Abstract
Objectives
Adenosine deaminase 2 deficiency (DADA2) is a genetic, neurologic, and systemic vasculitis syndrome, which can lead to recurrent strokes, typically lacunar. In the cohort of now 60 patients followed up at the NIH Clinical Center (NIH CC), no patient has had a stroke since starting tumor necrosis factor (TNF) blockade. We present a family with multiple affected children to highlight the importance of TNF blockade not just as secondary stroke prevention but also as primary stroke prevention in genetically affected but clinically asymptomatic patients.
Methods
A proband with recurrent cryptogenic strokes was referred for evaluation at the NIH CC. The parents and 3 clinically asymptomatic siblings were also evaluated.
Results
The proband was diagnosed with DADA2 based on biochemical testing; her antiplatelet therapies were discontinued, and she was started on TNF blockade for secondary stroke prevention. Her 3 asymptomatic siblings were subsequently tested and 2 were found to be biochemically affected. One of them elected to start TNF blockade for primary stroke prevention and the other sibling declined this approach and experienced a stroke. A second genetic sequence variant was subsequently identified in the ADA2 gene.
Discussion
This family illustrates the importance of testing for DADA2 in young patients with cryptogenic stroke, given the hemorrhagic risks with antiplatelet drugs in these patients and effectiveness of TNF blockade as secondary stroke prevention. In addition, this family highlights the importance of screening all siblings of affected patients because they may be presymptomatic, and we advocate starting TNF blockade for primary stroke prevention in those who are found to be genetically or biochemically affected.
Adenosine deaminase 2 deficiency (DADA2) is a genetic, neurologic, and systemic vasculitis syndrome, which can lead to recurrent strokes, typically lacunar.1-3 This disease responds to treatment with tumor necrosis factor (TNF) blockade.2,4 In the cohort of now 60 patients followed up at the NIH Clinical Center (NIH CC), no patient has had a stroke since starting TNF blockade.2,3 We present a family with multiple affected children to highlight the importance of TNF blockade not just as secondary stroke prevention but also as primary stroke prevention in genetically affected but clinically asymptomatic patients.
Methods
A patient and her immediate family members (parents and siblings) were evaluated at the NIH CC, under the National Human Genome Research Institute IRB-approved protocol 94-HG-0105 “An Exploratory Study of the Genetics, Pathophysiology, and Natural History of Autoinflammatory Diseases.” They underwent genetic sequencing, bloodwork, neuroimaging, and consultations with multiple subspecialties including neurology and cardiology.
Results
The proband is a now a 20-year-old woman who had an ischemic stroke at 8 years of age involving the posterior limb of the right internal capsule and thalamus. No etiology was identified, and she was placed on aspirin. At the age of 11 years, she experienced a second stroke involving the left paramedian midbrain. Clopidogrel was added. A conventional angiogram showed negative results for vasculopathy. She made a full neurologic recovery.
Subsequently, she developed livedo racemosa and some raised nodular skin lesions suggestive of polyarteritis nodosa; these were biopsied and consistent with vasculitis. Glucocorticoids and rituximab were added. Her rheumatologist referred her for an evaluation at the NIH CC. The parents and 3 clinically asymptomatic siblings were also evaluated.
One mutated allele in ADA2 was identified, although her ADA2 enzyme levels were measured to be in the deficiency range (Figure 1). The mutation of the second allele of ADA2 was elusive until recently and was found to be a loss-of-function variant in the upstream regulatory sequences.5 Her antiplatelets were discontinued, and she was started on etanercept. She has been neurologically stable for many years. Other features of her clinical phenotype include hepatosplenomegaly and peripheral vascular disease (MRA with irregularities and multiple stenoses of small arterial branches within the feet), which improved symptomatically with amlodipine, and hyposmia (UPSIT smell test 19–25/40 consistent with severe microsmia).
Figure 1. ADA Enzyme Activity.
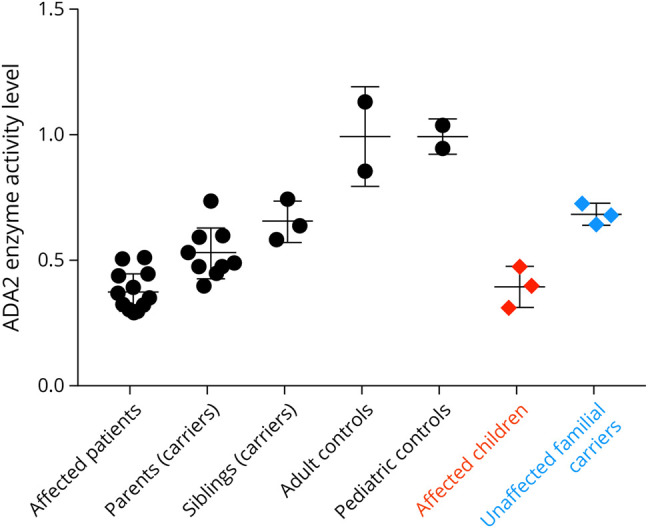
The presented family is represented in red and blue, while the other controls and patients are part of the larger NIH cohort.
Her siblings were subsequently tested because the age at onset of symptoms in DADA2 varies, so this practice has allowed us to identify presymptomatic siblings.3 She has 2 genetically affected siblings and 1 genetically unaffected sibling (Figure 2). Her genetically affected but neurologically asymptomatic older sister (a history of complicated migraines with normal neuroimaging) elected to start TNF blockade as per our clinical recommendation for primary stroke prevention in such cases. Her genetically affected but neurologically asymptomatic older adult brother (though on our evaluation, he endorsed a history of fevers, elevated inflammatory markers, livedo racemosa, a nodular rash biopsy proven as erythema nodosum, and right-sided sensorineural hearing loss) initially elected not to start TNF blockade but subsequently experienced a stroke at age 20 years (Figure 3) and is now on etanercept treatment.
Figure 2. Pedigree With Genetics.
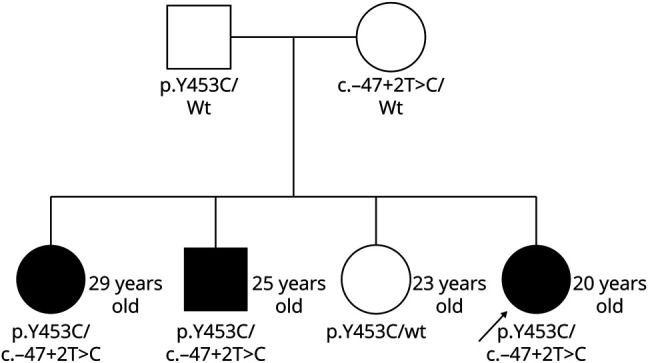
The arrow indicates the proband. Family members affected by DADA2 are represented by the dark shapes. DADA2 = ADA2 deficiency.
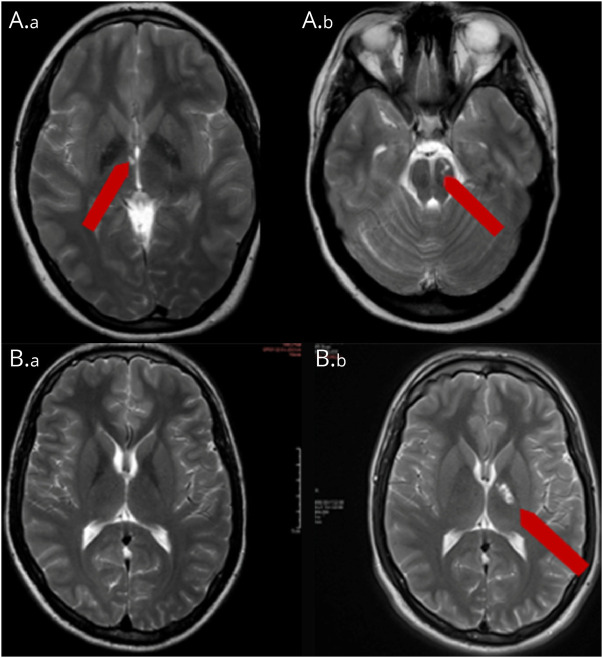
Figure 3. Neuroimaging.
(A.a, A.b) Proband's most recent brain MRI, 1/2019. Axial T2 showing old lacunar infarcts in the right internal capsule/thalamus and left midbrain (B.a, B.b) Male sibling's MRI axial T2: (B.a) Baseline, 3/2016, at 18 years of age and (B.b) follow-up, 3/2018, at 20 years of age showing lacunar infarct in the genu and posterior limb of the left internal capsule.
Discussion
DADA2 testing should be considered for any child or young adult with cryptogenic ischemic stroke. DADA2 can be diagnosed by biochemical testing with measuring the protein activity or genetic testing of the ADA2 gene (with the caveat that some ADA2-deficient patients may have an unidentifiable mutation on standard diagnostic sequencing). This diagnosis is important to make because the treatment is vastly different from other causes of ischemic stroke. Antiplatelets including low-dose aspirin are contraindicated due to the vascular friability and vasculitis in these patients leading to an increased risk of hemorrhage. In addition, the single effective stroke prevention for these patients is immunomodulation, specifically with TNF blockade. Rare patients have had to undergo hematopoietic stem cell transplant for refractory cytopenias.6,7
The patient's brother had some subtle symptoms of systemic inflammation, so it is unclear whether he was more at risk of stroke, given his disease activity. This is in contrast to the patient's sister who had complex migraines (which are overrepresented in the cohort even of DADA2 carriers, so not suggestive of an inflammatory basis). Thus, it is possible that the stroke in the brother reflects higher disease severity and that he may have experienced a stroke regardless or that vice versa the sister may have never experienced a stroke even without starting on therapy.
The illustrative example of this multiply affected family with DADA2 supports the treatment recommendation of genetic testing in clinically asymptomatic siblings and strongly considering starting TNF blockade to prevent the development of strokes. Further research is needed to conclusively elucidate the risk-benefit balance of this approach for asymptomatic affected patients. The use of long-term TNF blockade seems safe, but the mode of administration by injection is less convenient, and there are rare risks of reactivation of infections, such as mycobacterial and viral hepatitides, which require screening and monitoring, malignancies, demyelination, and financial considerations. In addition, some people with DADA2 have their first stroke in adulthood, so the ideal timing for primary prophylaxis is unclear because our ability to predict disease flares in these patients is not yet well established.
Acknowledgment
The authors thank the patient and her family for their ongoing participation in this study. This work was supported by the Intramural Research Programs of the National Human Genome Research Institute, the National Institute of Neurological Disorders and Stroke, and the National Heart, Lung, and Blood Institute.
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
No disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Zhou Q, Yang D, Ombrello AK, et al. . Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370(10):911-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ombrello AK, Qin J, Hoffmann PM, et al. . Treatment strategies for deficiency of adenosine deaminase 2. N Engl J Med. 2019;380(16):1582-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barron KS, Aksentijevich I, Deuitch NT, et al. . The spectrum of the deficiency of adenosine deaminase 2: an observational Analysis of a 60 patient cohort. Front Immunol. 2021;12:811473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deuitch NT, Yang D, Lee PY, et al. . TNF inhibition in vasculitis management in adenosine deaminase 2 deficiency (DADA2). J Allergy Clin Immunol. 2022;149(5):1812-1816.e6. [DOI] [PubMed] [Google Scholar]
- 5.Schnappauf O, Zhou Q, Moura NS, et al. . Deficiency of adenosine deaminase 2 (DADA2): hidden variants, reduced penetrance, and unusual inheritance. J Clin Immunol. 2020;40(6):917-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashem H, Bucciol G, Ozen S, et al. . Hematopoietic cell transplantation cures adenosine deaminase 2 deficiency: report on 30 patients. J Clin Immunol. 2021;41(7):1633-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee PY, Kellner ES, Huang Y, et al. . Genotype and functional correlates of disease phenotype in deficiency of adenosine deaminase 2 (DADA2). J Allergy Clin Immunol. 2020;145(6):1664-1672.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]