Keywords: aging, Eph/ephrin, neuromuscular junction, skeletal muscle fiber type
Abstract
Individual limb muscles have characteristic representation and spatial distribution of muscle fiber types (one slow and up to three fast isoforms) appropriate to their unique anatomical location and function. This distribution can be altered by physiological stimuli such as training (i.e., for increased endurance or force) or pathological conditions such as aging. Our group previously showed that ephrin-A3 is expressed only on slow myofibers, and that adult mice lacking ephrin-A3 have dramatically reduced numbers of slow myofibers due to postnatal innervation of previously slow myofibers by fast motor neurons. In this study, fiber type composition of hindlimb muscles of aged and denervated/reinnervated C57BL/6 and ephrin-A3−/− mice was analyzed to determine whether the loss of slow myofibers persists across the lifespan. Surprisingly, fiber-type composition of ephrin-A3−/− mouse muscles at two years of age was nearly indistinguishable from age-matched C57BL/6 mice. After challenge with nerve crush, the percentage of IIa and I/IIa hybrid myofibers increased significantly in aged ephrin-A3−/− mice. While EphA8, the receptor for ephrin-A3, is present at all neuromuscular junctions (NMJs) on fast fibers in 3–6 mo old C57BL/6 and ephrin-A3−/− mice, this exclusive localization is lost with aging, with EphA8 expression now found on a subset of NMJs on some slow muscle fibers. This return to appropriate fiber-type distribution given time and under use reinforces the role of activity in determining fiber-type representation and suggests that, rather than being a passive baseline, the developmentally and evolutionarily selected fiber type pattern may instead be actively reinforced by daily living.
INTRODUCTION
Muscle Fiber Types in Mammals
Skeletal muscles are composed of a heterogeneous mixture of functionally distinct contractile muscle cells (myofibers) (1). Individual muscles have characteristic proportions and arrangements of myofibers expressing a range of myosin isoforms that determine the muscle’s metabolic and force-generating capacity, appropriate to the use of each muscle; these patterns are established and refined during fetal and early postnatal development. Postnatally, these fiber types are reinforced and maintained by motor neuron innervation: “slow” motor neurons innervate “slow” myofibers, and “fast” motor neurons innervate “fast” myofibers (2). Mature myofibers in adult skeletal muscles generally express one of several myosin heavy chain (MyHC) isoforms, and individual fiber types are classified based on the MyHC isoform they express [type I (slow) and type IIA, IIX, and IIB (fast)], which express MyHC I, MyHC IIa, MyHC IIx, and MyHC IIb, respectively (3). MyHC-IIa, -IIx, and -IIb isoforms are present in all adult mouse skeletal muscles, in varying proportions (4, 5). In general, MyHC-IIb is the predominant isoform expressed in adult mouse limb skeletal muscle (4). Several studies have documented the muscle fiber composition of C57BL/6 mice (5–10). However, to the best of our knowledge, muscle fiber composition of aged C57BL/6 muscles has not been published. In humans, MyHC I, MyHC IIA, and MyHC IIX are expressed in differing proportions in different muscles, whereas MyHC IIB is not detectable (1); specialized MyHCs also exist in nonlimb muscles such as the extraocular muscle (11).
The slowest contracting fibers in humans and other mammals express MyHC-I (Myh7), with successively faster-contracting fibers expressing MyHC-IIa (Myh2), MyHC-IIx (Myh1), and MyHC-IIb (Myh4). Most adult myofibers express a single MyHC isoform, however rare hybrid fibers also exist that express two or more MyHC isoforms (3). Hybrid myofibers express MyHC proteins that are adjacent on the continuum of slow to fast (i.e., type I/IIa) and the contractile properties of hybrid fibers are also intermediate with respect to pure fiber types (3). An increased number of hybrid myofibers is a typical feature of neuromuscular disruption and aging in skeletal muscle in human and animal models (12–15). Fibers co-expressing MyHC-IIa and -IIx are the most common hybrid fibers found in rat and mouse muscle, followed by fibers co-expressing MyHC-IIx and -IIb. Fibers co-expressing MyHC-I and -IIa (both slow and fast) are rare but have been observed in rat, mouse, and human muscle (16).
Denervation is associated with decreased expression of slow type I MyHC and increased expression of the faster MyHC isoforms in an animal model (17). Slow to fast MyHC isoform transformations have also been observed in human muscle after spinal cord injury, and the proportion of type II to type I fibers is elevated in the tibialis anteriors (TAs) of subjects with spinal cord injury (18, 19). Denervation also plays a role in the age-related shift in MyHC expression and frequent MyHC coexpression seen in aged muscle (20–22). In addition, a preferential loss of fast-type IIb neuromuscular synapses has been reported in aged and neuromuscular disease-affected mouse muscles (23). The presence of hybrid fibers is the most prominent phenotype in aged human samples (20). It has been suggested that the significantly greater amounts of MyHC co-expression in rats could indicate a fiber type shift as denervation outpaces reinnervation (22). Increases in fibers positive for nondominant adult MyHC isoforms in rat soleus and gastrocnemius appear to be largely due to the presence of hybrid fibers (21). In animal models, in the absence of innervation, slow muscles become faster and fast muscles become slower (3). Chronic low-frequency stimulation can also change in myosin heavy chain gene expression that leads to the replacement of fast by slow isoforms and increases in hybrid fibers in rat muscle (24).
Eph/Ephrin Signaling in Muscle Fiber-Type Specification
Eph receptors are the largest family of receptor tyrosine kinases and are divided into two classes based on their ligand specificity. Fourteen of them are present in mammals: EphA1 to A8 and A10, and EphB1 to B4 and B6. The ligands are also divided into two classes: ephrin-As are anchored to the extracellular side of the plasma membrane by a glycosylphophatidylinositol (GPI) linker, whereas ephrin-Bs have a transmembrane domain with a short cytoplasmic tail. Ephs and ephrins are expressed in almost every tissue and regulate multiple key processes during development, homeostasis, and regeneration, particularly in establishing tissue organization. They are best known for mediating cell migration and pathfinding by changing cell adhesion or promoting cell repulsion between Eph-expressing cells and cells expressing their ligands, the ephrins (25–27). Eph and ephrin-A proteins play important roles in muscle patterning and motor axonal guidance (28), directing a broad variety of cellular and intracellular processes (29–31) including topographic mapping, neurulation, axon guidance, axon fasciculation, dendritic pruning, neuronal migration, and synapse formation.
In an immunohistochemical screen of Eph and ephrin expression in adult muscle, we noted that of the eight ephrin ligands, only ephrin-A3 showed differential expression among individual myofibers (32). Correlating ephrin-A3 immunopositivity with sarcomeric MyHC expression in uninjured hindlimb muscles revealed that ephrin-A3 exclusively marked all MyHC-I+ve myofibers (33). Furthermore, when mice genetically lacking ephrin-A3 (34) were analyzed, we found that while muscle fiber-type patterning was indistinguishable from wild type (WT) during fetal and early postnatal development, slow myofiber identity was lost in ephrin-A3−/− muscles between 3 and 5 weeks of age as formerly type I myofibers changed their MyHC expression to type IIA. Our conclusion was that ephrin-A3 expressed on slow myofibers that are cell-autonomously specified during development acts to prevent inappropriate innervation by fast motor neurons during postnatal axon pruning via repulsive interactions. This would require the receptor for ephrin-A3 to be expressed at the motor axon terminus. We identified EphA8, a potential ephrin-A3 receptor, at all and only fast neuromuscular junctions, however surprisingly it was expressed not by the motor neuron itself but by associated terminal Schwann cells.
To ask whether physiological influences such as aging that influence muscle fiber type interact with the ephrin-A3−/− phenotype, this study analyzed fiber-type composition of hind limb muscles of aged and denervated/reinnervated C57BL/6 and ephrin-A3−/− mice to determine if the loss of slow myofibers is persistent after these challenges.
MATERIALS AND METHODS
Mice
All experiments involving mice were conducted in accordance with National Institutes of Health and University of Missouri Animal Care and Use Committee guidelines. Age-matched ephrin-A3 nontransgenic C57BL/6 mice were the control for ephrin-A3−/− mice (34) (Jackson Laboratories, Bar Harbor, ME). n = 3 C57BL/6 mice, 10 ephrin-A3−/− mice; both male and female mice were used in this study, and the data were analyzed separately for each group.
Sciatic Nerve Crush
Mice were placed under isoflurane anesthesia and laid on a heated plate to maintain body temperature. Fur was removed from the left leg and the skin was cleaned using betadine and 70% ethanol. The sciatic nerve was then exposed via an incision in the flank followed by separation of underlying musculature by blunt dissection. The nerve was crushed using Dumont No. 5 fine forceps at the level of the obturator tendon by firmly pressing the forceps for 10 s twice. The musculature was re-opposed and the incision was closed using wound clips. The mice were then placed back in their original cage and the cage was placed on a heated surface until the mice regained consciousness and were ambulatory. A successful nerve crush was indicated by the mouse dragging its foot. The sciatic nerve is a mixed nerve and provides motor, sensory, and autonomic innervation of the lower limb. As such, there is no need for postoperative analgesics as no pain can be perceived at or below the injury including the incision, and injured mice do not show signs consistent with pain perception such as guarding or vocalization when handling above the normal amount during recovery. Muscles were harvested for analysis 4 weeks after nerve crush injury.
Immunohistochemistry and Imaging
Muscles were dissected from the hindlimb, weighed, placed in Tissue-Tek OCT (optimal cutting temperature) Compound (Sakura Finetek USA Inc., Torrance, CA), frozen in liquid nitrogen cooled 2-methylbutane (Fisher Scientific, Fair Lawn, NJ), and stored at −80°C. Cryosections (10 µm) were cut on a Leica CM1900 cryostat (Leica Microsystems, Buffalo Grove, IL). For myosin heavy chain staining, cryosections were rehydrated for 10 min in Dulbecco’s phosphate buffered saline (DPBS) at 4°C. Sections were then blocked for 15 min at 4°C with 10% normal goat serum (NGS) with 1% Nonidet-P40 in DPBS. Primary antibodies and dilutions were: laminin (1:200, L9393; Sigma-Aldrich, St. Louis, MO), EPHA8 (1:200, sc-25460; Santa Cruz Biotechnology, Inc, Dallas, TX) and either mouse monoclonal antibodies to myosin heavy chain Type I (1:50, BA-D5), Type IIa (1:50, SC-71), Type IIb (1:5, BF-F3) (Developmental Studies Hybridoma Bank) or all three antibodies to identify the (unstained) Type IIx myofibers overnight at 4°C. Sections were washed twice for 5 min with DPBS, one time for 15 min with 0.5% Tween20 in DPBS, one time for 5 min with DPBS, and fixed for 10 min at 4°C in 4% paraformaldehyde (PFA, Fisher Scientific, Fair Lawn, NJ). Slides were washed three times for 5 min with DPBS before incubation with Alexa Fluor 555 goat anti-mouse IgG and Alexa Fluor 488 goat anti-rabbit IgG secondary antibodies (1:500 dilution, Life Technologies, Eugene, OR) in 10% NGS in DPBS for 2 h at RT.
For MyHC I/IIA hybrid fiber staining, slides were incubated with Alexa Fluor 488-conjugated AffiniPure goat anti-mouse IgG, Fcγ subclass 2b specific (1:200 dilution, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), Rhodamine Red-X-conjugated AffiniPure goat anti-mouse IgG, Fcγ subclass 1 specific (1:50 dilution, Jackson ImmunoResearch Laboratories), and Alexa Fluor 633 goat anti-rabbit IgG secondary antibodies (1:500, Life Technologies) in 10% NGS in DPBS for 3 h at RT.
Following secondary antibody incubation, slides were washed twice for 5 min with DPBS, once for 15 min with 0.5% Tween20 in DPBS, and once for 5 min with DPBS.
For α-bungarotoxin staining, Alexa Fluor 647 conjugate of α-bungarotoxin (1:500 dilution, Life Technologies) in DPBS was incubated for 15 min at RT.
Slides were rinsed with water and coverslips were mounted using Vectashield Mounting Medium for Fluorescence with DAPI (Vector Laboratories, Inc., Burlingame, CA). Slides were imaged with an Olympus BX61 microscope (Waltham, MA) with a QImaging Retiga EXi digital camera (Surrey, BC). Images were stitched in ImageJ (35). The number of type I, type II (a, x, and b), and total muscle fibers were quantified in cross sections.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software (GraphPad Software, Inc.). Data were expressed as means ± standard error (means ± SE). Comparisons between two groups were evaluated using a paired t test. For three or more groups, one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons was performed. For all tests, a P value of <0.05 was considered to be statistically significant.
RESULTS
Changes to Muscle Fiber Type-Composition in the Absence of Ephrin-A3
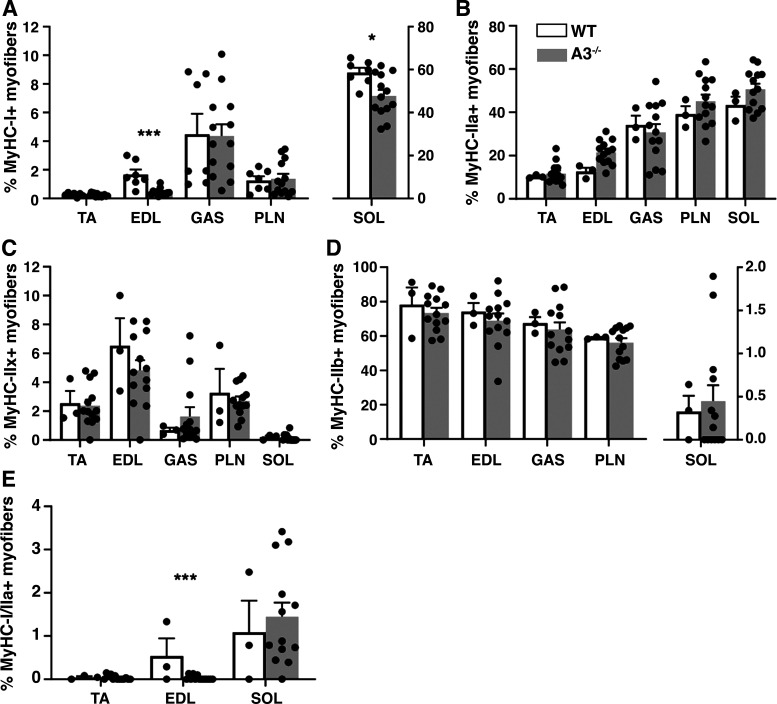
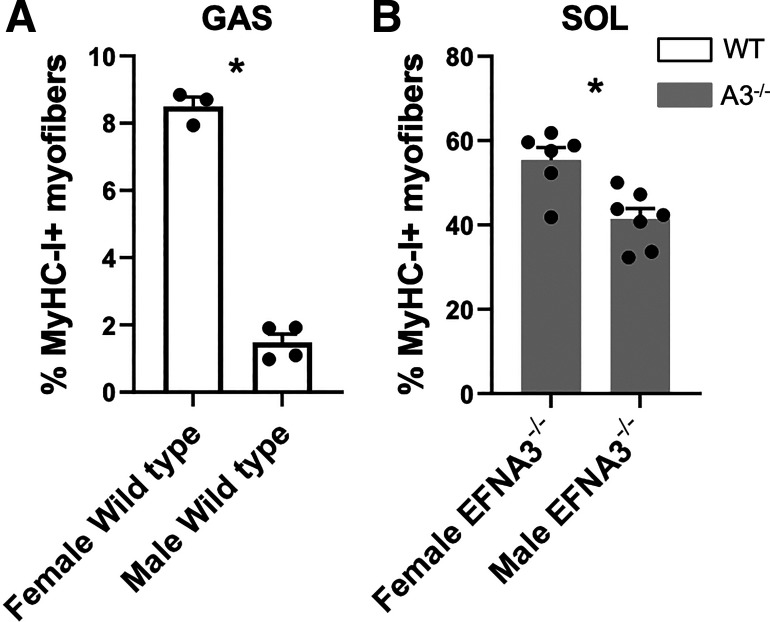
Fiber-type composition of hindlimb muscles from aged WT and ephrin-A3−/− mice was determined by immunohistochemistry (Fig. 1). Quantification of the percent of each fiber type in each of the hindlimb muscles examined are shown in Table 1; note that due to the presence of hybrid fibers coexpressing >1 MyHC the percentages are not expected to sum to 100%. Although there was no difference in the percentage of type I myofibers in the TA, gastrocnemius (GAS), and plantaris (PLN) muscles from aged ephrin-A3−/− and C57BL/6 mice, there was a significant decline from 1.68% to 0.44% of type I myofibers in extensor digitorum longus (EDL) from uninjured aged ephrin-A3−/− mice compared with C57BL/6 mice (P = 0.0007; Fig. 2A). A significant decline in the percentage of type I myofibers in the soleus (SOL) of aged uninjured ephrin-A3−/− mice was observed compared with C57BL/6 mice from 58% to 47% (P = 0.0498; Fig. 2A). In addition, sex-specific differences in the percentage of Type I myofibers in two muscles were noted: in gastrocnemius muscle, aged uninjured female WT muscle had 8.50% type I fibers whereas uninjured male WT muscle had 1.48% (P = 0.0405; appendix Fig. A1A). The percentage of type I myofibers in SOL of aged uninjured female ephrin-A3−/− mice was 55% whereas in aged uninjured male SOL muscle it was 41% (P = 0.0232; appendix Fig. A1B).
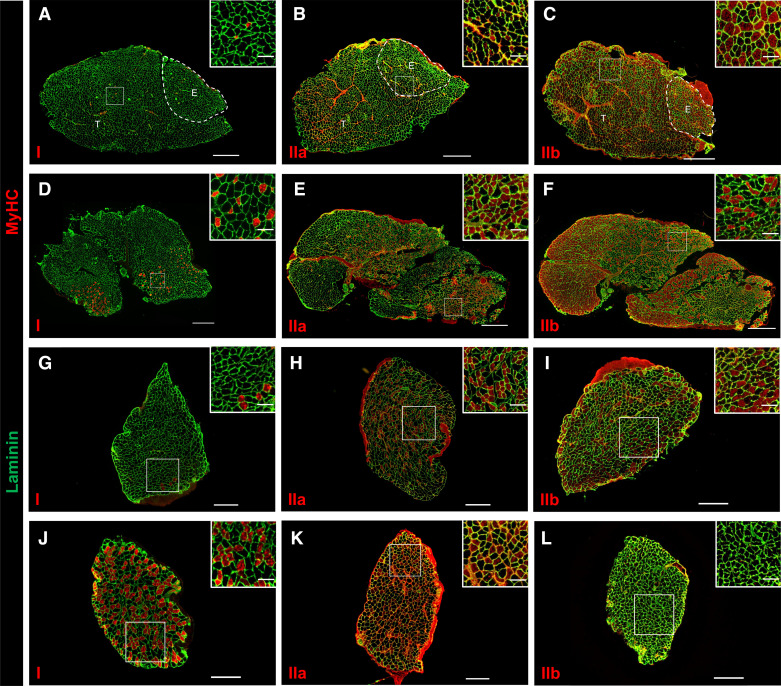
Figure 1.
Fiber-type representation in aged ephrin-A3−/− mouse muscles resembles aged wild-type (WT) mouse muscles. A–L: immunohistochemistry showing laminin (green) and myosin heavy chains (MyHCs) (red: MyHC-I, left; MyHC-IIa, middle; MyHC-IIb, right) expression in transverse cryosections from uninjured tibialis anterior (TA)-extensor digitorum longus (EDL) (A–C), gastrocnemius (GAS) (D–F), plantaris (PLN) (G–I), and soleus (SOL) muscles (J–L) from an aged ephrin-A3−/− mouse. A–F: scale bar, 600 µm. G–L: scale bar, 300 µm. Inset scale bar, 100 µm.
Table 1.
Muscle fiber type percentages in aged C57BL/6 and ephrin-A3−/− mice
| Muscle |
|||||
|---|---|---|---|---|---|
| MyHC | TA | EDL | GAS | PLN | SOL |
| Aged C57BL/6 uninjured | |||||
| I | 0.26 ± 0.05 | 1.68 ± 0.34 | 4.48 ± 1.43 | 1.26 ± 0.28 | 58.65 ± 2.18 |
| I/IIA | 0.04 ± 0.02 | 0.54 ± 0.41 | 1.09 ± 0.73 | ||
| IIA | 10.31 ± 0.42 | 12.64 ± 1.74 | 34.14 ± 4.28 | 39.19 ± 3.67 | 43.36 ± 3.85 |
| IIX | 2.54 ± 0.85 | 6.53 ± 1.91 | 0.69 ± 0.16 | 3.26 ± 1.66 | 0.20 ± 0.10 |
| IIB | 78.25 ± 9.99 | 74.21 ± 4.99 | 67.50 ± 3.52 | 59.02 ± 0.39 | 0.33 ± 0.18 |
| Aged ephrin-A3−/− uninjured | |||||
| I | 0.23 ± 0.03 | 0.44 ± 0.07 | 4.38 ± 0.80 | 1.38 ± 0.33 | 47.86 ± 2.71 |
| I/IIA | 0.03 ± 0.01 | 0.03 ± 0.01 | 1.45 ± 0.32 | ||
| IIA | 11.79 ± 1.31 | 21.52 ± 1.52 | 30.81 ± 3.77 | 45.17 ± 3.01 | 50.69 ± 2.55 |
| IIX | 2.39 ± 0.40 | 4.84 ± 0.69 | 1.65 ± 0.63 | 2.72 ± 0.29 | 0.14 ± 0.07 |
| IIB | 73.49 ± 2.89 | 69.04 ± 4.06 | 63.85 ± 4.07 | 56.21 ± 2.49 | 0.45 ± 0.18 |
| Aged C57BL/6 nerve crush | |||||
| I | 0.08 ± 0.04 | 1.57 ± 0.70 | 6.90 ± 0.96 | 1.85 ± 0.70 | 57.58 ± 0.59 |
| I/IIA | 0.05 ± 0.05 | 0.23 ± 0.12 | 7.99 ± 3.42 | ||
| IIA | 20.01 ± 3.50 | 26.14 ± 4.53 | 36.19 ± 5.27 | 47.09 ± 6.83 | 34.43 ± 3.99 |
| IIX | 2.47 ± 0.95 | 4.23 ± 2.07 | 0.84 ± 0.65 | 1.32 ± 0.54 | 0.17 ± 0.09 |
| IIB | 74.99 ± 4.68 | 73.97 ± 8.53 | 63.94 ± 0.54 | 61.40 ± 2.21 | 0.50 ± 0.27 |
| Aged ephrin-A3−/− nerve crush | |||||
| I | 0.29 ± 0.04 | 0.63 ± 0.13 | 5.25 ± 0.72 | 1.62 ± 0.45 | 50.02 ± 1.85 |
| I/IIA | 0.05 ± 0.02 | 0.05 ± 0.02 | 5.18 ± 0.68 | ||
| IIA | 25.18 ± 1.96 | 33.53 ± 2.93 | 44.98 ± 2.26 | 53.73 ± 3.36 | 44.79 ± 2.12 |
| IIX | 3.63 ± 0.65 | 7.34 ± 1.73 | 1.21 ± 0.30 | 2.07 ± 0.34 | 0.19 ± 0.09 |
| IIB | 74.85 ± 4.41 | 69.25 ± 5.09 | 68.27 ± 2.91 | 54.56 ± 4.64 | 0.61 ± 0.09 |
Values are means ± SE. EDL, extensor digitorum longus; GAS, gastrocnemius; MyHC, myosin heavy chain; PLN, plantaris; SOL, soleus; and TA, tibialis anterior.
Figure 2.
Muscle fiber-type composition of aged ephrin-A3−/− muscles is nearly indistinguishable from aged wild-type (WT) muscles. Percentage of type I (A), IIa (B), IIx (C), IIb (D), and I/IIa (E) hybrid fibers in tibialis anterior (TA), extensor digitorum longus (EDL), gastrocnemius (GAS), plantaris (PLN), and soleus (SOL) muscles. Data are presented as means ± SE. *P < 0.05; ***P < 0.001.
No significant differences in the percentage of IIa fibers in any of the hind limb muscles examined between ephin-A3−/− and C57BL/6 mice (Fig. 2B) was observed. Expression of MyHC-IIx was presumed in myofibers expressing neither Type I, IIa, nor IIb isoforms (36–38). No statistically significant differences in the percentages of Type IIx (Fig. 2C) or Type IIb (Fig. 2D) myofibers was observed in any of the muscles examined between ephrin-A3−/− and C57BL/6 mice. To determine the presence of type I/IIa hybrid myofibers, C57BL/6 and ephrin-A3−/− TA-EDL and SOL muscle sections were immunostained with antibodies for both type I and type IIa myofibers. In these experiments, a significant decrease in the percentage of I/IIa hybrid fibers (from 0.54 to 0.03%) was observed only in EDL from uninjured ephrin-A3−/− mice compared with uninjured WT mice (P = 0.0003; Fig. 2E).
Changes to Muscle Fiber Type Composition after Denervation
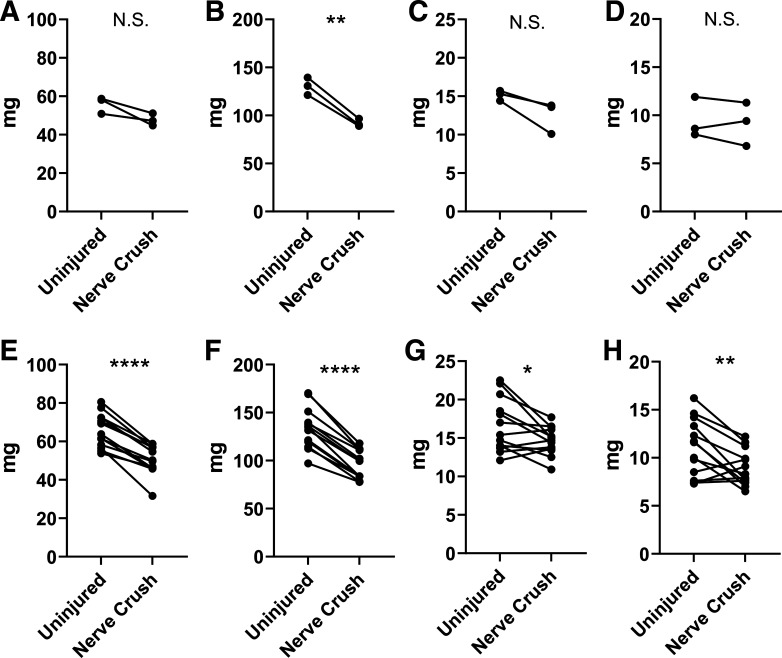
The reversion of aged ephrin-A3−/− muscles to “normal” fiber-type distributions with age was a surprising result, raising the question of which phenotype would be dominant after challenge by denervation/reinnervation. The model proposed in our prior publication posits that ephrin-A3 on slow myofibers prevents their inappropriate innervation by fast motor neurons. To test whether denervation followed by reinnervation would lead to recapitulation of the initial ephrin-A3−/− phenotype (e.g., a lack of slow myofibers) as would be predicted by this model, nerve crush was performed on aged WT and ephrin-A3−/− mice. Sciatic nerve crush causes innervated muscle to lose significant mass, and as would be expected decreased muscle mass was observed in the TA-EDL, GAS, PLN, and SOL muscles from both C57BL/6 and ephrin-A3−/− mice 4 weeks after nerve crush compared with uninjured muscle (appendix Fig. A2). Fiber-type composition of hindlimb muscles from aged WT and ephrin-A3−/− mice was quantified using immunohistochemistry following nerve crush (Table 1).
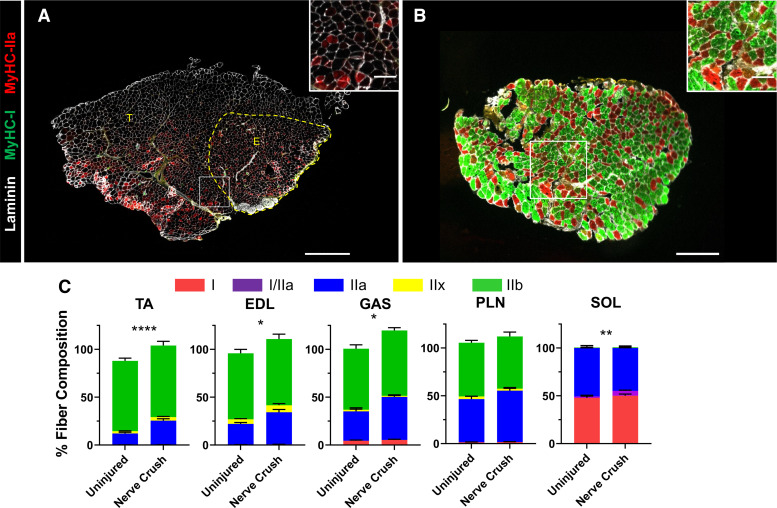
When fiber-type representation in TA, EDL, GAS, PLN, and SOL muscles from uninjured or nerve-crushed ephrin-A3−/− mice was scored, the percentage of IIa myofibers in aged ephrin-A3−/− mice was significantly increased in all muscles assayed (from 11% to 25% in TA, P < 0.0001; from 21% to 33% in EDL, P = 0.0106, 30% to 44%; P = 0.0421 in gastrocnemius, and 1.45% to 5.18%; P = 0.0021 in soleus; Fig. 3C). The cumulative fiber-type percentages were occasionally greater than 100% following nerve crush in the aged ephrin-A3−/− mice, suggesting an increased presence of hybrid fibers after reinnervation. Therefore, serial sections of TA-EDL and SOL muscle (Fig. 3, A and B) were assayed for I/IIa hybrid myofibers (as noted earlier, there is no reliable antibody for MyHC IIx and hybrid fibers tend to express adjacent MyHCs, thus because IIx is between IIa and IIb in the spectrum I/IIa are the only hybrid fibers that can be scored using this method). The percentage of I/IIa hybrid myofibers in the SOL was significantly increased after nerve crush in aged ephin-A3−/− mice compared with the uninjured (1.45% to 5.18%; P = 0.0021; Fig. 3C); it is probable that IIa/IIx and IIx/IIb hybrids are also increased but could not be scored.
Figure 3.
I/IIa hybrid and IIa myofibers increase in aged ephrin-A3−/− mice after nerve crush. Immunohistochemistry showing laminin (white), myosin heavy chain (MyHC)-I (green), and MyHC-IIa (red) expression in transverse cryosections from tibialis anterior (TA)-extensor digitorum longus (EDL) (A) and soleus (SOL) muscles from aged ephin-A3−/− mice 4 wk post-nerve crush (B). C: fiber-type distribution of the TA, EDL, gastrocnemius (GAS), plantaris (PLN), and SOL muscles of aged ephrin-A3−/− mice. Data are presented as means ± SE. *P < 0.05; **P < 0.01; ****P < 0.0001. A: scale bar, 600 µm. B: scale bar, 300 µm. Inset scale bar, 100 µm.
EphA8 Localization at Neuromuscular Junctions on Fast Myofibers is Maintained in Aged Mice, but Fast Myofiber Neuromuscular Junctions Lacking EphA8 Are Also Present
The neuromuscular junction (NMJ) is a tripartite synapse formed between motoneurons, muscle fibers, and terminal Schwann cells. Previous studies localized expression of several Ephs and ephrins at the neuromuscular junction (NMJ) during development and in adult muscle (39, 40). In our previous screen for mammalian EphA proteins at NMJs identified by acetylcholine receptors (AChRs) labeled with fluorochrome-conjugated α-bungarotoxin (BTX), it was noted that in young adult mice EphA8 was localized exclusively at NMJs associated with type II myofibers in the soleus (33). Further analysis using S100b-GFP transgenic mice (41) showed EphA8 expression on all and only terminal Schwann cells associated with fast fibers NMJs in adult WT mice (33). Using EphA8 on terminal Schwann cells as a proxy for motor neuron type, one would expect fast myofiber NMJs (innervated by a fast motor neuron) to be EphA8+ve, while slow myofiber NMJs (innervated by a slow motor neuron) to be EphA8−ve. However, for hybrid I/IIa myofibers, it would be possible to observe either EphA8+ve, slow myofiber NMJs or EphA8−ve, fast myofiber NMJs.
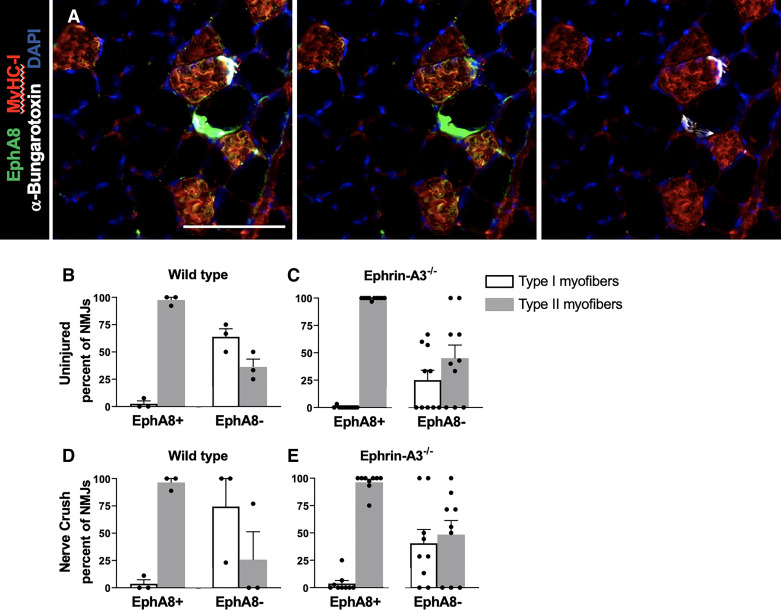
In this study, all identifiable NMJs in sections of gastrocnemius muscle from aged mice of both genotypes were scored for EphA8 and MyHC-I (Fig. 4A). Consistent with prior results, the majority of NMJs scored positive for EphA8 were on fast muscle fibers (MyHC-I−ve, red bars) in GAS muscle of aged uninjured WT (n = 56/57; Fig. 4B) as well as ephrin-A3−/− mice (n = 202/203; Fig. 4C), whereas EphA8+ve slow fibers (blue bars) were rare. However, unlike prior observations in 3–6 mo old mice, in aged mice of both genotypes some EphA8−ve NMJs were associated with fast myofibers (n = 4/12 in WT and n = 21/35 in ephrin-A3−/−).
Figure 4.
The majority of neuromuscular junctions (NMJs) on fast fibers in aged gastrocnemius (GAS) muscle express EphA8. A: section of GAS muscle (myosin heavy chain, MyHC-I+ve myofibers, red) showing expression of EphA8 (green) predominantly at fast myofiber NMJs (a-bungarotoxin, white). Scale bar, 100 µm. B and C: EphA8 expression is restricted to fast myofibers in young/adult mice, whereas slow fibers consistently lack EphA8 (33). In both wild type (WT) and ephrin-A3−/− mice, in uninjured muscle the great majority of EphA8+ve NMJs remain associated with fast myofibers (gray bars), but unlike young/adult mice EphA8−ve NMJs were not restricted to slow myofibers (white bars). n = 3 mice, two sections per sample in WT and n = 10 mice, two sections per sample in ephrin-A3−/−. D and E: after challenge by denervation/renervation, EphA8+ve NMJs in both WT and ephrin-A3−/− mice remained consistently associated with fast myofibers (gray bars) but the number of EphA8−ve fast myofibers was, surprisingly, even greater than in uninjured mice. n = 3 mice, two sections per sample in WT and n = 9 mice, two sections per sample in ephrin-A3−/−. Data are presented as means ± SE.
When EphA8 localization was scored four weeks after nerve crush, the majority of EphA8+ve NMJs remained associated with fast myofibers in aged WT (n = 60/62; Fig. 4D) and ephrin-A3−/− mice (n = 208/212; Fig. 4E). Surprisingly, however, EphA8−ve NMJs associated with fast myofibers not only persisted but increased, particularly in ephrin-A3−/− mice (n = 10/19 in WT, n = 32/49 in ephrin-A3−/−). Although this mismatch between fiber-type and EphA8 expression in aged ephrin-A3−/− mice was more prevalent than in aged WT, it did not reach statistical significance (P = 0.06).
DISCUSSION
At steady state in mature muscle, it is generally held that it is the frequency of electrical stimulation characteristic to each neuron type that specifies the fiber type and MyHC gene expression of myofibers (2). Although the fiber type representation and distribution in each muscle is established during development and reinforced by stabilization of NMJs postnatally, this pattern can also be modified by physiological stimuli such as training or pathological conditions such as disease or aging. In human muscle, the percentage of each fiber type appears not to change significantly with age (42–44). However, fiber type shifts toward a lower percentage of type II (fast-twitch) fibers with a corresponding increase in type I (slow-twitch) fibers with age (45), and increasing age leads to greater loss of fast-twitch than slow-twitch due to a reduction in the number and/or size of type II fibers (46).
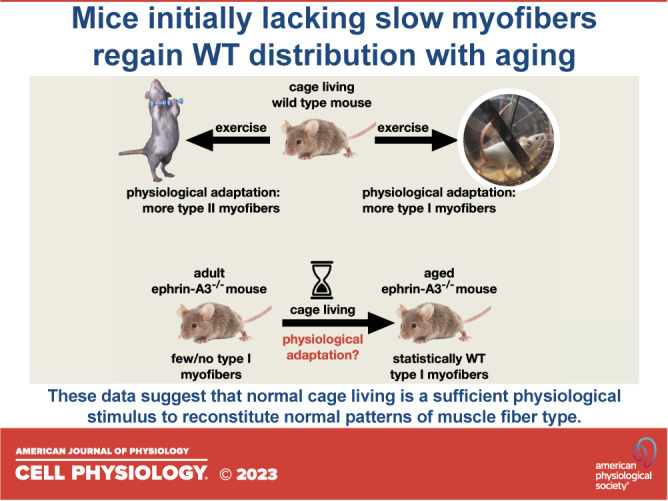
Our prior studies in ephrin-A3−/− adult mice (3–6 mo of age) noted a significant decrease in the number of MyHC-I+ve myofibers in all of the hindlimb muscles except the SOL (33). However, when slow fiber representation was re-examined in aged ephrin-A3−/− mice (23 mo of age), it was observed that while there remains a significant decrease in the percentage of type I myofibers in the EDL compared with aged C57BL/6 mice, there was no longer a significant difference in the number of slow fibers in the TA, GAS, or PLN. Sex-specific differences in muscle fiber type were not consistently observed from either mouse strain or muscle examined, nor were statistically significant differences between aged ephrin-A3−/− and C57BL/6 mice in the percentages of type IIa, type IIx, or type IIb myofibers in any of the muscles examined. We interpret these results as suggesting that over time and under even limited use (normal cage living), physiologically appropriate fiber-type distribution will become predominant even in the absence of ephrin-A3. The wild type distribution of fiber types, as selected for by evolution, could thus be considered an ongoing adaptation to mundane use, as fiber-type composition of lower hind limb muscles of ephrinA3−/− mice at two years of age was nearly indistinguishable from aged C57BL/6 mice.
All NMJs on fast fibers (MyHC-1−ve) in adult (3–6 mo) C57BL/6 and ephrin-A3−/− mice scored positive for EphA8 as did rare (n = 2/53) NMJs of MyHC-I+ve myofibers; all EphA8−ve NMJs were on slow fibers (33). This exclusive association of EphA8 with fast myofiber NMJs is lost with aging. Although the great majority of EphA8+ve NMJs remain associated with fast muscle fibers (MyHC-I−ve) in both aged C57BL/6 and ephrin-A3−/− mice, EphA8−ve fast myofiber NMJs became prevalent in both genotypes with aging and surprisingly increased following nerve crush. These myofibers may represent the MyHC-I/IIa hybrid population [usually ∼3% of myofibers; (47)] and/or could be undergoing fiber-type transition as the NMJs are destabilized with age. Muscles from elderly humans have increased numbers of fibers coexpressing multiple MyHC isoforms (12), with IIa and IIx hybrid fibers being especially frequent (48). In rat muscles, increases in fibers positive for nondominant adult MyHC isoform in SOL and GAS muscles with age are primarily due to presence of hybrid fibers (21); the significantly greater amount of coexpression in rats may indicate a fiber-type shift suggestive of denervation that outpaces reinnervation (22).
Denervation occurs in aging muscle as evidenced by the degeneration of endplates at the neuromuscular junction, leading eventually to a loss of muscle fibers (49). Neural cell adhesion molecule (NCAM) immunohistochemistry also demonstrated an increased number of denervated fibers in aged muscle (50), which is consistent with the muscle atrophy observed in aged mammals (15). Denervation also plays a role in the age-related shifts in MyHC expression seen in aging muscle (20–22), leading to an increase in MyHC-IIa as slow myofibers become faster and fast myofibers become slower (51). Denervation alone was shown to increase the percentage of type IIA fibers at the expense of type IIB and IIX fibers (24). Little change in the percentage of type I myofibers was observed following nerve crush in aged mice, which is in agreement with denervation via transected sciatic nerve (36).
The ephrin-A3−/− phenotype, characterized by a dramatic reduction of slow muscle fibers, becomes apparent at ∼3 weeks of age during the period of axon pruning when previously slow type I-MyHC expressing fibers are converted to type IIA myofibers (33). To determine whether denervation/reinnervation would recapitulate the developmental phenotype as motor neurons were forced to find and synapse with their target myofibers in the absence of ephrin-A3, nerve crush was performed. However, the representation of type I fibers 28 days after nerve crush actually increased slightly, although not significantly; the only significant change observed was an increase in the percentage of MyHC-IIa myofibers in TA, EDL, and GAS. This is consistent with observations that type IIA fibers are increased after denervation as well as with the increased occurrence of hybrid myofibers, which is also a characteristic of aged skeletal muscle (12–15, 20–22). We interpret these data as suggesting that due to the persistence of glial sheaths and other support structures, regenerating axons were guided to their original myofiber synapse independent of the effect of ephrin-A3.
The NMJ is a complex association of cell types including myofiber, motor neuron, and glia including terminal Schwann cells (52) and interactions between them determine the stability and connectivity of the synapse. An intriguing possibility suggested by these data is that the myofiber may play a more active role in determining fiber type and motor neuron matching during and after adaptation than is currently thought to be the case, possibly facilitated by the accumulating instability of the NMJ with age.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This study was supported by the National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases R01 AR067450 (to DDW Cornelison).
DISCLOSURES
C. L. Lorson is the co-founder and chief scientific officer for Shift Pharmaceuticals. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
R.W.A., T.C.M., K.L.H., D.A.S., and D.C. conceived and designed research; R.W.A., T.C.M., K.L.H., D.A.S., and E.V. performed experiments; R.W.A., T.C.M., K.L.H., D.A.S., and D.C. analyzed data; R.W.A., T.C.M., K.L.H., D.A.S., and D.C. interpreted results of experiments; R.W.A., T.C.M., and K.L.H. prepared figures; R.W.A. and D.C. drafted manuscript; R.W.A. and D.C. edited and revised manuscript; R.W.A., T.C.M., K.L.H., D.A.S., E.V., C.L.L., and D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The monoclonal antibodies to MyHC-I, MyHC-IIa, and MyHC-IIb developed by Stefano Schiaffino were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at the University of Iowa, Department of Biology, Iowa City, IA 52242.
Present addresses: T. C. Moritz, College of Veterinary Medicine, Iowa State Univ., Ames IA 50011, USA; K. L. Hahn, A.T. Still University’s Kirksville College of Osteopathic Medicine, Kirksville, MO 63501, USA; D. A. Stark, Ceva Animal Health LLC, Lenexa, KS 66215, USA; E. Villalón, Dept. of Biological Chemistry, Johns Hopkins Univ. School of Medicine, Baltimore, MD 21205, USA.
Appendix
Figure A1 show Data from male and female mice that were analyzed separately for sex-specific differences, and in almost all conditions there was no significant difference. However, in the GAS muscle in WT mice and the SOL muscle in ephrin-A3−/− mice, the percentage of Type I myofibers was significantly different in males vs. females. Figure 2A shows 4 weeks after nerve crush muscle mass is decreased, indicating successful denervation.
Figure A1.
Sex-specific differences in the percentage of type I myofibers in aged uninjured mouse muscles. Changes in type I muscle fiber-type percentage in aged wild-type (WT) gastrocnemius (GAS) (A) and aged ephrin-A3−/− soleus (SOL) muscles (B). Data are presented as means ± SE. *P < 0.05.
Figure A2.
Decreased muscle mass 4 wk post-nerve crush. Mass of tibialis anterior (TA)-extensor digitorum longus (EDL) (A), gastrocnemius (GAS) (B), plantaris (PLN) (C), and soleus (SOL) (D) muscles in aged wild-type (WT) mice. Mass of TA-EDL (E), GAS (F), PLN (G), and SOL (H) muscles in aged ephrin-A3−/− mice. *P < 0.05; **P < 0.01; ****P < 0.0001; N.S., not significant.
REFERENCES
- 1. Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531, 2011. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 2. Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci 33: 409–440, 2010. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- 3. Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech 50: 500–509, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 4. Agbulut O, Li Z, Mouly V, Butler-Browne GS. Analysis of skeletal and cardiac muscle from desmin knock-out and normal mice by high resolution separation of myosin heavy-chain isoforms. Biol Cell 88: 131–135, 1996. doi: 10.1016/S0248-4900(97)83528-9. [DOI] [PubMed] [Google Scholar]
- 5. Hämäläinen N, Pette D. The histochemical profiles of fast fiber Types IIB, ID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem 41: 733–743, 1993. doi: 10.1177/41.5.8468455. [DOI] [PubMed] [Google Scholar]
- 6. Dribin LB, Simpson SB. Histochemical and morphological study of dystrophic (C57BL/6J dy2j/dy2j) and normal muscle. Exp Neurol 56: 480–497, 1977. doi: 10.1016/0014-4886(77)90316-8. [DOI] [PubMed] [Google Scholar]
- 7. Parry DJ, Parslow HG. Fiber type susceptibility in the dystrophic mouse. Exp Neurol 73: 674–685, 1981. doi: 10.1016/0014-4886(81)90204-1. [DOI] [PubMed] [Google Scholar]
- 8. Parry DJ, Wilkinson RS. The effect of reinnervation on the distribution of muscle fibre types in the tibialis anterior muscle of the mouse. Can J Physiol Pharmacol 68: 596–602, 1990. doi: 10.1139/y90-086. [DOI] [PubMed] [Google Scholar]
- 9. Redenbach DM, Ovalle WK, Bressler BH. Effect of neonatal denervation on the distribution of fiber types in a mouse fast-twitch skeletal muscle. Histochemistry 89: 333–342, 1988. doi: 10.1007/BF00500634. [DOI] [PubMed] [Google Scholar]
- 10. Augusto V, Padovani CR, Campos GER. Skeletal muscle fiber types in C57Bl6J mice. Braz J morphol Sci 21: 89–94, 2004. [Google Scholar]
- 11. Wieczorek D, Periasamy M, Butler-Browne G, Whalen R, Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol 101: 618–629, 1985. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand 140: 55–62, 1990. doi: 10.1111/j.1748-1716.1990.tb08975.x. [DOI] [PubMed] [Google Scholar]
- 13. Krivickas LS, Yang J-I, Kim S-K, Frontera WR. Skeletal muscle fiber function and rate of disease progression in amyotrophic lateral sclerosis. Muscle Nerve 26: 636–643, 2002. doi: 10.1002/mus.10257. [DOI] [PubMed] [Google Scholar]
- 14. Palencia P, Quiroz-Rothe E, Rivero J-LL. New insights into the skeletal muscle phenotype of equine motor neuron disease: a quantitative approach. Acta Neuropathol 109: 272–284, 2005. doi: 10.1007/s00401-004-0940-1. [DOI] [PubMed] [Google Scholar]
- 15. Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One 7: e29082, 2012. doi: 10.1371/journal.pone.0029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7: e35273, 2012. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Talmadge RJ. Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve 23: 661–679, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 18. Martin TP, Stein RB, Hoeppner PH, Reid DC. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol (1985) 72: 1401–1406, 1992. doi: 10.1152/jappl.1992.72.4.1401. [DOI] [PubMed] [Google Scholar]
- 19. Rochester L, Barron MJ, Chandler CS, Sutton RA, Miller S, Johnson MA. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. II. Morphological and histochemical properties. Paraplegia 33: 514–522, 1995. doi: 10.1038/sc.1995.112. [DOI] [PubMed] [Google Scholar]
- 20. Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sport 13: 40–47, 2003. doi: 10.1034/j.1600-0838.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 21. Carter EE, Thomas MM, Murynka T, Rowan SL, Wright KJ, Huba E, Hepple RT. Slow twitch soleus muscle is not protected from sarcopenia in senescent rats. Exp Gerontol 45: 662–670, 2010. doi: 10.1016/j.exger.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 22. Snow LM, McLoon LK, Thompson LV. Adult and developmental myosin heavy chain isoforms in soleus muscle of aging Fischer brown Norway rat. Anat Rec A Discov Mol Cell Evol Biol 286: 866–873, 2005. doi: 10.1002/ar.a.20218. [DOI] [PubMed] [Google Scholar]
- 23. Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci 20: 2534–2542, 2000. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Windisch A, Gundersen K, Szabolcs M, Gruber H, Lømo T. Fast to slow transformation of denervated and electrically stimulated rat muscle. J Physiol 510: 623–632, 1998. doi: 10.1111/j.1469-7793.1998.623bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Himanen J-P, Saha N, Nikolov DB. Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell Biol 19: 534–542, 2007. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miao H, Wang B. EphA receptor signaling—complexity and emerging themes. Semin Cell Dev Biol 23: 16–25, 2012. doi: 10.1016/j.semcdb.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nikolov DB, Xu K, Himanen JP. Eph/ephrin recognition and the role of Eph/ephrin clusters in signaling initiation. Biochim Biophys Acta 1834: 2160–2165, 2013. doi: 10.1016/j.bbapap.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwamasa H, Ohta K, Yamada T, Ushijima K, Terasaki H, Tanaka H. Expression of Eph receptor tyrosine kinases and their ligands in chick embryonic motor neurons and hindlimb muscles. Dev Growth Differ 41: 685–698, 1999. doi: 10.1046/j.1440-169x.1999.00468.x. [DOI] [PubMed] [Google Scholar]
- 29. Kao T-J, Law C, Kania A. Eph and ephrin signaling: lessons learned from spinal motor neurons. Semin Cell Dev Biol 23: 83–91, 2012. doi: 10.1016/j.semcdb.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 30. Laussu J, Khuong A, Gautrais J, Davy A. Beyond boundaries—Eph:ephrin signaling in neurogenesis. Cell Adh Migr 8: 349–359, 2014. doi: 10.4161/19336918.2014.969990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lisabeth EM, Falivelli G, Pasquale EB. Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol 5: a009159, 2013. doi: 10.1101/cshperspect.a009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stark DA, Karvas RM, Siegel AL, Cornelison DDW. Eph/ephrin interactions modulate muscle satellite cell motility and patterning. Development 138: 5279–5289, 2011. doi: 10.1242/dev.068411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stark DA, Coffey NJ, Pancoast HR, Arnold LL, Walker JPD, Vallée J, Robitaille R, Garcia ML, Cornelison DDW. Ephrin-A3 promotes and maintains slow muscle fiber identity during postnatal development and reinnervation. J Cell Biol 211: 1077–1091, 2015. doi: 10.1083/jcb.201502036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carmona MA, Murai KK, Wang L, Roberts AJ, Pasquale EB. Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proc Natl Acad Sci USA 106: 12524–12529, 2009. doi: 10.1073/pnas.0903328106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Preibisch S, Saalfeld S, Tomancak P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25: 1463–1465, 2009. doi: 10.1093/bioinformatics/btp184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chakkalakal JV, Kuang S, Buffelli M, Lichtman JW, Sanes JR. Mouse transgenic lines that selectively label Type I, Type IIA, and Types IIX+B skeletal muscle fibers. Genesis 50: 50–58, 2012. doi: 10.1002/dvg.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martins KJB, Gordon T, Pette D, Dixon WT, Foxcroft GR, MacLean IM, Putman CT. Effect of satellite cell ablation on low-frequency-stimulated fast-to-slow fibre-type transitions in rat skeletal muscle. J Physiol 572: 281–294, 2006. doi: 10.1113/jphysiol.2005.103366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sartorius CA, Lu BD, Acakpo-Satchivi L, Jacobsen RP, Byrnes WC, Leinwand LA. Myosin heavy chains IIa and IId are functionally distinct in the mouse. J Cell Biol 141: 943–953, 1998. doi: 10.1083/jcb.141.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lai KO, Ip FCF, Cheung J, Fu AKY, Ip NY. Expression of Eph receptors in skeletal muscle and their localization at the neuromuscular junction. Mol Cell Neurosci 17: 1034–1047, 2001. doi: 10.1006/mcne.2001.0997. [DOI] [PubMed] [Google Scholar]
- 40. Yumoto N, Wakatsuki S, Kurisaki T, Hara Y, Osumi N, Frisén J, Sehara-Fujisawa A. Meltrin β/ADAM19 interacting with EphA4 in developing neural cells participates in formation of the neuromuscular junction. PLoS One 3: e3322, 2008. doi: 10.1371/journal.pone.0003322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zuo Y, Lubischer JL, Kang H, Tian L, Mikesh M, Marks A, Scofield VL, Maika S, Newman C, Krieg P, Thompson WJ. Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination. J Neurosci 24: 10999–11009, 2004. doi: 10.1523/JNEUROSCI.3934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol (1985) 85: 1337–1341, 1998. doi: 10.1152/jappl.1998.85.4.1337. [DOI] [PubMed] [Google Scholar]
- 43. Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, Schnohr P, Saltin B. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand 140: 41–54, 1990. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- 44. Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PWR. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol (1985) 78: 2033–2038, 1995. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- 45. Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol 46: 451–456, 1979. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 46. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50A: 11–16, 1995. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 47. Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol 115: 359–372, 2001. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- 48. Andersen JL, Terzis G, Kryger A. Increase in the degree of coexpression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve 22: 449–454, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 49. Gutmann E, Hanzlíková V. Motor unit in old age. Nature 209: 921–922, 1966. doi: 10.1038/209921b0. [DOI] [PubMed] [Google Scholar]
- 50. Urbanchek MG, Picken EB, Kalliainen LK, Kuzon WM. Specific force deficit in skeletal muscles of old rats is partially explained by the existence of denervated muscle fibers. J Gerontol A Biol Sci Med Sci 56: B191–B197, 2001. doi: 10.1093/gerona/56.5.b191. [DOI] [PubMed] [Google Scholar]
- 51. Ciciliot S, Schiaffino S. Regeneration of mammalian skeletal muscle: basic mechanisms and clinical implications. Curr Pharm Des 16: 906–914, 2010. [Erratum in Curr Pharm Des 21: 4657, 2015]. doi: 10.2174/138161210790883453. [DOI] [PubMed] [Google Scholar]
- 52. Sanes J, Lichtman J. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci 22: 389–442, 1999. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.