Understanding current menstrual technologies is essential to empowering menstrual well-being and identifying heavy menstrual bleeding.
Abstract
Menstruation is a personal and cultural experience with financial and health implications. Menstruation historically has been managed with disposable commodities, including tampons and pads. New technologies, including underwear and menstrual cups and discs, have emerged to address diverse menstrual needs such as prioritization of sustainability, discretion, and inclusivity. New technologies are not routinely integrated into history taking or validated questionnaires, which currently rely on traditional tampon and pad use for identifying individuals with heavy menstrual bleeding. Review of menstrual technologies and accessories provides insight to empower gynecologists and other clinicians to take comprehensive menstrual histories, including strategies for identification of heavy menstrual bleeding and troubleshooting menstrual disturbances, within the context of new menstrual technologies.
Menstrual equity remains a humanitarian and environmental priority among the World Health Organization, the United Nations Population Fund, and the World Bank, and understanding the available menstrual technologies and their use is an important tool for social change.1–3 Menstrual health is an important patient-reported outcome beyond its importance as a general indicator of health and fertility. The patient perspective on menstrual bleeding has even changed how we clinically define heavy menstrual bleeding. Prior definitions of heavy menstrual bleeding identified it as blood loss greater than 80 mL per cycle or episode.4 The more modern, patient-centered definition proposed by the U.K. National Institute for Health and Care Excellence and adopted by the International Federation of Gynecology and Obstetrics is, “excessive menstrual blood loss which interferes with a woman's physical, social, emotional and/or material quality of life.”5
Up to 53% of menstruating individuals report heavy menstrual bleeding.6,7 Sequalae of heavy menstrual bleeding include decreased quality of life, anemia, and mood disturbances.7–9 In addition to health concerns, heavy menstrual bleeding can be associated with negative social, economic, and environmental consequences.10,11 A menstruating individual without heavy menstrual bleeding is estimated to use up to 10,000 pads in their lifetime.12 Many of the new, reusable menstrual products offer alternatives for menstrual containment that reduce environmental waste, but little is known about how well they function for individuals with heavy menstrual bleeding. Additionally, how to best include use of new, reusable products in clinical assessments for heavy menstrual bleeding and history-taking is unknown.
Individuals historically have managed menstrual bleeding with sanitary napkins or pads or tampons. The growing menstrual technology market, including various reusable products, has changed the landscape of menstrual management. Given the key role of menstrual products in estimating blood loss and diagnosing heavy menstrual bleeding, a basic understanding of the use and absorbency of different products is important. This review outlines the technical considerations of menstrual technologies with a focus on volumetric estimations and describes new menstrual technologies to enable health care professionals to more accurately assess for heavy menstrual bleeding as well as to discuss or troubleshoot new menstrual hygiene products.
QUANTIFYING HEAVY MENSTRUAL BLEEDING
Quantification of menstrual bleeding and defining heavy menstrual bleeding is an ongoing process, with increasingly inclusive clinical definitions and new menstrual hygiene product innovations. Obtaining a clinical history is often the easiest and most economical way to screen for menstrual abnormalities such as heavy menstrual bleeding. Self-perception of “very heavy” menstrual bleeding has a sensitivity and specificity of 74% for predicting heavy menstrual bleeding.13 Quantitative methods, such as photometric alkaline hematin elution from cotton sanitary products, remain the gold standard to objectively quantify menstrual blood loss for research.14 This method, although accurate, is highly impractical, costly, and not accessible in most clinical and research settings. Semi-quantitative methods of measuring mean menstrual blood loss, such as the pictorial bleeding assessment chart, which are also used mostly in a research setting, quantify volume based on standardized patient recordings of number and amount of saturation of menstrual products.15 Although simpler and more accessible than the alkaline hematin method, the pictorial bleeding assessment chart is not without its own limitations. The pictorial bleeding assessment chart and other semi-quantitative methods fail to capture the current diversity in the menstrual hygiene market, leaving out people who use alternative items such as menstrual cups, menstrual discs, menstrual underwear, or multiple methods.16
INTERNAL ABSORBENT PRODUCTS: TAMPONS AND SPONGES
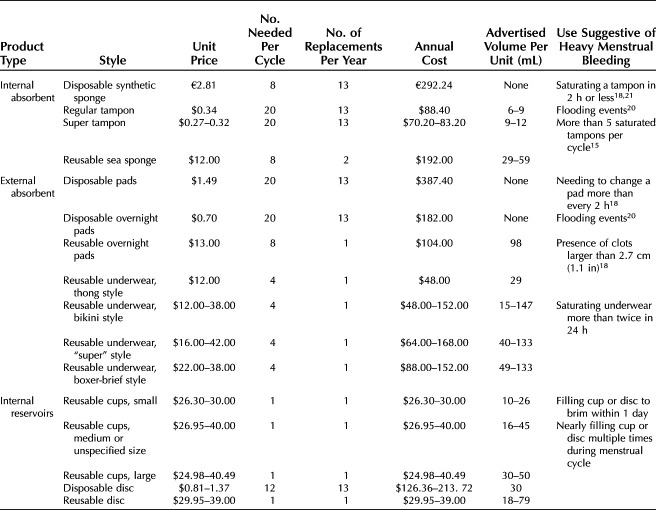
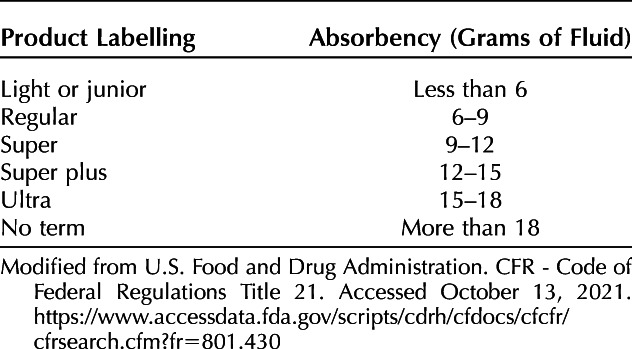
The disposable tampon has a legacy of regulation unique among menstrual technologies. To reduce the risk of toxic shock syndrome (TSS), the U.S. Food and Drug Administration's Tampon Task Force guided consumers to use the least absorbent product (Table 1). The Task Force chose gram weight of saline absorbed as the metric, although this remains controversial because heparinized blood is more absorbable than saline.17 Despite this limitation, one commonly accepted threshold at which an evaluation for heavy menstrual bleeding should be considered is when patients report saturating a tampon more frequently than every 2 hours or experiencing flooding around tampons.18–21
Table 1.
Disposable Tampon Labeling by Absorbency According to U.S. Food and Drug Administration Requirements
Recent surveys still demonstrate that just less than half of menstruating individuals report that tampons are their preferred product for menstrual management.22,23 Modern tampons generally are made of an absorbent cylinder of cotton, rayon, or polyester attached to a blended string made of cotton, polyester, or polypropylene.24 Tampons can be placed into the vagina digitally, with a disposable plastic or cardboard telescoping applicator or a reusable medical-grade silicone applicator. Public concerns continue to exist regarding both the environmental and personal exposure risk of the components included in tampons, although these do not appear to be elevated above background exposures.25
Manufacturers and environmental advocates have proposed sea sponges as an alternative to tampons since the 1980s. Early investigations found sea sponges to contain abrasives such as sand and contaminants such as mold and bacteria, including Staphylococcus aureus, the species most commonly associated with TSS.26 Some sea sponges are marketed as reusable, although cleaning specifications are not validated and sometimes contradictory. The U.S. Food and Drug Administration has not approved any sea sponge for menstrual use, but many user-identified strategies exist on the internet, including use of sponges advertised for cosmetic uses (eg, application of makeup). Internationally, several synthetic sponges are available in the European Union and Australian markets. A popular sponge from the Netherlands is made of rubber latex and purports its ability to be kept in place comfortably during intercourse. These come individually packed and are intended for single use.
EXTERNAL ABSORBENT PRODUCTS: PADS AND UNDERWEAR
Disposable pads are a ubiquitous menstrual technology. A 2019 study from St. Louis found that 59% of individuals used pads and 55% reported pads as the most preferred menstrual technology.22 The main advantages to pads are convenience, availability, and accessibility. Disposable menstrual pads are made of cotton combined with superabsorbent polymers, a common component of diapers. Superabsorbent polymers are nonbiodegradable petroleum products that have been implicated in dermatitis and TSS and pose a large environmental burden.27,28
Despite widespread use and a large market share, disposable pads lack any kind of regulation with regard to absorbency. Researchers estimate that a fully saturated “super” pad may hold 5 mL of fluid and that a nighttime pad may hold up to 15 mL.29 This absorbency capacity likely varies within and among brands, making it challenging to translate patient-reported product saturation into volumetric losses. Although patients' perception of bleeding heaviness has been shown to predict heavy menstrual bleeding, individuals who report saturating a regular or super pad within 2 hours also merit evaluation for heavy menstrual bleeding (Table 2).18
Table 2.
Comparison of Menstrual Products by Cost and Volume
Reusable menstrual pads are available as alternatives to disposable pads. Reusable pads are made of multiple layers of absorbent and wicking fabrics, held in place by snaps. Some of these reusable pads are purposefully in dark colors to reduce discoloration overtime, but that may obscure the user's ability to visually estimate menstrual blood loss. If quantification is desired, patients using reusable pads could weigh them with a kitchen scale instead of switching products, similar to strategies used to quantify blood loss in obstetric settings.30 For improved accuracy, it would be best to compare the weights of the saturated menstrual products with a product worn on a nonmenstruating day given the likely increased concomitant weight from absorption of vaginal, urinary, and diaphoretic secretions.31 If unabsorbed clots are visible, they can be photographed beside a standard item such as a coin for semi-quantitative estimation of menstrual blood loss.18
Menstrual-specific underwear has recently emerged as a more sustainable alternative to disposable pads. Menstrual underwear is marketed as having equivalent absorbency to tampons, but, as with traditional disposable pads, no regulations exist regarding underwear content or capacity. Menstrual underwear generally is manufactured from multiple layers of polyester designed to wick moisture away from the skin, with an outer liquid-repellant layer of nylon and lycra.32 The number of wicking layers varies by and within brands. Dark colored underwear can also obscure visual estimations of blood loss. Additionally, menstruating individuals who frequently pass clots report dissatisfaction with menstrual underwear because there may be decreased absorbency when menstrual blood is clotted.33 Until data emerge that address menstrual underwear absorbency and how to subjectively assess bleeding quantity, our expert opinion is to consider evaluation for heavy menstrual bleeding in menstrual underwear users who report needing to change underwear midday due to saturation, flooding events, or unabsorbed clots or who require a concurrent menstrual technology to protect clothing (Table 2).
Reusable products have increased menstrual hygiene inclusivity among populations historically excluded from the menstrual product market. The familiarity and diversity of menstrual underwear designs may destigmatize menstruation among teens; however, the cost and forethought required to have backup pairs available when not at home may be unachievable. For individuals with visual or mobility impairments, menstrual underwear may be a more accessible option than disposable pads.34 Boxer, brief, and hybrid styles of menstrual underwear are available and have been identified as a gender-affirming option for transgender individuals.35
INTERNAL RESERVOIRS: CUPS AND DISCS
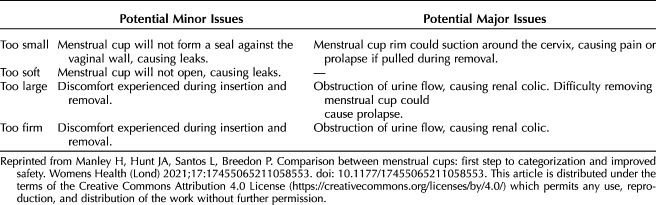
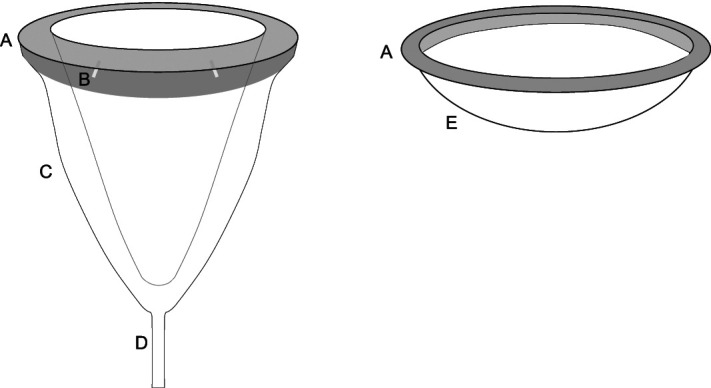
Menstrual cups and discs are reminiscent of contraceptive cervical caps and diaphragms, respectively. Menstrual cups generally are made of rubber or silicone and are reusable over multiple cycles, even years. Menstrual cups have four core components: rim, release holes, cup, and stem (Fig. 1). For placement, a user generally folds the rim, which collapses the diameter of the cup, and places the folded device into the vagina before releasing it. Multiple folding strategies are described online. Once released, the device expands to fill the vagina, where it can remain in place for up to 12 hours. When placed properly, the entire device, including the stem, is within the vagina. Users can ensure proper placement by both digitally verifying that there is no fold in the contour of the cup and gently tugging on the stem to make sure the cup remains in place. Improper fit can result in leaks or discomfort (Table 3). Rare case reports on renal colic and hydronephrosis in individuals using a menstrual cup have been published.36–40
Fig. 1. Components of reusable internal menstrual products. Menstrual cup (left) and menstrual disc (right). A. Rim. B. Channels for suction release. C. Cup. D. Stem. E. Disc reservoir.
Liberty. Menstrual Technology Innovations. Obstet Gynecol 2023.
Table 3.
Menstrual Cup Improper Fit Matrix
Menstrual cup placement may be facilitated by using a reusable device similar to a tampon applicator, wherein a folded cup is placed into a cylinder with a plunger (Fig. 2). To remove most cups, the user is advised to release the seal of the expanded rim by pinching the distal base of the cup and distorting the rim. Several tools, similar to hard plastic popsicle sticks, are available to facilitate removal (Fig. 2): the user places the device into the vagina and advances it to the body of the cup, then uses the device to depress the cup and therefore release the seal. Some cups exist with a tab attached to the rim that facilitates releasing suction without pinching the base of the cup. Removal before breaking this seal results in downward suction that can be uncomfortable but has not been associated with other side effects or adverse events. When the seal is released, the menstrual cup can be brought out of the body to dispose of the collected blood and then replaced or it can be cleaned with soap and water, dried, and stored until next use. Multiple accessories are available that can enable individuals to manage menstrual cups in shared restroom spaces (Box 1), but reusable menstrual products do not have to be washed with soap before replacing them within a 12-hour period. These accessories may increase the perceived cost and waste of menstrual cup maintenance.
Fig. 2. Example of menstrual cup insertion device (left) and menstrual cup removal device (right).
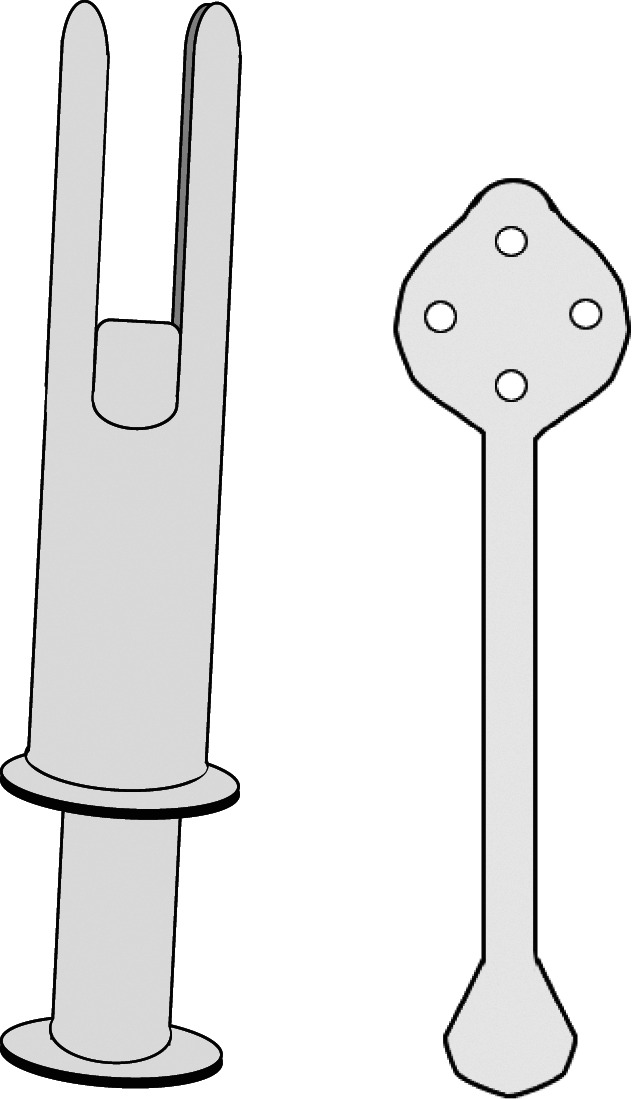
Liberty. Menstrual Technology Innovations. Obstet Gynecol 2023.
Box 1. Menstrual Cup and Disc Accessories.
Market debate exists regarding device “firmness” and how that might influence function and comfort, but no consensus has been reached, with cup size and firmness varying considerably across brands. The circumference of the rim likely affects not only comfort but also the probability of leaking or malposition. Multiple cup shapes are available, as well varying rim diameters, ranging from 31 mm to 54 mm. The volume of the cups ranges from 8 mL to 37 mL when measuring to the suction-release holes (Fig. 1B), because menstrual blood may leak out through those holes. The purpose of the suction-release holes is to facilitate ease of removal, but not all models include these. For individuals whose cervix-to-introitus distance cannot fully accommodate a cup with a stem, several cups without stems are available. For individuals with altered dexterity, alternatively shaped stems are available, which include loops or textures to improve grip. Several cups have stems that can be trimmed to fit an individual's needs. Stems can be as long as 27 mm, which yields a wide range of overall menstrual cup lengths, from 43 mm to 111 mm.
Menstrual discs have a rubber or silicone rim similar to a menstrual cup, with diameters ranging from 53 mm to 80 mm. Menstrual blood collection occurs in a flexible plastic or silicone collection basin, which is usually wider and shallower than a cup, holding between 30 and 79 mL. Neither menstrual cup nor disc use has been studied specifically in the context of heavy menstrual bleeding. Users who fill cups or discs are theoretically losing blood volumes consistent with heavy menstrual bleeding. Until additional research is available that explicitly includes these products, our expert opinion recommends screening for cup filling or overflow or both to inform evaluation for heavy menstrual bleeding (Table 2).
Menstrual discs generally are perceived as more challenging to use given the increased likelihood of spills with removal. The improved collapsibility and flexibility of the disc contribute to the sense that they might be more comfortable for some users. Some users advocate for concomitant disc use during sexual activity—for hygiene preferences rather than contraceptive purposes.41 Removal of a disc is accomplished by digitally reaching the rim or tabs extending from the rim. Menstrual discs have no suction component. Valsalva can tilt the disc and release the seal, increasing the chance of leaking. In some instances, users get reliable leaks during urination, when the decompressed bladder alters the disc’s position, a phenomenon called “auto dumping,” which has been advertised as an advantage of the menstrual disc.
There are several perceived benefits to menstrual cup or disc use. Although leaks and spills have proven a barrier to using menstrual cups for precise blood-loss quantification,42 individuals switching to the menstrual cup report fewer leaks compared with pads or tampons.42,43 People may perceive cups and discs as inert, therefore carrying lower risk of dysbiosis. Menstrual cup use has been identified as a risk factor for TSS through biofilm formation, although the risk remains similar to the risk of TSS associated with female barrier condoms and lower than the risk associated with tampon use.44–46 The reduced negative environmental impact of cups and discs has been cited as motivation for use.23 Future studies looking into the sustainability of cups and discs would benefit from understanding the longitudinal patient experience, because the greatest environmental benefit would come from reducing single-use products over time. Lastly, one potential benefit related to reusable products could be fewer supply chain disruptions interrupting access to menstrual technology.
There are several barriers to menstrual cup use. The upfront cost can range between $25 and $40 (Table 2), without a guarantee of successful use.43 Additionally, there are no formal data to guide individuals to find the optimal cup for their menstrual experiences. Informal internet guides fill this gap by polling individuals about their flow, parity, and comfort or ability to reach their own cervix to make recommendations of specific cups based on size, volume capacity, firmness, shape, and means of removal. The closest formal recommendation to evidence-based personalization of menstrual cup use came from a working group that identified firmness categories, ranging from “very soft” to “very firm,” as a first step to optimization of the user experience (Table 3).47 Some individuals are also advised against menstrual cup use, particularly if they have an intrauterine device (IUD). Approximately 11% of IUD users report concurrent menstrual cup use, although this represents a greater proportion of nonhormonal IUD users who also happen to experience ongoing or heavier menstrual bleeding.48 Evidence suggests that unintended IUD removal may be associated with menstrual cup use, although the mechanism of this is unknown.49 No data exist specific to menstrual disc use and IUD use, although the lack of a seal may decrease the odds of inadvertent removal.
CONCLUSION
Assessment of patients' menstrual health is standard of care for clinicians caring for menstruating individuals. Obstetricians and gynecologists have a critical role in educating patients on normal menstruation, assessing patients with symptoms that suggest a problem with menstruation, and providing guidance on evaluation for and treatment of abnormal uterine bleeding. To provide these assessments and guidance, it is important for obstetricians and gynecologists to ask patients about the type of menstrual products they use and to have up-to-date knowledge of all of the menstrual product options to answer questions, provide counseling related to their use, and understand what kinds of experiences (leaking or flooding around products or rate of change of products) may signal a problem with heavy menstrual bleeding that requires further assessment or treatment (Table 2).
In clinical care and research settings, the multiple means of evaluating individuals' menstrual flow are based on the use of specific menstrual products. Quantitative methods, such as the alkaline hematin method, and semiquantitative methods, such as the pictorial bleeding assessment chart, depend on collection or recording of used sanitary pads or napkins and tampons. Similarly, most bleeding-related quality-of-life questionnaires and common clinical questions used to structure a menstrual history require the patient or research participant to report how often they change fully saturated pads or tampons. Newer technologies to manage menstrual bleeding, such as period-specific undergarments and menstrual cups or discs, have not been incorporated into these measures and may not be clearly evaluated during clinical encounters. This may increasingly present challenges to appropriately determining whether or not an individual is experiencing heavy menstrual bleeding.
To address these challenges, improved data on emerging menstrual technologies’ absorbency and use and patient preferences is essential. With these data, clinicians and researchers could adapt how they evaluate and provide education and guidance to individuals by appropriately incorporating the specific type of menstrual products used by the individual patient or research participant. Increased variety in menstrual products gives people who menstruate increased ability to personalize menstrual management across the life course and across a variety of settings such that their menstrual health is not only less burdensome but, in some instances, empowering.
Footnotes
Supported by a grant from the Society of Family Planning.
Financial Disclosure Alison Edelman reports honoraria and travel reimbursement from ACOG, WHO, and Gynuity for committee activities and honoraria for peer review from the Karolinska Institute. She receives royalties from Up to Date, Inc. Oregon Health & Science University receives research funding from OHSU Foundation, Merck, HRA Pharma, and NIH, where Dr. Edelman is the principal investigator. The other authors did not report any potential conflicts of interest.
The authors thank Kim Rosas for her early feedback on menstrual technologies.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D79
REFERENCES
- 1.World Health Organization. UNESCO and WHO urge countries to make every school a health-promoting school. Accessed May 9, 2022, https://www.who.int/news/item/22-06-2021-unesco-and-who-urge-countries-to-make-every-school-a-health-promoting-school
- 2.World Bank. Menstrual hygiene management enables women and girls to reach their full potential. Accessed May 9, 2022, https://www.worldbank.org/en/news/feature/2018/05/25/menstrual-hygiene-management
- 3.United Nations Population Fund. Menstruation and human rights - frequently asked questions. Accessed May 9, 2022, https://www.unfpa.org/menstruationfaq
- 4.Fraser IS, Critchley HO, Munro MG, Broder M. A process designed to lead to international agreement on terminologies and definitions used to describe abnormalities of menstrual bleeding. Fertil Sterility 2007;87:466–76. doi: 10.1016/j.fertnstert.2007.01.023 [DOI] [PubMed] [Google Scholar]
- 5.Munro MG, Critchley HOD, Fraser IS, Committee FMD. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet 2018;143:393–408. doi: 10.1002/ijgo.12666 [DOI] [PubMed] [Google Scholar]
- 6.Schoep ME, Nieboer TE, van der Zanden M, Braat DDM, Nap AW. The impact of menstrual symptoms on everyday life: a survey among 42,879 women. Am J Obstet Gynecol 2019;220:569.e1–7. doi: 10.1016/j.ajog.2019.02.048 [DOI] [PubMed] [Google Scholar]
- 7.Weisberg E, McGeehan K, Fraser IS. Effect of perceptions of menstrual blood loss and menstrual pain on women's quality of life. Eur J Contraception Reprod Health Care 2016;21:431–5. doi: 10.1080/13625187.2016.1225034 [DOI] [PubMed] [Google Scholar]
- 8.Nur Azurah AG, Sanci L, Moore E, Grover S. The quality of life of adolescents with menstrual problems. J Pediatr Adolesc Gynecol 2013;26:102–8. doi: 10.1016/j.jpag.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 9.Weyand AC, Fitzgerald KD, McGrath M, Gupta V, Braun TM, Quint EH, et al. Depression in female adolescents with heavy menstrual bleeding. J Pediatric 2022;240:171–6. doi: 10.1016/j.jpeds.2021.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sebert Kuhlmann A, Teni MT, Key R, Billingsley C. Period product insecurity, school absenteeism, and use of school resources to obtain period products among high school students in St. Louis, Missouri. J Sch Nurs 2021:10598405211069601. doi: 10.1177/10598405211069601 [DOI] [PubMed] [Google Scholar]
- 11.Bauman D, Sommer A, Hamer T, Noy D, Elami M, Yogev SS, et al. Reduced activity and quality of life in women soldiers with heavy menstrual bleeding and dysmenorrhea. J Pediatr Adolesc Gynecol 2022;35:53–8. doi: 10.1016/j.jpag.2021.08.002 [DOI] [PubMed] [Google Scholar]
- 12.Peberdy E, Jones A, Green D. A study into public awareness of the environmental impact of menstrual products and product choice. Sustainability 2019;11:473. doi: 10.3390/su11020473 [DOI] [Google Scholar]
- 13.Janssen CA, Scholten PC, Heintz AP. A simple visual assessment technique to discriminate between menorrhagia and normal menstrual blood loss. Obstet Gynecol 1995;85:977–82. doi: 10.1016/0029-7844(95)00062-V [DOI] [PubMed] [Google Scholar]
- 14.Hallberg L, Nilsson L. Determination of menstrual blood loss. Scand J Clin Lab Invest 1964;16:244–8. [PubMed] [Google Scholar]
- 15.Higham JM, O'Brien PMS, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. BJOG 1990;97:734–9. doi: 10.1111/j.1471-0528.1990.tb16249.x [DOI] [PubMed] [Google Scholar]
- 16.Magnay JL, Nevatte TM, O'Brien S, Gerlinger C, Seitz C. Validation of a new menstrual pictogram (superabsorbent polymer-c version) for use with ultraslim towels that contain superabsorbent polymers. Fertil Sterility 2014;101:515–22.e5. doi: 10.1016/j.fertnstert.2013.10.051 [DOI] [PubMed] [Google Scholar]
- 17.Vostral S. Toxic shock syndrome, tampons and laboratory standard-setting. CMAJ 2017;189:E726–8. doi: 10.1503/cmaj.161479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warner PE, Critchley HO, Lumsden MA, Campbell-Brown M, Douglas A, Murray GD. Menorrhagia I: measured blood loss, clinical features, and outcome in women with heavy periods: a survey with follow-up data. Am J Obstet Gynecol 2004;190:1216–23. doi: 10.1016/j.ajog.2003.11.015 [DOI] [PubMed] [Google Scholar]
- 19.Center for Disease Control and Prevention. Heavy menstrual bleeding.Accessed September 20, 2022. https://www.cdc.gov/ncbddd/blooddisorders/women/menorrhagia.html
- 20.Philipp CS, Faiz A, Dowling NF, Beckman M, Owens S, Ayers C, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol 2008;198:163.e1–8. doi: 10.1016/j.ajog.2007.08.070 [DOI] [PubMed] [Google Scholar]
- 21.Screening and management of bleeding disorders in adolescents with heavy menstrual bleeding. ACOG Committee Opinion No. 785 [published erratum appears in Obstet Gynecol 2023;141:228]. American College of Obstetricians and Gynecologists. Obstet Gynecol 2019;134:e71–83. doi: 10.1097/aog.0000000000003411 [DOI] [PubMed] [Google Scholar]
- 22.Sebert Kuhlmann A, Peters Bergquist E, Danjoint D, Wall LL. Unmet menstrual hygiene needs among low-income women. Obstet Gynecol 2019;133:238–44. doi: 10.1097/aog.0000000000003060 [DOI] [PubMed] [Google Scholar]
- 23.Parent C, Tetu C, Barbe C, Bonneau S, Gabriel R, Graesslin O, et al. Menstrual hygiene products: a practice evaluation. J Gynecol Obstet Hum Reprod 2022;51:102261. doi: 10.1016/j.jogoh.2021.102261 [DOI] [PubMed] [Google Scholar]
- 24.Shaye C. In the red: a private economic cost and qualitative analysis of environmental and health implications for five menstrual products. Accessed August 15, 2022. https://cdn.dal.ca/content/dam/dalhousie/pdf/science/environmental-science-program/Honours%20Theses/2015/ThesisWeir.pdf
- 25.Archer JC, Mabry-Smith R, Shojaee S, Threet J, Eckert JJ, Litman VE. Dioxin and furan levels found in tampons. J Women's Health 2005;14:311–5. doi: 10.1089/jwh.2005.14.311 [DOI] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration. CPG Sec. 345.300 menstrual sponges. Accessed May 9, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-345300-menstrual-sponges
- 27.Sharma C. Making women's sanitary products safer and cheaper. Accessed March 25, 2022. https://www.elsevier.com/connect/making-womens-sanitary-products-safer-and-cheaper
- 28.Shibly MMH, Hossain MA, Hossain MF, Nur MG, Hossain MB. Development of biopolymer-based menstrual pad and quality analysis against commercial merchandise. Bull Natl Res Centre 2021/03/04 2021;45:50. doi: 10.1186/s42269-021-00504-2 [DOI] [Google Scholar]
- 29.Wyatt KM, Dimmock PW, Walker TJ, O'Brien PM. Determination of total menstrual blood loss. Fertil Sterility 2001;76:125–31. doi: 10.1016/s0015-0282(01)01847-7 [DOI] [PubMed] [Google Scholar]
- 30.Gudmundsdottir BR, Hjaltalin EF, Bragadottir G, Hauksson A, Geirsson RT, Onundarson PT. Quantification of menstrual flow by weighing protective pads in women with normal, decreased or increased menstruation. Acta Obstet Gynecol Scand 2009;88:275–9. doi: 10.1080/00016340802673162 [DOI] [PubMed] [Google Scholar]
- 31.Levin RJ, Wagner G. Absorption of menstrual discharge by tampons inserted during menstruation: quantitative assessment of blood and total fluid content. Int J Obstet Gynaecol 1986;93:765–72. doi: 10.1111/j.1471-0528.1986.tb08065.x [DOI] [PubMed] [Google Scholar]
- 32.University of Texas at Austin. Period products: the good, the bad, and the ugly. Accessed August 12, 2022. https://uthealthaustin.org/blog/period-products
- 33.Denton E. I tried free bleeding Into period panties and this is what happened. Accessed August 15, 2022. https://www.seventeen.com/health/sex-health/a39337/i-free-bled-into-period-panties-and-this-is-what-happened/
- 34.McGregor FA, Unsworth CA. Menstrual hygiene management strategies used by women who are blind or have low vision. Scand J Occup Ther 2021;29:598–610. doi: 10.1080/11038128.2021.1954995 [DOI] [PubMed] [Google Scholar]
- 35.Van De Graaff M. 5 trans-affirming period brands that make pads, tampons, cups and underwear for many bodies. Accessed August 17, 2022. https://www.wellandgood.com/transgender-period-products/
- 36.Stolz A, Meuwly JY, Roussel A, Nicodème Paulin E. An improperly positioned menstrual cup complicated by hydronephrosis: a case report. Case Rep Women's Health 2019;22:e00108. doi: 10.1016/j.crwh.2019.e00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nunes-Carneiro D, Couto T, Cavadas V. Is the menstrual cup harmless? A case report of an unusual cause of renal colic. Int J Surg Case Rep 2018;46:28–30. doi: 10.1016/j.ijscr.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umaramanan T, Hjort-Pedersen K, Besenbruch A, Krzak JM. Hydronephrosis caused by a menstrual cup [in Danish]. Ugeskr Laeger 2019;181:V04190222. [PubMed] [Google Scholar]
- 39.Wilhite S, Rogers D. Acute ureteral obstruction by deeply inserted menstrual cup. Urology 2020;139:e6–7. doi: 10.1016/j.urology.2020.02.012 [DOI] [PubMed] [Google Scholar]
- 40.Athiel Y, Benoit L, Pencolé L. Renal colic with ureterohydronephrosis due to menstrual cup. Urol Case Rep 2020;28:101058. doi: 10.1016/j.eucr.2019.101058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos-Longhurst A. Are menstrual discs the period product we’ve been waiting for? Accessed May 5, 2022. https://www.healthline.com/health/menstrual-disc#takeaway
- 42.Stewart K, Greer R, Powell M. Women's experience of using the Mooncup. J Obstet Gynaecol 2010;30:285–7. doi: 10.3109/01443610903572117 [DOI] [PubMed] [Google Scholar]
- 43.Howard C, Rose CL, Trouton K, Stamm H, Marentette D, Kirkpatrick N, et al. FLOW (finding lasting options for women): multicentre randomized controlled trial comparing tampons with menstrual cups. Can Fam Physician 2011;57:e208–15. [PMC free article] [PubMed] [Google Scholar]
- 44.van Eijk AM, Zulaika G, Lenchner M, Mason L, Sivakami M, Nyothach E, et al. Menstrual cup use, leakage, acceptability, safety, and availability: a systematic review and meta-analysis. Lancet Public Health 2019;4:e376–93. doi: 10.1016/s2468-2667(19)30111-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neumann C, Kaiser R, Bauer J. Menstrual cup-associated toxic shock syndrome. Eur J Case Rep Intern Med 2020;7:1825. doi: 10.12890/2020_001825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nonfoux L, Chiaruzzi M, Badiou C, Baude J, Tristan A, Thioulouse J, et al. Impact of currently marketed tampons and menstrual cups on Staphylococcus aureus growth and toxic shock syndrome toxin 1 production in vitro. Appl Environ Microbiol 2018;1584:e00351-18. doi: 10.1128/aem.00351-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manley H, Hunt JA, Santos L, Breedon P. Comparison between menstrual cups: first step to categorization and improved safety. Womens Health (Lond) 2021;17:174550652110585. doi: 10.1177/17455065211058553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnyer AN, Jensen JT, Edelman A, Han L. Do menstrual cups increase risk of IUD expulsion? A survey of self-reported IUD and menstrual hygiene product use in the United States. Eur J Contraception Reprod Health Care 2019;24:368–72. doi: 10.1080/13625187.2019.1643836 [DOI] [PubMed] [Google Scholar]
- 49.Seale R, Powers L, Guiahi M, Coleman-Minahan K. Unintentional IUD expulsion with concomitant menstrual cup use: a case series. Contraception 2019;100:85–7. doi: 10.1016/j.contraception.2019.03.047 [DOI] [PubMed] [Google Scholar]