Background:
In hypoplastic left heart syndrome, tricuspid regurgitation (TR) is associated with circulatory failure and death. We hypothesized that the tricuspid valve (TV) structure of patients with hypoplastic left heart syndrome with a Fontan circulation and moderate or greater TR differs from those with mild or less TR, and that right ventricle volume is associated with TV structure and dysfunction.
Methods:
TV of 100 patients with hypoplastic left heart syndrome and a Fontan circulation were modeled using transthoracic 3-dimensional echocardiograms and custom software in SlicerHeart. Associations of TV structure to TR grade and right ventricle function and volume were investigated. Shape parameterization and analysis was used to calculate the mean shape of the TV leaflets, their principal modes of variation, and to characterize associations of TV leaflet shape to TR.
Results:
In univariate modeling, patients with moderate or greater TR had larger TV annular diameters and area, greater annular distance between the anteroseptal commissure and anteroposterior commissure, greater leaflet billow volume, and more laterally directed anterior papillary muscle angles compared to valves with mild or less TR (all P<0.001). In multivariate modeling greater total billow volume, lower anterior papillary muscle angle, and greater distance between the anteroposterior commissure and anteroseptal commissure were associated with moderate or greater TR (P<0.001, C statistic=0.85). Larger right ventricle volumes were associated with moderate or greater TR (P<0.001). TV shape analysis revealed structural features associated with TR, but also highly heterogeneous TV leaflet structure.
Conclusions:
Moderate or greater TR in patients with hypoplastic left heart syndrome with a Fontan circulation is associated with greater leaflet billow volume, a more laterally directed anterior papillary muscle angle, and greater annular distance between the anteroseptal commissure and anteroposterior commissure. However, there is significant heterogeneity of structure in the TV leaflets in regurgitant valves. Given this variability, an image-informed patient-specific approach to surgical planning may be needed to achieve optimal outcomes in this vulnerable and challenging population.
Keywords: Fontan procedure, heart ventricles, hypoplastic left heart syndrome, tricuspid valve, tricuspid valve insufficiency
Clinical Perspective.
We created 3-dimensional models of tricuspid valve (TV) annuli, leaflets, papillary muscles, and right ventricles in 100 patients with hypoplastic left heart syndrome with a Fontan circulation. We investigated the association of TV structure and right ventricle volume to TV regurgitation. Greater leaflet billow volume, a more acute anterior papillary muscle angle, and a longer annular distance between the anterior septal leaflet commissure and the anterior posterior leaflet commissure are highly associated with moderate or greater tricuspid regurgitation. We calculated the mean shape of TV leaflets and the principal modes of variation and found that the leaflet shape is highly variable. Although there are meaningful associates of TV structure to valve function, the shape of the valves, and mechanism of valve dysfunction in hypoplastic left heart syndrome with a Fontan circulation are highly heterogeneous. Given this heterogeneity, patient-specific image-derived modeling may be informative for optimization of management and repair in this challenging population.
See Editorial by Hoganson and del Nido
In hypoplastic left heart syndrome (HLHS), the right ventricle (RV) acts as the systemic ventricle and the tricuspid valve (TV) functions as the atrioventricular valve. In this setting, hemodynamically significant tricuspid regurgitation (TR) is understandably associated with circulatory failure and death.1
In adults with biventricular hearts, 3-dimensional (3D) echocardiography (3DE) has significantly informed the understanding of the relationship between atrioventricular valve structure and dysfunction.2–4 Building upon this success, 3DE-based studies in HLHS have now characterized the TV annular and leaflet structure at different stages of repair and shown that the TV dynamics differ from normal biventricular hearts.5,6 Notably, aberrations in HLHS TV 3D structure have been associated with TR, likelihood of successful TV repair, and patient survival.5,7,8 However, there is a limited understanding of the relationship between 3D TV morphology and TV dysfunction in older patients with HLHS with a Fontan circulation.
In this study, we investigated the association of the 3D TV structure to TR in patients with HLHS with a Fontan circulation. To accomplish this, we created novel open-source software to model, visualize, and analyze the TV leaflets, annulus, and subvalvular structure. We used commercial software to assess RV function and 3D volume. We hypothesized that morphology of the TV in patients with hemodynamically significant (moderate or greater) TR would differ from those with mild or less TR and that greater RV volumes would be associated with TV dysfunction and greater anterior papillary muscle (APM) angles.
Methods
All code used for modeling is available open-source at www.github.com/SlicerHeart and implemented in the SlicerHeart extension for 3D Slicer.9 All code used for shape analysis and principal component analysis is available at salt.slicer.org and implemented in the SlicerSALT extension for 3D Slicer.10 Tutorials for application of principal component analysis using the Shape Variation Analyzer tool within SlicerSALT are available at salt.slicer.org.10 Detailed descriptions of the Euclidean metrics are available in the Appendix within the Supplemental Material. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects
In January 2016, acquisition of transthoracic 3DE images of the TV became part of the standard clinical echocardiography lab protocol for HLHS at the Children’s Hospital of Philadelphia. An institutional database was utilized to retrospectively identify patients with HLHS and a Fontan circulation in whom transthoracic 3DE of the TV had been previously performed. Exclusion criteria included presence of significant stitch artifact and lack of inclusion of the entire TV apparatus in the acquisition. Approximately 20% of patients were excluded due to inadequate image quality. No patients had significant arrhythmia. Demographic information on the patients are provided in Table 1 and Table S1. This study was performed according to a protocol approved by the institutional review board at the Children’s Hospital of Philadelphia.
Table 1.
Demographic and 2D Echo Characteristics by Tricuspid Regurgitation Group
Transthoracic Image Acquisition
3DE images of the TV were acquired as per lab protocol using EKG gated, Full Volume, or 3D Zoom mode. Transthoracic X7 or X5 probes were used with the Philips IE33 and EPIQ 7 ultrasound systems (Philips Medical, Cambridge, MA).
Assessment of TR Grade
TR was qualitatively assessed by a single observer (Y.W.) examining 2-dimensional echocardiography color-Doppler images. The severity of TR was graded as follows: trivial (no TR to narrow single jet), mild (multiple narrow jets), moderate (wide jet reaching the midportion of the right atrium), and severe (wide jet reaching the back wall of the right atrium). 2D vena contracta was measured in parasternal long axis and apical 4-chamber views by a single observer (Y.W.).
Annular Curve Modeling and Quantification
3DE images of patients with HLHS were imported into 3D Slicer using SlicerHeart as we have previously described.9,11,12 TV annular curve modeling was performed using a new TV preset for the Valve Annulus Analysis in the SlicerHeart extension.9,11 Annular modeling was performed in end-diastole (ED), mid-systole (MS), end-systole (ES), and mid-diastole. EKG was not available in Cartesian Digital Imaging and Communications in Medicine, so timing of cardiac cycle was determined by valve position as previously described.9,11 Quantification was performed using Euclidian geometry using our new TV Preset in the Valve Quantification Module in SlicerHeart.9,11 The distance along the annulus curve was measured as the circumferential distance between the leaflet’s two corresponding commissure points (Figure 1).
Figure 1.
Visualization of leaflet metrics. A, Anterior leaflet distance (anterior septal commissure [ASC] to anterior posterior commissure [APC]) along the annular curve. B, Tricuspid valve (TV) anterior leaflet area from the atrial view. C, Coaptation length (red), area (gray), and height (navy). D, Total leaflet billow volume. E, Total leaflet tenting volume; F, Papillary muscle angle (red) as measured between the plane of the annulus (navy) and the muscle chord. LV indicates left ventricle, and PSC, posterior septal commissure.
Leaflet and Papillary Muscle Modeling
After defining the annular curve, TV anterior, posterior, and septal leaflets were segmented in MS using the new TV preset in the Valve Segmentation Tool in SlicerHeart.9 Regions of the valve were divided into anterior, posterior, and septal leaflets. In cases where there appeared to be fusion (eg, bicuspid) they were divided at the area of apparent fusion. If there appeared to be more than three leaflets, those in the anatomic region of the traditional trileaflet designation were grouped, similar to emerging recommendations.13 Individual leaflet areas, total leaflet area, coaptation metrics (surface area, length, height), leaflet billow volume, and tenting volume were quantified using the Valve Quantification Module in SlicerHeart(Figure 1 and Video S1).9 APM angle relative to the annular plane was calculated using the Valve Papillary Analysis module as previously described (Figure 1).9,11
Valve Shape Analysis
Individual valve leaflet models were parameterized using SPHARM and SlicerSALT as previously detailed.10,14 Individual valves were viewed in the Shape Variation Analyzer tool in SlicerSALT (Video S2) and then analyzed using principal component analysis (Video S3).10 The mean TV model was visualized during variation of the major principle components from −3 to + 3 SDs (Video S3). Representative cases were compared with the mean shape of the TR group using vector distance diagram to visualize and quantify the differences between an individual regurgitant valve and the mean shape of trivially regurgitant valves (Figure 2). Finally, distance-weighted discrimination was applied to characterize differences in the shape of the TV with respect to TR grade.15 Distance-weighted discrimination is a statistical method that creates a separating hyperplane; a divider that neatly separates 2 groups, which in this case was TV with mild or less TR compared with moderate or greater TR. Individual valve morphologies most associated with trivial TR are furthest from one side of the hyperplane (left in Figure 3) and those most associated with severe TR are furthest from the opposite side of the hyperplane(right in Figure 3).
Figure 2.
Comparison of trivial regurgitation population mean shape and individual patient valves. A, Leaflet model of patient with significant billow and moderate or greater tricuspid regurgitation (TR); (B) Overlay of patient with billow leaflet model (red) on trivial population mean shape (gray); (C) Vector model displaying difference between billowing leaflet shape and mean shape of trivial TR population; (D) Leaflet model of patient with significant tenting and moderate or greater TR; (E) Overlay of patient with tenting leaflet model (blue) on trivial population mean shape (gray); (F) Vector model displaying difference between patient with tenting leaflet shape and mean shape of trivial TR population.
Figure 3.
Shape analysis of tricuspid valves by tricuspid regurgitation (TR) group. A, Trivial/mild TR mean population shape n=65 and moderate/severe population mean shape n=35; (B) Trivial population mean shape n=17 and severe population mean shape n=5; (C) Visual representation of the separating hyperplane (in red) created using distance-weighted discrimination that separates the valves into two regurgitant groups. Valves more distant from the hyperplane are statistically more likely to be in their assigned group using distance-weighted discrimination.
Right Ventricular Volume and Function Quantification
Qualitative 2D RV function was graded by a single observer (Y.W.). 2D-derived ventricular area, volumes, and ejection fraction were quantified using Tomtec 2D left ventricle Cardiac Performance Analysis (Image-Arena Version 4.6, Tomtec, Unterschleissheim, Germany). 3D right ventricular volumes and ejection fraction were calculated using the Tomtec 3D left ventricle package (Image-Arena Version 4.6, Tomtec, Unterschleissheim, Germany). The left ventricle package was chosen due to previous validation of this method for the RV in HLHS, and the more bullet shaped configuration of the single RV.16,17
Statistical Analysis
Annular and leaflet area measures and billow/tenting volumes were normalized to body surface area, linear measures were normalized to body surface area0.5, and ventricular volumes were normalized to body surface area1.3 as previously described.11,18 2D vena contracta was normalized to body surface area0.5 as previously described.11,19 Continuous variables are presented as median [interquartile range]. Comparisons of continuous variables between categorical groups were made using the Mann-Whitney U test and logistic regression. Linear regression was applied to determine the strength of the relationship between continuous variables; in preparation, quantile-quantile plots of residuals were examined for normality, and Box-Cox transformation was applied as needed. A multivariate logistic regression model was created using variables identified from univariate associates of moderate or greater TR (P<0.2). Intraobserver and interobserver for annular and papillary metrics in SlicerHeart have been previously described and shown to be highly reproducible in multiple populations.11,20,21 Intraobserver and interobserver agreement for new leaflet measurements was assessed using 10 3DE datasets in MS. The same observer remeasured all the parameters at least 1 month after the initial evaluation and a second observer performed all measurements without knowledge of the results of the first observer. Reproducibility was quantified using the interclass correlation coefficient (Table S2). All analysis was performed using R version 4.1.1 (2021-08-10) (R Foundation for Statistical Computing, Vienna, Austria).
Results
The median age of the patients at time of 3DE was 10.4 years [interquartile range, 6.0–14.8 years]. The complete TV annuli (ED, MS, ES, mid-diastole) and leaflets (MS) were modeled in a total of 100 patients with HLHS with a Fontan circulation. Thirty-five of the 100 patients had moderate or greater TR (Table 1). The 2D vena contracta in orthogonal views were both greater in the moderate or greater TR group compared with the mild or less group (P<0.001, Table 1). All continuous annular and leaflet metrics were indexed as described in the methods to account for the range of sizes in our HLHS Fontan population.
TV Structure in the HLHS Fontan Population
Annulus
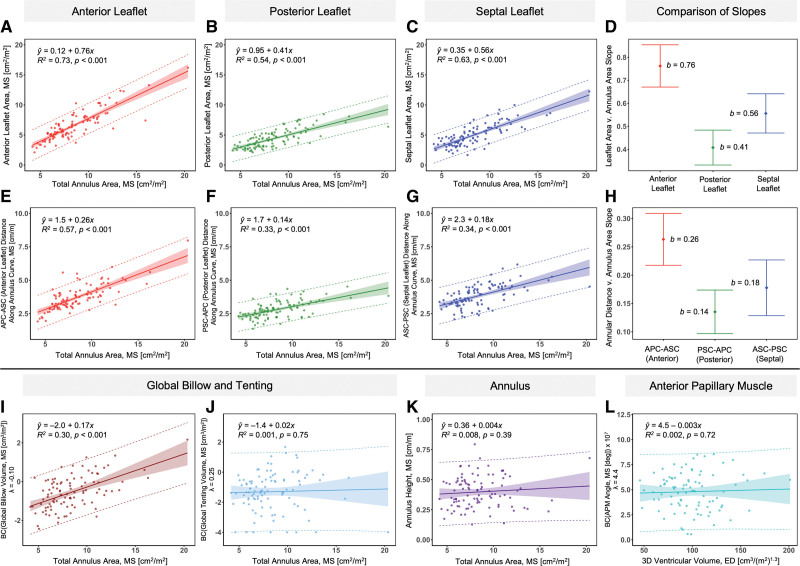
Greater 3D ventricular volume was associated with larger total annular area in both ED and ES (P<0.001, Figure S1). Annular height did not show an association with total annulus area (Figure 4) suggesting that the annulus is similarly round and flat independent of annular size.
Figure 4.
Leaflet and annular metrics versus total annulus area; anterior papillary muscle angle versus ventricular volume. A-C, Leaflet area versus total annulus area (mid-systole [MS]). D, Comparison of slopes of leaflet area versus total annulus area (MS). E-G. Leaflet distance along annulus curve versus total annulus area (MS). H, Comparison of slopes of leaflet distance along annulus curve versus total annulus area (MS). I-J, Transformed global billow and tenting volume versus total annulus area (MS). K, Annular height versus total annulus area (MS). L, Transformed anterior papillary muscle (APM) angle (MS) versus ventricular volume (end-diastole [ED]). Linear regression: solid line=best fit line, shaded area=95% confidence limits, dashed line=95% prediction limits, P values computed via linear regression t test. Whisker plot: b=slope in , bars=95% confidence limits of slope. 3D indicates 3-dimensional; APC, anterior posterior commissure; ASC, anterior septal commissure; BC, Box-Cox Transformation: ; and PSC, posterior septal commissure.
Leaflets
Greater total annulus area (MS) and ventricular volume (ED and ES) were associated with greater total and individual leaflet areas (P<0.001 for total annulus area and ED ventricular volume, Figure 4, Figure S2). The slope of the anterior leaflet area with respect to the total annulus area was greater than the slopes of the posterior and septal leaflet areas with respect to the total annulus area (Figure 4). Similarly, the slope of the anteroseptal commissure (ASC)–postero-septal commissure (PSC) annular chord (annulus associated with anterior leaflet) with respect to total annulus area was also greater than the slopes of annular chords associated with posterior and septal leaflets with respect to total annular area (Figure 4). Together, these findings suggest that the area of the anterior leaflet and the length of its associated annulus (distance from ASC to PSC) are proportionally greater in the setting of larger ventricular and annular size, relative to the posterior and septal leaflets.
Larger total annular area (MS) was associated with greater global billow volume (P<0.01, Figure 4, Figure S3) and individual leaflet billow volume (P<0.01, Figure S4). No significant associations were found between total annulus area or ventricular volume and leaflet tenting volume (Figure 4, Figures S3 and S5).
Papillary Muscle Angle
No significant associations were found between total annulus area (MS) or ventricular volume (ED and ES) and APM angle (Figure 4, Figure S6). No significant associations were found between APM angle and anterior or posterior leaflet area, billow volume, or tenting volume (Figures S7 and S8).
Association of TV Structure and RV Metrics to TR Grade
Annulus
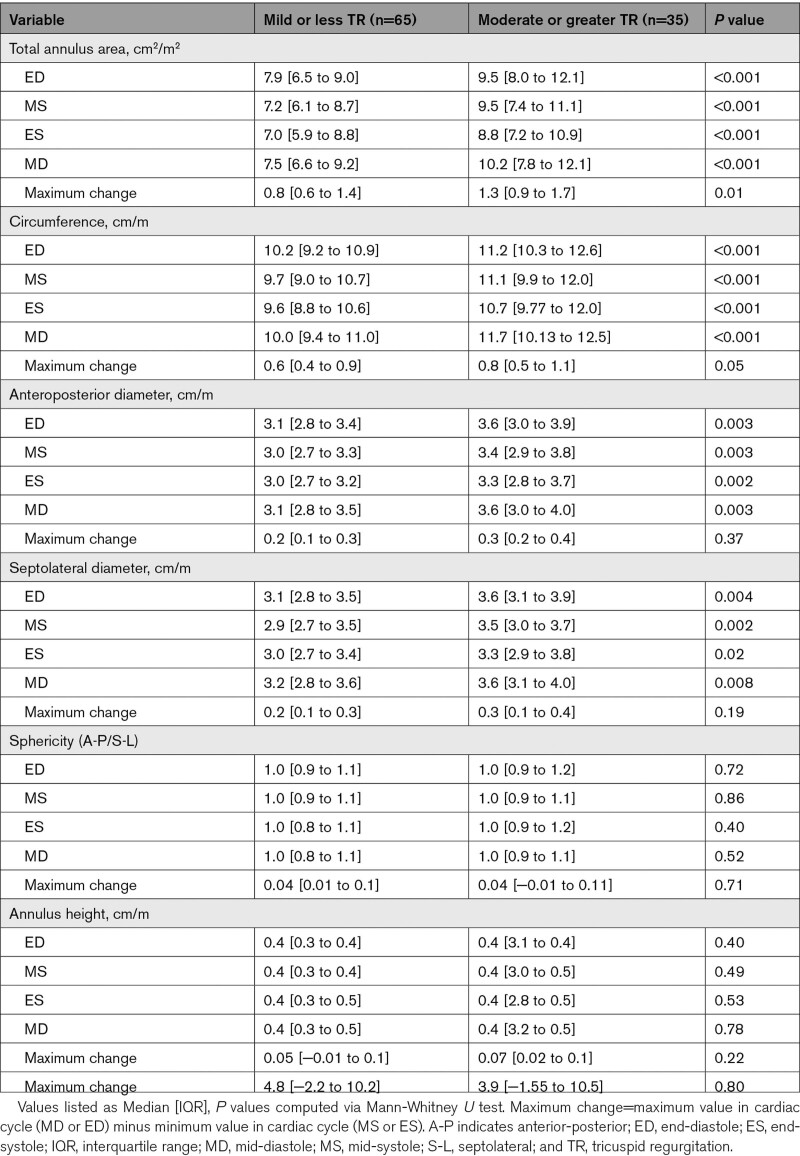
The moderate or greater TR group had larger annular area, circumference, anterior-posterior and septolateral diameter among all phases of the cardiac cycle compared to the mild or less TR group (Table 2). No differences were seen in the anterior-posterior or septolateral bending angles, or annular height between the 2 groups in any phase (Table 2). The moderate or greater TR group had a greater ASC-PSC annular chord (annulus associated with anterior leaflet) as well as a greater percentage of the total annular circumference compared to the mild or less TR group.
Table 2.
Annular Dimensions by TR Group
Leaflets and Papillary Muscles
The moderate or greater TR group had greater total leaflet area (Table 3) compared with the mild or less TR group, as well as greater individual leaflet area. Total and individual leaflet billow volume was greater in the moderate or greater TR group (Table 3). Neither total tenting volume nor individual leaflet tenting volumes differed between the 2 TR groups. No differences between the two TR groups were found in metrics of coaptation height or area among the three leaflets of the TV (Table 3). A lower, more acute, APM angle was associated with moderate or greater TR (Table 3). APM angle was not associated with anterior or posterior leaflet billow volume or tenting volume. Intraobserver variability for leaflet metrics was excellent, ranging from 0.82 to 0.99. Interobserver variability was also excellent, ranging from 0.78 to 0.98 (Table S2).
Table 3.
Leaflet, Papillary Muscle, and Ventricular Metrics by TR Group
Right Ventricle
The moderate or greater TR group had larger 3D ventricular volumes in ED and ES. 2D ventricular area was also found to be larger in the moderate or greater group (Table 3). 2D- and 3D-derived ejection fraction did not differ between the two TR groups (Table 3).
Univariate and Multivariate Logistic Regression
Odds ratios for univariate associates of TR were determined using logistic regression as shown in Table 4. In a multivariate logistic regression model, total billow volume, APM angle, and APC-ASC distance along the annulus curve were independently associated with moderate or greater TR (C statistic=0.85, model P<.001).
Table 4.
Univariate and Multivariate Structural Associates of Tricuspid Valve Regurgitation
Shape Analysis of the TV
The size normalized mean shape of the TVs by TR grade were calculated (Figure 3). While the mean shape between the 2 groups were not strikingly different (Figure 3A and 3B), there was substantial variation in the shape of the annulus and leaflets within the entire population, even within a given TR group (Video S2). We performed principal component analysis in order to characterize and quantify the marked variation in shape in this population with the results shown in Video S3. Principal component 1 qualitatively appeared strongly related to billow and tenting volume. The remaining principal components appeared related to relative leaflet size and shape. The results of distance-weighted discrimination are visually shown in Figure 3C. Valves with more tenting were more likely to appear on the Trivial/Mild side of the separating hyperplane, while those with more billow and a longer anterior leaflet annular chord were more likely to appear on the Moderate/Severe side of the separating hyperplane, consistent with the importance of billow demonstrated in our traditional analysis. However, even within a given TR group, valve structure varied markedly. For example, Figure 2A depicts a regurgitant valve with significant billow (red), whereas Figure 2D demonstrates a regurgitant valve with significant tenting/tethering (blue) exemplifying the heterogeneity of TV structure observed in the moderate or greater TR group during our analysis.
Discussion
We performed a detailed analysis of 3DE-derived quantification of the dynamic HLHS TV and RV in 100 patients, representing nearly all of the eligible HLHS Fontan patients imaged at our institution over a 5-year period. We believe this is the first quantitative 3D image–derived analysis of the TV complex and RV in HLHS at the Fontan stage, the anatomy where most patients with HLHS will spend the majority of their lives. We found that TV leaflet billow, a more acute APM angle, and greater annular distance between the ASC and APC were highly associated with moderate or greater TR. Despite statistically significant associations between TV structure and function, we found there was significant heterogeneity in the TV structure in patients with HLHS with a Fontan circulation and were able to characterize that variation using shape-based methods. Finally, we have released the novel valve modeling software we used to perform this research open-source in SlicerHeart to facilitate transparent and reproducible valve science.9
Relationship of RV Metrics to TV Structure and Function
We found that larger ventricular and annular size was associated with a proportionally larger annular distance from the ASC to the APC (annulus associated with the anterior leaflet) and a larger percentage of the leaflet area apportioned to the anterior leaflet. This novel finding suggests that the region of the annulus associated with the anterior leaflet disproportionally contributes to annular dilation in the setting of RV dilation.7,22
Although decreased RV function is a commonly invoked mechanism of TR in HLHS, no study to our knowledge has been able to demonstrate an association.22,23 In this study, with the additional benefit of 3D volumes, we again did not find an association between 2D and 3D metrics of RV function and TR grade (Table 3). However, consistent with prior studies, larger 3D RV volume was associated with moderate or greater TR.
APM angle has been associated with TV dysfunction in patients with HLHS.11 The APM is the insertion site for chords supplying the anterior and posterior TV leaflets. A more laterally directed angle is thought to pull these leaflets away from the septal leaflet, resulting in a coaptation gap.11,23 Notably this relationship is not seen in younger patients at the Glenn stage but develops by the Fontan stage.11,23 RV dilation is a logical mechanism by which APM angle may decrease with time (and engender TR), but no previous studies in this population have demonstrated this relationship.5,23 In this study, we did not find a correlation between 3D RV volume and APM angle, despite a clear association of lower APM angle to TR. This provokes the hypothesis that the APM angle or RV shape at birth could be a predictive factor for the development of TR over time, independent of RV dilation.7
Relationship of TV Structure to TV Regurgitation
In this study, we found that greater annular segment length between the ASC and PSC (annulus associated with the anterior leaflet) is associated with moderate or greater TR. Further, TV annulus was essentially round and flat, suggesting that by the Fontan stage, the TV leaflets in HLHS may no longer benefit from the structural protection against increased leaflet stress afforded by non-planar annular shapes.5,6,11,24 Together, these findings may have implications for annuloplasty design.
In previous work in the HLHS population, both greater leaflet billow and greater tenting have been associated with varying effects on TR in different stages of repair.7,8,22,23 In a neonatal HLHS population described by Kutty et al7 excessive tenting (tethering) was associated with TR and decreased survival. However, a series focused on patients with a Glenn circulation billow was associated with TR while leaflet tenting was not, suggesting significant changes in TV structure and mechanisms of TR over the period of half a year.23 In this study, we found that the greater leaflet billow volume is associated with TR in traditional and shape analysis(Figure 3). Increased leaflet billow may represent an adaptation secondary to TV leaflet expansion, which develops over time in response to systemic RV pressures. Future studies following TV leaflet stress and strain on individual patients using emerging techniques may yield further insights.25,26
Papillary muscle angles have also been associated with TR in HLHS children, although this varies with age and stage of repair. In younger patients with HLHS, APM angle was not associated with TR.7,23 However, Colen et al23 recently demonstrated a trend toward a lower (more laterally directed) APM angle from prestage 1 to presuperior cavopulmonary anastomosis, which they speculated may progress to significance with time. In our large Fontan cohort, we found that lower APM angle is indeed associated with moderate or greater TR.11
Relevance of Patient-Specific Modeling of the TV in HLHS
The prevailing theme of our work is that there is remarkable variation in TV structure across both individuals and stage of repair in HLHS. As such, patient-specific analysis of TV structure may be needed to determine optimal management and therapy in an individual patient.5,7,23 For example, application of image-derived modeling to a large population of TV could create a multiparameter structural bell curve. Metrics of an individual patient could be placed within that age-specific bell curve to risk stratify for TR progression, probability of successful repair, and even survival.7,8 However, to date, patient-specific TV modeling, including the modeling performed in this study, has been too laborious for routine clinical use. However, automatic segmentation methods, such as machine learning hold promise, and when combined with templates based on shape parameterization can enable unbiased and fully automatic quantification.27,28 Finally, image-derived biomechanical models may allow the testing and comparison of multiple different repair strategies in silico.25 Such methods could allow iterative optimization of repair before performing an actual surgical procedure, which may be particularly relevant to small but heterogeneous populations like HLHS which are less likely to benefit from iterative clinical refinement.
Limitations
Our study is retrospective. As such, we can only associate the suggested valvular differences to moderate or greater TR, but cannot determine if those changes caused TR, or if TR preceded and potentiated those changes. The number of patients with moderate, and particularly severe TR was limited. Similarly, quantitative assessment of TR severity in HLHS is difficult as there are no quantitative grading guidelines in children; even 2D TV vena contracta is of questionable reproducibility in HLHS.29 3D color images and hence 3D vena contracta were not consistently available in our retrospective cohort, and there were no coincident magnetic resonance images to determine TR fraction. We grouped >3 leaflets into traditional tricuspid leaflet categories, and TV variability may have appeared even greater with the use of emerging classifications.13 Only the APM was reliably visualized, consistent with the limitations seen in other studies.5,30 Our analyses were done semi-automatically, which is time-consuming and contributes to variability. In the future, automated methods, including machine learning, could improve these limitations.27
Conclusions
Moderate or greater TR in patients with HLHS with a Fontan circulation is associated with billow volume, a more acute papillary muscle angle, and greater annular distance between the anteroseptal and PSC. However, there is a high degree of structural heterogeneity in this cohort. Given the variability in TV structure, an image-derived, patient-specific approach to surgical planning may need to be adopted to achieve optimal outcomes in this challenging population.
Article Information
Sources of Funding
This work was supported by National Institutes of Health (NIH) R01HL153166, R01EB021391, R56EB021391, Big Hearts to Little Hearts, a Children’s Hospital of Philadelphia (CHOP) Cardiac Center Innovation Award, a Additional Ventures Single Ventricle Expansion Award, The Cora Topolewski Fund at the Pediatric Valve Center at CHOP, and the CHOP Pediatric Valve Frontier Program. Dr. Fichtinger is supported as a Canada Research Chair in Computer-Integrated Surgery.
Disclosures
Beatriz Paniagua, Jared Vicory, and David Allemang are employees of Kitware Inc. The other authors report no conflicts.
Supplemental Material
Supplemental Methods
Figures S1–S8
Tables S1–S2
Videos S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 2D
- 2-dimensional
- 3D
- 3-dimensional
- 3DE
- 3-dimensional echocardiography
- APM
- anterior papillary muscle
- ASC
- anteroseptal commissure
- ED
- end-diastole
- ES
- end-systole
- HLHS
- hypoplastic left heart syndrome
- MD
- mid-diastole
- MS
- mid-systole
- PSC
- postero septal commissure
- RV
- right ventricle
- TA
- tricuspid annulus (annuli)
- TR
- tricuspid regurgitation
- TV
- tricuspid valve
For Sources of Funding and Disclosures, see page 239.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCIMAGING.122.014671.
Contributor Information
Hannah H. Nam, Email: NAMH@EMAIL.CHOP.EDU.
Maura Flynn, Email: mauralynne99@gmail.com.
Andras Lasso, Email: lasso@queensu.ca.
Christian Herz, Email: herzc@chop.edu.
Patricia Sabin, Email: sabinp@chop.edu.
Yan Wang, Email: wangy1@chop.edu.
Alana Cianciulli, Email: cianciulla@chop.edu.
Chad Vigil, Email: chad.vigil5@gmail.com.
Jing Huang, Email: jing14@pennmedicine.upenn.edu.
Jared Vicory, Email: jared.vicory@kitware.com.
Beatriz Paniagua, Email: beatriz.paniagua@kitware.com.
David Allemang, Email: goldbergda@chop.edu.
David J. Goldberg, Email: goldbergda@chop.edu.
Mohammed Nuri, Email: nurim@chop.edu.
Meryl S. Cohen, Email: cohenm@email.chop.edu.
Gabor Fichtinger, Email: fichting@queensu.ca.
References
- 1.King G, Ayer J, Celermajer D, Zentner D, Justo R, Disney P, Zannino D, d’Udekem Y. Atrioventricular valve failure in Fontan palliation. J Am Coll Cardiol. 2019;73:810–822. doi: 10.1016/j.jacc.2018.12.025 [DOI] [PubMed] [Google Scholar]
- 2.Lee AP, Hsiung MC, Salgo IS, Fang F, Xie JM, Zhang YC, Lin QS, Looi JL, Wan S, Wong RH, et al. Quantitative analysis of mitral valve morphology in mitral valve prolapse with real-time 3-dimensional echocardiography: importance of annular saddle shape in the pathogenesis of mitral regurgitation. Circulation. 2013;127:832–841. doi: 10.1161/CIRCULATIONAHA.112.118083 [DOI] [PubMed] [Google Scholar]
- 3.Caravita S, Figliozzi S, Florescu DR, Volpato V, Oliverio G, Tomaselli M, Torlasco C, Muscogiuri G, Cernigliaro F, Parati G, et al. Recent advances in multimodality imaging of the tricuspid valve. Expert Rev Med Devices. 2021;18:1069–1081. doi: 10.1080/17434440.2021.1990753 [DOI] [PubMed] [Google Scholar]
- 4.Badano LP, Hahn R, Rodriguez-Zanella H, Araiza Garaygordobil D, Ochoa-Jimenez RC, Muraru D. Morphological assessment of the tricuspid apparatus and grading regurgitation severity in patients with functional tricuspid regurgitation: thinking outside the box. JACC Cardiovasc Imaging. 2019;12:652–664. doi: 10.1016/j.jcmg.2018.09.029 [DOI] [PubMed] [Google Scholar]
- 5.Nii M, Guerra V, Roman KS, Macgowan CK, Smallhorn JF. Three-dimensional tricuspid annular function provides insight into the mechanisms of tricuspid valve regurgitation in classic hypoplastic left heart syndrome. J Am Soc Echocardiogr. 2006;19:391–402. doi: 10.1016/j.echo.2005.10.025 [DOI] [PubMed] [Google Scholar]
- 6.Nii M, Roman KS, Macgowan CK, Smallhorn JF. Insight into normal mitral and tricuspid annular dynamics in pediatrics: a real-time three-dimensional echocardiographic study. J Am Soc Echocardiogr. 2005;18:805–814. doi: 10.1016/j.echo.2005.01.014 [DOI] [PubMed] [Google Scholar]
- 7.Kutty S, Colen T, Thompson RB, Tham E, Li L, Vijarnsorn C, Polak A, Truong DT, Danford DA, Smallhorn JF, et al. Tricuspid regurgitation in hypoplastic left heart syndrome: mechanistic insights from 3-dimensional echocardiography and relationship with outcomes. Circ Cardiovasc Imaging. 2014;7:765–772. doi: 10.1161/CIRCIMAGING.113.001161 [DOI] [PubMed] [Google Scholar]
- 8.Shigemitsu S, Mah K, Thompson RB, Grenier J, Lin LQ, Silmi A, Beigh MVR, Khoo NS, Colen T. Tricuspid valve tethering is associated with residual regurgitation after valve repair in hypoplastic left heart syndrome: a three-dimensional echocardiographic study. J Am Soc Echocardiogr. 2021;34:1199–1210. doi: 10.1016/j.echo.2021.06.007 [DOI] [PubMed] [Google Scholar]
- 9.Lasso A, Herz C, Nam H, Cianciulli A, Pieper S, Drouin S, Pinter C, St-Onge S, Vigil C, Ching S, et al. SlicerHeart: an open-source computing platform for cardiac image analysis and modeling. Front Cardiovasc Med. 2022;9:886549. doi: 10.3389/fcvm.2022.886549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vicory J, Pascal L, Hernandez P, Fishbaugh J, Prieto J, Mostapha M, Huang C, Shah H, Hong J, Liu Z, et al. SlicerSALT: Shape AnaLysis Toolbox. Shape Med Imaging (2018). 2018;11167:65–72. doi: 10.1007/978-3-030-04747-4_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen AV, Lasso A, Nam HH, Faerber J, Aly AH, Pouch AM, Scanlan AB, McGowan FX, Mercer-Rosa L, Cohen MS, et al. Dynamic three-dimensional geometry of the tricuspid valve annulus in hypoplastic left heart syndrome with a Fontan circulation. J Am Soc Echocardiogr. 2019;32:655–666.e13. doi: 10.1016/j.echo.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn RT, Weckbach LT, Noack T, Hamid N, Kitamura M, Bae R, Lurz P, Kodali SK, Sorajja P, Hausleiter J, et al. Proposal for a standard echocardiographic tricuspid valve nomenclature. JACC Cardiovasc Imaging. 2021;14:1299–1305. doi: 10.1016/j.jcmg.2021.01.012 [DOI] [PubMed] [Google Scholar]
- 14.Vicory J, Herz C, Allemang D, Nam HH, Cianciulli A, Vigil C, Han Y, Lasso A, Jolley MA, Paniagua B. Statistical shape analysis of the tricuspid valve in hypoplastic left heart sydrome. Stat Atlases Comput Models Heart. 2022;13131:132–140. doi: 10.1007/978-3-030-93722-5_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiao X, Zhang HH, Liu Y, Todd MJ, Marron JS. Weighted distance weighted discrimination and its asymptotic properties. J Am Stat Assoc. 2010;105:401–414. doi: 10.1198/jasa.2010.tm08487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutty S, Graney BA, Khoo NS, Li L, Polak A, Gribben P, Hammel JM, Smallhorn JF, Danford DA. Serial assessment of right ventricular volume and function in surgically palliated hypoplastic left heart syndrome using real-time transthoracic three-dimensional echocardiography. J Am Soc Echocardiogr. 2012;25:682–689. doi: 10.1016/j.echo.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 17.Sato T, Calderon RJ, Klas B, Pedrizzetti G, Banerjee A. Simultaneous volumetric and functional assessment of the right ventricle in hypoplastic left heart syndrome after Fontan palliation, utilizing 3-dimensional speckle-tracking echocardiography. Circ J. 2020;84:235–244. doi: 10.1253/circj.CJ-19-0926 [DOI] [PubMed] [Google Scholar]
- 18.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol (1985). 2005;99:445–457. doi: 10.1152/japplphysiol.01144.2004 [DOI] [PubMed] [Google Scholar]
- 19.Prakash A, Lacro RV, Sleeper LA, Minich LL, Colan SD, McCrindle B, Covitz W, Golding F, Hlavacek AM, Levine JC, et al. Challenges in echocardiographic assessment of mitral regurgitation in children after repair of atrioventricular septal defect. Pediatr Cardiol. 2012;33:205–214. doi: 10.1007/s00246-011-0107-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam HH, Dinh PV, Lasso A, Herz C, Huang J, Posada A, Aly AH, Pouch AM, Kabir S, Simpson J, et al. Dynamic annular modeling of the unrepaired complete atrioventricular canal annulus. Ann Thorac Surg. 2022;113:654–662. doi: 10.1016/j.athoracsur.2020.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nam HH, Herz C, Lasso A, Cianciulli A, Flynn M, Huang J, Wang Z, Paniagua B, Vicory J, Kabir S, et al. Visualization and quantification of the unrepaired complete atrioventricular canal valve using open-source software. J Am Soc Echocardiogr. 2022;35:985–996.e11. doi: 10.1016/j.echo.2022.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi K, Inage A, Rebeyka IM, Ross DB, Thompson RB, Mackie AS, Smallhorn JF. Real-time 3-dimensional echocardiography provides new insight into mechanisms of tricuspid valve regurgitation in patients with hypoplastic left heart syndrome. Circulation. 2009;120:1091–1098. doi: 10.1161/CIRCULATIONAHA.108.809566 [DOI] [PubMed] [Google Scholar]
- 23.Colen T, Kutty S, Thompson RB, Tham E, Mackie AS, Li L, Truong DT, Maruyama M, Smallhorn JF, Khoo NS. Tricuspid valve adaptation during the first interstage period in hypoplastic left heart syndrome. J Am Soc Echocardiogr. 2018;31:624–633. doi: 10.1016/j.echo.2017.11.020 [DOI] [PubMed] [Google Scholar]
- 24.Salgo IS, Gorman JH, 3rd, Gorman RC, Jackson BM, Bowen FW, Plappert T, St John Sutton MG, Edmunds LH, Jr. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation. 2002;106:711–717. doi: 10.1161/01.cir.0000025426.39426.83 [DOI] [PubMed] [Google Scholar]
- 25.Wu W, Ching S, Maas SA, Lasso A, Sabin P, Weiss JA, Jolley MA. A computational framework for atrioventricular valve modeling using open-source software. J Biomech Eng. 2022;144:101012. doi: 10.1115/1.4054485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Tallawi KC, Zhang P, Azencott R, He J, Herrera EL, Xu J, Chamsi-Pasha M, Jacob J, Lawrie GM, Zoghbi WA. Valve strain quantitation in normal mitral valves and mitral prolapse with variable degrees of regurgitation. JACC Cardiovasc Imaging. 2021;14:1099–1109. doi: 10.1016/j.jcmg.2021.01.006 [DOI] [PubMed] [Google Scholar]
- 27.Herz C, Pace DF, Nam HH, Lasso A, Dinh P, Flynn M, Cianciulli A, Golland P, Jolley MA. Segmentation of tricuspid valve leaflets from transthoracic 3D echocardiograms of children with hypoplastic left heart syndrome using deep learning. Front Cardiovasc Med. 2021;8:735587. doi: 10.3389/fcvm.2021.735587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pouch AM, Wang H, Takabe M, Jackson BM, Gorman JH, 3rd, Gorman RC, Yushkevich PA, Sehgal CM. Fully automatic segmentation of the mitral leaflets in 3D transesophageal echocardiographic images using multi-atlas joint label fusion and deformable medial modeling. Med Image Anal. 2014;18:118–129. doi: 10.1016/j.media.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Colen TM, Jani V, Barnes BT, Craft M, Tham E, Khoo NS, Smallhorn J, Danford DA, Kutty S. Dynamic systolic changes in tricuspid regurgitation vena contracta size and proximal isovelocity surface area in hypoplastic left heart syndrome: a three-dimensional color doppler echocardiographic study. J Am Soc Echocardiogr. 2021;34:877–886. doi: 10.1016/j.echo.2021.03.004 [DOI] [PubMed] [Google Scholar]
- 30.Stamm C, Anderson RH, Ho SY. The morphologically tricuspid valve in hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 1997;12:587–592. doi: 10.1016/s1010-7940(97)00184-x [DOI] [PubMed] [Google Scholar]