Key Points
-
•
Results from SOLACE-adults demonstrate the PK and PD properties of crizanlizumab in patients with SCD during long-term treatment.
-
•
Results also show that no new safety signals were identified and the efficacy of crizanlizumab was consistent with that seen in SUSTAIN.
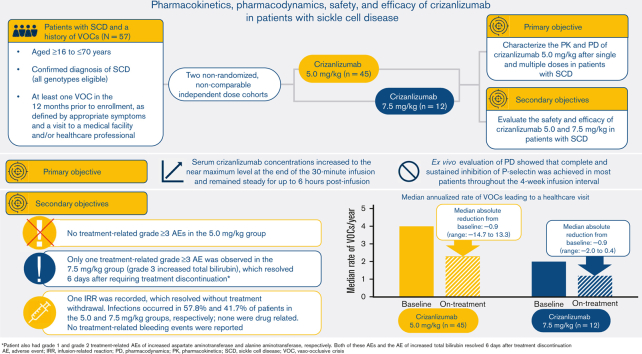
Visual Abstract
Abstract
Crizanlizumab is an anti–P-selectin monoclonal antibody indicated to reduce the frequency/prevent recurrence of vaso-occlusive crises (VOCs) in patients with sickle cell disease (SCD) aged ≥16 years. This analysis of an ongoing phase 2, nonrandomized, open-label study reports the pharmacokinetics (PK), pharmacodynamics (PD), safety, and efficacy of crizanlizumab 5.0 mg/kg (N = 45) and 7.5 mg/kg (N = 12) in patients with SCD with a history of VOCs. The median treatment duration was 104.7 and 85.7 weeks in the 5.0 and 7.5 mg/kg groups, respectively. For both doses, serum crizanlizumab concentrations rose to near maximum levels shortly after infusion, and near complete and sustained ex vivo P-selectin inhibition was observed. Grade ≥3 adverse events (AEs) occurred in 48.9% and 33.3% of patients in the 5.0 and 7.5 mg/kg groups, respectively; only 1 event was deemed treatment-related (7.5 mg/kg group). No treatment-related serious AEs occurred. One infusion-related reaction was recorded (5.0 mg/kg, grade 2 “pain during infusion”), which resolved without treatment withdrawal. Infections occurred in 57.8% and 41.7% of patients in the 5.0 and 7.5 mg/kg groups, respectively; none were drug-related. No treatment-related bleeding events were reported. No patients developed immunogenicity. The median (range) absolute reduction from baseline in the annualized rate of VOCs leading to a health care visit was −0.88 (−14.7 to 13.3) and −0.93 (−2.0 to 0.4) in the 5.0 and 7.5 mg/kg groups, respectively. Results here demonstrate the PK/PD properties of crizanlizumab in patients with SCD and the potential sustained efficacy and long-term safety of the drug after >12 months’ treatment. This trial was registered at www.clinicaltrials.gov as #NCT03264989.
Introduction
Acutely painful vaso-occlusive crises (VOCs) are the hallmark of sickle cell disease (SCD) and are the leading cause of emergency room visits and hospitalizations. They are associated with decreased quality of life, can lead to organ damage, and increase the risk of death.1,2
The pathophysiology of VOCs is thought to involve adhesive interactions between hemoglobin S–containing erythrocytes and activated endothelial cells, leukocytes, and platelets.3 Central to this process is P-selectin, a cell-adhesion protein expressed on activated platelets and endothelial cells.3,4
Crizanlizumab is a first-in-class humanized anti–P-selectin monoclonal antibody, initially manufactured by Reprixys (investigational name SelG1). In the phase 2 SUSTAIN study, crizanlizumab 5.0 mg/kg (SelG1) reduced the annualized rate of VOCs leading to a health care visit by 45.3% vs placebo (P = .01) in patients with SCD, regardless of SCD genotype or concomitant use of hydroxyurea (HU) (www.clinicaltrials.gov, #NCT01895361).5 Crizanlizumab was well tolerated; however, comprehensive data on adverse events (AEs) are so far unpublished.
Results from SUSTAIN led to the approval of crizanlizumab by the US Food and Drug Administration and European Medicines Agency to reduce the frequency/prevent recurrent VOCs in patients with SCD aged ≥16 years.
We report interim results from the phase 2, open-label SOLACE-adults study (#NCT03264989) evaluating the long-term pharmacokinetics (PK), pharmacodynamics (PD), safety, and efficacy of crizanlizumab 5.0 and 7.5 mg/kg, manufactured by Novartis under the investigational name SEG101, in patients with SCD and a history of VOCs. Based on the mechanism of action of crizanlizumab and considering it is a monoclonal antibody, AEs of special interest (AESI) include infections, infusion-related reactions (IRRs), effects on hemostasis (eg, bleeding), and immunogenicity. As the primary publication of the SUSTAIN trial did not describe AESI, these data are reported here together with the equivalent data from SOLACE-adults.
Methods
Patients
Patients were eligible to participate in SOLACE-adults if they met the key inclusion criteria: age ≥16 to ≤70 years, a confirmed diagnosis of SCD (all SCD genotypes were eligible), and at least 1 VOC in the 12 months before enrollment. Patients receiving HU or an erythropoietin-stimulating agent could be included, but they must have been receiving the drug for ≥6 months and continue at the same dose and schedule during the trial. Patients not already receiving HU could not initiate the drug during the trial. The trial definition of a VOC and key exclusion criteria are described in the supplemental materials.
Study design and treatment
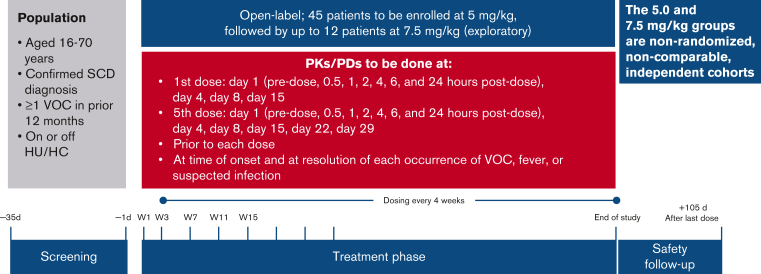
SOLACE-adults is an ongoing phase 2, multicenter, open-label study initiated in December 2017 (Figure 1). Data presented here are from an interim analysis (cutoff date: 1 August 2020). Patient enrollment and the dosing schedule are described in the supplemental materials.
Figure 1.
SOLACE-adults study design. HC, hydroxycarbamide.
Objectives and assessments
The primary objective of SOLACE-adults was to characterize the PK and PD of crizanlizumab (5.0 mg/kg) at the starting dose and at steady state in adults with SCD. PK and PD assessments were performed in all patients pre- and postdose on days 1, 2, 4, 8, and 15 after the first dose (week 1 day 1) and then on days 1, 2, 4, 8, 15, 22, and 29 after the fifth dose (week 15 day 1). For predose PK evaluation, serum concentrations were collected every 4 weeks from week 3 to week 51 and every 24 weeks thereafter. When possible, PK and PD were also assessed at the time of onset and resolution of each VOC, fever, or suspected infection.
Secondary objectives of SOLACE-adults were to evaluate the safety and efficacy of crizanlizumab at 5.0 and 7.5 mg/kg, with safety assessments performed in all patients who received ≥1 dose of crizanlizumab. As part of efficacy assessments, patients were continuously assessed for VOCs, with relevant medical treatment information collected in case of an occurrence. Self-reported VOCs managed at home were also collected, provided they were documented via a phone call to a relevant study site. VOCs were not included in safety assessments.
Statistical analyses
Descriptive statistics were used to summarize quantitative data, including demographic and baseline characteristics. All PK and ex vivo PD parameters are summarized descriptively for the PK/PD analysis sets. The PK analysis set 1 (PAS1)/PD analysis set 1 (PDS1) included all patients who provided at least 1 evaluable PK/PD profile. The PAS2/PDS2 included all patients who received ≥1 planned dose of crizanlizumab at 5.0 or 7.5 mg/kg and provided ≥1 corresponding evaluable PK concentration/PD assessment. The criteria for an evaluable PK/PD profile and definitions of the PK/PD parameters assessed are described in the supplemental materials.
AEs were evaluated based on the Medical Dictionary for Regulatory Activities (MedDRA) version 23.1, and AE severity was based on the Common Terminology Criteria for Adverse Events version 5.0. AESI included infections, IRRs, effects on hemostasis (eg, bleeding), and immunogenicity. Potential IRRs were assessed by searching the Novartis Case Retrieval Strategy database using standardized MedDRA queries and 4 different search strategies (standard, severe reactions, pain events, and complement-mediated), regardless of grade and causality, as deemed by the study investigators. Definitions for the IRR search strategies and the full lists of preferred terms used in each search are provided in the supplemental materials. As the evaluation of pain related to IRRs may be confounded by the occurrence of VOCs within 24 hours of infusion, a simultaneous exploratory analysis of VOCs occurring on the same day or the day after infusion was also conducted. Immunogenicity was evaluated by the occurrence of on-treatment antidrug antibodies to crizanlizumab.
The rate of VOCs leading to a health care visit for every patient was annualized and descriptively compared with the corresponding rate in the 12 months before study screening (baseline).
AESI and results from the exploratory analysis of VOCs occurring on the same day or the day after infusion in SUSTAIN are also reported here and were determined according to the same criteria described for SOLACE-adults, using MedDRA version 23.1 for AEs. Detailed information regarding the methodology and main results of SUSTAIN has been published previously.5
Ethics approval
SOLACE-adults was approved by the institutional review board or independent ethics committee of each participating center. This study was carried out in accordance with the recommendations of the Declaration of Helsinki with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Results
Study population
In total, 57 patients were enrolled in SOLACE-adults; 45 and 12 received 5.0 and 7.5 mg/kg of crizanlizumab, respectively (Table 1). In the 5.0 and 7.5 mg/kg cohorts, respectively, 41 (91.1%) and 11 (91.7%) patients were eligible for both PAS1 and PAS2, 38 (84.4%) and 11 (91.7%) were eligible for PDS1, and 40 (88.9%) and 11 (91.7%) were included in PDS2. All patients in both dose cohorts were included in the safety and efficacy analyses. Accordingly, there were differences between the dose groups in certain baseline characteristics. For instance, the proportions of patients with concomitant HU use and ≥5 VOCs in the 12 months before screening were higher in the 5.0 mg/kg group than in the 7.5 mg/kg group (Table 1). Baseline characteristics and demographics for patients enrolled in SUSTAIN have been described previously.5
Table 1.
Baseline characteristics and demographics of the SOLACE-adults study population
| Crizanlizumab, 5.0 mg/kg, N = 45 | Crizanlizumab, 7.5 mg/kg, N = 12 | |
|---|---|---|
| Age, y | ||
| Mean (SD) | 32 (12.7) | 27 (12.3) |
| Median (range) | 29 (17-65) | 21 (16-48) |
| Sex, n (%) | ||
| Female | 25 (55.6) | 6 (50.0) |
| Male | 20 (44.4) | 6 (50.0) |
| Race, n (%) | ||
| Black or African American | 44 (97.8) | 12 (100) |
| White and Black or African American∗ | 1 (2.2) | 0 |
| Ethnicity, n (%) | ||
| Not Hispanic or Latino | 33 (73.3) | 8 (66.7) |
| Hispanic or Latino | 3 (6.7) | 0 |
| Unknown | 9 (20.0) | 4 (33.3) |
| Genotype, n (%) | ||
| HbSS | 27 (60.0) | 4 (33.3) |
| HbSC | 10 (22.2) | 5 (41.7) |
| HbSβ0 thalassemia | 3 (6.7) | 0 |
| HbSβ+ thalassemia | 3 (6.7) | 0 |
| Other/unknown | 2 (4.4) | 3 (25.0) |
| Concomitant HU/hydroxycarbamide, n (%) | ||
| Yes | 33 (73.3) | 7 (58.3) |
| No | 12 (26.7) | 5 (41.7) |
| Patients’ VOCs in prior 12 mo, n (%) | ||
| <5 | 26 (57.8) | 9 (75.0) |
| ≥5 | 19 (42.2) | 3 (25.0) |
| Patients’ VOC type in prior 12 mo, n (%) | ||
| Uncomplicated sickle cell VOC | 44 (97.8) | 11 (91.7) |
| Acute chest syndrome | 4 (8.9) | 2 (16.7) |
| Priapism | 2 (4.4) | 0 |
HbSS, hemoglobin SS; SD, standard deviation.
Patients of mixed descent.
A total of 15 (26.3%) patients discontinued treatment during SOLACE-adults; 13 (28.9%) and 2 (16.7%) in the 5.0 and 7.5 mg/kg cohorts, respectively. Reasons for treatment discontinuation in the 5.0 mg/kg cohort were mostly related to patient (n = 7, 15.6%) and physician decision (n = 3, 6.7%). Other reasons for discontinuation in the 5.0 mg/kg group included AE (n = 1, 2.2%), pregnancy (n = 1, 2.2%), and death (n = 1, 2.2%). In the 7.5 mg/kg group, reasons for discontinuation were AE and death (both n = 1, 8.3%). For patients who discontinued treatment during the study, the median time to discontinuation was 86 and 16 weeks in the 5.0 and 7.5 mg/kg groups, respectively. Data for dose compliance are described in the supplemental materials.
At data cutoff, patients had received crizanlizumab for a median (range) of 104.7 (6-133) and 85.7 (9-92) weeks in the 5.0 and 7.5 mg/kg cohorts, respectively; 39 (86.7%) and 10 (83.3%) patients received treatment for ≥54 weeks. Patients received a median (range) of 26.0 (2-33) doses of crizanlizumab in the 5.0 mg/kg cohort and 21.5 (3-24) doses of crizanlizumab in the 7.5 mg/kg cohort. The study is ongoing.
Change in PK and PD parameters
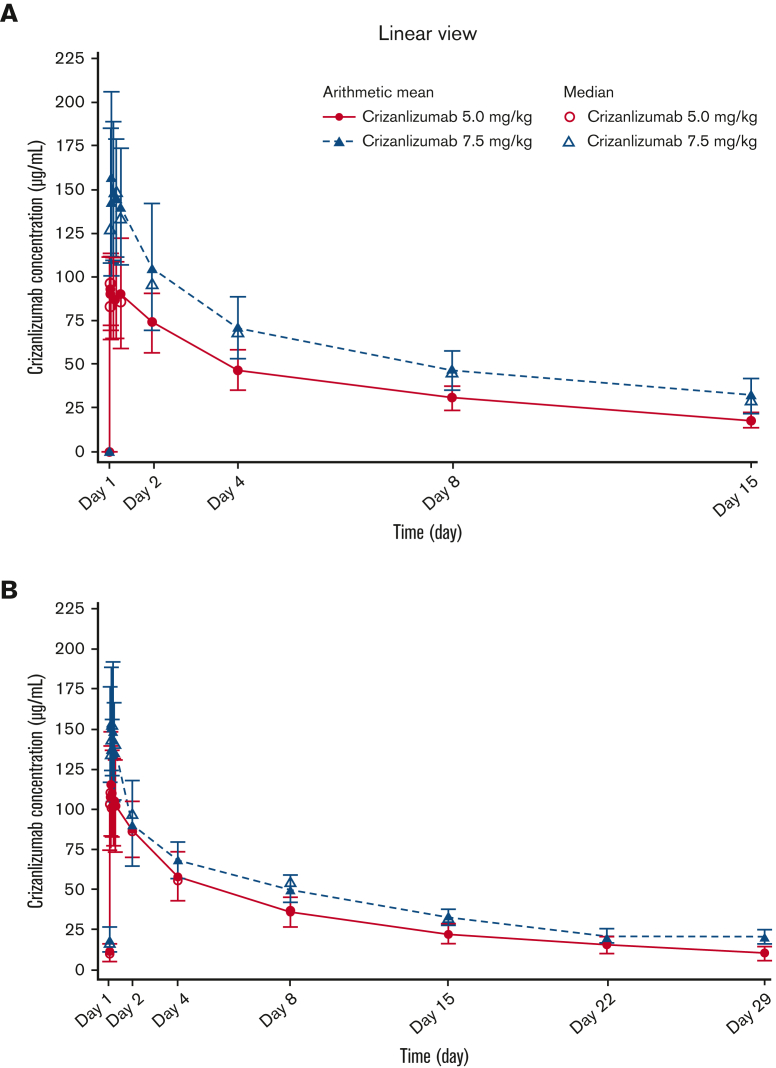
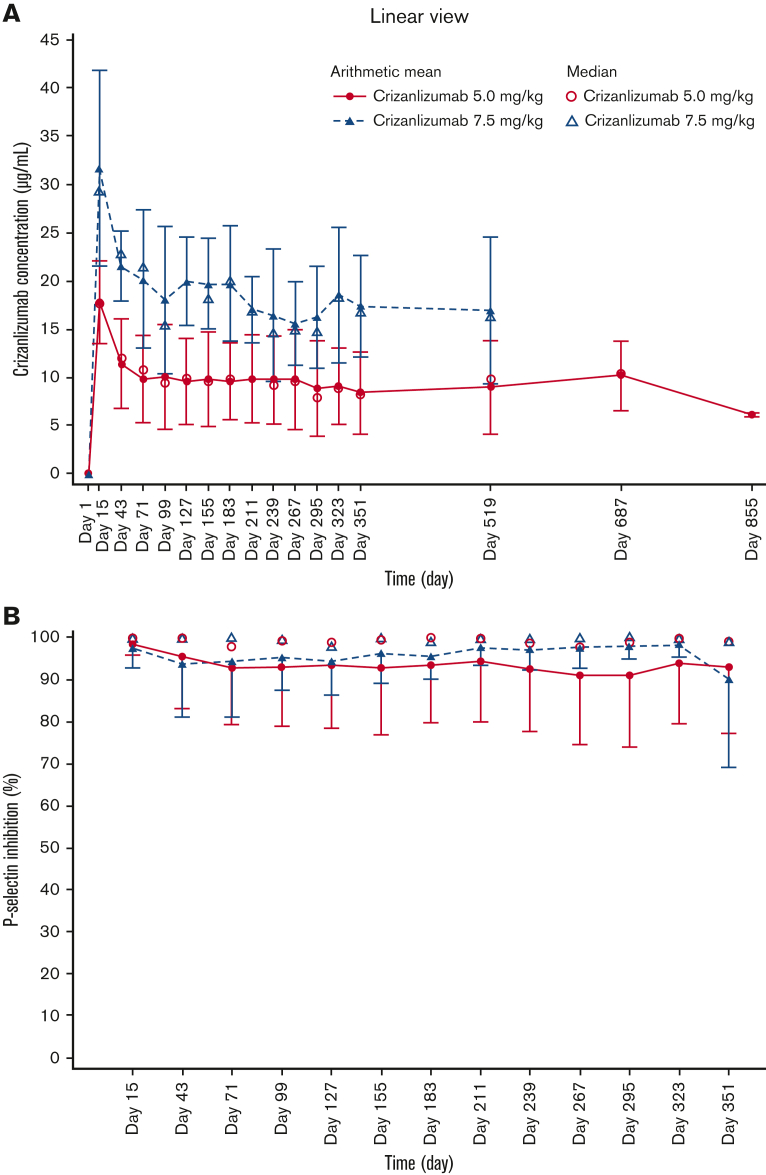
Serum crizanlizumab concentrations increased to near maximum levels at the end of the 30-minute infusion and remained steady for up to 6 hours after infusion, both at the starting dose and steady state, in the 5.0 and 7.5 mg/kg groups (Figure 2). PK parameters generally exhibited low interpatient variability (Table 2).
Figure 2.
Serum concentration-time profiles for crizanlizumab. Mean and median serum concentration-time profiles for the crizanlizumab 5.0 and 7.5 mg/kg dose groups at starting dose (A) and steady state (PAS2) (B).
Table 2.
PK and PD parameters for crizanlizumab at starting dose (week 1 day 1) and at steady state (week 15 day 1) in PAS1
| Crizanlizumab, 5.0 mg/kg, N = 41 | Crizanlizumab, 7.5 mg/kg, N = 11 | |
|---|---|---|
| Wk 1 d 1 | ||
| AUCd15 (h × μg/mL), n | 37 | 10 |
| Mean (SD) | 13 000 (2 770) | 19 800 (4 490) |
| CV% | 21.3 | 22.7 |
| Cmax (μg/mL), n | 40 | 10 |
| Mean (SD) | 99.9 (29.6) | 174 (49.9) |
| CV% | 29.6 | 28.7 |
| Tmax (h), n | 40 | 10 |
| Median (range) | 1.5 (0.4-26.1) | 1.1 (0.6-23.6) |
| PD-AUCd15 (h%), n | 37 | 10 |
| Mean (SD) | 33 200 (1 800) | 33 200 (2 530) |
| CV% | 5.4 | 7.6 |
| Wk 15 d 1 (steady state) | ||
| AUCtau (h × μg/mL), n | 35 | 9 |
| Mean (SD) | 20 900 (4 810) | 27 800 (2 780) |
| CV% | 23.0 | 10.0 |
| Cmax (μg/mL), n | 35 | 10 |
| Mean (SD) | 125 (36.1) | 168 (34.2) |
| CV% | 28.9 | 20.4 |
| Tmax (h), n | 35 | 10 |
| Median (range) | 1.9 (0.6-6.3) | 3.0 (0.6-23.4) |
| T½ (h), n | 35 | 10 |
| Mean (SD) | 269 (84.8) | 325 (57.7) |
| PD-AUCd29 (h%), n | 32 | 10 |
| Mean (SD) | 67 500 (5 900) | 61 600 (10 900) |
| CV% | 8.7 | 17.6 |
AUCd15, area under the curve from time 0 to the last measurable concentration sampling time at starting dose; AUCtau, area under the curve from time 0 to the last measurable concentration sampling time at steady state; Cmax, maximum observed serum drug concentration after dose administration; CV%, coefficient of variation; PD-AUCd15, area under the curve from time 0 to the last measurable PD sampling time at starting dose; PD-AUCd29, area under the curve from time 0 to the last measurable PD sampling time at steady state; Tmax, time to reach maximum observed serum drug concentration after dose administration; T½, elimination half-life associated with the terminal slope of a semilogarithmic scale.
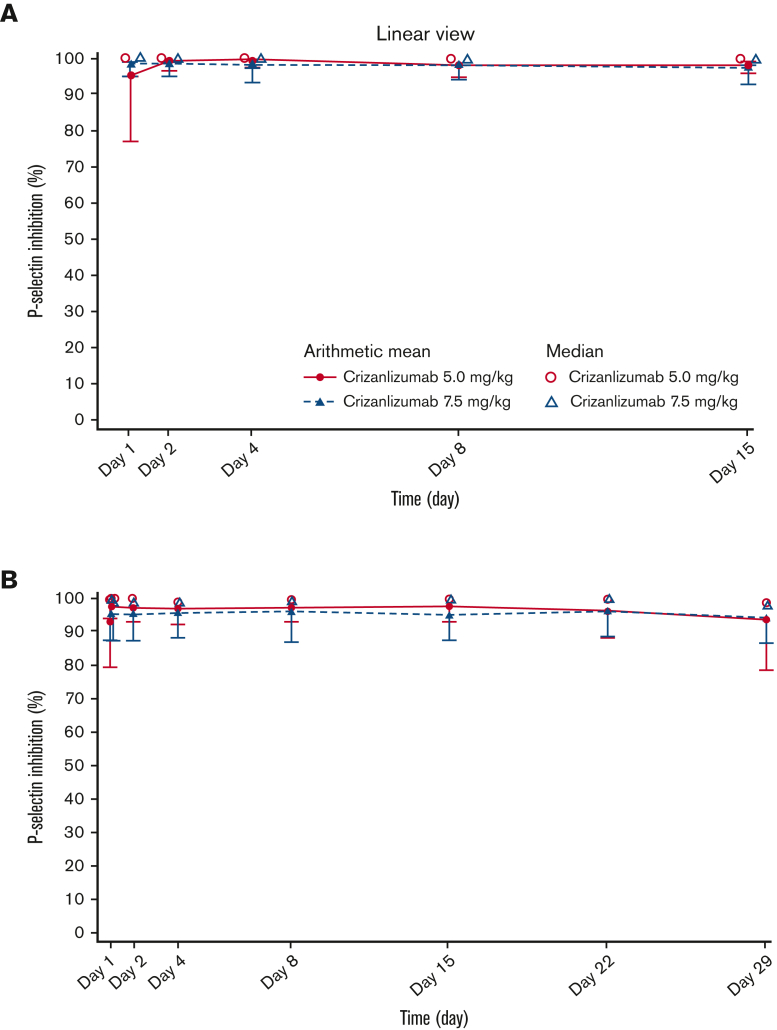
In both dose groups, ex vivo evaluation of PD showed that complete and sustained inhibition of P-selectin was achieved in most patients throughout the 4-week infusion interval (Figure 3). Primary PK and PD parameters at the starting dose and at steady state are described in Table 2.
Figure 3.
Serum PD-time profiles for crizanlizumab. Mean and median serum PD-time profiles for the crizanlizumab 5.0 and 7.5 mg/kg dose groups at starting dose (A) and steady state (PDS2) (B).
Mean (standard deviation) predose (trough) concentrations at weeks 3 and 7 in the 5.0 mg/kg group were 17.8 (4.3) μg/mL and 11.4 (4.7) μg/mL, respectively, and 31.7 (10.1) μg/mL and 21.6 (3.6) μg/mL in the 7.5 mg/kg group, respectively. Values at steady state ranged between 8.4 (4.2) μg/mL and 10.1 (5.5) μg/mL from weeks 11 to 51 in the 5.0 mg/kg group and between 15.5 (4.3) μg/mL and 20.2 (7.2) μg/mL in the 7.5 mg/kg group (Figure 4A). Predose PK data in the 5.0 mg/kg group from week 75 onward should be interpreted with caution, as the number of evaluable patients decreased from 20 at week 75 to 2 at week 123; data are only available up to week 75 in the 7.5 mg/kg group. Predose levels of P-selectin inhibition remained stable at 90% to 100% throughout the study in both dose groups (Figure 4B).
Figure 4.
Predose serum concentration-time and serum PD-time profiles for crizanlizumab. Mean and median predose serum concentration-time (A) and serum PD-time profiles (B) for crizanlizumab 5.0 and 7.5 mg/kg (PAS2 and PDS2, respectively).
Safety
The most common AEs in SOLACE-adults, regardless of relationship to study treatment, were pyrexia, headache, and hypokalemia (supplemental Table 1). Grade ≥3 AEs occurred in 22 (48.9%) patients in the 5.0 mg/kg cohort and in 4 patients (33.3%) in the 7.5 mg/kg cohort. Seven patients (all in the 5.0 mg/kg group; 15.6%) had grade ≥3 AEs of hypokalemia; none were considered treatment-related, led to dose interruption, or treatment discontinuation.
AEs deemed treatment-related were reported in 12 (26.7%) and 3 (25.0%) patients in the 5.0 and 7.5 mg/kg groups, respectively; none were of grade ≥3 severity in the 5.0 mg/kg group, with only 1 event of grade ≥3 severity in the 7.5 mg/kg group (increased serum bilirubin, which resolved after treatment discontinuation). The most common treatment-related AE was headache (5.0 mg/kg, n = 2, 4.4%; 7.5 mg/kg, n = 1, 8.3%). In the 5.0 mg/kg group, each treatment-related AE was reported in 1 or 2 patients only (a total of 15 events in 12 patients).
Serious AEs (SAEs) were reported in 17 (37.8%) and in 3 (25.0%) patients in the 5.0 and 7.5 mg/kg groups, respectively; none were drug-related. Only 1 SAE, pulmonary embolism, occurred in >1 patient (n = 2). Two patients died during the study; 1 in the 5.0 mg/kg group (multiorgan dysfunction) and 1 in the 7.5 mg/kg group (brain injury together with acute kidney injury, ischemic hepatitis, and cardiac arrest) (Table 3); none of the SAEs were considered to be related to treatment.
Table 3.
AESI, VOCs occurring within 24 hours of infusion (the same day or the day after infusion), and deaths in SOLACE-adults
| Safety topic | Crizanlizumab, 5.0 mg/kg, N = 45 |
Crizanlizumab, 7.5 mg/kg, N = 12 |
||
|---|---|---|---|---|
| All grades | Grade ≥3 | All grades | Grade ≥3 | |
| No. of patients with AESI, n (%) | ||||
| Infections (all) | 26 (57.8) | 8 (17.8) | 5 (41.7) | 1 (8.3) |
| Potential signs and symptoms of IRRs (standard search)∗ | 16 (35.6) | 0 | 3 (25.0) | 0 |
| IRR (severe reactions search)∗ | 1 (2.2) | 0 | 1 (8.3) | 1 (8.3) |
| Pain events∗ | 10 (22.2) | 1 (2.2) | 1 (8.3) | 0 |
| CARPA (complement-mediated search)∗ | 0 | 0 | 0 | 0 |
| Effect on hemostasis, hemorrhage | 8 (17.8) | 2 (4.4) | 1 (8.3) | 0 |
| Antidrug antibodies | 0 | 0 | 0 | 0 |
| VOCs | ||||
| Patients with a VOC on the same d or the d after infusion, n (%) | 19 (42.2) | 4 (33.3) | ||
| Total number of VOCs on the same d or the d after infusion, n | 32 | 6 | ||
| Proportion of infusions associated with a VOC on the same d or the d after infusion, % | 3.0 | 2.6 | ||
| Deaths, n (%) | ||||
| On-treatment deaths | 1 (2.2) | 1 (8.3) | ||
| Deaths deemed related to treatment | 0 | 0 | ||
| Deaths deemed unrelated to treatment | 1 (2.2) | 1 (8.3) | ||
AEs were evaluated based on MedDRA version 23.1.
CARPA, complement activation–related pseudoallergy.
As the different searches are not mutually exclusive (ie, some events are captured in multiple searches), the total number of patients in the combined search is less than the sum of the individual search categories.
One patient in the 5.0 mg/kg group discontinued treatment because of breast cancer (not considered related to treatment). One patient discontinued treatment in the 7.5 mg/kg group because of a grade 3 AE of elevated serum bilirubin, which was deemed treatment-related and resolved without sequelae.
AESI
Infections, potential effects on hemostasis (eg, bleeding), immunogenicity, and IRRs were AESI (Table 3). Infections were reported in 26 patients in the 5.0 mg/kg group (57.8%; grade ≥3, n = 8, 17.8%), most commonly upper respiratory tract infection (n = 9, 20.0%), pharyngitis (n = 4, 8.9%), gastroenteritis, pneumonia, and urinary tract infection (each n = 3, 6.7%). Five patients in the 7.5 mg/kg group (41.7%; grade ≥3, n = 1, 8.3%) had AEs of infection, most commonly upper respiratory tract infection (n = 2, 16.7%). No AEs of infection were considered treatment-related or led to treatment discontinuation.
The search for potentially severe IRRs, which used preferred terms that did not specify a relationship to treatment, identified events in 2 patients: 1 in the 5.0 mg/kg group (2.2%) and 1 in the 7.5 mg/kg group (8.3%). The event in the 5.0 mg/kg group was of grade 2 severity; it was documented as “pain during infusion,” was deemed treatment-related, and resolved on the same day without drug interruption or hospitalization, without sequelae, and without the need for any premedication. The event in the 7.5 mg/kg group was a grade 3 anaphylactic reaction that occurred 22 days after the last infusion; it was deemed related to the administration of an antibiotic and resolved without any action.
The search for other potential signs and symptoms that may be indicative of, but not specific to, IRRs (standard search) identified events in 16 (35.6%) and 3 (25.0%) patients in the 5.0 and 7.5 mg/kg groups, respectively; none of these events were of grade ≥3 severity. Four patients had potential signs and symptoms of IRRs that were considered related to treatment (5.0 mg/kg, n = 2 [4.4%; IRR and headache]; 7.5 mg/kg, n = 2 [16.7%; headache and diarrhea]). Pain events occurring within 24 hours of infusion were reported in 10 (22.2%) patients in the 5.0 mg/kg group, comprising headache (n = 7, 15.6%), arthralgia (n = 2, 4.4%), and bone pain (n = 1, 2.2% [grade 3; deemed not related to treatment]); none were considered treatment-related except for 1 event of headache, which resulted in a temporary dose interruption. No events were identified from the complement-mediated search. None of the events identified from any of the IRR searches led to treatment discontinuation.
A search for AEs indicative of potential bleeding or disturbances of hemostasis identified events in 8 (17.8%) patients in the 5.0 mg/kg group and in 1 (8.3%) patient in the 7.5 mg/kg cohort and they were mostly related to laboratory abnormalities, considered as expected events that are consistent with SCD itself. In the 5.0 mg/kg group, the most common AEs were decrease in hemoglobin from baseline and contusion (both n = 2, 4.4%). No patients developed antidrug antibodies during the study. Other AEs of potential interest are described in the supplemental materials.
AESI in SUSTAIN are described in supplemental Table 2. No apparent differences in the rate of infections, pain events, or effects on hemostasis (eg, bleeding) were observed between the 2 crizanlizumab arms and compared with placebo.
Pain caused by an IRR may be confounded by the occurrence of VOCs and/or chronic pain. Therefore, in addition to evaluating the potential pain-related IRRs, an exploratory analysis of VOCs occurring on the same day or the day after crizanlizumab infusion was conducted for SUSTAIN and SOLACE-adults (supplemental Table 3).
In SUSTAIN, VOCs occurring on the same day or the day after infusion were reported for 4.7% of infusions in the placebo arm compared with 2.0% and 2.8% of infusions in the crizanlizumab 2.5 and 5.0 mg/kg arms, respectively, suggesting that IRRs related to crizanlizumab did not manifest as VOCs in SUSTAIN (supplemental Table 3). Similar results for VOCs occurring on the same day or the day after infusion were observed in SOLACE-adults (Table 3).
Efficacy
In SOLACE-adults, the median (range) annualized rate of VOCs leading to a health care visit in the 5.0 mg/kg group was 4.0 (1.0-25.0) in the 12 months before screening and 2.25 (0.0-17.3) on-treatment (median [range] absolute reduction from baseline, −0.88 [−14.7 to 13.3]). In the 7.5 mg/kg group, the corresponding rates were 2.0 (1.0-9.0) and 1.16 (0.0-7.9) (median [range] absolute reduction from baseline, −0.93 [−2.0 to 0.4]). Nine (20.0%) patients in the 5.0 mg/kg group and 5 (41.7%) in the 7.5 mg/kg group were VOC-free during the first 12 months of treatment in each respective group.
The median (range) annualized rate of VOCs leading to a health care visit in the 5.0 mg/kg cohort in year 1 (n = 45) and year 2 (n = 39), respectively, was 3.0 (0-21.0) and 1.38 (0-17.0) on-treatment (median [range] absolute reduction from baseline, −1.0 [−14.0 to 17.0] and −1.0 [−16.0 to 10.0]). In the 7.5 mg/kg group, the corresponding rate for year 1 (n = 12) was 2.0 (0.0-7.9) (median [range] absolute reduction from baseline, −1.0 [−2.0 to 1.0]); the duration of follow-up was not long enough in the 7.5 mg/kg group to evaluate the rate for year 2.
Discussion
Crizanlizumab at 5.0 mg/kg is an effective therapy for reducing the frequency of VOCs that lead to a health care visit in patients with SCD, with a generally favorable safety profile, as demonstrated in the prior SUSTAIN study5 and reinforced here. Published PK/PD data for crizanlizumab in patients with SCD are limited. This analysis evaluated the PK and PD, as well as the safety and efficacy of crizanlizumab at 5.0 and 7.5 mg/kg in patients with SCD and a history of VOCs. Results from SOLACE-adults demonstrate that the PK/PD profiles of crizanlizumab at 5.0 and 7.5 mg/kg (SEG101) in patients with SCD are generally consistent with those observed for crizanlizumab (SelG1) in healthy volunteers and in SUSTAIN.5,6 Mean elimination half-life for the 5.0 mg/kg dose (SEG101) in SOLACE-adults was 269 hours (11.2 days) vs 254 hours (10.6 days) for the same dose (SelG1) in healthy volunteers.6 Exposure to crizanlizumab at 5.0 and 7.5 mg/kg (SEG101) increased in a roughly dose-proportional manner in SOLACE-adults, similar to findings for the 2.5 and 5.0 mg/kg doses in SUSTAIN,5 and exposure to crizanlizumab (SelG1) over a dose range of 0.2 to 8.0 mg/kg increased in a nonlinear manner in healthy volunteers.6,7 Notably, prior PK/PD analyses of crizanlizumab (SelG1) in healthy volunteers mostly evaluated single doses in small patient cohorts (N ranging from 3-6). For both doses in SOLACE-adults, maximum observed serum drug concentrations at starting dose and at steady state were similar, indicating a lack of accumulation. Moreover, both doses resulted in almost complete and sustained ex vivo inhibition of P-selectin throughout the 4-week infusion interval, consistent with previous findings in healthy volunteers and in SUSTAIN.6,7
There were no new or unexpected safety signals in SOLACE-adults after >12 months’ treatment in each dose group. The rate and severity of AEs in SOLACE-adults were generally consistent with those observed in the SUSTAIN trial,5 although safety comparisons between the 2 studies should be interpreted with caution, given the different durations of exposure to crizanlizumab in each trial. The frequency and severity of AEs were similar in the 5.0 and 7.5 mg/kg dose groups, with the most commonly occurring AE being pyrexia. Very few AEs led to treatment discontinuation (1 patient per dose group), indicating that most AEs were manageable. Importantly, the results here demonstrate the favorable safety profile of crizanlizumab during long-term treatment, compared with SUSTAIN, with patients in the 5.0 mg/kg group receiving crizanlizumab for a median of ∼24 months and ∼87% completing >12 months of treatment.
Infections, IRRs, bleeding events, and immunogenicity are AESI for crizanlizumab. By reporting the incidence of AESI both in SUSTAIN and SOLACE-adults, we provide the most in-depth analysis to date on the potential occurrence and severity of these AEs during treatment with crizanlizumab. Notably, no AESI led to treatment discontinuation in SUSTAIN or SOLACE-adults.
IRRs related to monoclonal antibody infusions are caused by an immune reponse8 and commonly present as allergic-type reactions.9,10 The cause of potential IRRs associated with crizanlizumab remains unclear and is undergoing evaluation. The incidence of IRRs in SOLACE-adults and SUSTAIN, evaluated using 4 different search strategies, was rare: ∼3% of patients in SUSTAIN and ∼2% of those in SOLACE-adults in the 5.0 mg/kg groups (severe reactions search criteria). No cases of severe hypersensitivity/anaphylaxis occurred. The frequency and severity of pain events (not VOCs) on the day of infusion with crizanlizumab in SOLACE-adults and/or SUSTAIN were comparable with that in the placebo arm of SUSTAIN, suggesting that the incidence of these AEs is probably related to the underlying disease or concurrent conditions/treatments. There were fewer VOCs occurring on the same day or the day after infusion with crizanlizumab in both SUSTAIN and SOLACE-adults vs the placebo arm of SUSTAIN.
The search for potential complement activation–related pseudoallergy found no events in either SOLACE-adults or SUSTAIN, suggesting that the occurrence of these events is very rare. Individuals with SCD may be at risk of complement activation–related pseudoallergy irrespective of crizanlizumab because complement activation has previously been reported in patients with SCD, with 1 study demonstrating that sC5b-9 levels are natively increased in ∼61% of untreated patients.11
Although no severe treatment-related IRRs presenting as pain events were observed in SUSTAIN or SOLACE-adults, such events have been observed in the postmarketing setting.6,12,13 Additional studies, particularly in the real-world setting in larger patient cohorts,13 are ongoing and will ascertain the real-world incidence of IRRs with crizanlizumab relative to that observed in clinical trials. Regardless, patients receiving crizanlizumab should be vigilantly monitored for and advised of the potential signs and symptoms indicative of IRRs (eg, headache, fever, and chills).6 As it can be difficult to distinguish the pain associated with IRRs from VOCs and/or chronic pain, patients should receive crizanlizumab infusions in a monitored environment under the supervision of an SCD specialist. In the rare event that an IRR occurs, based on the data presented here, appropriate action should be taken, including temporary treatment interruption and the administration of necessary medications (eg, analgesics, nonsteroidal anti-inflammatory drugs) for mild/moderate IRRs and the discontinuation of crizanlizumab in addition to the initiation of appropriate therapy for severe IRRs.6 Importantly, corticosteroids for the treatment of pain associated with IRRs should be avoided where possible, as any exposure to these medications may cause rebound pain and other SCD-related complications.14
Efficacy analyses in SOLACE-adults were a secondary objective. However, results from SOLACE-adults corroborate findings from SUSTAIN, demonstrating a reduction from baseline in the annualized rate of VOCs leading to a health care visit with both the 5.0 and 7.5 mg/kg doses. Importantly, findings from SOLACE-adults demonstrate the sustained efficacy of crizanlizumab after >12 months of treatment, with the median absolute change in the annualized rate of VOCs leading to a health care visit reduced by a consistent amount in years 1 and 2 of treatment with the 5.0 mg/kg dose. Moreover, 20% and 42% of patients in the 5.0 and 7.5 mg/kg groups, respectively, were VOC-free during the first 12 months of treatment. The proportion of patients who were VOC-free in each dose group, as well as comparisons with the corresponding data in SUSTAIN, should be interpreted with caution. It should also be noted that the 5.0 and 7.5 mg/kg dose groups were nonrandomized, noncomparable, independent cohorts, resulting in differences between the 2 dose groups in certain baseline patient characteristics, and only 12 patients were included in the exploratory 7.5 mg/kg dose group. Comparisons of efficacy between the 2 dose groups are therefore infeasible. The efficacy of crizanlizumab at 5.0 vs 7.5 mg/kg in patients with SCD is being investigated in the ongoing phase 3 STAND study (#NCT03814746), which will also elucidate the potential sustained efficacy of crizanlizumab for an even longer period than demonstrated in this study, with patients receiving treatment for up to 5 years. The exploratory analysis of VOCs occurring on the same day or the day after infusion in SUSTAIN and SOLACE-adults may have identified events that started before the infusion.
Conclusion
The therapeutic landscape for SCD has been devoid of treatment options until recent years. As various new therapeutics emerge from clinical development, it is of paramount importance that evidence on the use of these treatments in patients continues to grow. Here, we show that no new safety signals were identified in the population of patients with SCD treated with crizanlizumab at 5.0 or 7.5 mg/kg in SOLACE-adults. Stable predose concentrations were observed for both doses throughout the study, with a lack of accumulation. The elimination half-life for the 5.0 mg/kg dose was ∼11 days, consistent with findings in healthy volunteers. Exposure to crizanlizumab increased in a roughly dose-proportional manner from the 5.0 to 7.5 mg/kg dose. Consistent with the SUSTAIN trial, crizanlizumab reduced the annualized rate of VOCs leading to a health care visit from baseline in SOLACE-adults. Importantly, results from this study suggest the potential sustained efficacy and long-term safety of crizanlizumab after >12 months of treatment in patients with SCD and a history of VOCs.
Clinical trial registration
Pharmacokinetics and pharmacodynamics study of SEG101 (crizanlizumab) in patients with SCD with VOCs (SOLACE-adults) (NCT03264989); study to assess safety and impact of SelG1 with or without HU therapy in patients with SCD with pain crises (SUSTAIN) (NCT01895361).
Conflict-of-interest disclosure: J.K. reports consultancy for Novartis, Forma Therapeutics, Graphite Bio, and Fulcrum Therapeutics and participation in an advisory board meeting for Novartis, Forma Therapeutics, Fulcrum Therapeutics, Novo Nordisk, and Beam Therapeutics. R.C.B. reports consultancy for Global Blood Therapeutics, Imara, Novartis, and Novo Nordisk and research funding for Global Blood Therapeutics, Imara, Novartis, Forma Therapeutics, and Pfizer. C.N. reports participation in an advisory board meeting for Global Blood Therapeutics and Forma Therapeutics and research funding for Global Blood Therapeutics and Novartis. A.K. reports consultancy for Novartis and Guidepoint; research funding for Global Blood Therapeutics and Forma Therapeutics; participation in a speakers bureau for Global Blood Therapeutics; membership on a scientific committee for bluebird bio and Graphite Bio; participation in an advisory board meeting for Novartis; and participation in an adjudication committee for Vertex. D.M. reports consultancy for Novartis, Global Blood Therapeutics, bluebird bio, and Forma Therapeutics; honoraria from the Research Triangle Institute and the Cure Sickle Cell Initiative, each on behalf of the National Heart, Lung and Blood Institute, National Institutes of Health; and research funding from Grifols. N.S. reports consultancy for Novartis and Global Blood Therapeutics; research funding from Novartis; participation in an advisory board meeting for Global Blood Therapeutics and Forma Therapeutics; and participation in a speakers bureau for Novartis and Global Blood Therapeutics. C.T., G.S.-O., and U.A. are employees of Novartis Pharma AG. S.B. was an employee of IQVIA at the time of writing. The remaining authors declare no competing financial interests.
The current affiliation for R.C.B. is Cytel, London, United Kingdom.
The current affiliation for S.B. is Global Blood Therapeutics, South San Francisco, CA.
Acknowledgments
The authors thank the patients who participated in the SOLACE-adults and SUSTAIN trials, site investigators, studies’ coordinators, and Thomas Walker from Mudskipper Business Ltd, United Kingdom, who provided medical writing support funded by Novartis Pharmaceuticals Corporation in accordance with Good Publication Practice (GPP3) guidelines.
The SOLACE-adults study is sponsored by Novartis.
Authorship
Contribution: J.K., R.C.B., C.N., A.K., D.M., N.S., S.M.N., and D.L. were study investigators and contributed to the interpretation of data and manuscript development; C.T., G.S.-O., U.A., and S.B. contributed to the interpretation of data and manuscript development.
Footnotes
Novartis is committed to sharing with qualified external researchers, access to patient-level data, and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel based on scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Shah N, Bhor M, Xie L, et al. Sickle cell disease complications: prevalence and resource utilization. PLoS One. 2019;14(7) doi: 10.1371/journal.pone.0214355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Tuijn CFJ, van Beers EJ, Schnog J-JB, et al. Pain rate and social circumstances rather than cumulative organ damage determine the quality of life in adults with sickle cell disease. Am J Hematol. 2010;85(7):532–535. doi: 10.1002/ajh.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Prim. 2018;4:18010. doi: 10.1038/nrdp.2018.10. [DOI] [PubMed] [Google Scholar]
- 4.Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood. 2013;122(24):3892–3898. doi: 10.1182/blood-2013-05-498311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429–439. doi: 10.1056/NEJMoa1611770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adakveo Prescribing information. 2020. https://www.ema.europa.eu/en/documents/product-information/adakveo-epar-product-information_en.pdf Novartis.
- 7.Stocker JW, Mandarino D, Kawar Z, et al. Placebo-controlled, double-blind, first-in-human, ascending single dose and multiple dose, healthy subject study of intravenous-administered SelG1, a humanized anti-P-selectin antibody in development for sickle cell disease [abstract] Blood. 2013;122(21):970. [Google Scholar]
- 8.Brennan FR, Morton LD, Spindeldreher S, et al. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. MAbs. 2010;2(3):233–255. doi: 10.4161/mabs.2.3.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doessegger L, Banholzer ML. Clinical development methodology for infusion-related reactions with monoclonal antibodies. Clin Transl Immunology. 2015;4(7):e39. doi: 10.1038/cti.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cáceres MC, Guerrero-Martín J, Pérez-Civantos D, et al. The importance of early identification of infusion-related reactions to monoclonal antibodies. Ther Clin Risk Manag. 2019;15:965–977. doi: 10.2147/TCRM.S204909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roumenina LT, Chadebech P, Bodivit G, et al. Complement activation in sickle cell disease: dependence on cell density, hemolysis and modulation by hydroxyurea therapy. Am J Hematol. 2020;95(5):456–464. doi: 10.1002/ajh.25742. [DOI] [PubMed] [Google Scholar]
- 12.Kanter J, Shah A, Joshi V, et al. Rare cases of infusion-related reactions (IRRs) presenting as pain events during or after crizanlizumab infusion in patients (pts) with sickle cell disease (SCD): a systematic evaluation of post-marketing (PM) reports [abstract] Blood. 2021;138(suppl 1):3112. Abstract. [Google Scholar]
- 13.Kanter J, Hellemann G, Cohen A, et al. Early evaluation of the use of crizanlizumab in sickle cell disease: a National Alliance of Sickle Cell Centers Study [abstract] Blood. 2021;138(suppl 1):3113. Abstract. [Google Scholar]
- 14.Brandow AM, Carroll CP, Creary S, et al. American Society of Hematology 2020 guidelines for sickle cell disease: management of acute and chronic pain. Blood Adv. 2020;4(12):2656–2701. doi: 10.1182/bloodadvances.2020001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.