Summary
Australian Genomics is a national collaborative partnership of more than 100 organizations piloting a whole-of-system approach to integrating genomics into healthcare, based on federation principles. In the first five years of operation, Australian Genomics has evaluated the outcomes of genomic testing in more than 5,200 individuals across 19 rare disease and cancer flagship studies. Comprehensive analyses of the health economic, policy, ethical, legal, implementation and workforce implications of incorporating genomics in the Australian context have informed evidence-based change in policy and practice, resulting in national government funding and equity of access for a range of genomic tests. Simultaneously, Australian Genomics has built national skills, infrastructure, policy, and data resources to enable effective data sharing to drive discovery research and support improvements in clinical genomic delivery.
We describe outcomes of a 5-year national program to accelerate the implementation of genomics into healthcare. We evaluated genomic testing in more than 5,200 individuals across 19 rare disease and cancer flagship studies. Simultaneously, we built skills, infrastructure, policy, and data resources to drive discovery research and support clinical genomic delivery.
Introduction
Genomics is transforming healthcare across disciplines and life stages, from rare disease to cancer, infectious disease, and screening. Rapid, effective, and equitable implementation is dependent on whole-of-system change: building evidence, workforce, and infrastructure simultaneously while addressing ethical, legal, access, and data management challenges.1 In the past 10 years, many governments have invested in large-scale projects to harness the power of genomics and accelerate implementation.2 Program design has varied, from population-based sequencing initiatives through to projects developing infrastructure, fostering discovery, or assessing utility in clinical cohorts.3,4,5 Few have reported outcomes to date, with Genomics England recently reporting preliminary results from the rare disease pilot of the 100,000 genomes project, encompassing 2,500 families.6
We synthesize the outcomes of the first five years of a government-funded national program led by Australian Genomics, a partnership of more than 100 organizations, including clinical and laboratory genetics services, hospitals, research institutes, professional bodies, and patient organizations.7 This research collaboration aimed to evaluate and accelerate the implementation of genomic testing into healthcare, with the decentralized nature of the Australian healthcare system necessitating a national approach based on federation principles. Australian Genomics was designed to deliver two inter-related bodies of work: exemplar clinical flagship studies prospectively providing genomic testing across a broad range of rare diseases and cancers and interdependent programs to advance the diagnostic, data management, regulatory, ethical, policy, and workforce infrastructure necessary for the integration of genomics in the health system. During the first five years, Australian Genomics has generated a significant body of work to inform policy and practice nationally and internationally. Here we reflect on the principal outcomes and future directions.
Informing evidence-based implementation: Genomic testing in rare disease and cancer
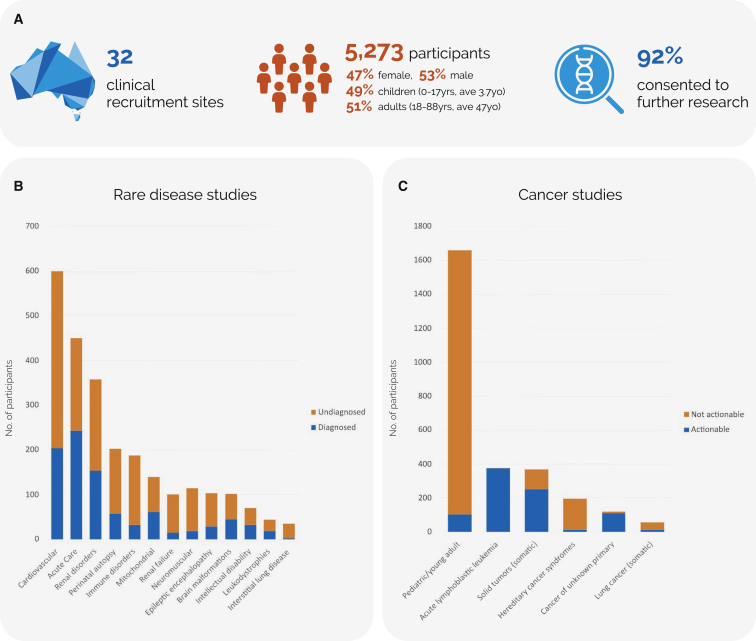
Genomic testing was performed in 5,273 individuals with rare diseases and cancers and 2,399 relatives to evaluate diagnostic and clinical outcomes across a broad range of clinical indications. Participants were recruited prospectively from all states and territories as part of 19 flagship studies (Figure 1 and Table S1) with multi-site Human Research Ethics approval (HREC/16/MH/251). The first tranche of studies originated from existing centers of research excellence, while the second tranche were selected using a competitive process. Cohort sizes ranged from 35 to 1,659 participants. Genetic counsellors were pivotal for the delivery of the program, with approximately one full-time equivalent position funded per 240 participants recruited. In addition, genetic counsellors contributed to the design of the program and led research into establishing best practices in genomic counseling, particularly in the critical care setting.8,9,10,11,12 A variety of sequencing approaches were assessed over the course of the program, including in comparative studies and studies designed to evaluate adjunct modalities such as transcriptome sequencing and high-throughput functional assays. Most testing was clinically accredited and used a singleton, rather than family-based, approach to optimize resource use.
Figure 1.
Informing evidence-based implementation of genomic testing in rare disease and cancer
(A) National recruitment sites, participant demographics, and willingness for data to be used for further research.
(B and C) Diagnostic outcomes of genomic testing across (B) 13 rare disease and (C) 6 cancer studies.
The average diagnostic yield in the rare disease flagships was 33%, ranging from 17% to 54%13,14,15,16; 48% of findings in the cancer flagships were clinically actionable (range 6%–100%). The relatively high diagnostic yield likely reflects robust patient selection criteria, focusing on patients without prior sequencing tests. The average time from patient consent to result was 5 months; one study specifically evaluating rapid genomic testing in critical care consistently achieved a time to result of 3 days on a national scale.14
Beyond the diagnostic and clinical outcomes of genomic testing for patients, the importance of non-clinical and process outcomes for both patients and families is increasingly appreciated.17 These include ending the diagnostic odyssey and associated uncertainty, providing prognostic information, restoring reproductive confidence, and enabling access to peer support. A key research priority for Australian Genomics has been the development and application of innovative health economic methodologies to measure the economic value of all key outcomes of genomic testing (referred to as welfare gain) and to evaluate cost effectiveness. Analysis of parental and child quality-of-life data from over 200 participants in the rare disease flagships demonstrated significant parental health spillover effects.18 Preference-elicitation studies with 3,255 members of the Australian public and study participants highlighted the high economic value placed on the outcomes of genomic testing, with an average welfare gain ranging from AUD$1,570 (USD$1,080) to $11,500 (USD$7,917) per test, depending on clinical context.19,20,21,22 Cost-effectiveness and cost-benefit evaluations in specific patient cohorts have continued to demonstrate that genomic testing is both less costly and more effective than conventional diagnostic approaches.23,24,25 For example, in pediatric mitochondrial disease, genomic testing was estimated to lead to a cost saving of AUD$8,800 (USD$6,082) per child tested and a net benefit of AUD$14,700 (USD$10,059).23 A budget impact assessment estimated an annual cost-saving of AUD$7.3M (USD$5M) for the Australian healthcare system through the implementation of rapid genomic testing for critically ill children alone.26
The results of these studies provided a robust evidence base to inform the transition of genomic testing to healthcare system funding. Applications for public funding for multiple clinical indications (syndromic and non-syndromic intellectual disability, genetic cardiac disorders, genetic kidney diseases, and a range of cancers) have undergone successful health technology assessment through the Medical Services Advisory Committee (MSAC) and have been funded, with many more applications in process.
National data resources: Supporting research and clinical genomic delivery
Genomic sequencing generates data of unprecedented scale and complexity. Successful implementation necessitates substantial investment in high-performance computing, data storage infrastructure, and local and national services for data access and exchange. It is imperative that data are collected, stored, and shared using internationally recognized standards and interoperable formats. Responsible management needs to ensure participant privacy, data security, and rigorous governance, while optimizing the accessibility and value of data for healthcare and research benefit. Federated systems present further challenges in this context, with the real danger of creating data siloes. Australian Genomics is a driver project for the Global Alliance for Genomics and Health (GA4GH) placing an emphasis on integrating GA4GH standards and frameworks to support international interoperability.27 Australian Genomics also co-leads the GA4GH National Initiatives Forum with Genomics England, building a community to align standards and share practical experience internationally.
One of the principal legacies of Australian Genomics is the creation of several integrated national data resources to support a virtuous cycle between clinical and research genomic delivery. These are informing prototypes and recommendations for developing a national genomic information management ecosystem. Available resources include a Genomic Data Repository (GDR), containing genomic data from flagship studies, hosted on commercial cloud. Data sharing for secondary research uses, where appropriate consent and ethics are in place, is supported by a data governance framework, including policies and access processes developed by Australian Genomics.
The national clinical consent form and supporting materials developed by Australian Genomics specifically incorporate consent to national and international data sharing for the benefit of healthcare and advancing knowledge. In addition, Australian Genomics has built the CTRL (control) dynamic consent web application as a means for participants to better engage with research and to enable granular decision making over time in relation to return of results and secondary use of data.28 CTRL incorporates the GA4GH Data Use Ontology29 so that an individual’s dataset can be tagged with their own permissions and restrictions on use. The pilot implementation facilitated evaluation of participation and usage rates, participant perspectives, and exploration of the barriers to implementation. CTRL was further developed to manage online decision support, recruitment, and consent of 18,000 individuals for the Australian Reproductive Genetic Carrier Screening Project (Mackenzie’s Mission) and has attracted widespread national and international interest in adaptation. To support uptake, the CTRL codebase is a freely available open-source code via Australian Genomics’ GitHub repository (https://github.com/Australian-Genomics/CTRL) for the research community to adopt and participate in its improvement.
To promote sharing of knowledge about genes and variants associated with disease, Australian Genomics has deployed two platforms, PanelApp Australia30 and Shariant.31 These serve to facilitate harmonization of gene and variant curation efforts between laboratories, promoting consistency in data interpretation, a key driver in improving diagnostic accuracy and patient outcomes.
To further gene and virtual gene panel curation, Australian Genomics deployed a local instance of the PanelApp software in collaboration with Genomics England.30 It currently contains clinical validity assessments for 5,403 genes and hosts 272 virtual gene panels developed by Australian research studies and clinical and laboratory services. PanelApp Australia consolidates virtual panels between Australian diagnostic laboratories with the aim of promoting collaborative discordance resolution and harmonization. Internationally, a systematic comparison of the content of 80 virtual panels between PanelApp Australia and Genomics England PanelApp resulted in the identification and review of 2,144 discordant gene-disease assessments,30 improving diagnostic outcomes and providing a blueprint for expanding participation in international harmonization efforts via the Gene Curation Coalition.32
Shariant is a controlled access platform, supporting real-time, two-way automated sharing of variant interpretations and associated evidence between Australian diagnostic laboratories, with submitters notified and encouraged to resolve discrepant variant interpretations. Shariant currently contains 14,000 variant entries, with 11% (28/260) of variants submitted by more than one laboratory found to be discrepant.31 The platform supports automation of data sharing with the ClinVar public archive, enabling Australian laboratories to contribute internationally.
Building a cohesive national approach to genomic implementation: Whole-of-system change
Data and evidence alone are not enough to ensure effective translation of a complex technology such as genomic testing into an already complex healthcare system. Australian Genomics has supported a broad program of implementation science research to identify methods and strategies that facilitate the uptake of evidence-based genomics practice by both practitioners and policymakers. Theory-informed interviews helped researchers to understand individual and organizational barriers (and enablers) to implementation as pre-requisites to informing interventions.33,34 These identified a shift on the implementation journey from preadoption through to adoption and implementation, highlighting the importance of demonstrating the value of clinical genomics in the initial phases. The role of leadership35 and clear organizational priorities that include clinical genomics are key success drivers in the latter phases.33 Process mapping with non-genetic physicians was used to better understand the complexity of delivering genomic testing, enabling the development of implementation strategies to support scaling up while allowing for local variation with national standardization.36
Arguably, one of the most significant achievements of Australian Genomics has been to strategically build a connected, collaborative national network to enable collective learning. A social network analysis conducted in 2018 found a doubling of professional ties from 2,925 ties before 2016 to 6,381 ties in 2018, and an increase in relationship density (0.020–0.043).37 Shared leadership across the country and multi-site participation were encouraged in all projects, and the national network of more than 60 project coordinators was central to facilitating connections within and between individual projects and working groups.
To support workforce development, Australian Genomics conducted research to understand needs and preferences for education and training and to develop tools to support evidence-based genomics education. A national survey captured experiences and perspectives of more than 400 medical practitioners from varied specialties, career stages, and work settings.38 Over half (54%) reported that they already practiced genomic medicine. There was a significant preference for a service model that includes support from genetics services, either through referral or providing pre- and post-test support (p < 0.001). The survey data provide important insights into the preferred models of practice and education needs of physicians, to guide genomic educators on the content, mode, and level of genomic education required. It also validated the importance of experiential learning and of specialty-based “genomics champions,” highlighted previously in interviews with 86 medical specialists.39 To connect genomics educators as a community of practice, Australian Genomics established the Genomics Education Network of Australasia (GENA) in 2018. Finally, to support best practice in genomics education, Australian Genomics developed a program logic model to articulate the required inputs and processes for planning, delivery, and evaluation40 and standards to enable consistent reporting of genomics education interventions and their evaluation.41 Both were developed with international experts and the reporting standards have been endorsed by international genomics and reporting standards networks. These are now informing the development and delivery of continuing professional development courses as well as the integration of genomics into the training of the current and emerging Australian workforce.
Engaging more broadly, the Australian Genomics Community Advisory Group has led key projects, including Genomics in the Community, whereby representatives of patient support and advocacy groups worked together to develop resources on genomic testing for the public (genomicsinfo.org). Australian Genomics has also actively engaged with mainstream and social media to promote positive, accurate messaging about genomics to the broader public (Facebook and Twitter: @AusGenomics).
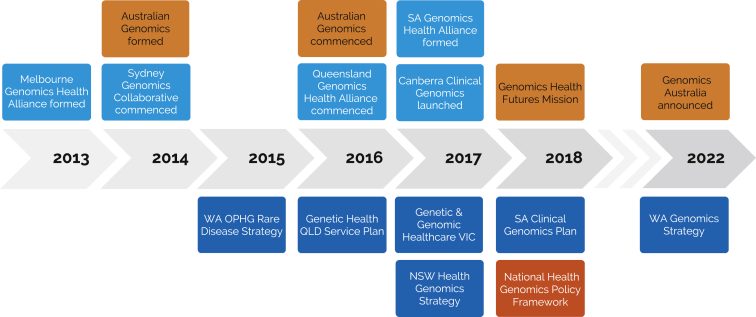
At the government level, Australian Genomics has operated in a complex political ecosystem, where the delivery of healthcare is a shared responsibility between the Australian federal government and the State and Territory jurisdictions. Policy frameworks and service plans have been developed both at national and jurisdictional levels, with several jurisdictions funding their own collaborative genomic alliances (Figure 2).1,42 Australian Genomics has partnered with these state-based efforts to maximize research funding through shared support of projects, facilitating expansion of single-jurisdiction projects to national, multi-site research programs. As evidence is generated through these projects, the Australian Genomics National Implementation Committee has served as a forum for jurisdictional health departments to consider the healthcare system implementation of nationally significant translational projects.
Figure 2.
The Australian landscape of national and state/territory-based policies, frameworks, and genomic initiatives
National, orange; state/territory, blue. SA, South Australia; WA, Western Australia; OPHG, Office of Population Health Genomics; QLD, Queensland; VIC, Victoria; NSW, New South Wales.
Key challenges, limitations, and solutions
Australian Genomics has produced a large body of work from 2016 to 2021 for a relatively modest level of investment (AUD$25M, USD$17M over 5 years) compared with similar programs internationally. This has been achieved through leveraging more than AUD$100M (USD$69M) in additional federal and state investments in genomics and building an engaged genomics community. The long lead-in time for multi-site ethics approvals43 impacted on reaching some recruitment targets and affected equity of access to the program across Australia. Toward the end of the program, the COVID-19 pandemic hindered reagent supplies, sample collection, and transport but promoted a compensatory shift toward telehealth, electronic test ordering, and electronic consent. As with many other genomic studies, participants living in non-urban areas, from culturally and linguistically diverse groups, are underrepresented. This specifically includes Australia’s First Nations people, Aboriginal and Torres Strait Islanders, and has now led to significant investment in strategies to engage and ensure broad participation and benefit.
Future directions
The Australian Government has invested AUD$500M (USD$344M) in a research fund, the Genomics Health Futures Mission (GHFM, 2018–2028) and a further AUD$15M (USD$10M) to support Australian Genomics (2021–2023). One of the principal objectives of the current Australian Genomics funding is to improve the efficiency, reach, and timeliness of genomic research projects. Australian Genomics currently supports 27 GHFM-funded projects, to an estimated in-kind value of AUD$12M (USD$8M). Examples of GHFM-funded initiatives include Mackenzie’s Mission, a national reproductive carrier screening project recruiting >9,000 couples, and the establishment of the Australian Functional Genomics Network (AFGN) connecting research groups and clinicians to facilitate variant interpretation and novel gene discovery. As the outcomes of these research projects are published, the second aim of Australian Genomics will be to support government health departments in clinical implementation by refining and communicating evidence to inform policy. Recently, the Australian Government announced the establishment in 2024 of a new national government entity, Genomics Australia, with the remit to build upon the strong foundations laid by Australian Genomics in ensuring a cohesive national approach to genomics implementation into mainstream healthcare.
Acknowledgments
We would like to thank all participants, families, advisory board members, and research collaborators. The Australian Genomics Health Alliance (Australian Genomics) project was funded by an NHMRC Targeted Call for Research grant (GNT1113531). Mackenzie’s Mission, the Australian Genomics Cardiovascular Genetics Disorders Flagship, Massimo’s Mission Leukodystrophy Flagship, and the Acute Care Flagship were funded by the Australian Government’s Medical Research Futures Fund.
Declaration of interests
R.L.W. is the Chair of the Medical Services Advisory Committee; the views in this paper are not representing those of the Commonwealth of Australia. I.E.S. has served on scientific advisory boards for BioMarin, Chiesi, Eisai, Encoded Therapeutics, GlaxoSmithKline, Knopp Biosciences, Nutricia, Rogcon, Takeda Pharmaceuticals, UCB, and Xenon Pharmaceuticals; has received speaker honoraria from GlaxoSmithKline, UCB, BioMarin, Biocodex, Chiesi, Liva Nova, Nutricia, Zuellig Pharma, and Eisai; has received funding for travel from UCB, Biocodex, GlaxoSmithKline, Biomarin, and Eisai; has served as an investigator for Anavex Life Sciences, Cerecin Inc, Cerevel Therapeutics, Eisai, Encoded Therapeutics, EpiMinder Inc, Epygenyx, ES-Therapeutics, GW Pharma, Marinus, Neurocrine BioSciences, Ovid Therapeutics, Takeda Pharmaceuticals, UCB, Ultragenyx, Xenon Pharmaceuticals, Zogenix, and Zynerba; and has consulted for Care Beyond Diagnosis, Epilepsy Consortium, Atheneum Partners, Ovid Therapeutics, UCB, Zynerba Pharmaceuticals, BioMarin, Encoded Therapeutics, and Biohaven Pharmaceuticals; and is a Non-Executive Director of Bellberry Ltd and a Director of the Australian Academy of Health and Medical Sciences and the Australian Council of Learned Academies Limited. She may accrue future revenue on pending patent WO61/010,176 (filed: 2008): Therapeutic Compound; has a patent for SCN1A testing held by Bionomics Inc and licensed to various diagnostic companies; and has a patent molecular diagnostic/theranostic target for benign familial infantile epilepsy (BFIE) (PRRT2) 2,011,904,493 & 2,012,900,190 and PCT/AU2012/001,321 (TECH ID:2012-009).
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2023.01.018.
Contributor Information
Zornitza Stark, Email: zornitza.stark@vcgs.org.au.
Kathryn N. North, Email: kathryn.north@mcri.edu.au.
Supplemental information
Data and code availability
The datasets supporting the current study have not been deposited in a public repository due to consent restrictions. De-identified genomic and associated data from this study are available for ethically approved research. The online access application process is administered by the Australian Genomics Data Access Committee.
CTRL dynamic consent platform codebase is available open-source via Australian Genomics’ GitHub repository (https://github.com/Australian-Genomics/CTRL).
References
- 1.Gaff C.L., M Winship I., M Forrest S., P Hansen D., Clark J., M Waring P., South M., H Sinclair A. Preparing for genomic medicine: a real world demonstration of health system change. NPJ Genom. Med. 2017;2:31. doi: 10.1038/s41525-017-0028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stark Z., Dolman L., Manolio T.A., Ozenberger B., Hill S.L., Caulfied M.J., Levy Y., Glazer D., Wilson J., Lawler M., et al. Integrating genomics into healthcare: a global responsibility. Am. J. Hum. Genet. 2019;104:13–20. doi: 10.1016/j.ajhg.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.All of Us Research Program Investigators. Denny J.C., Rutter J.L., Goldstein D.B., Philippakis A., Smoller J.W., Jenkins G., Dishman E. The "All of Us" research program. N. Engl. J. Med. 2019;381:668–676. doi: 10.1056/NEJMsr1809937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turnbull C., Scott R.H., Thomas E., Jones L., Murugaesu N., Pretty F.B., Halai D., Baple E., Craig C., Hamblin A., et al. The 100 000 Genomes Project: bringing whole genome sequencing to the NHS. BMJ. 2018;361:k1687. doi: 10.1136/bmj.k1687. [DOI] [PubMed] [Google Scholar]
- 5.Fatumo S., Yakubu A., Oyedele O., Popoola J., Attipoe D.A., Eze-Echesi G., Modibbo F.Z., Ado-Wanka N., et al. 54gene Team. NCD-GHS Consortium Promoting the genomic revolution in Africa through the Nigerian 100K Genome Project. Nat. Genet. 2022;54:531–536. doi: 10.1038/s41588-022-01071-6. [DOI] [PubMed] [Google Scholar]
- 6.100000 Genomes Project Pilot Investigators. Smedley D., Smith K.R., Martin A., Thomas E.A., McDonagh E.M., Cipriani V., Ellingford J.M., Arno G., Tucci A., et al. 100,000 Genomes pilot on rare-disease diagnosis in health care - preliminary report. N. Engl. J. Med. 2021;385:1868–1880. doi: 10.1056/NEJMoa2035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stark Z., Boughtwood T., Phillips P., Christodoulou J., Hansen D.P., Braithwaite J., Newson A.J., Gaff C.L., Sinclair A.H., North K.N. Australian genomics: a federated model for integrating genomics into healthcare. Am. J. Hum. Genet. 2019;105:7–14. doi: 10.1016/j.ajhg.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayres S., Gallacher L., Stark Z., Brett G.R. Genetic counseling in pediatric acute care: reflections on ultra-rapid genomic diagnoses in neonates. J. Genet. Couns. 2019;28:273–282. doi: 10.1002/jgc4.1086. [DOI] [PubMed] [Google Scholar]
- 9.Lynch F., Nisselle A., Gaff C.L., McClaren B. Rapid acute care genomics: challenges and opportunities for genetic counselors. J. Genet. Couns. 2021;30:30–41. doi: 10.1002/jgc4.1362. [DOI] [PubMed] [Google Scholar]
- 10.Lynch F., Nisselle A., Stark Z., Gaff C.L., McClaren B. Genetics follow up after rapid genomic sequencing in intensive care: current practices and recommendations for service delivery. Eur. J. Hum. Genet. 2022;30:1276–1282. doi: 10.1038/s41431-022-01168-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch F., Nisselle A., Stark Z., Gaff C.L., McClaren B. Parents' experiences of decision making for rapid genomic sequencing in intensive care. Eur. J. Hum. Genet. 2021;29:1804–1810. doi: 10.1038/s41431-021-00950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brett G.R., Martyn M., Lynch F., de Silva M.G., Ayres S., Gallacher L., Boggs K., Baxendale A., Schenscher S., King-Smith S., et al. Parental experiences of ultrarapid genomic testing for their critically unwell infants and children. Genet. Med. 2020;22:1976–1985. doi: 10.1038/s41436-020-0912-4. [DOI] [PubMed] [Google Scholar]
- 13.Scheffer I.E., Bennett C.A., Gill D., de Silva M.G., Boggs K., Marum J., Baker N., Australian Genomics DEE Flagship. Palmer E.E., Howell K.B. Exome sequencing for patients with developmental and epileptic encephalopathies in clinical practice. Dev. Med. Child Neurol. 2023;65:50–57. doi: 10.1111/dmcn.15308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Australian Genomics Health Alliance Acute Care Flagship. Lunke S., Eggers S., Wilson M., Patel C., Barnett C.P., Pinner J., Sandaradura S.A., Buckley M.F., Krzesinski E.I., et al. Feasibility of ultra-rapid exome sequencing in critically ill infants and children with suspected monogenic conditions in the Australian public health care system. JAMA. 2020;323:2503–2511. doi: 10.1001/jama.2020.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Temple S.E.L., Ho G., Bennetts B., Boggs K., Vidic N., Mowat D., Christodoulou J., Schultz A., Gayagay T., Roscioli T., et al. The role of exome sequencing in childhood interstitial or diffuse lung disease. Orphanet J. Rare Dis. 2022;17:350. doi: 10.1186/s13023-022-02508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne A.B., Arts P., Ha T.T., Kassahn K.S., Pais L.S., O’Donnell-Luria A., Broad Institute Center for Mendelian Genomics. Babic M., Frank M.S.B., Feng J., Wang P., Lawrence D.M., Eshraghi L., Arriola L., Toubia J., Nguyen H., Genomic Autopsy Study Research Network. McGillivray G., Pinner J., McKenzie F., Morrow R., Lipsett J., Manton N., Khong T.Y., Moore L., Liebelt J.E., Schreiber A.W., King-Smith S.L., Hardy T.S.E., Jackson M.R., Barnett C.P., Scott H.S. A genomic autopsy identifies underlying causes of pregnancy loss and perinatal death and provides options to prevent recurrence. Nat. Med. 2023;29:180–189. doi: 10.1038/s41591-022-02142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Best S., Stark Z., Phillips P., Wu Y., Long J.C., Taylor N., Braithwaite J., Christodoulou J., Goranitis I. Clinical genomic testing: what matters to key stakeholders? Eur. J. Hum. Genet. 2020;28:866–873. doi: 10.1038/s41431-020-0576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y., Al-Janabi H., Mallett A., Quinlan C., Scheffer I.E., Howell K.B., Christodoulou J., Leventer R.J., Lockhart P.J., Stark Z., et al. Parental health spillover effects of paediatric rare genetic conditions. Qual. Life Res. 2020;29:2445–2454. doi: 10.1007/s11136-020-02497-3. [DOI] [PubMed] [Google Scholar]
- 19.Goranitis I., Best S., Christodoulou J., Boughtwood T., Stark Z. Preferences and values for rapid genomic testing in critically ill infants and children: a discrete choice experiment. Eur. J. Hum. Genet. 2021;29:1645–1653. doi: 10.1038/s41431-021-00874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goranitis I., Best S., Christodoulou J., Stark Z., Boughtwood T. The personal utility and uptake of genomic sequencing in pediatric and adult conditions: eliciting societal preferences with three discrete choice experiments. Genet. Med. 2020;22:1311–1319. doi: 10.1038/s41436-020-0809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goranitis I., Best S., Stark Z., Boughtwood T., Christodoulou J. The value of genomic sequencing in complex pediatric neurological disorders: a discrete choice experiment. Genet. Med. 2021;23:155–162. doi: 10.1038/s41436-020-00949-2. [DOI] [PubMed] [Google Scholar]
- 22.Meng Y., Clarke P.M., Goranitis I. The value of genomic testing: a contingent valuation across six child- and adult-onset genetic conditions. Pharmacoeconomics. 2022;40:215–223. doi: 10.1007/s40273-021-01103-9. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y., Balasubramaniam S., Rius R., Thorburn D.R., Christodoulou J., Goranitis I. Genomic sequencing for the diagnosis of childhood mitochondrial disorders: a health economic evaluation. Eur. J. Hum. Genet. 2021;30:577–586. doi: 10.1038/s41431-021-00916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stark Z., Schofield D., Alam K., Wilson W., Mupfeki N., Macciocca I., Shrestha R., White S.M., Gaff C. Prospective comparison of the cost-effectiveness of clinical whole-exome sequencing with that of usual care overwhelmingly supports early use and reimbursement. Genet. Med. 2017;19:867–874. doi: 10.1038/gim.2016.221. [DOI] [PubMed] [Google Scholar]
- 25.Stark Z., Schofield D., Martyn M., Rynehart L., Shrestha R., Alam K., Lunke S., Tan T.Y., Gaff C.L., White S.M. Does genomic sequencing early in the diagnostic trajectory make a difference? A follow-up study of clinical outcomes and cost-effectiveness. Genet. Med. 2018;21:173–180. doi: 10.1038/s41436-018-0006-8. [DOI] [PubMed] [Google Scholar]
- 26.Goranitis I., Wu Y., Lunke S., White S.M., Tan T.Y., Yeung A., Hunter M.F., Martyn M., Gaff C., Stark Z. Is faster better? An economic evaluation of rapid and ultra-rapid genomic testing in critically ill infants and children. Genet. Med. 2022;24:1037–1044. doi: 10.1016/j.gim.2022.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Rehm H.L., Page A.J.H., Smith L., Adams J.B., Alterovitz G., Babb L.J., Barkley M.P., Baudis M., Beauvais M.J.S., Beck T., et al. GA4GH: International policies and standards for data sharing across genomic research and healthcare. Cell Genom. 2021;1:100029. doi: 10.1016/j.xgen.2021.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas M.A., Teare H., Prictor M., Ceregra G., Vidgen M.E., Bunker D., Kaye J., Boughtwood T. 'CTRL': an online, dynamic consent and participant engagement platform working towards solving the complexities of consent in genomic research. Eur. J. Hum. Genet. 2021;29:687–698. doi: 10.1038/s41431-020-00782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson J., Cabili M.N., Kerry G., Boughtwood T., Thorogood A., Alper P., Bowers S.R., Boyles R.R., Brookes A.J., Brush M., et al. The Data Use Ontology to streamline responsible access to human biomedical datasets. Cell Genom. 2021;1:100028. doi: 10.1016/j.xgen.2021.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark Z., Foulger R.E., Williams E., Thompson B.A., Patel C., Lunke S., Snow C., Leong I.U.S., Puzriakova A., Daugherty L.C., et al. Scaling national and international improvement in virtual gene panel curation via a collaborative approach to discordance resolution. Am. J. Hum. Genet. 2021;108:1551–1557. doi: 10.1016/j.ajhg.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tudini E., Andrews J., Lawrence D.M., King-Smith S.L., Baker N., Baxter L., Beilby J., Bennetts B., Beshay V., Black M., et al. Shariant platform: enabling evidence sharing across Australian clinical genetic testing laboratories to support variant interpretation. Am. J. Hum. Genet. 2022;109:1960–1973. doi: 10.1016/j.ajhg.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiStefano M.T., Goehringer S., Babb L., Alkuraya F.S., Amberger J., Amin M., Austin-Tse C., Balzotti M., Berg J.S., Birney E., et al. The Gene Curation Coalition: A global effort to harmonize gene-disease evidence resources. Genet. Med. 2022;24:1732–1742. doi: 10.1016/j.gim.2022.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Best S., Long J.C., Gaff C., Braithwaite J., Taylor N. Organizational perspectives on implementing complex health interventions: clinical genomics in Australia. J. Health Organ. Manag. 2021;35:825–845. doi: 10.1108/JHOM-12-2020-0495. ahead-of-print. [DOI] [PubMed] [Google Scholar]
- 34.Best S., Long J.C., Gaff C., Braithwaite J., Taylor N. Investigating the adoption of clinical genomics in Australia. An Implementation Science Case Study. Genes. 2021;12 doi: 10.3390/genes12020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Best S., Stark Z., Brown H., Long J.C., Hewage K., Gaff C., Braithwaite J., Taylor N. The leadership behaviors needed to implement clinical genomics at scale: a qualitative study. Genet. Med. 2020;22:1384–1390. doi: 10.1038/s41436-020-0818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Best S., Long J.C., Braithwaite J., Taylor N. Standardizing variation: scaling up clinical genomics in Australia. Genet. Med. 2022:100109. doi: 10.1016/j.gim.2022.01.004. online ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Long J.C., Pomare C., Best S., Boughtwood T., North K., Ellis L.A., Churruca K., Braithwaite J. Building a learning community of Australian clinical genomics: a social network study of the Australian Genomic Health Alliance. BMC Med. 2019;17:44. doi: 10.1186/s12916-019-1274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nisselle A., King E.A., McClaren B., Janinski M., Metcalfe S., Gaff C., Australian Genomics Workforce. Education Working Group Measuring physician practice, preparedness and preferences for genomic medicine: a national survey. BMJ Open. 2021;11:e044408. doi: 10.1136/bmjopen-2020-044408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClaren B.J., Crellin E., Janinski M., Nisselle A.E., Ng L., Metcalfe S.A., Gaff C.L. Preparing medical specialists for genomic medicine: continuing education should include opportunities for experiential learning. Front. Genet. 2020;11:151. doi: 10.3389/fgene.2020.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nisselle A., Martyn M., Jordan H., Kaunein N., McEwen A., Patel C., Terrill B., Bishop M., Metcalfe S., Gaff C. Ensuring best practice in genomic education and evaluation: a program logic approach. Front. Genet. 2019;10:1057. doi: 10.3389/fgene.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nisselle A., Janinski M., Martyn M., McClaren B., Kaunein N., Maguire J., Riggs E.R., Barlow-Stewart K., Belcher A., Bernat J.A., Best S. Ensuring best practice in genomics education and evaluation: reporting item standards for education and its evaluation in genomics (RISE2 Genomics) Genet. Med. 2021;23:1356–1365. doi: 10.1038/s41436-021-01140-x. [DOI] [PubMed] [Google Scholar]
- 42.Vidgen M.E., Williamson D., Cutler K., McCafferty C., Ward R.L., McNeil K., Waddell N., Bunker D. Queensland Genomics: an adaptive approach for integrating genomics into a public healthcare system. NPJ Genom. Med. 2021;6:71. doi: 10.1038/s41525-021-00234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haas M.A., Boughtwood T.F., Quinn M.C., Australian Genomics The ethics approval process for multisite research studies in Australia: changes sought by the Australian Genomics initiative. Med. J. Aust. 2019;211:440–444.e1. doi: 10.5694/mja2.50397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the current study have not been deposited in a public repository due to consent restrictions. De-identified genomic and associated data from this study are available for ethically approved research. The online access application process is administered by the Australian Genomics Data Access Committee.
CTRL dynamic consent platform codebase is available open-source via Australian Genomics’ GitHub repository (https://github.com/Australian-Genomics/CTRL).