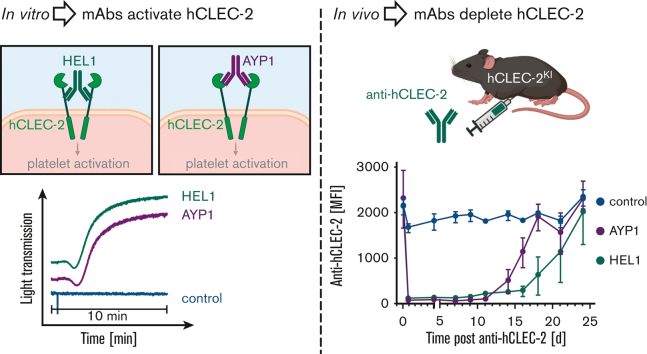
Visual Abstract
TO THE EDITOR:
C-type lectin-like receptor 2 (CLEC-2) is a unique platelet activation receptor signaling through a single YXXL sequence, representing half of an immunoreceptor tyrosine-based activation motif (hemITAM).1,2 CLEC-2 and its endogenous ligand podoplanin are crucial for normal development, with mice deficient in either showing defective blood–lymphatic vessel separation.3, 4, 5 This interaction also has a role in tumor metastasis.6,7
CLEC-2 is important in thrombosis, particularly in immunothrombosis and smaller vascular beds,2,8, 9, 10, 11, 12 with CLEC-2 deficiency reducing vessel occlusion in several in vivo thrombosis models with little effect on hemostasis.13, 14, 15 Occlusion is unaltered in CLEC-2 Y7A signaling null mice, in which the receptor is normally expressed, suggesting that it is the presence of CLEC-2 itself, rather than CLEC-2-induced platelet activation, that has a role in thrombus stability.14 Furthermore, immunodepletion of CLEC-2 from the platelet surface using the monoclonal antibody INU1 has similar effects on thrombus formation, with depletion lasting up to 6 days and accompanied by transient thrombocytopenia.8,13
As a result, CLEC-2 has been suggested as a potential antithrombotic target. However, the in vivo role of human CLEC-2 cannot be readily investigated experimentally in humans, meaning there are limited methods to assess the relevance of human CLEC-2 in arterial thrombosis or tools to test potential therapeutics preclinically. In addition, although antibodies against human CLEC-2, such as AYP1,16 exist, it is unknown if human, like mice, CLEC-2 can be immunodepleted. Here, we have generated a humanized CLEC-2 mouse model that can be used to test potential antihuman CLEC-2 therapeutics in vivo.
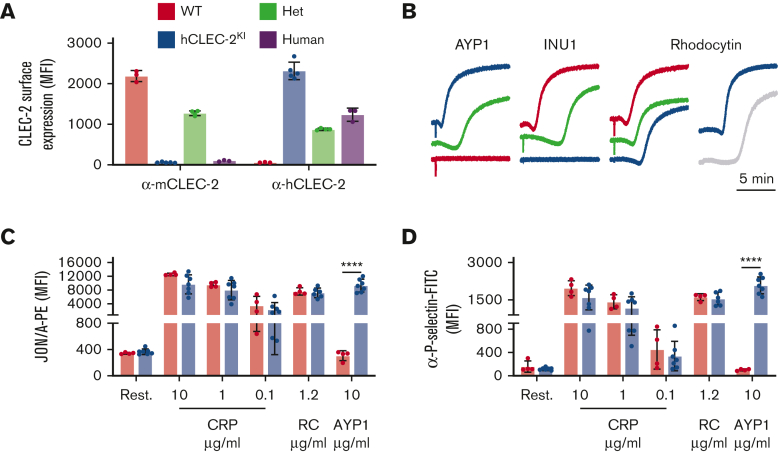
Humanized CLEC-2 (hCLEC-2KI) mice were generated using CRISPR to replace the mouse gene with the human variant (supplemental Figure 1). These mice are viable, fertile, and born in the Mendelian ratio (supplemental Table 1). There were no obvious signs of blood lymphatic defects, and both platelet and megakaryocyte counts were comparable to wild-type (WT) mice (supplemental Table 2; supplemental Figure 2). This is in contrast to other mouse lines in which CLEC-2 or podoplanin have been genetically modified3,4,14 and suggests that human CLEC-2 can compensate for loss of the mouse protein and the interaction with murine podoplanin is sufficient for blood–lymphatic vessel separation. Human but not mouse CLEC-2 could be detected on platelets from hCLEC-2KI mice with heterozygotes expressing half each of human and mouse CLEC-2 (Figure 1A; supplemental Figure 3). The surface abundance of CLEC-2 on hCLEC-2KI platelets was approximately double that on human platelets (Figure 1A). The surface abundance of all other glycoprotein receptors was comparable to WT platelets (supplemental Table 3) as were platelet activation and aggregation for G protein–coupled receptors and GPVI agonists (Figure 1B-D; supplemental Figure 4). However, there was a slight increase in lag time before aggregation with RC, similar to that seen in human platelets (Figure 1B). Thrombus formation on collagen under flow at 1200 s−1 was unaltered in hCLEC-2KI mice (supplemental Figure 5). Platelet spreading was comparable to WT on both fibrinogen and collagen-related peptide. However, it was slightly reduced on mouse podoplanin for hCLEC-2KI platelets because, although they formed both filopodia and lamellipodia, there were few fully spread platelets (supplemental Figure 6). Overall, this suggests that there are no major differences in platelet function in hCLEC-2KI compared with WT mice.
Figure 1.
hCLEC-2KImice have normal CLEC-2 expression and platelet activation. (A) Surface expression of mouse and human CLEC-2 by flow cytometry using INU1 and AYP1 antibodies, respectively. Heterozygous mice expressed half human and half mouse CLEC-2, whereas hCLEC-2KI mice expressed only hCLEC-2 on their platelet surface. (B) Light transmission aggregometry with washed platelets shows that AYP1 (10 μg/mL) and INU1 (10 μg/mL) cause aggregation of hCLEC-2KI and WT platelets, respectively, and reduced aggregation in hCLEC-2KI heterozygous platelets. RC-induced (0.24 μg/mL) aggregation has a longer lag time not only in hCLEC-2KI platelets but also in human platelets. (C) Platelet integrin activation measured by JON/A-PE antibody binding in flow cytometry shows no difference in (hem)ITAM-mediated platelet activation in hCLEC-2KI platelets and hCLEC-2–specific activation by AYP1. (D) Platelet granule secretion measured using an anti–P-selectin antibody in flow cytometry was unaltered in hCLEC-2KI following platelet activation by (hem)ITAM agonists, whereas AYP1 causes hCLEC-2–specific granule secretion. Data analyzed by two-way analysis of variance followed by a Sidak multiple comparison test. ∗∗∗∗P < .001. Results are shown as mean ± standard deviation, with each circle representing 1 individual and are representative of 3 independent experiments. CRP, collagen-related peptide; FITC, fluorescein isothiocyanate; Het, heterozygous; MFI, mean fluorescent intensity; PE, phycoerythrin; RC, rhodocytin.
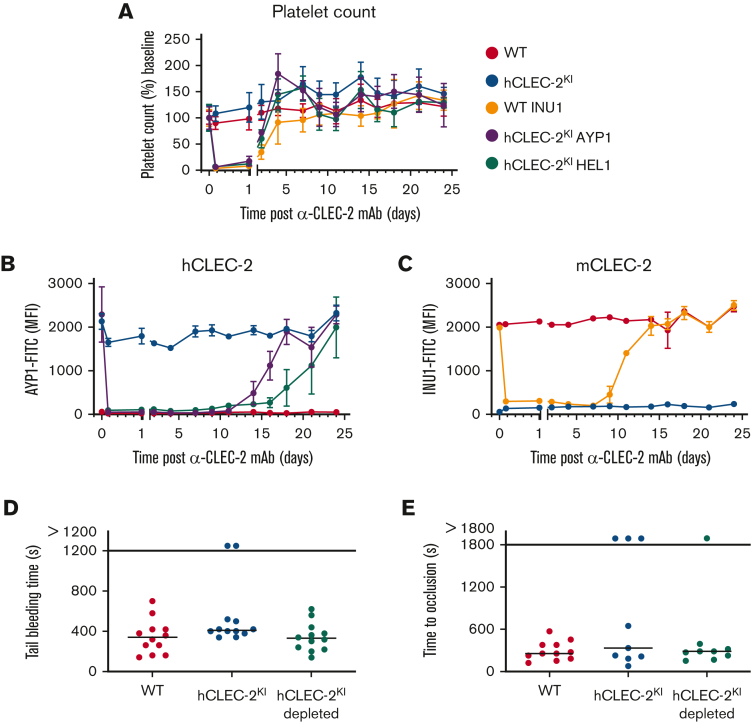
The novel antibody HEL1 was generated by hybridoma technology following immunization of Wistar rats with hCLEC-2 immunoprecipitated from human platelet lysates. HEL1 is specific to hCLEC-2 and can be used in flow cytometry, western blotting, and immunoprecipitation (supplemental Figure 7A-C). It binds to a different epitope on CLEC-2 than AYP1 because no competition between the 2 antibodies was observed (supplemental Figure 7D), although both antibodies cause hCLEC-2KI platelet aggregation (Figure 1B; supplemental Figure 7E). Furthermore, HEL1 Fab fragments neither block RC-induced platelet aggregation, unlike AYP1 Fab fragments,16 nor block AYP1 IgG-induced aggregation of hCLEC-2KI platelets (supplemental Figure 7F). This not only shows that AYP1 and HEL1 act at different sites on CLEC-2 but also shows that CLEC-2 dimerization at either site is sufficient to trigger platelet activation because both antibodies, but not their Fab fragments, stimulate aggregation. To investigate whether human, like mice, CLEC-2 can be immunodepleted in vivo, AYP1 or HEL1 were injected intraperitoneally, and CLEC-2 surface expression was determined by flow cytometry. Both antibodies depleted CLEC-2 for at least 11 days, with levels returning to normal by 18 days for AYP1 and 24 days for HEL1 (Figure 2B; supplemental Figure 8). In both cases, there was a transient thrombocytopenia lasting up to 4 days (Figure 2A). This is consistent with immunodepletion of mCLEC-2 using INU1; however, the length of the CLEC-2 depletion is prolonged (Figure 2C).8,17
Figure 2.
hCLEC-2 can be immunodepleted using HEL1 or AYP1, with little effect on hemostasis. (A) Platelet count following intraperitoneal injection of INU1, AYP1, or HEL1 antibody (3 μg/g bodyweight). Transient thrombocytopenia lasting up to 4 days after injection can be seen for all antibody-treated groups compared with the untreated controls. Platelet count was determined by flow cytometry and is shown as the percentage of the baseline count. (B) hCLEC-2 surface expression determined by flow cytometry following depletion by either AYP1 or HEL1. For both antibodies, CLEC-2 could not be detected on the platelet surface for at least 11 days. (C) mCLEC-2 surface expression determined by flow cytometry following depletion by INU1 shows that CLEC-2 was absent for at least 7 days after injection. Measurements with anti-rat and anti-mouse IgG excluded the possibility that the abolished anti-CLEC-2-FITC binding was due to the presence of remaining anti-CLEC-2 antibodies on the platelets (supplemental Figure 8). (D) Depletion of CLEC-2 (using HEL1) had no effect on tail bleeding time. P > .05, Fisher exact test. Horizontal lines represent the median time to cessation of bleeding, with each circle representing 1 mouse. (E) Depletion of CLEC-2 had no effect on occlusive thrombus formation following mechanical injury of the abdominal aorta, and hCLEC-2KI mice were comparable to WT. P > .05, Fisher exact test. Horizontal lines represent the median time to vessel occlusion, with each circle representing 1 mouse. For all experiments, a minimum of 5 mice were tested per group. FITC, fluorescein isothiocyanate; mAb, monoclonal antibody; MFI, mean fluorescent intensity.
The effect of hCLEC-2 depletion was investigated in in vivo thrombosis and hemostasis models. Depletion had no effect on tail bleeding time (Figure 2D), which adds further support for CLEC-2 having a minor role in hemostasis.13 In the mechanical injury of the abdominal aorta thrombosis model, there was no difference in the time to vessel occlusion in CLEC-2–depleted hCLEC-2KI mice compared with untreated controls, and neither group differed significantly from WT mice (Figure 2E). Notably, in the ferric chloride–induced injury of the mesenteric arterioles, hCLEC-2KI mice displayed an increased time to occlusion with 9 of 16 vessels failing to occlude. This was not the case for the hCLEC-2KI–depleted mice, in which only 3 of 14 vessels failed to occlude (supplemental Figure 9). Thus, hCLEC-2KI mice apparently resemble CLEC-2–depleted WT mice, as mice lacking CLEC-2 were protected in this model of arterial thrombosis.8,13 These results are in line with previous studies suggesting that the contribution of CLEC-2 to thrombosis differs depending on the type of injury or the vascular bed.8,13,14 In addition, our data suggest that there is a difference in the roles of mouse and humanized CLEC-2 in arterial thrombosis, with mouse CLEC-2 contributing to thrombus stability. We speculate that this discrepancy to previous reports on CLEC-2–deficient mice that displayed reduced thrombus formation8,13,15,18 could be attributed to the postulated intravascular CLEC-2 ligand. This could explain the reduction in vessel occlusion in untreated hCLEC-2KI mice because the human receptor cannot interact with the mouse ligand to the same extent as the mouse receptor. In line with this hypothesis, it has recently been confirmed that mouse CLEC-2 contributes to arterial thrombosis and thrombus formation on collagen in vitro, whereas competitive inhibition of human CLEC-2 had no effect on in vitro thrombus formation.18 It is also possible that human CLEC-2 itself has, at least to some extent, an antithrombotic effect, which could explain the difference in vessel occlusion between hCLEC-2KI and hCLEC-2KI–depleted mice. Notably, hCLEC-2 can compensate for mCLEC-2 during development and in hemostasis, suggesting a conserved interaction between CLEC-2 and podoplanin in hCLEC-2KI mice.
Here, we have demonstrated that hCLEC-2 can be immunodepleted and provide proof of principle that the hCLEC-2KI mouse can be used to study anti-hCLEC-2 agents in vivo.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
Acknowledgments: The authors thank Stefanie Hartmann, Juliana Goldmann, and Ewa Stepien-Bötsch for their excellent technical assistance.
This project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement number 766118. This work was supported by the CRC1525 (project number 453989101) and the TR240 (project number 374031971) of the Deutsche Forschungsgemeinschaft (German Research Foundation) and the Rudolf Virchow Center.
Contribution: H.C.B. performed experiments, analyzed data, and wrote the manuscript; S.B., S.N., E.M.S.J., and J.K. performed experiments and analyzed data; Y.D. and V.M. provided vital reagents and proofread the manuscript; S.G.T., S.P.W., and B.N. supervised the research and edited the manuscript; and D.S. supervised the research, performed experiments, analyzed the data, and wrote the manuscript.
Footnotes
The data that support the plots within this article and other findings of this study are available upon reasonable request from the corresponding author, David Stegner (stegner@virchow.uni-wuerzburg.de).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Fuller GL, Williams JA, Tomlinson MG, et al. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282(17):12397–12409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rayes J, Watson SP, Nieswandt B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J Clin Invest. 2019;129(1):12–23. doi: 10.1172/JCI122955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertozzi CC, Schmaier AA, Mericko P, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116(4):661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finney BA, Schweighoffer E, Navarro-Nunez L, et al. CLEC-2 and Syk in the megakaryocytic/platelet lineage are essential for development. Blood. 2012;119(7):1747–1756. doi: 10.1182/blood-2011-09-380709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki-Inoue K, Kato Y, Inoue O, et al. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282(36):25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 6.Kato Y, Kaneko MK, Kunitz A, et al. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 2008;99(1):54–61. doi: 10.1111/j.1349-7006.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki-Inoue K. Roles of the CLEC-2–podoplanin interaction in tumor progression. Platelets. 2018;29(8):786–792. doi: 10.1080/09537104.2018.1478401. [DOI] [PubMed] [Google Scholar]
- 8.May F, Hagedorn I, Pleines I, et al. CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood. 2009;114(16):3464–3472. doi: 10.1182/blood-2009-05-222273. [DOI] [PubMed] [Google Scholar]
- 9.Shao B, Hoover CM, Shi H, et al. Deletion of platelet CLEC-2 decreases GPIbα-mediated integrin αIIbβ3 activation and decreases thrombosis in TTP. Blood. 2022;139(16):2523–2533. doi: 10.1182/blood.2021012896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hitchcock JR, Cook CN, Bobat S, et al. Inflammation drives thrombosis after Salmonella infection via CLEC-2 on platelets. J Clin Invest. 2015;125(12):4429–4446. doi: 10.1172/JCI79070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beristain-Covarrubias N, Perez-Toledo M, Flores-Langarica A, et al. Salmonella-induced thrombi in mice develop asynchronously in the spleen and liver and are not effective bacterial traps. Blood. 2019;133(6):600–604. doi: 10.1182/blood-2018-08-867267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stegner D, Göb V, Krenzlin V, et al. Foudroyant cerebral venous (sinus) thrombosis triggered through CLEC-2 and GPIIb/IIIa dependent platelet activation. Nat Cardiovasc Res. 2022;1(2):132–141. doi: 10.1038/s44161-021-00017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bender M, May F, Lorenz V, et al. Combined in vivo depletion of glycoprotein VI and C-type lectin-like receptor 2 severely compromises hemostasis and abrogates arterial thrombosis in mice. Arterioscler Thromb Vasc Biol. 2013;33(5):926–934. doi: 10.1161/ATVBAHA.112.300672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haining EJ, Cherpokova D, Wolf K, et al. CLEC-2 contributes to hemostasis independently of classical hemITAM signaling in mice. Blood. 2017;130(20):2224–2228. doi: 10.1182/blood-2017-03-771907. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki-Inoue K, Inoue O, Ding G, et al. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010;285(32):24494–24507. doi: 10.1074/jbc.M110.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gitz E, Pollitt AY, Gitz-Francois JJ, et al. CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood. 2014;124(14):2262–2270. doi: 10.1182/blood-2014-05-572818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenz V, Stegner D, Stritt S, et al. Targeted downregulation of platelet CLEC-2 occurs through Syk-independent internalization. Blood. 2015;125(26):4069–4077. doi: 10.1182/blood-2014-11-611905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourne J, Smith C, Jooss N, et al. CLEC-2 supports platelet aggregation in mouse but not human blood at arterial shear. Thromb Haemost. 2022;122(22):1988–2000. doi: 10.1055/a-1896-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.