Keywords: early life adversity, exercise intervention, heart disease, hemodynamic, trauma
Abstract
Adverse childhood experiences (ACEs) are early-life psychosocial stressors that are associated with poorer mental health and increased cardiovascular disease (CVD) risk in a dose-dependent manner. We examined the feasibility of an 8-wk combined aerobic and resistance exercise training program to improve systolic (SBP) and diastolic blood pressure (DBP), serum endothelin-1 (ET-1), resilience, hope agency, and hope pathways in young women with ACEs. Forty-two healthy women (21 ± 3 yr) with ≥4 (ACE+; n = 28) or 0 ACEs (ACE−; n = 14) participated in this study. Women with ACEs were randomly assigned to an exercise (ACE+EXT; n = 14) or nonexercise control (ACE+CON; n = 14) group, whereas all ACE− participants were assigned to a nonexercise control (n = 14) group. Hope agency and DBP did not change in any group (P ≥ 0.43), but hope pathways improved only in ACE+EXT (means ± SE change; +1.6 ± 0.74 au, P = 0.032, Hedges’ g = 0.53). ET-1 decreased in ACE+EXT only (−0.31 ± 0.15 pg/mL, P = 0.043, g = 0.46). Although the interactions for resilience and SBP did not reach significance (P = 0.05–0.06), forced post hoc analyses indicated that resilience improved (+4.9 ± 1.9 au, P = 0.012, g = 0.64) and SBP tended to improve (−4.0 ± 2.0 mmHg, P = 0.053, g = 0.51) in ACE+EXT only. There were significant associations between changes in hope pathways and SBP (ρ = −0.43, P = 0.023) and ET-1 (ρ = −0.53, P = 0.005), and between changes in SBP and ET-1 (ρ = 0.49; P = 0.012) in the ACE+ group. In summary, structured exercise training reduces serum ET-1 levels, improves positive psychological coping, and may improve SBP in young women with ACEs. The relationships among the changes in hope pathways, SBP, and ET-1 suggest a cardiovascular psychophysiological relationship in young women with ACEs.
NEW & NOTEWORTHY This randomized controlled pilot trial shows, for the first time, that 8 wk of structured, progressive exercise training lowers serum endothelin-1 (ET-1) and improves positive psychological coping in young women with significant early-life psychosocial stress. Furthermore, the observed associations among changes in psychological attributes, ET-1, and systolic blood pressure signify a potential interplay between positive psychology and cardiovascular disease risk among women with adverse childhood experiences.
INTRODUCTION
Adverse childhood experiences (ACEs) are psychosocial stressors such as physical, sexual, and emotional abuse; neglect; and interpersonal and household dysfunction that occur during childhood and adolescence (1, 2). Recent prevalence data indicate that ∼60% of Americans have been exposed to at least one ACE, and approximately one in every six Americans has been exposed to four or more ACEs (3, 4). Several epidemiological and cohort studies have shown that ACEs increase cardiovascular disease (CVD) risk in a dose-dependent manner (5–7). For example, in the Centers for Disease Control and Prevention (CDC) ACE study, Felitti et al. (6) reported a graded association between ACE exposure and risk of ischemic heart disease in adults, such that individuals with exposure to four or more ACEs display a 2.2-fold increase in risk compared with those with no ACEs. These findings have since been replicated and extended in additional cohorts and to other CVDs such as myocardial infarction and stroke (7–14). Although ACEs have increasingly been recognized as a risk factor for CVD, they remain understudied relative to the potent detrimental impact they have on cardiovascular health.
Although it has been proposed that lifestyle factors may mediate the association of ACEs with CVD risk, a growing body of evidence suggests that this relationship is largely independent of traditional lifestyle factors (15). Instead, evidence indicates that the association between ACE exposure and CVD is mediated more strongly by psychological risk factors than by traditional lifestyle risk factors such as smoking, inactivity, and obesity (7), suggesting an important psychophysiological connection between psychological attributes and cardiovascular health in those with ACEs. Notably, ACE exposure is strongly associated with an increased risk of psychopathology (16, 17) and has been linked with lower positive psychological coping such as hope and resilience. Hope assesses an individual’s own perception of their ability to develop strategies to achieve a goal (e.g., hope pathways) and to direct the mental energy required to pursue the goal (e.g., hope agency; 18, 19). Accordingly, hope is recognized as one of the most salient attributes in positive psychology and is considered as a positive psychological strength that promotes well-being and healthy, adaptive behaviors (20, 21). In the only prior study that has examined the association of ACEs with hope, to our knowledge, Baxter et al. (20) reported that caregivers with prior ACE exposure scored lower on hope than caregivers who had no prior exposure to ACEs. Similar to hope, ACE exposure has been linked to lower resilience (22, 23), an attribute that describes the capacity to negotiate, manage, and adapt to stress or trauma (24, 25). Furthermore, low resilience in early life has been linked with an increased risk of hypertension in adulthood (26). Thus, there is a need to identify effective interventions to improve positive psychological coping in individuals with ACEs at young ages, which could also promote improvements in cardiovascular health (22).
Hypertension is a major modifiable risk factor for CVD, and the association of blood pressure (BP) with CVD risk is continuous, even in the normotensive range. Age-related increases in BP normally occur across the lifespan, with the most dramatic increases occurring near or during middle adulthood (27). However, Su et al. (28) reported that individuals with a history of ACEs display earlier and greater increases in BP beginning during the third decade of life when compared with those without exposure to ACEs. As with CVD risk, these associations are also dose dependent, such that individuals with exposure to four or more ACEs have the greatest increases in BP, and this association is largely independent of socioeconomic status, sex, body mass index (BMI), or risky lifestyle behaviors (28). Endothelin-1 (ET-1), a peptide produced by the vascular endothelium with potent vasoconstrictive effects, has been shown to be responsive to psychosocial stress (29, 30), mediates BP increases to acute stress (30), and is elevated in the presence of psychosocial risk factors (31). Therefore, alterations in the ET-1 pathway, such as elevations in ET-1 production, may be a mechanism through which ACEs increase BP in adulthood, a hypothesis that is supported by initial evidence in both preclinical models and humans (32, 33). As such, BP represents an important modifiable risk factor by which ACE exposure promotes CVD, perhaps by increasing circulating ET-1.
Together, these data suggest that there is an interplay between positive psychological coping, ET-1, and BP, which may promote CVD risk in individuals with ACEs. Furthermore, due to the temporal development of elevated BP in individuals with ACEs, interventions deployed during periods of life adjacent to the exposure, but before overt elevations in BP (e.g., early young adulthood), may be particularly effective for reducing future CVD risk in this population. Given that exercise is recommended as a first-line treatment for elevated BP (34) and the evidence that it has a host of psychological benefits (35), we hypothesized that exercise training would be a novel intervention to improve cardiovascular and psychophysiological outcomes in young adult women with a history of moderate-to-severe ACE exposure. In this study, we chose to study only women because more women have been exposed to ACEs than men and because women are more likely to experience adverse psychological outcomes following ACE exposure (36, 37). Therefore, we examined the feasibility of 8 wk of progressive, combined aerobic interval and resistance exercise training for improving psychological hope and resilience, BP, and ET-1 in young adult women with moderate-to-severe ACE exposure. We also examined the associations of changes in hope, resilience, BP, and ET-1 to understand whether improvements in positive psychological attributes are related to improvements in cardiovascular biomarkers among young women with ACEs.
METHODS
Participants
Forty-two otherwise physically healthy, untrained, young adult females volunteered for and completed this trial. Before participating, all volunteers were screened and completed written informed consent and a health history questionnaire, which asked about disease status, prescription medication, and family history. To be eligible, participants must have been a female between the ages of 18 and 29 yr, answered “no” to all questions on a Physical Activity Readiness Questionnaire for people aged 15–69 yr, had a BMI of 18.5–40 kg/m2, and had an ACE score of either 0 or ≥4. An ACE score of ≥4 was chosen because the original CDC-ACE study indicated that individuals with exposure to four or more ACEs have the greatest risk for CVD and depression (6). Exclusion criteria included achieving the 2018 Physical Activity Guidelines (38) or having participated in structured exercise training for 6 mo before enrollment; having been diagnosed with chronic cardiovascular, renal, metabolic, pulmonary, or musculoskeletal disease according to their responses on the health history questionnaire; having been prescribed or were taking anti-inflammatory (including habitual NSAID use), antioxidant, or lipid-lowering medications at the time of enrollment; or having been enrolled in another clinical trial within 30 days of enrollment. Table 1 contains baseline characteristics for the participants stratified by group. The study procedures were approved by the university’s Institutional Review Board (IRB) in accordance with the Declaration of Helsinki (IRB Approval No. ED-17–157, approved February 2nd, 2018).
Table 1.
Baseline characteristics at preintervention for the ACE+EXT, ACE+CON, and ACE− groups
| n | ACE+EXT |
ACE+CON |
ACE− |
P Value | |
|---|---|---|---|---|---|
| (n = 14) | (n = 14) | (n = 14) | |||
| ACE score | 42 | 6 ± 1* | 6 ± 1* | 0 ± 0 | <0.001 |
| Age, yr | 42 | 21 ± 3 | 20 ± 2 | 21 ± 3 | 0.46 |
| Bodyweight, kg | 42 | 75 ± 18 | 66 ± 7 | 74 ± 17 | 0.28 |
| BMI, kg/m2 | 42 | 28 ± 6 | 24 ± 3 | 26 ± 6 | 0.23 |
| Race, n, % | 42 | 0.26 | |||
| White | 10 (71%) | 13 (93%) | 10 (71%) | ||
| Black | 1 (7%) | 1 (7%) | 0 (0%) | ||
| Other | 3 (21%) | 0 (0%) | 4 (29%) | ||
| PA, MET·min/wk | 41 | 449 ± 603 | 340 ± 217 | 405 ± 383 | 0.80 |
| kcal/day, kcal | 41 | 1,673 ± 619 | 1,525 ± 547 | 1,523 ± 533 | 0.74 |
| Resting HR, beats/min | 39 | 73 ± 13 | 72 ± 12 | 77 ± 13 | 0.53 |
| CRP, mg/L | 40 | 2.04 ± 2.4 | 1.85 ± 2.4 | 1.7 ± 2.1 | 0.93 |
Values are means ± SD; bold P values indicate significance (P ≤ 0.05) from the one-way ANOVA or χ2 test. ACE, adverse childhood experiences; ACE−, ACE− control group; ACE+CON, ACE+ control group; ACE+EXT, ACE+ exercise training group; BMI, body mass index; CRP, C-reactive protein; HR, heart rate; kcal, kilocalories; MET·min/wk, metabolic equivalent minutes per week; PA, physical activity.
*Significantly different to ACE−.
Experimental Design
Using a repeated-measures study design, participants who were eligible and who reported four or more ACEs (ACE+; n = 28) were randomly assigned to either an ACE+ exercise (ACE+EXT; n = 14) group or an ACE+ nonexercise control group (ACE+CON; n = 14). Randomization was performed using a freely available, online research randomizer (randomizer.org). Those eligible who reported 0 ACEs were assigned to a nonexercise ACE−control group (ACE−; n = 14). All participants completed pre- and posttesting during which body weight, hope agency, hope pathways, resilience, heart rate (HR), BP, serum ET-1, and C-reactive protein (CRP) were measured. Pre- and posttesting visits were separated by an 8-wk intervention period, during which participants in ACE− and ACE+CON group were asked to maintain baseline physical activity levels, and ACE+EXT completed an 8-wk exercise training program. Baseline testing was completed during the early follicular phase of the menstrual cycle. To accomplish this, participants were initially scheduled for their baseline visit based on their estimated cycle start date. Before arrival at the laboratory, participants confirmed whether their cycle had begun and their visit was confirmed or rescheduled, as appropriate. All participants began exercise training within 7 days of testing, and posttesting was completed 48–96 h after completing the exercise training intervention. The menstrual cycle phase was not assessed at posttesting. Maintenance of baseline physical activity levels among the ACE− and ACE+CON groups was confirmed via International Physical Activity Questionnaire (IPAQ). All groups were asked to maintain their typical diet throughout the intervention period, which was confirmed via analysis of dietary food logs. This pilot trial was preregistered on clinicaltrials.gov (NCT03521401), and data from this study used to test a different hypothesis have been published elsewhere (1). Of note, the outcomes measured here were primarily secondary outcomes. The assessment of ET-1 was not originally planned but was added during analyses of blood samples based on the emerging evidence, suggesting a role for ET-1 in psychosocial stress-related CVD risk.
Adverse Childhood Experiences
The ACE questionnaire (6) was used to determine the degree of ACE exposure. The questionnaire contained 10 detailed questions regarding exposure to childhood abuse (physical, emotional, or sexual), neglect (e.g., feeling unloved, not having enough to eat, not having clean clothes, not having protection), and household dysfunction (e.g., substance abuse, mental illness, domestic violence, incarceration of a household member, parental marital discord, or death) occurring before the age of 18 yr. Each exposure to a specific category of childhood adversity was coded as an ACE. The composite ACE score was then used to indicate the degree of exposure to ACEs.
Exercise Intervention
Women in ACE+EXT completed 8 wk of supervised exercise training, which we have described previously (1, 39). In brief, the intervention consisted of 2 days per week of resistance exercise and 2 days per week of aerobic interval training. All exercise sessions were supervised by a research team member, who provided feedback related to the exercise form and tracked exercise performance. The resistance exercise program provided a full-body stimulus and included the following nine exercises: bench press, latissimus pulldown, lateral raise, seated row, leg press, leg extension, leg curl, biceps curl, and triceps extension. On weeks 1–4, participants performed 2 sets of 15 repetitions for each exercise at ∼60% of their one repetition maximum (1RM). During weeks 5–8, they performed 3 sets of 12 repetitions at ∼70% of their 1RM. The program was progressive in nature whereby the load was increased by 5%–10% for the following session if the participant was able to perform two or more additional repetitions on their last set in two consecutive training sessions. During the aerobic interval sessions, participants cycled at a low intensity for 5 min to warm up, and then completed 10 separate, 30-s intervals with a 90-s active recovery period between each interval on an air bike with a belt-driven steel fan (Rogue Echo Bike, Rogue Fitness, Columbus, OH). Participants’ ratings of perceived exertion (RPE; 40) were used to determine the intensity for the interval and the recovery periods, which were set at an RPE of 7 and 3 out of 10, respectively. After completing the last interval, participants completed a 5-min cooldown. Both the warm-up and cooldown were also performed at an RPE of 3 out of 10. All ACE+EXT participants were encouraged to improve their exercise performance throughout the exercise training period. In addition, all participants were asked to refrain from engaging in any exercise training outside of the confines of this study.
Exercise Capacity and Muscle Strength
Exercise capacity was quantified as the mean power (MP; W) for the intervals completed on the air bike during each of the 16 aerobic interval sessions for ACE+EXT group. In addition, to verify that MP increased due to increased fitness and not increased effort alone, MP was also expressed relative to the average heart rate (HR) during the intervals (MPHR; W/beats/min). Because participants in ACE+CON and ACE− groups did not complete exercise training, they completed an aerobic interval session both before and following the 8-wk control period to quantify exercise capacity. Muscle strength was assessed by estimating 1RM in each of the nine exercises used during the exercise training program pre- and postintervention in all groups. The estimated 1RM for all exercises was summated to provide a total strength score (kg).
Psychological Coping
Hope was assessed using the Adult Hope Scale, which contains 12 items on a four-point scale from 1 (definitely false) to 4 (definitely true). The scale contains eight hope items (four assessing hope pathways and four assessing hope agencies) and four fillers. The Adult Hope Scale demonstrates internal consistency, temporal stability, and discriminate and construct validity (41). Psychological resilience was assessed using the 25-item Connor–Davidson Resilience Scale (CD-RISC25) (25). In brief, participants responded to 25 items on a five-point scale from 0 to 4, with lower scores (e.g., 0) representing lower resilience and higher scores (e.g., 4) representing greater resilience. The scale has previously been shown to be reliable and valid (25) and has since been administered in a wide range of populations, including young adults with ACEs, where it has been shown to mediate the association of ACEs with subjective well-being (42).
Cardiovascular Hemodynamic Variables
BP was assessed in accordance with American Heart Association (AHA) BP measurement recommendations (43). In brief, participants sat quietly in an upright position with their feet flat on the floor in a thermoneutral environment for 5 min before BP assessments. An appropriately sized BP cuff was then placed on the participant’s upper arm, and the arm was allowed to rest on a table during measurements. An automatic BP monitor (Omron 3 Series Upper Arm Blood Pressure Monitor, BP7100, Omron Healthcare, Bannockburn, IL) was used to get three BP readings in accordance with the AHA guidelines (43) and the average of the BP readings was recorded and used for the analysis of systolic BP (SBP) and diastolic BP (DBP).
HR was measured using a chest strap and an HR monitor (Polar H1, Polar, Kempele, Finland). Participants rested quietly in a supine position on a padded examination table for 5 min in a quiet, darkened room before 5 min of HR data were collected. The average HR during this 5-min period was recorded as the participant’s resting HR. The rate-pressure product (RPP) was then determined for each participant as the product of HR and SBP as previously described (44).
Serum Endothelin-1 and CRP Measurement
A butterfly needle was inserted into the antecubital vein and blood samples were collected into serum separator tubes (BD Vacutainer, Becton Dickinson, Franklin Lakes, NJ) in the morning following an overnight fast. Samples were inverted upon collection and allowed to clot for 45 min before being centrifuged at 2,500 rpm (700 RCF) for 12 min to separate serum. The serum was then transferred to 1.7-mL microcentrifuge tubes for storage at −20°C. Deidentified samples were then transported to the Integrative Immunology Center Laboratory at the University of Oklahoma School of Community Medicine on dry ice for ET-1 (Endothelin-1 Quantikine ELISA Kit, R&D Systems Inc., Minneapolis, MN) and CRP [V-PLEX Human CRP kit, Meso Scale Diagnostics (MSD), Rockville, MD] quantification. ET-1 was assessed using enzyme-linked immunoassay (ELISA) in accordance with the manufacturer’s instructions. CRP was assessed using MSD electrochemiluminescence immunoassays and a MESO QuickPlex SQ 120 instrument. The detection range of the ET-1 ELISA kit was 0.4–25 pg/mL with a sensitivity of 0.207 pg/mL. The detection range of the CRP immunoassay kit was 1.33–49,608 pg/mL with a sensitivity of 1.33 pg/mL. The average intra-assay coefficient of variation (CV) across all duplicates in the ET-1 ELISA plates was 4.3% and all duplicates had a CV ≤13.9%. The average intra-assay CV across all duplicates in the CRP immunoassay kit was 1.4% and all duplicates had a CV ≤8.0%. All analyses were performed with the technician masked to the subject’s condition. We were unable to obtain blood samples from one participant in the ACE+EXT group at either pre- or posttesting, and thus our n for the ACE+EXT group was 13 for ET-1 and CRP analyses.
Statistical Analyses
ACEs-related differences and lifestyle control.
To examine baseline differences between ACE+EXT, ACE+CON, and ACE− groups, one-way ANOVAs were used. Independent samples t tests were also used to examine baseline differences between participants in the ACE+ and ACE− groups. To ensure outside physical activity levels and nutrition were maintained across the intervention, IPAQ-derived physical activity levels (MET·min/wk) and dietary food log-derived caloric intake were examined using two-way mixed-effects analyses (condition × time).
Exercise intervention effects.
Two-way ANOVAs, or where there were missing data points, two-way mixed-effects analyses, were used to examine the between- (ACE+EXT vs. ACE+CON vs. ACE−) and within-group (pre- vs. postintervention) interaction for MP, MPHR, hope agency, hope pathways, resilience, SBP, DBP, RPP, ET-1, and CRP. When condition × time interactions were <0.075 but ≥0.05, forced post hoc tests were used to probe pre- to postintervention changes within each group to inform future studies of changes that may be of interest and importance. These decisions were also supported by effect size estimates, which were calculated as the unbiased within-subjects Hedges’ g effect size, with 95% confidence intervals (45). Partial eta squared effect sizes () were calculated and are presented for interaction effects (i.e., condition × time). Spearman rank correlation analyses were also used to examine the relation of absolute changes from pre- to postintervention (Δ) in hope pathways, and resilience versus SBP and ET-1, and in ET-1 versus SBP.
Data are reported as means ± SD, except when otherwise denoted. Statistical analyses were performed using GraphPad Prism for macOS (v. 8.4.3), effect sizes were calculated using R for MacOS (v. 4.2.2), and significance was set at P ≤ 0.05. All figures were made using GraphPad Prism for macOS (v. 8.4.3).
RESULTS
Attrition
Eighty-nine women were screened for participation in this study, among whom 21 were ineligible and 24 were eligible and enrolled but dropped out soon after enrollment because they either were no longer interested in participating, realizing that the time commitment would not be feasible for them, or were otherwise lost to follow up. Therefore, 44 participants were eligible, enrolled, and completed at least one experimental visit. Of these 44 participants, two discontinued participation in the ACE+EXT group due to scheduling difficulties.
Baseline ACEs-Related Differences and Group Differences
There were no differences in age, BMI, physical activity, SBP, DBP, or race between the ACE+ and ACE− groups (all P ≥ 0.57). Furthermore, hope pathways (25 ± 4 vs. 28 ± 2, P = 0.038), hope agency (25 ± 4 vs. 28 ± 2, P = 0.011), and resilience (70 ± 14 vs. 82 ± 7, P = 0.003) were all lower in ACE+ group than in ACE−group. There were also no differences between the ACE+ and ACE− groups for calories consumed per day, resting HR, RPP, CRP, or ET-1 (all P ≥ 0.15). Baseline characteristics at preintervention for the ACE+EXT, ACE+CON, and ACE− groups are displayed in Table 1.
Nutrition, Physical Activity, and Bodyweight Control Variables
There were no condition × time interactions for bodyweight (P = 0.31), average calories consumed per day (P = 0.65), or MET·min/wk (P = 0.41).
Exercise Capacity and Muscle Strength
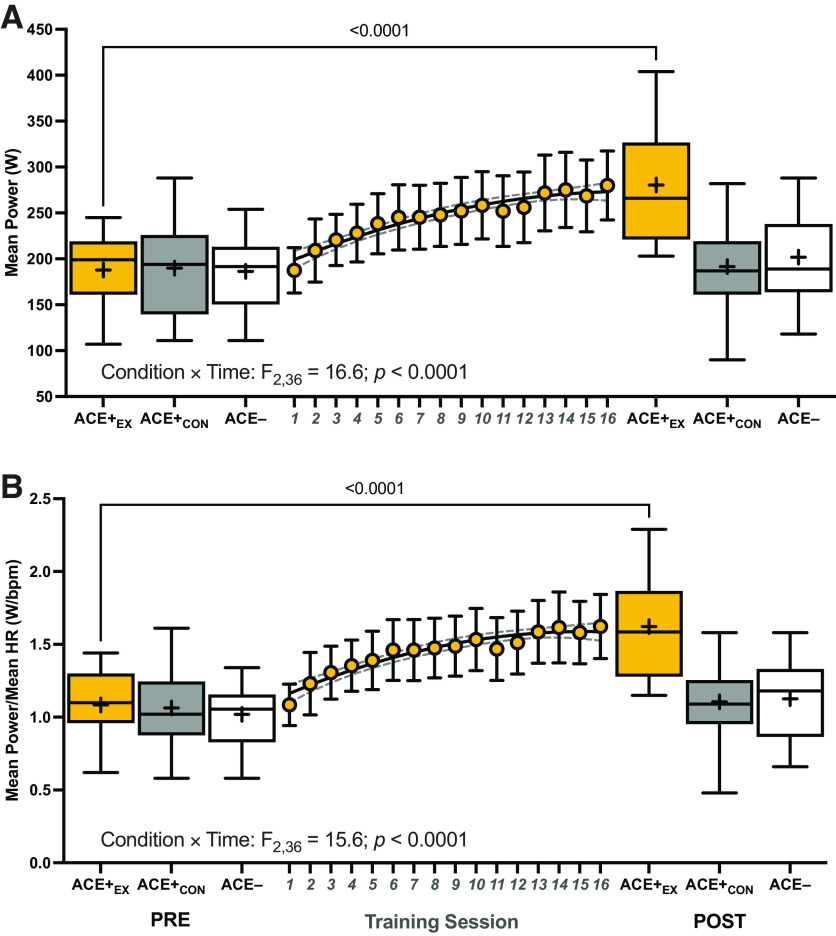
The condition × time interactions for MP (F2,36 = 16.6; P < 0.0001; η2 = 0.48) and MPHR (F2,36 = 15.6; P < 0.0001; η2 = 0.46) were significant. Both MP and MPHR increased from pre- to postintervention in ACE+EXT (both P < 0.001) group, but not in ACE+CON or ACE− (all p ≥ 0.25) groups. Furthermore, the increases in MP and MPHR in ACE+EXT group exhibited quadratic polynomial increases (R2 = 0.94 and 0.93, respectively) throughout the training period (Fig. 1). There was also a condition × time interaction for total strength (F2,39 = 73.8; P < 0.0001; η2 = 0.79), where total strength increased in ACE+EXT (means ± SE of difference; +85 ± 6 kg; P < 0.0001) group, but not in ACE+CON or ACE− (both P ≥ 0.14) groups.
Figure 1.
The mean power (A) and mean power expressed relative to average session HR before (PRE) and after (POST) the 8-wk exercise (or control) intervention (B). Also depicted by the gold circles [±95% confidence intervals (CIs)] are the mean power and mean power relative to HR values across each of the 16 training sessions for ACE+EXT. P values from two-way mixed-effects analyses (n = 14/group; two missing values) are provided where the within-group PRE to POST change in mean power or mean power relative to HR is significant. Box and whisker plots are plotted, where the middle of the box is the median, the “+” indicates the mean, the box extends from the 25th to 75th percentiles, and the whiskers extend to the minimum and maximum observed values. ACE−, ACE− control group; ACE+CON, ACE+ control group; ACE+EX, ACE+ exercise training group; bpm, beats per minute; HR, heart rate; W, watts.
Psychological Coping
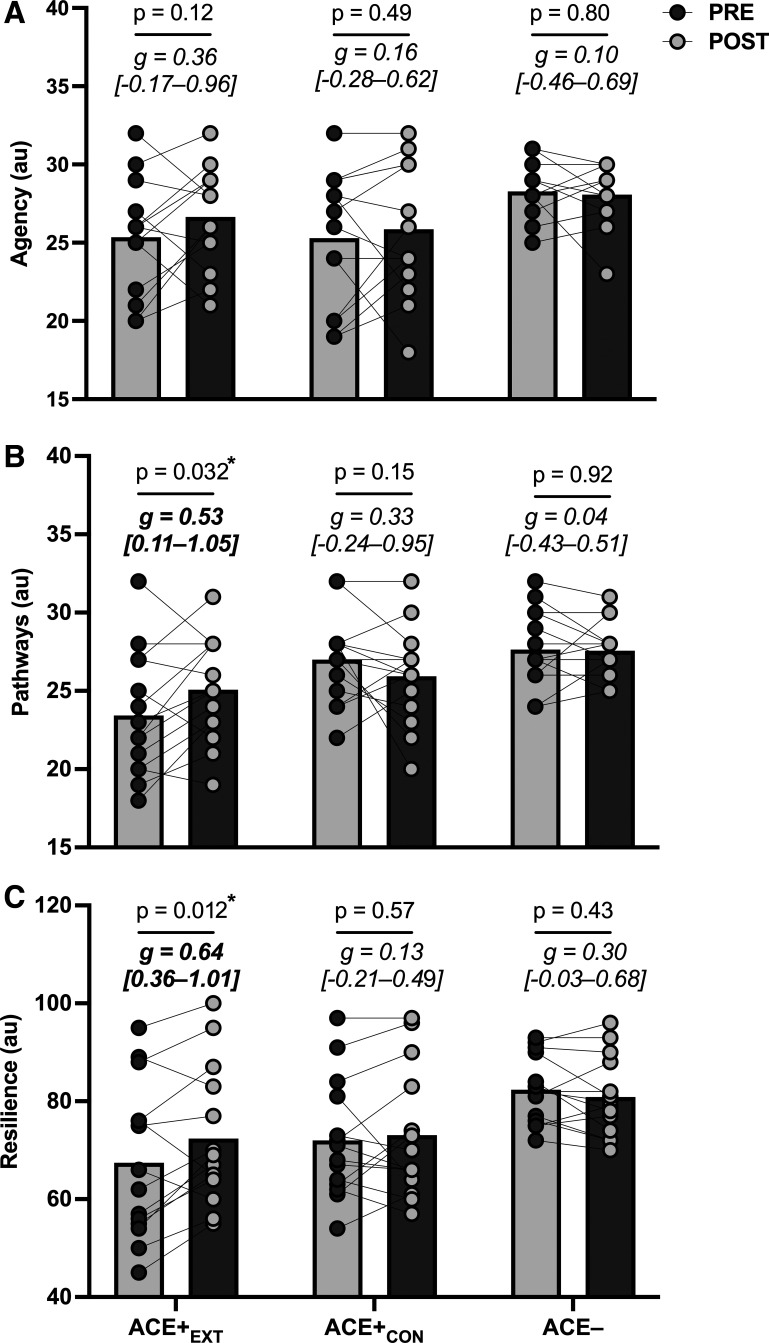
The condition × time interaction for hope pathways was significant (P = 0.041; = 0.15), but there was no interaction for hope agency (P = 0.44). The interaction for hope pathways was driven by an improvement in ACE+EXT (P = 0.032; Fig. 2B) group, but not in ACE+CON or ACE− group. The condition × time interaction for resilience was not significant (P = 0.06; = 0.13), but forced post hoc analyses indicated that resilience improved from pre- to postintervention in ACE+EXT alone (P = 0.012; Fig. 2C).
Figure 2.
Psychological coping including hope agency (A), hope pathways (B), and resilience (C) before (PRE) and after (POST) the 8-wk exercise training (or control) intervention (n = 42). P values are for the PRE to POST change within groups (n = 14/group). g Values are within-subjects Hedges’ g effect sizes (95% confidence intervals). *Significant within-group changes. ACE−, ACE− control group; ACE+CON, ACE+ control group; ACE+EXT, ACE+ exercise training group; au, arbitrary units.
Cardiovascular hemodynamic outcomes.
The condition × time interactions for DBP (P = 0.43) and resting HR (P = 0.24) were not significant. The condition × time interactions for both SBP and RPP were also not significant (P = 0.053 and 0.050, = 0.15 and 0.15, respectively), but were suggestive of a training-related effect (Fig. 3, A and C). Forced post hoc tests indicated that SBP tended to improve in ACE+EXT group only [P = 0.053; g = 0.51 (0.09–1.01)], whereas RPP improved in ACE+EXT [P = 0.002; g = 0.78 (0.16–1.53)] and ACE− [P = 0.026; g = 0.52 (−0.02–1.15)] groups.
Figure 3.
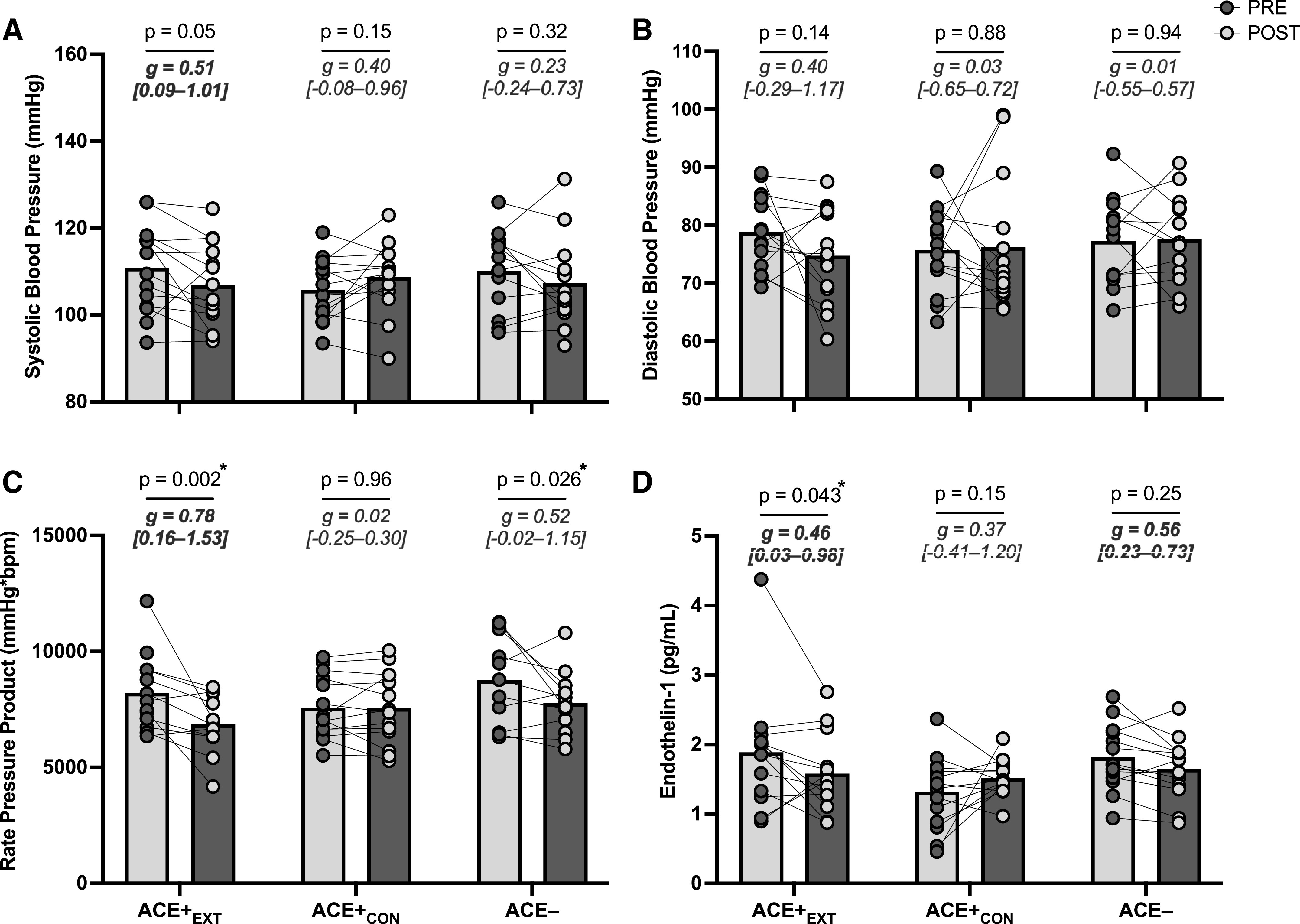
Cardiovascular outcomes including systolic blood pressure (n = 14/group; two missing values; A), diastolic blood pressure (n = 14/group; two missing values; B), rate pressure product (n = 14/group; six missing values; C), and serum endothelin-1 levels (n = 14/group except, ACE+EXT, where n = 13; one missing value; D) before (PRE) and after (POST) the 8-wk exercise training (or control) intervention. P values are for the PRE to POST change within groups. g Values are within-subjects Hedges’ g effect sizes (95% confidence intervals). *Significant within-group changes. ACE− = ACE− control group; ACE+CON, ACE+ control group; ACE+EXT, ACE+ exercise training group.
Endothelin-1 and C-reactive protein.
The condition × time interaction for ET-1 was significant (P = 0.044; = 0.15). This interaction was driven by a decrease in ET-1 in ACE+EXT group only [P = 0.043; g = 0.46 (0.03–0.98); Fig. 3D]. There was no condition × time interaction for CRP (P = 0.58).
Correlation analyses.
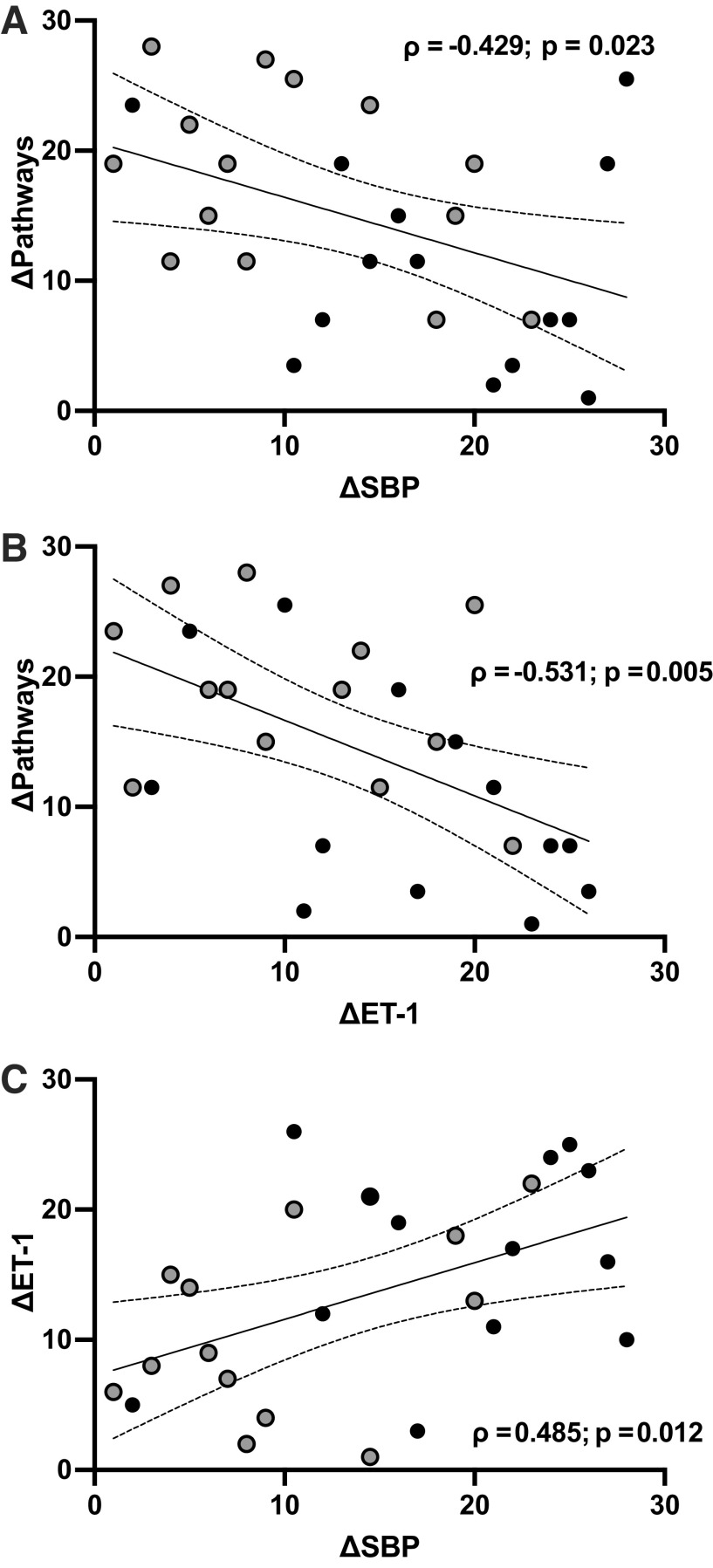
Correlation analyses conducted on the changes across the intervention period within those with ACEs (i.e., ACE+) revealed that ΔSBP (ρ = −0.43, P = 0.023) and ΔET-1 (ρ = −0.53, P = 0.005) were inversely associated with Δhope pathways (Fig. 4, A and B). In addition, ΔSBP was directly associated with ΔET-1 (ρ = 0.49; P = 0.012; Fig. 4C). The Δresilience was not significantly related to ΔSBP or ΔET-1 (ρ = −0.23 and −0.09, respectively). The were no significant associations observed in the ACE− group as expected.
Figure 4.
Spearman rank correlations with 95% confidence intervals between the changes in hope pathways and SBP (n = 28; A), hope pathways and ET-1 (n = 27; B), and ET-1 and SBP (n = 27; C) from pre- to postintervention in participants with ACEs, with ACE+EXT represented by black and ACE+CON represented by gray circles. Note that units are rank orders, such that for SBP and ET-1, higher values represent greater improvements (decreases) and for hope pathways, lower values indicate greater improvements (increases). P values ≤ 0.05 indicate significant relationships. ACE, adverse childhood experience; ACE+CON, ACE+ control group; ACE+EXT, ACE+ exercise training group; ET-1, endothelin-1; SBP, systolic blood pressure.
DISCUSSION
As expected, the 8-wk structured progressive exercise training program improved the exercise capacity and muscle strength of participants in ACE+EXT group, but not in ACE+CON or ACE− group. The primary finding though was that participants in ACE+EXT group experienced improvements in hope pathways and serum ET-1, and potentially in resilience and SBP. Also of note, the changes in the hope pathways were associated with changes in serum ET-1 and SBP in those with ACEs, suggesting a potential cardiovascular psychophysiological connection in women with a history of ACEs. Furthermore, these findings provide initial evidence that progressive exercise training may be an effective intervention to improve cardiovascular psychophysiological outcomes in young women with ACEs.
A robust body of evidence demonstrates that ACEs increase not only the risk for development of mental health disorders but also CVD. In fact, a dose-response relationship exists such that greater cumulative exposure to ACEs is associated with greater cardiovascular morbidity and mortality (6, 33, 46). Importantly, the effects of ACEs on clinical indices of cardiovascular health, such as BP, are not readily apparent in childhood or even emerging adulthood until the age of ∼30 yr (15, 28). In agreement, there were no significant baseline differences in SBP, DBP, resting HR, RPP, ET-1, or CRP in ACE+ versus ACE− group adult women in this cohort who had a mean age of 21 yr (1). In contrast, those with ACEs displayed lower resilience and lower scores in the two hope subdimensions, pathways and agency. Therefore, ACE exposure was associated with lower positive psychological coping in young adult women, but not overt baseline differences in traditional clinical cardiovascular risk markers.
Our data support and extend findings from prior studies, suggesting that ACEs are associated with lower resilience and hope. However, ours is the first to examine the effectiveness of exercise training for improving positive psychological coping in individuals with substantial childhood adversity. In accordance with our hypothesis, the hope pathways dimension improved, by 8.2%, in ACE+EXT group alone (P = 0.012; Fig. 2B). Similarly, although the overall interaction was not significant (P = 0.06), forced post hoc analysis indicated that resilience also improved, by 8.9%, in ACE+EXT (P = 0.032; Fig. 2C) group. In contrast, the hope agency dimension did not improve in any group. These data suggest that progressive exercise training, initiated without a supporting psychological intervention, may be able to improve hope pathways and resilience, but not hope agency in young adult women with ACEs. Hope involves agency—or the resolve to pursue one’s goals and the belief in one’s ability to reach them—and pathways—the knowledge that there are many ways to achieve one’s goals and the flexibility to shift paths when another is not working. Whereas hope agency is thought of as the motivational aspect of hope, pushing one determinedly forward, hope pathways involve behavioral flexibility and willingness to shift when a pathway does not yield the desired outcome (18). Moving forward, it will be important to test whether exercise training alongside psychological interventions designed to improve resilience or psychological flexibility, such as acceptance and commitment therapy (ACT), can promote more robust changes in these psychological coping mechanisms and improvements in hope agency and hope pathways. The findings herein suggest that exercise training alone is sufficient to impact hope pathways and underscores the importance of flexibility; however, perhaps the augmentation of exercise training with a flexibility-focused intervention such as ACT could improve hope agency, along with hope pathways, and resilience.
Previous studies have suggested that ET-1 is responsive to psychosocial stress and thus may play a mechanistic role in the relationship between ACEs and CVD risk (30). As previously discussed, baseline differences in BP and RPP were not expected in our sample based on prior evidence that the effects of ACEs on BP are not apparent until near the age of 30 yr (28). However, our lack of baseline differences in ET-1 between young adult women in the ACE+ and ACE− groups in this study is in disagreement with the prior study by Su et al. (33), who reported that ET-1 is higher in adolescents and young adults with a history of ACEs. The much larger (n = 221) and more diverse sample in the study by Su et al. (33) could possibly explain our differing findings. Nevertheless, exercise training decreased serum ET-1 by 16% (Fig. 3D) in ACE+EXT group. Our findings also suggest that exercise training decreased SBP by 4% (∼4 mmHg, P = 0.053), which was a moderate effect [g = 0.51 (0.09–1.01)] and is likely a clinically meaningful reduction (47, 48), although this effect did not reach the a priori significance level (Fig. 3A). Furthermore, the changes from pre- to postintervention in ET-1 and SBP were related in the ACE+ women (ρ = 0.49). Perhaps more surprisingly, the changes in both ET-1 and SBP were associated with improvements in hope pathways (ρ = −0.53 and −0.43, respectively). Despite no differences in baseline ET-1 or SBP between ACE+ and ACE− groups, the findings that exercise training induced improvements in ET-1 and potentially SBP in ACE+EXT group are important because they demonstrate, for the first time, that ET-1 and SBP may be modifiable in this population. This is important as the association of BP with CVD risk is linear even in the normotensive range. Thus, improvements in SBP are likely to be preventative and may improve CVD risk profiles in those with ACEs. Finally, the association among changes in ET-1, SBP, and hope pathways suggests an important cardiovascular psychophysiological connection in young adult women with ACEs, supporting prior evidence that psychological risk factors may mediate the association of ACEs with CVD risk and suggesting that exercise may represent an effective transdiagnostic intervention in individuals with ACEs.
There are several limitations to this study worth acknowledging. First, as this is the first study to examine the effects of an exercise training intervention on psychophysiological outcomes in young adult women with moderate-to-severe ACE exposure, our sample size was determined based on the ability to observe a moderate effect (f = 0.25) with a power of 0.9 and correlation among repeated measures of 0.7 for the repeated-measures, within-between interaction. It is likely that we were in fact underpowered for some of the outcomes reported herein that were secondary or tertiary outcomes. However, these preliminary data are suggestive of a potential transdiagnostic effect of progressive exercise training on cardiovascular psychophysiological outcomes in young adult women with ACEs, and this study should be replicated with a larger sample size to further investigate this possibility. Second, our BP measurements were limited to a single resting value in the morning at pre- and postintervention. Future studies may wish to use more robust BP monitoring protocols, such as 24-h ambulatory BP monitoring and studying beat-by-beat BP variability. Finally, circulating ET-1 levels do not directly reflect the status of the ET-1 system in the endothelium, where ET-1 levels are 100-fold greater than in circulation (49). Future studies with direct vascular assessment of ET-1 system activity are needed to understand the role of ET-1 in ACEs-related CVD risk.
In conclusion, our findings indicate that progressive exercise training represents a feasible transdiagnostic intervention capable of improving positive psychological attributes such as hope pathways, as well as circulating ET-1, and potentially resilience and SBP. However, it should be noted that the interactions for resilience and SBP were below the threshold for significance (P = 0.061 and 0.053, respectively), despite there being significant moderate effect size improvements (g ≥ 0.5) in the ACE+EXT group alone. Our study is also the first to show that improvements in positive psychological coping are observed alongside, and associated with, improvements in biomarkers of CVD risk among women with ACEs. These data reveal a potential cardiovascular psychophysiological association in individuals that have been exposed to ACEs and have important implications for the development of interventions to lower CVD risk in this population.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This study was supported by the Center for Integrative Research on Childhood Adversity Grant P20GM109097, through the National Institute of General Medical Sciences (to N.D.M.J., S.R.E., A.T., and T.K.T.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.F.B. and N.D.M.J. conceived and designed research; E.M.R., N.F.B., P.M.T., C.M.S., A.T., T.K.T., and N.D.M.J. performed experiments; E.M.R., N.F.B., A.T., T.K.T., and N.D.M.J. analyzed data; E.M.R., N.F.B., S.R.E., and N.D.M.J. interpreted results of experiments; E.M.R., N.F.B., and N.D.M.J. prepared figures; E.M.R., N.F.B., and N.D.M.J. drafted manuscript; E.M.R., N.F.B., P.M.T., C.M.S., S.R.E., E.B.K.T., A.T., T.K.T., and N.D.M.J. edited and revised manuscript; E.M.R., N.F.B., P.M.T., C.M.S., S.R.E., E.B.K.T., A.T., T.K.T., and N.D.M.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank all the women who graciously dedicated time to participate in this study. We also thank Brenda Davis and Ahlam Alarbi for performing the C-reactive protein (CRP) and endothelin-1 (ET-1) analyses, respectively, at the University of Oklahoma Integrative Immunology Center. Finally, we thank Hannah Bryan and Sam Hart for all of their help in training participants.
Graphical abstract created with BioRender and published with permission.
REFERENCES
- 1. Jenkins NDM, Rogers EM, Banks NF, Tomko PM, Sciarrillo CM, Emerson SR, Taylor A, Teague TK. Childhood psychosocial stress is linked with impaired vascular endothelial function, lower SIRT1, and oxidative stress in young adulthood. Am J Physiol Heart Circ Physiol 321: H532–H541, 2021. doi: 10.1152/ajpheart.00123.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petruccelli K, Davis J, Berman T. Adverse childhood experiences and associated health outcomes: a systematic review and meta-analysis. Child Abuse Negl 97: 104127, 2019. doi: 10.1016/j.chiabu.2019.104127. [DOI] [PubMed] [Google Scholar]
- 3. Giano Z, Wheeler DL, Hubach RD. The frequencies and disparities of adverse childhood experiences in the U.S. BMC Public Health 20: 1327, 2020. doi: 10.1186/s12889-020-09411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merrick MT, Ford DC, Ports KA, Guinn AS. Prevalence of adverse childhood experiences from the 2011-2014 behavioral risk factor surveillance system in 23 states. JAMA Pediatr 172: 1038–1044, 2018. [Erratum in JAMA Pediatr 172: 1104, 2018]. doi: 10.1001/jamapediatrics.2018.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riley EH, Wright RJ, Jun HJ, Hibert EN, Rich-Edwards JW. Hypertension in adult survivors of child abuse: observations from the Nurses’ Health Study II. J Epidemiol Community Health 64: 413–418, 2010. doi: 10.1136/jech.2009.095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14: 245–258, 1998. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 7. Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation 110: 1761–1766, 2004. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 8. Basu A, McLaughlin KA, Misra S, Koenen KC. Childhood maltreatment and health impact: the examples of cardiovascular disease and type 2 diabetes mellitus in adults. Clin Psychol (New York) 24: 125–139, 2017. doi: 10.1111/cpsp.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loucks EB, Almeida ND, Taylor SE, Matthews KA. Childhood family psychosocial environment and coronary heart disease risk. Psychosom Med 73: 563–571, 2011. doi: 10.1097/PSY.0b013e318228c820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pierce JB, Kershaw KN, Kiefe CI, Jacobs DR Jr, Sidney S, Merkin SS, Feinglass J. Association of childhood psychosocial environment with 30-year cardiovascular disease incidence and mortality in middle age. J Am Heart Assoc 9: e015326, 2020. doi: 10.1161/JAHA.119.015326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deschênes SS, Kivimaki M, Schmitz N. Adverse childhood experiences and the risk of coronary heart disease in adulthood: examining potential psychological, biological, and behavioral mediators in the Whitehall II cohort study. J Am Heart Assoc 10: e019013, 2021. doi: 10.1161/JAHA.120.019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roy A, Janal MN, Roy M. Childhood trauma and prevalence of cardiovascular disease in patients with type 1 diabetes. Psychosom Med 72: 833–838, 2010. doi: 10.1097/PSY.0b013e3181eafc2d. [DOI] [PubMed] [Google Scholar]
- 13. Scott KM, Von Korff M, Angermeyer MC, Benjet C, Bruffaerts R, de Girolamo G, Haro JM, Lépine JP, Ormel J, Posada-Villa J, Tachimori H, Kessler RC. Association of childhood adversities and early-onset mental disorders with adult-onset chronic physical conditions. Arch Gen Psychiatry 68: 838–844, 2011. doi: 10.1001/archgenpsychiatry.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bellis MA, Hughes K, Leckenby N, Hardcastle KA, Perkins C, Lowey H. Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: a national survey. J Public Health (Oxf) 37: 445–454, 2015. doi: 10.1093/pubmed/fdu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jenkins NDM, Robinson AT. How do adverse childhood experiences get under the skin to promote cardiovascular disease? A focus on vascular health. Function (Oxf) 3: zqac032, 2022. doi: 10.1093/function/zqac032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang YC, Moya Guerola M, Lin YC, Hsieh YP, Strong C, Tsai MC, Lin CY. Effects of childhood adversity and resilience on Taiwanese youth health behaviors. Pediatr Neonatol 60: 368–376, 2019. doi: 10.1016/j.pedneo.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 17. Dolbier CL, Haley EN, Conder L, Guiler W. Adverse childhood experiences and adult psychopathological symptoms: the moderating role of dispositional mindfulness. J Contextual Behav Sci 21: 73–79, 2021. doi: 10.1016/j.jcbs.2021.06.001. [DOI] [Google Scholar]
- 18. Snyder CR. Target article: hope theory: rainbows in the mind. Psychol Inq 13: 249–275, 2002. doi: 10.1207/S15327965PLI1304_01. [DOI] [Google Scholar]
- 19. Allan BA. Balance among character strengths and meaning in life. J Happiness Stud 16: 1247–1261, 2015. doi: 10.1007/s10902-014-9557-9. [DOI] [Google Scholar]
- 20. Baxter MA, Hemming EJ, McIntosh HC, Hellman CM. Exploring the relationship between adverse childhood experiences and hope. J Child Sex Abus 26: 948–956, 2017. doi: 10.1080/10538712.2017.1365319. [DOI] [PubMed] [Google Scholar]
- 21. Cotton Bronk K, Hill PL, Lapsley DK, Talib TL, Finch H. Purpose, hope, and life satisfaction in three age groups. J Posit Psychol 4: 500–510, 2009. doi: 10.1080/17439760903271439. [DOI] [Google Scholar]
- 22. Morgan CA, Chang YH, Choy O, Tsai MC, Hsieh S. Adverse childhood experiences are associated with reduced psychological resilience in youth: a systematic review and meta-analysis. Children (Basel) 9: 27, 2021. doi: 10.3390/children9010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daníelsdóttir HB, Aspelund T, Thordardottir EB, Fall K, Fang F, Tómasson G, Rúnarsdóttir H, Yang Q, Choi KW, Kennedy B, Halldorsdottir T, Lu D, Song H, Jakobsdóttir J, Hauksdóttir A, Valdimarsdóttir UA. Adverse childhood experiences and resilience among adult women: a population-based study. Elife 11: e71770, 2022. doi: 10.7554/eLife.71770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Windle M, Haardörfer R, Getachew B, Shah J, Payne J, Pillai D, Berg CJ. A multivariate analysis of adverse childhood experiences and health behaviors and outcomes among college students. J Am Coll Health 66: 246–251, 2018. doi: 10.1080/07448481.2018.1431892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety 18: 76–82, 2003. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 26. Crump C, Sundquist J, Winkleby MA, Sundquist K. Low stress resilience in late adolescence and risk of hypertension in adulthood. Heart 102: 541–547, 2016. doi: 10.1136/heartjnl-2015-308597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gurven M, Blackwell AD, Rodríguez DE, Stieglitz J, Kaplan H. Does blood pressure inevitably rise with age?: longitudinal evidence among forager-horticulturalists. Hypertension 60: 25–33, 2012. doi: 10.1161/HYPERTENSIONAHA.111.189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation 131: 1674–1681, 2015. doi: 10.1161/CIRCULATIONAHA.114.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Treiber FA, Kapuku GK, Davis H, Pollock JS, Pollock DM. Plasma endothelin-1 release during acute stress: role of ethnicity and sex. Psychosom Med 64: 707–713, 2002. doi: 10.1097/01.psy.0000021952.59258.1c. [DOI] [PubMed] [Google Scholar]
- 30. Fox BM, Becker BK, Loria AS, Hyndman KA, Jin C, Clark H, Johns R, Yanagisawa M, Pollock DM, Pollock JS. Acute pressor response to psychosocial stress is dependent on endothelium-derived endothelin-1. J Am Heart Assoc 7: e007863, 2018. doi: 10.1161/JAHA.117.007863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yammine L, Kang DH, Baun MM, Meininger JC. Endothelin-1 and psychosocial risk factors for cardiovascular disease: a systematic review. Psychosom Med 76: 109–121, 2014. doi: 10.1097/PSY.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 32. Loria AS, D'Angelo G, Pollock DM, Pollock JS. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Am J Physiol Regul Integr Comp Physiol 299: R185–R191, 2010. doi: 10.1152/ajpregu.00333.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Su S, Wang X, Kapuku GK, Treiber FA, Pollock DM, Harshfield GA, McCall WV, Pollock JS. Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension 64: 201–207, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barone Gibbs B, Hivert MF, Jerome GJ, Kraus WE, Rosenkranz SK, Schorr EN, Spartano NL, Lobelo F; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Physical activity as a critical component of first-line treatment for elevated blood pressure or cholesterol: who, what, and how?: a scientific statement from the American Heart Association. Hypertension 78: e26–e37, 2021. doi: 10.1161/HYP.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 35. Hassmén P, Koivula N, Uutela A. Physical exercise and psychological well-being: a population study in Finland. Prev Med 30: 17–25, 2000. doi: 10.1006/pmed.1999.0597. [DOI] [PubMed] [Google Scholar]
- 36. Almuneef M, ElChoueiry N, Saleheen HN, Al-Eissa M. Gender-based disparities in the impact of adverse childhood experiences on adult health: findings from a national study in the Kingdom of Saudi Arabia. Int J Equity Health 16: 90, 2017. doi: 10.1186/s12939-017-0588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verma R, Balhara YP, Gupta CS. Gender differences in stress response: role of developmental and biological determinants. Ind Psychiatry J 20: 4–10, 2011. doi: 10.4103/0972-6748.98407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA 320: 2020–2028, 2018. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jenkins NDM, Banks NF, Rogers EM, Sciarrillo CM, Koemel NA, Colquhoun RJ, Emerson SR. Resistance exercise attenuates postprandial metabolic responses to a high-fat meal similarly in younger and older men. Nutr Res 83: 73–85, 2020. doi: 10.1016/j.nutres.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 40. Ciolac EG, Mantuani SS, Neiva CM, Verardi C, Pessôa-Filho DM, Pimenta L. Rating of perceived exertion as a tool for prescribing and self regulating interval training: a pilot study. Biol Sport 32: 103–108, 2015. doi: 10.5604/20831862.1134312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Snyder CR, Harris C, Anderson JR, Holleran SA, Irving LM, Sigmon ST, Yoshinobu L, Gibb J, Langelle C, Harney P. The will and the ways: development and validation of an individual-differences measure of hope. J Pers Soc Psychol 60: 570–585, 1991. doi: 10.1037//0022-3514.60.4.570. [DOI] [PubMed] [Google Scholar]
- 42. Kelifa MO, Yang Y, Carly H, Bo W, Wang P. How adverse childhood experiences relate to subjective wellbeing in college students: the role of resilience and depression. J Happiness Stud 22: 2103–2123, 2021. doi: 10.1007/s10902-020-00308-7. [DOI] [Google Scholar]
- 43. Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, Urbina EM, Viera AJ, White WB, Wright JT Jr.. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension 73: e35–e66, 2019. doi: 10.1161/HYP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. White WB. Heart rate and the rate-pressure product as determinants of cardiovascular risk in patients with hypertension. Am J Hypertens 12: 50S–55S, 1999. doi: 10.1016/s0895-7061(98)00280-5. [DOI] [PubMed] [Google Scholar]
- 45. Goulet-Pelletier J-C, Cousineau D. A review of effect sizes and their confidence intervals, Part I: The Cohen’s d family. TQMP 14: 242–265, 2018. doi: 10.20982/tqmp.14.4.p242. [DOI] [Google Scholar]
- 46. Rodriguez-Miguelez P, Looney J, Blackburn M, Thomas J, Pollock JS, Harris RA. The link between childhood adversity and cardiovascular disease risk: role of cerebral and systemic vasculature. Function (Oxf) 3: zqac029, 2022. doi: 10.1093/function/zqac029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA; American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc 36: 533–553, 2004. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 48. Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J; National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 288: 1882–1888, 2002. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 49. Stauffer BL, Westby CM, DeSouza CA. Endothelin-1, aging and hypertension. Curr Opin Cardiol 23: 350–355, 2008. doi: 10.1097/HCO.0b013e328302f3c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.