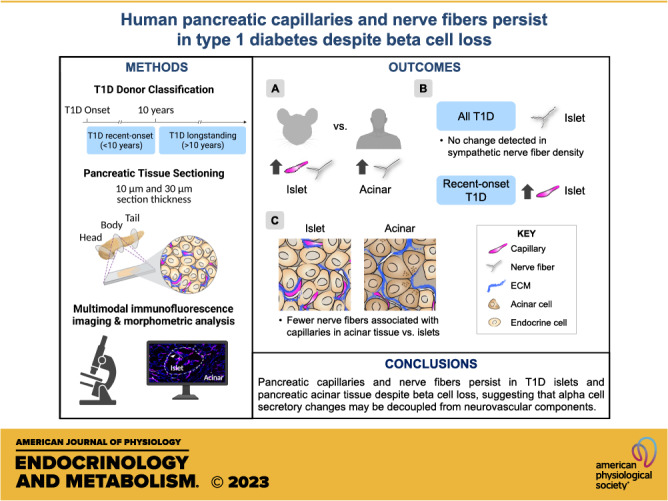
Keywords: blood vessels, exocrine tissue, islets, nerve fibers, type 1 diabetes
Abstract
The autonomic nervous system regulates pancreatic function. Islet capillaries are essential for the extension of axonal projections into islets, and both of these structures are important for appropriate islet hormone secretion. Because beta cells provide important paracrine cues for islet glucagon secretion and neurovascular development, we postulated that beta cell loss in type 1 diabetes (T1D) would lead to a decline in intraislet capillaries and reduction of islet innervation, possibly contributing to abnormal glucagon secretion. To define morphological characteristics of capillaries and nerve fibers in islets and acinar tissue compartments, we analyzed neurovascular assembly across the largest cohort of T1D and normal individuals studied thus far. Because innervation has been studied extensively in rodent models of T1D, we also compared the neurovascular architecture between mouse and human pancreas and assembled transcriptomic profiles of molecules guiding islet angiogenesis and neuronal development. We found striking interspecies differences in islet neurovascular assembly but relatively modest differences at transcriptome level, suggesting that posttranscriptional regulation may be involved in this process. To determine whether islet neurovascular arrangement is altered after beta cell loss in T1D, we compared pancreatic tissues from non-diabetic, recent-onset T1D (<10-yr duration), and longstanding T1D (>10-yr duration) donors. Recent-onset T1D showed greater islet and acinar capillary density compared to non-diabetic and longstanding T1D donors. Both recent-onset and longstanding T1D had greater islet nerve fiber density compared to non-diabetic donors. We did not detect changes in sympathetic axons in either T1D cohort. Additionally, nerve fibers overlapped with extracellular matrix (ECM), supporting its role in the formation and function of axonal processes. These results indicate that pancreatic capillaries and nerve fibers persist in T1D despite beta cell loss, suggesting that alpha cell secretory changes may be decoupled from neurovascular components.
NEW & NOTEWORTHY Defining the neurovascular architecture in the pancreas of individuals with type 1 diabetes (T1D) is crucial to understanding the mechanisms of dysregulated glucagon secretion. In the largest T1D cohort of biobanked tissues analyzed to date, we found that pancreatic capillaries and nerve fibers persist in human T1D despite beta cell loss, suggesting that alpha cell secretory changes may be decoupled from neurovascular components. Because innervation has been studied extensively in rodent T1D models, our studies also provide the first rigorous direct comparisons of neurovascular assembly in mouse and human, indicating dramatic interspecies differences.
INTRODUCTION
Hormone secretion from pancreatic islets of Langerhans is essential to maintain blood glucose levels within a narrow physiological range. Vascular and neuronal inputs to the islet aid in the regulation of coordinated hormone secretion (1–5). Alpha cells within the islet secrete glucagon to counterbalance insulin secretion by beta cells and prevent hypoglycemia. In type 1 diabetes (T1D), alpha cell function is dysregulated with loss of glucagon secretion in response to hypoglycemia (6, 7), which interestingly precedes a secretory impairment of a major alpha cell stimulus, epinephrine, produced by adrenal chromaffin cells (7). Paradoxically, individuals with T1D secrete more glucagon during mixed-meal stimulation (8, 9). Recurrent hypoglycemic episodes, which correlate with progressive beta cell loss in T1D, lead to an impairment of sympathoadrenal responses and the risk of a life-threatening hypoglycemia unawareness syndrome, also known as hypoglycemia-associated autonomic failure (10). Additionally, autonomic dysfunction could be further exacerbated with disease duration, and progressive diabetic neuropathy may affect autonomic innervation of the pancreatic islet (1, 7, 8, 11). Thus, defining the neurovascular architecture in the pancreas of individuals with T1D is crucial to understanding the mechanisms of dysregulated glucagon secretion.
Studies from rodent and human T1D pancreatic tissues provide a spectrum of inferences on disease-associated intraislet innervation phenotypes. Work first done in rodent models of T1D, such as the BioBreeder (BB) rat and the nonobese diabetic (NOD) mouse, showed a loss of intraislet sympathetic nerve fibers associated with insulitis and decreased glucagon secretion in response to tyramine-mediated norepinephrine release (12–16). By contrast, a recent study used optically cleared tissue from NOD mice to show an increase in islet nerve fibers (17). Other studies, however, revealed considerable interspecies differences in intraislet nerve fiber and capillary densities, thus raising concerns about whether the data from rodent models reflect the findings about human islet neurovascular architecture in T1D (18–21). Moreover, two recent studies of human pancreatic sympathetic innervation in short-duration T1D reached opposing conclusions as well: one study indicated an early decline in islet sympathetic nerve density in T1D (22), whereas the other showed no significant differences between non-diabetic and short-duration T1D donor islets (23). Interestingly, the latter study found that pancreata from autoantibody-positive (AAb+) human donors had lower sympathetic nerve fiber density, specifically within pancreatic islets (23). In addition, Lundberg et al. (24) showed that the second arm of the autonomic nervous system, parasympathetic nerve fibers, may also be altered in human T1D. Considering the developmental and functional links between vascularization and innervation (25–27), it is noteworthy that islet vessel density was found to be greater in short-duration T1D than in non-diabetic donors (28).
Thus, there is a need to systematically define changes in the human neurovascular architecture across a broader range of T1D duration and a large cohort, including not only islet but also acinar tissue compartment, since both appear to be influenced in the disease process (29, 30). Because innervation has been studied extensively in rodent T1D models, we first compared the islet neurovascular architecture between mouse and human. We found dramatic interspecies differences that were not easily explained by transcriptomic profiles of factors guiding angiogenesis and neuronal development. Next, we performed high-throughput morphometric analyses of the islet neurovascular architecture in thin human pancreatic tissue sections from a large cohort of biobanked tissues, including 15 recent-onset and 12 longstanding T1D donors, and compared them to 11 non-diabetic control donors. We found that islets and acinar tissue had greater capillary density in recent-onset T1D. This was accompanied by overall increases in islet nerve fiber densities visualized by a panneuronal marker in both early-onset and longstanding T1D. We did not detect changes in sympathetic axon densities in T1D tissues. Further assessment of three-dimensional (3-D) reconstructions from thicker sections (30 μm vs. 10 μm) highlighted the persistence of intraislet nerves in T1D donors and a relationship between pancreatic nerves, blood vessels, and extracellular matrix (ECM). Collectively, our data indicate that islet capillaries and nerve fibers persist in T1D, suggesting that alpha cell secretory changes may be decoupled from neurovascular components.
RESEARCH DESIGN AND METHODS
Mice
Animal studies were approved by the Institutional Animal Care and Use Committee at Vanderbilt University Medical Center, and animals were kept in facilities monitored by the Vanderbilt University Division of Animal Care. Pancreata from anesthetized C57BL/6 wild-type mice (N = 10 mice; adult males >10 wk old) were collected, lightly paraformaldehyde (PFA) fixed in 4% PFA in 1× PBS, cryoprotected in 30% (wt/wt) sucrose, and frozen in Tissue-Tek Optimal Cutting Temperature (OCT) compound before cryosectioning (26).
Human Donors
Pancreata from non-diabetic (N = 11 donors) and T1D (N = 27 donors) donors were obtained through partnerships with the International Institute for Advancement of Medicine (IIAM), the National Disease Research Interchange (NDRI), the Human Pancras Analysis Program (HPAP), and local organ procurement organizations. Pancreata were processed in Pittsburgh by R. Bottino or at the University of Pennsylvania through the HPAP T1D program for both islet isolation and histological analysis, as previously described (31, 32). Donor information is detailed in Table 1 and Supplemental Table S1 Deidentified medical records provided information for T1D staging. The Vanderbilt University Institutional Review Board declared that studies on deidentified human pancreatic specimens do not qualify as human subject research.
Table 1.
Overview of donor cohort characteristics
| Donor Group | N | Age, yr | Disease Duration, yr | Sex (F/M) | BMI, kg/m² | HbA1c, % | C-Peptide, ng/mL |
|---|---|---|---|---|---|---|---|
| Non-diabetic | 11 | 31 ± 15 | N/A | 3/11 | 30 ± 13 | 5.57 ± 0.30 | 13.18 ± 11.41 |
| T1D Recent-onset (<10 yr) | 15 | 25 ± 14 | 6 ± 2 | 5/15 | 24 ± 6 | 9.49 ± 0.168 | 0.11 ± 0.13* (vs. ND and T1D >10 yr) |
| T1D Longstanding (>10 yr) | 12 | 45 ± 14 | 31 ± 11 | 3/12 | 27 ± 5 | 8.9 ± 1.45 | < 0.02 or UD* (vs. ND and T1D <10 yr) |
Averages are displayed as means ± SD (N = sample size) when applicable for non-diabetic (ND) and type 1 diabetes (T1D) donors. For statistical purposes, C-peptide values < 0.02 ng/mL (the lower limit of assay detection) and undetectable C-peptide levels were set to 0. For recent-onset T1D, 4 donors had C-peptide values < 0.02 ng/mL and 3 donors had undetectable values. For longstanding T1D, 7 donors had C-peptide levels < 0.02 ng/mL and 4 donors had undetectable values. The longstanding T1D donor with a C-peptide level of 1.78 was determined to be an outlier and excluded from analyses. BMI, body mass index; F, female; HbA1c, hemoglobin A1c; M, male; UD, undetectable. *Statistically significant, P value < 0.05 for comparisons between donor groups assessed by Mann–Whitney tests.
Human Pancreas Procurement and Immunohistochemical Analysis
Pancreata from non-diabetic and T1D donors (see Table 1 and Supplemental Table 1 for donor information) were received within 18 h from cross clamp, maintained in cold preservation solution on ice, and processed as described previously (31, 32). Importantly, fixation methodologies were similar between mouse and human pancreatic tissue. Multiple 10-μm and 30-μm serial cryosections from the pancreatic head, body, and tail regions of 27 T1D and 11 age-matched non-diabetic donors were lightly PFA postfixed in 1% PFA in 1× PBA and then labeled for immunofluorescence as described previously (31, 32). Primary and secondary antibodies and their working dilutions are listed in Supplemental Tables S2 and S3. Acinar tissue was visualized by DAPI nuclear counterstain, and islet location was defined by hormone markers. Insulin and glucagon labeling was visualized on the same fluorescence channel unless otherwise indicated because a maximum of four imaging channels was available per section for this immunofluorescence paradigm.
Morphometric Analysis of 2-D Images
Digital images of 10-μm cryosections were acquired with a ScanScope FL (Aperio) for human tissue and a Leica DMI6000 B Microscope (Leica Biosystems) for mouse tissue. These images were then analyzed with MetaMorph v.7.10 software (Molecular Devices LLC). Analyses were run on manually annotated islets (region of interest, ROI) identified by hormone staining. Three equally sized ROIs, outside of islet annotations, were randomly selected per image for acinar measurements. Large nerve fiber bundles and vessels around pancreatic ducts and ganglia were excluded from formal analyses. Intensity thresholding was set per image per individual fluorescence channel to collect object data with the Integrated Morphometry Analysis function (MetaMorph).
For innervation measurements, a pixel filter was set to ≥0.463 μm to avoid the inclusion of single pixels as neural fibers. A pixel filter was set to ≥2.000 μm for vasculature analysis to prevent the inclusion of single pixels and nonvascular structures while also accounting for average islet vessel diameters determined by previous studies to be between 2 and 10 μm (20, 28, 33). Islet and acinar nerve fiber length, nerve fiber density, and vascular density were calculated by dividing MetaMorph output by the total ROI area. The analyzed islet area was similar between groups (Supplemental Fig. S11). We analyzed an average of 34 ± 1 islets with cross-sectional diameter >50 μm/donor/condition. For a donor to be included in aggregate analyses, a minimum of 10 islets needed to be assessed. Detailed metrics can be found in respective figure legends.
Analysis of ECM Overlap with Nerve Fibers
Digital images of 30-μm sections were acquired with Zeiss LSM880 (Zeiss Microscopy Ltd) or Olympus FV3000 (Olympus) microscopes. Z stacks were assembled to generate 3-D reconstructions and maximum-intensity projections with cellSens (Olympus) and ZEN (Zeiss) software. To quantify the overlap between the panneuronal marker [tubulin beta 3 (TUBB3)] and the ECM marker [collagen IV (COLIV)], we used Manders’ overlap coefficients generated from the ImageJ plugin “Just Another Co-localization Plugin” (https://imagej.nih.gov/ij/plugins/track/jacop2.html).
Image Processing
All confocal images underwent deconvolution and were then displayed as maximum-intensity projections. Nearest neighbor-constrained iterative algorithms were employed with cellSens v.3.1 (Olympus). Z stack and 3-D reconstruction videos were made with cellSens, Imaris v.9.8 (Oxford Instruments), or ImageJ software and exported as mp4 files.
Mouse vs. Human Transcriptomic Profile Comparison
Previously published data were queried to determine general expression profiles of angiogenic, neuronal, and morphogenic pathways and extracellular matrix components in mouse and human islet cells. Gene lists were compiled by authors based on previous publications (25, 34) and HUGO Gene Nomenclature Committee (HGNC)-curated gene group pages (35). Normalized count matrices were accessed from bulk RNA sequencing (RNA-seq) data sets as follows: mouse beta and endothelial cells, GSE163825 (34); human endothelial cells, GSE157546 (36); human beta cells (37). Raw single-cell (sc) data sets were accessed as follows: mouse, GSE159844 (38); human, GSE183568 (39). See Supplemental Table S4 for information on experimental parameters, sample sizes, and specific samples utilized. For the sc mouse data set, wild-type samples were processed and visualized by uniform manifold alignment projection (UMAP) using 30 principal component analysis (PCA) dimensions, and K-nearest neighbor (KNN)-based clustering was performed with a resolution of 0.5 in Seurat (40), yielding 18 clusters. A total of 6 clusters were removed based on strong expression of exocrine markers or mixed cell type assignment, and then further clustering of remaining cells (30 dimensions at resolution = 1) yielded 21 clusters. Cell type assignment was determined with canonical markers (see Supplemental Table S4) for endocrine, endothelial, and immune cell populations, and multiple clusters expressing the same canonical markers were combined as applicable. Stellate cells could not be resolved from endothelial cells in this data set. For the sc human data set, clustering was performed as previously described (39), with technical replicates combined and donor values averaged. In Fig. 1, G and H, Fig. 2G, and Supplemental Fig. 5, for clarity only genes expressed in >2% of cells in cell types were kept (sc), and log2 expression >8 (bulk) were graphed. All values will be provided in a data file upon publication.
Figure 1.
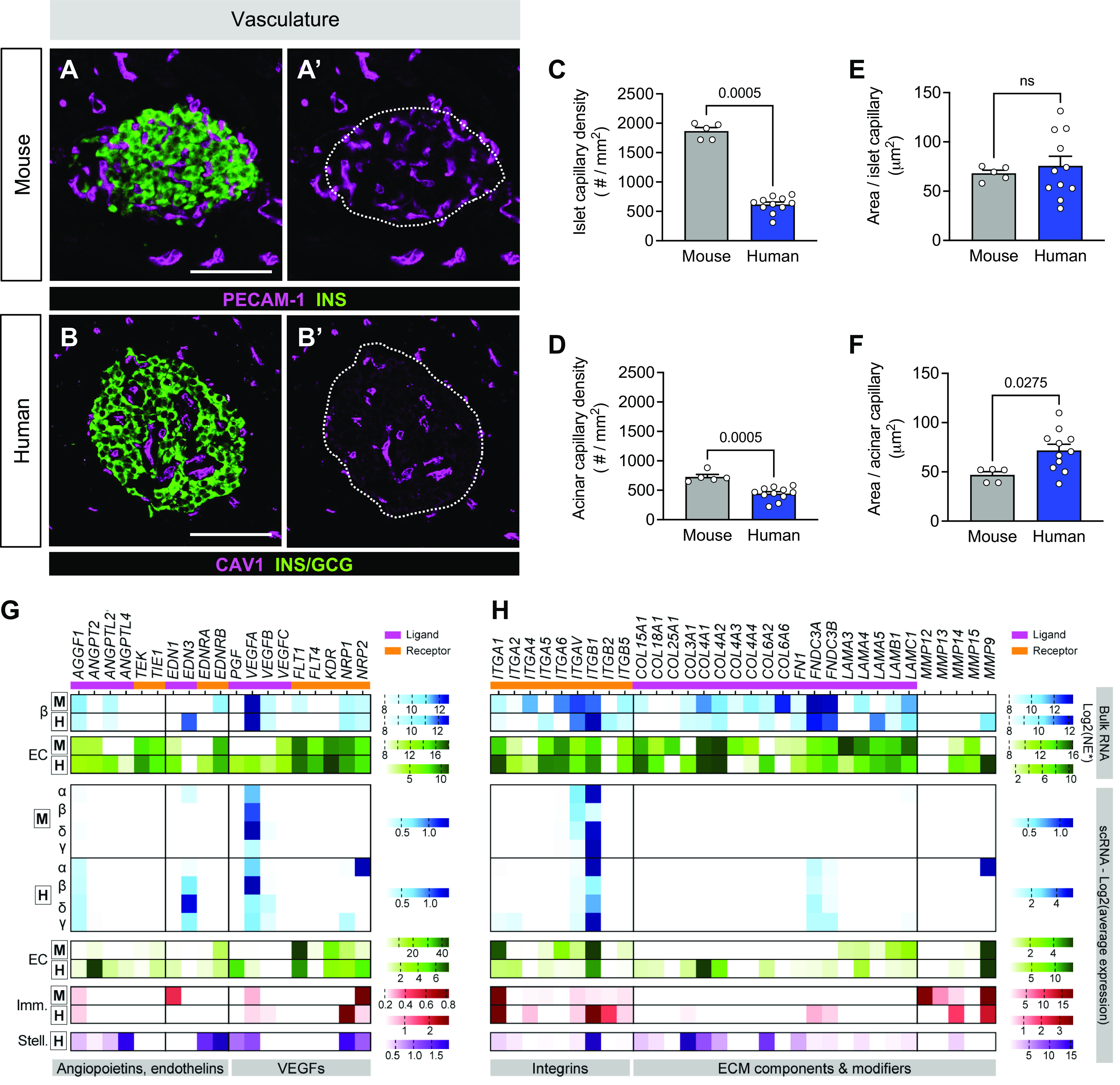
Human islets are less vascularized than mouse islets. A–F: representative immunofluorescent staining (A and A′, B and B′) and quantification (C–F) of vasculature as measured by endothelial cell staining [platelet endothelial cell adhesion molecule 1 (PECAM-1) or caveolin-1 (CAV1); magenta]. Islets are visualized by hormones [insulin (INS), glucagon (GCG); green], and A′ and B′ show PECAM-1 or CAV1 only with islet area outlined. Scale bars, 100 μm. Quantification (mean ± SE) includes capillary density (C and D) and area per capillary in islets (E) and acinar tissue (F) from mouse (gray; N = 5 mice; 129 total islets, 127 total acinar measurements) and human (dark blue; N = 11 donors; 488 total islets, 1,392 total acinar measurements). Symbols on bar graphs represent individual mouse or human donors. Statistically significant P values (<0.05) are stated for Mann–Whitney tests. G and H: transcript levels of select angiogenic factors and receptors (G) and extracellular matrix (ECM) molecules (H) detected in islet endocrine cells, endothelial cells, and immune cells as measured by RNA sequencing (RNA-seq). From top to bottom: mouse bulk data sets (34); human bulk beta (β) (37); human bulk endothelial (EC) (36); single-cell (sc) data sets: mouse (38) and human (39). Boxed “M” and “H” label mouse and human data sets, respectively. Only values > 0.2 (sc) and log2 > 8 (bulk) are graphed. Normalized expression (NE) units vary by study; see research design and methods. Imm., immune; Stell., stellate; VEGF, vascular endothelial growth factor.
Figure 2.
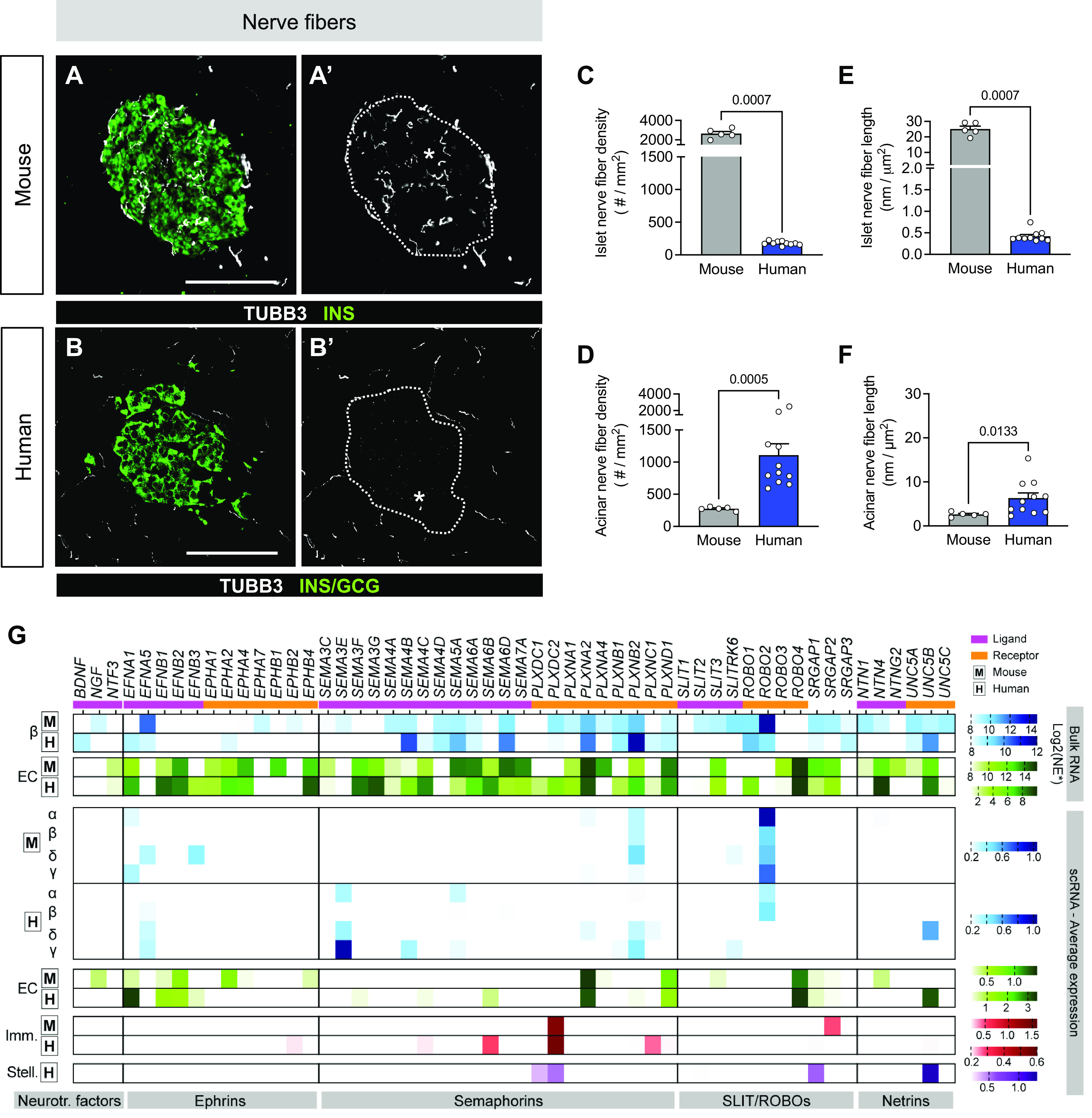
Human islets are less innervated than mouse islets. A–F: representative immunofluorescent staining (A and A′, B and B′) and quantification (C–F) of nerve fibers, as measured by tubulin beta 3 (TUBB3; white). Islets are visualized by hormones [insulin (INS), glucagon (GCG); green], and A′ and B′ show TUBB3 only with islet area outlined. Scale bars, 100 μm. Quantification (mean ± SE) includes TUBB3+ fiber density (C and D) and length in islets (E) and acinar tissue (F) from mouse (N = 5 mice; 128 total islets, 262 total acinar measurements) and human (N = 11 donors; 547 total islets, 1,830 total acinar measurements). Symbols on bar graphs represent individual mouse or human donors. Statistically significant P values (< 0.05) are stated for Mann–Whitney tests. G: transcript levels of select neuronal ligands and receptors as measured by RNA sequencing (RNA-seq). From top to bottom: mouse bulk data sets (34); human bulk beta (β) (37); human bulk endothelial (EC) (36); single-cell (sc) data sets: mouse (38) and human (39). Boxed “M” and ‘H’ label mouse and human data sets, respectively. Neurotr. factors, neurotrophic factor genes. Only values > 0.2 (sc) and log2 > 8 (bulk) are graphed. Normalized expression (NE) units vary by study; see research design and methods. Imm., immune; Stell., stellate.
Statistical Analysis
Statistical tests are described in the figure legends and text where appropriate. Data are represented as mean ± standard error (SE), with each mouse or donor considered as N = 1. Outliers were determined and excluded from analyses with the Robust regression and Outlier removal (ROUT) method with Q = 1%. P values < 0.05 were considered significant, and nonsignificant P values are designated with “ns” or not denoted. Statistical comparisons were performed with GraphPad Prism software 8.0–9.3 and R version 4.1.2.
RESULTS
Neurovascular Architecture of Pancreatic Islets and Acinar Tissue Differs Significantly between Mouse and Human
To better understand prior reports of interspecies differences in neurovascular architecture, we first defined the neurovascular architecture in non-diabetic mouse pancreas, focusing on both islets and acinar tissue, and compared it to human. Blood vessels were visualized by immunofluorescence labeling for platelet endothelial cell adhesion molecule 1 (PECAM-1), nerve fibers were labeled for panneuronal marker tubulin beta 3 (TUBB3), and islet beta and alpha cells were colabeled for insulin (INS) and glucagon (GCG), respectively. To confirm TUBB3 immunolabeling of nerve fibers in human tissue, we assessed 30 laser-scanning confocal microscopy images in which individual image tiles (captured at ×10 magnification) were stitched to visualize the entire pancreatic tissue sections. Additionally, we reviewed >1,000 whole slide images (captured at ×20 magnification) that were used for our high-throughput morphometric analyses. We found many TUBB3+ interlobular nerve fiber bundles throughout our tissues, demonstrating the utility of this neuronal marker (Supplemental Fig. 1). We then utilized morphometric analyses to measure capillary and nerve fiber structural characteristics (Supplemental Fig. S2, a–c). Using this approach, we found striking interspecies differences; capillaries were much more abundant in mouse islets compared to human (Fig. 1, A and B), with capillary density being greater in islets than acinar tissue in both species (Supplemental Fig. S3, a and b). The capillary size was similar in the islet and acinar tissue compartments in mouse and human (Fig. 1, E and F, Supplemental Fig. S3, c and d).
To investigate possible explanations for the species differences in vascularization with emerging islet bulk and single-cell RNA sequencing (RNA-seq) data, we compared the transcriptional profile of angiogenic factors and their cognate receptors in cells from mouse and human islets using existing resources and, for the first time in this context, comprehensively assessed mouse and human islet data sets side by side. The cell-specific expression of angiogenic ligand and receptor transcripts was similar in bulk and single-cell (sc) RNA-seq data sets from mouse (34, 38) and human (36, 37, 39) islets (Fig. 1G), notably with high vascular endothelial growth factor A (VEGFA) expression in endocrine cells and associated receptor kinase insert domain receptor (KDR) highly expressed in endothelial cells. Transcripts encoding ECM components, including integrins, collagens, and laminins, were detected at high levels in pooled islet endothelial cells from both species, as well as single stellate cells from human islets (Fig. 1H).
Nerve fiber densities were 15-fold greater and nerve fiber lengths were 63-fold greater in mouse compared with human (Fig. 2, A and B). In contrast, human acinar tissue had greater nerve fiber density and length compared with mouse (Fig. 2, D and F). Expression profiles of molecules that directly provide signals for axon growth, survival, and guidance cues, such as neurotrophic factors, ephrins, and semaphorins, and their associated receptors were relatively consistent between mouse and human islets, whereas SLIT/ROBO pathway components were detected at higher levels in mouse beta cells compared with human (Fig. 2G). Overall, the nerve fibers in the human pancreas were surprisingly more abundant and longer in acinar tissue than in islets (Supplemental Fig. S3, e–h), and there were modest differences in neuronal signaling pathways by transcriptomic analysis (Supplemental Fig. S5). Additionally, percent capillary and nerve fiber area analyses revealed similar findings as described above (Supplemental Fig. S4). Our results demonstrate that the neurovascular architecture of islets and acinar tissue differs considerably between mouse and human, supporting prior findings on pancreatic interspecies differences (17–19). We also showed that despite significant interspecies differences in islet neurovascular architecture, transcriptomic profiles of factors guiding angiogenesis and neuronal development in islets were quite similar, suggesting other cross-species regulatory relationships.
Capillary Density Is Greater in Islet and Acinar Tissue of Recent-Onset T1D
Utilizing the largest T1D cohort to date to assess neurovascular architecture, we investigated whether architectural changes are associated with or related to T1D disease duration. Because the frequency of neuropathy increases with longer diabetes duration and the heterogeneity of T1D tissues, we subdivided a T1D donor cohort of 27 donors into two groups (Fig. 3A): 1) relatively recent-onset (<10 yr of disease) and 2) longstanding (>10 yr of disease) T1D (31). Recent-onset T1D donors had low but measurable C-peptide levels, whereas the levels in longstanding T1D donors were mostly below the detection limit (Table 1). Additionally, 33% (6 recent-onset; 3 longstanding) of these T1D donors were previously phenotyped for endocrine composition and/or islet function (31, 41, 42). The deidentified record of these donors did not specify whether the donors had autonomic or peripheral neuropathy (donor summary in Table 1 and Supplemental Table S1).
Figure 3.
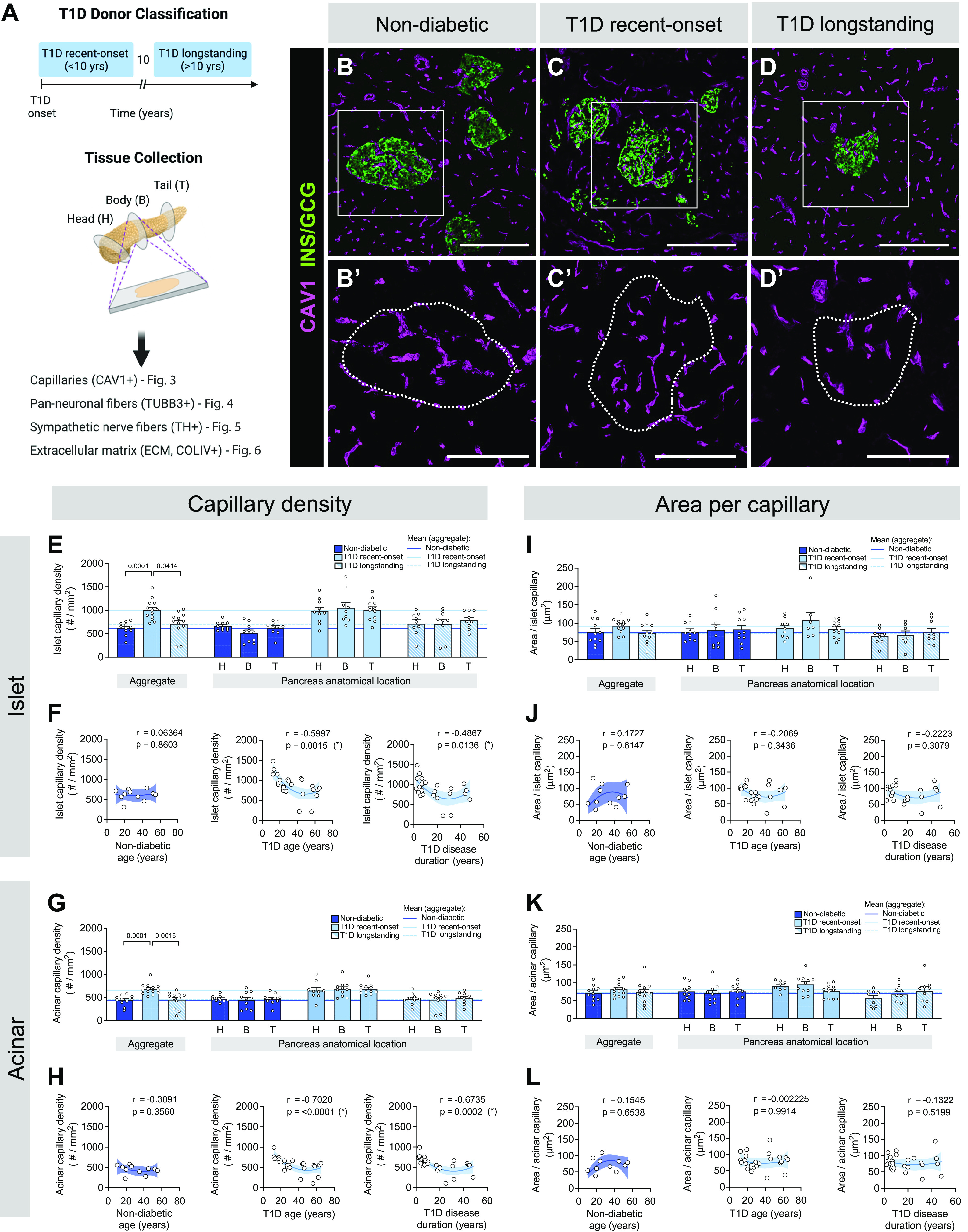
Islets in recent-onset type 1 diabetes (T1D) have greater vascular density compared with controls. A: schematic illustrating donor classification and experimental approach. B–D: representative immunofluorescent staining of vasculature labeled with caveolin-1 (CAV1; magenta) in pancreatic tissue sections from non-diabetic (B), recent-onset T1D (C), and longstanding T1D (D) donors. GCG, glucagon; INS, insulin. B′–D′: insets of B–D, CAV1 channel only, with islet area outlined. Scale bars, 100 μm. E–L: quantification (mean ± SE) of capillary density (E–H) and area per capillary (I–L) in islet (E, F, I, and J) and acinar (G, H, K, and L) tissue from pancreata of non-diabetic (dark blue; N = 11 donors; 488 total islets, 1,392 total acinar measurements), recent-onset T1D (light blue; N = 14 donors; 471 total islets, 1,332 total acinar measurements), and longstanding T1D (light blue striped; N = 12 donors; 316 total islets, 867 total acinar measurements) donors. Each symbol represents an individual donor; “aggregate” graph values reflect the average data from all pancreas regions, which are stratified (H, head; B, body; T, tail) on right. Horizontal lines represent mean values and are colorized based on the donor group. F and H: islet (F) and acinar (H) capillary density in non-diabetic and T1D tissue, plotted as a function of donor age and disease duration. J and L: islet (J) and acinar (L) area per capillary in non-diabetic and T1D tissue, plotted as a function of donor age and disease duration. Statistically significant P values (<0.05) are stated for the following statistical tests: E, I, G, and K: aggregate, Kruskal–Wallis 1-way ANOVA with Dunn’s multiple comparisons tests; anatomical location, mixed-effects 1-way ANOVA with Holm–Sidak’s multiple comparison tests; F, J, H, and L: nonlinear regression analysis with lines of best fit and Spearman correlation r values denoted on each plot.
The density of pancreatic capillaries, visualized by immunofluorescence labeling for caveolin-1 (CAV1) with 10-μm tissue sections, was greater in recent-onset T1D than in non-diabetic and longstanding T1D tissue in both pancreatic compartments (Fig. 3, B–D), with no significant difference among anatomical regions (Fig. 3, E–H). As with non-diabetic donors, T1D donors showed greater islet capillary density than in the acinar tissue (Supplemental Fig. S3b, Supplemental Fig. S6, a and b), whereas area per capillary did not vary by T1D subgroup (Fig. 3, I–L) or between the islet and acinar compartments (Supplemental Fig. S3d and Supplemental Fig. S6, c and d). Additionally, we found a negative correlation between the capillary density of islets (Fig. 3F) and acinar tissue (Fig. 3H) and age and disease duration in T1D donors. Quantification of percent capillary area revealed similar findings as described above (Supplemental Fig. S7).
Pancreatic Islet and Acinar Nerve Fibers Persist in T1D
To investigate whether nerve fiber density is altered in T1D, we visualized all pancreatic nerve fibers with TUBB3. Genetic ablation of intraislet capillaries through inactivation of a major angiogenic factor produced by beta cells, vascular endothelial growth factor A (VEGF-A), leads to extensive islet nerve fiber loss, thus highlighting coordinated interactions between islet innervation and vasculature and the importance of studying these two islet compartments simultaneously (25). To our knowledge, the simultaneous comparison of nerve fibers and capillaries has not been done at this scale in human T1D tissues. Like non-diabetic pancreata, T1D nerve fibers projected from the acinar compartment into islets but were less frequent within islets compared to acinar tissue (Fig. 4, Supplemental Videos S1–S3). Interestingly, fiber density in islets (Fig. 4D), but not acinar tissue (Fig. 4F), was greater in both recent-onset and longstanding T1D compared with non-diabetic donor tissue. Acinar nerve fiber length did not differ among groups (Fig. 4J). Within each group, both nerve fiber density and length were greater in acinar tissue compared with islets (Supplemental Fig. S3, e–h, Supplemental Fig. S8, a and b). However, neither nerve fiber density nor length correlated to donor age or T1D duration in either compartment (Fig. 4, E and I and G and K).
Figure 4.
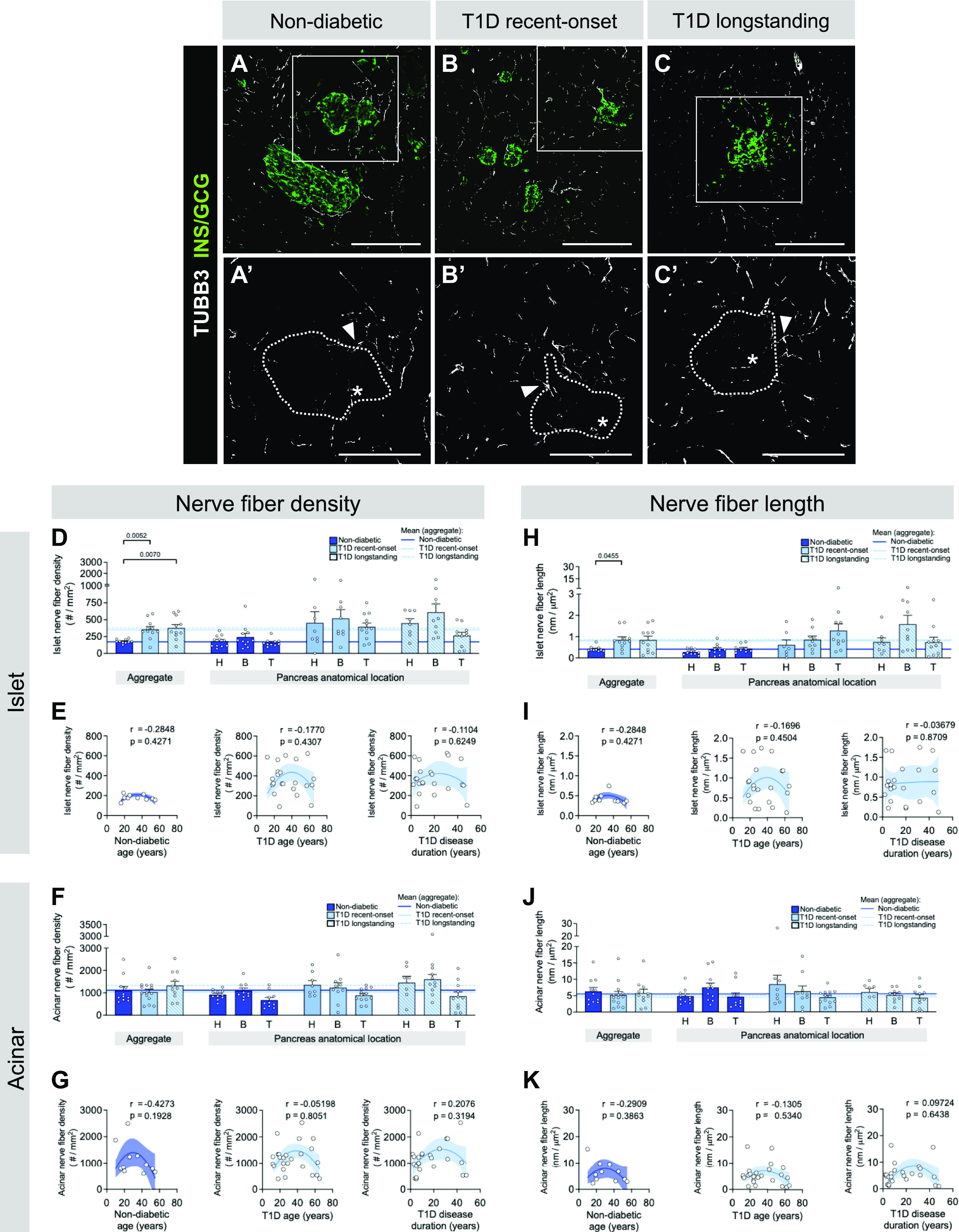
Islets in type 1 diabetes (T1D) have greater nerve fiber density compared with controls. A–C: representative immunofluorescent staining of nerve fibers labeled with tubulin beta 3 (TUBB3; white) in pancreatic tissue sections from non-diabetic (A), recent-onset T1D (B), and longstanding T1D (C) donors. GCG, glucagon; INS, insulin. A′–C′: insets of A–C, TUBB3 channel only, with islet area outlined. Scale bars, 100 μm. D–K: quantification (mean ± SE) of nerve fiber density (D–G) and nerve fiber length (H–K) in islet (D, E, H, and I) and acinar (F, G, J, and K) tissue from pancreata of non-diabetic (dark blue; N = 11 donors; 536 total islets, 1,830 total acinar measurements), recent-onset T1D (light blue; N = 14 donors; 417 total islets, 1,258 total acinar measurements), and longstanding T1D (light blue striped; N = 11 donors; 345 total islets, 1,044 total acinar measurements) donors. Each symbol represents an individual donor; “aggregate” graph values reflect the average data from all pancreas regions, which are stratified (H, head; B, body; T, tail) on right. Horizontal lines represent mean values and are colorized based on the donor group. E and G: islet (E) and acinar (G) nerve fiber density in non-diabetic and T1D tissue, plotted as a function of donor age and disease duration. I and K: islet (I) and acinar (K) nerve fiber length in non-diabetic and T1D tissue, plotted as a function of donor age and disease duration. Statistically significant P values (<0.05) are stated for the following statistical tests: D, H, F, and J: aggregate, Kruskal–Wallis 1-way ANOVA with Dunn’s multiple comparisons tests; anatomical location, mixed-effects 1-way ANOVA with Holm–Sidak’s multiple comparison tests; E, I, G, and K: nonlinear regression analysis with lines of best fit and Spearman correlation r values denoted on each plot.
Sympathetic Nerve Fibers Are Similar in T1D and Non-diabetic Pancreata
The autonomic nervous system is important for initiating alpha cell response to hypoglycemia, particularly through sympathetic fibers (1). Early loss of rodent sympathetic nerve fibers in models of T1D has been hypothesized to lead to the dysregulation of glucagon secretion (12–14). Therefore, we assessed T1D pancreatic sympathetic nerve fibers utilizing the marker tyrosine hydroxylase (TH). In contrast to findings in T1D rodent models, we did not detect a change in sympathetic islet or acinar fiber density in recent-onset or longstanding T1D (Fig. 5) (13, 14). In line with our findings with the panneuronal marker TUBB3, the density and length of TH+ sympathetic nerve fibers were greater in the acinar tissue compared with islets of both ND and T1D donors (Supplemental Fig. S8c). Furthermore, sympathetic fibers predominated in the islet compartment (Supplemental Fig. S9, a and b) but made up only a subset of TUBB3+ panneuronal fibers in the acinar compartment (Supplemental Fig. S9, c and d). There was a negative correlation between TH+ islet fiber density and length versus T1D cohort age but not disease duration (Fig. 5, E and I). Quantification of percent nerve fiber area revealed similar findings as described above (Supplemental Fig. S10).
Figure 5.
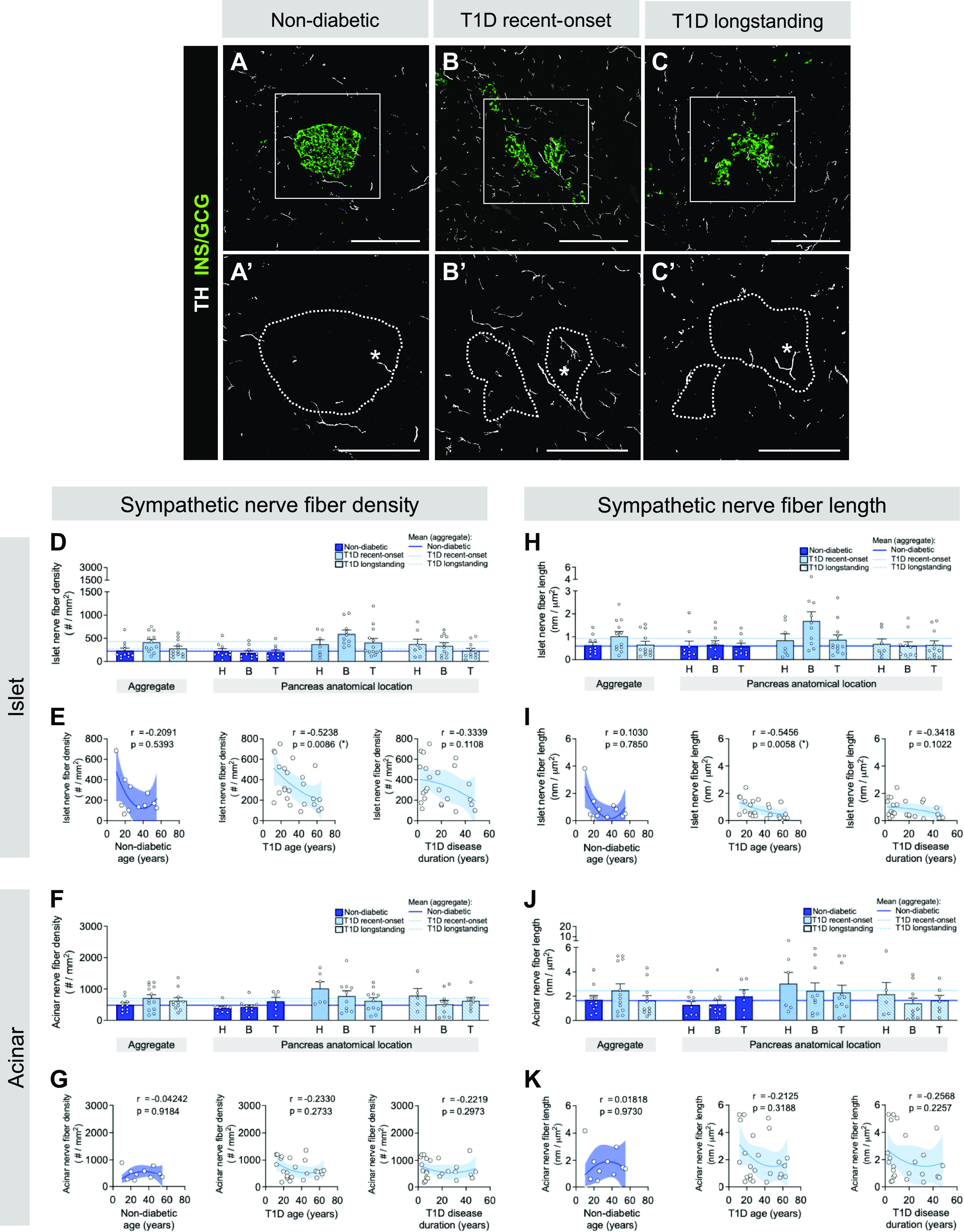
Sympathetic nerve fibers are not reduced in human type 1 diabetic (T1D) compared with non-diabetic (ND) islets. A–C: representative immunofluorescent staining of sympathetic nerve fibers labeled with tyrosine hydroxylase (TH; white) in pancreatic tissue sections from ND (A), recent-onset T1D (B), and longstanding T1D (C) donors. GCG, glucagon; INS, insulin. A′–C′: insets of A–C, TH channel only, with islet area outlined. Scale bars, 100 μm. D–K: quantification (mean ± SE) of sympathetic nerve fiber density (D–G) and nerve fiber length (H–K) in islet (D, E, H, and I) and acinar (F, G, J, and K) tissue from pancreata of ND (dark blue; N = 11 donors; 514 total islets, 789 total acinar measurements), recent-onset T1D (light blue; N = 13 donors; 469 total islets, 864 total acinar measurements), and longstanding T1D (light blue striped; N = 12 donors; 325 total islets, 504 total acinar measurements) donors. Each symbol represents an individual donor; “aggregate” graph values reflect the average data from all pancreas regions, which are stratified (H, head; B, body; T, tail) on right. Horizontal lines represent mean values and are colorized based on the donor group. E and G: islet (E) and acinar (G) acinar sympathetic nerve fiber density in ND and T1D tissue, plotted as a function of donor age and disease duration. I and K: islet (I) and acinar (K) acinar sympathetic nerve fiber length in ND and T1D tissue, plotted as a function of donor age and disease duration. Statistically significant P values (<0.05) are stated for the following statistical tests: D, H, F, and J: aggregate, Kruskal–Wallis 1-way ANOVA with Dunn’s multiple comparisons tests; anatomical location, mixed-effects 1-way ANOVA with Holm–Sidak’s multiple comparison tests; E, I, G, and K: nonlinear regression analysis with lines of best fit and Spearman correlation r values denoted on each plot.
Nerve Fibers in Islets and Acinar Tissue Overlap with the Extracellular Matrix
The ECM is an essential part of the vasculature by providing structural blood vessel support and regulating vascular phenotype and function (43, 44). At the same time, it also regulates neuronal migration, the formation of axonal processes, and their function (43, 44). To understand the structural relationship between pancreatic nerve fibers, capillaries, and the ECM, we determined nerve fiber and ECM patterning in non-diabetic, recent-onset T1D, and longstanding T1D tissues. Three-dimensional (3-D) reconstructions of TUBB3+ nerve fibers and ECM show that nerve fibers follow ECM within acinar tissue before extending into islets (Fig. 6, A–F, Supplemental Videos S1–S3). By visualizing capillaries, nerve fibers, and ECM with PECAM-1, TUBB3, and collagen IV (COLIV), respectively, we found that nerve fibers associated with the ECM in the acinar and islet compartments regardless of disease state (Fig. 6, A–F). Our image analyses showed ∼40% overlap of TUBB3+ nerve fiber area with COLIV+ ECM area (Fig. 6G). These results demonstrate that there is a strong spatial association between nerve fibers and ECM (Fig. 6, D′, E′, and F′), whereas in acinar tissue they follow ECM generated by acinar cells in areas devoid of capillaries (Fig. 6, D′′ E′′, F′′). Overall, these data indicate that pancreatic capillaries and nerve fibers persist in longstanding T1D (Fig. 7), contrasting with the loss of pancreatic innervation in rodent T1D models and thus emphasizing potentially distinct mechanisms of intrinsic alpha cell defects in dysregulated human glucagon secretion.
Figure 6.
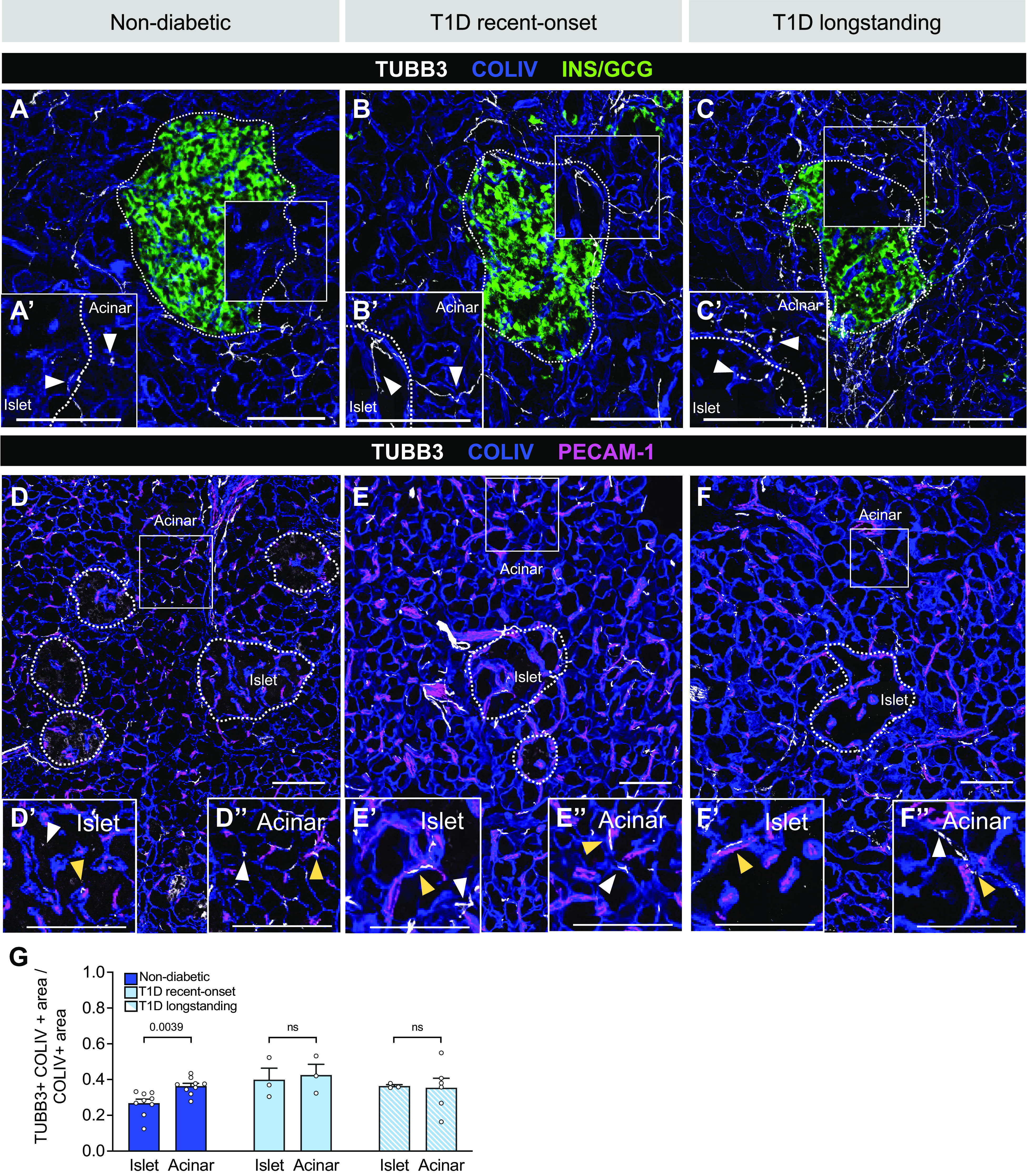
Pancreatic nerve fibers associate with endothelial cell- or acinar cell-derived extracellular matrix (ECM) in human pancreatic tissue. A–C: representative images of pancreatic tissue from non-diabetic and T1D donors costained for nerves [tubulin beta 3 (TUBB3); white], ECM [collagen IV (COLIV); blue], and islets [insulin (INS)/glucagon (GCG); green]. Insets (A′–C′) show nerve fibers and ECM overlapping (white arrowheads) in both endocrine and exocrine compartments; the endocrine channel is removed for clarity. D–F: costaining for nerves, ECM, and endothelial cells [platelet endothelial cell adhesion molecule 1 (PECAM-1); magenta]. Insets from islet (D′–F′) and acinar (D′′–F′′) regions show nerve fibers and ECM overlapping with endothelial cells (yellow arrowheads), whereas white arrowheads distinguish regions where there is no overlap with the vasculature. Islets are outlined in white. Scale bars, 100 μm. G: quantification (mean ± SE) of colocalization between nerve fibers (TUBB3+) and ECM (COLIV+) of islet and acinar tissue from non-diabetic (dark blue; N = 9 donors; 35 total islets, 46 total acinar measurements), recent-onset T1D (light blue; N = 3 donors; 11 total islets, 13 total acinar measurements), and longstanding T1D (light blue striped N = 6 donors; 12 total islets, 19 total acinar measurements). Manders’ overlap coefficients range between 0 and 1, where 1 indicates 100% colocalization. P values were calculated by paired Wilcoxon t tests, where ns indicates a P value > 0.05.
Figure 7.
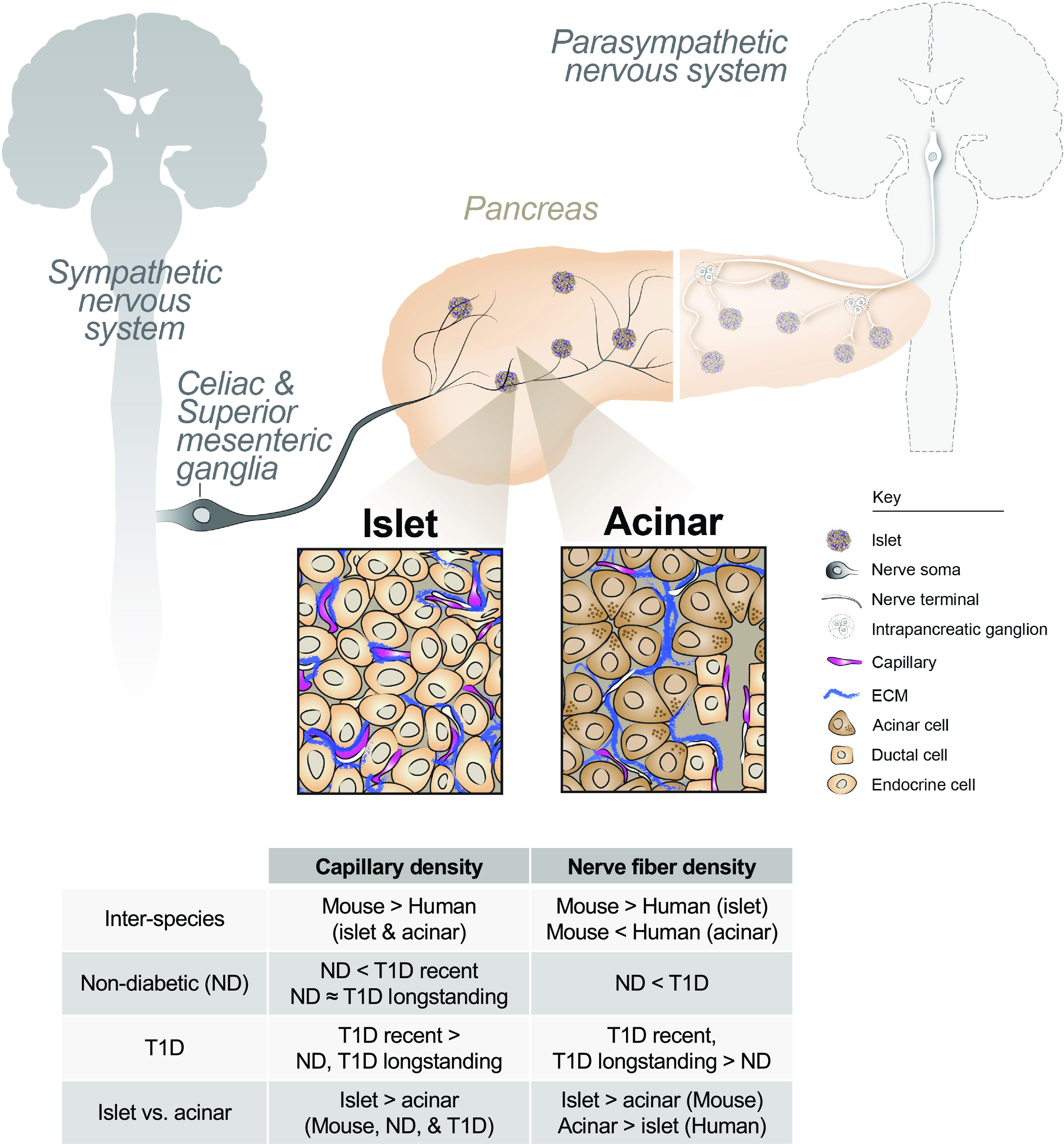
Human pancreatic neurovascular landscape in health and type 1 diabetes (T1D). The pancreas is intricately connected to the central nervous system and vasculature through branches of the autonomic nervous system and branching vessels. Nerve fibers and vessels can directly influence pancreatic function. Sympathetic projections originate in the spinal cord before extending through distal ganglia and intrapancreatic ganglia. Islets (see left inset) receive vascular and nerve fiber inputs that overlap with each other. Acinar tissue (see right inset) is innervated and vascularized, with these components extending around acini. Pancreatic nerve fibers are associated with extracellular matrix (ECM) laid down by either intraislet vessels or acini. There exist interspecies and disease differences in the neurovascular patterns in the pancreas, as described by the summary table. ND, non-diabetic. The key denotes pertinent neuronal components (nerve soma and nerve terminals), capillaries, and ECM.
DISCUSSION
Hypoglycemia is a major limitation of insulin therapy in T1D, and impaired glucagon secretion is a major contributor. Using biobanked pancreatic tissues from T1D donors that spanned a wide range of diabetes duration, we investigated whether impaired glucagon secretion could be attributed, at least in part, to the reduced islet innervation as suggested by studies in rodent models of T1D. To understand changes in the neurovascular architecture of human T1D pancreatic tissue, we systematically assessed innervation and vascularization in both islet and acinar tissue compartments of recent-onset and longstanding T1D donors and age-matched control donors. As part of this study, we also analyzed the neurovascular architecture in mouse pancreas, a widely used model of human disease, and compared it to that of non-diabetic human control donors. We found that human islets are much less vascularized and innervated than mouse islets (>15-fold difference). In contrast to mouse islets, human islets are less innervated compared with surrounding acinar tissue. Human islets have fewer intraislet capillaries with sparse nerve fibers, with these in close association with ECM. We also found that both nerve fibers and capillaries were present with greater capillary density in recent-onset T1D islets and acinar tissue. Surprisingly, we found that islet innervation was similar in T1D donors and controls, indicating that the islet neurovascular structures persist and are relatively unchanged in human T1D. These results, in conjunction with reports from others, suggest the need for alternative hypotheses other than reduced innervation to explain the dysregulated glucagon secretion in human T1D (18–20, 45).
The mechanisms responsible for the marked difference in vascularization and innervation in the mouse and human pancreas are unknown. Since in islets innervation is tightly linked to vascularization with the vascular network generating an ECM scaffold for nerve fiber migration (26), one possible explanation is that greater vascularization in the mouse islets promotes greater innervation. This could explain the difference in innervation patterns between mouse and human islets. Our comparative analysis of transcriptome data available for mouse and human islet endocrine and endothelial cells found overall similar transcriptomic profiles of factors guiding angiogenesis and neuronal development in islets suggesting other regulatory mechanisms, including possibly posttranscriptional processes (25, 26, 43, 46–49). For example, the generation of different isoforms of a master regulator of islet vascularization, VEGF-A, by alternative splicing (46) across different species could lead to isoforms with varying levels of activity due to their regulation by ECM, which could further influence growth factor availability, islet vascularization, and morphogenesis (20, 21, 50–53). Because vascularization and innervation are complex processes, it is also possible that a combinatorial difference in the ligand-receptor expression could yield different neurovascular patterning between mouse and human. Alternatively, stromal and/or immune cells could further modulate signaling in a species-specific manner during the development of neurovascular assembly (54, 55). Furthermore, the greater innervation in the human exocrine compartment compared with the mouse indicates that the degree of innervation and vascularization are not always linked (25, 26, 43, 46–49). The interspecies differences in islet vascularization and innervation are reminiscent of other differences, such as islet cell composition and spatial arrangement, basement membrane organization, proliferative capacity, and basal insulin secretion, but the responsible molecular mechanisms for these are also not well defined, underscoring the caution when translating findings from model systems to human disease (50, 51, 56, 57).
Although the fundamental ability of the autonomic nervous system to activate pancreatic secretion is present in mice and humans, each species may integrate and respond to these signals differently (2–4). Rodent models of T1D show an early loss of islet nerve fiber density dependent on the degree of insulitis, suggesting a role for the immune system in perturbing these structures in conjunction with targeting beta cells (13, 14, 58) leading to an impaired sympathetic nerve fiber activation of glucagon secretion (14). In human T1D tissues, we did not detect a reduction or loss of sympathetic nerve fibers. Instead, we found the persistence of these nerve fibers in islets. These differences between human and rodent models may be due to interspecies differences in the islet-related T1D pathogenesis, with much more profound insulitis in NOD mice compared with human (2–4, 23, 58). Other nerve fiber type densities not assessed here because of reagent availability, such as parasympathetic or sensory nerves, may also change and influence secretion dysfunction seen in T1D (24, 59). Furthermore, a previous study found differences in the islet microvasculature between T1D insulin-positive and insulin-negative islets that we did not capture in the present study because of our immunolabeling paradigms (28). Additionally, an upregulation in vascular gene sets such as angiogenesis and blood vessel morphogenesis found in T1D islets (60) parallels the histological capillary changes reported in our present study. Therefore, the heterogeneous nature of beta cell loss across T1D islets may lead to variabilities in T1D islet microvasculature and nerve fiber densities that need to be further studied (28). Overall, our studies of many human tissues do not support the hypothesis that glucagon secretion defects in T1D result from decreased islet sympathetic nerve fiber density.
Some differences exist between our findings and prior reports, including tissue thickness, the number of donors assessed, and the nerve fiber markers utilized. One experimental difference is that we utilized both thin (10 μm) and thick (30 μm) cryosections from human tissue that were gently fixed to maximally preserve cellular and anatomical structures. Compared with optically cleared sections, 10-μm sections do not always capture the full length of some nerve fibers or capillaries. In contrast to much thicker optically cleared tissue sections, though, 10-μm sections allowed for high-throughput morphometric analysis of ∼3,400 islets in total from a unique biobanked cohort of 38 non-diabetic and T1D donors. We sought to circumvent this limitation by studying a tremendous number of islets from a large cohort of donors. Our high-throughput analysis also allowed us to overcome high variability in human samples, particularly in T1D donor tissues. Other differences between this and other studies may be due to donor variability, sample size, and/or technical differences during tissue handling, which may affect the downstream quantification of delicate structures such as nerve fibers. We used the panneuronal marker TUBB3, whereas others have used neural cell adhesion molecule 1 (NCAM), neurofilament 200 (NF200), and ubiquitin carboxy-terminal hydrolase isozyme (UCHL1 or PGP9.5) (17, 23, 59, 61) with a similar distribution of nerve fibers in human islets and acinar tissue compared with PGP9.5 marker, thus further underscoring the appropriate utilization of TUBB3 in this study (61). It is important to note that TUBB3+ intrapancreatic ganglia could not be identified in either full-section confocal images or >1,000 whole slide images that we used for our high-throughput analyses. Therefore, nerve fiber bundles served as a positive control for TUBB3 immunolabeling of pancreatic nerve fibers in our study. Additionally, we used the sympathetic nerve fiber marker TH, which labels throughout the axon to the nerve terminal and allows direct comparison to other studies utilizing this same marker.
The preservation of neurovascular architecture in T1D islets suggests other explanations for impaired glucagon secretion, such as intrinsic nerve fiber or capillary dysfunction and/or an intrinsic alpha cell defect. Recent studies using isolated islets and tissues from the same organ donors provide significant support for intrinsic alpha cell defect in T1D at both molecular and functional levels, showing that impaired glucagon secretion was associated with reduced expression of key alpha cell-enriched regulators, including transcription factors MAFB and ARX and pointing to changes in metabolic pathways related to glycolysis and oxidative phosphorylation (31, 41, 62). Although we show that neurovascular architecture is preserved in T1D, one cannot exclude an islet nerve fiber or capillary dysfunction, which are far more difficult to elucidate in human. For example, hyperglycemia could cause neurotransmitter receptor oxidation and reduced acetylcholine receptor activity in sympathetic neurons (63). Conversely, hypoglycemia sensed in the brain promotes counterregulatory responses from the adrenal gland and alpha cells. Dysfunctional central nervous system (CNS) hypoglycemia sensing in T1D could disrupt sympathetic activation in islets, further impairing glucagon secretion (64). Given prior findings that sympathetic nerve fibers preferentially innervate smooth muscle cells within the islet, disruptions in the autonomic modulation of intraislet vascular contractile components in T1D may also contribute to diminished glucagon secretion during hypoglycemia (65). The recent development of pancreatic slice physiology, which preserves vascular cytoarchitecture, highlighted the importance of islet pericytes in modulating local vasodilation and constriction (33, 66).
Additional research is needed to determine whether the dysregulated glucagon secretion in T1D results primarily from alpha cell-intrinsic defect and whether it is further compounded by neuronal dysfunction. It is important that such studies examine a broad range of T1D duration since mechanisms may differ with disease stages. New technologies, approaches, and models to probe neurovascular function in islets are emerging, and these include the islet-on-a-chip platform, pancreatic slices, islet transplantation models, and new imaging approaches (67–71). For example, examining in vivo the processes of both innervation and vascularization of human islets transplanted into immunodeficient mice may provide insights into the molecular mechanisms of such processes.
DATA AND RESOURCE AVAILABILITY
The raw data sets analyzed in this study are already available or will be provided to the journal upon publication (Supplemental Table S4). Processed Seurat object data is publicly available (https://doi.org/10.5281/zenodo.7626110).
SUPPLEMENTAL DATA
Supplemental Figs. S1–S11 and Supplemental Tables S1–S4: https://doi.org/10.6084/m9.figshare.21824502.
Supplemental Video S1: https://doi.org/10.6084/m9.figshare.21111625.
Supplemental Video S2: https://doi.org/10.6084/m9.figshare.21111622.
Supplemental Video S3: https://doi.org/10.6084/m9.figshare.21111628.
GRANTS
This work was supported by the Human Islet Research Network (RRID:SCR_014393), the Human Pancreas Analysis Program (RRID:SCR_016202), DK106755, DK123716, DK123743, DK120456, DK104211, DK108120, DK112232, DK117147, DK112217, EY032442, and DK020593 [Vanderbilt Diabetes Research and Training Center (DRTC)], The Leona M. and Harry B. Helmsley Charitable Trust, JDRF, the US Department of Veterans Affairs (BX000666), and the National Science Foundation Graduate Research Fellowship (1937963). Whole slide imaging was performed in the Islet and Pancreas Analysis Core of the Vanderbilt DRTC (DK20593). Some confocal imaging studies were performed in part through the Vanderbilt Cell Imaging Shared Resource (supported by NIH Grants CA068485, DK020593, DK058404, DK059637, and EY008126).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.M.R., D.C.S., R.H., A.C.P., and M.B. conceived and designed research; T.M.R., R.H., R.B.R., J.P., and R.A. performed experiments; T.M.R., D.C.S., S.S., J.-P.C. S.P., and H.K. analyzed data; T.M.R. and D.C.S. prepared figures; T.M.R., D.C.S., A.C.P., and M.B., drafted manuscript; T.M.R., D.C.S., R.H., S.S., J.-P.C., R.B.R., J.P., R.B., R.A., A.M.B., R.J., S.P., H.K., A.C., A.C.P., and M.B. edited and revised manuscript; T.M.R., D.C.S., R.H., S.S., J.-P.C., R.B.R., J.P., R.B., R.A., A.M.B., R.J., S.P., H.K., H.P.A.P., A.C., A.C.P., and M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the organ donors and their families for the invaluable donations and the Organ Procurement Organizations, International Institute for Advancement of Medicine (IIAM) and National Disease Research Interchange (NDRI) for partnership in studies of human pancreatic tissue.
The graphical abstract was made, in part, with BioRender.com.
REFERENCES
- 1. Faber CL, Deem JD, Campos CA, Taborsky GJ Jr, Morton GJ. CNS control of the endocrine pancreas. Diabetologia 63: 2086–2094, 2020. doi: 10.1007/s00125-020-05204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahrén B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes 50: 1030–1038, 2001. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- 3. Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 43: 393–410, 2000. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 4. Havel PJ, Ahren B. Activation of autonomic nerves and the adrenal medulla contributes to increased glucagon secretion during moderate insulin-induced hypoglycemia in women. Diabetes 46: 801–807, 1997. doi: 10.2337/diab.46.5.801. [DOI] [PubMed] [Google Scholar]
- 5. Hampton RF, Jimenez-Gonzalez M, Stanley SA. Unravelling innervation of pancreatic islets. Diabetologia 65: 1069–1084, 2022. doi: 10.1007/s00125-022-05691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 182: 171–173, 1973. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- 7. Bolli G, de Feo P, Compagnucci P, Cartechini MG, Angeletti G, Santeusanio F, Brunetti P, Gerich JE. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus: interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes 32: 134–141, 1983. doi: 10.2337/diab.32.2.134. [DOI] [PubMed] [Google Scholar]
- 8. Brown RJ, Sinaii N, Rother KI. Too much glucagon, too little insulin: time course of pancreatic islet dysfunction in new-onset type 1 diabetes. Diabetes Care 31: 1403–1404, 2008. doi: 10.2337/dc08-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sherr J, Tsalikian E, Fox L, Buckingham B, Weinzimer S, Tamborlane WV, White NH, Arbelaez AM, Kollman C, Ruedy KJ, Cheng P, Beck RW; Diabetes Research in Children Network. Evolution of abnormal plasma glucagon responses to mixed-meal feedings in youth with type 1 diabetes during the first 2 years after diagnosis. Diabetes Care 37: 1741–1744, 2014. doi: 10.2337/dc13-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 369: 362–372, 2013. doi: 10.1056/NEJMra1215228. [DOI] [PubMed] [Google Scholar]
- 11. Taborsky GJ Jr, Ahrén B, Havel PJ. Autonomic mediation of glucagon secretion during hypoglycemia: implications for impaired alpha-cell responses in type 1 diabetes. Diabetes 47: 995–1005, 1998. doi: 10.2337/diabetes.47.7.995. [DOI] [PubMed] [Google Scholar]
- 12. Mei Q, Mundinger TO, Lernmark A, Taborsky GJ. Early, selective, and marked loss of sympathetic nerves from the islets of BioBreeder diabetic rats. Diabetes 51: 2997–3002, 2002. [Erratum in Diabetes 51: 3591, 2002]. doi: 10.2337/diabetes.51.10.2997. [DOI] [PubMed] [Google Scholar]
- 13. Mundinger TO, Mei Q, Figlewicz DP, Lernmark A, Taborsky GJ. Impaired glucagon response to sympathetic nerve stimulation in the BB diabetic rat: effect of early sympathetic islet neuropathy. Am J Physiol Endocrinol Metab 285: E1047–E1054, 2003. doi: 10.1152/ajpendo.00136.2003. [DOI] [PubMed] [Google Scholar]
- 14. Taborsky GJ Jr, Mei Q, Hackney DJ, Figlewicz DP, LeBoeuf R, Mundinger TO. Loss of islet sympathetic nerves and impairment of glucagon secretion in the NOD mouse: relationship to invasive insulitis. Diabetologia 52: 2602–2611, 2009. doi: 10.1007/s00125-009-1494-5. [DOI] [PubMed] [Google Scholar]
- 15. Tominaga M, Maruyama H, Vasko MR, Baetens D, Orci L, Unger RH. Morphologic and functional changes in sympathetic nerve relationships with pancreatic α-cells after destruction of β-cells in rats. Diabetes 36: 365–373, 1987. doi: 10.2337/diab.36.3.365. [DOI] [PubMed] [Google Scholar]
- 16. Persson-Sjögren S, Holmberg D, Forsgren S. Remodeling of the innervation of pancreatic islets accompanies insulitis preceding onset of diabetes in the NOD mouse. J Neuroimmunol 158: 128–137, 2005. doi: 10.1016/j.jneuroim.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 17. Alvarsson A, Jimenez-Gonzalez M, Li R, Rosselot C, Tzavaras N, Wu Z, Stewart AF, Garcia-Ocaña A, Stanley SA. A 3D atlas of the dynamic and regional variation of pancreatic innervation in diabetes. Sci Adv 6: eaaz9124, 2020. doi: 10.1126/sciadv.aaz9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 14: 45–54, 2011. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang SC, Shen CN, Lin PY, Peng SJ, Chien HJ, Chou YH, Chamberlain CE, Pasricha PJ. Pancreatic neuro-insular network in young mice revealed by 3D panoramic histology. Diabetologia 61: 158–167, 2018. doi: 10.1007/s00125-017-4408-y. [DOI] [PubMed] [Google Scholar]
- 20. Brissova M, Shostak A, Fligner CL, Revetta FL, Washington MK, Powers AC, Hull RL. Human islets have fewer blood vessels than mouse islets and the density of islet vascular structures is increased in type 2 diabetes. J Histochem Cytochem 63: 637–645, 2015. doi: 10.1369/0022155415573324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. Unique basement membrane structure of human pancreatic islets: implications for β‐cell growth and differentiation. Diabetes Obes Metab 10: 119–127, 2008. doi: 10.1111/j.1463-1326.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 22. Mundinger TO, Mei Q, Foulis AK, Fligner CL, Hull RL, Taborsky GJ. Human type 1 diabetes is characterized by an early, marked, sustained, and islet-selective loss of sympathetic nerves. Diabetes 65: 2322–2330, 2016. doi: 10.2337/db16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campbell-Thompson M, Butterworth EA, Boatwright JL, Nair MA, Nasif LH, Nasif K, Revell AY, Riva A, Mathews CE, Gerling IC, Schatz DA, Atkinson MA. Islet sympathetic innervation and islet neuropathology in patients with type 1 diabetes. Sci Rep 11: 6562, 2021. doi: 10.1038/s41598-021-85659-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lundberg M, Lindqvist A, Wierup N, Krogvold L, Dahl-Jørgensen K, Skog O. The density of parasympathetic axons is reduced in the exocrine pancreas of individuals recently diagnosed with type 1 diabetes. PLoS One 12: e0179911, 2017. doi: 10.1371/journal.pone.0179911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reinert RB, Brissova M, Shostak A, Pan FC, Poffenberger G, Cai Q, Hundemer GL, Kantz J, Thompson CS, Dai C, McGuinness OP, Powers AC. Vascular endothelial growth factor-A and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes 62: 4154–4164, 2013. doi: 10.2337/db13-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reinert RB, Cai Q, Hong JY, Plank JL, Aamodt K, Prasad N, Aramandla R, Dai C, Levy SE, Pozzi A, Labosky PA, Wright CV, Brissova M, Powers AC. Vascular endothelial growth factor coordinates islet innervation via vascular scaffolding. Development 141: 1480–1491, 2014. doi: 10.1242/dev.098657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plank JL, Mundell NA, Frist AY, LeGrone AW, Kim T, Musser MA, Walter TJ, Labosky PA. Influence and timing of arrival of murine neural crest on pancreatic beta cell development and maturation. Dev Biol 349: 321–330, 2011. doi: 10.1016/j.ydbio.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Canzano JS, Nasif LH, Butterworth EA, Fu DA, Atkinson MA, Campbell-Thompson M. Islet microvasculature alterations with loss of beta-cells in patients with type 1 diabetes. J Histochem Cytochem 67: 41–52, 2019. doi: 10.1369/0022155418778546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Campbell-Thompson ML, Filipp SL, Grajo JR, Nambam B, Beegle R, Middlebrooks EH, Gurka MJ, Atkinson MA, Schatz DA, Haller MJ. Relative pancreas volume is reduced in first-degree relatives of patients with type 1 diabetes. Diabetes Care 42: 281–287, 2019. doi: 10.2337/dc18-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wright JJ, Saunders DC, Dai C, Poffenberger G, Cairns B, Serreze DV, Harlan DM, Bottino R, Brissova M, Powers AC. Decreased pancreatic acinar cell number in type 1 diabetes. Diabetologia 63: 1418–1423, 2020. doi: 10.1007/s00125-020-05155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brissova M, Haliyur R, Saunders D, Shrestha S, Dai C, Blodgett DM, Bottino R, Campbell-Thompson M, Aramandla R, Poffenberger G, Lindner J, Pan FC, von Herrath MG, Greiner DL, Shultz LD, Sanyoura M, Philipson LH, Atkinson M, Harlan DM, Levy SE, Prasad N, Stein R, Powers AC. α Cell function and gene expression are compromised in type 1 diabetes. Cell Reports 22: 2667–2676, 2018. doi: 10.1016/j.celrep.2018.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haliyur R, Tong X, Sanyoura M, Shrestha S, Lindner J, Saunders DC, Aramandla R, Poffenberger G, Redick SD, Bottino R, Prasad N, Levy SE, Blind RD, Harlan DM, Philipson LH, Stein RW, Brissova M, Powers AC. Human islets expressing HNF1A variant have defective β cell transcriptional regulatory networks. J Clin Invest 129: 246–251, 2019. doi: 10.1172/JCI121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Almaça J, Weitz J, Rodriguez-Diaz R, Pereira E, Caicedo A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab 27: 630–644.e4, 2018. doi: 10.1016/j.cmet.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saunders DC, Aamodt KI, Richardson TM, Hopkirk AJ, Aramandla R, Poffenberger G, Jenkins R, Flaherty DK, Prasad N, Levy SE, Powers AC, Brissova M. Coordinated interactions between endothelial cells and macrophages in the islet microenvironment promote β cell regeneration. NPJ Regen Med 6: 22, 2021. doi: 10.1038/s41536-021-00129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tweedie S, Braschi B, Gray K, Jones TE, Seal RL, Yates B, Bruford EA. Genenames.org: the HGNC and VGNC resources in 2021. Nucleic Acids Res 49: D939–D946, 2021. doi: 10.1093/nar/gkaa980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jonsson A, Hedin A, Müller M, Skog O, Korsgren O. Transcriptional profiles of human islet and exocrine endothelial cells in subjects with or without impaired glucose metabolism. Sci Rep 10: 22315, 2020. doi: 10.1038/s41598-020-79313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walker JT, Saunders DC, Rai V, Dai C, Orchard P, Hopkirk AL, Reihsmann CV, Tao Y, Fan S, Shrestha S, Varshney A, Wright JJ, Pettway YD, Ventresca C, Agarwala S, Aramandla R, Poffenberger G, Jenkins R, Hart NJ, Greiner DL, Shultz LD, Bottino R, Human Pancreas Analysis Program, Liu J, Parker SC, Powers AC, Brissova M. RFX6-mediated dysregulation defines human β cell dysfunction in early type 2 diabetes (Preprint). bioRxiv 2021.12.16.466282, 2021. doi: 10.1101/2021.12.16.466282. [DOI]
- 38. Erener S, Ellis CE, Ramzy A, Glavas MM, O’Dwyer S, Pereira S, Wang T, Pang J, Bruin JE, Riedel MJ, Baker RK, Webber TD, Lesina M, Blüher M, Algül H, Kopp JL, Herzig S, Kieffer TJ. Deletion of pancreas-specific miR-216a reduces beta-cell mass and inhibits pancreatic cancer progression in mice. Cell Rep Med 2: 100434, 2021. doi: 10.1016/j.xcrm.2021.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shrestha S, Saunders DC, Walker JT, Camunas-Soler J, Dai XQ, Haliyur R, Aramandla R, Poffenberger G, Prasad N, Bottino R, Stein R, Cartailler JP, Parker SC, MacDonald PE, Levy SE, Powers AC, Brissova M. Combinatorial transcription factor profiles predict mature and functional human islet α and β cells. JCI Insight 6: e151621, 2021. doi: 10.1172/jci.insight.151621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hao Y, Hao S, Andersen-Nissen E, Mauck WM 3rd, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, Hoffman P, Stoeckius M, Papalexi E, Mimitou EP, Jain J, Srivastava A, Stuart T, Fleming LM, Yeung B, Rogers AJ, McElrath JM, Blish CA, Gottardo R, Smibert P, Satija R. Integrated analysis of multimodal single-cell data. Cell 184: 3573–3587.e29, 2021. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Doliba NM, Rozo AV, Roman J, Qin W, Traum D, Gao L, Liu J, Manduchi E, Liu C, Golson ML, Vahedi G, Naji A, Matschinsky FM, Atkinson MA, Powers AC, Brissova M, Kaestner KH, Stoffers DA; HPAP Consortium. α Cell dysfunction in islets from non-diabetic, glutamic acid decarboxylase autoantibody-positive individuals. J Clin Invest 132: e156243, 2022. doi: 10.1172/JCI156243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Damond N, Engler S, Zanotelli VR, Schapiro D, Wasserfall CH, Kusmartseva I, Nick HS, Thorel F, Herrera PL, Atkinson MA, Bodenmiller B. A map of human type 1 diabetes progression by imaging mass cytometry. Cell Metab 29: 755–768.e5, 2019. doi: 10.1016/j.cmet.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Myers JP, Santiago‐Medina M, Gomez TM. Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions. Dev Neurobiol 71: 901–923, 2011. doi: 10.1002/dneu.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fässler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E. The vascular basement membrane: a niche for insulin gene expression and β cell proliferation. Dev Cell 10: 397–405, 2006. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 45. Chiu YC, Hua TE, Fu YY, Pasricha PJ, Tang SC. 3-D imaging and illustration of the perfusive mouse islet sympathetic innervation and its remodelling in injury. Diabetologia 55: 3252–3261, 2012. doi: 10.1007/s00125-012-2699-6. [DOI] [PubMed] [Google Scholar]
- 46. Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature 436: 193–200, 2005. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 47. Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS, Nicholson W, Bader DM, Jetton T, Gannon M, Powers AC. Pancreatic islet production of vascular endothelial growth factor-A is essential for islet vascularization, revascularization, and function. Diabetes 55: 2974–2985, 2006. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 48. Cai Q, Brissova M, Reinert RB, Pan FC, Brahmachary P, Jeansson M, Shostak A, Radhika A. L, Poffenberger G, Quaggin SE, Jerome WG, Dumont DJ, Powers AC. Enhanced expression of VEGF-A in Β cells increases endothelial cell number but impairs islet morphogenesis and Β cell proliferation. Dev Biol 367: 40–54, 2012. doi: 10.1016/j.ydbio.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol 2: a001875, 2010. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 53: 1087–1097, 2005. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 51. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 103: 2334–2339, 2006. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dolenšek J, Rupnik MS, Stožer A. Structural similarities and differences between the human and the mouse pancreas. Islets 7: e1024405, 2015. doi: 10.1080/19382014.2015.1024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Korpos É, Kadri N, Kappelhoff R, Wegner J, Overall CM, Weber E, Holmberg D, Cardell S, Sorokin L. The peri-islet basement membrane, a barrier to infiltrating leukocytes in type 1 diabetes in mouse and human. Diabetes 62: 531–542, 2013. doi: 10.2337/db12-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hajizadeh-Saffar E, Tahamtani Y, Aghdami N, Azadmanesh K, Habibi-Anbouhi M, Heremans Y, De Leu N, Heimberg H, Ravassard P, Shokrgozar MA, Baharvand H. Inducible VEGF expression by human embryonic stem cell-derived mesenchymal stromal cells reduces the minimal islet mass required to reverse diabetes. Sci Rep 5: 9322, 2015.doi: 10.1038/srep09322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ghezelayagh Z, Zabihi M, Kazemi Ashtiani M, Ghezelayagh Z, Lynn FC, Tahamtani Y. Recapitulating pancreatic cell-cell interactions through bioengineering approaches: the momentous role of non-epithelial cells for diabetes cell therapy. Cell Mol Life Sci 78: 7107–7132, 2021. doi: 10.1007/s00018-021-03951-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Virtanen I, Banerjee M, Palgi J, Korsgren O, Lukinius A, Thornell LE, Kikkawa Y, Sekiguchi K, Hukkanen M, Konttinen YT, Otonkoski T. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia 51: 1181–1191, 2008. doi: 10.1007/s00125-008-0997-9. [DOI] [PubMed] [Google Scholar]
- 57. Dai C, Brissova M, Hang Y, Thompson C, Poffenberger G, Shostak A, Chen Z, Stein R, Powers AC. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia 55: 707–718, 2012. doi: 10.1007/s00125-011-2369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. In’t Veld PI. Insulitis in human type 1 diabetes: a comparison between patients and animal models. Semin Immunopathol 36: 569–579, 2014. doi: 10.1007/s00281-014-0438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chien HJ, Chiang TC, Peng SJ, Chung MH, Chou YH, Lee CY, Jeng YM, Tien YW, Tang SC. Human pancreatic afferent and efferent nerves: mapping and 3-D illustration of exocrine, endocrine, and adipose innervation. Am J Physiol Gastrointest Liver Physiol 317: G694–G706, 2019. doi: 10.1152/ajpgi.00116.2019. [DOI] [PubMed] [Google Scholar]
- 60. Granlund L, Hedin A, Korsgren O, Skog O, Lundberg M. Altered microvasculature in pancreatic islets from subjects with type 1 diabetes. PLoS One 17: e0276942, 2022. doi: 10.1371/journal.pone.0276942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tang SC, Baeyens L, Shen CN, Peng SJ, Chien HJ, Scheel DW, Chamberlain CE, German MS. Human pancreatic neuro-insular network in health and fatty infiltration. Diabetologia 61: 168–181, 2018. doi: 10.1007/s00125-017-4409-x. [DOI] [PubMed] [Google Scholar]
- 62. Chakravarthy H, Gu X, Enge M, Dai X, Wang Y, Damond N, Downie C, Liu K, Wang J, Xing Y, Chera S, Thorel F, Quake S, Oberholzer J, MacDonald PE, Herrera PL, Kim SK. Converting adult pancreatic islet α cells into β cells by targeting both Dnmt1 and Arx. Cell Metab 25: 622–634, 2017. doi: 10.1016/j.cmet.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Campanucci V, Krishnaswamy A, Cooper E. Diabetes depresses synaptic transmission in sympathetic ganglia by inactivating nAChRs through a conserved intracellular cysteine residue. Neuron 66: 827–834, 2010. doi: 10.1016/j.neuron.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 64. Belfort-DeAguiar R, Gallezot JD, Hwang JJ, Elshafie A, Yeckel CW, Chan O, Carson RE, Ding YS, Sherwin RS. Noradrenergic activity in the human brain: a mechanism supporting the defense against hypoglycemia. J Clin Endocrinol Metab 103: 2244–2252, 2018. doi: 10.1210/jc.2017-02717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 14: 45–54, 2011. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gonçalves LM, Almaça J. Functional characterization of the human islet microvasculature using living pancreas slices. Front Endocrinol (Lausanne) 11: 602519, 2021. doi: 10.3389/fendo.2020.602519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Glieberman AL, Pope BD, Zimmerman JF, Liu Q, Ferrier JP, Kenty JH, Schrell AM, Mukhitov N, Shores KL, Tepole AB, Melton DA, Roper MG, Parker KK. Synchronized stimulation and continuous insulin sensing in a microfluidic human Islet on a Chip designed for scalable manufacturing. Lab Chip 19: 2993–3010, 2019. doi: 10.1039/C9LC00253G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Speier S, Rupnik M. A novel approach to in situ characterization of pancreatic β-cells. Pflugers Arch 446: 553–558, 2003. doi: 10.1007/s00424-003-1097-9. [DOI] [PubMed] [Google Scholar]
- 69. Speier S, Nyqvist D, Cabrera O, Yu J, Molano RD, Pileggi A, Moede T, Köhler M, Wilbertz J, Leibiger B, Ricordi C, Leibiger IB, Caicedo A, Berggren PO. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med 14: 574–578, 2008. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rodriguez-Diaz R, Speier S, Molano RD, Formoso A, Gans I, Abdulreda MH, Cabrera O, Molina J, Fachado A, Ricordi C, Leibiger I, Pileggi A, Berggren PO, Caicedo A. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proc Natl Acad Sci USA 109: 21456–21461, 2012. doi: 10.1073/pnas.1211659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Morini S, Brown ML, Cicalese L, Elias G, Carotti S, Gaudio E, Rastellini C. Revascularization and remodelling of pancreatic islets grafted under the kidney capsule. J Anat 210: 565–577, 2007. doi: 10.1111/j.1469-7580.2007.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1–S11 and Supplemental Tables S1–S4: https://doi.org/10.6084/m9.figshare.21824502.
Supplemental Video S1: https://doi.org/10.6084/m9.figshare.21111625.
Supplemental Video S2: https://doi.org/10.6084/m9.figshare.21111622.
Supplemental Video S3: https://doi.org/10.6084/m9.figshare.21111628.
Data Availability Statement
The raw data sets analyzed in this study are already available or will be provided to the journal upon publication (Supplemental Table S4). Processed Seurat object data is publicly available (https://doi.org/10.5281/zenodo.7626110).


