Abstract
BACKGROUND:
Postextubation stridor (PES) is an imminently life-threatening event. Maximizing patient safety requires a systematic approach to screen patients for PES risk factors and a standardized test to evaluate that risk. This retrospective study of adult subjects was based on quality assurance data including standardized surveillance screening criteria and a volume-based cuff leak test (CLT) to evaluate PES risk among predominantly surgical-trauma and neurotrauma subjects. Data characterizing PES subjects also were collected.
METHODS:
Data were collected between May 2010–December 2017 for all intubated subjects in our surgical-trauma, neurotrauma, and medical ICUs. Respiratory therapists were trained in performing both PES risk assessment surveillance and a volume-based CLT. A pre hoc cutoff leak volume of < 110 mL defined a true positive test result when associated with PES, and a leak ≥ 110 mL defined a true negative test if PES was absent. Multiple comparisons were analyzed by Kruskal-Wallis tests and dichotomous variables assessed by Fisher exact tests. Alpha was set at 0.05.
RESULTS:
In 681 pre-extubation CLTs ∼85% produced true-negative results and 15% consisted of true-positive (∼4%), false-negative (∼5%), and false-positive (∼6%) results. Positive and negative predictive values were 0.42 (0.32–0.54) and 0.94 (0.92-0.96), respectively. The PES likelihood ratio was 7.0, and correct classification was 89%. Of the 115 PES incidences occurring in 112 PES cases, 67% were female and 48% had suffered acute brain injury.
CONCLUSIONS:
Among predominantly surgical-trauma and neurotrauma subjects with a CLT, leak volume of ≥ 110 mL was associated with a PES risk of ∼6%, whereas the risk of PES was 7 times greater when the leak volume was < 110 mL.
Keywords: airway management, airway extubation, airway obstruction, postextubation stridor, cuff leak test, laryngeal edema
Introduction
Postextubation stridor (PES) is an imminently life-threatening event often requiring definitive control of the airway. The reported incidence of PES ranges widely from 2–30%,1-3 with re-intubation required in ∼15% of cases.3 Methodological differences across studies likely account not only for PES incidence variation but also the predictive value of the cuff leak test (CLT) that assess the likelihood for developing PES. Methodological factors include (1) sample size, (2) critical care setting and subject characteristics (most importantly the presence, number, and relative severity of PES risk factors), (3) intubation history and duration,1 (4) endotracheal tube (ETT) size relative to body size and sex,4 (5) variations in technique (eg, quantitative vs qualitative, negative vs positive-pressure ventilation),5 (6) determination of cutoff values used to assess PES (eg, pre hoc decision vs post hoc analysis),5 and (7) duration of monitoring for PES (eg, hours vs days). A relatively recent meta-analysis observed that in contrast to the widely varying (and often poor) positive predictive value of CLT to assess PES risk the negative predictive value was consistently and substantially higher across most studies.5 This suggests that patient safety should focus not on the ability to accurately predict PES but rather shift toward a high likelihood of its absence.
Thus, systematically screening and grading PES risk in all intubated patients along with a uniform, quantitative method for evaluating that risk is likely the best pragmatic approach to optimize patient safety. This study describes this approach and reports its findings based on a 7.5-year quality assurance initiative aimed at minimizing PES risk among all intubated critically ill subjects.
QUICK LOOK.
Current Knowledge
Volume-based cuff leak tests (CLTs) are used to predict postextubation stridor (PES). Previous studies have found that leaks between 110–150 mL were associated with widely variable positive predictive values but consistently high negative predictive values. However, most studies that examined the CLT in a relatively limited number of subjects often reported a relatively high incidence of PES and lacked formal risk stratification by which overall subject risk could be assessed.
What This Paper Contributes to Our Knowledge
This study found that CLT performed with specific ventilator settings and sedation requirements and standardized risk surveillance in a large number of adult subjects reaffirmed that a volume-based CLT producing a leak of ≥ 110 mL produces a very strong negative predictive value consistent with previous studies and meta-analyses. Moreover, in the context of a standardized risk surveillance program, it is unnecessary to routinely perform CLT prior to extubation in the absence of obvious or potential risk factors.
Methods
This retrospective study examined prospectively collected quality assurance data coinciding with implementation of a formal policy governing PES risk screening, testing, and treatment regimens to minimize PES incidences and ameliorate its impact. We appraised the utility of a modified, volume-based CLT previously studied in an adult medical ICU setting6 to assess PES risk among adult subjects in a predominantly surgical-trauma and neurotrauma ICU setting. Characteristics of PES subjects also were examined.
In May of 2010 San Francisco General Hospital instituted a quality improvement program to reduce PES risk following a sentinel event. This involved systematically screening all intubated patients in the trauma-surgical, neurotrauma, and medical ICUs for PES risk based upon published literature and cofactors that might enhance airways resistance posed by laryngeal or tracheal injury or edema (eg, severe obesity, fluid overload).
All CLTs performed in subjects deemed at high risk were reviewed by the quality assurance director. The primary focus was CLTs done within ∼12 h preceding extubation. Additional data were collected on all subjects who developed PES. This was done anticipating the potential need to enhance future quality improvement monitoring based upon the characteristics of subjects who developed PES. Post hoc usage of quality assurance data was approved by the University of California, San Francisco Committee on Human Research (approval #18-24329).
Assessment Protocol
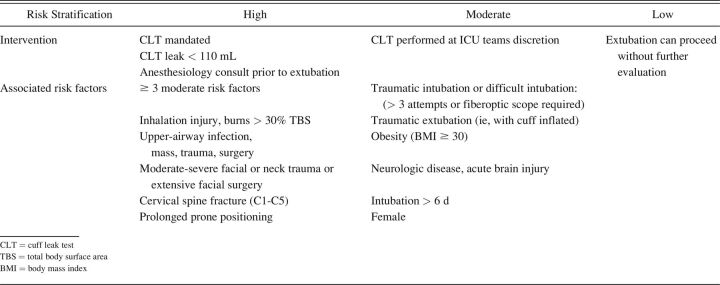
All intubated adult subjects admitted to any ICU underwent standardized screening for PES risk. In those requiring a prolonged course of mechanical ventilation (> 48 h), rescreening occurred during the weaning phase when extubation was to follow a successful spontaneous breathing trial. Subjects were categorized as being at low, medium, or high risk for PES depending upon the absence or presence of specific risk factors.
Identification and stratification of risk factors were based upon subject history, diagnostic tests, and anatomic considerations (Table 1). In regard to upper-airway anatomy, overall risk assessment included consideration of anatomic features likely to make potential re-intubation difficult (Supplementary Table 1, see related supplementary materials at http://www.rcjournal.com). This assessment also was based upon the initial intubation note that dictated whether an anesthesiologist should be present at extubation. This included the presence of an emergency airway cart and/or other respiratory therapies or whether to perform extubation in an operating room.
Table 1.
Screening Form to Stratify Risk for Postextubation Stridor to Evaluate Need for Performing a Cuff Leak Test
Modification of a Volume-Based Cuff Leak Test
A standardized CLT was performed when specific risk factors for stridor were present or at the discretion of the ICU team. Performance of a CLT required a separate physician review and order. In the original study by Miller and Cole,6 the difference between digital displays of inspired and expired tidal volume (VT) during volume control ventilation was used to calculate leak volume. The ventilator brand used in that study displayed inspired volume as reflecting the additive effects of gas conditioning and compressible volume compensation. This value was compared to the average expired VT taken from 3 breaths.
Adopting this technique for practical usage involved consideration that a large number of clinicians and different ventilator brands would introduce potential confounding factors. Therefore, we modified their CLT technique as follows. First, only the change in expired VT between conditions of ETT cuff inflation and deflation was used. This is because ventilator brands do not uniformly calculate and display inspired VT in the same manner. Second, we reasoned that a clinician’s ability to adequately judge expired VT stability would likely be as accurate as calculating the average VT that itself might introduce error. Third, we used standardized volume control ventilation settings to remove technique variation as a potential confounder (Table 2). CLT was performed with subjects in the semi-Fowler position between 20–30 degrees per hospital policy.
Table 2.
Modified Cuff Leak Test Procedure
Test preparation required one of 2 strategies, maximizing the likelihood of passive ventilation during CLT (ie, eliminating the potential confounding variable of spontaneous breathing efforts on the measured leak volume). When indicated, subjects received supplemental sedation to produce a minimum Richmond Agitation-Sedation Scale (RASS) of −1, or CLT was performed when subjects were asleep and additional sedation was not needed to achieve the desired RASS score. Although data were not collected regarding sedation at our institution, low-dose propofol (eg, 0.5–1.0 mg/kg loading dose and 0.5 mg/kg every 3–5 min) is used for brief procedural sedation that would include CLT.
Definitions
It is common to define a positive CLT as one producing a sufficient cuff leak. However, diagnostic testing is used to detect the presence of disease so that a positive CLT is one in which a gas leak is either absent or deemed insufficient. Therefore, we adopted the cutoff value reported by Miller and Cole,6 wherein a cuff leak ≥ 110 mL constituted a negative CLT if PES was absent following extubation and a leak < 110 mL as a positive CLT when PES occurred following extubation. Likewise, a false-negative test was one in which a pre-extubation CLT ≥ 110 mL was associated with PES and a false-positive test was one in which pre-extubation CLT was < 110 mL and PES did not occur.
Data Collection
Data were collected prospectively from May 1, 2010–December 31, 2017. Screening forms were collected and analyzed weekly. The quality assurance director (RHK) organized and analyzed the data in 3 ways: (1) according to those with and without risk factors documented on screening forms, (2) those with risk factors requiring CLT prior to extubation, and (3) information on all reported PES cases. Screening forms were kept in binders in every ICU along with a classification chart and CLT policy and procedure (Tables 1-2). All respiratory therapists were trained on risk assessment performance in consultation with the ICU physician staff. As part of the therapists’ training, stridor was reviewed and defined as a high-pitched monophonic sound during inspiration.7 In addition, copies of the classification chart were attached to each ventilator for ease of bedside access and as a constant visual reinforcement of the policy.
All PES incidences were captured regardless of whether a CLT had been performed. These were recorded on both the surveillance forms and in respiratory care flow sheets. Narrative reports documenting PES incidences were required of respiratory therapists. Both physician and nursing documentation were reviewed as an additional source of information. All documented incidences of upper-airway obstruction were reviewed and adjudicated by the quality assurance director, which in some instances when the description was unclear included direct communication with the bedside clinicians involved.
CLT data were obtained from reviewing respiratory care flow sheets in the electronic medical record. The data used to assess predictive values were limited to CLTs done within ∼12 h preceding extubation.
Monitored Variables
CLT variables included expired VT both with the cuff inflated and deflated. The recoded leak volume represented the difference between these readings and also as a percentage of the expired VT preceding cuff deflation. ETT size also was recorded.
Other pertinent data included tracheal intubation history (ie, difficult or traumatic intubation); incidences of unplanned traumatic extubation (ie, ETT removal with an inflated cuff); previous episodes of failed extubation; prior PES incidences; inhalation injury; and the presence of significant facial or neck trauma, surgery, or infection.
Subjects undergoing multiple CLTs were analyzed for test-to-test reproducibility (relative to clinical status). Poor reproducibility was defined as disparate test results occurring within a relatively brief time period (eg, ∼24 h) without corresponding changes in subject conditions (eg, diuresis, steroid administration). High reproducibility was defined as either unvarying CLT results mirroring an unchanged status or those in which an improved leak was consistent with improving clinical trajectory/response to therapy.
Statistical Analysis
Subject characteristics are reported as percentages and continuous variables as median and 25–75% interquartile range (IQR) as data were found to be non-normally distributed when analyzed by Kolmogorov-Smirnov test. Multiple comparisons were done by the Kruskal-Wallis test and Dunn post test, whereas between-group comparisons were done using Mann-Whitney test. Two-sided Fisher exact tests were used to assess dichotomous categorical variables as well as calculating sensitivity, specificity, positive and negative predictive values, and likelihood ratios. Area under the receiver operating curve was analyzed using the Wilson-Brown method. Multivariate logistic regression modeling was done on the subset of subjects in whom PES occurrence could be matched with both CLT data and a limited number of specific risk factor categorizations used for all subjects who underwent CLT. All analyses were done using Prism version 9.0 statistical software (GraphPad, San Diego, California.). Alpha was set at 0.05.
Results
Database Characteristics
Overall, 74% of subjects were managed in the surgical-trauma and neurotrauma ICUs. In the 7.5-year data collection period, 5,314 screening forms were completed, of which 43% of subjects had no discernable PES risk factors (low risk), 37% had 1–2 moderate risk factors, and 19% had either significant individual risk factors or ≥ 3 moderate risk factors. Of the 1,031 high-risk subjects screened, CLT data were not available for 241 subjects due to protocol noncompliance (Fig. 1). Of these, 43 developed PES (18%) and 11 (5%) developed supralaryngeal obstruction. Four additional PES cases occurred among low-risk subjects (< 0.2%). Overall protocol compliance was 71%.
Fig. 1.
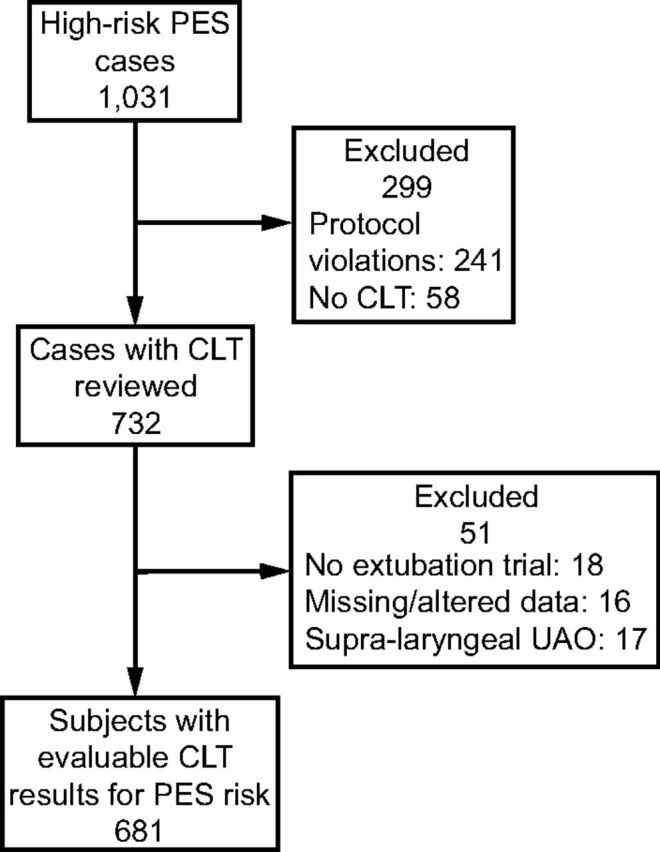
Flow chart. PES = postextubation stridor; CLT = cuff leak test; UAO = upper airway obstruction.
There were 732 cases in which a CLT trial was performed. Each case represented a discreet period of intubation. As such, 46 subjects (6.3%) represented 2 separate cases, of whom 19 (46%) developed PES. Of the 732 evaluable CLT trial cases, 18 subsequently underwent tracheostomy or were transferred to another institution prior to extubation (Fig. 1). Sixteen CLT trial cases were excluded because of missing leak data or altered CLT techniques. Consequently, 681 CLT trial cases were available to assess CLT accuracy in predicting PES. The largest cohort (∼85%) consisted of true-negative CLT results; the remainder was almost equally divided into CLT cohorts of true-positive (∼4%), false-negative (∼5%), and false-positive results (∼6%).
Although not required by protocol, respiratory therapists documented 17 cases in which acute upper-airway obstruction following extubation from loss of upper-airway muscle function. This was differentiated empirically and deductively from PES by both the low pitch and other attributes (eg, noncontinuous, intermittent sound production) and its relief after subject repositioning and/or placement of a nasal or oral airway as described by clinicians and reviewed by the quality assurance director. These data were analyzed separately.
Baseline Subject Characteristics
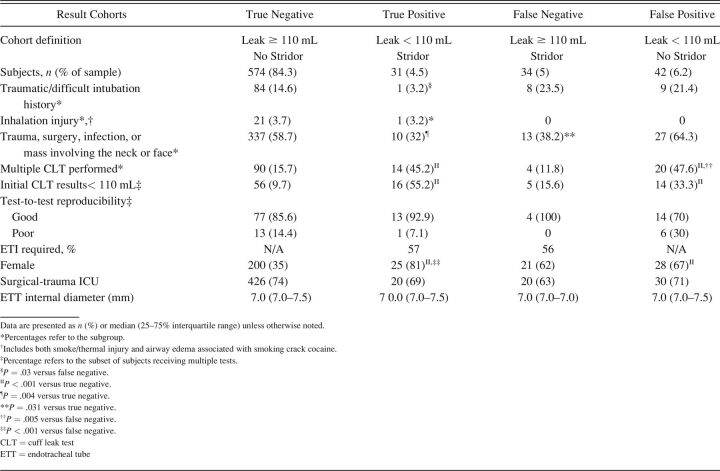
All cohorts had a sizable proportion of subjects with significant facial/neck surgery, trauma, or infection (Table 3). Females constituted the majority of subjects tested across most cohorts (67–100%), the exception being the true-negative cohort (38%). Interestingly, the true-positive cohort had a lower history of difficult or traumatic intubation, differing only with the false-negative cohort.
Table 3.
Characteristics and Results From 681 Subjects in Whom Evaluable Cuff Leak Tests Were Done Prior to a Trial of Extubation to Assess Stridor Risk
Of the 681 CLT trial cases, only 128 (∼19%) had multiple CLTs performed, with 91 initial findings of an absent or insufficient leak (∼13% of all CLT trial cases). Multiple CLTs occurred largely among the true-positive and false-positive cohorts as well as those in whom the initial CLT results produced a leak < 110 mL. Test-to-test reproducibility was high in 108 of 129 multiple CLT cases (84%), with no difference found between cohorts. ETT size was not different between cohorts.
CLT Volume Measurements
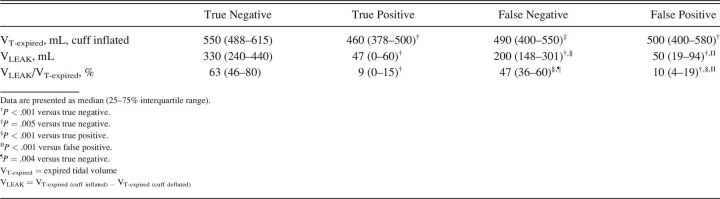
Substantially larger leak volumes (4.0–6.6-fold greater) were observed in both the true- and false-negative cohorts versus true- and false-positive cohorts (Table 4). Of note, the leak observed among true-negative subjects was significantly greater than that found among false-negative subjects by a factor of 1.7 (330 mL vs 200 mL).
Table 4.
Cuff Leak Test Volume Measurements Across Groups
Association Between CLT Results and PES
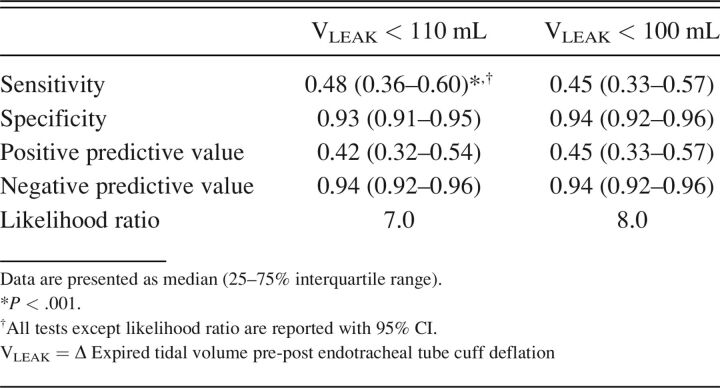
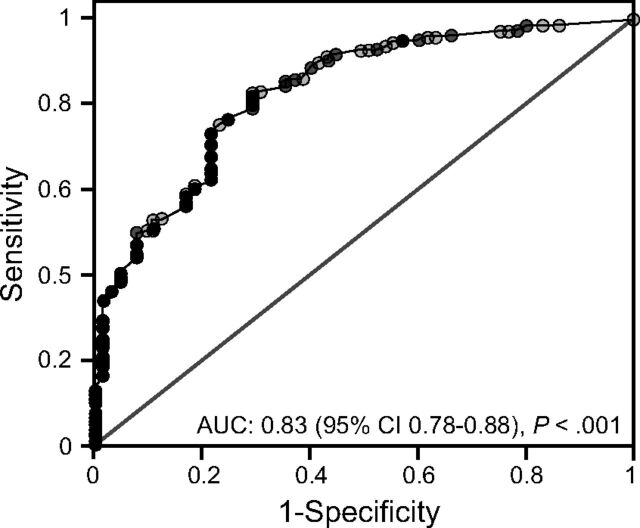
A leak of < 110 mL was only moderately sensitive in detecting PES, reflected in a positive predictive value below that of a coin flip. Nonetheless, it was associated with 7-fold higher PES risk (Table 5). More importantly, the specificity and negative predictive values of a leak ≥ 110 mL were very high. The 110 mL leak threshold produced a correct classification of 89% and area under the receiver operating curve of 0.83 (95% CI 0.78–0.88, P < .001) (Fig. 2). Because a leak cutoff < 100 mL would be easier for clinicians to remember, we reassessed the positive and negative predictive values and found them essentially unchanged (Table 5). Small improvements were found in both likelihood ratio (8.0) and correct classification (90.4%) at a leak threshold of 100 mL.
Table 5.
Predictive Characteristics Comparing Pre Hoc Cutoff Values for Leak Volume Following Cuff Deflation in Detecting Postextubation Stridor
Fig. 2.
Area under the receiver operating curve reflecting the 681 evaluable cuff leak tests for assessing postextubation stridor risk.
The limited multivariate logistic regression modeling revealed that females and those in whom the leak volume was < 110 mL were at increased risk for developing PES: odds ratio 4.03 (95% CI 2.16–7.91, P < .001) and odds ratio 7.45 (95% CI 3.83–15.56, P < .001), respectively. Paradoxically, the presence of major facial or neck injury was found protective: odds ratio 0.33 (95% CI 0.17–0.62, P < .001).
Association Between Leak Volume and Re-intubation Among PES Subjects
Among PES subjects, CLT leak volume did not distinguish those requiring re-intubation versus those in whom stridor either responded to therapy or resolved spontaneously: median 111 (IQR 33–170) mL versus median 62 (IQR 35–200) mL, P = .93). Likewise, a CLT leak of < 110 mL was not associated with a higher risk for re-intubation: odds ratio 0.77 (95% CI 0.26–2.13, P = .79) and area under the receiver operating curve of only 0.53 (95% CI 0.43–0.64, P = .52).
Overall Stridor Incidence Based Upon Risk Categorization
Of the 5,314 intubated patients, 3,006 (∼57%) had at least one PES risk factor, and 1,031 (∼19%) were classified as being at high risk. The overall incidence of PES was 2.2% (115/5,314) with incidences among cohorts of < 0.2% (low risk), 1.3% (moderate risk), and 7.8% (high-risk).
Characteristics of Stridor Subjects
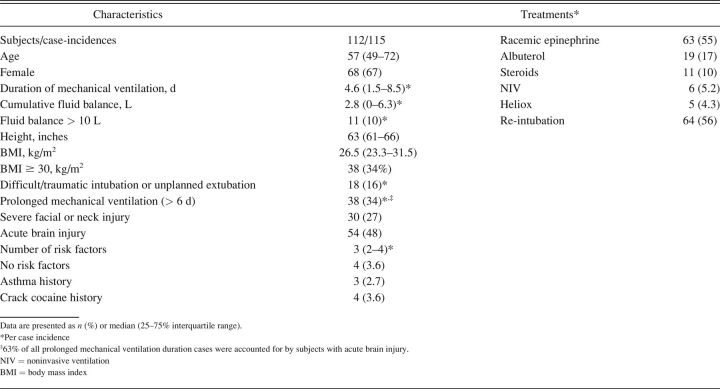
PES subjects were predominantly female (67%) despite females accounting for 43% of cases (Table 6). Approximately half (48%) suffered acute brain injury induced either by trauma or cerebral vascular accident. Subjects tended to be of shorter stature (median 63 [IQR 61–66] inches), older (median 57 [IQR 49–72] y), had multiple PES risk factors (median 3 [IQR 2–4]), and required a moderately prolonged course of intubation (median 4.6 [IQR 1.5–8.5] d). Only 34% of PES cases met our pre hoc risk threshold of ≥ 6 d of intubation.
Table 6.
Characteristics of Subjects Who Developed Postextubation Stridor and Associated Therapeutic Interventions
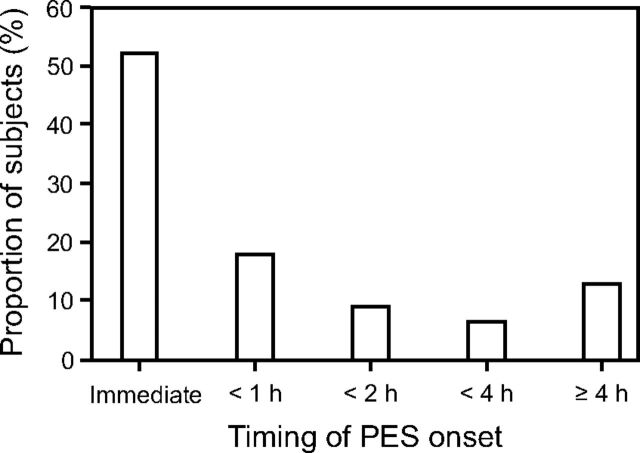
Other potential risk factors that were not prevalent included overhydration (10%) and difficult/traumatic intubation or traumatic (unplanned) extubation (16%). Severe facial/neck injury and ≥ Class 1 obesity were more prevalent (27% and 34%, respectively). Finally, onset of PES was rapid, with 71% of incidences occurring within the first hour following extubation and only a minority of cases (13%) developing stridor after 4 h (Fig. 3).
Fig. 3.
Timing of postextubation stridor onset. PES = postextubation stridor.
Comparing the characteristics between stridor subjects in the true-positive versus false-negative cohorts revealed no difference in those who required re-intubation (57% vs 56%, respectively, P > .99). And only in the false-negative cohort was a tendency toward a lower leak volume found in those requiring re-intubation (compared to those who responded to racemic epinephrine and other therapies or resolved spontaneously): 170 (128–270) mL versus 280 (174–314) mL, respectively, P = .058.
Although it was not possible to collect highly specific data on all subjects monitored during this continuous quality improvement project, those undergoing CLT were classified according to 4 prominent risk factor categories informing future project iterations: (1) difficult/traumatic intubation; (2) severe facial or neck trauma, surgery, or infection; (3) inhalation injury or crack cocaine use; and (4) initial poor CLT in those undergoing multiple CLTs.
Applied Therapies and Immediate Consequences of PES
The majority of PES cases (55%) received aerosolized racemic epinephrine, and a similar percentage (56%) required re-intubation. Re-intubation was achieved in all subjects without difficulty or adverse incident. Other therapies were seldom used (Table 6).
Forty-two percent of PES cases were subjectively judged by clinicians at the bedside to have had minor to moderate stridor and/or responded to therapy. Of the 30 cases treated with racemic epinephrine and did not require re-intubation, limited documentation described stridor as mild in 17% and moderate in 13% of cases. Additional documentation noted therapeutic efficacy in 20% of cases, whereas 7% cited either therapeutic ineffectiveness or spontaneous resolution of stridor. In 15 cases requiring neither re-intubation nor racemic epinephrine, PES was described as mild in 40% of cases and moderate in 13%. Overall, the CLT leak volume among PES cases reported as being mild-moderate was 160 (IQR 98–260) mL.
Discussion
Our study produced 2 main findings. First, a simplified CLT based only on changes in expired VT produced a strong signal for safely proceeding with extubation in an adult population of critically ill subjects in a predominantly surgical-trauma and neurotrauma ICU setting. This was apparent at a leak threshold of ≥ 110 mL. Post hoc analysis using a threshold of ≥ 100 mL did not alter the results. And in those undergoing multiple CLTs, the test-to-test reproducibility was judged to be high.
Second, the comprehensive PES risk screening protocol with specific, graded risk criteria allowed clinicians to accurately target CLT evaluations. This may explain both the overall low PES rate of 2.1% among the 5,314 screened subjects and the positioning of our results at the lowest end of the reported PES incidence of 2–30%.1-3 In addition, the seemingly paradoxical finding that major facial or neck injury was protective for PES in multivariate logistic modeling was puzzling. However, because severe facial or neck trauma is perhaps the most obvious signifier for potential PES, we surmised that clinicians more likely withheld extubation until a sufficient cuff leak was observed. Furthermore, the highest PES incidence occurred with protocol noncompliance among high-risk subjects (18%). This stands in stark contrast to the overall PES incidence of 7.8% among high-risk subjects. We interpret both these findings as prima facie evidence supporting the systematic screening and evaluation for PES risk.
Comparing our results to others using a volume-based CLT measured on volume control ventilation that reported the incidence of PES,1,6,8-26 5 (26%) used a 110 mL cutoff,6,11,15,17,24 2 (11%) used a leak of < 100 mL,16,18 4 (21%) used a similar but higher leak cutoff (≤ 140 mL),1,10,12,23 2 used a cutoff of ≥ 200 mL,13,22 and 5 (26%) reported a leakage cutoff as a percentage of VT ranging between 10– 24%.8,9,14,19,20 Thirteen (68%) studies used a preset VT similar to that used in our study (ie, 7–12 mL/kg),1,9,10,12-14,16-21 and 7 (41%) used expired VT to measure CLT leak.8,10,11,13,14,19,21 Grouped data revealed a median (IQR) PES incidence of 9.5% (7.8–12.3) and range of 1–18%. By comparison, when confined to 681 subjects who underwent CLT evaluation, our PES incidence was the same: 9.5% (65/681).
Of the 17 observational PES studies or therapeutic trials with a control arm reviewed, 9 (53%) had ≤ 100 subjects,6,9,13,16,19,20,23,24,26 and only 3 (18%) had > 115 subjects (n 432–524).11,15,22 PES incidence in these substantially larger studies were 1, 4, and 10%, suggesting an overestimation bias among smaller studies. This is consistent with our findings in which an overall PES incidence was 2.2% in over 5,000 screened subjects compared to a 7.8% incidence among the 19% of our high-risk subjects. This lends support to the opinion that “if used at all, the CLT should therefore be used in a high-risk population to select patients who may benefit from preventive treatment.”12
Another important finding from our study was the stark reduction in CLT leak volume and percentage of pre-deflation VT found among our true-positive cohort (medians of 40 mL and 9%, respectively). This is consistent with other observational studies in which the corresponding average values ranged from 23–91 mL in 7 studies8-10,16,21,26 and 7–10% in 2 studies8,9 that reported these data, In contrast, the corresponding median data observed in our true-negative cohort (330 mL and 63%) either exceeded or was similar to that found in all studies that reported these data, with average CLT leak volumes between 220–395 mL and percentages of average pre-deflation VT between 41–73%.6,9-11,13,16,18,21,23,26
Our very high negative predictive value was (with few exceptions) a consistent finding in the meta-analysis by Kuriyama et al,5 despite variations in technique. This suggests that clinicians’ focus should be the negative predictive power of CLT. Furthermore, our screening, evaluation, and management protocol along with our finding of likelihood ratios of 7–8 provides a sufficient signal for when to proceed cautiously with extubation when CLT is < 110 mL. This was reflected in our policy stipulating the presence of an anesthesiologist and an array of equipment and therapies at the bedside in these situations.
Adherence to our CLT protocol was 71%. Unfortunately, sparse documentation and subjective impressions only suggest plausible explanations. We encountered incidences when either the number of moderate risk factors was undercounted or individual high-risk factors were missed. In other cases, facial or neck injury was minor so that a CLT was unnecessary, and the ICU team justifiably declined a CLT. Unfortunately, there were situations when either the protocol was ignored or a different measurement technique was demanded. Despite these occurrences, there was a noticeable culture shift toward increased awareness of stridor risk. Most often when a CLT was declined, ICU team members remained at the bedside during extubation. There also were several instances when direct laryngoscopy was done prior to extubation in lieu of a CLT.
Among the 115 incidences of PES in 112 subjects, the most pronounced findings were a higher incidence among females (67%) and a majority of subjects (63%) having multiple (≥ 3) risk factors. Overall, a majority of these subjects (56%) developed stridor severe enough to require re-intubation. This likely was influenced by ICU teams deciding to proceed with extubation once we had accumulated sufficient data demonstrating the relatively poor positive predictive value of CLT and also by our policy of having both anesthesia and respiratory care personnel at the bedside in these situations. Contextually, in 9 studies reporting these data, the re-intubation rate among PES subjects ranged from 9–63%,6,8-11,15,18,20,21 with 6 reporting rates ≥ 25%, 3 of which reported re-intubation rates similar to ours (ie, ≥ 50%).6,10,18 Interestingly, the pre-extubation CLT leak volume was not associated with re-intubation risk among our PES subjects.
Lastly, only a small minority of subjects developed stridor without identifiable risk factors (∼4% of all PES cases, overall incidence < 0.2%), thus obviating the need to routinely perform CLT prior to extubation when a disciplined surveillance and testing program is in place.
The higher incidence of PES among females is consistent with other studies.1,11-14,18,19,21,22,24 Postmortem measurements in females found significantly smaller cricoid ring (internal) diameter and shorter distance between the cricoarytenoid joints, thus supporting the impressions that females are at higher risk for developing pressure necroses with standard ETT sizes.27 This impression recently was supported by a computer tomography study.4
Our PES subjects also shared other characteristics observed in some PES studies, these being: higher incidence in the trauma and neurotrauma surgical ICU setting, older age, moderately prolonged intubation (4.6 d), and a sizable minority (34%) meeting morbid obesity criteria.1,8,11,13,14
Study limitations include its retrospective nature based upon quality assurance data (with limited data collection capabilities), incomplete data (∼30% of required CLTs were not performed), and CLT performed by numerous respiratory therapists. In addition, as the methodology was altered for pragmatic reasons, individual CLTs may not have possessed the same precision as those done by a dedicated research team. As an example, we did not capture height for all subjects undergoing CLT testing that would have provided useful information regarding the relationship between ETT size and stridor risk. Notwithstanding these limitations, our results are consistent with other studies.
Conclusions
A simplified volume-based CLT in a large sample of adult, predominantly surgical-trauma and neurotrauma subjects, using a leak threshold of 110 mL, produced a very high negative predictive value similar to other studies. In addition, when multiple CLTs were required, our test-to-test reproducibility was high when standardized volume control ventilation settings and procedural passive ventilation were emphasized. Moreover, the absence of stridor in a substantial portion of intubated, critically ill adult subjects without risk factors obviates the need to routinely perform CLT prior to extubation but only when a protocolized surveillance, assessment, and treatment policy is in place.
Supplementary Material
Acknowledgments
We would like to acknowledge the contribution of our respiratory therapists whose diligence and professionalism was essential to our findings as well as our gratitude for the cooperation and support received from critical care physicians, nurses, and anesthesiologists that also were essential for the success of this quality assurance initiative.
Footnotes
See the Related Editorial on Page 442
Mr Kallet discloses a relationship with ContinuED. The remaining authors have disclosed no conflicts of interest.
A version of this paper was presented by Ms Matsushima at AARC Congress 2018, held in Las Vegas, Nevada, December 4–7, 2018.
Supplementary material related to this paper is available at http://www.rcjournal.com.
REFERENCES
- 1.Jaber S, Chanques G, Matecki S, Ramonatxo M, Vergne C, Souche B, et al. Postextubation stridor in intensive care unit patients. Risk factors evaluation and importance of the cuff leak test. Intensive Care Med 2003;29(1):69-74. [DOI] [PubMed] [Google Scholar]
- 2.Pluijms WA, van Mook WN, Wittekamp BH, Bergmans DC. Postextubation laryngeal edema and stridor resulting in respiratory failure in critically ill adult patients: updated review. Crit Care 2015;19:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wittekamp BH, van Mook WN, Tjan DH, Zwaveling JH, Bergmans DC. Clinical review: postextubation laryngeal edema and extubation failure in critically ill adult patients. Crit Care 2009;13(6):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinohara M, Iwashita M, Abe T, Takeuchi I. Association between postextubation upper-airway obstruction symptoms and airway size measured by computed tomography: a single-center observational study. BMC Emerg Med 2022;22(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuriyama A, Jackson JL, Kamei J. Performance of the cuff leak test in adults in predicting postextubation airway complications: a systematic review and meta-analysis. Crit Care 2020;24(1):640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller RL, Cole RP. Association between reduced cuff leak volume and postextubation stridor. Chest 1996;110(4):1035-1040. [DOI] [PubMed] [Google Scholar]
- 7.Kallet RH. Bedside assessment of the patient. In: Kacmarek RM, Stoller JK, Heuer AJ, editors. Egan’s fundamentals of respiratory care. North Andover, Massachusetts: Elsevier; 2017:338-339. [Google Scholar]
- 8.Sandhu RS, Pasquale MD, Miller K, Wasser TE. Measurement of endotracheal tube cuff leak to predict postextubation stridor and need for reintubation. J Am Coll Surg 2000;190(6):682-687. [DOI] [PubMed] [Google Scholar]
- 9.De Bast Y, De Backer D, Moraine JJ, Lemaire M, Vandenborght C, Vincent JL. The cuff leak test to predict failure of tracheal extubation for laryngeal edema. Intensive Care Med 2002;28(9):1267-1272. [DOI] [PubMed] [Google Scholar]
- 10.Keeratichananot W, Limthong T, Keeratichananot S. Cuff leak volume as a clinical predictor for identifying postextubation stridor. J Med Assox Thai 2012;95(6):752-755. [PubMed] [Google Scholar]
- 11.Kriner EJ, Shafazand S, Colice GL. The endotracheal tube cuff leak test as a predictor for postextubation stridor. Respir Care 2005;50(12):1632-1638. [PubMed] [Google Scholar]
- 12.Gros A, Holzapfel L, Marque S, Perard L, Demingeon G, Piralla B, et al. Intra-individual variation of the cuff leak test as a predictor of postextubation stridor. Respir Care 2012;57(12):2026-2031. [DOI] [PubMed] [Google Scholar]
- 13.Erginel S, Ucgun I, Yildirim H, Metintas M, Parspour S. High body mass index and long duration of intubation increase postextubation stridor in patients with mechanical ventilation. Tohoku J Exp Med 2005;207(2):125-132. [DOI] [PubMed] [Google Scholar]
- 14.Cheng KC, Hou CC, Huang HC, Lin SC, Zhang H. Intravenous injection of methylprednisolone reduces the incidence of postextubation stridor in intensive care unit patients. Crit Care Med 2006;34(5):1345-1350. [DOI] [PubMed] [Google Scholar]
- 15.Engoren M. Evaluation of the cuff leak test in a cardiac surgery population. Chest 1999;116(4):1029-1031. [DOI] [PubMed] [Google Scholar]
- 16.Lim SY, Suh GY, Kyung SY, An CH, Lee SP, Park JW, et al. Risk factors for extubation failure and analysis of cuff leak test as a predictor for postextubation stridor. Tuberc Respir Dis 2006;61(1):34-40. [Google Scholar]
- 17.Lee CH, Peng MJ, Wu CL. Dexamethasone to prevent postextubation airway obstruction in adults: a prospective, randomized, double-blind, placebo-controlled study. Crit Care 2007;11(4):R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang CL, Tsai YH, Huang CC, Wu YK, Ye MZ, Chou HM, et al. The role of the cuff leak test in predicting the effects of corticosteroid treatment on postextubation stridor. Chang Gung Med J 2007;30(1):53-61. [PubMed] [Google Scholar]
- 19.Shin SH, Heath K, Reed S, Collins J, Weireter LJ, Britt LD. The cuff leak test is not predictive of successful extubation. Am Surg 2008;74(12):1182-1185. [DOI] [PubMed] [Google Scholar]
- 20.Radhi S, Guerra D, Alalawi R, Raj R, Nugent K. Cuff leak test at the time of extubation correlates with voice quality assessment. ICU Dir 2012;3(1):27-30. [Google Scholar]
- 21.Sutherasan Y, Theerawit P, Hongphanut T, Kiatboonsri C, Kiatboonsri S. Predicting laryngeal edema in intubated patients by portable intensive care unit ultrasound. J Crit Care 2013;28(5):675-680. [DOI] [PubMed] [Google Scholar]
- 22.El-Baradey GF, El-Shmaa NS, Elsharawy F. Ultrasound-guided laryngeal air column width difference and the cuff leak volume in predicting the effectiveness of steroid therapy on postextubation stridor in adult. Are they useful? J Crit Care 2016;36:272-276. [DOI] [PubMed] [Google Scholar]
- 23.Sahbal MA, Mohamed KA, Zaghla HH, Kenawy MM. Laryngeal ultrasound versus cuff leak test in prediction of postextubation stridor. Egypt J Crit Care Med 2017;5(3):83-86. [Google Scholar]
- 24.Mikaeili H, Yazdchi M, Tarzamni MK, Ansarin K, Ghasemzadeh M. Laryngeal ultrasonography versus cuff leak test in predicting postextubation stridor. J Cardiovasc Thorac Res 2014;6(1):25-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettignano R, Holloway SE, Hyman D, LaBuz M. Is the leak test reproducible? South Med J 2000;93(7):683-685. [PubMed] [Google Scholar]
- 26.Chung YH, Chao TY, Chiu CT, Lin MC. The cuff leak test is a simple tool to verify severe laryngeal edema in patients undergoing long-term mechanical ventilation. Crit Care Med 2006;34(2):409-414. [DOI] [PubMed] [Google Scholar]
- 27.Randestad A, Lindholm CE, Fabian P. Dimensions of the cricoid cartilage and the trachea. Laryngoscope 2000;110(11):1957-1961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.