Abstract
The study of the metastatic cascade has revealed the complexity of the process and the multiple cellular states that disseminated cancer cells must go through. The tumor microenvironment and in particular the extracellular matrix (ECM) plays an important role in regulating the transition from invasion, dormancy to ultimately proliferation during the metastatic cascade. The time delay from primary tumor detection to metastatic growth is regulated by a molecular program that maintains disseminated tumor cells in a non-proliferative, quiescence state known as tumor cell dormancy. Identifying dormant cells and their niches in vivo and how they transition to the proliferative state is an active area of investigation and novel approaches have been developed to track dormant cells during dissemination.
In this review, we highlight the latest research on the invasive nature of disseminated tumor cells and their link to dormancy programs. We also discuss the role of the ECM in sustaining dormant niches at distant sites.
Keywords: Dormancy, intravital imaging, metastasis, extracellular matrix, migration, invasion
Introduction
Metastasis is a complex multistep process during which disseminated tumor cells (DTCs) go through different cellular states (migratory, quiescent, proliferative) while transitioning through multiple tissue microenvironments (tumor extracellular matrix, blood vessels, lymphatic vessels) before seeding at a secondary organ (i.e. lungs, bone, brain, liver)1-3. Patients diagnosed with metastatic lesions have limited treatment options, which emphasizes the immediate need for new therapeutic strategies that can prevent metastatic progression before the lesions become uncontrollable4. In this regard, understanding the biology of DTCs will pave the way for defining strategies that could eliminate DTCs, the seeds of metastasis, and prevent their growth in other organs.
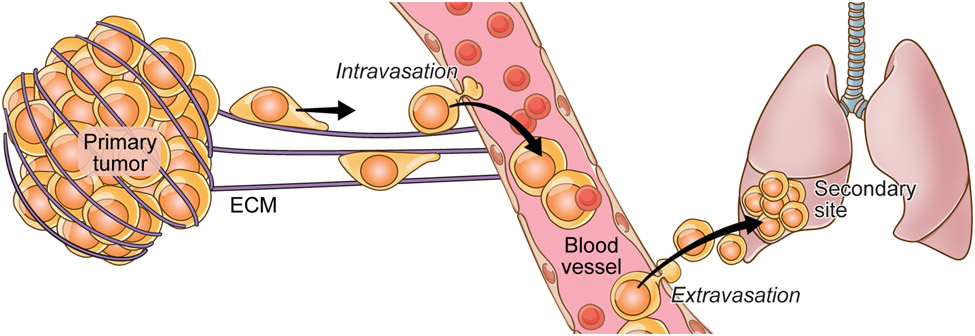
The early stages of metastatic progression can be divided into 1) migration/invasion into the stroma, 2) intravasation, 3) extravasation and 4) seeding (Figure 1). The acquisition of the invasive and migratory state by DTCs enables them to escape the primary tumor5 and has been well documented in breast6, melanoma7, and pancreatic8 tumors. While disseminating, DTCs have to overcome physical barriers, like the extracellular matrix (ECM) which plays an important role in all the metastatic stages from invasion to seeding9. DTCs need to degrade the ECM in order to invade the stroma, migrate along ECM to leave the tumors, and ultimately intravasate and extravasate by degrading the basement membrane along blood vessels.
Fig 1: The metastatic cascade.
Tumor cells leave the primary site of the tumor and intravasate into the neighboring vasculature followed by extravasation and eventual seeding at a secondary site. Several factors (extrinsic and intrinsic) regulate this process. The interaction between the tumor cell and its surrounding ECM at the primary site, vasculature, and secondary site primarily governs all the steps of this cascade.
Once DTCs leave the primary tumor, they must survive in circulation followed by extravasation at distant sites where they encounter a new and hostile microenvironment. This new metastatic microenvironment is very different from the site of primary tumors in composition, architecture, and tissue-resident cells. How do migratory DTCs survive in those organs before restoring growth? It has been documented that DTCs can enter a state of hibernation called tumor cell dormancy, during which their proliferation is interrupted.
While some tumors (e.g. lung) undergo rapid metastasis, others, such as breast and prostate, have a long metastatic latency. What is the underlying biology for this delay in metastatic growth? Can we take advantage of this time window to eliminate DTCs, the seeds of metastasis? Can metastasis be prevented by targeting DTCs? From a clinical perspective, preventing metastasis will be a better strategy than treating an already active metastasis given that very few treatment options are available for patients at that stage. But how can we prevent it? This question requires a deeper understanding of the biology of DTCs: 1) the mechanisms that govern their quiescence/non-proliferative cellular state, 2) the niches in which they reside and 3) the mechanisms that control their awakening.
In this review we will focus on the biology of DTCs, how they reach distant organs, how they control their fate and the importance of niches in which they reside with emphasis on the role that the ECM plays in sustaining their cell state. We will also discuss the different imaging approaches used to track various DTC states during metastasis.
Tumor mass dormancy and dormant disseminated single cancer cells
Dormancy (from Latin “dormire” or “to sleep”) is an evolutionary conserved mechanism that allow worms10 or plant seeds11 to enter a state of reversible growth arrest under unfavorable conditions. When conditions get better, growth is restored. Work done in Arabidopsis showed that dormancy is controlled by light, temperature, time of seed dry storage and genetic factors11. These studies in plants show how extrinsic and intrinsic factors can regulate the process of dormancy. Interestingly, there are a few parallels between seed dormancy and cancer cell dormancy and both processes share several aspects. As illustrated in the above example, DTCs too can enter a period of dormancy when seeding at distant sites, slowing growth and remaining in a non-proliferative state to overcome unfavorable conditions till better conditions favoring their reawakening and metastatic growth prevail. This tumor dormancy period is regulated by several extrinsic and intrinsic factors as described below.
Towards a definition of dormancy:
Rupert A. Willis in his seminal work “The Spread of Tumours in the Human Body” used the term dormancy for the first time to refer to cells that disseminate but are in growth arrest12. Later, in 1954, Hadfield published an article titled: “The dormant cancer cell” in which he referred to the dormant tumor cell as “malignant cells which, although remaining alive in the tissues for relatively long periods, show no evidence of multiplication during this time, yet retain all their former and vigorous capacity to multiply”13. In this publication, he alludes to some of the pressing questions like, 1) where do dormant cells reside? and 2) what are the factors inducing the growth of these dormant cells? Hadfield’s article stimulated a series of correspondence with other investigators in the British Medical Journal. In one of them, Worral stated that “The factors responsible for dormancy of cancer cells in situ as well as for restriction of tile growth of normal cells, must therefore be sought in the organism as a whoIe.” setting forth the relevance of the tissue microenvironment as a regulator of dormancy14. Work in the 50s and 60s showed the first clinical evidence of dormant tumors in humans. Early clinical studies described long remission times after primary tumor surgery for breast tumors15, suggesting that dormancy may have occurred in these tumors. Autopsies of seemingly healthy individuals uncovered small tumors in prostate16, thyroid17 and brain 18 revealing cells that did not develop into larger tumors by possibly entering some kind of pause that restricted their growth in these patients.
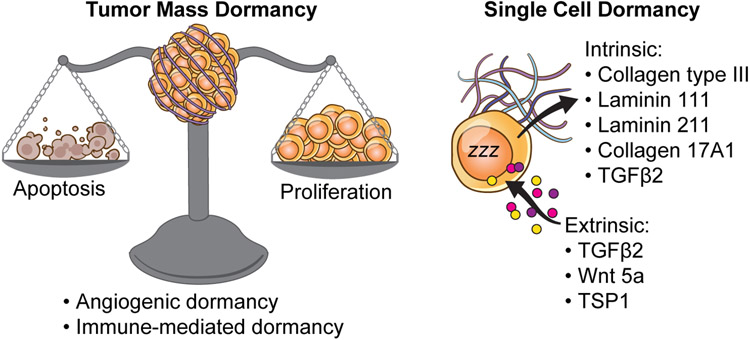
Work from multiple labs in the last 60 years have contributed to defining the hallmarks of dormancy, where dormant cells reside, and what drives their awakening. However, when we define tumor dormancy, we must make a distinction between two different types of dormancy (Figure 2):
Fig 2: Tumor mass dormancy and Single-cell dormancy.
Dormancy can imply one of the two situations shown here. Tumor cell dormancy is when there is a balance between cell proliferation and cell death such that the net change is zero. Factors governing tumor mass dormancy include an impaired angiogenic response (angiogenic dormancy) and a heightened cytotoxic immune response (immune-mediated dormancy). Single-cell dormancy on the other hand is marked by an arrest in proliferation and is a state of “hibernation” that the tumor cell adapts and escapes chemotherapeutic drugs and immunosurveillance. This is governed by several extrinsic and intrinsic factors. These include factors such as TGFβ2, WNT5a, several Laminins and Collagens.
1) Tumor mass dormancy, wherein metastatic outgrowth is suppressed by a balance of proliferation and apoptosis19-22, a concept first proposed in the 70s by Judah Folkman. This equilibrium can be achieved by angiogenic dormancy or immunosurveillance. Angiogenic dormancy refers to an impaired angiogenic response that keeps the tumor mass constant by balancing between proliferation and apoptosis19. Dormancy in this case is mainly attributed to both oxygen and nutrient deprivation23. Studies have shown that tumor cells need to undergo an “angiogenic switch” to be able to break from this regime of dormancy24,25. This switch can either be mediated by tumor intrinsic factors or by factors secreted by the stromal cells and tumor-associated 26-28. This in turn has led to the development and use of several anti-angiogenic drugs for treating tumors. On the other hand, during immune-mediated dormancy, effector T-cells, that are CD8+ and CD4+, mediate their effect via IFNγ and TNFα signaling and maintain the tumor cells in a state of dormancy29-34. Apart from this cytotoxic immune response that keeps the tumor mass at equilibrium, these CD8+/CD4+ T-cells are also known to release anti-angiogenic chemokines such as CXCL9/10 that downregulates angiogenesis and induces tumor dormancy35,36.
2) Cellular dormancy is defined as a reversible growth arrest (G0/G1) for DTCs 37,38 and refers solitary DTCs as well as DTC clusters of 10-20 cells. In this situation, cancer cells achieve dormancy by undergoing prolonged growth arrest without an increase in cell death.
For the purpose of this review, we will focus on the cellular dormancy of DTCs.
Are highly migratory cancer cells dormant DTCs?
When analyzing the steps of the metastatic cascade and the acquisition of a dormant state two possible scenarios can be considered: 1) cancer cells are reprogrammed into dormancy in the primary tumor itself and carry their phenotype to the secondary organs while disseminating or, 2) the metastatic microenvironment shapes the state of DTCs to become dormant. Below we summarize the studies that suggest that both these scenarios may co-exist.
The definition of dormancy by Willis combines two important cellular features: a) dissemination, meaning the ability of cancer cells to move away from the tumors, and b) growth arrest; (“…cells that disseminate but are in growth arrest”)12. Work by Raviraj et al., proposed that solitary cancer cells located at the perimeter of breast tumors are invasive and dormant39. By using laser microdissection technology, the authors profiled these cells and found that they are enriched in invasive (N-Wasp, WAVE, ROCK1, fascin) and anti-proliferative markers (p21, p27). They used in vitro models where cancer cells were embedded in dense collagen matrices to mimic the densities found in tumors of breast cancer patients. In these in vitro models they found that breast cancer cells became migratory, and growth was arrested in G0/G1 in a p27-dependent manner. Recent work by the Gligorijevic lab showed that matrix degradation through membrane degrading protrusion (invadopodia) is enhanced when breast cancer cells are in G1 suggesting that invasion is coupled with cell cycle progression40. Analysis of gene expression in several patient-derived xenograft models of prostate cancer cells established from bone metastasis also revealed that genes associated with cellular movement (CDC42, MYLK) were increased in dormant cells when compared with proliferative ones41 (Figure 3a).
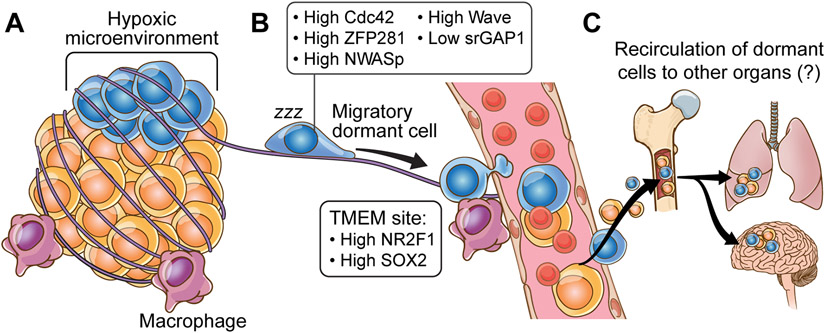
Fig 3: Salient features of dormancy.
A) The microenvironment of the primary site dictates the fate of the DTCs. Recent studies have helmed hypoxia as one such factor that regulates dormancy programs in the cells that eventually become DTCs and are characterized by changes in expression levels of Cdc42, ZFP281, NWASp, Wave, srGAP1 among others. B) Dynamic tripartite structure, known as “TMEM doorways” comprising of disseminated tumor cells, tumor-associated macrophages, and endothelial cells are at the core of the metastatic cascade. At these doorways, tumor cells have been profiled to have high levels of NR2F1 and SOX2, both of which are known mediators of cellular dormancy. C) Reseeding of cancer cells to tertiary organs is at the forefront of many metastasis studies. Research indicates that certain organs e.g., the bone marrow, can potentially act as reservoirs from where DTCs can spread to tertiary organs such as the lung and brain.
Evidence of early dissemination in breast tumors have been demonstrated and suggest that a highly motile population of cells is present in the early lesion and is capable of becoming dormant at distant sites, such as the bone marrow6,42. Similar results were reported using intravital imaging in a HER2+ mouse model, that showed dissemination of breast cancer cells in early lesions and intravasation of highly motile cells into the blood vessels 42,43. In this study43, HER2 expression activates programs of stemness, motility and invasion supporting the theory of early dissemination. Most of the early DTCs become dormant when they reach the lung supporting the notion that early DTCs are more prone to enter dormancy.
Can primary tumor microenvironments invigorate dormant DTCs?
In mouse models of breast cancer, analysis of hypoxic microenvironments within primary tumors revealed an increase in dormancy markers. Work by Fluegen et al., showed that hypoxic shock results in increased expression of dormancy markers in tumor cells 44. This study showed that hypoxic microenvironments within the primary tumors give rise to dormant cells and suggest that hypoxic DTCs may induce disease relapse (Figure 3a). By using an implantable microfabricated device to induce hypoxia in tumors (NANVID) the authors show that upon hypoxia induction, dormancy markers are elevated. These post-hypoxic cancer cells, when delivered intravenously into mice, became dormant at metastatic sites suggesting that reprogramming by hypoxic microenvironments trigger a dormancy program in these cells.
Work by Borriello et al., also showed that primary tumor microenvironments “prime” certain subpopulations of tumor cells to express dormancy markers45. By using breast cancer cells and intravital two-photon microscopy, the authors showed that spontaneous DTCs from mammary tumors express the dormancy markers NR2F1 and Sox9. Briefly, in patient samples from breast tumors, dormancy markers are associated with intravasation areas or TMEMs (tumor microenvironment of metastasis). TMEMs are formed by tumor cells, macrophages and endothelial cells and are defined as the ”doorways” for tumor cell intravasation46-48. The cancer cells at TMEM are positive for dormancy markers such as NR2F1. Moreover, macrophages can upregulate this expression of NR2F1 in tumor cells suggesting that the interaction with macrophages at TMEM sites may prime DTC dormancy. This study suggests that dormancy and dissemination phenotypes can be carried by DTCs from the primary tumor and migratory cancer cells can acquire programs of dormancy at the primary site that can impact metastasis progression (Figure 3b).
A recent study in breast tumors by Mondal et al.,49 suggested that highly motile cancer cells with low levels of srGAP1, a GTPase regulator, may be more prone to enter dormancy. In this study, the authors identified a population of srGAP1low cells within breast tumors that displayed a highly motile phenotype and entered dormancy when seeding at the lung through the upregulation of TGFβ2 secretion. The authors showed that TGFβ2 is not only important for intravasation, through regulation of matrix degradation by invadopodia, but also for establishing a dormancy niche at the lungs. They also reported that srGAP1low cells remain as single cells or small clusters upon dissemination at the lung and have increased dormancy markers such as p27.
Are dormant DTCs motile at metastatic sites?
Although there are some evidences showing that dormant DTCs are motile at distant sites, we don’t yet have a clear understanding on how motility pathways are regulated in dormant DTCs at distant sites. Work by Winker lab showed that dormant melanoma cells in the brain are highly motile and invade along pre-existing blood vessels50. Additionally, work by Sipkins lab showed that dormant breast cancer cells in the bone marrow can extravasate and enter into circulation without losing the dormant phenotype51. In this study, by using in vivo imaging, the authors showed that breast cancer dormant cells reside in E-selectin and SDF-1-rich perisinusoidal vascular regions and inhibition of E-selectin or CXCR4 receptor mobilizes these dormant cells into the circulation (Figure 3c). The authors suggest that mobilization of dormant cells from the niche may trigger apoptosis in these cells if the separation from the niche is prolonged and may result in elimination of dormant cells.
Motility and metastasis cross-seeding:
Analysis of metastatic lesions of prostate52,53, breast54 and ovarian55 cancer patients revealed that metastases are formed by multiple cells through reseeding from other metastatic sites. This process is known as metastatic cross-seeding or metastasis-to-metastasis seeding.
In the 70s Hoover and Ketcham probed metastasis-to-metastasis seeding by a technique known as parabiosis in which two mice are surgically joined which results in the development of a shared circulatory system. Hoover and Ketcham showed that by parabiosis, if one of the mice (the host) develops pulmonary metastasis from a primary sarcoma (that was eventually removed), the non-tumor bearing (guest) mouse eventually develops lung metastasis56. Work by Dr. Joshua Fidler also showed that cancer cells can recirculate from metastasis lesion in melanoma models57. In his studies, mice were inoculated intravenously with cancer cells followed by parabiosis a week later. The mice were eventually separated and the authors showed that the guest mouse developed metastasis in the lungs. Since cancer cells are eliminated from circulation one week after intravenous injection these results show that melanoma cells may have traveled from one metastatic organ of the host mice to another organ in the guest mouse.
Evidences supporting metastasis cross-seeding in humans has been documented in the context of organ transplant. Clinical studies have shown that recipients of organ transplant may develop multiple metastasis58. These transplanted organs may harbor DTCs that upon immunosuppressive treatment undergo reactivation in the recipients and has potential to metastasize to other organs in the body59.
Recently, intravital imaging has shed some light on the mechanisms of metastasis-to-metastasis seeding. By using microscopy techniques and photoconvertible fluorescent proteins it was shown that cancer cells in the lung60 or the lymph nodes61-63 can reseed to other metastatic organs. Borriello et al.,60 used a lung metastatic model consisting of cancer cells expressing Dendra2 photoconvertible fluorescent protein. Post tail-vein injection, these cells were allowed to form lung metastasis followed by photoconversion from green to red. On probing few weeks post-photoconversion, these red cells were found to be present at secondary sites such as bone and liver providing proof of recirculation of DTCs from lung metastasis.
The idea of metastasis reseeding hints at the possibility that certain organs could be a reservoir for dormant cells for further dissemination (Figure 3c). Several studies have reported that organs such as the bone marrow where dormant cells are found42,64,65 act as stores of dormant DTCs. If these niches are reservoirs for migratory dormant DTCs, we can speculate that dormant cells can travel and populate other organs from the bone marrow early during primary tumor development and also after primary tumor has been treated. Although this hypothesis hasn’t been proved there is evidentiary support for reseeding of cancer cells from the bone marrow to other organs. For example, in breast tumors, Zhang et al., showed that the bone marrow microenvironment invigorates metastatic cells for further seeding at distant sites by epigenetic reprogramming of cancer cells seeded in the bone through EZH2 activity66. In another study, Bacelli et al., showed, by using a patient derived CTC (circulating tumor cells) xenograft assay, that tumor cells from breast cancer patients injected in the femoral medullar cavity of mice develop metastasis in lungs, liver and other bones after a dormancy period. This study supports the idea that the bone marrow microenvironment serves as a niche to promote further dissemination of breast cancer cells67.
These studies highlight a signaling program that links migration and invasion with dormancy and suggest that the ability of cancer cells to invade and migrate may be part of a sophisticated epigenetic program that favors dormancy upon dissemination.
Adapting to the niche: Sensing the ECM and the implications for DTCs fate
Given the importance of ECM at every stage of cancer cell dissemination, it is not surprising that it is crucial in regulating the fate of DTCs. The ECM is the structural component of the tumor microenvironment that constantly undergoes remodeling (degradation, change in composition, etc) and several studies have demonstrated the dynamic interaction of ECM with DTCs. The ECM is comprised of fibrous proteins (such as collagen), proteoglycans (glycosylated proteins that are covalently linked to glucosaminoglycans), glycoproteins (such as Laminin, fibronectin, etc), and polysaccharides (such as hyaluronan). Over the years several studies have focused on the effects of ECM stiffness and its role in mediating the regulation of cell fate choices68-70.
What causes changes in stiffness:
Multiple factors regulate the stiffness of the ECM, and these range from changes in the composition of the ECM (eg. increase in the amount of Collagen I in ECM of certain tumor tissues) to changes in the alignment of the fibrillar collagens and fibronectin71,72 . Another crucial factor contributing to ECM stiffness is the amount of water which is determined by the hydration level of the proteoglycans and hyaluronan.
How is stiffness sensed by cells:
The different ways cells sense the stiffness of their surrounding tissue (mechanosensing) and the underlying biochemical pathway (mechanotransduction)73,74 have been studied extensively. The first step of mechanotransduction is the sensing of the ECM by the transmembrane integrin receptors. These receptors form the connection between the inside and the outside of the cell and mediate their effect through focal adhesions (FAs). FAs act as signaling hubs and are critical for the transmission of force information through their association with the cytoskeleton. Upon binding with the ECM, the integrin receptors form clusters and lead to the formation of nascent FAs on the inside. FAs are composed of proteins (such as focal adhesion kinase, talin, paxillin, vinculin, etc.) that contain binding domains for both integrins and actins and thus play an important role in relaying information from integrins to F-actin. The mechanism through which integrins bind the ECM has been well documented75. Non-muscle myosin II (NMII) present on the F-actin-bundles together forms actomyosin stress fibers, and these stress fibers are involved in the continuous probing of the visco-elastic properties of the ECM. Depending on the stiffness of the substrate, contractile forces lead to the maturation of the FAs and the further assembly of the actomyosin stress fibers. The RhoGTPase pathway is crucial for mechanosensing since it controls the interaction of NMII and the actin filament. Briefly, Rho A regulates the phosphorylation of the regulatory myosin light chain via Rho kinase (ROCK). ROCK directly phosphorylates the myosin light chain as well as inhibits the myosin light chain phosphatase. The phosphorylation of the regulatory myosin light chain increases the bundling of the actin filaments and generates more contractile forces. In addition, ROCK also inhibits the actin severing protein Cofilin (via LIM kinase) thereby stabilizing actin filaments76,77.
Apart from integrins, the other major class of receptors involved in sensing stiffness changes in the ECM are the Piezo1/2 ion channels. These mechanically activated ion channels were discovered only a decade ago and since then have been studied extensively78. The Piezo1 channel is expressed mostly in non-neuronal cells and is involved in mechanosensing/mechanotransduction while Piezo2 is expressed in sensory neurons and specialized cells, and is involved in nociception79-81. The common players in the mechanotransduction pathway activated by the Piezo1 channel include GPCRs (Gq/G11), cAMP, PI3K/Akt, and NFkB which ultimately results in transcriptional changes in the nucleus. While earlier reports suggested a role of Piezo1 in mediating lineage choice in human neural stem cells82, recent studies on Piezo1 focus on its role in the differentiation of bone tissue from mesenchymal stem cells83. Additionally, in endothelial cells Piezo1 plays important role in the transmission of shear stress and stretch signals as experienced by the cells of the blood vessel wall and in mediating iron homeostasis by blood cells84. It is also involved in regulating pulmonary circulation, in modulating bladder volume and osmoregulation in the urinary tract85,86 as well as differentiation of brown adipocytes87. Interestingly, loss of Piezo1 activity increases migration in non-small cell lung cancer and breast cancer. Despite the lack of direct evidence for the role of the Piezo1 channel in dormancy or reawakening of dormant DTCs, the critical contribution of Piezo 1 in determining cell fate choices warrants further investigation into its role in dormancy reversal/reawakening.
What happens once the mechanotransduction pathways are activated:
The visco-elastic properties of the ECM as determined by the actomyosin filaments are directly communicated to the nucleus through the LINC (linker of the nucleoskeleton and cytoskeleton) complex. It comprises of two distinct transmembrane proteins; KASH domain protein in the outer nuclear membrane and SUN domain protein in the inner nuclear membrane. In this arrangement, the KASH proteins interact directly with the actomyosin filaments, microtubules, and intermediate filaments (through nesprin proteins) whereas the SUN proteins interact with the nuclear lamina (via emerin protein)88,89. Together these domains control a myriad of functions ranging from the shape of the nucleus to the chromatin organization as well as the epigenetic regulation of gene expression90-93. Recent studies on the LINC complex components have shown that they play an important role in the differentiation of mesenchymal stem cells (MSCs) for e.g. the control of nuclear positioning by the action of nesprin-1 is critical for myogenic differentiation. Additionally, the adipogenic differentiation of MSCs via the localization of beta-catenin is mediated by the components of the LINC complex and nuclear envelope proteins. These and other similar studies suggest a central role for the LINC complex in mediating the effects of ECM stiffness in determining cell fate which could be pertinent to dormancy and reawakening of the dormant DTCs.
Nucleo-cytoplasmic shuttling of TFs on mechanical cues and epigenetic regulation of cell fate:
Apart from transmitting ECM stiffness information directly to the nucleus, the actomyosin cytoskeletal-Rho GTPase mechanotransduction pathway is also crucial for the nucleocytoplasmic shuttling of transcription factors downstream of force sensing94-96. The transcription factors that are widely studied in regards to mechanosensing are (i) the Myocardin-related Transcription Factor (MRTF) which upon actomyosin filament formation translocates to the nucleus and binds to its cognate partner Serum Response Factor (SRF), and (ii) the Yes-associated protein (YAP) and Transcriptional activator with PDZ-motif (TAZ)97,98. Both these families of transcription factors accumulate in the nucleus on activation and promote changes in gene expression by regulating the epigenetic landscape of the cells. Interestingly, a recent study by Speight et al. 99 showed the context-dependent interaction between MRTF and TAZ and reported that MRTF regulates the expression, cytoplasmic retention as well as nuclear mobility of TAZ depending on whether the signal is purely mechanical or mechano-chemical (involving TGFβ signaling). This regulation affects transcriptional programs important for EMT, wound healing, and fibrogenesis. Another elegant study by Joan Massague and colleagues100 showed that DTCs in order to colonize novel perivascular niches express L1CAM (an adhesion molecule expressed by the resident pericytes) which in turn activates YAP and MRTF. This activation of YAP and MRTF is crucial for subsequent growth of the DTCs and is also independent of the canonical Hippo pathway for YAP activation suggesting a critical and exclusive role for mechanosensing by the DTCs for their growth at a secondary site. Apart from survival and growth, MRTF-mediated stiffening of the nucleus, known as mechanosurveillance, rendered human melanoma cells vulnerable to cytotoxic T lymphocytes and NK cells101. Thus, there is enough evidence indicating that ECM stiffness controls a multitude of factors, which affect the survival of DTCs.
Comparison of the epigenetic landscape of cancer cells and cells from healthy tissue reveal key differences in the status of DNA methylation of promoter regions of key transcription factors, histone modifications, and chromatin organization, and these changes are known to be important for tumorigenesis102-105. Interestingly, research from the group of Ovijit Chaudhuri 106,107 revealed that ECM stiffness facilitates specific epigenomic changes such as increased chromatin accessibility which allows the exposed sites to bind to transcription factors (such as Sp1) and activate histone modification enzymes (such as HDACs) in 3D models of breast cancer. Another study by Jang et al.,108 showed that changing stiffness, by controlling alginate/Col I, changes the methylation status of the YAP promoter region and thereby controls YAP expression by a feedback loop. These studies, therefore, point to a very specialized form of ECM stiffness-regulated control of cancer cell fate. A recent review109 summarizes the mechanical regulation of cell cycle progression and cell division and demonstrates the importance of ECM stiffness and cellular mechanotransduction in governing cell fate choices. With regards to the epigenetic status of DTCs, work by Sosa et al.,110 and others111 revealed that the dormancy of DTCs is mediated by Nr2F/SMAD4 which ensures that DTCs remain in a quiescent state. ECM stiffness also controls dormancy of cancer cells. Combining these two parallel bodies of research on epigenetics in maintaining dormancy and stiffness-mediated control of cell fate, Liu et al showed that increase in stiffness results in translocation of the GTPase CDC42 into the nucleus promoting Tet2 expression, and leads to the hydroxymethylation of p21 and p71, ultimately driving melanoma cells into dormancy112
Establishing an ECM-niche supportive of dormancy
Determining where DTCs reside is important for understanding their behavior in metastatic tissues. The concept of niche (from French, nicher or “to rest”) was first used by Schofield in 1978 as a hypothesis to define the immortality of stem cells and the importance of their association with other cells to determine stem cell behavior. In this classical sense, the niche regulates the stem population of a tissue, its maturation and proliferation113.
A dormant niche can be defined as a physical space in which dormant cells reside and the niche itself contributes to sustain their quiescence state. Within the niche there are two components: 1) a cellular component including tissue resident cells that establishes contact with dormant cells and 2) an acellular component or the ECM. While both these components play an important role in influencing the behavior and fate of DTCs, in the next section we look beyond the stiffness-centric role of ECM in in regulating DTC behavior at metastatic sites.
The collection of ECM and ECM-related proteins is referred to as the matrisome and include two categories of proteins: core matrisome proteins (collagen, proteoglycans and glypotroteins) and matrisome-associated proteins (including secreted factors, ECM-regulators and ECM-affiliated molecules114-116. The assembly and remodeling of the ECM plays an important role is sustaining the dormant niche and is described below.
Early work by Ossowski and colleagues highlighted the importance of looking beyond the stiffness-centric role of integrin-ECM interactions in dormancy. By using head and neck cancer models they showed that cancer cells with low uPAR levels are incapable of properly interacting with the ECM and fail to activate proliferative signals, thus entering dormancy. On the contrary, interaction of uPAR with α5β1 integrin promotes binding to FN and activates ERK signaling, thereby triggering proliferation. Assembly of FN by uPARhigh cells suppress p38 activity whereas uPAR low cells have low ERK activity and cannot support assembly of FN fibrils117,118. This seminal work was the first proof of concept showcasing how tumor cell/ECM interaction controls tumor dormancy. Recent work on ECM has strengthened these ideas on the importance of other ECM molecules in regulating dormancy.
Collagens and dormancy:
Work by di Martino et al., using head and neck, and breast cancer models, identified Collagen type III as an important component of a self-assembled ECM that sustains the dormant state119. In this study, the authors showed that dormant cells establish a Type III collagen-rich niche that sustains dormancy through a DDR1/Stat1 signaling pathway. Other studies have also identified Collagen III as an important part of the dormancy program. Work by Sahai lab showed that in the lungs, AT1 cells are involved in sustaining dormancy of the disseminated breast cancer cells. The authors showed that interaction with AT1, among other things, triggers the expression of COL3A1 in breast cancer cells120. Recent work by Aguirre-Ghiso lab shows that early DTCs in a HER2+ breast cancer model overexpressed Collagen type III121 and work by Berskin lab identified Collagen III as a dormancy marker in ER+ tumors122. All these studies highlight the importance of COL3A1 as a novel dormancy regulator. Interestingly, early work on cell cycle arrest show that Collagen Type III is one of the genes induced when fibroblast enter in G0. In a study by Coppock et al.,123 the concept of quiescin is used to refer to the set of genes induced during quiescence and provide the first evidence of a collagen gene linked to quiescence. Similarly, we propose the term ECM-quiesces to refer to ECM molecules that regulate dormancy.
Apart from mediating dormancy, collagens also regulate the reawakening of dormant cells. Work by the Green lab showed that fibrotic environments enriched in Collagen I trigger awakening of dormant cells through remodeling of the actin cytoskeleton and induction of stress fiber formation. Collagen I leads signaling through integrin β1 induces phosphorylation of MLC by MLCK124. These results connect the previous hypothesis that the molecular programs of dormancy/awakening are linked to changes in migratory and invasive properties driven by actin cytoskeleton remodeling. Showcasing the importance of COL17A , Otha et al., identified a population of LRG5+ cancer stem cells that display dormancy and persist during chemotherapy in colon cancer models125. These LRG5+ cells overexpress COL17A1, and knockdown of COL17A1 eliminates the LRG5+ dormant cells, rendering the tumors sensitive to chemotherapy. Consequently, disruption of COL17A1 during chemotherapy breaks the dormancy of LRG5+ cells through activation of FAK/YAP.
Laminins and dormancy:
Many studies have reported the contribution of laminins in sustaining dormancy. For e.g. Albrengues et al., showed that remodeling of the lung ECM by neutrophils during inflammation triggers reactivation of dormant cells through MMP9 activation126. The MMP cleavage of Laminin-111 activates α3β1 signaling and triggers tumor dormant cell awakening. Another recent study on brain tumor dormancy demonstrated that Laminin 211 secreted by astrocytes regulates breast cancer dormancy in the brain69.
Matrisome-associated proteins and dormancy:
Work by the Aguirre-Ghiso lab identified TGFβ2, an ECM associated molecule, as a regulator of tumor dormancy64. Secretion of TGFβ2 by dormant cells can drive cancer cells into dormancy through TGFβRIII and increased expression of p27 through SMAD2 signaling. Also, In the bone marrow, Nestin+ MSCs regulate DTC dormancy through TGFβ2 secretion65. Another study that showcases the importance of matrisome-associated proteins as prime regulators of DTC dormancy uses an elegant organotypic microvascular niches to recapitulate bone and lung microenvironment. In doing so it was revealed that deposition of TSP-1 by the endothelial cells can sustain quiescence of DTCs127. Wnt, another secreted factor belonging to the class of matrisome-associated proteins has also been linked to dormancy. A seminal study by the Weeraratna lab showed that the aged lung microenvironment facilitates the growth of dormant DTCs128. Aged lung fibroblasts secrete the WNT5a inhibitor sFRP1, inhibiting WNT5a in melanoma cells and enabling metastatic growth.
A recent review by Elkholi et al.,129 summarizes different organ-specific mechanisms regulating tumor cell dormancy in the context of breast cancer. For example, in the bone marrow niche, a dormancy-permissive organ130 and a frequent site of metastasis for breast cancer, osteoblasts can promote dormancy while osteoclasts can drive reactivation131.
Imaging tools to detect DTCs in vivo
Different imaging techniques ranging from intravital multiphoton imaging (IVI) to whole animal imaging (MRI) have been used to study different stages of tumor progression. In the laboratory, pre-clinical and clinical settings these imaging techniques are widely used to understand the biology of tumor cells in their native environment and for diagnosis in cancer patients.
While MRI is routinely used to detect tumors and metastasis, its application in the context of single cell biology has not been extensively explored132-134. In a study by Heyn et al., the authors showed the potential of MRI to track breast cancer cells in a mouse model of brain metastasis at single cell resolution132. In this study, tumor cells were labeled with a fluorescently-tagged 0.9μm polystyrene MPIOs (MRI contrast agents, micron-sized iron oxide particles) with a labeling efficiency of almost 100%. Labeling tumor cells with MPIOs was shown to not affect cell proliferation or metastatic efficiency132and the authors were able to longitudinally track the growth of a single MPIO-labeled MDA-MB-231BR/EGFP cell into a tumor. From a clinical perspective it will be beneficial to develop tools that allow the detection of dormant cells in patients who have undergone primary tumor surgery and therapy, which could potentially guide treatment decision and allow more control on disease progression.
In mouse models, intravital imaging has been extensively used to understand the behavior of tumor cells within tumors of breast, pancreas, glioblastoma as well as lung, liver and lung metastasis135. Intravital two photon microscopy has also been used to determine changes in the ECM during dormancy and awakening119 by utilizing the second harmonic signature of ECM to study the tumor vasculature136. This imaging technology has also been applied to cancer patients to study blood vessels during tumorigenesis137.
To identify dormant disseminated tumor cells in vivo, several techniques are in place. The pulse-chase method is the oldest and one of the most widely used methods for identifying quiescent/slow-cycling stem cells from a mixed population (comprising both proliferative and quiescent cells). These experiments use nucleus-labeling dyes such as tritiated thymidine or BrdU (5-Bromo-2’-deoxyuridine) and cells are “pulsed” with the dye for a specified period followed by a “chase” phase. During this chase phase, the proliferative cells lose the dye as they undergo successive rounds of division, and the quiescent cells retain the dyes thus lending them the name “label-retaining cells”. Other dye-based methods used to study slow-cycling cells include membrane labeling dyes such as DiI and PKH67 that do not require cell permeabilization (as in the case of BrdU)138-141.
While dye-based methods are popular, there are issues with non-specific labeling and the inadvertent phagocytosis by neighboring cells. Thus, several fluorescent protein-based reporter systems, that are an improvement over conventional dye-based systems, have been developed and are being used to identify cells in a particular phase of the cell cycle. These consist of fluorescent protein fusions of either signaling proteins that regulate cell proliferation such as ERK, p3865,142,143 or proteins that are directly involved in the cell cycle such as the FUCCI system which identifies the phase of cell cycle based on the selective proteolysis of Geminin and Cdt1 proteins at different stages of the cell cycle144-146. Along the same line a p27 sensor has been developed by the same group which uses mVenus fused to a mutant version of p27 that lacks binding capabilities to cyclin-CDK but itself gets degraded normally as the cell cycle progresses147. Another example of fluorescent protein-based cell cycle reporter system is the CDK2 activity sensor developed by the Tobias Meyer group. The CDK2 activity sensor uses the nucleo-cytoplasmic shuttling of a chimeric DHB-venus construct as a proxy for CDK2 activity which in turn serves as a useful tool to identify specific stages of the cell-cycle148. This sensor was recently used in two recent studies to successfully identify dormant cells in vivo49,119 highlighting the robustness of cell-cycle reporter systems in identifying dormant DTCs.
The newest addition to this repertoire of sensors for identifying dormant cells is OSCAR. OSCAR is a ratiometric sensor comprising of WT mCherry and chimeric mVenus (VEN90, peptide seq NPKATPPQI inserted at 90thposition of mVenus) in tandem. This construct allows fluorescence of Ven90 to be dependent on phosphorylation of the peptide sequence by CDK9 thereby making identification of cells which are either transcriptionally high or low (dormant cells)149 plausible.
Clinical relevance of DTCs profiling
While imaging tools as described above remain the method of choice to detect dormant disseminated cells across different cancer types, several groups are working towards developing biomarkers that can be used to detect early DTCs in secondary sites as well as in the primary tumors. These studies use single cell RNA sequencing (scRNA seq) methods to identify rare DTCs and profile the expression of markers that can be used in the future for detection and isolation of DTCs. Some of the genes that have been associated with dormancy include tumor-intrinsic Type-1 IFN identified in prostate cancer cells150, AXL genes identified in myeloma cells151 and NR2F1, Cfh, Ogn, Gas6 and Mme in breast cancer cells from metastatic organs like the lungs and bone42,152. The ultimate goal of these studies is to determine genes that are important in controlling different aspects of cancer cell dormancy and use these for designing drugs that can be tested in clinical trials. This aspect of dormancy study has been discussed in a recent piece by Julio Aguirre-Ghiso, wherein he describes the current state of clinical trials for drugs targeting dormancy153. Another recent review154 describes the metabolic features of dormant tumor cells and provides an overview of the state of drug development/clinical trials for drugs targeting crucial metabolic pathways.
Apart from developing tools for identifying DTCs, a constant effort is ongoing to better characterize both the primary tumor and metastases. Case in point is a recent study155 aimed at identifying the molecular features associated with metastatic breast cancer using a multi-omic approach. By analyzing RNA seq, tumor/germline exome seq, whole genome seq and global methylation patterns of both primary and matched metastatic tumors, the study was able to pick out genetic and epigenetic changes that shows how tumors evolve. On similar lines, there are efforts focused on understanding the diversity in the tumor microenvironment especially with regards to immune cells using metabolomic, epigenomic, transcriptomic and proteomic approaches156,157. These approaches are sure to yield useful information that can ultimately be used to improve the prognosis of the disease in patients and thereby improve their quality of life.
Conclusions
In this review we have provided an overview of the recent tumor dormancy literature as well as some of the seminal concepts and studies that shaped the field. As we explore more the biology of dormant cells, new features and regulators are described and new opportunities for targeting these cells could be developed. Understanding the dormancy niche as well as how migratory and dormancy programs are regulated in DTCs will bring forth new targets that may allow us to eliminate these rare cells. Importantly, the use of in vivo imaging technology coupled with fluorescence reporters is a very exciting avenue to understand how dormant cells evolve and detect their vulnerabilities in a physiologically relevant setting.
Acknowledgments
We would like to thank Jill Gregory for her illustrations in Figures 1, 2 and 3 and Swagata Basu for critical reading and editing of the manuscript.
This work was supported by an NCI R01 (CA244780), NCI R03 (CA270679), the Irma T. Hirschl Trust, the Emerging Leader Award from the Mark Foundation (to J.J.B.C) and the Tisch Cancer Institute NIH Cancer Center grant (P30 CA196521).
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
REFERENCES
- 1.Massagué J & Obenauf AC Metastatic colonization by circulating tumour cells. Nature 529, 298–306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quail DF & Joyce JA Microenvironmental regulation of tumor progression and metastasis. Nat. Med 19, 1423–1437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bravo-Cordero JJ, Hodgson L & Condeelis J Directed cell invasion and migration during metastasis. Curr. Opin. Cell Biol 24, 277–283 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganesh K & Massagué J Targeting metastatic cancer. Nat. Med 27, 34–44 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mondal C, Di Martino JS & Bravo-Cordero JJ Actin dynamics during tumor cell dissemination. Int. Rev. Cell Mol. Biol 360, 65–98 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosseini H et al. Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werner-Klein M et al. Genetic alterations driving metastatic colony formation are acquired outside of the primary tumour in melanoma. Nat. Commun 9, 595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray A et al. Stromal architecture directs early dissemination in pancreatic ductal adenocarcinoma. JCI insight 7, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kai FB, Drain AP & Weaver VM The Extracellular Matrix Modulates the Metastatic Journey. Dev. Cell 49, 332–346 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J & Kim SK Global analysis of dauer gene expression in Caenorhabditis elegans. Development 130, 1621–1634 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Koornneef M, Bentsink L & Hilhorst H Seed dormancy and germination. Curr. Opin. Plant Biol 5, 33–36 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Willis RA The Spread of Tumours in the Human Body. (J. A. Churchill) (1934). [Google Scholar]
- 13.HADFIELD G The dormant cancer cell. Br. Med. J 2, 607–610 (1954). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worrall R The dormant Cancer Cell ( Correspondence). Br. Med. J 2, 813 (1954). [Google Scholar]
- 15.Bryant T AN ANALYSIS OF FORTY-SIX CASES OF CANCER OF THE BREAST WHICH HAVE BEEN OPERATED UPON AND SURVIVED THE OPERATION FROM 5 TO 32 YEARS, WITH REMARKS UPON THE TREATMENT OF RECURRENT GROWTHS, INCLUDING THE DISEASE OF THE SECOND BREAST, OPERATIVE AND OTHERWISE. Br. Med. J 1, 1200–1203 (1902). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashley DJ On the incidence of carcinoma of the prostate. J. Pathol. Bacteriol 90, 217–224 (1965). [DOI] [PubMed] [Google Scholar]
- 17.MORTENSEN JD, WOOLNER LB & BENNETT WA Gross and microscopic findings in clinically normal thyroid glands. J. Clin. Endocrinol. Metab 15, 1270–1280 (1955). [DOI] [PubMed] [Google Scholar]
- 18.BECKWITH JB & PERRIN EV IN SITU NEUROBLASTOMAS: A CONTRIBUTION TO THE NATURAL HISTORY OF NEURAL CREST TUMORS. Am. J. Pathol 43, 1089–1104 (1963). [PMC free article] [PubMed] [Google Scholar]
- 19.Holmgren L, O’Reilly MS & Folkman J Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med 1, 149–153 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Townson JL & Chambers AF Dormancy of solitary metastatic cells. Cell Cycle 5, 1744–1750 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Yeh AC & Ramaswamy S Mechanisms of Cancer Cell Dormancy--Another Hallmark of Cancer? Cancer Res. 75, 5014–5022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phan TG & Croucher PI The dormant cancer cell life cycle. Nat. Rev. Cancer 20, 398–411 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Naumov GN, Folkman J & Straume O Tumor dormancy due to failure of angiogenesis: role of the microenvironment. Clin. Exp. Metastasis 26, 51–60 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Naumov GN, Akslen LA & Folkman J Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle 5, 1779–1787 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Almog N et al. Prolonged dormancy of human liposarcoma is associated with impaired tumor angiogenesis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 20, 947–949 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Senft D & Ronai ZA Immunogenic, cellular, and angiogenic drivers of tumor dormancy--a melanoma view. Pigment Cell Melanoma Res. 29, 27–42 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Shchors K et al. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1beta. Genes Dev. 20, 2527–2538 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stockmann C, Schadendorf D, Klose R & Helfrich I The impact of the immune system on tumor: angiogenesis and vascular remodeling. Front. Oncol 4, 69 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koebel CM et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450, 903–907 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Schaller J & Agudo J Metastatic Colonization: Escaping Immune Surveillance. Cancers (Basel). 12, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghajar CM Metastasis prevention by targeting the dormant niche. Nature Reviews Cancer (2015). doi: 10.1038/nrc3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohme M, Riethdorf S & Pantel K Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol 14, 155–167 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Salmon H et al. Expansion and Activation of CD103+Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 44, 924–938 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahnke YD, Schwendemann J, Beckhove P & Schirrmacher V Maintenance of long-term tumour-specific T-cell memory by residual dormant tumour cells. Immunology 115, 325–336 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller-Hermelink N et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell 13, 507–518 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Wang H-F et al. Targeting Immune-Mediated Dormancy: A Promising Treatment of Cancer. Front. Oncol 9, 498 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linde N, Fluegen G & Aguirre-Ghiso JA The Relationship Between Dormant Cancer Cells and Their Microenvironment. Advances in Cancer Research 132, (Elsevier Inc., 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Risson E, Nobre AR, Maguer-Satta V & Aguirre-Ghiso JA The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat. cancer 1, 672–680 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raviraj V et al. Dormant but migratory tumour cells in desmoplastic stroma of invasive ductal carcinomas. Clin. Exp. Metastasis 29, 273–292 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Bayarmagnai B et al. Invadopodia-mediated ECM degradation is enhanced in the G1 phase of the cell cycle. J. Cell Sci 132, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruppender N et al. Cellular Adhesion Promotes Prostate Cancer Cells Escape from Dormancy. PLoS One 10, e0130565 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borgen E et al. NR2F1 stratifies dormant disseminated tumor cells in breast cancer patients. Breast Cancer Res. 20, 120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harper KL et al. Mechanism of early dissemination and metastasis in Her2+mammary cancer. Nature (2016). doi: 10.1038/nature20609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fluegen G et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat. Cell Biol (2017). doi: 10.1038/ncb3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borriello L et al. Primary tumor associated macrophages activate programs of invasion and dormancy in disseminating tumor cells. Nat. Commun 13, 626 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harney AS et al. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer Discov. 5, 932–943 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pignatelli J et al. Invasive breast carcinoma cells from patients exhibit MenaINV- and macrophage-dependent transendothelial migration. Sci. Signal 7, ra112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohan TE et al. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J. Natl. Cancer Inst 106, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mondal C et al. A proliferative to invasive switch is mediated by srGAP1 downregulation through the activation of TGF-β2 signaling. Cell Rep. 40, 111358 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kienast Y et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med 16, 116–122 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Price TT et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med (2016). doi: 10.1126/scitranslmed.aad4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gundem G et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong MKH et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat. Commun 6, 6605 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krøigård AB et al. Genomic Analyses of Breast Cancer Progression Reveal Distinct Routes of Metastasis Emergence. Sci. Rep 7, 43813 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarz RF et al. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: a phylogenetic analysis. PLoS Med. 12, e1001789 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoover HCJ & Ketcham AS Metastasis of metastases. Am. J. Surg 130, 405–411 (1975). [DOI] [PubMed] [Google Scholar]
- 57.Hart IR & Fidler IJ Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res. 40, 2281–2287 (1980). [PubMed] [Google Scholar]
- 58.Strauss DC & Thomas JM Transmission of donor melanoma by organ transplantation. Lancet. Oncol 11, 790–796 (2010). [DOI] [PubMed] [Google Scholar]
- 59.Stephens JK et al. Fatal transfer of malignant melanoma from multiorgan donor to four allograft recipients. Transplantation 70, 232–236 (2000). [PubMed] [Google Scholar]
- 60.Borriello L, Condeelis J, Entenberg D & Oktay MH Breast Cancer Cell Re-Dissemination from Lung Metastases-A Mechanism for Enhancing Metastatic Burden. J. Clin. Med 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Condeelis JS & Entenberg D Hematogenous Dissemination of Breast Cancer Cells From Lymph Nodes Is Mediated by Tumor MicroEnvironment of Metastasis Doorways. 10, 1–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pereira ER et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 359, 1403–1407 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown M et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 359, 1408–1411 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Bragado P et al. TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat. Cell Biol (2013). doi: 10.1038/ncb2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nobre AR et al. Bone marrow NG2(+)/Nestin(+) mesenchymal stem cells drive DTC dormancy via TGFβ2. Nat. cancer 2, 327–339 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang W et al. The bone microenvironment invigorates metastatic seeds for further dissemination. Cell 184, 2471–2486.e20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baccelli I et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol 31, 539–544 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Nemec S & Kilian KA Materials control of the epigenetics underlying cell plasticity. Nat. Rev. Mater 6, 69–83 (2021). [Google Scholar]
- 69.Dai J et al. Astrocytic laminin-211 drives disseminated breast tumor cell dormancy in brain. Nat. cancer 3, 25–42 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nallanthighal S, Heiserman JP & Cheon D-J The Role of the Extracellular Matrix in Cancer Stemness. Front. cell Dev. Biol 7, 86 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barney LE et al. Tumor cell-organized fibronectin maintenance of a dormant breast cancer population. Sci. Adv 6, eaaz4157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karamanos NK et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 288, 6850–6912 (2021). [DOI] [PubMed] [Google Scholar]
- 73.Sun Z, Guo SS & Fässler R Integrin-mediated mechanotransduction. J. Cell Biol 215, 445–456 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolfenson H, Yang B & Sheetz MP Steps in Mechanotransduction Pathways that Control Cell Morphology. Annu. Rev. Physiol 81, 585–605 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kechagia JZ, Ivaska J & Roca-Cusachs P Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol 20, 457–473 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Burridge K, Monaghan-Benson E & Graham DM Mechanotransduction: from the cell surface to the nucleus via RhoA. Philos. Trans. R. Soc. London. Ser. B, Biol. Sci 374, 20180229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohashi K, Fujiwara S & Mizuno K Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J. Biochem 161, 245–254 (2017). [DOI] [PubMed] [Google Scholar]
- 78.Coste B et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y & Xiao B The mechanosensitive Piezo1 channel: structural features and molecular bases underlying its ion permeation and mechanotransduction. J. Physiol 596, 969–978 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geng J et al. A Plug-and-Latch Mechanism for Gating the Mechanosensitive Piezo Channel. Neuron 106, 438–451.e6 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Qin L et al. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 9, 44 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pathak MM et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. U. S. A 111, 16148–16153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun X et al. Bone Piezoelectricity-Mimicking Nanocomposite Membranes Enhance Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by Amplifying Cell Adhesion and Actin Cytoskeleton. J. Biomed. Nanotechnol 17, 1058–1067 (2021). [DOI] [PubMed] [Google Scholar]
- 84.Wang F et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J. Physiol 595, 79–91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lai A et al. Mechanosensing by Piezo1 and its implications for physiology and various pathologies. Biol. Rev. Camb. Philos. Soc 97, 604–614 (2022). [DOI] [PubMed] [Google Scholar]
- 86.Wu J, Lewis AH & Grandl J Touch, Tension, and Transduction - The Function and Regulation of Piezo Ion Channels. Trends Biochem. Sci 42, 57–71 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kenmochi M et al. Involvement of mechano-sensitive Piezo1 channel in the differentiation of brown adipocytes. J. Physiol. Sci 72, 13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang N, Tytell JD & Ingber DE Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol 10, 75–82 (2009). [DOI] [PubMed] [Google Scholar]
- 89.Bouzid T et al. The LINC complex, mechanotransduction, and mesenchymal stem cell function and fate. J. Biol. Eng 13, 68 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kalukula Y, Stephens AD, Lammerding J & Gabriele S Mechanics and functional consequences of nuclear deformations. Nat. Rev. Mol. Cell Biol 23, 583–602 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hamouda MS, Labouesse C & Chalut KJ Nuclear mechanotransduction in stem cells. Curr. Opin. Cell Biol 64, 97–104 (2020). [DOI] [PubMed] [Google Scholar]
- 92.Alam SG et al. The mammalian LINC complex regulates genome transcriptional responses to substrate rigidity. Sci. Rep 6, 38063 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang W, Worman HJ & Gundersen GG Accessorizing and anchoring the LINC complex for multifunctionality. J. Cell Biol 208, 11–22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharili AS & Connelly JT Nucleocytoplasmic shuttling: a common theme in mechanotransduction. Biochem. Soc. Trans 42, 645–649 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Kofler M & Kapus A Nucleocytoplasmic Shuttling of the Mechanosensitive Transcription Factors MRTF and YAP /TAZ. Methods Mol. Biol 2299, 197–216 (2021). [DOI] [PubMed] [Google Scholar]
- 96.Jang J-W, Kim M-K & Bae S-C Reciprocal regulation of YAP/TAZ by the Hippo pathway and the Small GTPase pathway. Small GTPases 11, 280–288 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miranda MZ, Lichner Z, Szászi K & Kapus A MRTF: Basic Biology and Role in Kidney Disease. Int. J. Mol. Sci 22, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shreberk-Shaked M & Oren M New insights into YAP/TAZ nucleo-cytoplasmic shuttling: new cancer therapeutic opportunities? Mol. Oncol 13, 1335–1341 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Speight P, Kofler M, Szászi K & Kapus A Context-dependent switch in chemo/mechanotransduction via multilevel crosstalk among cytoskeleton-regulated MRTF and TAZ and TGFβ-regulated Smad3. Nat. Commun 7, 11642 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Er EE et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat. Cell Biol 20, 966–978 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tello-Lafoz M et al. Cytotoxic lymphocytes target characteristic biophysical vulnerabilities in cancer. Immunity 54, 1037–1054.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zabransky DJ, Jaffee EM & Weeraratna AT Shared genetic and epigenetic changes link aging and cancer. Trends Cell Biol. 32, 338–350 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naik S & Fuchs E Inflammatory memory and tissue adaptation in sickness and in health. Nature 607, 249–255 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Skvortsova K, Stirzaker C & Taberlay P The DNA methylation landscape in cancer. Essays Biochem. 63, 797–811 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dawson MA & Kouzarides T Cancer epigenetics: from mechanism to therapy. Cell 150, 12–27 (2012). [DOI] [PubMed] [Google Scholar]
- 106.Stowers R & Chaudhuri O Epigenetic regulation of mechanotransduction. Nat. Biomed. Eng 5, 8–10 (2021). [DOI] [PubMed] [Google Scholar]
- 107.Stowers RS et al. Matrix stiffness induces a tumorigenic phenotype in mammary epithelium through changes in chromatin accessibility. Nat. Biomed. Eng 3, 1009–1019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jang M et al. Matrix stiffness epigenetically regulates the oncogenic activation of the Yes-associated protein in gastric cancer. Nat. Biomed. Eng 5, 114–123 (2021). [DOI] [PubMed] [Google Scholar]
- 109.Gupta VK & Chaudhuri O Mechanical regulation of cell-cycle progression and division. Trends Cell Biol. 32, 773–785 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sosa MS et al. NR2F1 controls tumour cell dormancy via SOX9- and RARβ-driven quiescence programmes. Nat. Commun 6, 6170 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singh DK et al. Epigenetic reprogramming of DCCs into dormancy suppresses metastasis <em>via</em> restored TGFβ–SMAD4 signaling. bioRxiv 2021.08.01.454684 (2021). doi: 10.1101/2021.08.01.454684 [DOI] [Google Scholar]
- 112.Liu Y et al. 3D Fibrin Stiffness mediates Dormancy of Tumor-repopulating cells via a Cdc42-driven Tet2 Epigenetic Program. Cancer Res. canres.3719.2017 (2018). doi: 10.1158/0008-5472.CAN-17-3719 [DOI] [PubMed] [Google Scholar]
- 113.Schofield R The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25 (1978). [PubMed] [Google Scholar]
- 114.Socovich AM & Naba A The cancer matrisome: From comprehensive characterization to biomarker discovery. Seminars in Cell and Developmental Biology (2019). doi: 10.1016/j.semcdb.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 115.Naba A et al. The Matrisome: In Silico Definition and In Vivo Characterization by Proteomics of Normal and Tumor Extracellular Matrices. Mol. Cell. Proteomics 11, M111.014647–M111.014647 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hynes RO & Naba A Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol 4, a004903 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K & Ossowski L Urokinase Receptor and Fibronectin Regulate the ERKMAPK to p38MAPK Activity Ratios That Determine Carcinoma Cell Proliferation or Dormancy In Vivo. Mol. Biol. Cell (2001). doi: 10.1091/mbc.12.4.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aguirre Ghiso JA, Kovalski K & Ossowski L Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell Biol 147, 89–103 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Di Martino JS et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat. Cancer 3, 90–107 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Montagner M et al. Crosstalk with lung epithelial cells regulates Sfrp2-mediated latency in breast cancer dissemination. Nat. Cell Biol 22, 289–296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nobre AR et al. ZFP281 drives a mesenchymal-like dormancy program in early disseminated breast cancer cells that prevents metastatic outgrowth in the lung. Nat. cancer (2022). doi: 10.1038/s43018-022-00424-8 [DOI] [PubMed] [Google Scholar]
- 122.Aouad P et al. Epithelial-mesenchymal plasticity determines estrogen receptor positive breast cancer dormancy and epithelial reconversion drives recurrence. Nat. Commun 13, 4975 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coppock DL, Kopman C, Scandalis S & Gilleran S Preferential gene expression in quiescent human lung fibroblasts. Cell growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res 4, 483–493 (1993). [PubMed] [Google Scholar]
- 124.Barkan D et al. Metastatic growth from dormant cells induced by a Col-I-enriched fibrotic environment. Cancer Res. 70, 5706–5716 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohta Y et al. Cell-matrix interface regulates dormancy in human colon cancer stem cells. Nature 608, 784–794 (2022). [DOI] [PubMed] [Google Scholar]
- 126.Albrengues J et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. 4227, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ghajar CM et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol 15, 807–817 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fane ME et al. Stromal changes in the aged lung induce an emergence from melanoma dormancy. Nature 606, 396–405 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elkholi IE, Lalonde A, Park M & Côté J-F Breast Cancer Metastatic Dormancy and Relapse: An Enigma of Microenvironment(s). Cancer Res. 82, 4497–4510 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sosa MS, Bragado P & Aguirre-Ghiso JA Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611–22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Russo S, Scotto di Carlo F & Gianfrancesco F The Osteoclast Traces the Route to Bone Tumors and Metastases. Front. cell Dev. Biol 10, 886305 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heyn C et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn. Reson. Med 56, 1001–1010 (2006). [DOI] [PubMed] [Google Scholar]
- 133.Heyn C et al. In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn. Reson. Med 55, 23–29 (2006). [DOI] [PubMed] [Google Scholar]
- 134.Shapiro EM, Sharer K, Skrtic S & Koretsky AP In vivo detection of single cells by MRI. Magn. Reson. Med 55, 242–249 (2006). [DOI] [PubMed] [Google Scholar]
- 135.Di Martino JS, Mondal C & Bravo-Cordero JJ Textures of the tumour microenvironment. Essays Biochem. 63, 619–629 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fukumura D, Duda DG, Munn LL & Jain RK Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation 17, 206–225 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fisher DT et al. Intraoperative intravital microscopy permits the study of human tumour vessels. Nat. Commun 7, 10684 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moore N & Lyle S Quiescent, slow-cycling stem cell populations in cancer: a review of the evidence and discussion of significance. J. Oncol 2011, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cotsarelis G, Sun TT & Lavker RM Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990). [DOI] [PubMed] [Google Scholar]
- 140.Potten CS, Kellett M, Roberts SA, Rew DA & Wilson GD Measurement of in vivo proliferation in human colorectal mucosa using bromodeoxyuridine. Gut 33, 71–78 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schillert A, Trumpp A & Sprick MR Label retaining cells in cancer--the dormant root of evil? Cancer Lett. 341, 73–79 (2013). [DOI] [PubMed] [Google Scholar]
- 142.Aguirre-Ghiso JA, Ossowski L & Rosenbaum SK Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 64, 7336–7345 (2004). [DOI] [PubMed] [Google Scholar]
- 143.Regot S, Hughey JJ, Bajar BT, Carrasco S & Covert MW High-sensitivity measurements of multiple kinase activities in live single cells. Cell 157, 1724–1734 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yano S, Tazawa H, Kagawa S, Fujiwara T & Hoffman RM FUCCI Real-Time Cell-Cycle Imaging as a Guide for Designing Improved Cancer Therapy: A Review of Innovative Strategies to Target Quiescent Chemo-Resistant Cancer Cells. Cancers (Basel). 12, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sakaue-Sawano A & Miyawaki A Visualizing spatiotemporal dynamics of multicellular cell-cycle progressions with fucci technology. Cold Spring Harb. Protoc 2014, (2014). [DOI] [PubMed] [Google Scholar]
- 146.Sakaue-Sawano A et al. Visualizing Spatiotemporal Dynamics of Multicellular Cell-Cycle Progression. Cell 132, 487–498 (2008). [DOI] [PubMed] [Google Scholar]
- 147.Oki T et al. A novel cell-cycle-indicator, mVenus-p27K-, identifies quiescent cells and visualizes G0-G1 transition. Sci. Rep 4, 4012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Spencer SL et al. XThe proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 155, 369–383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Freter R et al. Establishment of a fluorescent reporter of RNA-polymerase II activity to identify dormant cells. Nat. Commun 12, 3318 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Owen KL et al. Prostate cancer cell-intrinsic interferon signaling regulates dormancy and metastatic outgrowth in bone. EMBO Rep. 21, e50162 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Khoo WH et al. A niche-dependent myeloid transcriptome signature defines dormant myeloma cells. Blood 134, 30–43 (2019). [DOI] [PubMed] [Google Scholar]
- 152.Ren Q et al. Gene expression predicts dormant metastatic breast cancer cell phenotype. Breast Cancer Res. 24, 10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aguirre-Ghiso JA Translating the Science of Cancer Dormancy to the Clinic. Cancer Res. 81, 4673–4675 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pranzini E, Raugei G & Taddei ML Metabolic Features of Tumor Dormancy: Possible Therapeutic Strategies. Cancers (Basel). 14, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Garcia-Recio S et al. Multiomics in primary and metastatic breast tumors from the AURORA US network finds microenvironment and epigenetic drivers of metastasis. Nat. cancer (2022). doi: 10.1038/s43018-022-00491-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tan Z et al. Mapping Breast Cancer Microenvironment Through Single-Cell Omics. Front. Immunol 13, 868813 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma R-Y, Black A & Qian B-Z Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 43, 546–563 (2022). [DOI] [PubMed] [Google Scholar]