Abstract
Background
Gastric cancer (GC) is a life-threatening malignant tumor with high incidence rate. Despite great progress, there are still many GC sufferers that cannot benefit from the existing anti-GC treatments. Therefore, it is still necessary to develop novel medicines against GC. Emetine, a natural small molecule isolated from Psychotria ipecacuanha, has been broadly used for medicinal purposes including cancer treatment. Here, we conducted a comprehensive study on the anti-GC effects of emetine and the related mechanisms of action.
Methods
The cell viability was evaluated by MTT and colony formation assay. Cellular proliferation and apoptosis were analyzed by edu incorporation assay and Annexin V-PI staining, respectively. Moreover, wound healing assay and transwell invasion assay were conducted to detect cell migration and invasion after treatment with emetine. To elucidate the molecular mechanism involved in the anti-GC effects of emetine, RNA sequencing and functional enrichment analysis were carried out on MGC803 cells. Then, the western blot analysis was performed to further verify the anti-GC mechanism of emetine. In vivo anti-tumor efficacy of emetine was evaluated in the MGC803 xenograft model.
Results
MTT and colony formation assay exhibited a strong potency of emetine against GC cell growth, with IC50 values of 0.0497 μM and 0.0244 μM on MGC803 and HGC-27 cells, respectively. Further pharmacodynamic studies revealed that emetine restrained the growth of GC cells mainly via proliferation inhibition and apoptosis induction. Meanwhile, emetine also had the ability to block GC cell migration and invasion. The results of RNA sequencing and western blot showed that emetine acted through regulating multiple signaling pathways, including not only MAPKs and Wnt/β-catenin signaling axes, but also PI3K/AKT and Hippo/YAP signaling cascades that were not found in other tumor types. Notably, the antitumor efficacy of emetine could also be observed in MGC803 xenograft models.
Conclusion
Our data demonstrate that emetine is a promising lead compound and even a potential drug candidate for GC treatment, deserving further structural optimization and development.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00280-023-04521-y.
Keywords: Gastric cancer, Emetine, YAP, PI3K, AKT, Wnt, MAPKs
Introduction
Gastric cancer (GC) remains one of the most important malignancies worldwide. There were over one million new GC cases and approximately 769,000 GC-caused deaths in 2020 globally, making GC the fifth incidence rate and fourth mortality cancer in the world [1]. There are great regional and gender differences in GC incidence, and adenocarcinoma accounts for about 95% of all types of GC [1, 2]. GC patients with early-stage disease could be treated and even cured by surgical resection [3, 4]. However, due to the inconspicuous symptoms of early GC, many sufferers are diagnosed at advanced stages and systemic treatment must be adopted for such patients. Chemotherapy, radiotherapy, targeted therapy and immunotherapy are the main systemic therapies for patients with advanced GC [5–8]. Despite great progress in GC treatments recently, primary resistance and tumor relapse limited the efficacies of the therapeutic agents or approaches [9–12]. Therefore, it is still necessary to develop anti-GC medicines to benefit more patients with advanced GC.
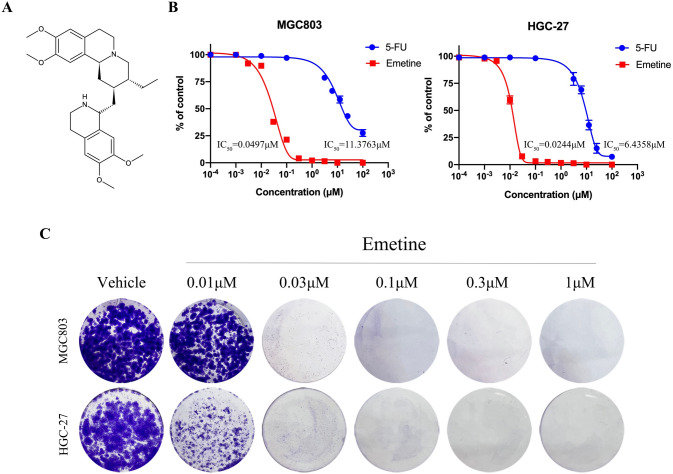
Emetine (Fig. 1A) is an alkaloid that exists only in three plant families Rubiaceae, Icacinaceae, and Alangiaceae [13]. It is mainly isolated from Cephaelis ipecacuanha Rich (Rubiaceae) which is also known as Psychotria ipecacuanha Stokes [13]. Since introduced into Europe, ipecacuanha alkaloids have been extensively used for medicinal purposes. As one of the major active alkaloids from Psychotria ipecacuanha, emetine has a long history for inducing emesis against toxic substances, treating amoebiasis (a protozoan infection), and eliminating phlegm [14]. Recently, it is also reported to be potent against multiple viruses, including Zika virus, Ebola virus and SARS-CoV-2 virus [15, 16].
Fig. 1.
Emetine effectively inhibited GC cell growth in vitro. A Chemical structure of emetine. B Anti-viability activity of emetine was determined using MTT assay after treatment with different concentrations of emetine for 72 h. Data were presented as mean ± SD (n = 3). C Colony formation assay in MGC803 and HGC-27 cells treated with serial dilutions of emetine for 10 days
Evidence for the anti-tumor effects of emetine was first reported early in 1900s [17]. Subsequently, emetine was evaluated in the treatment of a variety of malignant tumors, including leukemia, lung cancer, hepatocytes and breast cancer [18–20]. In 2011, Akinboye ES et al. summarized in their review that emetine exerted anti-cancer activity mainly by promoting apoptosis; regulation of pro-apoptotic factors, DNA interaction, and suppression of protein biosynthesis were the major mechanisms of action involved [21]. In the last decade, studies investigating the role of emetine in intervening in various functions of tumor cells and the corresponding molecular mechanisms were widely conducted. In the identification of agents that could interrupt bone marrow stromal cells (BMSCs)-mediated protection for chronic lymphocytic leukemia (CLL) cells, emetine was found to be active against the interaction between CLL cells and BMSCs. It acted via reducing HIF-1α expression and disrupting intracellular redox homeostasis [22]. Sun et al. revealed that the upstream components of Wnt/β-catenin signaling (including LRP6 and DVL) could be targeted by emetine in breast cancer cells, which resulted in the blockade of this signaling pathway. This pharmacological action of emetine enabled it to be effective in inhibiting cell viability and stemness, as well as inducing apoptosis of breast cancer cells [23]. Additionally, emetine also showed inhibitory effects on the growth of non-small cell lung cancer (NSCLC) cells through targeting mitogen-activated protein kinases (MAPKs) and Wnt/β-catenin signaling [24, 25]. The combination of emetine with cisplatin exhibited synergistic effects against NSCLC cells and cisplatin-resistant cells [24].
In this study, we found GC cell lines were very sensitive to emetine, with half inhibitory concentrations at the nanomolar level, and the molecular mechanisms involved blocking multiple signaling pathways including inhibition of Hippo/YAP and PI3K/AKT signaling cascades, which are not reported in other studies. In this paper, we reported the comprehensive anti-GC effects of emetine in vitro and in vivo, as well as the related mechanisms of action.
Materials and methods
Chemicals and reagents
Emetine hydrochloride and positive agent 5-Fu were acquired from a commercial source. The antibodies against β‐catenin, LEF1, Cyclin D1, Axin 2, GSK3 beta, YAP1 and Survivin were obtained from Abcam. The antibodies against p-p38 MAPK (T180/Y182), p-p44/42 MAPK (ERK1/2 T202/T204), p‐JNK (T183/Y185), non-phospho (active) β‐catenin (S37/T41), p38 MAPK, ERK1/2, JNK, AKT were purchased from Cell Signaling Technology. Antibodies for p‐AKT (Ser473), CTGF and the secondary antibody were obtained from Proteintech.
Cell culture and reagents
The human MGC803 and HGC-27 cells were acquired from the National Platform of Experimental Cell Resources for Sci-Tech (China) and were grown in the recommended medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. All cell lines were cultured at 37 °°C in an incubator supplied with 5% (v/v) CO2 and experimented within 3 months of receipt or resuscitation.
Cell viability assay
MGC803 and HGC-27 cells were seeded in 96-well plates at 2000–3000 cells/well and cultured overnight in the incubator. Then, the cells were treated with emetine or 5-Fu at different concentrations for 72 h. After treatment, MTT reagent (5 mg/mL, 20 μL/well) was added to the plates, which were then incubated at 37 °C for another 3 h. The formazan crystals were dissolved overnight with 50 μL of acidified SDS (20%, w/v). The absorbance was detected at 570 nm with SpectraMax iD5-multifunctional microplate reader, and IC50 values were calculated using GraphPad Prism v9.0 software.
Colony formation assay
MGC803 and HGC-27 cells were seeded in 12-well plates at a density of 5000 cells/well and cultured overnight. The next day, different concentrations of emetine were added to the plates. Cells were then incubated at 37 °C, and the medium containing emetine was replaced every 3 days. After treatment for 10 days, the cells were washed with PBS and then fixed with methanol for 20 min and stained with crystal violet solution (0.05%, w/v) for another 20 min. Pictures were taken with a camera after natural drying.
Edu incorporation assay
MGC803 and HGC-27 cells were cultured in 96-well plates. Serial dilutions of emetine were added to the plates when the cells reached about 80% confluence. After treatment for 18 h, cell proliferation detection was conducted according to the instruction of BeyoClick™ EdU Cell Proliferation Kit with DAB. Pictures were taken using an OLYMPUS light microscope.
Flow cytometry for apoptosis analysis
MGC803 and HGC-27 cells were seeded in 6-well plates and treated with indicated concentrations of emetine the next day for 24 h. The cells were then harvested and washed twice with precooled PBS, followed by staining with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) according to the manufacturer’s instructions. The cell suspension was incubated for 15 min in the dark at room temperature. Cellular apoptosis was analyzed by flow cytometry (NovoCyto 2070R) within 1 h.
Wound healing assay
MGC803 and HGC-27 cells were seeded in 12-well plates and cultured to a density of about 90%. Then a straight scratch was made on the monolayer cells using a sterilized pipette tip, and the floating cells were softly rinsed with PBS. Serial dilutions of emetine were added to the plate to treat GC cells for 12 h. The wound healing was observed and photographed with an OLYMPUS microscope.
Transwell invasion assay
The Corning transwell chambers precoated with diluted matrigel were inserted into a 24-well plate and placed at 37 °C to allow the matrigel to solidify. Then, GC cells resuspended with serum-free medium were seeded in the upper chamber and treated with emetine. The complete medium was added to the outer bottom of the chamber to induce cell invasion. After treatment for 12 h at 37 °C, the invaded cells were fixed with methanol for 20 min and stained with crystal violet (0.05%, w/v) for another 20 min. Pictures were taken under an OLYMPUS upright microscope.
RNA sequencing and differentially expressed genes analysis
MGC803 cells seeded in a 6-well plate were treated with 0.1 μM emetine for 18 h and then harvested. Total RNA was extracted according to the protocol of mirVana miRNA Isolation Kit (Ambion). The libraries were constructed with TruSeq Stranded mRNA LTSample Prep Kit (Illumina, USA) and sequenced on Illumina HiSeq X Ten sequencing platform. The DEGs between emetine-treated and control groups were analyzed using the DESeq R package. We set the adjusted p value < 0.05 and fold change > 2 or fold change < 0.5 as the threshold of significant difference in gene expression. Hierarchical cluster analysis of DEGs was performed to analyze the gene expression pattern in different groups. KEGG enrichment analysis of DEGs was carried out using R based on the hypergeometric distribution.
Western blotting
GC cells were treated with indicated concentrations of emetine for 18 h and then lysed with RIPA buffer (Solarbio, China) containing phosphatase and protease inhibitors. The proteins extracted from cells were separated using 10% SDS-PAGE, and then transferred to PVDF membranes (Millipore, USA). After blocking in 5% nonfat milk for 2 h, the PVDF membranes were incubated with the diluted specific primary antibodies overnight at 4 °C and then incubated with the secondary antibody for 1 h at 37 °C. Then the membranes were washed 3 times with TBST and developed using ECL Enhanced Kit (Biosharp, China). Specific proteins were detected by the ChemiScope 6000 Fluorescence Chemiluminescence Imaging System (CLiNX, China).
Xenograft studies
All animal studies were approved and guided by the Animal Care and Use Committee of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital (Chengdu, Sichuan, China). Five-week-old BALB/c nude mice were adaptively fed for 1 week. MGC803 cells were collected and washed 3 times with serum-free medium, and then subcutaneously injected into the posterior axillary region of each mouse at a concentration of 5 × 106 cells/100 μL. When the xenograft tumors grew to about 100 mm3, the mice were randomly divided into three groups (n = 5 each): vehicle treatment group, emetine 10 mg/kg treatment group, and 5-FU 30 mg/kg treatment group. All agents were administered by intraperitoneal injection every other day. The tumor volumes and body weights of the mice were also monitored every 2 days. After treatment for 3 weeks, mice were killed, and tumors as well as main organs were obtained for further analysis. Tumor volume was calculated as (a2 × b)/2 (a = width, b = length). The tumor growth inhibition rate was calculated using this formula: 100% × {1−[(tumor volumefinal−tumor volumeinitial) for the drug-treated group]/[(tumor volumefinal−tumor volumeinitial) for the vehicle-treated group]}.
H&E staining and immunohistochemistry staining
The tumors and major organs obtained from the animal experiment were fixed in 4% paraformaldehyde and then embedded in Paraffin. Sections of the main organs were subjected to H&E staining according to the standard protocols. Immunohistochemical analysis was performed on tumor tissue sections to detect the expression of Ki67 and TUNEL. Representative images were captured under a Leica microscope.
Statistical analysis
GraphPad Prism v9.0 was used for statistical analysis. All experiments were repeated at least three times and data were shown as mean ± standard deviation (SD). For statistical analysis, Student’s t test was used to assess comparison between two groups, and one-way ANOVA was performed to assess comparison among multiple groups. Statistical significance was presented as *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Emetine inhibits GC cell viability in vitro
Two representative GC cell lines MGC803 (low differentiated adenocarcinoma) and HGC-27 (poorly differentiated) were selected for the anti-GC pharmacodynamic study of emetine. MTT assay was conducted to detect the anti-viability activity of emetine on GC cells. As indicated in Fig. 1B, emetine effectively restrained the viability of MGC803 and HGC-27 cell lines, with IC50 values of 0.0497 μM and 0.0244 μM, respectively. As a positive control, the anti-viability activities of the chemotherapeutic agent 5-fluorouracil (5-FU) were 11.3763 μM and 6.4358 μM on MGC803 and HGC-27, respectively, displaying a weaker toxicity to GC cells compared with emetine. To further visually evaluate the effect of long-term emetine treatment on GC cells, a colony formation assay was carried out on MGC803 and HGC-27 cells. As shown in Fig. 1C, emetine remarkably reduced the colonies of both MGC803 and HGC-27 cells at concentrations greater than 0.03 μM. These data showed that emetine could potently inhibit GC cell growth in vitro.
Emetine suppresses the growth of GC cells via proliferation inhibition and apoptosis induction
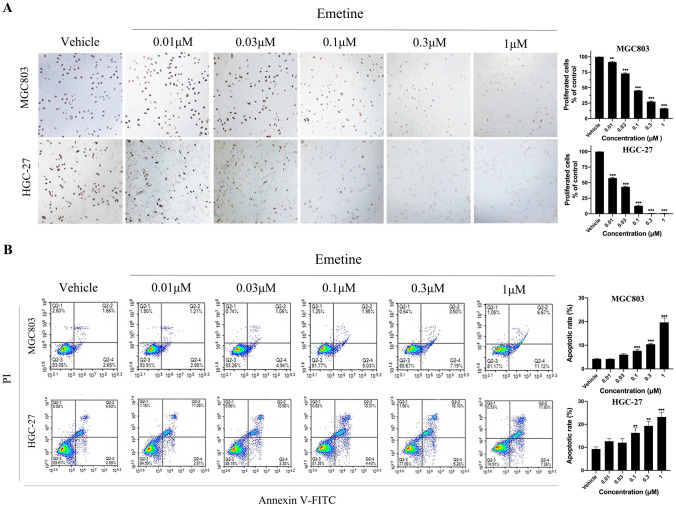
To explore the specific pharmacodynamic mechanisms of action of emetine in suppressing the growth of GC cells, we measured the influence of emetine on cellular proliferation, apoptosis, and cell cycle. In the Edu cell proliferation assay with DAB, emetine decreased the number of Edu-labeled brown cells (proliferative cells) in a dose-dependent manner, which was consistent with its anti-viability activity (Fig. 2A). In flow cytometry assays, the number of Annexin V-positive cells (apoptotic cells) were significantly elevated in emetine-treated groups, with apoptosis rate of 7.63% (0.1 μM), 10.43% (0.3 μM) and 19.63% (1 μM) in MGC803 cells, and 16.27% (0.1 μM), 19.31% (0.3 μM) and 23.17% (1 μM) in HGC-27 cells (Fig. 2B). Notably, we did not find apparent effect of emetine on cell cycle arrest in flow cytometry analysis (Supplementary Fig. 1). This is different from the results observed in emetine-treated breast cancer cells [26]. Taken together, these data indicated that emetine could inhibit GC cell growth through suppression of cellular proliferation and induction of apoptosis.
Fig. 2.
Anti-proliferative and pro-apoptotic effects of emetine in GC cells. A Edu incorporation assay in MGC803 and HGC-27 cells after emetine treatment for 18 h (100 ×). The number of proliferative GC cells (Edu-labeled brown cells) in different treatment groups was counted for statistics, and data were shown as mean ± SD (n = 3). B Annexin V-FITC/PI apoptosis assay in MGC803 and HGC-27 cells treated with emetine for 24 h. Apoptotic rates (the percentage of Annexin V-positive cells) in different treatment groups were quantified for statistics, and data were shown as mean ± SD (n = 3). **p < 0.01 vs vehicle, ***p < 0.001 vs vehicle
Emetine inhibits the migration and invasion of GC cells in vitro
To assess the influence of emetine on cellular migration and invasion (the critical biological processes of cancer metastasis), wound healing assay and transwell invasion assay were carried out. As presented in Fig. 3A, emetine potently repressed the migration of GC cells in a concentration-dependent manner. Meanwhile, cellular invasion could also be dramatically suppressed after treatment with emetine at concentrations higher than 0.01 μM (Fig. 3B). As compared with vehicle group, there were significant differences in the number of both migrated cells and invaded cells in emetine-treated groups.
Fig. 3.
Emetine restrained the migration and invasion of GC cells. A Representative images (100 ×) of scratch migration assay in emetine-treated MGC803 and HGC-27 cells. Images were captured at 0 and 12 h after treatments. The statistical chart displayed the amounts of migrated cells in different treatment groups, and data were shown as mean ± SD (n = 3). B Representative images (100 ×) of transwell invasion assay in emetine-treated MGC803 and HGC-27 cells. Images were captured after 12 h treatments. The statistical chart presented the amounts of invaded cells in different treatment groups, and data were shown as mean ± SD (n = 3). **p < 0.01 vs vehicle, ***p < 0.001 vs vehicle
Identification of differentially expressed genes (DEGs) and functional enrichment analysis in emetine-treated GC cells
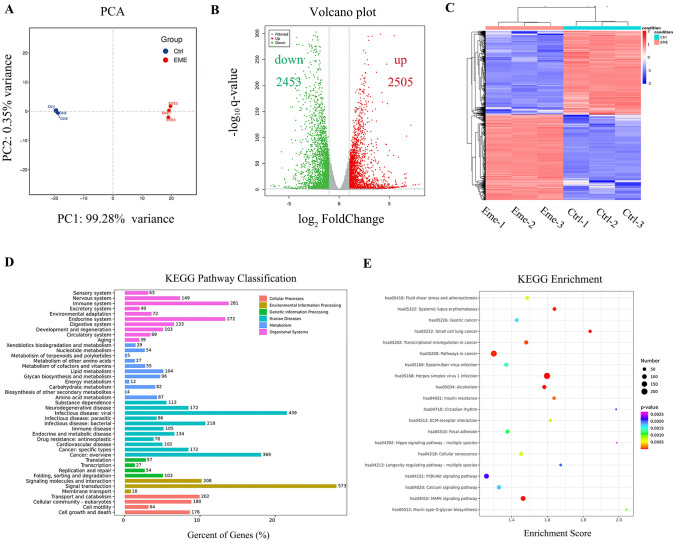
To further elucidate the molecular mechanism involved in the anti-GC effects of emetine, RNA sequencing was carried out in MGC803 cells treated with emetine for 18 h. As shown in Fig. 4A, there is a prominent difference between emetine-treated and untreated (control) GC cells according to the results of principal component analysis (PCA). After analyzing the sequencing data, we finally got 4958 DEGs. The volcano plots described the distribution of DEGs, including 2505 elevated genes and 2453 down-regulated genes (Fig. 4B). The heatmap revealed that DEGs could precisely cluster samples into emetine and control groups (Fig. 4C).
Fig. 4.
Differentially expressed genes (DEGs) and functional enrichment analysis. MGC803 cells were treated with emetine for 18 h and then used for RNA sequencing analysis. A Principal component analysis (PCA) was performed based on gene expression. B Volcano plot revealed the significantly elevated (red dots) and down-regulated (green dots) genes in emetine-treated cells. C Heatmap of DEGs between emetine and control groups. Two groups were well clustered. D KEGG pathway classification of DEGs. E KEGG enrichment analysis (Top 20) of DEGs
Functional enrichment analysis was further conducted to better understand the interactions among DEGs. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway classification analysis showed that DEGs were mainly distributed in the related pathways of signal transduction, cancers and viral infectious disease (Fig. 4D). In the top 20 KEGG enrichment pathways, nearly half of them were related to cancers. Of these, the pathways associated with tumor cell signal transduction mainly included Hippo, PI3K/AKT and MAPKs signaling cascades (Fig. 4E). In addition, significant enrichment of Wnt signaling was also observed in the KEGG enrichment analysis for gastric cancer (Supplementary Fig. 2).
Emetine suppresses GC cell growth via regulation of multiple signaling pathways
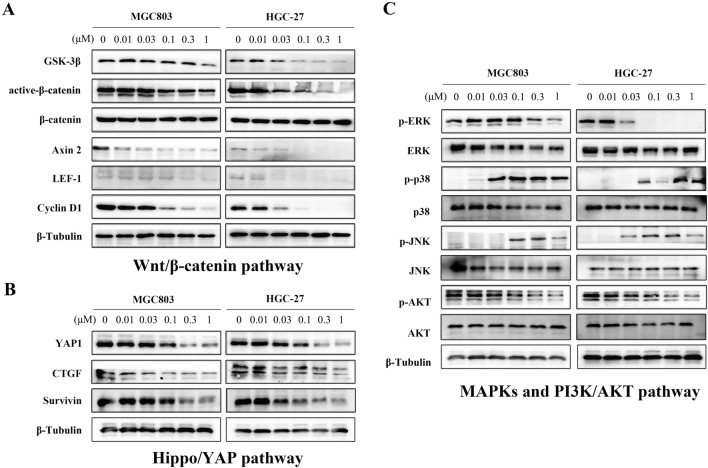
Due to the enrichment of DEGs in Hippo, PI3K/AKT, MAPKs, and Wnt signals that play important roles in tumor biological regulation, western blotting was conducted to further verify the effects of emetine on such signaling pathways. As exhibited in Fig. 5A, emetine effectively down-regulated the amounts of pivotal proteins in the Wnt/β-catenin signaling cascade (including GSK-3β, active-β-catenin, Axin2, LEF1, and the downstream target protein Cyclin D1), particularly in HGC-27 cells, in which Wnt pathway was significantly inhibited by emetine even at a low dose of 0.03 μM. For the Hippo signaling pathway, emetine suppressed the expression of its downstream effector YAP1 in a concentration-dependent manner (Fig. 5B). YAP1 is considered as an oncoprotein in various malignancies and functions as a transcriptional co-activator that regulates the transcription of multiple target genes related to cancer cell survival, such as CTGF, Cyr61, Survival etc. The inhibitory effects were also observed in the expression of CTGF and Survivin in emetine-treated cells (Fig. 5B). Additionally, emetine also had inhibitory activity against MAPKs and PI3K/AKT cascades, the classical signaling pathways involved in cancer (Fig. 5C). It potently reduced the phosphorylated ERK and AKT in both cell lines, and had no impact on the total ERK and AKT. Notably, the phosphorylation of another MAPK family members p38 and JNK were elevated after treatment with emetine. This phenomenon also occurred in other cells, and activation of p38 and JNK was also beneficial for inducing cellular apoptosis [27, 28]. Taken together, these data indicated that emetine exerted anti-GC effects through regulation of multiple signaling cascades, including Wnt/β-catenin, Hippo/YAP, MAPKs, and PI3K/AKT signaling pathways.
Fig. 5.
Emetine acted via blockade of multiple signaling cascades. MGC803 and HGC-27 cells were treated with different concentrations of emetine for 18 h, and then the proteins were extracted for western blot. A Western blot assay was used to verify the effects of emetine against Wnt/β-catenin signaling pathway. B Inhibitory effects of emetine on the downstream effector and target proteins of Hippo signaling pathway. C Regulation of emetine on the pivotal proteins of MAPKs and PI3K/AKT signaling axes
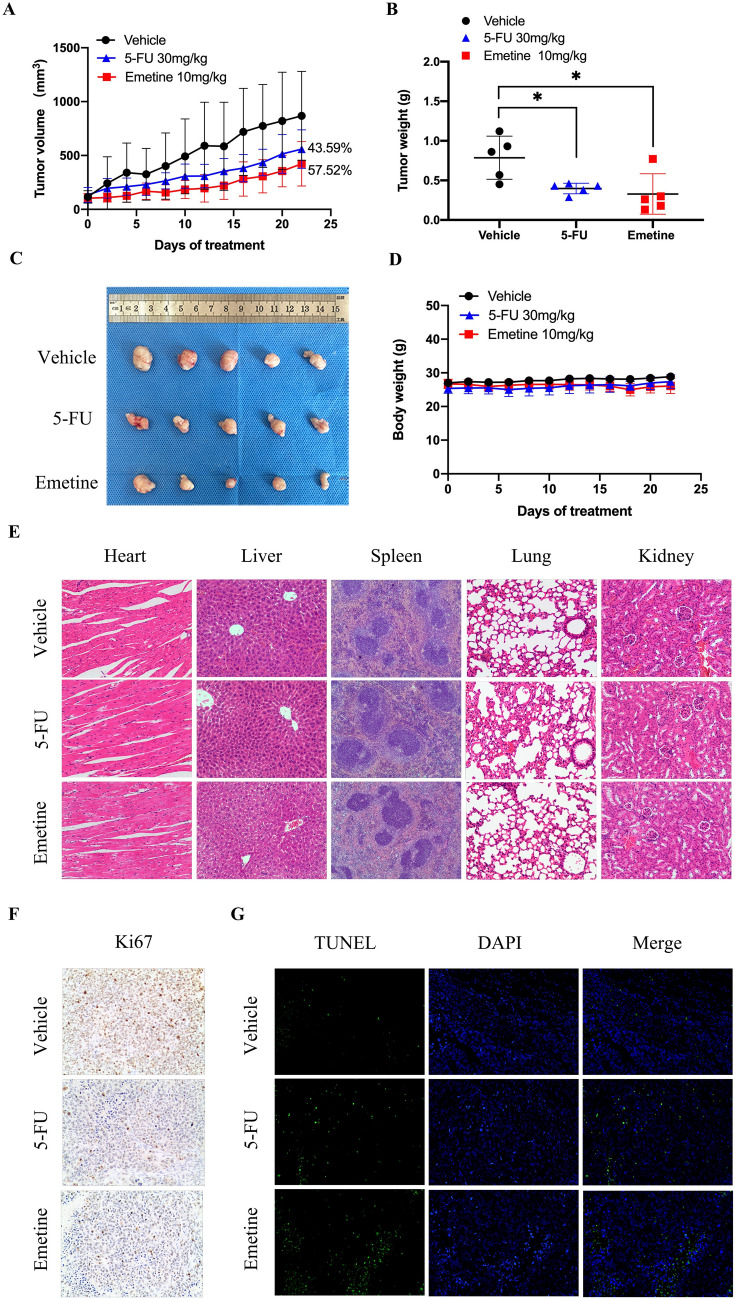
Antitumor effects of emetine in MGC803 xenograft model
The in vivo anti-GC efficacy of emetine was evaluated in the MGC803 xenograft model. Tumor growth was efficaciously blocked by emetine at 10 mg/kg administered every other day, with a tumor growth inhibition rate of 57.52% (Fig. 6A). As a positive control, 5-FU treatment (30 mg/kg, qod) also obviously inhibited the growth of MGC803 xenograft tumors, with a tumor growth inhibition rate of 43.59%, which is more potent compared with its anti-viability activity in vitro. The enhanced anti-tumor activity of 5-FU in vivo may be attributed to its mechanism of action; it will be more cytotoxic and inhibits DNA synthesis after metabolism and transformation in vivo [29]. The anti-tumor effect of emetine can also be seen intuitively from the tumor weight statistics (Fig. 6B) and tumor picture (Fig. 6C). At the end of the treatment, the average tumor weight in the emetine group was only half that of the vehicle group. Emetine (10 mg/kg) and 5-FU (30 mg/kg) alike did not result in significant weight loss in mice during the treatment (Fig. 6D). Meanwhile, no pathological phenotypes were observed in the HE staining of major organs including heart, liver, spleen, lung, and kidney, demonstrating the low toxicities of the treatment agents at such dosages (Fig. 6E). Immunohistochemical assays were conducted to test the effects of emetine on cellular proliferation and apoptosis in vivo. As shown in Fig. 6F, the Ki67-positive proliferative cells were remarkably reduced in both emetine and 5-FU treatment groups, as compared with vehicle group. Moreover, treatment with both emetine and 5-FU significantly increased the number of TUNEL-positive apoptotic cells in xenografts (Fig. 6G). To sum up, emetine could still exhibit anti-GC effects by inhibiting cell proliferation and inducing apoptosis in vivo.
Fig. 6.
In vivo anti-GC effects of emetine in MGC803 xenograft model. A Tumor inhibition curves of emetine (10 mg/kg, qod) and 5-Fu (30 mg/kg, qod) in MGC803 xenograft model. All agents were administered intraperitoneally. Data were presented as mean tumor volume ± SD (n = 5). B Statistical chart of tumor weight in each treatment group. Data were presented as mean tumor weight ± SD (n = 5). C Tumor image obtained after the pharmacodynamic studies. D Body weights of the mice in different treatment groups. Data were presented as mean body weight ± SD (n = 5). E Representative images (100 ×) of H&E staining of the main organs collected from each treatment group. F Representative images (200 ×) of Ki67 immunohistochemical staining (brown color) conducted on the tumor sections of each treatment group. G Representative images (200 ×) of TUNEL immunofluorescence staining (green color) conducted on the tumor sections of each treatment group. *p < 0.05 vs vehicle
Discussion
The anti-tumor effects of emetine have been assessed in several solid tumors, including ovarian cancer, lung cancer, bladder cancer, prostate cancer, osteosarcoma, and breast cancer, but not GC [30, 31]. In the present study, we found emetine was highly potent against GC cells, with IC50 values at nanomolar range in cell viability assay. It could block GC cell growth both in vitro and in vivo by inhibiting cellular proliferation and inducing apoptosis. Meanwhile, it also had the ability to repress the migration and invasion of GC cells, even at concentrations lower than 0.1 μM, indicating a potential anti-metastasis effect of emetine.
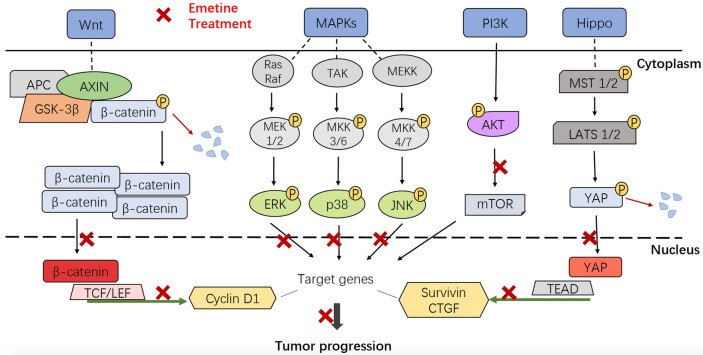
The molecular mechanisms concerning the antitumor activity of emetine are complicated and differ in diverse types of tumors. In the present study, the sequencing analysis and immunoblot revealed that emetine exhibited anti-GC effects via regulation of multiple signaling pathways, including MAPKs, Wnt/β‑catenin, Hippo and PI3K/AKT signaling axes. Of these, the regulation of emetine on MAPKs and Wnt/β‑catenin signaling was also observed in previous studies conducted in other types of tumors [23–25, 31], but the results were slightly different. First, emetine inhibited the phosphorylation of ERK and JNK, and activated p38 in human U2OS osteosarcoma cells [31]; whereas in emetine-treated GC cells, only ERK activity was restrained, and phosphorylated p38 and JNK levels increased. As the three major MAPKs, down-regulated ERK activity as well as enhanced activity of p38 and JNK can be coupled with proliferation inhibition and apoptosis induction in GC cells [27, 28, 32]. Therefore, the regulation of emetine on MAPKs is one of the important mechanisms by which it exerts anti-GC activity (Fig. 7). Second, emetine efficaciously depressed the signal transduction of Wnt/β‑catenin pathway in GC cells. However, it could not produce a synergistic effect by targeting such signaling when combined with 5-Fu (data not shown), which is inconsistent with that observed in NSCLC cells [24]. The synergistic effect may depend on tumor types and drugs used for combination. Due to the promoting effect of Wnt/β‑catenin signaling in GC cell proliferation, migration, and invasion [33, 34], it is implied that targeting this pathway also contributes to the comprehensive anti-GC effects of emetine (Fig. 7).
Fig. 7.
The anti-GC molecular mechanisms of emetine involved blockade of multiple signaling cascades, mainly including Wnt/β-catenin, Hippo/YAP, MAPKs and PI3K/AKT signaling pathways
In addition to MAPKs and Wnt/β‑catenin signaling, emetine also displayed inhibitory activity against PI3K/AKT and Hippo/YAP signaling cascades in GC cells, which has not been reported in other cancers treated by emetine. Activated PI3K/AKT axis can trigger a series of intracellular processes in GC cells, which drive oncogenicity of GC cells, mainly manifested by inhibition of apoptosis and promotion of proliferation, invasion, metastasis as well as drug resistance [35, 36]. Inhibiting PI3K/AKT signaling has always been regarded as an effective strategy for the treatment of GC. Several small molecule antagonists targeting PI3K/AKT axis have advanced to clinical trials for GC therapy, such as ipatasertib, capivasertib, and afuresertib [35, 37, 38]. Moreover, Hippo pathway is also implicated in tumorigenesis and progression. As a downstream effector of Hippo pathway, YAP is considered to be an oncoprotein and its nuclear expression is closely associated with the poor prognosis of GC patients [39, 40]. Accumulating evidence indicated that YAP inhibition could create many pharmacological effects against GC, such as repression of cellular proliferation and self-renewal of GC stem cells, and conquering drug resistance [41–43]. Therefore, targeting PI3K/AKT and Hippo/YAP axes are also pivotal molecular mechanisms of emetine in suppressing GC cell growth, migration, and invasion (Fig. 7).
Emetine is thus a multi‑target anticancer agent. Compared with target-specific inhibitors, multi‑target inhibitors have advantages in antitumor efficacy and conquering resistance due to the biologically heterogeneous and complexity of malignant tumors. However, side effect is a major challenge for such inhibitors and emetine is no exception. Although no obvious toxicity of emetine was observed in the animal experiment of this study, it cannot be confirmed that the side effects do not exist because of the limitations of the experimental design. Actually, emetine was assessed for tumor therapy in the clinic in the early 1970s [44, 45]. These clinical trials did not drive emetine to be approved since they revealed some side effects, such as cardiac toxicity, and limited efficacy in the evaluated patients. Therefore, the researchers concluded that combination therapy was a potential approach for the application of emetine in cancer treatment, which may improve the curative effect, tolerance, and safety when using relatively low doses of emetine [44]. Alternatively, optimizing the chemical structure and identifying the applicable tumor types and population are promising strategies to further explore the medicinal value of this natural small molecule in antitumor therapy.
Conclusion
In conclusion, the major findings of this study were that emetine effectively suppressed the viability, proliferation, migration, and invasion of GC cells, as well as induced cellular apoptosis, resulting in anti-GC effects both in vitro and in vivo. It acted through the blockade of multiple signaling cascades, including not only MAPK/ERK and Wnt/β-catenin signaling networks that were also found in other tumor types, but also the newly revealed PI3K/AKT and Hippo/YAP signaling pathways. The findings of this study together with the antitumor effects of emetine disclosed in the previous studies support it as a lead compound and even a drug candidate for GC therapy, which deserves further structural optimization and development.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Conception and design: XP and LZ; development of methodology: JS and XZ; acquisition of data: XP and ZZ; analysis and interpretation of data: XP, JS and LZ; technical and material supports: LZ, XZ and RT; writing and review of the manuscript: XP, LZ and XZ; study supervision: LZ and XZ.
Funding
This work was supported by the Research Funds of Science & Technology Department of Sichuan Province (2021YJ0134, 2019YFS0514), the Research Project of Chengdu Science and Technology Bureau (2022-YF05-01611-SN), the Fundamental Research Funds for the Central Universities (ZYGX2019J106), the Open Project of Radiation Oncology Key Laboratory of Sichuan Province (2020FSZLX04), and the National Key Research and Development Program of China (2020YFC2005500).
Availability of data and material
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Animal Care and Use Committee of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital (Chengdu, Sichuan, China).
Informed consent
Formal consent is not required for this type of study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuerun Peng and Jianyou Shi contributed equally to this work.
Contributor Information
Xiaonan Zhang, Email: xiaonan.zhang@igp.uu.se.
Lei Zhong, Email: zhl7235301@163.com.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:167–192. doi: 10.6004/jnccn.2022.0008. [DOI] [PubMed] [Google Scholar]
- 3.Bollschweiler E, Berlth F, Baltin C, et al. Treatment of early gastric cancer in the Western World. World J Gastroenterol. 2014;20:5672–5678. doi: 10.3748/wjg.v20.i19.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39:1179–1203. doi: 10.1007/s10555-020-09925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong H-M, Wang Q, Wang W-L, et al. A clinical analysis of systemic chemotherapy combined with radiotherapy for advanced gastric cancer. Medicine (Baltimore) 2018;97:e10786. doi: 10.1097/MD.0000000000010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, Fang H. Efficacy of paclitaxel and S-1 combined with apatinib in the conversion therapy for unresectable advanced gastric cancer. J BUON. 2021;26:1485–1490. [PubMed] [Google Scholar]
- 7.Patel TH, Cecchini M. Targeted therapies in advanced gastric cancer. Curr Treat Options Oncol. 2020;21:70. doi: 10.1007/s11864-020-00774-4. [DOI] [PubMed] [Google Scholar]
- 8.Liao Y, Chen W, Shi W, Zha H. Targeting cPLA2α inhibits gastric cancer and augments chemotherapy efficacy via suppressing Ras/MEK/ERK and Akt/β-catenin pathways. Cancer Chemother Pharmacol. 2021;88:689–697. doi: 10.1007/s00280-021-04322-1. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y, Zheng S, Wu Q, et al. Long noncoding RNA (lncRNA) EIF3J-DT induces chemoresistance of gastric cancer via autophagy activation. Autophagy. 2021;17:4083–4101. doi: 10.1080/15548627.2021.1901204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng R, Chen Y, Wei L, et al. Resistance to FGFR1-targeted therapy leads to autophagy via TAK1/AMPK activation in gastric cancer. Gastric Cancer. 2020;23:988–1002. doi: 10.1007/s10120-020-01088-y. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Li Y, Tan B, et al. Progress and current status of molecule-targeted therapy and drug resistance in gastric cancer. Drugs Today (Barc) 2020;56:469–482. doi: 10.1358/dot.2020.56.7.3112071. [DOI] [PubMed] [Google Scholar]
- 12.Spirina LV, Avgustinovich AV, Afanas’ev SG, et al. Molecular mechanism of resistance to chemotherapy in gastric cancers, the role of autophagy. Curr Drug Targets. 2020;21:713–721. doi: 10.2174/1389450120666191127113854. [DOI] [PubMed] [Google Scholar]
- 13.Wiegrebe W, Kramer WJ, Shamma M. The emetine alkaloids. J Nat Prod. 1984;47:397–408. doi: 10.1021/np50033a001. [DOI] [Google Scholar]
- 14.Lambert AC. The treatment of amoebic dysentery with emetine and bismuth iodide. Br Med J. 1918;1:116–118. doi: 10.1136/bmj.1.2978.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S, Xu M, Lee EM, et al. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discov. 2018;4:31. doi: 10.1038/s41421-018-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang A, Sun Y, Liu Q, et al. Low dose of emetine as potential anti-SARS-CoV-2 virus therapy: preclinical in vitro inhibition and in vivo pharmacokinetic evidences. Mol Biomed. 2020;1:14. doi: 10.1186/s43556-020-00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewisohn R. Action of emetine on malignant tumors. J Am Med Assoc. 1918;70:9–10. doi: 10.1001/jama.1918.02600010007002. [DOI] [Google Scholar]
- 18.Kane RC, Cohen MH, Broder LE, et al. Phase I-II evaluation of emetine (NSC-33669) in the treatment of epidermoid bronchogenic carcinoma. Cancer Chemother Rep. 1975;59:1171–1172. [PubMed] [Google Scholar]
- 19.Meijerman I, Blom WM, de Bont HJ, et al. Induction of apoptosis and changes in nuclear G-actin are mediated by different pathways: the effect of inhibitors of protein and RNA synthesis in isolated rat hepatocytes. Toxicol Appl Pharmacol. 1999;156:46–55. doi: 10.1006/taap.1998.8616. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y-D, Kim Y-P, Mohammed KA, et al. Terpenoid tetrahydroisoquinoline alkaloids emetine, klugine, and isocephaeline inhibit the activation of hypoxia-inducible factor-1 in breast tumor cells. J Nat Prod. 2005;68:947–950. doi: 10.1021/np050029m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akinboye ES, Bakare O. Biological activities of emetine. Open Nat Prod J. 2011;411:8–15. doi: 10.2174/1874848101104010008. [DOI] [Google Scholar]
- 22.Yosifov DY, Idler I, Bhattacharya N, et al. Oxidative stress as candidate therapeutic target to overcome microenvironmental protection of CLL. Leukemia. 2020;34:115–127. doi: 10.1038/s41375-019-0513-x. [DOI] [PubMed] [Google Scholar]
- 23.Sun Q, Fu Q, Li S, et al. Emetine exhibits anticancer activity in breast cancer cells as an antagonist of Wnt/β-catenin signaling. Oncol Rep. 2019 doi: 10.3892/or.2019.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu T-H, Chang S-Y, Shih Y-L, et al. Emetine synergizes with cisplatin to enhance anti-cancer efficacy against lung cancer cells. Int J Mol Sci. 2019;20:E5914. doi: 10.3390/ijms20235914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JH, Cho EB, Lee J, et al. Emetine inhibits migration and invasion of human non-small-cell lung cancer cells via regulation of ERK and p38 signaling pathways. Chem Biol Interact. 2015;242:25–33. doi: 10.1016/j.cbi.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Busonero C, Leone S, Acconcia F. Emetine induces estrogen receptor alpha degradation and prevents 17β-estradiol-induced breast cancer cell proliferation. Cell Oncol (Dordr) 2017;40:299–301. doi: 10.1007/s13402-017-0322-z. [DOI] [PubMed] [Google Scholar]
- 27.Xia Z, Dickens M, Raingeaud J, et al. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Q, Guo Y, Chen S, et al. Irinotecan induces autophagy-dependent apoptosis and positively regulates ROS-related JNK- and P38-MAPK pathways in gastric cancer cells. Onco Targets Ther. 2020;13:2807–2817. doi: 10.2147/OTT.S240803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 30.Uzor PF. Recent developments on potential new applications of emetine as anti-cancer agent. EXCLI J. 2016;15:323–328. doi: 10.17179/excli2016-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Son J, Lee SY. Emetine exerts anticancer effects in U2OS human osteosarcoma cells via activation of p38 and inhibition of ERK, JNK, and β-catenin signaling pathways. J Biochem Mol Toxicol. 2021 doi: 10.1002/jbt.22868. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Choo G, Yoo E, et al. Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells. Oncol Rep. 2019 doi: 10.3892/or.2019.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li K, Dan Z. Research progress of Wnt/β-catenin signaling pathway in prevention and treatment of gastric cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:1852–1856. [PubMed] [Google Scholar]
- 34.Koushyar S, Powell AG, Vincan E, Phesse TJ. Targeting Wnt signaling for the treatment of gastric cancer. Int J Mol Sci. 2020;21:E3927. doi: 10.3390/ijms21113927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang BW, Chau I. Molecular target: pan-AKT in gastric cancer. ESMO Open. 2020;5:e000728. doi: 10.1136/esmoopen-2020-000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baghery Saghchy Khorasani A, Pourbagheri-Sigaroodi A, Pirsalehi A, et al. The PI3K/Akt/mTOR signaling pathway in gastric cancer; from oncogenic variations to the possibilities for pharmacologic interventions. Eur J Pharmacol. 2021;898:173983. doi: 10.1016/j.ejphar.2021.173983. [DOI] [PubMed] [Google Scholar]
- 37.Bang Y-J, Kang Y-K, Ng M, et al. A phase II, randomised study of mFOLFOX6 with or without the Akt inhibitor ipatasertib in patients with locally advanced or metastatic gastric or gastroesophageal junction cancer. Eur J Cancer. 2019;108:17–24. doi: 10.1016/j.ejca.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Kim ST, Kim K, et al. Tumor genomic profiling guides patients with metastatic gastric cancer to targeted treatment: the VIKTORY umbrella trial. Cancer Discov. 2019;9:1388–1405. doi: 10.1158/2159-8290.CD-19-0442. [DOI] [PubMed] [Google Scholar]
- 39.Jiao S, Wang H, Shi Z, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Kang W, Tong JHM, Chan AWH, et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17:2130–2139. doi: 10.1158/1078-0432.CCR-10-2467. [DOI] [PubMed] [Google Scholar]
- 41.Seeneevassen L, Giraud J, Molina-Castro S, et al. Leukaemia inhibitory factor (LIF) inhibits cancer stem cells tumorigenic properties through hippo kinases activation in gastric cancer. Cancers (Basel) 2020;12:E2011. doi: 10.3390/cancers12082011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi J, Li F, Yao X, et al. The HER4-YAP1 axis promotes trastuzumab resistance in HER2-positive gastric cancer by inducing epithelial and mesenchymal transition. Oncogene. 2018;37:3022–3038. doi: 10.1038/s41388-018-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seeneevassen L, Dubus P, Gronnier C, Varon C. Hippo in gastric cancer: from signalling to therapy. Cancers (Basel) 2022;14:2282. doi: 10.3390/cancers14092282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panettiere F, Coltman CA. Experience with emetine hydrochloride (NSC 33669) as an antitumor agent. Cancer. 1971;27:835–841. doi: 10.1002/1097-0142(197104)27:4<835::aid-cncr2820270413>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 45.Mastrangelo MJ, Grage TB, Bellet RE, Weiss AJ. A phase I study of emetine hydrochloride (NSC 33669) in solid tumors. Cancer. 1973;31:1170–1175. doi: 10.1002/1097-0142(197305)31:5<1170::aid-cncr2820310520>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.