Abstract
Spinal cord injury (SCI) dysregulates metabolic homeostasis. Metabolic homeostasis is optimized across the day by the circadian system. Despite the prevalence of metabolic pathologies after SCI, post-SCI circadian regulation of metabolism remains understudied. Here, we hypothesized that SCI in rats would disrupt circadian regulation of key metabolic organs, leading to metabolic dysregulation. Female and male Sprague-Dawley rats received moderate thoracic (T)-9 contusion SCI (or sham surgery). First, SCI disrupted diurnal rhythms in two metabolic behaviors: fecal production and food intake rhythms were ablated acutely. SCI also expedited whole–gut transit time. In parallel, acute SCI increased plasma glucose. Diurnal glucose storage-release cycles regulated by the liver were disrupted by SCI, which also increased liver glucose metabolism messenger RNAs (mRNAs). Further, SCI disrupted liver clock gene expression and suppressed inflammatory gene rhythms. Together, our novel data suggest that SCI disrupts typical metabolic and circadian function. Improving post-SCI metabolic function could enhance recovery of homeostasis.
Keywords: bowel dysfunction, circadian rhythm, liver, metabolism, spinal cord injury
Introduction
The spinal cord communicates and integrates bidirectional sensory, motor, and autonomic information between supraspinal centers and peripheral tissues. Given the central role of the spinal cord in information integration, it follows that spinal cord injury (SCI) disrupts functions throughout the body.1 For instance, SCI can elicit long-lasting deficits in bowel function2 and metabolism.3 Thus, SCI initiates pathological processes that likely worsen recovery by disrupting the body's ability to maintain a healthy and stable equilibrium (“homeostasis”).
To efficiently maintain homeostasis, internal functions are linked to daily predictable fluctuations in the external environment via the circadian system. Diurnal rhythms (rhythms synchronized to day–night cycles) are entrained to light by the suprachiasmatic nucleus, which coordinates peripheral clocks and related gene expression present in all cells to optimize function across the day.4,5
The circadian system tightly regulates metabolic function. One key organ involved in metabolism is the liver, which processes absorbed nutrients including glucose. During feeding, glucose in the bloodstream is increased, and insulin release supports its storage in liver as glycogen; conversely, during fasting, glucagon release stimulates the liver to break down glycogen to glucose.6 In this manner, the liver helps maintain more constant circulating glucose levels across the day.7 Glucose storage–release cycles are linked with typical feed–fast times through circadian variation of several glucose metabolism genes.8,9 Thus, the homeostatic regulation of liver metabolism is supported by systemic and local circadian rhythms.7
There is evidence that SCI dysregulates metabolic and circadian systems. Individuals with SCI are susceptible to impaired glucose tolerance,10 and those with high glucose at the time of SCI display worsened neurologic outcomes.11 SCI also perturbs typical fecal output: a study by Squair and colleagues demonstrated that 96% of SCI patients at hospital discharge had challenges related to defecation.12 In addition, SCI disrupts function of the circadian system. For instance, rats with SCI have dampened daily body temperature rhythms,13 and individuals with chronic SCI have disrupted melatonin, blood pressure, activity, and sleep–wake rhythms.14–19 Despite accumulating data suggesting that SCI dysregulates metabolic and circadian function, it remains unclear whether SCI-elicited circadian disruption contributes to post-SCI metabolic pathology.
Here, we hypothesized that SCI in rats would disrupt circadian regulation of key metabolic organs including the bowel and liver, leading to metabolic dysregulation. Female and male rats received sham surgery or contusion SCI. SCI disrupted diurnal rhythms in fecal production, and SCI increased fecal output, expedited whole–gut transit time, and dysregulated feeding patterns. Post-SCI, rats also exhibited increased and perturbed diurnal rhythms of plasma glucose. In addition, SCI disrupted rhythmic expression of circadian, glucose-related, and immune genes in the liver. Our data show that clinically relevant SCI in rats disrupts metabolic function at the behavioral and molecular levels, revealing that central nervous system trauma has widespread detrimental consequences for peripheral tissue physiology and metabolism.
Methods
Surgery and animal care
The University of Colorado Boulder Institutional Animal Care and Use Committee approved all housing, surgery, and postoperative care, and this study was conducted in accordance with Animal Research: Reporting of In Vivo Experiments guidelines. All animals were fed standard chow and filtered tap water ad libitum and maintained on a 12:12 light/dark cycle. Rats were pair housed for most studies, but were housed individually for the entire fecal collection study to obviate the stress-related confound of moving pair-housed rats to individual cages. For all experiments, sham/SCI surgeries occurred throughout the light phase. Female and male Sprague-Dawley rats (females: 200–250 g, males: 320–380 g; 2–3 months old; Harlan Laboratories) were anesthetized with isoflurane inhalation anesthesia, and both sham and SCI rats were treated with prophylactic antibiotics (gentamicin sulfate (Butler Schein), 1.25 mg subcutaneously in 0.25 mL sterile water). A partial T8 laminectomy was performed prior to SCI. The periosteum, but not the dura, was removed for all surgeries.
Animals were subjected to a moderate contusion injury (150 kDyne, 1 sec dwell)20 at T9 spinal cord using the Infinite Horizon device (Precision Systems and Instrumentation). Post-operative animal care included daily administration of intraperitoneal gentamicin (both sham and SCI rats; 1 mL/day for 5 days), subcutaneous injection of saline (5, 5, 4, 3, 2 mL on each of the first 5 days post-injury [dpi], respectively, for both sham and SCI rats) to prevent dehydration, and manual voiding of bladders twice daily (until recovery of bladder function at 2–3 weeks).21,22 Animals were monitored daily for infection or other signs of suboptimal recovery. A dim red headlamp was used for all manipulations that occurred during the animals' dark phase to limit circadian disruption.4
Metabolic behavior studies
To study fecal output and food intake, individually housed rats were relocated from their home cages to hanging metal cages (dimensions l × w × h: 41 cm × 24 cm × 18 cm) for 24 h at a time, then returned to their home cages. All rats were acclimated to this system for 24 h (no data collection in this period) at least 1 week before the initiation of experiments. Rats were placed in the hanging cages at Zeitgeber time (ZT) 9 (ZT0-12 = lights on; ZT12-24 = lights off). The rats had a 15 cm × 15 cm ceramic platform to stand or lie on and had ad libitum access to water and food. At all times in our facility, rats were provided Teklad 8460 diet (energy density: 12.6 kJ/g, 3.0 kcal/g). Fecal output was measured by collecting fecal pellets from each animal (fell through the cage onto a tray), then counting and weighing the pellets (every 3 h throughout each 24 h test, to enable studying diurnal rhythms of fecal output). Water (at end of 24 h) and food weight (every 3 h) also were examined. Pellet wet mass (immediate weighing) and dry masses (pellets weighed after air dried in covered cage ∼48 h) were assessed. Percent water content of fecal matter was calculated using this equation: 100 – (dry mass/wet mass).
Three separate fecal output studies were conducted and are presented together here for clarity. The first study consisted of female rats (sham, n = 6; SCI: n = 5); the second study comprised male rats (sham, n = 7; SCI: n = 5); and the third study consisted of female and male rats run concurrently (female sham, n = 8; female SCI, n = 10; male sham, n = 8; male SCI, n = 9). In studies one and two, fecal pellets were collected prior to surgery (“pre-”), then at 2, 7, and 14 days post-surgery. Having the SCI rats in the hanging cages for fecal collections so soon and frequently after SCI (as in studies one and two) caused minor fur wetness and cutaneous sores, so for subsequent studies we avoided frequent acute time-points in these hanging wire cages. In study three, fecal pellets were collected prior to surgery, then at 7 days post-surgery (to confirm consistency with studies 1 and 2) and at 42 days post-surgery (to study fecal output at a chronic time-point).
For whole–gut transit time (performed simultaneously with fecal output studies), unanesthetized rats were first acclimated to handling related to gavage. On test days, rats were gavaged with Evan's blue dye (2% Evan's blue, 5% gum Arabic in 2 mL tap water)23 using a flexible feeding tube (FTP-18-75; Instech Laboratories, Plymouth Meeting, PA) around ZT8.5, and then placed in the hanging cages for fecal pellet analysis. The time until blue fecal pellet production was assessed every 3 h as an indication of whole–gut transit time.
Collection and measurement of plasma glucose
Rats were pair-housed; cage mates had the same surgery (at start: female sham n = 6; female SCI n = 10; male sham n = 6; male SCI n = 10); two female SCI rats and one male SCI rat died. Rats were acclimated to handling for 5 days. After acclimation to handling, blood samples were collected from immobilized unanesthetized rats via tail nick (< 500 μL/24 h). Blood was collected pre-surgery, and 2, 7, and 14 days post-surgery at ZT0, 6, 12, and 18 (starting at ZT0). Samples were centrifuged (10,000 g/10 min) to isolate plasma. Plasma was used for glucose testing (Bayer Contour). Several samples (various rats and time-points) had insufficient plasma, and therefore were excluded.
Circadian regulation of gene expression
For the polymerase chain reaction (PCR) study in surgically naïve rats, tissues were collected from uninjured female/male rats across the day (ZT0, ZT6, ZT12, ZT18; n = 6/sex/time-point for a total of 48 rats). Rats received intraperitoneal pentobarbital overdose, then were perfused with 0.9% saline. Tissues were flash frozen for PCR.
For the sham/SCI PCR study, female/male rats received sham/SCI surgery (n = 6/sex/surgery). Prior to surgery, rats were handled to minimize post-surgery bladder care stress. SCI rats received post-surgery bladder care and sham rats received similar handling (control for stress); the bladder care immediately prior to tissue collection was omitted to limit circadian disruption. Tissue from sham/SCI rats was collected for PCR analysis in the middle of the light phase (ZT6; ∼48 h post-surgery) and the middle of the dark phase (ZT18; ∼60 h post-surgery) at 2 days post-surgery. Two days post-surgery was chosen as an acute post-surgery time-point for two reasons: first, earlier studies in this manuscript suggested that circadian measures were most strongly disrupted soon after injury, and second, early post-SCI inflammatory and pathologic events likely influence the trajectory of recovery at later times.24–27 Tissue collections were completed within 1–2 h of the time-point (e.g., ZT6 – tissue collected ZT5-7). Sub-groups of males and females were collected on the same days to minimize between-day differences and to enable sex comparisons (total of 48 rats included in this circadian-SCI experiment).
Quantitative real-time PCR was completed as previously described28 using a BioRad iQ5 Real Time PCR Detection Instrument. Primers (Invitrogen) spanned exons (see Table 1 for sequences). Gene expression was assessed in duplicate and is presented relative to β-actin. There were no significant differences in β-actin expression between groups, and male, female, sham, and SCI rat samples were all run on the same plates to ensure consistency. PCR results were analyzed using 2-ΔΔCt and normalized with female sham-ZT6 set to 1.
Table 1.
Primer Sequences of Assessed RNAs
| Gene | Protein function | Primer sequences |
|---|---|---|
| b-actin | Housekeeping control | F: TTCCTTCCTGGGTATGGAAT |
| R: GAGGAGCAATGATCTTGATC | ||
| Glut2 | Facilitated glucose transport | F: CTGGGTCTGCAATTTTGTCA |
| R: TGTAAACAGGGTGAAGACCA | ||
| G6pt1 | Glucose transport into endoplasmic reticulum; glucose homeostasis | F: GTGGGTCCTGGACACTGACT |
| R: ACCTCAGAACAGTCCGTAA | ||
| Pck1 | Enzyme critical for gluconeogenesis | F: AGGAGGAAGAAAGGTGGCACCAG |
| R: GGCAGAGAAGTCCAGACCATTATGC | ||
| AldoB | Roles in glycolysis and gluconeogenesis | F: TTGCCAATGGGAAGGGTA |
| R: ATCCTCTGTAGGCGGTTTCC | ||
| Per2 | Circadian clock gene – with Cry, represses Bmal & Clock transcription | F: ACAAGCGGCTGCAGTAGTGA |
| R: TTCAAGGTTGCCAGCGTGCT | ||
| Cry1 | Circadian clock gene – with Per, represses Bmal and Clock transcription | F: GTGGTGGCGGAAACTGCTCTC |
| R: ACTCTGTGCGTCCTCTTCCTGA | ||
| Bmal1 | Circadian clock gene – with Clock, activates Per and Cry transcription | F: AAAATGCAAGGGAGGCCCAC |
| R: TCTAACTTCCGGGACATCGC | ||
| Rev-erba (Nr1d1) | Circadian clock gene – represses expression of core clock proteins | F: AGACGCTGTGCGTTTTGGAC |
| R: TGTGGGAACTGAGAGAAGCC | ||
| IL-1b | Pro-inflammatory cytokine; secreted mainly by microglia/macrophages | F: CCTTGTGCAAGTGTCTGAAG |
| R: GGGCTTGGAAGCAATCCTTA | ||
| Tnfa | Pro-inflammatory cytokine; secreted mainly by microglia/macrophages | F: CAAGGAGGAGAAGTTCCCA |
| R: TTGGTGGTTTGCTACGACG | ||
| Cd68 | Inflammation – expressed by microglia/macrophages; activation marker; cell homing and adhesion | F: CAAGCAGCACAGTGGACATTC |
| R: CAAGAGAAGCATGGCCCGAA | ||
| Iba1 (AIF1) | Inflammation – expressed by all microglia/macrophages; increased with activation; binds calcium & actin | F: GGCAATGGAGATATCGATAT |
| R: AGAATCATTCTCAAGATGGC |
Statistical analysis
Data were analyzed (SigmaPlot 13.0; Systat Software, San Jose, CA) using Student's t-test or non-parametric Mann-Whitney U test, or analyses of variance (ANOVAs; one- two- or three-way ANOVA, as appropriate). Holm-Sidak post hoc tests were completed. Circadian data were analyzed using CircWave (http://www.euclock.org/results/item/circ-wave.html), which assessed circadian waveforms and acrophases (rhythm peaks). Researchers were blind to experimental group. Data were significant when p < 0.05. Data plotted as mean ± standard error of the mean.
Results
Spinal cord injury disrupts typical diurnal defecation rhythms, overall fecal output, and whole–gut transit
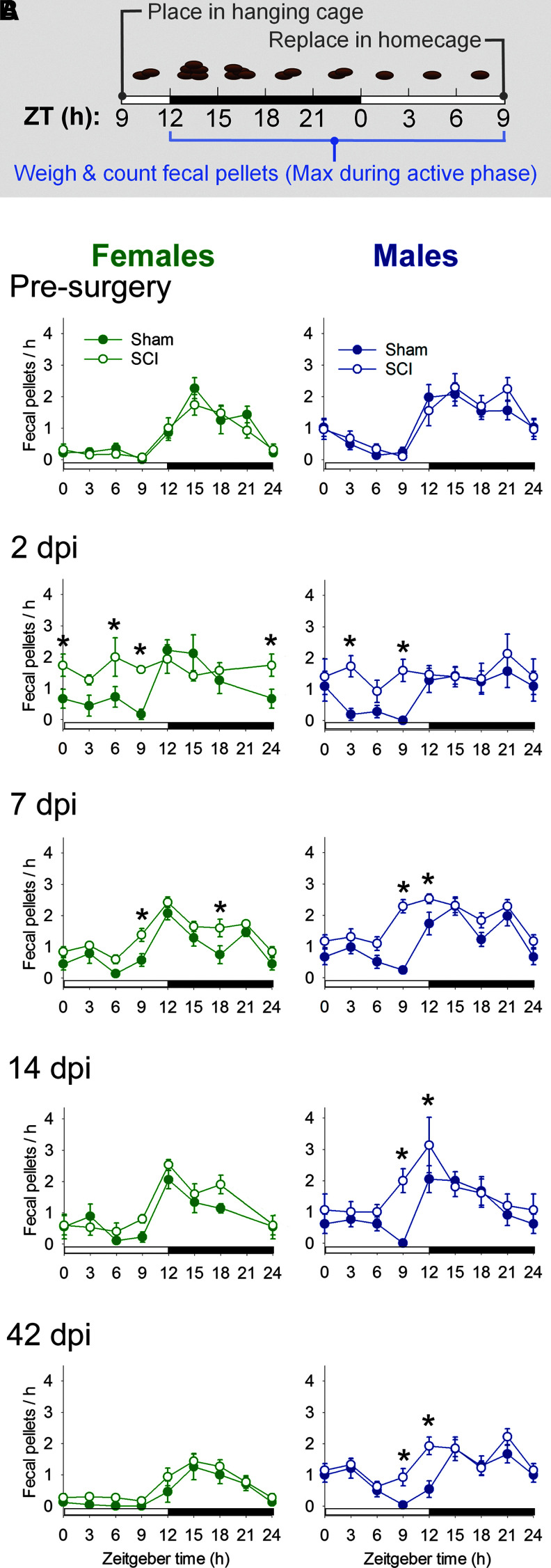
First, we sought to establish whether SCI altered diurnal rhythms of a key metabolic behavior, defecation. Fecal pellets were counted and weighed every 3 h over a 24-h period before and several times after surgery (Fig. 1A). Prior to surgery, fecal pellet output from female and male rats showed the expected daily pattern: fecal pellet output was increased at the beginning of the active (dark) phase (ZT12-24; Fig. 1B). In sham rats, this pattern was maintained after surgery. In contrast, SCI disrupted diurnal rhythms of fecal pellet output (i.e., more constant output throughout 24 h) in female and male rats at 2 dpi and 7 dpi (2 days post-surgery, female, sham vs. SCI: p < 0.05 at ZT0, ZT6, and ZT9, and male, sham vs. SCI: main effect, F1,95 = 10.033, p = 0.01; p < 0.05 at ZT3 and ZT9) (7 d post-surgery: female, sham v. SCI: main effect, F1,220 = 14.080, p < 0.001; p < 0.05 at ZT9 and ZT18, and male, sham vs. SCI: main effect; F1,231 = 35.199, p < 0.001; p < 0.05 at ZT9 and ZT12). Males, but not females, also showed persistent disruption of fecal output rhythms at 14 dpi (male, sham vs. SCI: p < 0.05 at ZT9 and ZT12) and at a chronic time-point, 42 dpi (male, sham vs. SCI: main effect, F1,135 = 5.181, p < 0.05; p < 0.05 at ZT9 and ZT12). Disrupted post-SCI diurnal rhythms in pellet number were closely mirrored by differences in mass output (Supplementary Fig. 1; see online supplementary material at http://www.liebertpub.com).
FIG. 1.
In female and male rats, spinal cord injury (SCI) disrupted daily fecal output rhythms. (A) Timeline of fecal collection studies. Rats were placed in the hanging cage at Zeitgeber time (ZT) 9 (3 h prior to lights-off; ZT12). Fecal pellets were collected, counted, and weighed every 3 h for 24 h prior to and after surgery (sham or SCI). Typical fecal output from a healthy rat over the course of a day is schemetized in this figure. After 24 h in the hanging cage, rats were returned to their homecage. (B) SCI rats produced significantly more fecal pellets, particularly during the inactive (light) phase. Fecal output rhythms were disrupted by SCI (in both females and males) at 2 days post-injury (dpi) and 7 dpi. In male rats, SCI-elicited diurnal disruption of pellet production persisted at 14 and 42 dpi. *p < 0.05 for sham vs. SCI at that time-point. Color image is available online.
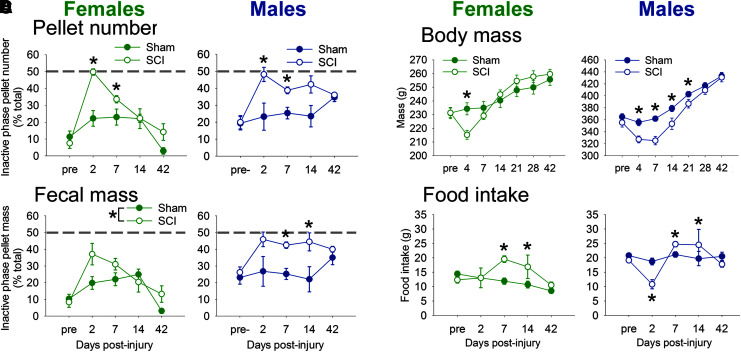
Further analysis highlighted that SCI increased relative fecal pellet and mass output from rats during the inactive phase—the time when healthy rats are typically excreting fewer pellets (Fig. 2A, 2B). Prior to injury, rats in the SCI group produced few pellets in their inactive phase (females: 8% ± 3% pellets in inactive phase; males: 20% ± 4% pellets in inactive phase). At acute times post-SCI (2 and 7 dpi), female and male rats produced 35–50% of their pellets during their inactive phase (gray dashed line = 50% produced in inactive phase; main effect, female: F1,95 = 12.582, p < 0.001, and male: F1,103 = 10.565, p < 0.005; female, sham vs. SCI: 2 dpi, p = 0.01; 7 dpi, p < 0.05, and male, sham vs. SCI: 2 dpi, p < 0.05 ; 7 dpi, p < 0.005). In addition, SCI in males significantly disrupted rhythms of fecal mass excretion (Fig. 2B; main effect, male: F1,103 = 12.269, p < 0.005; 7 dpi, p < 0.005; 14 dpi, p < 0.05). At 42 days post-surgery, females had reduced fecal output (no difference between sham or SCI), which likely contributed to the unusually low inactive phase pellet output. Thus, diurnal rhythmic defecation in rats was abolished soon after SCI, and mostly recovered by a chronic time-point (42 dpi).
FIG. 2.
In female and male rats, spinal cord injury (SCI) disrupted rhythmic fecal output, body mass, and food intake. (A, B) Uninjured and pre-injury rats produced 5–25% of fecal pellets (A) and mass (B) during the inactive phase. SCI caused significant disruption at acute times, with up to 50% of pellets and mass being produced in the inactive phase (gray dotted line; representing random inactive-active fecal output). Abnormal fecal output rhythms recovered by 14–42 days post-injury (dpi). (C) After SCI, rats had reduced body mass compared with shams for 4 dpi (females) and 21 dpi (males). (D) Changes in body mass were accompanied by altered food intake: Compared with sham rats, female rats with SCI increased food intake at 7 and 14 dpi; male SCI rats showed reduced food intake at 2 dpi, then increased food intake at 7 and 14 dpi. *p < 0.05 for sham vs. SCI at that time-point. Color image is available online.
SCI in female and male rats also transiently increased overall fecal output compared with sham rats (pellet number and mass; Supplementary Fig. 2; see online supplementary material at http://www.liebertpub.com). Pellet excretion by SCI female and male rats was increased at 2 dpi and 7 dpi; fecal mass by SCI rats was increased at 7 and 14 dpi (females) and at 14 dpi (males); and SCI output was comparable to shams by 42 dpi. Wet (shown) and dry fecal mass showed an identical increase in post-SCI excretion patterns.
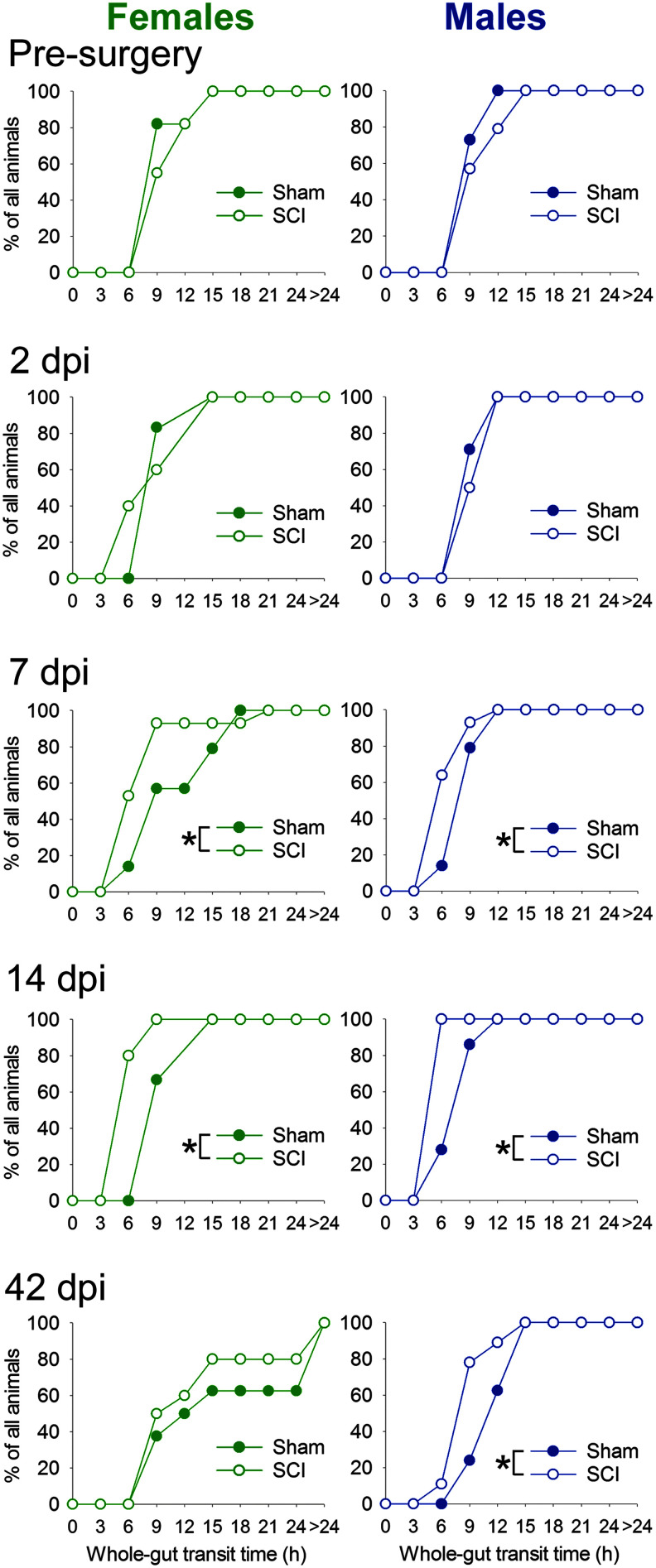
To determine whether SCI affects whole–gut transit time—which could potentially influence nutrient/fluid absorption and overall health—we assessed the latency to pass gavaged Evan's blue dye from stomach to fecal pellets (Fig. 3). T9 contusion SCI accelerated whole–gut transit time: at 7 and 14 dpi, both female and male SCI rats had shorter whole–gut transit times compared with sham rats. At 42 dpi, only male rats had significantly shorter whole–gut transit time (though there was a similar but non-significant pattern in females). SCI did not appear to alter fecal water content (Supplementary Fig. 3,; see online supplementary material at http://www.liebertpub.com). Although pellets in this study were air-dried at room temperature (in the low-humidity environment of Colorado) rather than in a convection oven, fecal water content reported here are similar to values reported by others.29,30 Thus, our results suggest that SCI expedited whole–gut transit without causing diarrhea.
FIG. 3.
Female and male rats with spinal cord injury (SCI) have reduced whole–gut transit time. Rats were gavaged with 2% Evan's blue dye; then, fecal pellets were monitored for the appearance of blue pellets (“whole–gut transit time”). Cumulative distributions show the time at which each rat first released a blue pellet. Prior to injury, rats in the sham and SCI groups had similar whole–gut transit times. By 7 days post-injury (dpi), both female and male SCI rats had accelerated whole–gut transit time; this persisted through 14 dpi in females and through 14 and 42 dpi in males. Color image is available online.
Spinal cord injury causes transient reduction in body mass and altered food intake
To establish whether SCI affects body mass or food intake, sham and SCI rats and food were weighed. Both female and male rats at 4 dpi had reduced body mass, which recovered with time post-injury (Fig. 2C). These body mass changes after SCI coincided with altered food intake (Fig. 2D). Female rats with SCI increased daily food intake over sham rats at 7 and 14 dpi. Male rats showed reduced food intake at 2 dpi, but increased food intake over sham rats at 7 and 14 dpi. There also were differences in post-SCI diurnal food intake: male SCI rats showed reduced active phase food intake at 2 dpi, and male and female SCI rats both ate more than shams at 7 dpi (Supplementary Fig. 4; see online supplementary material at http://www.liebertpub.com). Thus, an initial SCI-elicited loss in body mass appears to be compensated for by increased food intake.
Spinal cord injury dysregulates liver expression of glucose metabolism machinery
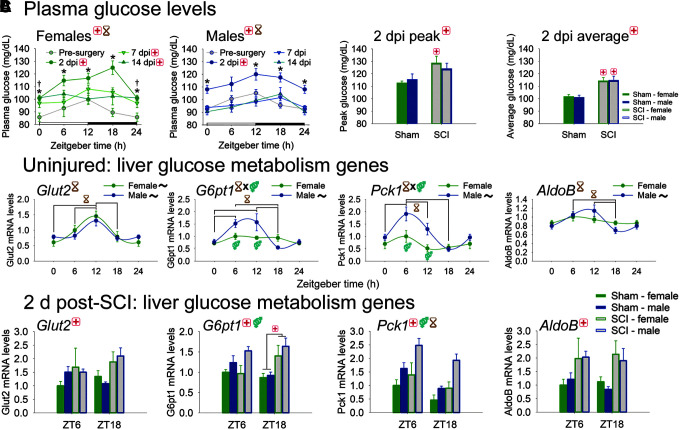
Given that SCI disrupted diurnal rhythms of functional metabolic outputs (including fecal output and whole–gut transit), we sought to assess how SCI affected diurnal function in a key organ involved in metabolism. Metabolism of glucose in liver is regulated across the day and glucose tolerance is reduced after SCI,10 so we focused on post-SCI diurnal regulation of glucose and related messenger RNA (mRNA) machinery as prototypical metabolic outputs. Plasma glucose rhythms were assessed before surgery, and at 2, 7, and 14 d post-surgery (Fig. 4A). Prior to surgery, female and male rats exhibited expected rhythms of plasma glucose expression, with peak expression around the beginning of the active phase (time of increased feeding and glucose intake; peak expression [acrophases]: female ZT11.0 ± 3.6, male ZT10.5 ± 3.7). SCI significantly increased plasma glucose levels overall (dpi main effect: females F3,124 = 17.46, p < 0.001; males F3,140 = 11.88, p < 0.001) and at 2 dpi (2 dpi vs. pre-surgery: females p < 0.001, males p < 0.001). Females showed persistent effects of SCI at 7 and 14 dpi (both p < 0.01), whereas males with SCI showed similar glucose regulation to pre-surgery by 7 dpi. At 2 dpi, SCI (vs. sham) significantly increased peak glucose (main effect and in females, p < 0.05) and average glucose (main effect and in both females and males, p < 0.005).
FIG. 4.
Diurnal regulation of glucose metabolism genes in liver from uninjured rats, and from sham/ spinal cord injury (SCI) rats at 2 days post-injury (dpi). (A) Plasma glucose in female and male rats is significantly increased by SCI. Plasma glucose was increased in females and males at 2 dpi. Females, but not males, showed persistent SCI-induced dysregulation at 7 and 14 dpi. (B) Female and male rat livers display circadian rhythms in glucose metabolism gene expression. Glut2 and AldoB showed significant time-of-day regulation; G6pt1 and Pck1 had significant interaction between Zeitgeber time (ZT) and sex (males > females at specific times). (C) SCI disrupts glucose metabolism gene expression in the liver. SCI increased expression of glucose metabolism genes, including Glut2, G6pt1, Pck1, and AldoB. G6pt1 was also differentially regulated by sex, and Pck1 was also differentially expressed by sex and by Zeitgeber time. Black ∼ indicates that females or males for that gene show significant rhythm; red + indicates injury difference (sham vs. SCI), p < 0.05; yellow hourglass indicates time difference, p < 0.05; blue gender symbol indicates sex difference, p < 0.05; * indicates difference between 2 dpi and pre-SCI, p < 0.05; † indicates significant difference between 14 dpi and pre-SCI, p < 0.05. Symbols at the top of each graph indicates significant main effect; symbols above/below data indicate significant interactions. Color image is available online.
Several glucose metabolism-related genes in the liver are expressed rhythmically across the day, enabling appropriately timed glucose storage and release; this helps maintain more stable peripheral glucose levels across daily feed-fast (wake-sleep) cycles.7 Indeed, in the uninjured female and male rat liver, several glucose-related genes were expressed in a diurnal manner (Fig. 4B). Glut2 (glucose transporter 2; facilitates glucose transport), G6pt1 (glucose-6-phosphate translocase; plays roles in glucose transport into endoplasmic reticulum and glucose homeostasis), Pck1 (phosphoenolpyruvate carboxykinase 1; is a pivotal enzyme in gluconeogenesis), and AldoB (aldolase-B; plays roles in glycolysis and gluconeogenesis) were all rhythmically regulated in liver across the day (Glut2, AldoB: main effect of time, p < 0.005; G6pt1, Pck1: significant time-sex interaction, p < 0.05; acrophases: Glut2 female ZT11.2 ± 2.8, male ZT11.4 ± 3.3; G6pt1 female ZT11.3 ± 3.4, male ZT8.6 ± 2.7; Pck1 female ZT5.4 ± 3.2, male ZT6.9 ± 2.6; AldoB female ZT9.9 ± 3.5, male ZT8.9 ± 3.3).
Given that SCI alters plasma glucose levels, we predicted that SCI would dysregulate expression of glucose metabolism genes in the liver (Fig. 4C; Table 2). SCI significantly upregulated glucose metabolism genes, including Glut2, G6pt1, Pck1, and AldoB (main effect, sham vs. SCI, all p < 0.05). G6pt1 was also regulated by sex (main effect, males>females, p < 0.05), and Pck1 was regulated by both sex (main effect, males >females, p < 0.001) and ZT (main effect, ZT6 > ZT18, p < 0.005).
Table 2.
Expression of additional Clock (Per1, Clock), Inflammatory (Mhc II, IL-6), and Glucose Metabolism (G6pc, Gck, Hnf1a) Genes at 2 dpi in the Liver
| Female | Male | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ZT6 | ZT18 | ZT6 | ZT18 | Significant differences | ||||||
| Gene | Sham | SCI | Sham | SCI | Sham | SCI | Sham | SCI | Main effect | Interactions |
| Per1 | 1.00 ± 0.40 | 2.83 ± 0.88 | 0.85 ± 0.37 | 2.27 ± 0.56 | 1.35 ± 0.22 | 2.80 ± 0.43 | 1.02 ± 0.30 | 3.21 ± 0.76 | SCI | N.S. |
| Clock | 1.00 ± 0.05 | 0.89 ± 0.08 | 0.63 ± .14 | 0.89 ± .10 | 1.19 ± .11 | 1.29 ± 0.19 | 0.56 ± 0.04 | 0.85 ± 0.12 | ZT | ZT6 × sex |
| Mhc II | 1.00 ± 0.14 | 0.65 ± 0.09 | 0.97 ± .06 | 0.77 ± .10 | 1.07 ± 0.83 | 0.83 ± .08 | 0.91 ± 0.23 | 0.90 ± 0.24 | N.S. | N.S. |
| IL-6 | 1.00 ± 0.26 | 1.28 ± 0.66 | 0.31 ± .08 | 0.35 ± .07 | 2.13 ± 0.64 | 0.36 ± .07 | 0.68 ± 0.23 | 0.69 ± 0.23 | ZT | N.S. |
| G6pc | 1.00 ± 0.32 | 1.19 ± 0.25 | 1.70 ± .79 | 1.37 ± .25 | 4.46 ± 1.5 | 2.10 ± .95 | 0.67 ± 0.18 | 2.44 ± 0.29 | Sex | Sham ZT6 × sex |
| Gck | 1.00 ± 0.12 | 1.19 ± 0.40 | 1.84 ± .37 | 1.13 ± .46 | 0.53 ± 0.11 | 0.87 ± .22 | 0.83 ± 0.16 | 0.74 ± 0.35 | Sex | N.S. |
| Hnf1a | 1.00 ± 0.11 | 0.77 ± 0.14 | 0.83 ± .13 | 1.05 ± .16 | 0.97 ± 0.12 | 1.26 ± .18 | 0.72 ± 0.10 | 1.15 ± 0.12 | N.S. | N.S. |
Data only for genes not shown in figures. For statistical comparisons (far right column): “SCI” = significant main effect of SCI vs. sham; “ZT” = significant main effect of time (ZT6 vs. ZT18); “sex” = significant main effect of sex; “Sham x ZT” = significant interaction between sham and ZT.
dpi, days post-injury; SCI, spinal cord injury; N.S., no significant difference; ZT, Zeitgeber time.
Spinal cord injury disrupts diurnal regulation of clock and inflammatory gene expression in liver
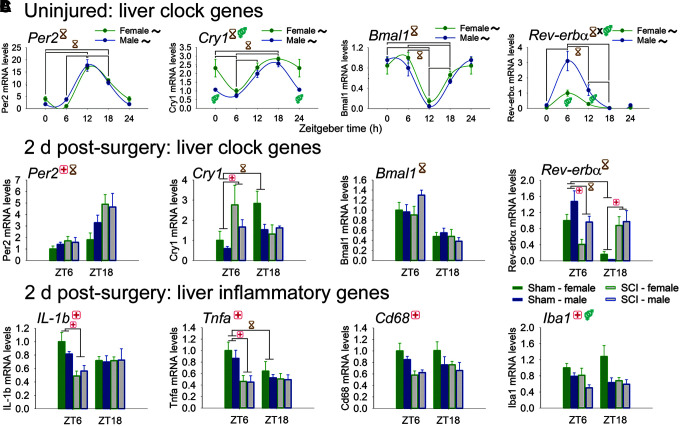
Next, we sought to reveal whether SCI more broadly perturbs circadian gene networks and related inflammatory functions. Liver tissue from healthy uninjured rats displayed strong rhythms of clock gene expression (Fig. 5A). Per2, Cry1, and Bmal1, and Rev-erbα were all robustly regulated in liver by time (Per2, Bmal1, Cry1: main effect of time, p < 0.001; Rev-erbα: significant time–sex interaction, F3,47 = 6.48, p = 0.001; Cry1: sex, main effect and ZT0, p < 0.005). Circwave analysis established that these clock genes were expressed rhythmically across the day (all in both females and males; acrophases: Per2 female ZT14.6 ± 1.9, male ZT13.6 ± 1.9; Cry1 female ZT17.9 ± 3.0, male ZT16.2 ± 2.6; Bmal1 female ZT1.7 ± 2.7, male ZT1.1 ± 2.3; Rev-erbα female ZT6.9 ± 1.0, male ZT7.2 ± 1.1).
FIG. 5.
Diurnal regulation of genes in liver from uninjured rats, and from sham/spinal cord injury (SCI) rats at 2 days post-injury. (A) Female and male rat livers express clock genes, and clock gene expression varies through the day. Per2, Cry1, Bmal1, and Rev-erbα displayed rhythmic expression in liver. Cry1 expression was differentially regulated by sex; Rev-erbα showed significant interaction between Zeitgeber time (ZT) and sex. (B) SCI disrupts clock gene expression in the liver. Liver from sham rats showed clock gene variation between ZT6 and ZT18. SCI dysregulated expression of several clock genes, including Per2, Cry1, and Rev-erbα. SCI also ablated time-of-day differences in expression of Cry1 and Rev-erbα. (C) SCI reduced expression in the liver of inflammatory genes, including IL-1b, Tnfa, Cd68, and Iba1 (main effect). SCI also significantly reduced IL-1b and Tnfa at ZT6. For Iba1, there was also a significant main effect of sex (females higher). Black ∼ indicates that females or males for that gene show significant rhythm; red + indicates injury difference (sham vs. SCI), p < 0.05; yellow hourglass indicates time difference, p < 0.05; blue gender symbol indicates sex difference, p < 0.05. Symbols at top of each graph indicates significant main effect; symbols above/below data indicate significant interactions. Color image is available online.
SCI dysregulated expression of circadian clock genes in liver (Fig. 5B). Per2 in liver was upregulated by SCI (vs. sham) (main effect, F1,45 = 8.17, p < 0.01), and was higher at ZT18 vs. ZT6 (main effect). Cry1 also was upregulated by SCI (at ZT6, p = 0.005); Cry1 expression showed time-of-day differences in sham rats that were ablated by SCI. Bmal1 displayed significant main effects of Zeitgeber time (ZT6 > ZT18), but was not significantly altered by SCI. Rhythms of Rev-erbα expression observed in sham rats were ablated by SCI (at ZT6 [p < 0.005] and ZT18 [p < 0.001]). (In addition, Per1 was increased by SCI, and Clock was regulated by time; Supplementary Table 1.) Thus, SCI altered clock gene expression and circadian regulation in the liver.
SCI also dysregulated liver expression of inflammatory genes (Fig. 5C). SCI significantly downregulated liver expression of pro-inflammatory cytokines IL-1b and Tnfa (vs. sham; main effects, F1,44 = 6.45, 11.64, respectively; both p < 0.05 and both at ZT6). SCI also reduced expression of the macrophage activation mRNA Cd68 and of the macrophage-specific mRNA Iba1 (main effects, F1,46 = 10.61, 8.528, respectively; both p < 0.01). Iba1 was also regulated by sex (females > males; main effect). (Liver expression of MhcII and IL-6 were also assessed, but were not regulated by SCI; Supplementary Table 1.) These data suggest that moderate SCI elicits robust peripheral changes that include dysregulated expression of glucose machinery, clock, and inflammatory genes in the liver.
Discussion
This study revealed that SCI disrupted diurnal rhythms of behaviors and gene expression related to metabolism. SCI in female and male rats had abnormal arrhythmic defecation patterns: whereas sham rats produced ∼80% of fecal output during their active phase, SCI rats at 2 days post-surgery produced 50% of fecal output during their active phase (representing complete loss of typical diurnal rhythms—random production during active/inactive phases). SCI also increased fecal output, expedited whole–gut transit time, and dysregulated food intake. In parallel, SCI altered diurnal and metabolic regulation in the liver. SCI disrupted metabolism-related outcomes: acute post-SCI rats had increased plasma glucose; this coincided with increased and arrhythmic post-SCI liver expression of genes encoding glucose machinery. In parallel, SCI dampened diurnal expression of liver clock and inflammatory genes. Together, these changes show that SCI worsens function of distinct peripheral tissues and processes related to health and metabolism. Our data suggest that moderate thoracic SCI causes a widespread, multi-organ shift away from physiologic homeostasis.
SCI dampened diurnal rhythms of metabolic behavior: defecation as an output of metabolism
We found that daily rhythms of fecal output in female and male rats were completely abolished at 2 dpi, when 50% of fecal pellets were produced during the SCI rats' inactive phase. The diurnal regulation of fecal output gradually recovered to a more typical pattern, though male rats showed persistent disruption through 42 dpi.
Time of defecation in rodents and humans is linked to time of peak glucocorticoids (i.e., typically the beginning of the active phase).31,32 T9 SCI partially denervates the adrenal glands, which are major sources of glucocorticoids. Glucocorticoids are transiently upregulated and dysregulated across the day soon after SCI, and adrenalectomy ameliorates SCI-elicited increases in glucocorticoids.33,34 Therefore, it is possible that acute post-SCI glucocorticoid induction could increase fecal output across the day, thereby disrupting typical diurnal defecation rhythms.
SCI increased fecal output and expedited whole–gut transit
SCI increased fecal output, including both pellet number and mass. Increased pellet number was observed by 2 dpi. Post-SCI increased fecal output was not related to increased water content. It is possible that acute SCI influenced fecal output through reduced absorption of nutrients (i.e., more release of nutrients in feces, which could relate to observed expedited whole–gut transit, increased feeding, and dysregulated glucose metabolism) and/or altered microbiome composition.35,36 In addition, increased daily fecal mass output was observed at 7 dpi (females only) and 14 dpi (both females and males); this is likely related to expedited whole–gut transit time and increased food intake at these post-injury times.
Whole–gut transit was expedited in female and male rats after moderate T9 contusion SCI. As mentioned above, acute SCI increases glucocorticoids across the day, which could drive more frequent and arrhythmic defecation, and accelerated whole–gut transit. In contrast, other studies in rats showed that severe 300-kDyne T3 (but not T9) contusion SCI caused significant constipation (i.e., protracted whole–gut transit time).37,38 In the severe SCI model, T9 contusion also appeared to cause some constipation (though not significant),38 suggesting that increased sparing with our moderate 150-kDyne T9 SCI may differentially influence bowel function compared with the more severe injury (lesion epicenter: 150-kDyne, 1 sec dwell ∼24% spared tissue versus 300-kDyne ∼12% spared tissue). In humans, no known published articles describe whole–gut transit time at acute SCI time-points; however, patients with chronic SCI often show extended whole–gut transit times.39 Constipation and incontinence are common, persistent post-SCI issues that can co-exist in patients40 and can worsen patients' quality-of-life and psychological well-being.41–45 Future studies could further elucidate how SCI at different severities and segments influences bowel function over time.
SCI dysregulates diurnal rhythms of glucose metabolism machinery
Given that diurnal rhythms critically control expression of metabolic machinery,46 it is possible that SCI-elicited circadian disruption could elicit and/or exacerbate post-SCI metabolic dysfunction. The liver helps orchestrate whole–body physiologic homeostasis, including diurnal rhythms of glucose, so we assessed at acute post-SCI plasma glucose and liver levels of glucose machinery mRNAs. Plasma glucose strongly increased at 2 dpi, and gradually recovered over time (though females showed persistent elevations). Glucose dysregulation after SCI has functional consequences. Upregulating glucose in mice prior to SCI significantly reduces functional recovery, and normalizing glucose levels using insulin improves recovery; in parallel, SCI patients admitted with hyperglycemia have worsened locomotor outcomes.11 Glucose release into circulation is gated by the liver, which normalizes plasma glucose through the day via specific transporters and enzymes whose expression is regulated diurnally.7,8,47,48 Diurnal regulation of glucose metabolism machinery helps ensure more steady glucose levels across the day (dampens peaks after feeding and nadirs during fasting); disrupting rhythms of genes involved in glucose machinery amplifies plasma glucose rhythms to more strongly mirror feed–fast times (causing potentially harmful spikes and crashes).7,9
We revealed that SCI increased liver expression of glucose metabolism genes Glut2, G6pt1, Pck1, and AldoB. Gene products of Pck1, G6pt1, and Glut2 critically regulate liver glucose storage/release and therefore define the liver's contribution to physiologic glucose homeostasis. During nutrient absorption, GLUT2 transports D-glucose into the hepatocyte, and glucokinase (encoded by Pck1) phosphorylates D-glucose into D-glucose 6-phosphate to promote glycogen storage and energy metabolism.49,50 Conversely, during fasting, glucose 6-phosphate transporter-1 (G6Pase; encoded by G6pt1) converts D-glucose 6-phosphate to D-glucose for efflux into circulation via GLUT2.50 GLUT2 has a particularly important role in glucose efflux49 and reducing G6Pase limits susceptibility to diabetes51; therefore, SCI-elicited increases in expression of these genes could contribute to post-SCI hyperglycemia. Thus, normalizing liver diurnal rhythms could accelerate recovery of glucose homeostasis, which could benefit neurologic recovery.
SCI alters diurnal rhythms of circadian clock and inflammatory genes in liver
To establish whether SCI influenced liver diurnal rhythms more broadly, we assessed expression in healthy and post-SCI liver of clock genes. Clock genes are expressed rhythmically through the day via interlocking feedback loops; most are transcription factors that link expression of other genes to time of day.46 In liver, SCI increased expression of the clock genes Per1, Per2, and Cry1, and abolished rhythms of Cry1 and Rev-erbα. To our knowledge, no one has assessed post-SCI clock gene expression in any tissue; thus, our novel data reveal that SCI causes broad shifts in clock gene expression in liver.
In addition, there were post-SCI decreases in most inflammatory genes examined, suggesting acute immune suppression in the liver. This is consistent with SCI-elicited peripheral immunosuppression, which contributes to susceptibility to infection (e.g., pneumonia).34 Conversely, others have reported chronic post-SCI accumulation of fat and inflammatory factors in liver52; it is possible that acute post-SCI immunosuppression in liver (observed here) is followed by chronic inflammation. In addition, Tnfa in sham rats was significantly higher at ZT6 than at ZT18. This time-of-day difference in Tnfa was abolished after SCI, suggesting that SCI dysregulates diurnal rhythms of some inflammatory genes.
Interestingly, clock and inflammatory factors can reciprocally regulate each others' expression, suggesting the existence of a coordinated clock-inflammatory gene network.53–55 For instance, tumor necrosis factor α and interleukin 1β can downregulate Per2 (as well as Per1, Per3, and other clock genes).56 In support of this relationship, we found that in shams Per2 was higher at ZT18, whereas IL-1b and Tnfa were higher at ZT6. SCI increased Per2, while decreasing IL-1b and Tnfa. Thus, coordinated shifts in post-SCI gene expression highlight the potential link between clock and inflammatory gene regulation after SCI. Manipulating these clock-inflammatory gene networks represents a promising post-SCI therapeutic strategy.
Sex differences and similarities after SCI
Females and males were included in all studies herein. Potential sex differences have been largely overlooked in past pre-clinical SCI studies, although there is increasing focus on their importance.57 Here, we found that males and females displayed largely similar patterns in SCI-elicited circadian dysfunction and recovery between the sexes. In all measures, the most robust SCI-elicited changes occurred acutely after injury, and recovered by the chronic time-point. There were subtle differences suggesting that males had worsened recovery curves: compared with shams, SCI males (but not females) showed protracted flattening of fecal output rhythms and expedited whole–gut transit into chronic SCI, and they had delayed body mass recovery. Females and males had similar post-SCI alterations in glucose-related, inflammatory, and clock genes in the liver at 2 days post-surgery. In a study completed in parallel with the current study, we found that females and males subjected to this same SCI severity (150 kDyne, 1-sec dwell midline T9 SCI) had similar lesion size, percent spared tissue, and open-field locomotor scores,58 suggesting that differential lesion size likely did not contribute to observed differences presented here.
Our data showing that post-SCI recovery was similar between the sexes (if slightly worse in males) parallels others' findings in rodents and humans. Swartz and colleagues59 used Sprague-Dawley rats and found that females (vs. males) subjected to 150 kDyne SCI (Infinite Horizon device) had significantly reduced injury length and improved tissue sparing. Females have higher levels of endogenous estrogen (dependent on the time of estrous cycle), which can be protective in models of SCI60 and other neuroinflammatory conditions.61 In humans, improvements of American Spinal Injury Association Impairment Scale (AIS) grade and neurologic function between time of SCI and 1 year later were broadly similar between males and females, although females had slightly better neurologic scores (AIS), whereas males had slightly higher Functional Independence Measure scores.62 Thus, major outcomes after SCI are typically quite similar between the sexes; however, studying post-SCI responses in both sexes remains important because these studies could reveal sex-specific mechanisms and/or therapies.
Future directions and conclusions
This study has several implications. First, it highlights how SCI disrupts whole–body metabolic function: SCI disrupted diurnal defecation rhythms, expedited whole–gut transit time, increased fecal output, altered expression of glucose and glucose metabolism mRNAs in liver, and disrupted diurnal rhythms of liver gene expression. Here, a moderate T9 spinal cord contusion was used; future studies could establish whether more severe injuries or injuries at other spinal levels (e.g., cervical or high thoracic) differentially alter post-injury metabolic outcomes. Second, since post-SCI fecal output was increased, whole–gut transit was accelerated, and glucose metabolism was perturbed, treatments that optimize post-SCI nutrient absorbance and/or energy intake could potentially improve recovery.39, 63–65 Nutritional health (e.g., nitrogen balance)66 is worsened in SCI patients for up to 2 months post-injury, despite persistent nutrient support.35, 65 Thus, accelerating recovery of nutrition and metabolism could improve recovery. Future studies could explore how experimental SCI affects nutrition balance, and whether optimizing nutrient balance after SCI boosts repair. Finally, our data suggest that a “circadian rehabilitation” regimen could help accelerate recovery of circadian rhythms—which could improve metabolic homeostasis and overall health. In humans, several strategies could strengthen post-SCI circadian rhythms, including brighter daytime light (e.g., window facing the outside in the room),67 dark nights with fewer interruptions (to increase sleep quality68), and optimized time of feeding and exercise.69–71
In conclusion, we have used a clinically relevant rat spinal contusion model to assess how SCI affects metabolic behavior, liver metabolism measures, and peripheral diurnal rhythms.
Our results demonstrate that moderate thoracic SCI increases and disrupts diurnal fecal output, alters food intake patterns, increases plasma glucose and related glucose machinery mRNAs, and dampens liver rhythms of glucose-related, circadian, and inflammatory genes. This study reveals that SCI broadly disrupts physiologic homeostasis in diverse peripheral organs. Future discovery and clinical research could develop therapeutic approaches to augment post-SCI metabolic function and diurnal rhythms, which could ultimately help regain homeostasis and improve recovery.
Supplementary Material
Acknowledgments
The authors thank the Muenzinger husbandry staff for excellent animal care, and the Biological Sciences Initiative (BSI) and Undergraduate Research Opportunities Program (UROP) at CU-Boulder for undergraduate research support. This work was supported by the United States Department of Defense (LRW: W81XWH-13-1-0277/SC120066). Additional support was provided by Paralyzed Veterans of America (LRW: #3004), the Craig H. Nielsen Foundation (SFM), and the Wings for Life Foundation (LRW/ADG).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Collinger J.L., Boninger M.L., Bruns T.M., Curley K., Wang W., and Weber D.J. (2013). Functional priorities, assistive technology, and brain-computer interfaces after spinal cord injury. J. Rehab. Res. Dev. 50, 145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stiens S.A., Bergman S.B., and Goetz L.L. (1997). Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch. Phys. Med. Rehabil. 78, S86–S102. [DOI] [PubMed] [Google Scholar]
- 3. Gorgey A.S., Dolbow D.R., Dolbow J.D., Khalil R.K., Castillo C., and Gater D.R. (2014). Effects of spinal cord injury on body composition and metabolic profile—part I. J. Spinal Cord Med. 37, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fonken L.K. and Nelson R.J. (2014). The effects of light at night on circadian clocks and metabolism. Endocrine Rev. 35, 648–670. [DOI] [PubMed] [Google Scholar]
- 5. Bedrosian T.A., Fonken L.K., and Nelson R.J. (2016). Endocrine effects of circadian disruption. Annual Rev. Physiol. 78, 109–131. [DOI] [PubMed] [Google Scholar]
- 6. Adeva-Andany M.M., Perez-Felpete N., Fernandez-Fernandez C., Donapetry-Garcia C., and Pazos-Garcia C. (2016). Liver glucose metabolism in humans. Biosci. Rep. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gatfield D. and Schibler U. (2008). Circadian glucose homeostasis requires compensatory interference between brain and liver clocks. Proc. Natl. Acad. Sci. U. S. A. 105, 14753–14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akhtar R.A., Reddy A.B., Maywood E.S., Clayton J.D., King V.M., Smith A.G., Gant T.W., Hastings M.H., and Kyriacou C.P. (2002). Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr. Biol. 12, 540–550. [DOI] [PubMed] [Google Scholar]
- 9. Lamia K.A., Storch K.F., and Weitz C.J. (2008). Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. U. S. A. 105, 15172–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elder C.P., Apple D.F., Bickel C.S., Meyer R.A., and Dudley G.A. (2004). Intramuscular fat and glucose tolerance after spinal cord injury–a cross-sectional study. Spinal Cord 42, 711–716. [DOI] [PubMed] [Google Scholar]
- 11. Kobayakawa K., Kumamaru H., Saiwai H., Kubota K., Ohkawa Y., Kishimoto J., Yokota K., Ideta R., Shiba K., Tozaki-Saitoh H., Inoue K., Iwamoto Y., and Okada S. (2014). Acute hyperglycemia impairs functional improvement after spinal cord injury in mice and humans. Sci. Transl. Med. 6, 256ra137. [DOI] [PubMed] [Google Scholar]
- 12. Squair J.W., le Nobel G., Noonan V.K., Raina G., and Krassioukov A.V. (2015). Assessment of clinical adherence to the international autonomic standards following spinal cord injury. Spinal Cord 53, 668–672. [DOI] [PubMed] [Google Scholar]
- 13. West C.R., Popok D., Crawford M.A., and Krassioukov A.V. (2015). Characterizing the temporal development of cardiovascular dysfunction in response to spinal cord injury. J. Neurotrauma 32, 922–930. [DOI] [PubMed] [Google Scholar]
- 14. Giannoccaro M.P., Moghadam K.K., Pizza F., Boriani S., Maraldi N.M., Avoni P., Morreale A., Liguori R., and Plazzi G. (2013). Sleep disorders in patients with spinal cord injury. Sleep Med. Rev. 17, 399–409. [DOI] [PubMed] [Google Scholar]
- 15. Claydon V.E., Steeves J.D., and Krassioukov A. (2006). Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 44, 341–351. [DOI] [PubMed] [Google Scholar]
- 16. Kneisley L.W., Moskowitz M.A., and Lynch H.G. (1978). Cervical spinal cord lesions disrupt the rhythm in human melatonin excretion. J. Neural. Transm. Suppl. 311–323. [PubMed] [Google Scholar]
- 17. Krum H., Louis W.J., Brown D.J., Jackman G.P., and Howes L.G. (1991). Diurnal blood pressure variation in quadriplegic chronic spinal cord injury patients. Clin. Sci. (Lond.) 80, 271–276. [DOI] [PubMed] [Google Scholar]
- 18. Scheer F.A., Zeitzer J.M., Ayas N.T., Brown R., Czeisler C.A., and Shea S.A. (2006). Reduced sleep efficiency in cervical spinal cord injury; association with abolished night time melatonin secretion. Spinal Cord 44, 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeitzer J.M., Ayas N.T., Shea S.A., Brown R., and Czeisler C.A. (2000). Absence of detectable melatonin and preservation of cortisol and thyrotropin rhythms in tetraplegia. J. Clin. Endocrinol. Metab. 85, 2189–2196. [DOI] [PubMed] [Google Scholar]
- 20. Scheff S.W., Rabchevsky A.G., Fugaccia I., Main J.A., and Lumpp J.E., Jr. (2003). Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma 20, 179–193. [DOI] [PubMed] [Google Scholar]
- 21. Gaudet A.D., Ayala M.T., Schleicher W.E., Smith E.J., Bateman E.M., Maier S.F., and Watkins L.R. (2017). Exploring acute-to-chronic neuropathic pain in rats after contusion spinal cord injury. Exp. Neurol. 295, 46–54. [DOI] [PubMed] [Google Scholar]
- 22. Gaudet A.D., Sweet D.R., Polinski N.K., Guan Z., and Popovich P.G. (2015). Galectin-1 in injured rat spinal cord: implications for macrophage phagocytosis and neural repair. Mol. Cell. Neurosci. 64, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bashashati M., Nasser Y., Keenan C.M., Ho W., Piscitelli F., Nalli M., Mackie K., Storr M.A., Di Marzo V., and Sharkey K.A. (2015). Inhibiting endocannabinoid biosynthesis: a novel approach to the treatment of constipation. Br. J. Pharmacol. 172, 3099–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaudet A.D. and Fonken L.K. (2018). Glial cells shape pathology and repair after spinal cord injury. Neurotherapeutics 15, 554–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., and Popovich P.G. (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCreedy D.A., Lee S., Sontag C.J., Weinstein P., Olivas A.D., Martinez A.F., Fandel T.M., Trivedi A., Lowell C.A., Rosen S.D., and Noble-Haeusslein L.J. (2018). Early targeting of L-selectin on leukocytes promotes recovery after spinal cord injury, implicating novel mechanisms of pathogenesis. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noble L.J., Donovan F., Igarashi T., Goussev S., and Werb Z. (2002). Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J. Neurosci. 22, 7526–7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fonken L.K., Frank M.G., Gaudet A.D., D'Angelo H.M., Daut R.A., Hampson E.C., Ayala M.T., Watkins L.R., and Maier S.F. (2018). Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav. Immun. 70, 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han S.H., Park K., Kim E.Y., Ahn S.H., Lee H.S., and Suh H.J. (2017). Cactus (Opuntia humifusa) water extract ameliorates loperamide-induced constipation in rats. BMC Complement. Altern. Med. 17, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu X., Liu Z., Qu H., Niu W., Gao L., Wang Y., Zhang A., and Bai L. (2016). The effect and mechanism of electroacupuncture at LI11 and ST37 on constipation in a rat model. Acupunct. Med. 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fukudo S., Nomura T., and Hongo M. (1998). Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut 42, 845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang L., Sundaresh S., Elliott J., Anton P.A., Baldi P., Licudine A., Mayer M., Vuong T., Hirano M., Naliboff B.D., Ameen V.Z., and Mayer E.A. (2009). Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol. Motil. 21, 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lucin K.M., Sanders V.M., Jones T.B., Malarkey W.B., and Popovich P.G. (2007). Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp. Neurol. 207, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pruss H., Tedeschi A., Thiriot A., Lynch L., Loughhead S.M., Stutte S., Mazo I.B., Kopp M.A., Brommer B., Blex C., Geurtz L.C., Liebscher T., Niedeggen A., Dirnagl U., Bradke F., Volz M.S., DeVivo M.J., Chen Y., von Andrian U.H., and Schwab J.M. (2017). Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat. Neurosci. 20, 1549–1559. [DOI] [PubMed] [Google Scholar]
- 35. Rodriguez D.J., Clevenger F.W., Osler T.M., Demarest G.B., and Fry D.E. (1991). Obligatory negative nitrogen balance following spinal cord injury. JPEN. J. Parenter. Enteral Nutr. 15, 319–322. [DOI] [PubMed] [Google Scholar]
- 36. Kigerl K.A., Hall J.C., Wang L., Mo X., Yu Z., and Popovich P.G. (2016). Gut dysbiosis impairs recovery after spinal cord injury. J. Exp. Med. 213, 2603–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qualls-Creekmore E., Tong M., and Holmes G.M. (2010). Time-course of recovery of gastric emptying and motility in rats with experimental spinal cord injury. Neurogastroenterol. Motil. 22, 62–69, e27–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tong M. and Holmes G.M. (2009). Gastric dysreflexia after acute experimental spinal cord injury in rats. Neurogastroenterol. Motil. 21, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krogh K., Mosdal C., and Laurberg S. (2000). Gastrointestinal and segmental colonic transit times in patients with acute and chronic spinal cord lesions. Spinal Cord 38, 615–621. [DOI] [PubMed] [Google Scholar]
- 40. Glickman S. and Kamm M.A. (1996). Bowel dysfunction in spinal-cord-injury patients. Lancet 347, 1651–1653. [DOI] [PubMed] [Google Scholar]
- 41. Anderson K.D. (2004). Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383. [DOI] [PubMed] [Google Scholar]
- 42. Bloemen-Vrencken J.H., Post M.W., Hendriks J.M., De Reus E.C., and De Witte L.P. (2005). Health problems of persons with spinal cord injury living in the Netherlands. Disabil. Rehabil. 27, 1381–1389. [DOI] [PubMed] [Google Scholar]
- 43. Ditunno P.L., Patrick M., Stineman M., and Ditunno J.F. (2008). Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord 46, 500–506. [DOI] [PubMed] [Google Scholar]
- 44. Noreau L., Noonan V.K., Cobb J., Leblond J., and Dumont F.S. (2014). Spinal cord injury community survey: a national, comprehensive study to portray the lives of canadians with spinal cord injury. Top. Spinal Cord Inj. Rehabil. 20, 249–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simpson L.A., Eng J.J., Hsieh J.T., and Wolfe D.L. (2012). The health and life priorities of individuals with spinal cord injury: a systematic review. J. Neurotrauma 29, 1548–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang E.E. and Kay S.A. (2010). Clocks not winding down: unravelling circadian networks. Nat. Rev. Mol. Cell Biol. 11, 764–776. [DOI] [PubMed] [Google Scholar]
- 47. Panda S., Antoch M.P., Miller B.H., Su A.I., Schook A.B., Straume M., Schultz P.G., Kay S.A., Takahashi J.S., and Hogenesch J.B. (2002). Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320. [DOI] [PubMed] [Google Scholar]
- 48. Reddy A.B., Karp N.A., Maywood E.S., Sage E.A., Deery M., O'Neill J.S., Wong G.K., Chesham J., Odell M., Lilley K.S., Kyriacou C.P., and Hastings M.H. (2006). Circadian orchestration of the hepatic proteome. Curr. Biol. 16, 1107–1115. [DOI] [PubMed] [Google Scholar]
- 49. Karim S., Adams D.H., and Lalor P.F. (2012). Hepatic expression and cellular distribution of the glucose transporter family. World J. Gastroenterol. 18, 6771–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leturque A., Brot-Laroche E., and Le Gall M. (2009). GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am. J. Physiol. Endocrinol. Metab. 296, E985–E992. [DOI] [PubMed] [Google Scholar]
- 51. Sloop K.W., Showalter A.D., Cox A.L., Cao J.X., Siesky A.M., Zhang H.Y., Irizarry A.R., Murray S.F., Booten S.L., Finger E.A., McKay R.A., Monia B.P., Bhanot S., and Michael M.D. (2007). Specific reduction of hepatic glucose 6-phosphate transporter-1 ameliorates diabetes while avoiding complications of glycogen storage disease. J. Biol. Chem. 282, 19113–19121. [DOI] [PubMed] [Google Scholar]
- 52. Sauerbeck A.D., Laws J.L., Bandaru V.V., Popovich P.G., Haughey N.J., and McTigue D.M. (2015). Spinal cord injury causes chronic liver pathology in rats. J. Neurotrauma 32, 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nguyen K.D., Fentress S.J., Qiu Y., Yun K., Cox J.S., and Chawla A. (2013). Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341, 1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Segal J.P., Tresidder K.A., Bhatt C., Gilron I., and Ghasemlou N. (2018). Circadian control of pain and neuroinflammation. J. Neurosci. Res. 96, 1002–1020. [DOI] [PubMed] [Google Scholar]
- 55. Fonken L.K., Kitt M.M., Gaudet A.D., Barrientos R.M., Watkins L.R., and Maier S.F. (2016). Diminished circadian rhythms in hippocampal microglia may contribute to age-related neuroinflammatory sensitization. Neurobiol. Aging 47, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cavadini G., Petrzilka S., Kohler P., Jud C., Tobler I., Birchler T., and Fontana A. (2007). TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc. Natl. Acad. Sci. U. S. A. 104, 12843–12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clayton J.A. and Collins F.S. (2014). Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gaudet A.D., Fonken L.K., Ayala M.T., Bateman E.M., Schleicher W.E., Smith E.J., D'Angelo H.M., Maier S.F., and Watkins L.R. (2018). Spinal cord injury in rats disrupts diurnal rhythms. J. Neurotrauma 2018. Dec 1; Epub ahead of print. [Google Scholar]
- 59. Swartz K.R., Fee D.B., Joy K.M., Roberts K.N., Sun S., Scheff N.N., Wilson M.E., and Scheff S.W. (2007). Gender differences in spinal cord injury are not estrogen-dependent. J. Neurotrauma 24, 473–480. [DOI] [PubMed] [Google Scholar]
- 60. Chaovipoch P., Jelks K.A., Gerhold L.M., West E.J., Chongthammakun S., and Floyd C.L. (2006). 17beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J. Neurotrauma 23, 830–852. [DOI] [PubMed] [Google Scholar]
- 61. Roof R.L. and Hall E.D. (2000). Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma 17, 367–388. [DOI] [PubMed] [Google Scholar]
- 62. Sipski M.L., Jackson A.B., Gomez-Marin O., Estores I., and Stein A. (2004). Effects of gender on neurologic and functional recovery after spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1826–1836. [DOI] [PubMed] [Google Scholar]
- 63. Jeong M.A., Plunet W., Streijger F., Lee J.H., Plemel J.R., Park S., Lam C.K., Liu J., and Tetzlaff W. (2011). Intermittent fasting improves functional recovery after rat thoracic contusion spinal cord injury. J. Neurotrauma 28, 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ramer L.M., Ramer M.S., and Bradbury E.J. (2014). Restoring function after spinal cord injury: towards clinical translation of experimental strategies. Lancet Neurol. 13, 1241–1256. [DOI] [PubMed] [Google Scholar]
- 65. Thibault-Halman G., Casha S., Singer S., and Christie S. (2011). Acute management of nutritional demands after spinal cord injury. J. Neurotrauma 28, 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barbosa-Silva M.C. (2008). Subjective and objective nutritional assessment methods: what do they really assess? Curr. Opin. Clin. Nutr. Metab. Care 11, 248–254. [DOI] [PubMed] [Google Scholar]
- 67. Engwall M., Fridh I., Bergbom I., and Lindahl B. (2014). Let there be light and darkness: findings from a prestudy concerning cycled light in the intensive care unit environment. Crit. Care Nurs. Q. 37, 273–298. [DOI] [PubMed] [Google Scholar]
- 68. Li S.Y., Wang T.J., Vivienne Wu S.F., Liang S.Y., and Tung H.H. (2011). Efficacy of controlling night-time noise and activities to improve patients' sleep quality in a surgical intensive care unit. J. Clin. Nurs. 20, 396–407. [DOI] [PubMed] [Google Scholar]
- 69. Stokkan K.A., Yamazaki S., Tei H., Sakaki Y., and Menaker M. (2001). Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493. [DOI] [PubMed] [Google Scholar]
- 70. Schroeder A.M., Truong D., Loh D.H., Jordan M.C., Roos K.P., and Colwell C.S. (2012). Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J. Physiol. 590, 6213–6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Youngstedt S.D., Kline C.E., Elliott J.A., Zielinski M.R., Devlin T.M., and Moore T.A. (2016). Circadian phase-shifting effects of bright light, exercise, and bright light + exercise. J. Circadian Rhythms 14, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.