Abstract
Corticospinal neurons (CSN) are centrally required for skilled voluntary movement, which necessitates that they establish precise subcerebral connectivity with the brainstem and spinal cord. However, molecular controls regulating specificity of this projection targeting remain largely unknown. We previously identified that developing CSN subpopulations exhibit striking axon targeting specificity in the spinal white matter. These CSN subpopulations with segmentally distinct spinal projections are also molecularly distinct; a subset of differentially expressed genes between these distinct CSN subpopulations regulate differential axon projection targeting. Rostrolateral CSN extend axons exclusively to bulbar-cervical segments (CSNBC-lat), while caudomedial CSN (CSNmedial) are more heterogeneous, with distinct, intermingled subpopulations extending axons to either bulbar-cervical or thoraco-lumbar segments. Here, we report, in male and female mice, that Cerebellin 1 (Cbln1) is expressed specifically by CSN in medial, but not lateral, sensorimotor cortex. Cbln1 shows highly dynamic temporal expression, with Cbln1 levels in CSN highest during the period of peak axon extension toward thoraco-lumbar segments. Using gain-of-function experiments, we identify that Cbln1 is sufficient to direct thoraco-lumbar axon extension by CSN. Misexpression of Cbln1 in CSNBC-lat either by in utero electroporation, or by postmitotic AAV-mediated gene delivery, redirects these axons past their normal bulbar-cervical targets toward thoracic segments. Further, Cbln1 overexpression in postmitotic CSNBC-lat increases the number of CSNmedial axons that extend past cervical segments into the thoracic cord. Collectively, these results identify that Cbln1 functions as a potent molecular control over thoraco-lumbar CSN axon extension, part of an integrated network of controls over segmentally-specific CSN axon projection targeting.
SIGNIFICANCE STATEMENT Corticospinal neurons (CSN) exhibit remarkable diversity and precision of axonal projections to targets in the brainstem and distinct spinal segments; the molecular basis for this targeting diversity is largely unknown. CSN subpopulations projecting to distinct targets are also molecularly distinguishable. Distinct subpopulations degenerate in specific motor neuron diseases, further suggesting that intrinsic molecular differences might underlie differential vulnerability to disease. Here, we identify a novel molecular control, Cbln1, expressed by CSN extending axons to thoraco-lumbar spinal segments. Cbln1 is sufficient, but not required, for CSN axon extension toward distal spinal segments, and Cbln1 expression is controlled by recently identified, CSN-intrinsic regulators of axon extension. Our results identify that Cbln1, together with other regulators, coordinates segmentally precise CSN axon targeting.
Keywords: axon extension, cerebellin, cervical-thoracic transition, cortical neuron diversity, spinal segmental axon projection
Introduction
For skilled motor control, the cerebral cortex must precisely and accurately connect with specific spinal segments (Sahni et al., 2020). How corticospinal neuron (CSN) axonal projection targeting is established during development underlies motor function, CNS organization, and species differences in orofacial and forelimb dexterity during evolution. Prior work in the field has identified molecular controls over CSN specification, development, and projection targeting (Pang et al., 2000b; Arlotta et al., 2005; Chen et al., 2005, 2008; Molyneaux et al., 2005; Joshi et al., 2008; Kwan et al., 2008; Lai et al., 2008; Tomassy et al., 2010; McKenna et al., 2011; Han et al., 2011; Shim et al., 2012; Lodato et al., 2014; Muralidharan et al., 2017; Diaz et al., 2020; Sahni et al., 2021a, b).
We recently identified that developing CSN subpopulations exhibit striking axon targeting specificity in the spinal white matter, and that this establishes the foundation for durable specificity of adult corticospinal circuitry. CSNBC-lat, which reside in rostro-lateral cortex, are relatively homogeneous, with projections to only bulbar-cervical segments. In contrast, CSN residing in medial sensorimotor cortex (CSNmedial) are more heterogeneous, with distinct interdigitated subpopulations extending axons to either bulbar-cervical or thoraco-lumbar segments: CSNBC-med extend axons only to bulbar-cervical segments, while CSNTL extend axons past cervical cord to thoracic and lumbar spinal segments. We further identified that these segmentally distinct CSN subpopulations are molecularly distinct from early development, enabling molecular delineation and prospective identification even before eventual axon-targeting decisions are evident in the spinal cord: (1) Klhl14 expression delineates Klhl14-positive CSNBC-lat from Klhl14-negative CSNBC-med; (2) all CSNTL are Klhl14-negative; and (3) nearly all CSNTL express Crim1 (schematized in Fig. 1A; Sahni et al., 2021a).
Figure 1.
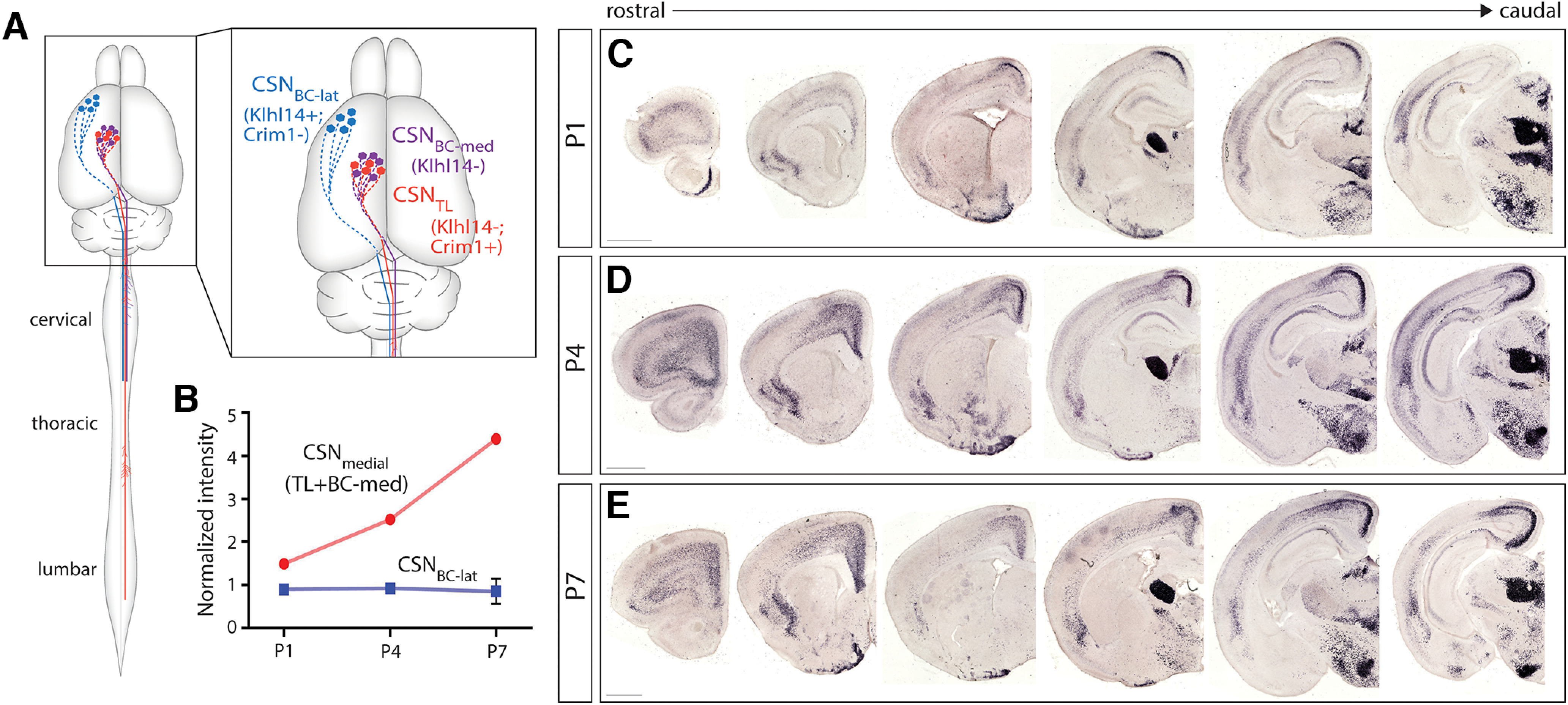
Cbln1 is specifically expressed by CSN residing in medial sensorimotor cortex. A, Schematic of the mouse brain and spinal cord, with inset delineating the three spatially, segmentally, and molecularly distinct CSN subpopulations: CSNBC-lat (blue) reside in rostro-lateral sensorimotor cortex and extend axons only to bulbar-cervical segments; CSNTL (red) reside in medial sensorimotor cortex and extend axons to thoraco-lumbar spinal segments; and CSNBC-med (purple) also reside in medial sensorimotor cortex and extend axons only to bulbar-cervical segments. CSNTL and CSNBC-med are both located in medial sensorimotor cortex, cannot be spatially distinguished, and are collectively referred to as CSNmedial. Klhl14 expression delineates Klhl14-positive CSNBC-lat from Klhl14-negative CSNmedial. Nearly all CSNTL express Crim1 while CSNBC-lat are Crim1-negative. B, Prior transcriptomic analysis comparing CSNBC-lat and CSNmedial gene expression identified Cbln1 as a gene that is not expressed by CSNBC-lat (blue) but whose expression increases from P1 to P7 in CSNmedial (red; Sahni et al., 2021a). C–E, In situ hybridization confirms that Cbln1 is expressed in Layer V, where CSN reside. Cbln1 expression increases from P1 to P7 and is restricted to medial Layer V throughout the rostro-caudal extent of sensorimotor cortex. Scale bars are 1 mm.
Crim1 and Klhl14 direct differential CSN axon segmental targeting by these subpopulations (Sahni et al., 2021b), indicating that the diversity of CSN axonal targeting is controlled in part by CSN-intrinsic mechanisms. Crim1 is both necessary and sufficient for CSNTL axon extension to thoracic and lumbar segments. Crim1 misexpression is sufficient to redirect a subset of CSNBC-lat axons into the caudal thoracic cord. However, this effect of Crim1 misexpression, though striking, only affects a minority of the overall CSNBC-lat subpopulation, with the majority of CSNBC-lat axons terminating in the cervical cord. In addition, although a subset of CSNTL axons, which normally extend past the cervical cord, fail to extend to caudal thoraco-lumbar segments in Crim1 null mice, ∼50% of CSNTL axons still reach the lumbar cord. This indicates that some CSNTL axons can extend to distal spinal targets independent of Crim1 function. Collectively, these results indicate that there are likely additional regulators that direct CSNTL axon extension to distal spinal segments.
Here, we identify Cbln1 as a novel regulator of CSN axon targeting to thoraco-lumbar spinal segments. Cbln1 is a member of the C1q superfamily, which includes proteins critically essential for normal function of the immune and nervous systems (Ghai et al., 2007; Stevens et al., 2007; Yuzaki, 2011). Cbln1 has been extensively characterized in the cerebellum, where it is required for synapse formation and synapse stabilization between parallel fibers of granule cells and Purkinje cell dendrites (Hirai et al., 2005; Matsuda et al., 2010; Uemura et al., 2010; Elegheert et al., 2016; Ibata et al., 2019; Takeo et al., 2021). More recently, it also has been shown to play roles in synapse formation in the striatum and the hippocampus (Kusnoor et al., 2010; Seigneur and Südhof, 2018). There is no previously reported function for Cbln1 in corticospinal connectivity.
We find that within CSN, Cbln1 is expressed specifically by CSNTL, and its expression coincides with the peak period of CSN axon extension toward thoraco-lumbar spinal segments. Misexpression of Cbln1 in CSNBC-lat is sufficient to redirect axons past their normal targets in the cervical cord toward distal thoracic segments. We also identify that Cbln1 overexpression in CSNmedial can increase the number of axons extending past the cervical cord toward thoraco-lumbar segments. This suggests that Cbln1 is sufficient to direct thoraco-lumbar extension by CSN axons in multiple contexts. Further, this effect on CSN axon extension occurs before axon collateralization and synapse formation, establishing a novel function for Cbln1 in directing axon extension, independent of its known functions in synapse formation established elsewhere in the central nervous system. Together, these results identify Cbln1 as a novel, CSN-intrinsic determinant of thoraco-lumbar segmental axon targeting specificity.
Materials and Methods
Mice
CD-1 mice (Charles River Laboratories) were used for gene expression analysis, in utero electroporation, and AAV injections. Fezf2 null mice were generated previously (Hirata et al., 2004) and have been described (Molyneaux et al., 2005). Cbln1 null mice were generated and described previously (Hirai et al., 2005). Embryonic day (E)0.5 was set as the day of the vaginal plug, and post-natal day (P)0 was set as the day of birth. Mice received food and water ad libitum, and were housed on a 12/12 h light/dark cycle. All mouse studies were approved by the IACUC at Harvard University and at Weill Cornell Medicine. All studies were performed in accordance with institutional and federal guidelines.
Tissue collection and preparation
Mice were anesthetized by hypothermia [postnatal day (P)0–P4] or with an intraperitoneal injection (P7–adult) of 0.015 ml/g body weight Avertin (1.25% 2-2-2 tribromoethanol in a solvent that contains 0.63% isoamyl alcohol by weight in ddH2O). Mice were perfused transcardially, first with PBS then with 4% paraformaldehyde (PFA) for fixation. The skull, musculature, limbs, and internal organs (viscera) were removed from the thoracic and abdominal cavities. The remaining skeletal structures were postfixed overnight in 4% PFA at 4°C. The brains were also dissected out, and postfixed overnight in 4% PFA at 4°C. The following day, the spinal cords were dissected out of the vertebral column. The brains and spinal cords were then washed in 1× PBS, and stored in 1× PBS at 4°C.
To collect embryonic tissue (E18.5), timed pregnant females were anesthetized with an intraperitoneal injection of 1 ml Avertin, and euthanized with an additional 1-ml intracardiac injection of Avertin. Embryos were dissected from the uterine horn and decapitated, and the entire head was fixed overnight in 4% PFA at 4°C. The following day, the brains were dissected out, washed in 1× PBS, and stored in 1× PBS at 4°C.
For immunocytochemistry and in situ hybridization, brains or spinal cords were placed in Tissue-Tek OCT Compound (Sakura Finetek) for sectioning using a cryostat (Leica CM3050 S). Before sectioning, the cerebellum, pons, and medulla were removed with a razor blade, leaving the forebrain; 50-µm coronal brain sections, or 50-µm axial or sagittal spinal cord sections, were obtained using a cryostat. All sections were stored in 1× PBS at 4°C.
In situ hybridization and immunocytochemistry
Chromogenic in situ hybridization was performed as previously described (Arlotta et al., 2005). The primer sequences used to generate the chromogenic in situ hybridization probes are from the Allen Brain Atlas (http://www.brain-map.org). The Crim1 and Cbln1 dual fluorescence in situ hybridization combined with Ctip2 immunocytochemistry was performed using RNAscope probes (Advanced Cell Diagnostics; Crim1, catalog #550751; Cbln1, catalog #428551-C2) per the manufacturer's instructions. Briefly, 50-µm free floating sections were mounted on slides. Sections were then processed using the RNAscope Multiplex Fluorescent v2 kit (Advanced Cell Diagnostics; catalog #323100), with integration of the RNA protein co-detection workflow using the RNA-Protein Co-detection Ancillary kit (Advanced Cell Diagnostics; catalog #323180). The sections were treated for target retrieval followed by incubation overnight with rat anti-Ctip2 antibody, 1:100 (Abcam; catalog #ab18465; RRID:AB_2064130) at 4°C. The following day, we followed the RNAscope protocol for detecting Crim1 (C1, Opal 520, FP1487001KT; Akoya Biosciences) and Cbln1 (C2, Opal 650, FP1496001KT; Akoya Biosciences) gene expression followed by incubation with anti-rat Alexa Fluor 546 (Invitrogen; catalog #A-11081) for 3 h at room temperature. Sections were then coverslipped using Fluoromount-G (Southern Biotech; catalog #0100-01) for imaging.
For immunocytochemistry, brains and spinal cords were fixed and stained using standard methods (Molyneaux et al., 2005), with the primary antibody rabbit anti-GFP, 1:500 (Invitrogen; catalog #A-1112; RRID:AB_221569). For Cbln1 immunocytochemistry, we performed antigen retrieval as previously described (Kusnoor et al., 2010). Briefly, 50-µm sections were incubated in 10 mm sodium citrate buffer containing 0.05% Tween 20 at 80°C for 30 min before proceeding with primary antibody incubation. The following primary antibodies were used: rat anti-Ctip2, 1:250 (Abcam; catalog #ab28448; RRID:AB_1140055), and rabbit anti-Cbln1 (E3), 1:200 (Bao et al., 2005; Wei et al., 2007).
Retrograde labeling of corticospinal neurons
CSN that project to lumbar spinal segments were retrogradely labeled at P5 with an Alexa Fluor 555-conjugated cholera toxin subunit B (CTB-555) recombinant retrograde tracer (Invitrogen). For these injections, mice were anesthetized under ice for 4 min, then visualized by Vevo 770 ultrasound backscatter microscopy (VisualSonics) using Aquasonic 100 ultrasound gel (Parker Laboratories). Four slow-pulse injections of 60 nl of CTB-555 (2 mg/ml) were deposited on each side of the midline at L1–L2 using a pulled glass micropipette with a nanojector (Nanoject II, Drummond Scientific) to obtain bilateral labeling. The mice were placed on a heating pad for recovery. Mice were euthanized at P7, allowing the retrograde tracers 2 d for transport.
Anterograde labeling of corticospinal neurons
P28 mice were anesthetized using isofluorane anesthesia (2.5% in 100% O2), and injected in caudomedial cortex with the anterograde tracer biotinylated dextran amine (BDA) using the following stereotactic coordinates −1.0 mm lateral to the midline at bregma at a depth of 0.8 mm. A glass capillary micropipette was filled with a 10% solution of BDA (10,000 MW; Thermo Fisher Scientific) which was delivered into the cortex by iontophoresis using constant current conditions (8 µA; 7 s on 7 s off) for a total period of 20 min. Mice were perfused at P35. The injection site and labeled axons were visualized using DAB staining (Vector Laboratories).
In utero electroporation
Surgeries were performed as previously described (Molyneaux et al., 2005; Greig et al., 2016). To generate the Cbln1 overexpression construct, Cbln1 cDNA was cloned 3′ to an EGFP coding sequence, which was driven by the CAG promoter, and the two ORFs were separated by the t2A linker sequence. In the control plasmid, the Cbln1 cDNA was replaced with a STOP codon 3′ to the t2A linker sequence.
AAV-mediated gene delivery
Constructs expressing GFP or Cbln1 were packaged into AAV 2/1, a serotype known to be specific to neuronal expression, by the Massachusetts General Hospital Virus Core using established protocols. At P0, the appropriate viral mixture (103.5 ng of AAV, 0.05% DiI, and 0.08% Fast Green in 1× PBS) was injected at 23 nl per injection into specific cortical subregions using the same set-up described previously for in utero electroporation (Molyneaux et al., 2005; Greig et al., 2016). Briefly, for all intracortical AAV injections, we ensured localized injection and minimal injection into the ventricles by inserting the needle only into the cortical parenchyma under ultrasound guided backscatter microscopy. AAV particles were then injected in increments of 23 nl at a rate of 23 nl/s. This approach ensures localized intracortical injections in neonatal mice. Before analysis, we also used whole mount images of the cortex to ensure that cortical injection sites were well-matched between control and experimental mice. All viral work was approved by the Harvard Committee on Microbiologic Safety, and the Institutional Biosafety Committee at Weill Cornell Medicine; all work was conducted according to institutional guidelines.
Imaging and quantification
4× and 10× images of brain and spinal cord sections were obtained on a ANDOR Clara DR328G camera mounted on a Nikon Eclipse 90i microscope or a Zeiss Axioimager M2 using Stereo Investigator software (MBF Biosciences); 63× confocal Z stacks of brain sections were obtained on a Leica SP8 confocal microscope. For counts of Cbln1+, Crim1+, and Ctip2+ cells in Figure 4 and counts of Cbln1+, Ctip2+ cells in Figure 5, confocal images were obtained of medial Layer V at the rostral-caudal level indicated in Figures 4B and 5A. Cells were counted using the cell counter function in ImageJ. For Figure 4C, percentages represent the mean ± SEM across four mice.
Figure 4.
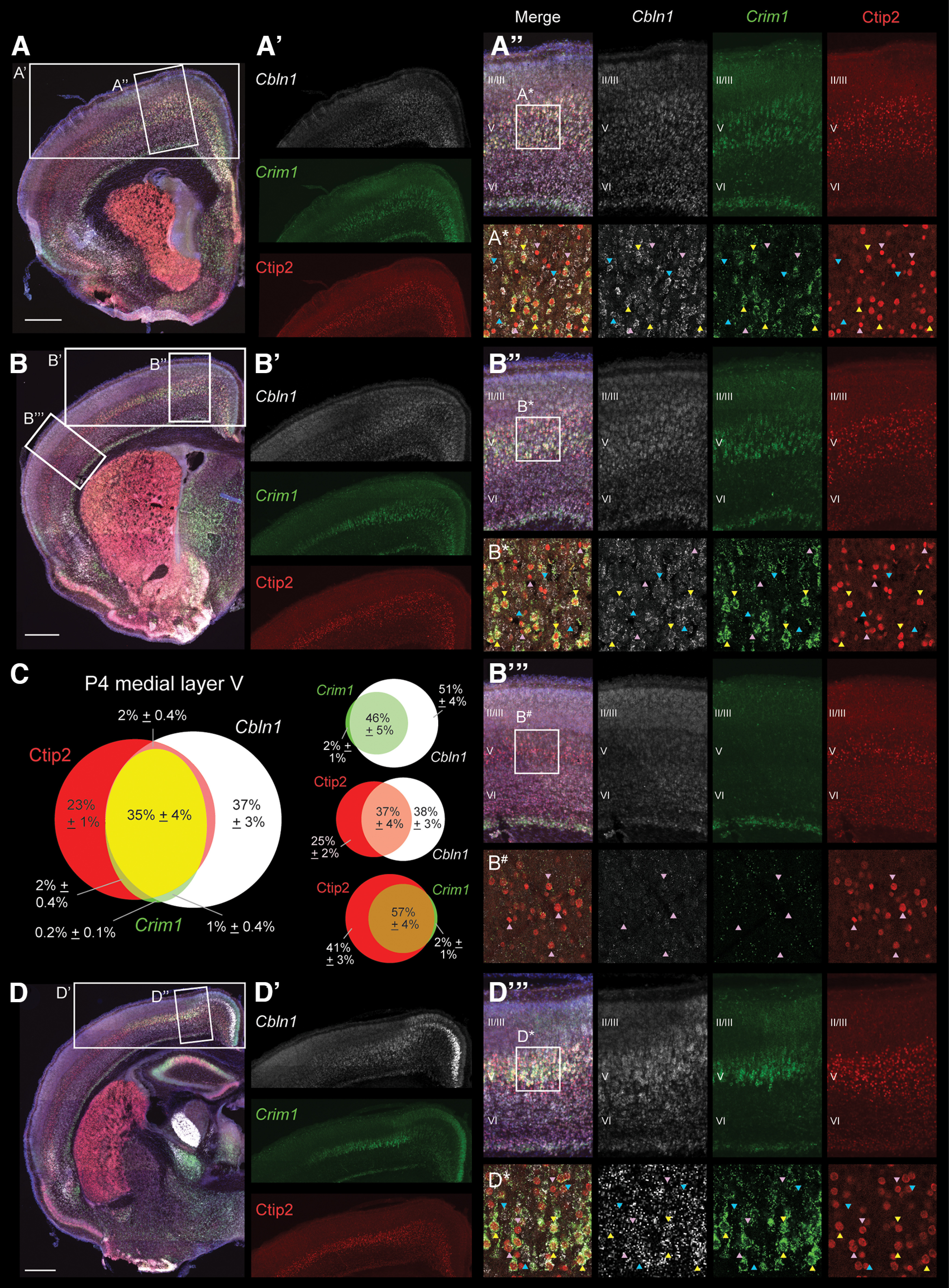
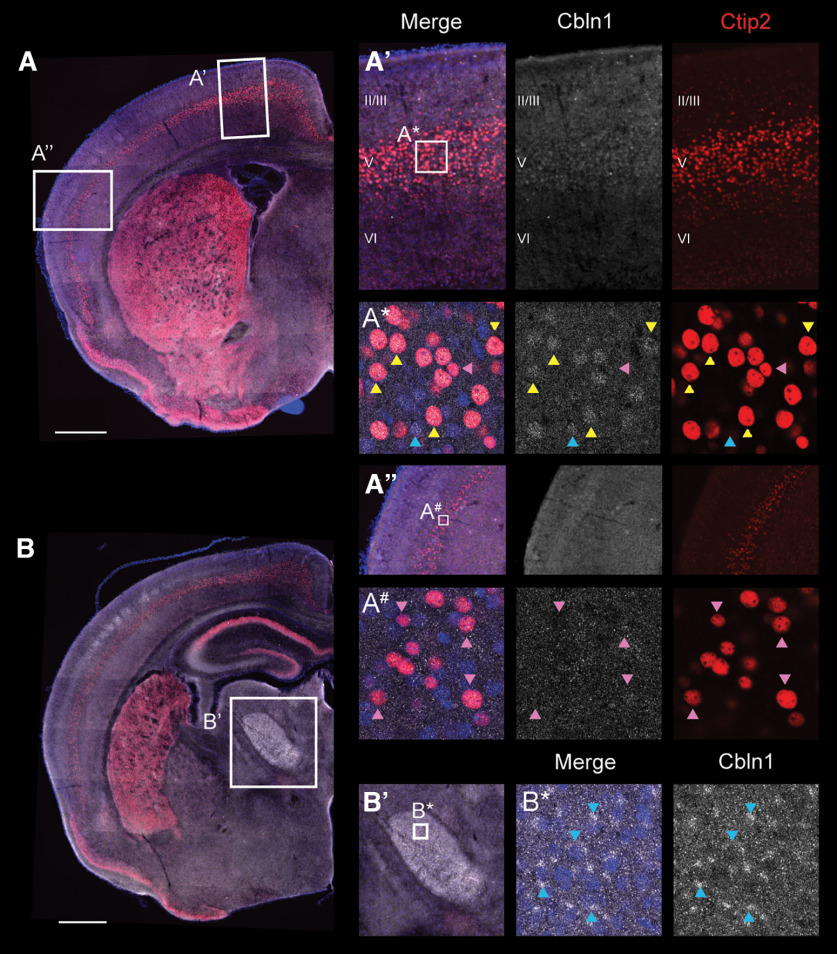
Cbln1 is expressed by CSNTL and not by CSNBC-med. Dual fluorescence in situ hybridization for Cbln1 (white) and Crim1 (green; Crim1 expression marks CSNTL) and immunocytochemistry for Ctip2 (red; high Ctip2+ marks all CSN) were performed on coronal WT brain sections at P4. Sections were also stained for DAPI (blue, nuclei). Representative sections across the rostral-caudal extent of sensorimotor cortex are shown (A, B, D). In confocal insets (A*, B*, B#, D*), yellow arrowheads indicate examples of Cbln1+, Crim1+, high Ctip2+ cells (CSNTL), while blue arrowheads indicate examples of Cbln1+, Crim1−, Ctip2− cells. Finally, pink arrowheads indicate examples of Cbln1−, Crim1−, and high Ctip2+ neurons in either medial Layer V (A*, B*, D*), which correspond to CSNBC-med, or in lateral Layer V (B#), which correspond to CSNBC-lat. The overlap of Cbln1, Crim1, and Ctip2 expression was quantified in four mice in medial Layer V (C). We find that within CSNmedial (high Ctip2+), Cbln1+ neurons are almost exclusively Crim1+, indicating that Cbln1+ CSN are CSNTL. Scale bars are 500 µm.
Figure 5.
Cbln1 protein is present in CSN. (A) Cbln1 protein is detected in medial (A′) but not lateral (A″′) Layer V, and co-localizes with high Ctip2+ neurons (CSN). (B) As a positive control, we observe Cbln1 protein in the parafascicular nucleus of the thalamus, consistent with prior reports using the Cbln1 E3 antibody (Wei et al., 2007; Kusnoor et al., 2010). In confocal insets (A*, A#, B*), yellow arrowheads indicate examples of Cbln1+, Ctip2+ cells (CSNTL), blue arrowheads indicate examples of Cbln1+, Ctip2− cells, and pink arrowheads indicate examples of Cbln1−, Ctip2+ cells (CSNBC-med). Apparent Cbln1 antibody staining in the upper layers of the somatosensory cortex is not specific when compared with secondary antibody only negative control staining. Sections were also stained for DAPI (blue, nuclei). Scale bars are 500 µm.
For all CST quantification on axial sections, 60× or 63× confocal Z stacks of the entire CST in the dorsal funiculus were obtained on either a Biorad Radiance 2100 confocal microscope, a Zeiss LSM 880 confocal microscope, or a Leica SP8 confocal microscope. Cervical, thoracic, and/or lumbar cord axial sections were imaged using identical parameters.
For counts of CSN retrogradely labeled from lumbar L1–L2, we first examined the spinal cord to confirm the matched spinal level of the retrograde tracer injection. Following this confirmation, we imaged and analyzed matched coronal sections for each of three specific rostro-caudal levels in wild-type (WT), Cbln1 heterozygous, and Cbln1 null mice. Cbln1 wild-type and Cbln1 heterozygous mice are indistinguishable in this line (Hirai et al., 2005). The labeled neurons were counted using the cell counter function in ImageJ (National Institutes of Health). In all mice, regardless of the genotype, labeled neurons were only found in the medial cortex on retrograde injection in L1–L2.
For counts of anterogradely labeled, BDA+ axons in the cervical, thoracic, and lumbar spinal cords, we first examined coronal sections of the brain to confirm matched sites of anterograde tracer injection. We then counted the number of axons present at the cervical C1–C2, thoracic T1–T2, and lumbar L1–L2 levels from 40× brightfield images of three axial sections per spinal level using the cell counter function in ImageJ.
For EGFP+ axon counts in axial sections, three axial sections were imaged at both cervical C1–C2 and thoracic T1–T2 levels per mouse, and the axon counts were averaged from three separate sections. For axon intensity measurements in axial sections, at least three axial sections were imaged for each mouse at cervical C1–C2, thoracic T1–T2, and lumbar L1–L2. Background fluorescence intensity was measured from the maximum intensity projection of each Z stack image, and subtracted from the Z stack using ImageJ, such that the intensity of parts of the section without labeled axons was zero. The dorsal funiculus was then selected as the region of interest, and the intensity was measured in each Z stack image. The top three measurements from the Z stack were averaged as the fluorescence intensity for that section. These measurements were then averaged for at least three axial sections at each spinal cord segmental level.
For axon extension experiments, the thoracic cord was sectioned sagittally, and every section that contained a labeled axon was imaged. Each such section was imaged in its entirety, from rostral to caudal and throughout the medio-lateral z-axis. Z stacks for each such section were collapsed to a single two-dimensional plane using the “create focused image” function on the NIS-Elements acquisition software (Nikon Instruments). The collapsed sections were then further combined into one two-dimensional image per mouse by aligning the edges of each section and performing a maximum intensity projection across all sections in Adobe Photoshop using the “Lighten” mode with 100% opacity. This single two-dimensional image per mouse was converted into a monochrome image. To quantify axon extension, we cropped the single image per mouse into dorso-ventral rectangular regions at five rostro-caudal locations (rostral-most, 25% caudal, 50% caudal, 75% caudal, and caudal-most). In each such rectangular region, the CST was then selected as the region-of-interest, and fluorescence intensity was measured in ImageJ. Background fluorescence intensity was measured at an immediately adjacent location in the image and subtracted from this measurement. Intensity at each rostro-caudal level was then normalized to the intensity at the rostral-most limit of the thoracic cord. If no labeled axons were present at the rostro-caudal limit in an individual case, the intensity was set to 0 in that case.
For all of the experiments, the experimenter analyzing the images remained blinded to the experimental conditions.
Experimental design and statistical analysis
Data are presented as mean ± SEM, with n indicating the number of mice used in each group for comparison. The Student's t test was used to assess whether the proportion of CSN axons that reach thoracic T1–T2 from cervical C1–C2 or that reach lumbar L1–L2 from cervical C1–C2 was significantly different between mice injected with GFP (control) or Cbln1. We used the two-tailed Student's t test for the in utero electroporation experiment, then used the one-tailed Student's t test in the AAV injection experiments to test the hypothesis that Cbln1 promotes axon extension into the thoracic spinal cord. To model the distribution of the proportion of axons that reach T1–T2 from cervical C1–C2 (T1/C1) following injection with either control AAV-EGFP or AAV-Cbln1, we fit a mixture model of two Gaussians using the R package mixtools. The two-tailed Student's t test was also used to compare the proportion of retrogradely-labeled neurons at distinct rostral-caudal levels when comparing WT and Cbln1 null mice. We used a two-way ANOVA with repeated measures followed by Fisher's least significant difference post hoc test for the axon extension analyses. Data distribution was assumed to be normal, but this was not formally tested. Male and female mice were used without distinction in experiments.
Results
Cbln1 is expressed by CSN in medial, but not lateral, sensorimotor cortex during early postnatal development
We previously performed differential gene expression analysis to identify potential candidate molecular controls over CSN axon targeting to bulbar-cervical versus thoraco-lumbar spinal segments (Sahni et al., 2021a). We compared gene expression at three critical timepoints, P1, P4, and P7, between CSN in lateral sensorimotor cortex (CSNBC-lat), which extend projections exclusively to bulbar-cervical spinal segments, and CSN in medial sensorimotor cortex (CSNmedial), with two interspersed and molecularly distinct subpopulations that extend projections to both bulbar-cervical (CSNBC-med) and thoraco-lumbar (CSNTL) segments (schematized in Fig. 1A). By P4, CSNTL, in contrast to CSNBC-lat and CSNBC-med, extend axon projections toward distal spinal cord segments (Bareyre et al., 2005; Kamiyama et al., 2015; Sahni et al., 2020, 2021a). To identify candidate molecular controls that control CSNTL axonal targeting specifically within CSNmedial, we previously investigated genes that exhibit significant differential expression between CSNBC-lat and CSNmedial at P4 (Sahni et al., 2021a, b). This work identified multiple candidate molecular regulators of axon targeting and collateralization, including Crim1 as both a unique identifier of CSNTL, and a molecular control that is both necessary and sufficient to direct CSN axon extension to thoraco-lumbar segments. This further validated the original differential gene expression analyses to identify critical regulators over segmentally-specific CSN axon targeting.
Another candidate molecular control identified by this approach is Cbln1, which is specifically expressed by CSNmedial but not by CSNBC-lat, with peak differential expression at P4 and P7 (Fig. 1B). This peak coincides with the period when CSNTL axons extend past the cervical cord toward thoraco-lumbar segments. This time course of significant differential expression along with known roles for Cbln1 and its family members in synaptogenesis (Hirai et al., 2005; Stevens et al., 2007; Kusnoor et al., 2010; Matsuda et al., 2010; Uemura et al., 2010; Seigneur and Südhof, 2018; Ibata et al., 2019; Takeo et al., 2021) suggested that Cbln1 might function in controlling these processes by some or all CSNmedial.
To investigate this hypothesis, we first confirmed these transcriptomic data by investigating Cbln1 expression in the developing sensorimotor cortex using chromogenic in situ hybridization. We examined expression at the three developmental times analyzed by differential gene expression analysis, P1, P4, and P7. These experiments identify that, at all three developmental times, Cbln1 is expressed in medial, but not lateral, Layer V (Fig. 1C–E). Cbln1 expression remains restricted to medial Layer V throughout the rostro-caudal extent of the sensorimotor cortex (Fig. 1C–E).
To confirm that Cbln1 is expressed by CSN in Layer V, we investigated Cbln1 expression in Fezf2 null mice, which completely lack CSN (Chen et al., 2005; Molyneaux et al., 2005). At P7, Cbln1 expression in Layer V is completely abolished in Fezf2 null mice, confirming that Cbln1 is expressed by CSN in Layer V (Fig. 2). The abolition of Cbln1 expression in medial Layer V in Fezf2 null mice was also observed at P1 and P4 (data not shown). As expected, Cbln1 expression in layers II/III and VI remains unchanged in Fezf2 null mice (Fig. 2). The expression of Cbln1 by CSNmedial throughout early postnatal development suggests that Cbln1 might function in a CSN subpopulation-specific manner.
Figure 2.

Cbln1 is expressed by CSN in Layer V. In situ hybridization at P7 shows that Cbln1 is expressed in medial Layer V in Fezf2 WT (A, C) but not in Fezf2 null (B, D) mice. Fezf2 null mice completely lack CSN (Chen et al., 2005; Molyneaux et al., 2005). This indicates that Cbln1 is expressed by CSN in Layer V in medial sensorimotor cortex. Scale bars are 100 µm for insets and 500 µm for other images.
Cbln1 is expressed in neocortical and subcortical regions during early postnatal development
To rigorously investigate the spatial and temporal course of Cbln1 expression in sensorimotor cortex from development into maturity, we performed chromogenic in situ hybridization for Cbln1 in WT mice at E18.5, P1, P4, P7, P10, P14, P21, P28, and adult (more than three months old). At E18.5, Cbln1 is not expressed in the sensorimotor cortex (data not shown), indicating that Cbln1 is not required for early CSN development. At P1, Cbln1 is expressed in medial Layer V throughout the rostral-caudal extent of sensorimotor cortex (Fig. 3A,B). Cbln1 expression then steadily increases in medial Layer V from P4 to P7 (Figs. 2A,C, 3C,D). This expression decreases slightly at P10 (Fig. 3E,F). Cbln1 is expressed at very low levels in Layer V by P14, and is absent in Layer V by P21 (Fig. 3G–K). Cbln1 expression was never observed in lateral Layer V at any time point (Fig. 3). These results confirm the initial observations of differential Cbln1 expression by CSNmedial versus CSNBC-lat (Sahni et al., 2021a). Further, these results also highlight the temporal dynamics of Cbln1 expression with peak expression around P7 and declining expression levels thereafter.
Figure 3.

Time course of Cbln1 expression. Cbln1 is expressed throughout the rostro-caudal extent of sensorimotor cortex in medial but not lateral Layer V at P1 (A, B), P4 (C, D), P10 (E, F), and P14 (G, H). Cbln1 expression in Layer V is absent in P21 (I), P28 (J), and in more than three-month-old (K) mice. Scale bars are 100 µm for insets and 500 µm for all other images.
Within the cortex, Cbln1 is also expressed in other layers in the neocortex and in subcerebral regions during postnatal development. In Layer VI, Cbln1 is expressed at low levels rostrolaterally. Cbln1 expression in Layer VI increases in caudomedial sensorimotor cortex from P1 until P10, and is present at low levels by P14 (Figs. 2, 3). Cbln1 is expressed in medial Layers II/III, with a higher expression level in caudal versus rostral sensorimotor cortex (Fig. 3). Cbln1 is also highly expressed in the cingulate and piriform cortex from P1 to P28, with expression decreasing with age (Fig. 3). Consistent with previous reports (Iijima et al., 2007; Kusnoor et al., 2010; Otsuka et al., 2016), we find Cbln1 is also very highly expressed outside the neocortex, in the thalamus and the hypothalamus from E18.5 into adulthood, and in the septum at P10 and P14 (Fig. 3).
Although Cbln1 is expressed in many neocortical and subcerebral regions, its specific restriction within Layer V to medial sensorimotor cortex combined with its distinct time course of expression suggests that Cbln1 might perform CSNTL-specific functions. Interestingly, Cbln1 expression in Layer V is largely confined to early postnatal development, with expression increasing from P1 to P7 and then decreasing by P14 (Figs. 2, 3). This corresponds with the time course of CSNTL axon extension to caudal spinal segments during development. CSNTL first extend axons toward the lumbar cord by P5, with the number of axons reaching the lumbar cord steadily increasing from P7 to P14, with continued extension up to P28 (Bareyre et al., 2005; Kamiyama et al., 2015; Sahni et al., 2020, 2021a). The restriction of Cbln1 expression to medial CSN with peak expression coincident with the time period of CSNTL axon extension to thoracic and lumbar segments, suggests that Cbln1 might function during CSNTL axon extension. Further, the temporal restriction of Cbln1 expression in Layer V to early postnatal development suggests that the function of Cbln1 within CSN is potentially distinct from the function of Cbln1 in the cerebellum, where it is constitutively expressed throughout development and adulthood, and is required for both the proper formation and maintenance of synapses between Purkinje neurons and parallel fibers (Hirai et al., 2005).
Within CSNmedial, Cbln1 is specifically expressed by CSNTL and not by CSNBC-med
CSNmedial are comprised of two interdigitated subpopulations: CSNBC-med, which extend axons to bulbar-cervical segments, and CSNTL, which extend axons past the cervical cord to thoraco-lumbar segments (Sahni et al., 2021a). We previously identified that CSNTL specifically express Crim1, while CSNBC-med are Crim1-negative (Sahni et al., 2021a, b). To investigate whether Cbln1 is expressed by CSNBC-med, CSNTL, or both subpopulations, we combined fluorescence in situ hybridization for both Cbln1 and Crim1, along with immunocytochemistry for Ctip2, which is expressed by all CSN (Arlotta et al., 2005), on coronal brain sections from WT mice at P4 (Fig. 4). As expected, we observe high Ctip2 expression throughout the rostral-caudal and medial-lateral extent of Layer V, but Crim1 expression is restricted to medial Layer V (compare Fig. 4B” and B”') where Crim1 specifically labels CSNTL (Sahni et al., 2021a,b). Consistent with the Cbln1 expression observed from chromogenic in situ hybridization (Figs. 1–3), Cbln1 expression in Layer V is also restricted to the medial sensorimotor cortex.
We next quantified cells that expressed Cbln1, Crim1, or Ctip2 in medial Layer V of sensorimotor cortex (Fig. 4C). High Ctip2+ neurons can be segregated into two subpopulations that correspond to the previously described CSN subpopulations within medial Layer V (Sahni et al., 2021a): Crim1-positive CSNTL that comprise of Ctip2+ neurons and Crim1-negative CSNBC-med that comprise the remaining of Ctip2+ neurons. Further, we observe that of Crim1-positive neurons are high Ctip2+, consistent with specific expression of Crim1 by CSNTL (Sahni et al., 2021a).
Surprisingly, we find two Cbln1-expressing populations in Layer V: a high Ctip2+ population ( of Cbln1+ cells) and a Ctip2-negative population ( of Cbln1+ cells; Fig. 4C). This is in contrast to the absence of Cbln1 expression in Layer V of the Fezf2 null cortex (which lacks all CSN) by chromogenic in situ hybridization (Fig. 2). The lack of Cbln1 expression in Layer V of the Fezf2 null cortex suggests that all Cbln1+ cells would be high Ctip2+, which makes the identification of a Cbln1+, Ctip2− population rather surprising. This could potentially indicate cell nonautonomous effects by Cbln1+ CSN on Cbln1 expression in non-CSN cells; that Cbln1 is also expressed by other, currently unidentified cells that require Fezf2 expression in Layer V, such as Fezf2-expressing callosal projection neuron (CPN; Tantirigama et al., 2014, 2016); or that Cbln1 expression in non-CSN cells is below the detection limit of chromogenic in situ hybridization. Nevertheless, we find that of the Cbln1+, Ctip2+ neurons (Cbln1-expressing CSN) are also Crim1+, and of the Crim1+, Ctip2+ neurons (CSNTL) are also Cbln1+ (Fig. 4C). This indicates that although Cbln1 expression in medial Layer V is not restricted to CSN, Cbln1 expression within CSNmedial is almost entirely restricted to Crim1+ CSNTL and excluded from CSNBC-med.
Cbln1 protein is present in CSNTL
To determine whether Cbln1 is translated in CSNTL, we performed immunocytochemistry for Cbln1 and Ctip2 on coronal WT brain sections at P4 (Fig. 5). As a positive control, we observe high levels of Cbln1 protein in the parafascicular nucleus (PF) of the thalamus (Fig. 5B) as previously reported (Kusnoor et al., 2010); this is consistent with high Cbln1 expression levels in the PF by fluorescence in situ hybridization (Fig. 4D). In the neocortex, Cbln1 protein is found in both the cingulate cortex and in medial Layer V, and excluded from lateral Layer V as expected (Fig. 5A). We find that a majority of Cbln1-expressing neurons in medial Layer V are high Ctip2+ (Fig. 5A). Intriguingly, unlike in the PF where Cbln1 is found in the cytoplasm (Fig. 5B), Cbln1 appears relatively localized within the nucleus in CSN (Fig. 5A), which has not been previously reported (see Discussion). Taken together, the localization, temporal trajectory, and cell type identity of Cbln1 expression and protein levels suggest that Cbln1 might function during CSNTL axon extension.
Other cerebellin family members are not expressed by CSN
Cbln1 forms homo- and hetero-complexes with itself or with other members of the Cbln family, and these complexes are known to perform varied, compensatory, and redundant functions (Pang et al., 2000a; Bao et al., 2006; Iijima et al., 2007; Miura et al., 2009; Joo et al., 2011; Rong et al., 2012; Seigneur and Südhof, 2018; Seigneur et al., 2018). To determine whether other Cbln family members are expressed by CSNmedial and might potentially interact with Cbln1 in CSNmedial, we examined previously published gene expression datasets and performed in situ hybridization in wild-type mice.
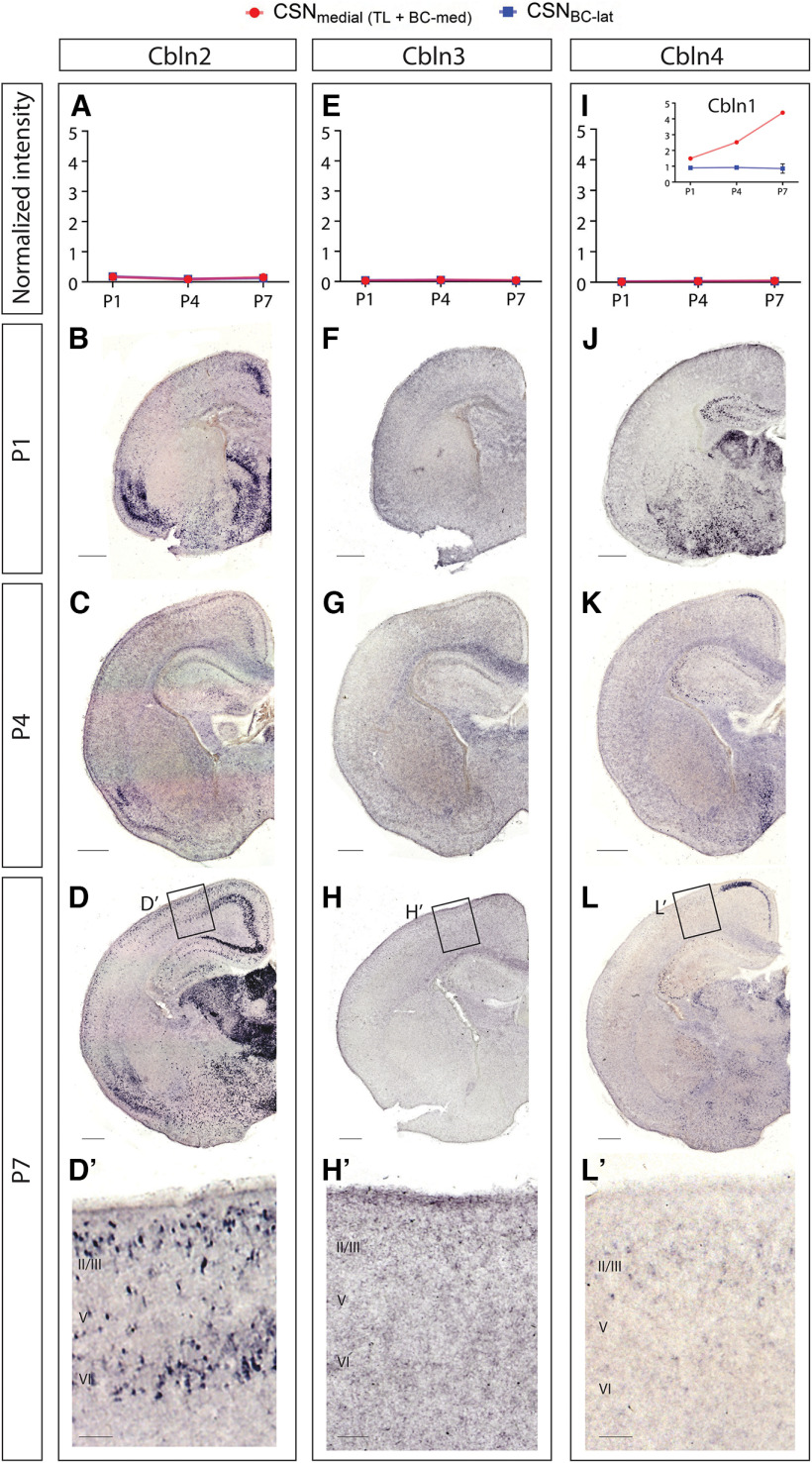
Differential gene expression analysis comparing CSNBC-lat and CSNmedial indicates that Cbln2, Cbln3, and Cbln4 are not expressed, or expressed at very low levels, by either CSNBC-lat or CSNmedial during early postnatal development (Sahni et al., 2021a; Fig. 6A,E,I). Accordingly, there is also no detectable difference between Cbln2, Cbln3, or Cbln4 expression in CSNBC-lat compared with CSNmedial, suggesting that these genes are unlikely to function in a CSN subpopulation-specific manner. Interestingly, prior gene expression comparisons at late embryonic and early postnatal times found that Cbln2 expression is enriched in a different neocortical projection neuron subtype, callosal projection neurons (CPNs), as compared with CSN, and that Cbln3 and Cbln4 are not expressed by CPN or by CSN (Arlotta et al., 2005). Together, these datasets suggest that Cbln1 functions independently of other Cbln family members within CSNmedial.
Figure 6.
Other Cbln family members are not expressed by developing CSN. Prior differential gene expression analysis (Sahni et al., 2021a) identified Cbln1 (inset in I) as expressed by CSNmedial. In that dataset, Cbln2 (A), Cbln3 (E), and Cbln4 (I) exhibit no expression by CSNmedial (blue) or CSNBC-lat (red) at P1, P4, and P7. Chromogenic in situ hybridization at P1 (B, F, J), P4 (C, G, K), and P7 (D, H, L) confirms that Cbln3 and Cbln4 are not expressed in Layer V of the developing neocortex and that Cbln2 is mostly excluded from Layer V. Neurons expressing Cbln2 in Layer V are likely callosal projection neurons (Arlotta et al., 2005). Scale bars are 100 µm for insets and 500 µm for other images.
Chromogenic in situ hybridization in wild-type mice confirmed the differential gene expression analyses. As reported previously (Miura et al., 2006; Seigneur and Südhof, 2017), Cbln2 is expressed at high levels in cingulate and piriform cortex, and at intermediate levels in Layer VI at P1, P4, and P7 (Fig. 6B–D). A small fraction of Layer V neurons express Cbln2 at P1 (Fig. 6B), but these are likely CPN based on the previous transcriptomic analysis (Arlotta et al., 2005). Cbln3 is not expressed within sensorimotor cortex at P1, P4, or P7 (Fig. 6F–H). Similarly, Cbln4 is not expressed in Layer V, but is expressed in the cingulate at P4 and P7 (Fig. 6J–L). Together with the transcriptomic analyses, these results indicate that Cbln2, Cbln3, and Cbln4 expression is likely absent in CSN, and thus, suggest that Cbln1 function in CSNmedial during early postnatal development is independent of other Cbln family members.
Cbln1 is not required for CSNTL axon extension to the thoraco-lumbar spinal cord
Since Cbln1 expression levels coincide with the peak period of CSNTL axon extension to thoracic and lumbar segments, we next investigated whether Cbln1 is necessary for CSNTL axon extension to these distal spinal segments. We analyzed Cbln1 null mice using anterograde and retrograde labeling (Hirai et al., 2005). We injected the retrograde tracer cholera toxin subunit B conjugated with Alexa-Fluor 555 (CTB-555) into lumbar L1–L2 in Cbln1 wild-type (WT), Cbln1 heterozygous (Het), and Cbln1 null mice at P5, at which time the first CSNTL axons have reached the lumbar cord, and analyzed the number of retrogradely labeled CSN in sensorimotor cortex at P7 (Fig. 7A). As expected, labeled CSN are located caudomedially in sensorimotor cortex. We observe no qualitative difference in the overall distribution or number of retrogradely labeled CSN in Cbln1 null mice (Fig. 7B–H).
Figure 7.

Cbln1 is not required for axon extension to lumbar L1–L2. A, Experimental outline: Retrograde labeling with injection of CTB-555 at lumbar L1–L2 was performed in Cbln1 WT, Cbln1 Het, and Cbln1 null mice at P5, and the number of retrogradely labeled neurons throughout the rostro-caudal extent of medial sensorimotor cortex was analyzed at P7. B, There is no qualitative difference in the proportion of retrogradely labeled CSN in medial sensorimotor cortex at rostral, middle, or caudal levels between Cbln1 WT or Het () and Cbln1 null () mice. C–H, Representative rostral, middle, and caudal sections from a Cbln1 Het and a Cbln1 null mouse. Scale bars are 100 µm for insets and are 500 µm for all other images. I, Experimental outline. Anterograde labeling via biotinylated dextran amine (BDA) iontophoresis into caudomedial sensorimotor cortex was performed in Cbln1 Het and Cbln1 null mice at P28. The number of BDA-labeled axons at cervical C1–C2, thoracic T1–T2, and lumbar L1–L2 was counted at P35 for three axial sections per spinal level, and the average number of counts is plotted. J, There is no qualitative difference in the proportion of axons that reach thoracic T1–T2 or lumbar L1–L2 from cervical C1–C2 between Cbln1 Het and Cbln1 null mice. K–P, Representative axial sections from cervical C1–C2, thoracic T1–T2, and lumbar L1–L2 in Cbln1 Het and Cbln1 null mice. Scale bars are 100 µm.
Next, we injected the anterograde tracer biotinylated dextran amine (BDA) into the caudomedial sensorimotor cortex at P28 and analyzed the brain and spinal cord at P35 to determine whether Cbln1 might be required for the maintenance of lumbar axon extension (Fig. 7I). Once again, there is no qualitative difference in the proportion of CSN axons at cervical C1–C2 that reach thoracic T1–T2 or lumbar L1–L2 between Cbln1 WT and Cbln1 null mice (Fig. 7J–P). These results suggest that Cbln1 is not necessary for CSNTL axon extension to the thoracic and lumbar cord.
Misexpression of Cbln1 in CSNBC-lat leads to aberrant axon extension past the cervical cord toward distal thoraco-lumbar spinal segments
Although Cbln1 is not required for CSNTL axon extension to distal thoraco-lumbar spinal segments, we wondered whether Cbln1 might be sufficient to direct long CSN axon extension. We had previously established that misexpression of Crim1, a CSNTL-specific control, in CSNBC-lat can redirect a subset of their axons to caudal thoracic segments (Sahni et al., 2021b). We therefore investigated whether Cbln1 is similarly sufficient to direct thoraco-lumbar axon extension by CSNBC-lat, which normally do not express Cbln1. To test this hypothesis, we introduced a plasmid expressing Cbln1 and an EGFP reporter into CSNBC-lat via in utero electroporation at E12.5. Control mice received a plasmid expressing EGFP alone (schematized in Fig. 6A). CSNBC-lat axons normally reach the caudal cervical cord by P1 and never extend past the rostral-most segments in the thoracic cord (Sahni et al., 2021a). We examined electroporated mice at P4, by which time the most distally extending CSNBC-lat axons have normally terminated within the caudal cervical or rostral-most segments of the thoracic cord.
We first confirmed that all electroporations were restricted to lateral sensorimotor cortex where CSNBC-lat reside (Fig. 8B,C). We next investigated the percentage of CSNBC-lat axons that reach the rostral thoracic cord (T1–T2) from the rostral cervical cord (C1–C2). Strikingly, when Cbln1 is misexpressed in CSNBC-lat, a sixfold higher percentage of axons reach T1–T2 from C1–C2 (T1/C1) compared with the control ( for Cbln1 misexpression, for the control; ; Fig. 8D–H). This indicates that Cbln1 misexpression aberrantly directs a higher percentage of CSNBC-lat axons to extend past their normal cervical targets into the thoracic cord by P4.
Figure 8.
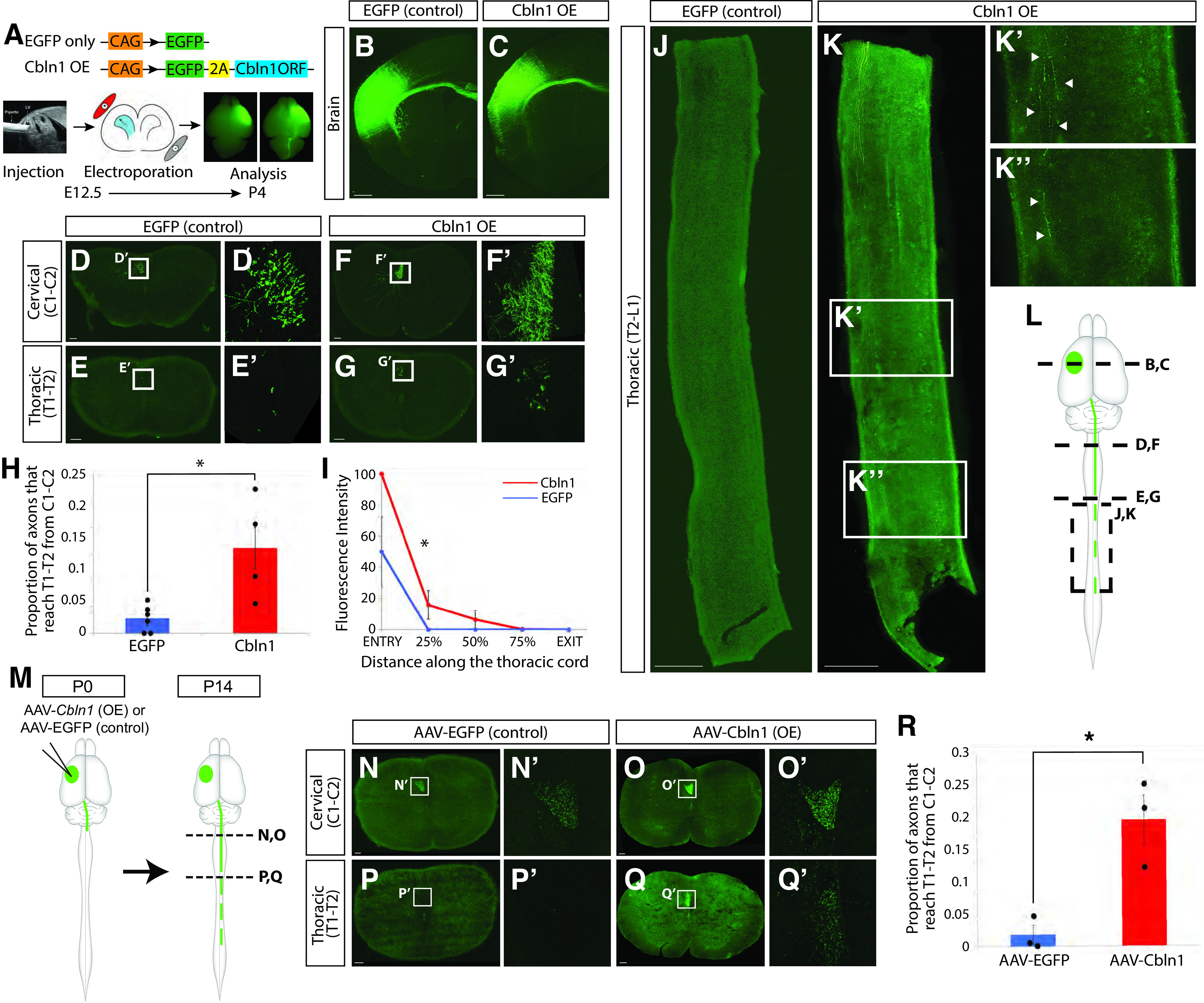
Cbln1 overexpression in CSNBC-lat is sufficient to redirect axon extension to distal thoracic spinal segments. A, Experimental outline: In one set of experiments, plasmids were designed to express either EGFP alone, or both EGFP and Cbln1 (Cbln1 OE). The constructs were delivered to developing CSNBC-lat in lateral cortex using in utero electroporation at E12.5, and tissue was collected at P4 for analysis. CSNBC-lat axons were visualized using immunocytochemistry for EGFP. B, C, Electroporation location and distribution in the brain are well-matched between EGFP (control) and Cbln1 OE mice. D–G, The number of CSNBC-lat axons at cervical C1–C2 and thoracic T1–T2 were counted in axial sections of the spinal cord in EGFP and Cbln1 OE mice. H, The proportion of individual axons that reach thoracic T1–T2 from cervical C1–C2 is significantly higher in Cbln1 OE (red, ) compared with EGFP (control; blue, ) mice ( by two-tailed Student's t test). I, CST fluorescence intensity was quantified along the rostro-caudal extent of the thoracic cord and normalized to the fluorescence intensity at thoracic T2. Significantly more Cbln1-expressing CSNBC-lat axons extend into the distal thoracic cord when compared with EGFP controls ( by two-way ANOVA with repeated measures followed by Fisher's least significant difference post hoc test). J, K, CSNBC-lat axons extend into the distal thoracic cord in thoracic sagittal sections in Cbln1 OE (arrowheads in K', K”) but not in EGFP (control) mice. L, Schematic indicating anatomic location of sections displayed in B–K. M, Experimental outline: In a second set of experiments, AAV particles engineered to express either EGFP alone (AAV-EGFP) or both EGFP and Cbln1 (AAV-Cbln1) were injected into rostrolateral sensorimotor cortex at P0 to test whether Cbln1 is sufficient to redirect axon extension by postmitotic CSNBC-lat. AAV-injected mice were then analyzed at P14. N–Q, The number of axons that reach cervical C1–C2 and thoracic T1–T2 were counted in axial sections of the spinal cord in AAV-EGFP and AAV-Cbln1 mice. R, There are significantly more axons that reach thoracic T1–T2 from cervical C1–C2 in AAV-Cbln1 (red, ) compared with AAV-EGFP (blue, ; by one-tailed Student's t test). Scale bars are 100 µm for D–G and N–Q and 500 µm for B, C, J, and K. Each data point in H and R is the axon count averaged over three axial sections per spinal level per mouse.
We next investigated whether these redirected axons from Cbln1-expressing CSNBC-lat extend further caudally toward more distal thoracic segments. As expected, in control mice, CSNBC-lat axons do not extend past the rostral-most segments of the thoracic cord (Fig. 8J). In striking contrast, not only do a significantly larger number of CSNBC-lat axons reach the rostral thoracic cord upon Cbln1 misexpression, a subset of these axons reach the distal-most segments of the thoracic cord (T13) at P4 (Fig. 8K). Quantification of CST fluorescence intensity along the rostro-caudal extent of the thoracic cord (normalized to the fluorescence intensity at thoracic T2), reveals that significantly more Cbln1-expressing CSNBC-lat axons extend into the distal thoracic cord when compared with controls (; Fig. 8I). Indeed, no axons even reached T2 in 3 of the 6 control samples (Fig. 8I). Interestingly, although axon collateralization by CSNBC-lat is well underway by P4 (Bareyre et al., 2005), we do not observe any collateralization by the aberrantly extended CSNBC-lat axons on Cbln1 misexpression in the thoracic cord (Fig. 8K). Together, these data indicate that Cbln1 is sufficient to redirect CSNBC-lat axon extension past the cervical cord toward thoracic spinal segments but does not promote axon collateralization.
Postnatal misexpression of Cbln1 in CSNBC-lat leads to aberrant long CSNBC-lat axon extension
The time course of Cbln1 expression suggests that its function is required specifically during the period of CSNTL axon extension. However, since misexpression by in utero electroporation begins in progenitors and continues into postmitotic neurons, there remained the unlikely possibility that the effect of Cbln1 misexpression on CSNBC-lat axon extension is because of alterations in early CSNBC-lat specification before their axons reach the spinal cord, ultimately causing their aberrant axon extension later at P4. To directly investigate this possibility, we performed misexpression in CSNBC-lat at P0 via AAV-mediated gene delivery. We generated AAV particles engineered to express Cbln1 and an EGFP reporter (AAV-Cbln1). Control mice received AAV particles engineered to express EGFP alone (AAV-EGFP). We injected these particles into the rostrolateral cortex of P0 mice under ultrasound guided backscatter microscopy and examined CSNBC-lat axon extension at P14 (schematized in Fig. 8M), at which point CSN axonal projections have been pruned and the adult connectivity pattern of the CST is largely established (Bareyre et al., 2005; Sahni et al., 2020, 2021a).
As with embryonic misexpression of Cbln1 in CSNBC-lat, postnatal Cbln1 misexpression leads to aberrant CSNBC-lat axon extension past the cervical cord into the thoracic spinal cord. We quantified the percentage of axons that reach thoracic T1–T2 from cervical C1–C2 in axial sections from mice injected with either AAV-EGFP or AAV-Cbln1 (Fig. 8N–R). There is a significant increase in the percentage of axons that reach thoracic T1–T2 at P14 compared with controls ( for Cbln1 misexpression, for the control; ; Fig. 8R). As with the in utero electroporation experiments, these aberrantly extended CSNBC-lat axons on Cbln1 misexpression at P0 also fail to collateralize in the thoracic cord (data not shown). Therefore, postnatal misexpression of Cbln1 at P0 is sufficient to direct CSNBC-lat axons past the cervical cord and into the thoracic cord. Together, these data combined with the results from in utero misexpression experiments indicate that Cbln1 regulates axon extension without affecting CSN axon collateralization. This is in contrast to known functions of Cbln1 as a critical synaptic organizer in the cerebellum, striatum, and other brain regions (Hirai et al., 2005; Kusnoor et al., 2010; Seigneur and Südhof, 2018), and represents a unique function of Cbln1 in controlling CSN axon extension independent of effects on axon collateralization or synapse formation.
Postnatal overexpression of Cbln1 in CSNmedial is sufficient to drive long CSN axon extension to the thoraco-lumbar spinal cord
The experiments above indicate that CSNTL axon extension to caudal thoracic and lumbar segments does not require Cbln1 function, but that Cbln1 is sufficient to redirect CSNBC-lat axons to the caudal thoracic cord. Unlike CSN in lateral sensorimotor cortex, which only project to bulbar-cervical segments (CSNBC-lat), CSNmedial include distinct subpopulations with projections to bulbar-cervical (CSNBC-med) or thoraco-lumbar segments (CSNTL). Previous work has established that CSNBC-lat, CSNBC-med, and CSNTL are distinct, molecularly delineated subpopulations that can be distinguished before CSN axons have even reached the spinal cord (schematized in Fig. 1A; Sahni et al., 2021a).
Cbln1 is specifically expressed by CSNTL and not by CSNBC-med within CSNmedial (Fig. 4). Since Cbln1 misexpression in CSNBC-lat promotes long axon extension, we next investigated whether Cbln1 is also sufficient to redirect axon extension by CSNBC-med, a molecularly and spatially distinct subpopulation from CSNBC-lat. Since CSNBC-med and CSNTL reside in a spatially interdigitated manner in caudomedial sensorimotor cortex (Sahni et al., 2021a), we overexpressed Cbln1 in medial cortex. We reasoned that if Cbln1 misexpression redirected CSNBC-med axons past the cervical cord toward caudal thoracic and lumbar segments, we would observe an increase in the proportion of axons that extend to thoracic T1–T2 from cervical C1–C2, similar to our observations with CSNBC-lat. However, if Cbln1 overexpression only had an effect in CSNTL, we might observe an increase in axon extension to lumbar L1–L2 but not an increase in axon extension to thoracic T1–T2 from cervical C1–C2.
We overexpressed Cbln1 at P0 via AAV-mediated gene delivery in medial sensorimotor cortex, where Cbln1 is normally expressed by only CSNTL. We injected the medial cortex of P0 mice with either AAV particles engineered to express Cbln1 and an EGFP reporter (AAV-Cbln1) or AAV particles engineered to express EGFP alone (AAV-EGFP). We examined CSNmedial axon extension at P14 by quantifying CST fluorescence intensity at thoracic T1–T2 and lumbar L1–L2 as a proportion of the fluorescence intensity at cervical C1–C2 in axial sections from mice injected with either AAV-Cbln1 or AAV-EGFP (schematized in Fig. 9A).
Figure 9.

Cbln1 overexpression in CSNmedial is sufficient to increase the number of axons extending past the cervical spinal cord. A, Experimental outline: AAV particles engineered to express either EGFP alone (AAV-EGFP, control) or both EGFP and Cbln1 (AAV-Cbln1) were injected into medial sensorimotor cortex at P0. AAV-injected mice were analyzed at P14. B, CST intensity was quantified in three axial sections per spinal level (cervical C1–C2, thoracic T1–T2, and lumbar L1–L2) per mouse, and the mean value across three axial sections is plotted. The proportion of axons that reach thoracic T1–T2 from cervical C1–C2 (T1/C1) is significantly higher in AAV-Cbln1 (red, ) compared with AAV-EGFP (blue, ) ( by one-tailed Student's t test). In contrast, the proportion of axons that reach lumbar L1–L2 from cervical C1–C2 is not significantly different between AAV-Cbln1 and AAV-EGFP mice ( by one-tailed Student's t test). C, We modeled the distribution of T1/C1 in control AAV-EGFP or AAV-Cbln1-injected mice as a mixture of two Gaussians. The distribution of T1/C1 in control AAV-EGFP-injected mice appears bimodal with one Gaussian centered at and the other at , likely reflecting variability in the proportion of CSNBC-med or CSNTL labeled by each injection. In contrast, the distribution of T1/C1 in AAV-Cbln1-injected mice appears unimodal with one Gaussian centered at and the other centered at . These Gaussians are similar to the Gaussian with control AAV-EGFP injections centered at 0.57, which is likely comprised of a higher proportion of CSNTL compared with CSNBC-med. This suggests that Cbln1 overexpression might specifically shift the segmental targeting of CSNBC-med past the bulbar-cervical cord into the thoraco-lumbar cord, but not overtly affect the segmental targeting of CSNTL. D–I, Representative axial sections from cervical C1–C2, thoracic T1–T2, and lumbar L1–L2 from control AAV-EGFP and AAV-Cbln1-injected mice. Scale bars are 100 µm.
Since there is no positive molecular identifier for CSNBC-med, we investigated their axon targeting as a subset of the broader CSNmedial subpopulation. Given the two-population diversity of CSNmedial, injections in medial sensorimotor cortex will label varying proportions of CSNBC-med and CSNTL, since both subpopulations reside interdigitated in medial sensorimotor cortex. Therefore, we expected these injections to result in greater variability in the proportion of labeled CSNmedial axons that reach thoracic T1–T2 from cervical C1–C2 (we refer to this proportion as T1/C1) and, as a result, analyzed a larger number of mice to account for this variability ( for AAV-Cbln1 injections; for control AAV-EGFP injections). Indeed, we observe high variance in the distribution of T1/C1 following injection with control AAV-EGFP (Fig. 9B). Importantly, the AAV-EGFP-injected mice clustered broadly into two groups, likely reflecting the predicted differences in the extent of labeling between CSNBC-med and CSNTL in each individual mouse. Therefore, we modeled the distribution of T1/C1 as a mixture of two Gaussians (Fig. 9C). The distribution of T1/C1 following the injection of control AAV-EGFP appears bimodal, with one Gaussian centered at , likely reflecting a higher proportion of CSNBC-med relative to CSNTL in these injections, and the other Gaussian at , likely reflecting a higher proportion of CSNTL relative to CSNBC-med.
Despite these differences in the relative numbers of CSNBC-med and CSNTL labeled by each injection, we investigated whether Cbln1 overexpression is sufficient to alter the T1/C1 proportion in AAV-Cbln1-injected mice. As with misexpression of Cbln1 in CSNBC-lat, we find that overexpression of Cbln1 in CSNmedial leads to long CSN axon extension past the cervical cord to thoraco-lumbar spinal segments (Fig. 9B–I). There is a significant increase in T1/C1 ( for Cbln1 overexpression, for the control; ). Intriguingly, we do not find a similar increase in the proportion of axons that reach lumbar L1–L2 from cervical C1–C2 ( for Cbln1 overexpression, for the control; ) on Cbln1 overexpression in CSNmedial.
In contrast with the T1/C1 distribution in control AAV-EGFP-injected mice, which appears bimodal, the distribution of T1/C1 following Cbln1 overexpression appears unimodal with the center of both Gaussian distributions around 0.57 (Fig. 9C). This is similar to the Gaussian distribution in the control T1/C1 distribution that is likely enriched for CSNTL. Although this analysis does not entail a priori cell identification, it suggests that Cbln1 overexpression might specifically shift the segmental targeting of CSNBC-med past the bulbar-cervical cord into the thoraco-lumbar cord, but might not substantially affect the segmental targeting of CSNTL. Together with our findings that Cbln1 misexpression in CSNBC-lat promotes axon extension past their normal cervical targets, these results indicate that Cbln1 is sufficient to drive axon extension past the cervical cord by multiple spatially and molecularly distinct CSN subpopulations.
Cbln1 is regulated by Klhl14 but acts independently of Crim1 to control CSN long axon extension
We previously identified Klhl14 and Crim1 as molecular controls expressed by CSNBC-lat and CSNTL, respectively (Sahni et al., 2021a, b). Klhl14 functions to limit CSNBC-lat axons to proximal segments in the cervical spinal cord during early postnatal development. Knock-down of Klhl14 by shRNA leads to aberrant CSNBC-lat axon extension toward distal thoracic segments at P4. This aberrant axon extension is accompanied by an upregulation of Crim1 by CSNBC-lat.
To determine whether Klhl14 might also similarly modulate Cbln1 expression, we examined coronal brain sections from mice at P4 in which Klhl14 shRNA was introduced into CSNBC-lat via in utero electroporation at E12.5 (Fig. 10A). Strikingly, Cbln1 is ectopically expressed in Layer V in rostrolateral sensorimotor cortex where Klhl14 is reduced, but not in the contralateral cortex where Klhl14 expression is normal (Fig. 10B). This strongly suggests that Cbln1 and Crim1 expression are both repressed by Klhl14 in CSNBC-lat to regulate CSN axon extension.
Figure 10.
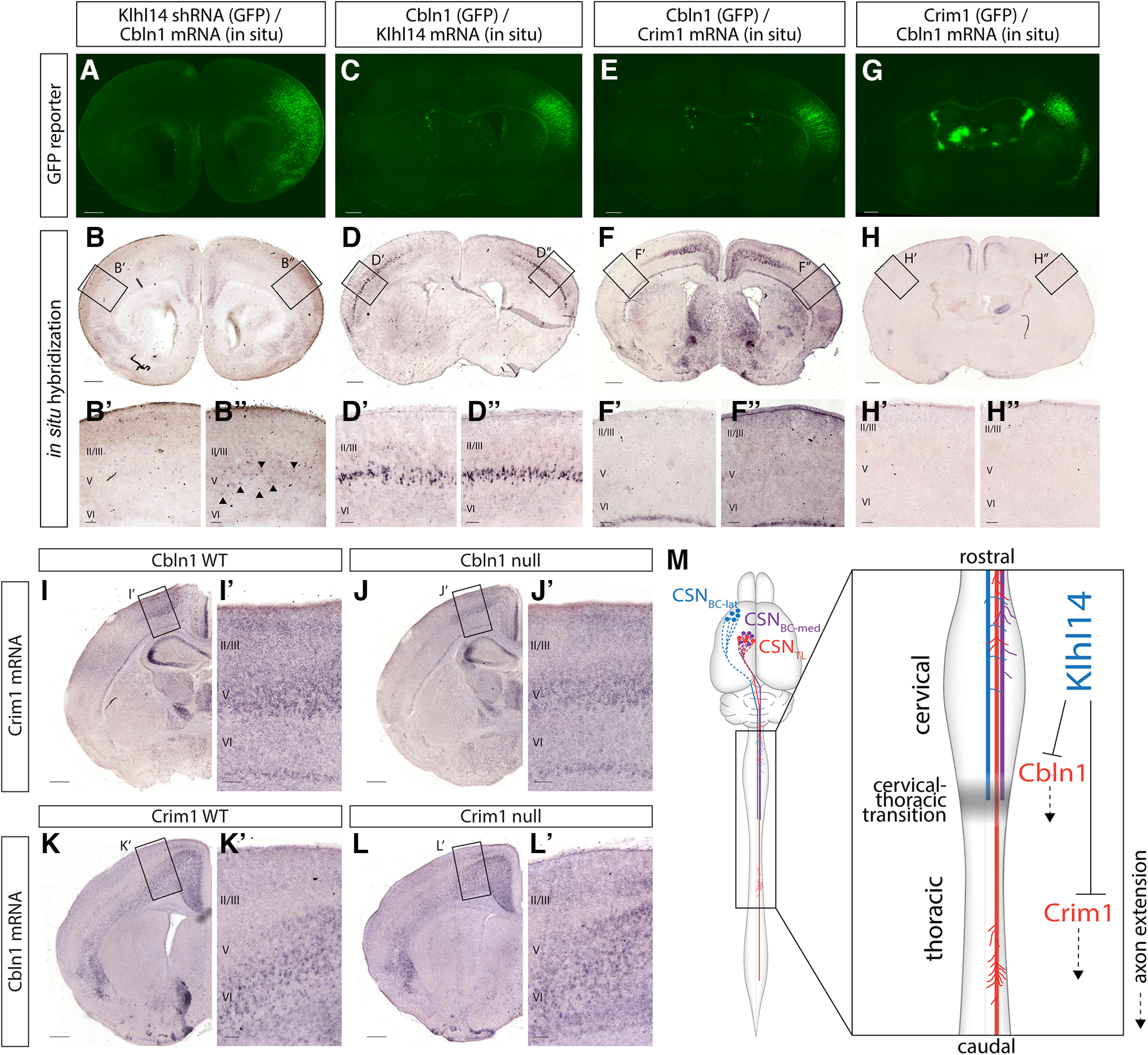
Cbln1 expression is regulated by Klhl14 but not by Crim1. A, B, Coronal section of a P4 brain that was electroporated in utero at E12.5 with Klhl14 shRNA. A, EGFP fluorescence (green) shows the site of electroporation in lateral cortex. B, In situ hybridization image of the same section in (A) shows Cbln1 expression. Cbln1 is normally restricted to medial cortex. However, Klhl14 knock-down by shRNA causes ectopic Cbln1 expression in lateral cortex (arrowheads in B”) in the electroporated cortical hemisphere (compare B” with contralateral B'). C, D, Coronal section of a P4 brain that was electroporated in utero at E12.5 with a plasmid containing Cbln1 and EGFP (Cbln1-EGFP). C, EGFP fluorescence (green) shows the site of electroporation in lateral cortex. D, In situ hybridization image of the same section in C showing Klhl14 expression in Cbln1-misexpressing CSNBC-lat remains unchanged, indicating that Klhl14 expression in lateral Layer V is unaffected by Cbln1 misexpression. E, F, Coronal section of a P4 brain that was electroporated in utero at E12.5 with Cbln1-EGFP. E, EGFP fluorescence (green) shows the site of electroporation in lateral cortex. F, In situ hybridization image of the same section in E showing that there is no ectopic Crim1 expression in Cbln1-misexpressing CSNBC-lat. G, H, Coronal section of a P4 brain that was electroporated in utero at E12.5 with Crim1-EGFP. Crim1 misexpression in CSNBC-lat can redirect axons toward caudal thoracic spinal segments (Sahni et al., 2021b). G, EGFP fluorescence (green) shows the site of electroporation in lateral cortex. H, In situ hybridization image of the same section in (G) showing that there is no ectopic Cbln1 expression in Crim1-misexpressing CSNBC-lat. I, J, Crim1 expression in medial Layer V does not differ between Cbln1 WT and Cbln1 null mice. K, L, Cbln1 expression in medial Layer V does not differ between Crim1 WT and Crim1 null mice. M, Summary schematic displaying molecular controls over CSN axon extension both at, and beyond, the transition between cervical and thoracic spinal segments. Together with the previous investigations identifying Crim1 and Klhl14 function (Sahni et al., 2021b), our data suggest a model whereby Cbln1 directs CSN axon extension from the cervical into the thoracic cord, whereas Crim1 directs those CSN axons that cross this transition zone to extend further toward caudal thoracic and lumbar spinal segments. This indicates that CSN segmental axon targeting toward thoracic and lumbar segments involves multiple, distinct molecular regulators acting at distinct spinal levels. Klhl14, which is specifically expressed in CSNBC-lat and restricts CSNBC-lat axon extension to the bulbar-cervical segments, represses the expression of both Cbln1 and Crim1 in CSNBC-lat. This indicates that Klhl14 represses a broad program of thoraco-lumbar directed axon extension in CSNBC-lat. This program, mediated by multiple independent molecular controls, would otherwise direct CSN axons past the cervical cord toward caudal thoracic and lumbar segments. Scale bars are 100 µm for insets and 500 µm for all other images.
We also examined whether Cbln1 overexpression can regulate Klhl14 expression. Coronal brain sections from mice electroporated with Cbln1 in lateral cortex at E12.5 and analyzed at P4 were examined for Klhl14 expression via in situ hybridization (Fig. 10C). There is no difference in Klhl14 expression in the electroporated versus contralateral cortex (Fig. 10D). This strongly suggests that Klhl14 acts upstream of Cbln1 to repress Cbln1 expression by CSNBC-lat.
Gain-of-function experiments show that Crim1 and Cbln1 function at distinct levels of the spinal cord. Crim1 misexpression does not increase the proportion of CSNBC-lat axons that reach thoracic T1–T2, but it does redirect the small minority of CSNBC-lat axons that reach the thoracic cord to extend farther into the thoracic cord toward caudal thoracic segments (Sahni et al., 2021b). In contrast, Cbln1 misexpression significantly increases axon extension to thoracic T1–T2 by both CSNBC-lat and CSNmedial. These data suggest that Cbln1 and Crim1 function in distinct pathways, and that Klhl14 acts as an upstream regulator of both Cbln1 and Crim1.
These results led us to investigate whether Cbln1 and Crim1 control CSN long axon extension via the same or distinct genetic pathways. We first examined Crim1 expression in sensorimotor cortex in Cbln1 WT and Cbln1 null mice. We detect no differences in Crim1 expression (Fig. 10I,J). Similarly, we detect no difference in Cbln1 expression in sensorimotor cortex between Crim1 WT and Crim1 null mice (Fig. 10K,L). We further investigated whether Cbln1 misexpression in CSNBC-lat via in utero electroporation at E12.5 might modulate Crim1 expression when analyzed at P4, and vice versa. There is no difference in Crim1 expression between Cbln1-expressing CSNBC-lat compared with the contralateral cortex (Fig. 10E,F). There is also no difference in Cbln1 expression between Crim1-expressing CSNBC-lat and the contralateral cortex (Fig. 10G,H). Together, these data indicate that Crim1 and Cbln1 act via distinct genetic pathways to control CSN axon extension.
Discussion
Previous work has identified that CSN subpopulations exhibit striking axon extension specificity during development, and that this specificity is durably maintained into maturity (Sahni et al., 2021a,b). CSN subpopulations with distinct spinal segmental targets are molecularly distinct from the earliest stages of axon extension, even before their axons reach the spinal cord. We previously investigated two molecular controls, Klhl14 and Crim1, that both prospectively identify CSN subpopulations with segmentally distinct projections, and control these projections. We identified their critical functions in directing CSN axons to appropriate spinal segmental levels, with dual-directional, complementary regulation toward thoraco-lumbar extension (by Crim1) and limiting axon extension past bulbar-cervical segments (by Klhl14). These results indicate that CSN-intrinsic molecular controls, at least in part, govern CSN axonal targeting specificity (Sahni et al., 2021a,b).
Here, we build on this work to identify a novel role for a member of the cerebellin family, Cbln1, in controlling CSN segmental axonal projection targeting. We find that within CSN, Cbln1 is expressed specifically by CSNTL in medial sensorimotor cortex. The time course of Cbln1 expression by CSN closely aligns with the period of CSNTL axon extension to thoracic and lumbar segments. Misexpression of Cbln1 in CSNBC-lat via either in utero electroporation at E12.5 or AAV injection at P0 redirects CSNBC-lat axons past their normal cervical targets to distal segments in the thoraco-lumbar cord. Similarly, Cbln1 overexpression in CSNmedial is sufficient to increase the proportion of CSNmedial axons that extend to thoracic spinal segments. These results indicate that Cbln1 can direct long axon extension by multiple CSN subpopulations residing in spatially distinct locations in sensorimotor cortex.
This represents a novel function for Cbln1 in axon extension, independent of its well-described function as a synaptic organizer. Cbln1 has been characterized extensively in the cerebellum, where it is localized and secreted at presynaptic terminals of cerebellar granule cells, and is instructive for synapse formation between Purkinje cells and the parallel fibers of granule cells (Hirai et al., 2005; Matsuda et al., 2010; Uemura et al., 2010; Ibata et al., 2019). Outside of the cerebellum, Cbln1 has also been shown to play critical roles in both synapse formation and synapse maintenance in a number of other brain regions, including the hippocampus, striatum, and the ventral tegmental area of the midbrain (Kusnoor et al., 2010; Krishnan et al., 2017; Seigneur and Südhof, 2018).
Strikingly, we find that its function in CSN is quite distinct. Cbln1 expression by CSN is strongest during the time period of axon extension, before axon collateralization or synapse formation. Moreover, although Cbln1 misexpression in CSNBC-lat redirects these axons to the thoracic cord, these redirected axons do not collateralize in the thoracic cord at either P4 or P14. This suggests that the function and mechanism of Cbln1 in axon extension occurs independent of synapse formation by CSN, raising the possibility that Cbln1 and other classical synaptic organizers might perform currently unappreciated functions in axon extension during development.
Cbln1 is normally thought to be trafficked to presynaptic terminals where it directly affects synapse formation and maintenance in multiple brain regions (Matsuda et al., 2010; Otsuka et al., 2016). It is possible that Cbln1 is similarly trafficked along CSN axons to directly regulate axon extension. Interestingly, our immunocytochemistry results identify Cbln1 protein in the nucleus of CSNTL; Cbln1 has not been previously detected in the nucleus in any other contexts. This could potentially suggest a novel nuclear function for Cbln1. For instance, Cbln1 may act as a nuclear scaffold, similar to known functions of C1q family members as scaffolds at the plasma membrane and at the synapse (Innamorati et al., 2002; Elegheert et al., 2016). We note that the Cbln1 E3 antibody used in this study detects the full-length form of Cbln1 (specifically, the globular C1q domain) and not the cleaved cerebellin peptide (Bao et al., 2005). Future investigation into the cellular localization and function of the full-length Cbln1 protein and the smaller cerebellin peptide will likely elucidate: (1) a potentially new role for Cbln1 in the nucleus; (2) whether Cbln1 or its cleaved cerebellin peptide is trafficked along CSN axons; and (3) how Cbln1 or the cerebellin peptide might function to promote long CSN axon extension.
Understanding Cbln1 localization will also be important to guide future work to identify proteins that interact with Cbln1 to mediate its function in regulating CSN axon extension. Cbln1 interactors such as GluR2 and -neurexins have been identified in the cerebellum (Matsuda et al., 2010; Uemura et al., 2010; Cheng et al., 2016; Elegheert et al., 2016), and there is evidence that, in the cerebral cortex, where GluR2 is not expressed, Cbln1 instead interacts with GluR1 (Matsuda et al., 2010; Rong et al., 2012; Wei et al., 2012; Yasumura et al., 2012). GRID1 and GRID2 (which encode GluR1 and GluR2, respectively), as well as neurexin family members, are expressed in the human spinal cord (GTEx Consortium, 2013). These interactors have not been implicated previously in axon extension; however, it is possible that they might also interact with Cbln1 to promote long axon extension in this novel context.
Although Cbln1 overexpression robustly directs long CSN axon extension, we do not observe any qualitative defects of CSNTL axon extension to thoracic and lumbar spinal segments in Cbln1 null mice. Given our limited sample size because of complexities related to the COVID-19 pandemic, it is possible that Cbln1 might have more subtle roles over CSN axon extension that might be elucidated by future studies. Nevertheless, our results suggest that Cbln1 is sufficient but not overtly necessary for CSN long axon extension. What might compensate for Cbln1 function in CSNTL in Cbln1 null mice? The other cerebellin family members, Cbln2, Cbln3, and Cbln4, are known to interact with Cbln1 and perform compensatory or redundant functions (Pang et al., 2000a; Bao et al., 2006; Iijima et al., 2007; Miura et al., 2009; Joo et al., 2011; Rong et al., 2012; Seigneur and Südhof, 2018; Seigneur et al., 2018). For instance, Cbln1 and Cbln2 are normally expressed in the cerebellum. Whereas Cbln1 null mice are ataxic and have disrupted synaptic connectivity, Cbln2 null mice display no functional or anatomic deficits in the cerebellum. Interestingly, however, Cbln2 overexpression in the cerebellum of Cbln1 null mice can rescue their ataxic phenotype, suggesting partial redundancy of function (Rong et al., 2012). Although compensation by other cerebellins presents a tempting hypothesis, we find that Cbln2, Cbln3, and Cbln4 are not normally expressed by CSNmedial at the time when their axons are normally extending to distal spinal segments. It is possible that molecular controls other than Cbln protein family members might compensate for loss of Cbln1 in CSNmedial. For instance, Cbln1 and neuroligin-3 have been shown to partially compensate for each other at calyx of Held synapses (Yuzaki, 2017; Zhang et al., 2017). Future studies will be needed to elucidate molecular mechanisms that might compensate for the loss of Cbln1 function in directing CSN axon extension.
We also investigated whether Cbln1 might regulate or be regulated by previously identified molecular controls over CSN axon extension. We previously identified that Klhl14, which is specifically expressed by CSNBC-lat and limits their axon extension to the cervical cord, acts at least in part to repress Crim1 expression by CSNBC-lat. Crim1 misexpression in CSNBC-lat is sufficient to redirect their axons to distal thoracic levels (Sahni et al., 2021b). In the work presented here, we find that Klhl14 also represses Cbln1 expression by CSNBC-lat, suggesting that Klhl14 acts as a broad transcriptional repressor to suppress multiple molecular controls that otherwise would direct CSN axons past the cervical cord. However, we find that Cbln1 and Crim1 are not in the same genetic pathway in CSN. In either Cbln1 null or Crim1 null mice, there is no change in the expression of the other molecular control. Likewise, when either Cbln1 or Crim1 is misexpressed in CSNBC-lat, the other molecular control is not ectopically expressed.
Indeed, Cbln1 and Crim1 perform similar, but distinct, functions in directing long CSN axon extension. Misexpression of Cbln1, but not Crim1 (Sahni et al., 2021b), in CSNBC-lat increases the proportion of axons that reach thoracic T1–T2. Further, although both Cbln1 and Crim1 misexpression in CSNBC-lat is sufficient to redirect those axons that reach T1–T2 to extend further into the thoracic cord, their effects on axon extension within the thoracic cord are distinct. While 100% of mice in which Crim1 was misexpressed in CSNBC-lat had axons that reached at least halfway through the thoracic cord (Sahni et al., 2021b), this was true of only 50% of mice in which Cbln1 was misexpressed in lateral cortex. This suggests that Cbln1 might serve as a regulator of axon targeting at the transition between cervical and thoracic spinal segments, whereas Crim1 primarily functions to drive axon extension distal to this transition (schematized in Fig. 10M).
Additionally, CSNBC-lat axons that aberrantly extend in the thoracic cord upon either Cbln1 or Crim1 misexpression fail to collateralize in the thoracic spinal gray matter. It is likely that distinct molecular controls are required to interact with extracellular cues specific to the thoraco-lumbar spinal cord to promote axon collateralization. This is consistent with previous indications that axon extension and collateralization are distinctly regulated processes (Kalil and Dent, 2014; Itoh et al., 2021).
Interestingly and potentially relevant, motor neuron diseases (MNDs) such as amyotrophic lateral sclerosis (ALS) and hereditary spastic paraplegia (HSP) do not affect all CSN equally (Bruijn et al., 2004; Strong and Gordon, 2005; Salinas et al., 2008). In bulbar forms of ALS, e.g., brainstem-projecting CSN are affected, while in HSP, lumbar-projecting CSN preferentially degenerate. Although multiple proteins including SOD1 and TDP-43 have been implicated in MND (Hardiman et al., 2017), it remains unclear why certain CSN subpopulations preferentially degenerate in distinct MND subtypes. Identification and characterization of molecular controls that govern axonal development and connectivity of specific CSN subpopulations might provide insight regarding molecular mechanisms underlying preferential vulnerability of specific CSN subpopulations to degeneration.
Finally, our results suggest that Cbln1 might be a relevant molecular control for potential application in spinal cord repair and/or regeneration, and might elucidate broader organizing principles for establishing diverse connectivity by other neocortical projection neuron subtypes. Reactivating developmental controls to regulate CSN axon extension and sprouting might offer a promising approach to reestablish with some specificity damaged connectivity following spinal cord injury. More broadly, identification of molecular controls over development of anatomically and functionally diverse CSN subpopulations might elucidate fundamental principles of evolutionary diversification within originally more homogeneous neuronal populations and circuitry, while also offering potentially novel avenues for regeneration and/or repair of diseased and/or damaged neocortical or other nervous system circuitry.
Footnotes
This work was supported by the National Institutes of Health (NIH) Grant R01 NS045523, the Emily and Robert Pearlstein Fund, and a grant from the Travis Roy Foundation (J.D.M.), the NIH Grant NTRAIN/NICHD K12HD093427, grants from the Wings for Life-Spinal Cord Injury Foundation and the Craig Neilsen Foundation, as well as additional infrastructure support from the Burke Foundation (V.S.). C.R. was partially supported by a postdoctoral fellowship from the Spinal Cord Injury Trust Fund through New York State Department of Health (SCIRB C33613GG). Additional infrastructure support from NIH Grants DP1 NS106665 and NS075672, Max and Anne Wien Professor of Life Sciences Fund, and the DEARS Foundation (J.D.M.). We thank Julia Kaiser for designing the schematics first shown in Figure 1A; James Morgan at St. Jude's Research Hospital for generously sharing the Cbln1 E3 antibody and Cbln1 null mice; and members of the Macklis and Sahni Labs for useful discussions and comments on the manuscript.
The authors declare no competing financial interests.
References
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD (2005) Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45:207–221. 10.1016/j.neuron.2004.12.036 [DOI] [PubMed] [Google Scholar]
- Bao D, Pang Z, Morgan JI (2005) The structure and proteolytic processing of Cbln1 complexes. J Neurochem 95:618–629. [DOI] [PubMed] [Google Scholar]
- Bao D, Pang Z, Morgan MA, Parris J, Rong Y, Li L, Morgan JI (2006) Cbln1 is essential for interaction-dependent secretion of Cbln3. Mol Cell Biol 26:9327–9337. 10.1128/MCB.01161-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR (2005) Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med 11:1355–1360. 10.1038/nm1331 [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 27:723–749. 10.1146/annurev.neuro.27.070203.144244 [DOI] [PubMed] [Google Scholar]
- Chen B, Schaevitz LR, McConnell SK (2005) Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci U S A 102:17184–17189. 10.1073/pnas.0508732102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK (2008) The Fezf2–Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc Natl Acad Sci U S A 105:11382–11387. 10.1073/pnas.0804918105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Seven AB, Wang J, Skiniotis G, Özkan E (2016) Conformational plasticity in the transsynaptic neurexin-cerebellin-glutamate receptor adhesion complex. Structure 24:2163–2173. 10.1016/j.str.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz JL, Siththanandan VB, Lu V, Gonzalez-Nava N, Pasquina L, MacDonald JL, Woodworth MB, Ozkan A, Nair R, He Z, Sahni V, Sarnow P, Palmer TD, Macklis JD, Tharin S (2020) An evolutionarily acquired microRNA shapes development of mammalian cortical projections. Proc Natl Acad Sci U S A 117:29113–29122. 10.1073/pnas.2006700117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elegheert J, Kakegawa W, Clay JE, Shanks NF, Behiels E, Matsuda K, Kohda K, Miura E, Rossmann M, Mitakidis N, Motohashi J, Chang VT, Siebold C, Greger IH, Nakagawa T, Yuzaki M, Aricescu AR (2016) Structural basis for integration of GluD receptors within synaptic organizer complexes. Science 353:295–299. 10.1126/science.aae0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai R, Waters P, Roumenina LT, Gadjeva M, Kojouharova MS, Reid KBM, Sim RB, Kishore U (2007) C1q and its growing family. Immunobiology 212:253–266. 10.1016/j.imbio.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Greig LC, Woodworth MB, Greppi C, Macklis JD (2016) Ctip1 controls acquisition of sensory area identity and establishment of sensory input fields in the developing neocortex. Neuron 90:261–277. 10.1016/j.neuron.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GTEx Consortium (2013) The Genotype-Tissue Expression (GTEx) project. Nat Genet 45:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Kwan KY, Shim S, Lam MMS, Shin Y, Xu X, Zhu Y, Li M, Sestan N (2011) TBR1 directly represses Fezf2 to control the laminar origin and development of the corticospinal tract. Proc Natl Acad Sci U S A 108:3041–3046. 10.1073/pnas.1016723108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z, van den Berg LH (2017) Amyotrophic lateral sclerosis. Nat Rev Dis Primers 3:17071. 10.1038/nrdp.2017.71 [DOI] [PubMed] [Google Scholar]
- Hirai H, Pang Z, Bao D, Miyazaki T, Li L, Miura E, Parris J, Rong Y, Watanabe M, Yuzaki M, Morgan JI (2005) Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat Neurosci 8:1534–1541. 10.1038/nn1576 [DOI] [PubMed] [Google Scholar]
- Hirata T, Suda Y, Nakao K, Narimatsu M, Hirano T, Hibi M (2004) Zinc finger gene fez-like functions in the formation of subplate neurons and thalamocortical axons. Dev Dyn 230:546–556. 10.1002/dvdy.20068 [DOI] [PubMed] [Google Scholar]
- Ibata K, Kono M, Narumi S, Motohashi J, Kakegawa W, Kohda K, Yuzaki M (2019) Activity-dependent secretion of synaptic organizer Cbln1 from lysosomes in granule cell axons. Neuron 102:1184–1198.e10. 10.1016/j.neuron.2019.03.044 [DOI] [PubMed] [Google Scholar]
- Iijima T, Miura E, Matsuda K, Kamekawa Y, Watanabe M, Yuzaki M (2007) Characterization of a transneuronal cytokine family Cbln–regulation of secretion by heteromeric assembly. Eur J Neurosci 25:1049–1057. 10.1111/j.1460-9568.2007.05361.x [DOI] [PubMed] [Google Scholar]
- Innamorati G, Whang MI, Molteni R, Le Gouill C, Birnbaumer M (2002) GIP, a G-protein-coupled receptor interacting protein. Regul Pept 109:173–179. 10.1016/S0167-0115(02)00201-X [DOI] [PubMed] [Google Scholar]
- Itoh Y, Sahni V, Shnider SJ, Macklis JD (2021) Lumican regulates cervical corticospinal axon collateralization via non-autonomous crosstalk between distinct corticospinal neuron subpopulations. bioRxiv 437104. 10.1101/2021.03.26.437104. [DOI] [Google Scholar]
- Joo JY, Lee SJ, Uemura T, Yoshida T, Yasumura M, Watanabe M, Mishina M (2011) Differential interactions of cerebellin precursor protein (Cbln) subtypes and neurexin variants for synapse formation of cortical neurons. Biochem Biophys Res Commun 406:627–632. [DOI] [PubMed] [Google Scholar]
- Joshi PS, Molyneaux BJ, Feng L, Xie X, Macklis JD, Gan L (2008) Bhlhb5 regulates the postmitotic acquisition of area identities in layers ii-v of the developing neocortex. Neuron 60:258–272. 10.1016/j.neuron.2008.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K, Dent EW (2014) Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nat Rev Neurosci 15:7–18. 10.1038/nrn3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama T, Kameda H, Murabe N, Fukuda S, Yoshioka N, Mizukami H, Ozawa K, Sakurai M (2015) Corticospinal tract development and spinal cord innervation differ between cervical and lumbar targets. J Neurosci 35:1181–1191. 10.1523/JNEUROSCI.2842-13.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Stoppel DC, Nong Y, Johnson MA, Nadler MJ, Ozkaynak E, Teng BL, Nagakura I, Mohammad F, Silva MA, Peterson S, Cruz TJ, Kasper EM, Arnaout R, Anderson MP (2017) Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1. Nature 543:507–512. 10.1038/nature21678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnoor SV, Parris J, Muly EC, Morgan JI, Deutch AY (2010) Extracerebellar role for Cerebellin1: modulation of dendritic spine density and synapses in striatal medium spiny neurons. J Comp Neurol 518:2525–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Lam MMS, Krsnik Z, Kawasawa YI, Lefebvre V, Šestan N (2008) SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Natl Acad Sci U S A 105:16021–16026. 10.1073/pnas.0806791105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JRL, Macklis JD (2008) SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron 57:232–247. 10.1016/j.neuron.2007.12.023 [DOI] [PubMed] [Google Scholar]
- Lodato S, Molyneaux BJ, Zuccaro E, Goff LA, Chen HH, Yuan W, Meleski A, Takahashi E, Mahony S, Rinn JL, Gifford DK, Arlotta P (2014) Gene co-regulation by Fezf2 selects neurotransmitter identity and connectivity of corticospinal neurons. Nat Neurosci 17:1046–1054. 10.1038/nn.3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, Fukazawa Y, Ito-Ishida A, Kondo T, Shigemoto R, Watanabe M, Yuzaki M (2010) Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science 328:363–368. 10.1126/science.1185152 [DOI] [PubMed] [Google Scholar]
- McKenna WL, Betancourt J, Larkin KA, Abrams B, Guo C, Rubenstein JLR, Chen B (2011) Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. J Neurosci 31:549–564. 10.1523/JNEUROSCI.4131-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura E, Iijima T, Yuzaki M, Watanabe M (2006) Distinct expression of Cbln family mRNAs in developing and adult mouse brains. Eur J Neurosci 24:750–760. 10.1111/j.1460-9568.2006.04950.x [DOI] [PubMed] [Google Scholar]
- Miura E, Matsuda K, Morgan JI, Yuzaki M, Watanabe M (2009) Cbln1 accumulates and colocalizes with Cbln3 and GluRdelta2 at parallel fiber-Purkinje cell synapses in the mouse cerebellum. Eur J Neurosci 29:693–706. 10.1111/j.1460-9568.2009.06632.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD (2005) Fezl is required for the birth and specification of corticospinal motor neurons. Neuron 47:817–831. 10.1016/j.neuron.2005.08.030 [DOI] [PubMed] [Google Scholar]
- Muralidharan B, Khatri Z, Maheshwari U, Gupta R, Roy B, Pradhan SJ, Karmodiya K, Padmanabhan H, Shetty AS, Balaji C, Kolthur-Seetharam U, Macklis JD, Galande S, Tole S (2017) LHX2 interacts with the NuRD complex and regulates cortical neuron subtype determinants Fezf2 and Sox11. J Neurosci 37:194–203. 10.1523/JNEUROSCI.2836-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka S, Konno K, Abe M, Motohashi J, Kohda K, Sakimura K, Watanabe M, Yuzaki M (2016) Roles of Cbln1 in non-motor functions of mice. J Neurosci 36:11801–11816. 10.1523/JNEUROSCI.0322-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z, Zuo J, Morgan JI (2000a) Cbln3, a novel member of the precerebellin family that binds specifically to Cbln1. J Neurosci 20:6333–6339. 10.1523/JNEUROSCI.20-17-06333.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z, Zuo J, Morgan JI (2000b) IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. J Neurosci 9:6333–6339. [DOI] [PubMed] [Google Scholar]
- Rong Y, Wei P, Parris J, Guo H, Pattarini R, Correia K, Li L, Kusnoor SV, Deutch AY, Morgan JI (2012) Comparison of Cbln1 and Cbln2 functions using transgenic and knockout mice. J Neurochem 120:528–540. 10.1111/j.1471-4159.2011.07604.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni V, Engmann A, Ozkan A, Macklis JD (2020) Motor cortex connections, Ed 2. Amsterdam: Elsevier. [Google Scholar]
- Sahni V, Shnider SJ, Jabaudon D, Song JHT, Itoh Y, Greig LC, Macklis JD (2021a) Corticospinal neuron subpopulation-specific developmental genes prospectively indicate mature segmentally specific axon projection targeting. Cell Rep 37:109843. 10.1016/j.celrep.2021.109843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni V, Itoh Y, Shnider SJ, Macklis JD (2021b) Crim1 and Kelch-like 14 exert complementary dual-directional developmental control over segmentally specific corticospinal axon projection targeting. Cell Rep 37:109842. 10.1016/j.celrep.2021.109842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas S, Proukakis C, Crosby A, Warner TT (2008) Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. Lancet Neurol 7:1127–1138. 10.1016/S1474-4422(08)70258-8 [DOI] [PubMed] [Google Scholar]
- Seigneur E, Südhof TC (2017) Cerebellins are differentially expressed in selective subsets of neurons throughout the brain. J Comp Neurol 525:3286–3311. 10.1002/cne.24278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneur E, Südhof TC (2018) Genetic ablation of all cerebellins reveals synapse organizer functions in multiple regions throughout the brain. J Neurosci 38:4774–4790. 10.1523/JNEUROSCI.0360-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneur E, Polepalli JS, Südhof TC (2018) Cbln2 and Cbln4 are expressed in distinct medial habenula-interpeduncular projections and contribute to different behavioral outputs. Proc Natl Acad Sci U S A 115:E10235–E10244. 10.1073/pnas.1811086115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S, Kwan KY, Li M, Lefebvre V, Sestan N (2012) Cis-regulatory control of corticospinal system development and evolution. Nature 486:74–79. 10.1038/nature11094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131:1164–1178. 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]
- Strong MJ, Gordon PH (2005) Primary lateral sclerosis, hereditary spastic paraplegia and amyotrophic lateral sclerosis: discrete entities or spectrum? Amyotroph Lateral Scler Other Motor Neuron Disord 6:8–16. 10.1080/14660820410021267 [DOI] [PubMed] [Google Scholar]
- Takeo YH, Shuster SA, Jiang L, Hu MC, Luginbuhl DJ, Rülicke T, Contreras X, Hippenmeyer S, Wagner MJ, Ganguli S, Luo L (2021) GluD2- and Cbln1-mediated competitive interactions shape the dendritic arbors of cerebellar Purkinje cells. Neuron 109:629–644.e8. 10.1016/j.neuron.2020.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantirigama MLS, Oswald MJ, Duynstee C, Hughes SM, Empson RM (2014) Expression of the developmental transcription factor Fezf2 identifies a distinct subpopulation of layer 5 intratelencephalic-projection neurons in mature mouse motor cortex. J Neurosci 34:4303–4308. 10.1523/JNEUROSCI.3111-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantirigama ML, Oswald MJ, Clare AJ, Wicky HE, Day RC, Hughes SM, Empson RM (2016) Fezf2 expression in layer 5 projection neurons of mature mouse motor cortex. J Comp Neurol 524:829–845. [DOI] [PubMed] [Google Scholar]
- Tomassy GS, Leonibus ED, Jabaudon D, Lodato S, Alfano C, Mele A, Macklis JD, Studer M (2010) Area-specific temporal control of corticospinal motor neuron differentiation by COUP-TFI. Proc Natl Acad Sci U S A 107:3576–3581. 10.1073/pnas.0911792107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Lee S-J, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, Mishina M (2010) Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell 141:1068–1079. 10.1016/j.cell.2010.04.035 [DOI] [PubMed] [Google Scholar]
- Wei P, Smeyne RJ, Bao D, Parris J, Morgan JI (2007) Mapping of Cbln1-like immunoreactivity in adult and developing mouse brain and its localization to the endolysosomal compartment of neurons. Eur J Neurosci 26:2962–2978. 10.1111/j.1460-9568.2007.05913.x [DOI] [PubMed] [Google Scholar]
- Wei P, Pattarini R, Rong Y, Guo H, Bansal PK, Kusnoor SV, Deutch AY, Parris J, Morgan JI (2012) The Cbln family of proteins interact with multiple signaling pathways. J Neurochem 121:717–729. 10.1111/j.1471-4159.2012.07648.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumura M, Yoshida T, Lee S-J, Uemura T, Joo J-Y, Mishina M (2012) Glutamate receptor 1 induces preferentially inhibitory presynaptic differentiation of cortical neurons by interacting with neurexins through cerebellin precursor protein subtypes. J Neurochem 121:705–716. [DOI] [PubMed] [Google Scholar]
- Yuzaki M (2011) Cbln1 and its family proteins in synapse formation and maintenance. Curr Opin Neurobiol 21:215–220. 10.1016/j.conb.2011.01.010 [DOI] [PubMed] [Google Scholar]
- Yuzaki M (2017) The C1q complement family of synaptic organizers: not just complementary. Curr Opin Neurobiol 45:9–15. 10.1016/j.conb.2017.02.002 [DOI] [PubMed] [Google Scholar]
- Zhang B, Seigneur E, Wei P, Gokce O, Morgan J, Südhof TC (2017) Developmental plasticity shapes synaptic phenotypes of autism-associated neuroligin-3 mutations in the calyx of Held. Mol Psychiatry 22:1483–1491. 10.1038/mp.2016.157 [DOI] [PMC free article] [PubMed] [Google Scholar]