end point is summarized by the proportion of subjects who experienced a PAE.
The primary effectiveness end point was analyzed in the per-protocol analysis population, enrolled subjects who met eligibility criteria and had insertion of the study catheter. Early success of effectiveness outcomes was determined by a piecewise exponential model for the time to failure during the 9-month (39 weeks) postblanking period, with vague gamma prior distributions used for the hazard rates. Early success of the primary effectiveness end point was reached if the posterior probability of the effectiveness success rate being greater than the predetermined threshold of 50% was >0.9975.
Kaplan-Meier estimates and plots were used to characterize the time to primary effectiveness in Wave II subjects and freedom from documented symptomatic AF/atrial tachycardia/atrial flutter recurrence in Wave I and Wave II subjects.
All statistical analyses were performed using SAS 9.4 or SAS Studio 3.8 (SAS Institute, Inc, Cary, NC).
Results
Study Subjects
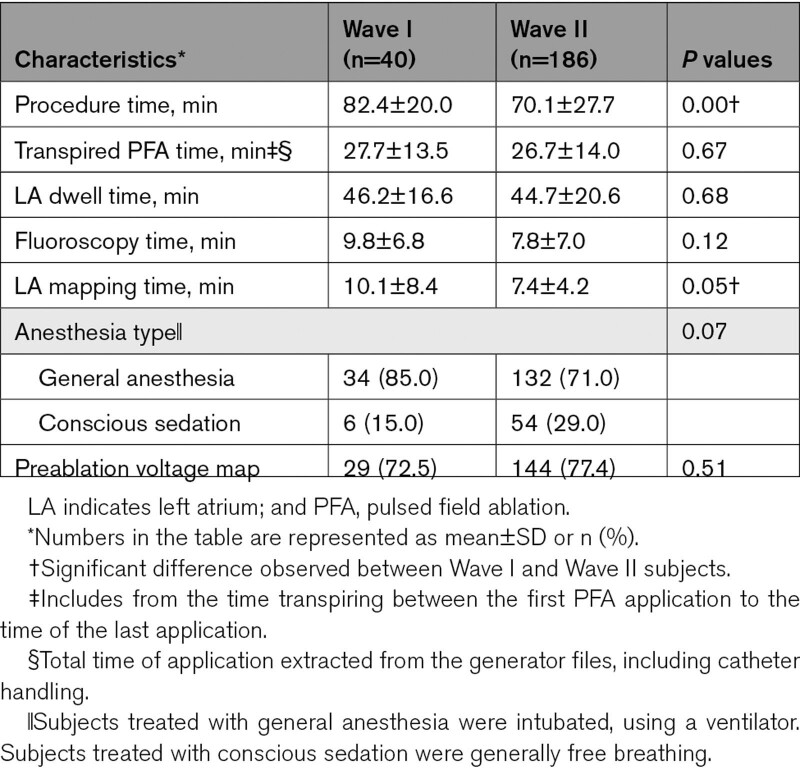
Across 13 centers in Europe and Canada, a total of 272 subjects were enrolled between August 2020 and May 2022 where 226 subjects met eligibility criteria for safety and effectiveness evaluations (Wave I, 40; Wave II, 186; Figure 2; Table S1). When an independent Data Monitoring Committee declared early success for the study, 83 of the Wave II subjects had 12-month follow-up data, and all subjects passed the 3-month window. Baseline characteristics and medical history are summarized in Table 1. Wave I and Wave II subjects were largely similar except for a higher number of male patients, shorter symptomatic AF durations, and fewer subjects with congestive heart failure in Wave II. Patients were generally young (mean age, 58.4–59.4 years), with a mean CHA2DS2-VASc score (1.3–1.8), and the most common comorbidity was hypertension (46.2%–52.5%).
Figure 2.
inspIRE trial (Study for Treatment of Paroxysmal Atrial Fibrillation [PAF] by Pulsed Field Ablation [PFA] System With Irreversible Electroporation [IRE]) patient population flowchart. inspIRE indicates Study for Treatment of Paroxysmal Atrial Fibrillation (PAF) by Pulsed Field Ablation (PFA) System With Irreversible Electroporation (IRE); mITT, modified intention to treat; PFA, pulsed field ablation; and PV, pulmonary vein.
Table 1.
Baseline Patient Characteristics
Procedural Data
Procedural data are summarized in Table 2. Procedure efficiencies generally improved in Wave II compared with Wave I. Specifically in Wave II, the mean total procedure time was 70.1±27.7 minutes, left atrial dwell time was 44.7±20.6 minutes, transpired PFA time was 26.7±14.0 minutes, and fluoroscopy time at the end of the procedure was 7.8±7.0 minutes, while preablation voltage maps were acquired in 77.4% (144/186) of subjects. Learning curve analysis showed the transpired PFA time per procedure decreased 13.6 minutes from the first procedure after analyzing all Wave I cases (Figure S1).
Table 2.
Procedural Characteristics
Safety
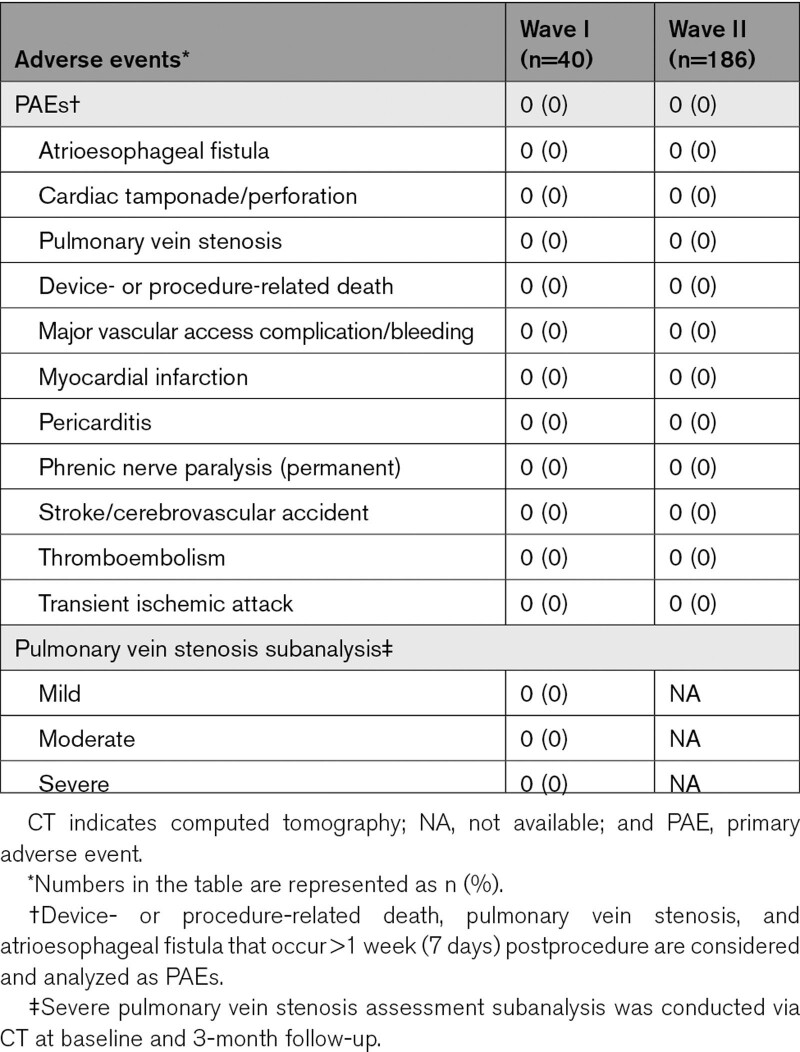
In either cohort, there were no reports of PAEs (Table 3). There were also no esophageal lesions of thermal origin, PV stenosis (including mild to severe), or evidence of coronary spasm in Wave I subjects. The safety objective was met (ie, the posterior probability that the PAE rate was <14% was >0.975).
Table 3.
Summary of PAEs
Thirty-nine Wave I subjects completed precerebral and postcerebral magnetic resonance imaging, with images assessed by a core laboratory. SCLs were detected in 4 of the first 6 subjects (4 lesions, 1.5–5.9 mm), after which workflow enhancements, including a 10-s pause between PFA applications, were implemented, resulting in detection of only 4 SCLs in the remaining 33 subjects (6 lesions, 1.2–3.7 mm). Of note, in addition to the longer pause between lesions, there were other general procedural modifications including minimizing catheter exchanges and stricter adherence to the anticoagulation regimen before and during the procedure.14 All lesions were asymptomatic and transient, resolving by 3 months of follow-up, and no participants reported subsequent new or worsening neurologic events, with a National Institutes of Health Stroke Scale score of 0 by 3-month follow-up.
Effectiveness
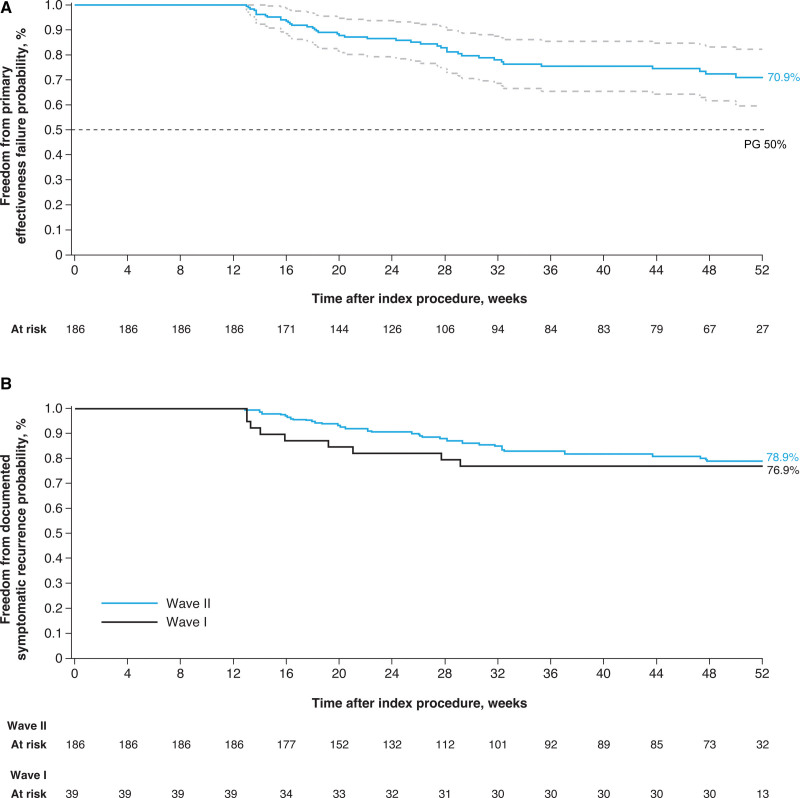
Confirmation of entrance block was achieved in all 226 Wave I and Wave II subjects who underwent ablation with the PFA system (100%). PVI without acute reconnection was achieved in 96.0% (Wave I) and 97.1% (Wave II) of targeted veins. At the time of early success interim analysis, the Kaplan-Meier estimate for Wave II freedom from the primary effectiveness end point was 70.9% (99.5% CI, 59.6%–82.2%; Figure 3A). The effectiveness objective was also met (ie, the posterior probability that the effectiveness proportion was >50% was >0.9975). The Kaplan-Meier estimates for clinical success at 12 months were 76.9% (95% CI, 63.7%–90.1%) and 78.9% (95% CI, 71.9%–85.9%) for Wave I and Wave II, respectively (Figure 3B). Twelve-month freedom from repeat ablation after the blanking period for the study arrhythmia was between 92.5% (95% CI, 84.3%–100.0%; Wave I) and 92.3% (95% CI, 87.6%–96.9%; Wave II). In those patients who presented for repeat ablation (3 Wave I and 10 Wave II subjects), 9 of 10 (90.0%, Wave I) and 27 of 39 (69.2%, Wave II) previously isolated PVs were electrically reconnected at the repeat procedure.
Figure 3.
Freedom from primary effectiveness and documented symptomatic atrial fibrillation/atrial tachycardia/atrial flutter recurrence (clinical success) in the per-protocol population. Kaplan-Meier analysis in the per-protocol population of (A) primary effectiveness in Wave II subjects and (B) freedom from documented symptomatic atrial fibrillation/atrial tachycardia/atrial flutter recurrence in Wave I and Wave II subjects. PG indicates performance goal.
At baseline, Class I/III antiarrhythmic drugs were used in 87.5% (35/40) and 81.7% (152/186) in Wave I and Wave II patients, respectively; by the 12-month follow-up, only 22.5% (9/40) and 33.3% (32/96) of patients were still using Class I/III antiarrhythmic drugs.
Discussion
This first clinical investigation of the fully integrated biphasic PFA system with a VLCC in combination with a PFA generator and 3D mapping system demonstrated a promising safety profile, with no PAEs and no postprocedural esophageal injury, PV stenosis, or neurological symptoms. The 12-month primary effectiveness using stringent monitoring was 71%, with that of clinical success mounting up to 79%.
Safety
The results from this clinical study are consistent with the preclinical safety findings demonstrating that the PFA system creates transmural lesions without collateral damage to neighboring structures, such as the esophagus or phrenic nerves.12 This previous evaluation of the fully integrated PFA system in a porcine model demonstrated that effective and durable ablation of cardiac tissue could be achieved with no collateral damage to the phrenic nerve, esophagus, or mitral valve. This was true despite these structures being deliberately targeted by more extensive ablation applications than would be expected for therapeutic ablation in patients with AF, such as ablating inside the PV lumen and directly to the mitral valve and with multiple applications at each site.
The safety margin, as compared with existing ablative energy modalities, such as radiofrequency and cryoablation, is the result of the tissue preference of PFA under the appropriate energy-delivery setting. Beyond a 0% PAE rate, notable from the safety results is the absence of esophageal injury, despite systematic endoscopy postablation, and PV stenosis with routine imaging. This is consistent with recent real-world clinical experience of a different PFA technology.15,16 In a retrospective survey of 24 clinical centers performing PFA in 1758 patients with AF using a penta-spline PFA catheter, there were no post-PFA esophageal complications, evidence of clinical PV stenosis, or persistent phrenic nerve injury.15 The SCL rate observed in our study is in line with rates seen with published data.14–17 The occurrence of SCLs is a known risk of catheter ablation with multifactorial contributing factors (eg, anticoagulation regimen, catheter exchanges, potentially microbubbles) but with unknown clinical significance.15,18,19 Taken together, although the overall safety result is encouraging, the current study is the first of this novel PFA system. Thus, it is important to continue to be vigilant with applying good workflow during PFA to minimize any procedure-related complications including SCLs.
Twelve-Month Effectiveness
Earlier preclinical investigation of the study device demonstrated complete circumferential cell necrosis achieved by PFA as visualized by histological staining of PV sections 30 days after ablation.12 It has been suggested that circumferential ablation lesions with a higher degree of necrosis compared with edema are associated with a lower risk of AF recurrence due to the irreversibility of tissue injury.13 Additionally, an in vivo animal study demonstrated the ability of the study device to isolate the PVs and perform large linear lesions. At 1-month follow-up, the persistence of the lesions was confirmed by electroanatomical mapping. The histological analysis of the tissues, at the same follow-up time point, highlighted the disappearance of the signs of inflammation and the complete healing of the endothelium.13
The 12-month success Kaplan-Meier rates observed from the pivotal Wave II, namely 79% freedom from symptomatic arrhythmia and 92% freedom from repeat ablation, are comparable to those reported from multicenter experience of radiofrequency ablation technologies. For example, the 12-month success rates from 2 large tag-index ablation studies were 78% and 82% with freedom from repeat ablation between 90% and 94%.19,20 As a new ablation energy modality, there have been few reports to date of 12-month effectiveness of PFA in patients with paroxysmal AF; these have ranged between 79% and 87%.8–10 It is important to note, however, that these studies incorporated a mandatory remap and reablation strategy at 3 to 4 months regardless of recurrence, which may have overestimated the true long-term single-procedure success rate.
The PV reconnection rates in both Wave I and Wave II must be interpreted in the proper context. Unlike in several prior PFA reports, our study did not include protocol-mandated invasive remapping procedures. Indeed, these patients presented for repeat procedures because of clinical recurrences. That is, it is quite reasonable to expect that this would be a group that would be enriched for PV reconnections. Indeed, this is supported by the high rate of freedom from repeat ablation at 12 months (92%). Future studies with protocol-mandated systematic remapping are necessary to determine the true PV reconnection rate.
Procedure Efficiency and 3D Electroanatomical Mapping Integration
Considering that this study was the initial clinical experience using this new PFA system, the total procedure time was short (70.1±27.7 minutes) and consistent with those reported with PFA procedures in other studies, which ranged from 65 to 96 minutes.1,8,10,15 Likewise, the catheter dwell time of 45 minutes reported in the current study is short and comparable to the 34 minutes reported in studies with other PFA ablation catheter and 40 minutes reported in studies with the radiofrequency balloon catheter and shorter than the 74 minutes reported in a recent study with the cryoballoon.8,10,21 As operators gain additional experience with the PFA system, overall procedure efficiencies may continue to improve as seen with other mature technologies with experienced operators. For example, the procedure time associated with focal or balloon ablations has now decreased substantially to 70 to 80 minutes in recent studies.21,22
The current PFA system features the integration of the ablation catheter with a 3D electroanatomical mapping system. The practical advantages of this integrated system include the ability to map and track the placement of lesion sets and validate voltage postablation with minimal fluoroscopy use. Using a 3D mapping system helped reduce unnecessary energy deliveries, by allowing operators to prospectively identify overlapping electrodes and adjust the catheter accordingly to eliminate such overlap, while ensuring energy application was delivered around the entire vein without gaps. The 3D mapping system helped ensure coaxial positioning of the catheter to the vein as well. As observed, fluoroscopy time in this study was low (7.2 minutes), which was likely partly attributable to navigation with 3D electroanatomical mapping and the option to utilize intracardiac echocardiography.
Limitations
The current study is a single-arm study, and, therefore, direct comparisons to other PFA technologies or ablative energy sources would require prospective randomized controlled studies. Factors associated with effectiveness failures need to be evaluated in future studies. The study population consisted of relatively young subjects with less severe disease inferred by the CHA2DS2-VASc score and comorbidities; treatment outcome in a wider population with more severe disease will need to be investigated further. The absence of continuous monitoring with implanted devices raises the possibility that certain clinical recurrences were not counted and prevents an understanding of changes in AF burden. Although maximum energy setting was recommended, not all patients were treated following the recommendation. Future standardization of the energy level may help optimize ablation outcome.
Conclusions
These initial results of paroxysmal AF ablation using the novel fully integrated biphasic PFA system with VLCC in combination with a PFA generator and 3D mapping system showed a strong safety profile with no reported device-related serious adverse events, esophageal injury, or PV stenosis. The 12-month efficacy is comparable to that achieved with early experience of novel radiofrequency ablation technologies.
Article Information
Acknowledgments
The authors would like to express their deepest gratitude to all investigators, including coinvestigators and guest operating investigators. They wish to thank the following individuals for their support in trial design, execution, statistical analysis, medical writing, and additional value to developing the manuscript: Carmen Rousseeuw, Sarah Rabau, Liesbeth Vanderlinden, Lee Ming Boo, Christina Kaneko, Jaclyn Alcazar, Tara Gomez, Tiffany Tan, Melissa Mert, Guixia Huang, Xiaoxue Gu, Puneet Jatana, Rebecca Beals, and Dror Berman (all employees of Biosense Webster, Inc.). Michelle Hughes, Bsc, with Lumanity Communications Inc (Yardley, PA), provided medical writing and editorial support, in accordance with Good Publication Practice guidelines, which were funded by Biosense Webster, Inc, under direction of the authors.
Sources of Funding
This study was sponsored by Biosense Webster, Inc (Irvine, CA).
Disclosures
Dr Duytschaever has served on the speaker’s bureau, is a consultant for Biosense Webster, Inc, and has received research support from Biosense Webster, Inc. Dr De Potter has received consulting fees, honorarium for lectures, and presentation fees from Biosense Webster and Adagio Medical (all payments were directed to the institution). Dr Anic has received consulting fees and has contracted research with Farapulse, Boston Scientific, Galaxy Medical, and Biosense Webster. Dr Grimaldi has an unrelated patent agreement with Biosense Webster, Inc. Dr Neuzil has received grant support from Biosense Webster, Inc. Dr Van Herendael has received support from Biosense Webster for congress-related activities. Dr Verma has received grants from Biosense Webster, Medtronic, Bayer, and Biotronik; has received consulting fees from Biosense Webster, Medtronic, Adagio Medical, Galaxy Medical, Ablacon, and Thermedical; and has received honorarium for lectures from Biosense Webster and Medtronic. Dr Skanes has served on the speaker’s bureau for Biosense Webster and has received research support from Biosense Webster, Inc. Dr Scherr has received grant support from Biosense Webster. Dr Pürerfellner has received consulting fees from Biosense Webster, Abbott, Boston Scientific, Biotronik, and Medtronic and has received payment or honorarium for lectures or presentations from Biosense Webster, Abbott, Boston Scientific, Biotronik, and Medtronic. Dr Jais has received a LIRYC research grant from Biosense Webster; has received speaker fees from Biosense Webster; is a share holder of Farapulse/Affera; and has also received speaker fees and research grants from Boston Scientific, Medtronic, and Abbott. Dr Reddy is a consultant for Biosense Webster, Inc; he also has additional disclosures unrelated to this article that are listed in the Supplemental Material. The other authors report no conflicts.
Supplemental Material
Supplemental Methods
Table S1
Figure S1
Supplemental Disclosures I
Supplementary Material
Appendix
Andrea Natale, Luigi Di Biase, Sebastien Knecht, Jan Petru, Georgios Kollias, Peter Lukac, Jim Hansen, and Thomas Phlips.
Nonstandard Abbreviations and Acronyms
- 3D
- 3 dimensional
- AF
- atrial fibrillation
- PAE
- primary adverse event
- PFA
- pulsed field ablation
- PV
- pulmonary vein
- PVI
- pulmonary vein isolation
- SCL
- silent cerebral lesion
- VLCC
- variable-loop circular catheter
A list of all inspIRE Trial Investigators is given in the Appendix.
For Sources of Funding and Disclosures, see page 127.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.122.011780.
Contributor Information
Mattias Duytschaever, Email: Mattias.Duytschaever@azsintjan.be.
Tom De Potter, Email: tom.de.potter@olvz-aalst.be.
Massimo Grimaldi, Email: fiatric@hotmail.com.
Ante Anic, Email: anteanic@gmail.com.
Johan Vijgen, Email: johan.vijgen@jessazh.be.
Petr Neuzil, Email: pneuzil@seznam.cz.
Hugo Van Herendael, Email: hugo.vanherendael@zol.be.
Atul Verma, Email: atul.verma@mcgill.ca.
Allan Skanes, Email: askanes@uwo.ca.
Daniel Scherr, Email: daniel.scherr@medunigraz.at.
Helmut Pürerfellner, Email: helmut.puererfellner@ordensklinikum.at.
Gediminas Rackauskas, Email: gediminas.rackauskas@santa.lt.
Pierre Jaïs, Email: pierre.jais@chu-bordeaux.fr.
Collaborators: Andrea Natale, Luigi Di Biase, Sebastien Knecht, Jan Petru, Georgios Kollias, Peter Lukac, Jim Hansen, and Thomas Phlips
References
- 1.Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky I, Hebeler R, Labrousse L, Barandon L, Kralovec S, et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol. 2018;4:987–995. doi: 10.1016/j.jacep.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 2.Verma A, Boersma L, Haines DE, Natale A, Marchlinski FE, Sanders P, Calkins H, Packer DL, Hummel J, Onal B, et al. First-in-human experience and acute procedural outcomes using a novel pulsed field ablation system: the PULSED AF pilot trial. Circ Arrhythm Electrophysiol. 2022;15:e010168. doi: 10.1161/CIRCEP.121.010168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koruth J, Kuroki K, Iwasawa J, Enomoto Y, Viswanathan R, Brose R, Buck ED, Speltz M, Dukkipati SR, Reddy VY. Preclinical evaluation of pulsed field ablation: electrophysiological and histological assessment of thoracic vein isolation. Circ Arrhythm Electrophysiol. 2019;12:e007781. doi: 10.1161/CIRCEP.119.007781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart MT, Haines DE, Verma A, Kirchhof N, Barka N, Grassl E, Howard B. Intracardiac pulsed field ablation: proof of feasibility in a chronic porcine model. Heart Rhythm. 2019;16:754–764. doi: 10.1016/j.hrthm.2018.10.030 [DOI] [PubMed] [Google Scholar]
- 5.Yavin H, Brem E, Zilberman I, Shapira-Daniels A, Datta K, Govari A, Anic A, Wazni O, Anter E. Circular multielectrode pulsed field ablation catheter Lasso pulsed field ablation: lesion characteristics, durability and effect on neighboring structures. Circ Arrhythm Electrophysiol. 2021;14:e009229. doi: 10.1161/CIRCEP.120.009229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yavin H, Shapira-Daniels A, Barkagan M, Sroubek J, Shim D, Melidone R, Anter E. Pulsed field ablation using a lattice electrode for focal energy delivery: biophysical characterization, lesion durability, and safety evaluation. Circ Arrhythm Electrophysiol. 2020;13:e008580. doi: 10.1161/CIRCEP.120.008580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loh P, van Es R, Groen MHA, Neven K, Kassenberg W, Wittkampf FHM, Doevendans PA. Pulmonary vein isolation with single pulse irreversible electroporation: a first in human study in 10 patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13:e008192. doi: 10.1161/CIRCEP.119.008192 [DOI] [PubMed] [Google Scholar]
- 8.Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H, Sediva L, Chovanec M, Dukkipati SR, Jais P. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019;74:315–326. doi: 10.1016/j.jacc.2019.04.021 [DOI] [PubMed] [Google Scholar]
- 9.Reddy VY, Anter E, Rackauskas G, Peichl P, Koruth JS, Petru J, Funasako M, Minami K, Natale A, Jais P, et al. Lattice-tip focal ablation catheter that toggles between radiofrequency and pulsed field energy to treat atrial fibrillation: a first-in-human trial. Circ Arrhythm Electrophysiol. 2020;13:e008718. doi: 10.1161/CIRCEP.120.008718 [DOI] [PubMed] [Google Scholar]
- 10.Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako M, Cochet H, Minami K, Breskovic T, Sikiric I, et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol. 2021;7:614–627. doi: 10.1016/j.jacep.2021.02.014 [DOI] [PubMed] [Google Scholar]
- 11.Yavin H, Brem E, Zilberman I, Shapira-Daniels A, Datta K, Govari A, Altmann A, Anic A, Wazni O, Anter E. Circular multielectrode pulsed field ablation catheter LASSO pulsed field ablation: lesion characteristics, durability, and effect on neighboring structures. Circ Arrhythm Electrophysiol. 2021;14:e009229. doi: 10.1161/CIRCEP.120.009229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu JC, Gibson D, Banker R, Doshi SK, Gidney B, Gomez T, Berman D, Datta K, Govari A, Natale A. In vivo porcine characterization of atrial lesion safety and efficacy utilizing a circular pulsed-field ablation catheter including assessment of collateral damage to adjacent tissue in supratherapeutic ablation applications. J Cardiovasc Electrophysiol. 2022;33:1480–1488. doi: 10.1111/jce.15522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimaldi M, Di Monaco A, Gomez T, Berman D, Datta K, Sharma T, Govari A, Altmann A, Di Biase L. Time course of irreversible electroporation lesion development through short- and long-term follow-up in pulsed-field ablation-treated hearts. Circ Arrhythm Electrophysiol. 2022;15:e010661. doi: 10.1161/CIRCEP.121.010661 [DOI] [PubMed] [Google Scholar]
- 14.Grimaldi M, Swarup V, DeVille B, Sussman J, Jais P, Gaita F, Duytschaever M, Ng GA, Daoud E, Lakkireddy DD, et al. Importance of anticoagulation and postablation silent cerebral lesions: subanalyses of REVOLUTION and reMARQable studies. Pacing Clin Electrophysiol. 2017;40:1432–1439. doi: 10.1111/pace.13205 [DOI] [PubMed] [Google Scholar]
- 15.Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner A, Hansen J, Blaauw Y, Maury P, Arentz T, et al. Multi-National Survey on the Methods, Efficacy, and Safety on the Post-Approval Clinical Use of Pulsed Field Ablation (MANIFEST-PF). Europace. 2022;24:1256–1266. doi: 10.1093/europace/euac050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt B, Bordignon S, Tohoku S, Chen S, Bologna F, Urbanek L, Pansera F, Ernst M, Chun KRJ. 5S study: safe and simple single shot pulmonary vein isolation with pulsed field ablation using sedation. Circ Arrhythm Electrophysiol. 2022;15:e010817. doi: 10.1161/CIRCEP.121.010817 [DOI] [PubMed] [Google Scholar]
- 17.Haeusler KG, Eichner FA, Heuschmann PU, Fiebach JB, Engelhorn T, Blank B, Callans D, Elvan A, Grimaldi M, Hansen J, et al. MRI-detected brain lesions and cognitive function in patients with atrial fibrillation undergoing left atrial catheter ablation in the randomized AXAFA-AFNET 5 trial. Circulation. 2022;145:906–915. doi: 10.1161/CIRCULATIONAHA.121.056320 [DOI] [PubMed] [Google Scholar]
- 18.Arujuna A, Karim R, Caulfield D, Knowles B, Rhode K, Schaeffter T, Kato B, Rinaldi CA, Cooklin M, Razavi R, et al. Acute pulmonary vein isolation is achieved by a combination of reversible and irreversible atrial injury after catheter ablation: evidence from magnetic resonance imaging. Circ Arrhythm Electrophysiol. 2012;5:691–700. doi: 10.1161/CIRCEP.111.966523 [DOI] [PubMed] [Google Scholar]
- 19.Duytschaever M, Vijgen J, De Potter T, Scherr D, Van Herendael H, Knecht S, Kobza R, Berte B, Sandgaard N, Albenque JP, et al. Standardized pulmonary vein isolation workflow to enclose veins with contiguous lesions: the multicentre VISTAX trial. Europace. 2020;22:1645–1652. doi: 10.1093/europace/euaa157 [DOI] [PubMed] [Google Scholar]
- 20.Di Biase L, Monir G, Melby D, Tabereaux P, Natale A, Manyam H, Athill C, Delaughter C, Patel A, Gentlesk P, et al. ; SURPOINT Postapproval Trial Investigators. Composite index tagging for PVI in paroxysmal AF: a prospective, multicenter postapproval study. JACC Clin Electrophysiol. 2022;8:1077–1089. doi: 10.1016/j.jacep.2022.06.007 [DOI] [PubMed] [Google Scholar]
- 21.Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J, Roux JF, Yung D, Skanes A, Khaykin Y, et al. ; EARLY-AF Investigators. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–315. doi: 10.1056/NEJMoa2029980 [DOI] [PubMed] [Google Scholar]
- 22.Berte B, Kobza R, Toggweiler S, Schupfer G, Duytschaever M, Hoop V, Lehnick D, Santangeli P, Purerfellner H. Improved procedural efficiency of atrial fibrillation ablation using a dedicated ablation protocol and lean management. JACC Clin Electrophysiol. 2021;7:321–332. doi: 10.1016/j.jacep.2020.08.023 [DOI] [PubMed] [Google Scholar]