Abstract
Objective
Angiogenesis plays an important role in various physiological and pathological conditions and is essential for tumor growth and metastasis. The aim of this study was to evaluate the effect of a combination of vandetanib and celecoxib on angiogenic tube formation and its effect on angiogenic genes (MMP-2 and MMP-9) using an in vitro model of human umbilical vein endothelial cells (HUVECs).
Methods
HUVECs were cultured and verified by flow cytometry. HUVECs were then treated with vandetanib, celecoxib, and the combination of both drugs. Then, we investigated cell viability and cell apoptosis by MTT assays and flow cytometry. The process of angiogenesis was analyzed by tube formation assays, and the effect on angiogenic genes was determined by RT-qPCR.
Results
HUVECs were positive for CD144 and negative for CD14. Vandetanib, celecoxib, and their combination inhibited HUVEC viability in a dose-dependent manner (p < 0.001). The rate of apoptosis was 13.1%, 9%, and 23.7% (p < 0.001) when treated with vandetanib, celecoxib, or the combination of both drugs, respectively. Vandetanib inhibited tube formation by 43.7%, celecoxib by 21%, and their combination by 77.3% (p < 0.001), respectively. RT-qPCR revealed that both vandetanib and celecoxib reduced the expression levels of MMP-2 and MMP-9, and their combination resulted in an even greater extent of reduction in expression levels (p < 0.001).
Conclusion
Celecoxib enhanced the effect of vandetanib in inhibiting in vitro angiogenesis and the combination of these two drugs led to even greater extents of inhibition than vandetanib alone.
Keywords: Angiogenesis, Celecoxib, HUVEC, MMP-2, MMP-9, Vandetanib
المخلص
أهداف البحث
يلعب تولد الأوعية دورا مهما في مختلف الحالات الفسيولوجية والمرضية. إنه ضروري لنمو الورم ورم خبيث. الهدف من هذه الدراسة هو تقييم تأثير تركيبة فانديتانيب وسيليكوكسيب على تكوين الأنبوب الوعائي وتأثيره على الجينات المولدة للأوعية (م.م.ب -2 و م.م.ب -9) باستخدام نموذج مختبري للإنسان. الخلايا البطانية للوريد السري.
طرق البحث
تم استنبات الخلايا البطانية للوريد السري البشري والتحقق منها عن طريق قياس التدفق الخلوي. عولجت الخلايا البطانية في الوريد السري البشري بفانديتانيب وسيليكوكسيب ومزيج من العقارين ، وتم تحديد قابلية بقاء الخلية وموت الخلايا المبرمج باستخدام مقايسة م.ت.ت وقياس التدفق الخلوي. تم تحليل عملية تكوين الأوعية باستخدام اختبار تكوين الأنبوب، وتم تحديد التأثير على الجينات المولدة للأوعية باستخدام النسخ العكسي لتفاعل البلمرة المتسلسل الكمي في الوقت الحقيقي.
النتائج
كانت الخلايا البطانية للوريد السري البشري موجبة لـ سي.دي-144 وسلبية لـ سي.دي-14. أدى فانديتانيب وسيليكوكسيب وتركيبهما إلى تثبيط بقاء الخلايا البطانية للوريد السري البشري بطريقة تعتمد على الجرعة. كان معدل موت الخلايا المبرمج 13.1٪، 9٪ و 23.7٪، عند العلاج بـ فانديتانيب، سيليكوكسيب، أو مزيج من كلا العقارين. منع فانديتانيب تشكيل الأنبوب بنسبة 43.7٪ ، والسيليكوكسيب بنسبة 21٪، ومزيجهم بنسبة 77.3٪ على التوالي. النسخ العكسي من تفاعل البوليميراز المتسلسل الكمي في الوقت الحقيقي. أظهر أن كلا من فاندتانيب وسيليكوكسيب يقللان من مستويات التعبير عن م.م.ب -2 و م.م.ب -9، وأظهر الجمع بينهما مستوى أعلى من الانخفاض في التعبير المستوى الذي وجد أنه ذو دلالة إحصائية.
الاستنتاجات
سيليكوكسيب يعزز تأثير فانديتانيب في تثبيط تكوين الأوعية في المختبر، وقد أظهر الجمع بينهما مستويات أعلى من التثبيط مقارنة باستخدام فانديتانيب وحده.
الكلمات المفتاحية: تولد الأوعية, سيليكوكسيب, فانديتانيب, الخلايا البطانية للوريد السري البشري, م.م.ب -2, م.م.ب -9
Introduction
Angiogenesis is defined as the process used to form new blood vessels from pre-existing vasculature.1 Angiogenesis is a naturally occurring process that takes place throughout the body and can be divided into physiological and pathological angiogenesis. Physiological angiogenesis is necessary for tissue development, reproduction and wound repair while pathological angiogenesis is required for tumor growth and metastasis.2,3 Angiogenesis is considered as a main target for treating cancer because it is essential for supplying nutrients and oxygen for the growth of tumors.4
Matrix metalloproteinases (MMPs) are zinc-containing proteolytic enzymes that are mostly involved in degradation of the extracellular matrix to promote cellular invasion, migration and events such as angiogenesis.5 In humans, there are 23 members of the MMP family. Of these, MMP-2 and MMP-9 are predominantly expressed in various human cancers (breast, lung, gastric, bladder, prostate and ovarian cancer) and play a vital role in tumor angiogenesis and invasion. Therefore, the targeting of MMP-2 and MMP-9 is considered as an ideal approach for the treatment of cancer.6, 7, 8
Vandetanib is a multi-kinase inhibitor that inhibits angiogenesis by targeting vascular endothelial growth factor receptor 2, the epidermal growth factor receptor, proto-oncogene RET9 and MMPs. Vandetanib is mainly used for treating medullary thyroid cancer but has also shown beneficial effects for other cancers, such as breast, colon and lung cancer, and hepatocellular carcinoma.10, 11, 12
Celecoxib belongs to a class of non-steroidal anti-inflammatory drugs (NSAIDs) that inhibits the process of inflammation by targeting the cyclooxygenase-2 (COX-2) enzyme.13 Celecoxib is commonly used for treating arthritis, although various studies have also demonstrated its beneficial effect in the treatment of human cancers. Studies have also reported that celecoxib possesses an anti-cancer effect and is also capable of inhibiting angiogenesis by targeting VEGF and MMPs. Different clinical trials are underway to investigate the effect of celecoxib in combination with other drugs for treating cancer.14
As vandetanib and celecoxib both possess anticancer and antiangiogenic effects, they have never been used in combination. In this study, we investigated the effect of vandetanib and celecoxib, as single agents and in combination, on angiogenesis using HUVECs as an in vitro model.
Materials and Methods
Vandetanib (Caprelsa, Sanofi) and celecoxib (celbexx, Getz Pharma) stock solutions were serially diluted in culture medium to obtain final concentrations of vandetanib (1, 2 and 4 μM) and celecoxib (1.5, 3 and 4.5 μg/mL).
Isolation and primary culture of HUVECs
The umbilical cord, which is normally discarded after childbirth, was collected with the informed consent of patients from the gynecology wards of Dow University Hospital. HUVECs were isolated from umbilical cords using methods described previously.15 The cells were cultured in EBM-2 media (Lonza, Walkersville, MD) supplemented with 100 U/mL penicillin/streptomycin and 15% FBS (Sigma–Aldrich). The cells were maintained at 37 °C in a cell culture incubator in the presence of 5% CO2 and 95% humidified air. The culture media was changed on alternate days until the cells reached a confluent state. The cells were then trypsinized using Tryple Express (Life Technologies) and sub-cultured till P2.
HUVEC verification (flow cytometry)
HUVECs were verified by flow cytometry using the BD FAC Celesta. Detached cells were resuspended in 500 μL of PBS and then incubated for 30 min at 4 °C with the following antibodies (BD Pharmingen): Horizon V450-A-conjugated anti-CD144 and Horizon v500-A-conjugated anti-CD14. Unstained cells were used as a negative control and the obtained data were analyzed using FACSDiva software 8.0.
Cell viability (MTT) assays
An MTT assay (methylthiazolyldiphenyl tetrazolium bromide) (Sigma–Aldrich) was performed to investigate the effect of drugs on the growth of HUVECs. In a 96-well plate, cells were seeded at a density of 15,000 cells per well and cultured continuously for 24 h. After 24 h, the media was replaced with fresh culture medium containing 2% FBS and then treated with different concentrations of vandetanib (1, 2 and 4 μM), celecoxib (1.5, 3 and 4.5 μg/mL), and their combination for 48 h. Cells without the drug were used as controls. After 48 h, MTT solution (5 mg/mL) was added to each well, and the incubation was continued for another 4 h. The culture medium was then removed and 150 μL of dimethyl sulfoxide (DMSO) was added to each well; this enabled MTT crystals to completely dissolve. Plates were read at a wavelength of 560 nm using a spectrophotometer, and the inhibition rates of cell growth were calculated using the following formula: Growth rate (%) = (ODControl group − ODTreatment group)/(ODControl group) × 100%.
Cell apoptosis (flow cytometry)
HUVEC apoptosis was examined by flow cytometry (BD FACS Celesta) using an annexin apoptosis detection kit (BD Pharmingen). HUVECs (30,000 cells/well) were plated in 6-well plates and then incubated for 24 h. After 24 h, the cells were treated with vandetanib (1 μM), celecoxib (1.5 μg/mL), and the combination of both drugs, for 48 h. After 48 h, the cells were collected by centrifugation at 10,000 rpm, resuspended in 500 μL of binding buffer, and incubated for 15 min at room temperature. After that, 5 μL of propidium iodide and 5 μL of Annexin V-FITC were added and then incubated for another 15–20 min at room temperature. The data were analyzed by flow cytometry using FACSDiva Software 8.0 (BD Bioscience).
Tube formation assays
The process of angiogenesis was demonstrated using a tube formation assay, as described by Liu et al.16 The 96-well plate, pre-chilled at −20 °C, was carefully filled with 60 μL per well of liquid geltrex at 4 °C with a pre-chilled pipette, avoiding bubbles. The geltrex was then polymerized for 1 h at 37 °C. HUVECs (15,000 cells per well) were suspended in 200 μL of complete medium supplemented with 2% FBS in the absence or presence of vandetanib, celecoxib, or their combinations, at the indicated concentrations and carefully layered on the top of the polymerized geltrex. After 8 h of incubation, the effect on the formation of endotubes was inspected using a phase contrast microscope, and images were recorded at 10× magnification. The data were analyzed by Image J software 1.52.
Real-time quantitative reverse transcriptase polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells treated with vandetanib, celecoxib, and the combination of both drugs, by TRIzol reagent (Life Technologies), as described in the manufacturer's protocol. Approximately 1 μg of RNA was reverse transcribed using a High-Capacity RNA-to-cDNA kit (Applied Biosystems). Then, qPCR was performed using the SYBR Green PCR Master Mix (Qiagen). The primer sequences were as follows: MMP-2: 5′-CAGGCTCTTCTCCTTTCACAAC-3′ and 5′-AAGCCACGGCTTGGTTTTCCTC-3′; MMP-9: 5′-TGGGCTACGTGACCTATGACAT-3′ and 5′-GCCCAGCCCACCTCCACTCCTC-3′; β-actin: 5′-CTACAATGAGCTGCGTGTGG-3′ and 5′-AGCTCTTCTCCAGGGAGGA-3′. The 2−ΔΔCT method was used to calculate the mRNA expression level after normalizing to β-actin.
Statistical analysis
Statistical analysis was performed using SPSS software 16.0. ANOVA and Tukey's post hoc test were applied to compare the control and treatment groups. A p value < 0.05 was considered statistically significant.
Results
HUVEC culture and verification
HUVECs exhibited typical cobblestone morphology under phase contrast microscopy, as shown in Figure 1A. The purity of HUVECs was determined by flow cytometry using positive and negative markers. HUVECs were positive for CD144 and negative for CD14, as shown in Figure 1B.
Figure 1.
HUVEC primary culture and verification. (A) The typical cobblestone morphology of HUVECs under phase contrast microscopy. (B) Verification of HUVECs by flow cytometry. Negative control cells are shown in black and the test sample is shown in pink (n = 3).
Effect of vandetanib, celecoxib, and their combination, on the viability of HUVECs
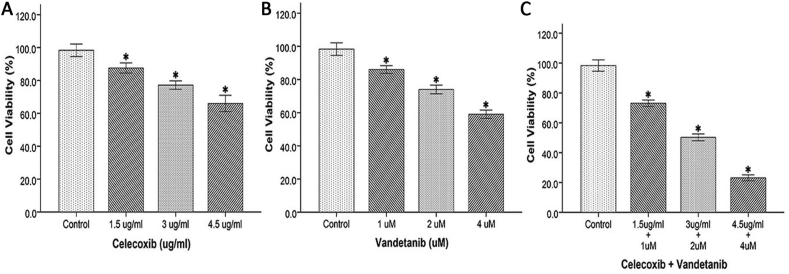
HUVEC viability in the presence of vandetanib was 87% at 1 μM, 75% at 2 μM, and 60% at 4 μM, respectively. Similarly, when treated with celecoxib, HUVEC viability was 89% at 1.5 μg/mL, 78% at 3 μg/mL, and 68% at 4.5 μg/mL, respectively. HUVEC viability at 1 μM vandetanib and 1.5 μg/mL celecoxib was 74%. At 2 μM vandetanib and 3 μg/mL of celecoxib, the viability of HUVECs was 51%. At 4 μM vandetanib and 4.5 μg/mL of celecoxib, the viability of HUVECs was 24%. These findings revealed a dose-dependent reduction in HUVEC viability (p < 0.001), as shown in Figure 2.
Figure 2.
Effect of drugs on the viability of HUVECs. The effect of (A) celecoxib, (B) vandetanib, and (C) the combination of these two drugs on the viability of HUVECs. Each bar represents the mean ± SD of three independent experiments. ∗p < 0.05 as compared to controls (n = 3).
Effect of vandetanib, celecoxib, and their combination, on apoptosis in HUVECs
The rates of apoptosis were 13.1%, 9%, and 23.7%, when HUVECs were treated with vandetanib, celecoxib, or the combination of both drugs. These results further confirmed that at the initial doses, vandetanib (1 μM), celecoxib (1.5 μg/mL), and their combination, showed a mild to moderate effect on HUVEC apoptosis (p < 0.001) and were considered safe, as shown in Figure 3.
Figure 3.
Effect of drugs on apoptosis in HUVECs. (A) The effect of celecoxib (1.5 μg/mL), vandetanib (1 μM), and their combination on apoptosis in HUVECs as compared to controls. (B) Analysis of HUVEC apoptosis. Each bar represents the mean ± SD of three independent experiments. ∗p < 0.05 as compared to controls (n = 3).
Effect of vandetanib, celecoxib, and their combination, on HUVEC tube formation
Total tube length decreased by 43.7% (p < 0.001) when treated with vandetanib. Similarly, when treated with celecoxib, the total tube length was reduced by 21% (p < 0.001). The total tube length was significantly reduced by 77.3% (p < 0.001) when treated with their combination in comparison with the control, as shown in Figure 4. These results suggest that vandetanib and celecoxib are both effective in inhibiting tube formation, and that their combination showed more pronounced inhibition than either drug alone.
Figure 4.
Effect of drugs on HUVEC tube formation. (A) The effect of celecoxib (1.5 μg/mL), vandetanib (1 μM), and their combination on HUVEC tube formation as compared to controls. Image taken at 10× magnification. Scale bar = 1000 μm. (B) Analysis of HUVEC tube formation. Each bar represents the mean ± SD of three independent experiments. ∗p < 0.05 as compared to controls (n = 3).
Effect of vandetanib, celecoxib, and their combination, on angiogenic genes (MMP-2 and MMP-9)
RT-qPCR was performed to investigate the effects of vandetanib, celecoxib, and their combination, on tumor angiogenic genes (MMP-2 and MMP-9). Analysis showed that vandetanib and celecoxib both reduced the expression levels of MMP-2 and MMP-9, and that their combination resulted in a greater extent of reduction in expression levels, as shown in Figure 5. Both MMP-2 and MMP-9 led to the inhibition of angiogenesis with the co-treatment of vandetanib and celecoxib (p < 0.001).
Figure 5.
Effect of drugs on angiogenic genes. The effect of celecoxib (1.5 μg/mL), vandetanib (1 μM), and their combination, on (A) MMP-2 and (B) MMP-9. Each bar represents the mean ± SD of three independent experiments. ∗p < 0.05 as compared to control (n = 3).
Discussion
In this study we evaluated the effect of vandetanib and celecoxib, and their combination, in terms of the inhibition of angiogenesis by using an in vitro model (HUVECs). HUVECs are frequently used for the in vitro investigation of angiogenesis because they are very compatible and comparatively easy to obtain.17
HUVECs were successfully isolated from umbilical cords and exhibited a typical cobblestone morphology, as reported in previous studies.18 HUVECs were further verified by flow cytometry using positive and negative markers. Analysis showed that HUVECs were positive for CD144 and negative for CD14; these findings were in accordance with previous research.15
As described in our previous literature review, vandetanib and celecoxib both possess anticancer effects; however, the effects of a combination of these drugs on the inhibition of angiogenesis has yet to be investigated.10,14 We hypothesized that the combination of vandetanib and celecoxib would have a more prominent effect in terms of inhibiting in vitro angiogenesis, thereby enhancing drug efficacy and reducing drug resistance.
Our findings showed that vandetanib, celecoxib, and their combination, inhibited the viability of HUVECs in a dose-dependent manner; these findings were consistent with the research reported in a previous study, which revealed 45–50% inhibition.19,20
Next, we evaluated the effects of vandetanib (1 μM), celecoxib (1.5 μg/mL), and their combination, on apoptosis in HUVECs by flow cytometry. Our findings showed that vandetanib (1 μM), celecoxib (1.5 μg/mL), and their combination, exhibited a mild to moderate effect on HUVEC apoptosis and were therefore considered to be safe. This finding is in complete agreement with previous studies.21
The effect of vandetanib, celecoxib, and their combination, on angiogenesis was investigated by performing tube formation assays; such assays are widely considered to be a reliable technique for investigating the angiogenic and antiangiogenic actions of a drug. Our results showed that vandetanib and celecoxib both inhibited tube formation; these findings are in accordance with those of previous studies, which reported 37% inhibition by vandetanib22 and 35.6% inhibition by celecoxibs.23 When the combination of vandetanib and celecoxib was used, a more pronounced inhibition of tube formation was observed.
Finally, we investigated the effect of the combination of vandetanib and celecoxib on the expression of two tumor angiogenic genes (MMP-2 and MMP-9). Our results revealed that vandetanib and celecoxib both inhibited the expression levels of MMP-2 and MMP-9; this finding was in complete agreement with previous studies by Giannelli et al.12 and Zhou et al.24 When vandetanib and celecoxib were combined, there was a significant inhibition in the expression levels of MMP-2 and MMP-9.
Conclusion
This study demonstrated that the combination of vandetanib and celecoxib exerted a significantly greater inhibitory effect on in vitro angiogenesis than vandetanib alone. The results suggest that the combination of these drugs might be an effective therapeutic option for treating tumors.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This study was conducted in Dow University of Health Sciences after receiving Institutional Review Board permission (IRB-1908/DUHS/Approval/2021/211).
Authors contributions
AQ and MA designed the study. AQ prepared the manuscript. AQ and DAS gathered and analyzed the data. MMA and SZ revised the manuscript. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
The authors would like to thank Dow University of Health Sciences for their kind support in data collection.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Zeng A., Wang S.-R., He Y.-X., Yan Y., Zhang Y. Progress in understanding of the stalk and tip cells formation involvement in angiogenesis mechanisms. Tissue Cell. 2021;73 doi: 10.1016/j.tice.2021.101626. [DOI] [PubMed] [Google Scholar]
- 2.Zhou W., Tang W., Sun Z., Li Y., Dong Y., Pei H., et al. Discovery and optimization of N-substituted 2-(4-pyridinyl)thiazole carboxamides against tumor growth through regulating angiogenesis signaling pathways. Sci Rep. 2016;6 doi: 10.1038/srep33434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao S., Zhang Z., Yao Z., Shao J., Chen A., Zhang F., et al. Tetramethylpyrazine attenuates sinusoidal angiogenesis via inhibition of hedgehog signaling in liver fibrosis. IUBMB Life. 2017 doi: 10.1002/iub.1598. [DOI] [PubMed] [Google Scholar]
- 4.Kim Y.S., Li F., O'Neill B.E., Li Z. Specific binding of modified ZD6474 (Vandetanib) monomer and its dimer with VEGF receptor-2. Bioconjug Chem. 2013;24(11):1937–1944. doi: 10.1021/bc400374t. [DOI] [PubMed] [Google Scholar]
- 5.Jana S., Chatterjee K., Ray A.K., DasMahapatra P., Swarnakar S. Regulation of matrix metalloproteinase-2 activity by COX-2-PGE2-pAKT axis promotes angiogenesis in endometriosis. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0163540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Farina P., Tabouret E., Lehmann P., Barrie M., Petrirena G., Campello C., et al. Relationship between magnetic resonance imaging characteristics and plasmatic levels of MMP2 and MMP9 in patients with recurrent high-grade gliomas treated by Bevacizumab and Irinotecan. J Neurooncol. 2017;132(3):433–437. doi: 10.1007/s11060-017-2385-0. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandran R.K., Sørensen M.D., Aaberg-Jessen C., Hermansen S.K., Kristensen B.W. Expression and prognostic impact of matrix metalloproteinase-2 (MMP-2) in astrocytomas. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0172234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H.A., Hyun S.A., Byun B., Chae J.H., Kim K.S. Electrophysiological mechanisms of vandetanib-induced cardiotoxicity: comparison of action potentials in rabbit Purkinje fibers and pluripotent stem cell-derived cardiomyocytes. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0195577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L., Yu J., Jiao S., Wang W., Zhang F., Sun S. Vandetanib (ZD6474) induces antiangiogenesis through mTOR-HIF-1 alpha-VEGF signaling axis in breast cancer cells. Onco Targets Ther. 2018;11:8543–8553. doi: 10.2147/ott.s175578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Indra R., Pompach P., Martínek V., Takácsová P., Vavrová K., Heger Z., et al. Identification of human enzymes oxidizing the anti-thyroid-cancer drug vandetanib and explanation of the high efficiency of cytochrome P450 3A4 in its oxidation. Int J Mol Sci. 2019;20(14) doi: 10.3390/ijms20143392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannelli G., Sgarra C., Porcelli L., Azzariti A., Antonaci S., Paradiso A. EGFR and VEGFR as potential target for biological therapies in HCC cells. Cancer Lett. 2008;262(2):257–264. doi: 10.1016/j.canlet.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z., Ma X., Jia X., Wang R., Liu L., Zhang M., et al. Prevention of severe acute pancreatitis with cyclooxygenase-2 inhibitors: a randomized controlled clinical trial. Am J Gastroenterol. 2020;115(3):473–480. doi: 10.14309/ajg.0000000000000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosas C., Sinning M., Ferreira A., Fuenzalida M., Lemus D. Celecoxib decreases growth and angiogenesis and promotes apoptosis in a tumor cell line resistant to chemotherapy. Biol Res. 2014;47:27. doi: 10.1186/0717-6287-47-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voltan R., Zauli G., Rizzo P., Fucili A., Pannella M., Marci R., et al. In vitro endothelial cell proliferation assay reveals distinct levels of proangiogenic cytokines characterizing sera of healthy subjects and of patients with heart failure. Mediators Inflamm. 2014;2014:257081. doi: 10.1155/2014/257081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Tian H., Blobe G.C., Theuer C.P., Hurwitz H.I., Nixon A.B. Effects of the combination of TRC105 and bevacizumab on endothelial cell biology. Invest New Drugs. 2014;32(5):851–859. doi: 10.1007/s10637-014-0129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittarella P., Squarzanti D.F., Molinari C., Invernizzi M., Uberti F., Reno F. NO-dependent proliferation and migration induced by Vitamin D in HUVEC. J Steroid Biochem Mol Biol. 2015;149:35–42. doi: 10.1016/j.jsbmb.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y., Yu T., Liang H., Wang J., Xie J., Shao J., et al. Nitric oxide inhibits hetero-adhesion of cancer cells to endothelial cells: restraining circulating tumor cells from initiating metastatic cascade. Sci Rep. 2014;4:4344. doi: 10.1038/srep04344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue K., Torimura T., Nakamura T., Iwamoto H., Masuda H., Abe M., et al. Vandetanib, an inhibitor of VEGF receptor-2 and EGF receptor, suppresses tumor development and improves prognosis of liver cancer in mice. Clin Cancer Res. 2012;18(14):3924–3933. doi: 10.1158/1078-0432.ccr-11-2041. [DOI] [PubMed] [Google Scholar]
- 20.Liu N.N., Zhao N., Cai N. The effect and mechanism of celecoxib in hypoxia-induced survivin up-regulation in HUVECs. Cell Physiol Biochem. 2015;37(3):991–1001. doi: 10.1159/000430225. [DOI] [PubMed] [Google Scholar]
- 21.Lin H.P., Kulp S.K., Tseng P.H., Yang Y.T., Yang C.C., Chen C.S. Growth inhibitory effects of celecoxib in human umbilical vein endothelial cells are mediated through G1 arrest via multiple signaling mechanisms. Mol Cancer Ther. 2004;3(12):1671–1680. [PubMed] [Google Scholar]
- 22.Conconi M.T., Marzaro G., Guiotto A., Urbani L., Zanusso I., Tonus F., et al. New vandetanib analogs: fused tricyclic quinazolines with antiangiogenic potential. Invest New Drugs. 2012;30(2):594–603. doi: 10.1007/s10637-010-9621-1. [DOI] [PubMed] [Google Scholar]
- 23.Kim J.Y., Chung S.W., Kim S.Y., Byun Y. Enhanced anti-angiogenic effect of low molecular weight heparin-bile acid conjugates by co-administration of a selective COX-2 inhibitor. Pharm Res. 2015;32(7):2318–2327. doi: 10.1007/s11095-015-1623-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X., Shi X., Ren K., Fan G.T., Wu S.J., Zhao J.N. Celecoxib inhibits cell growth and modulates the expression of matrix metalloproteinases in human osteosarcoma MG-63 cell line. Eur Rev Med Pharmacol Sci. 2015;19(21):4087–4097. [PubMed] [Google Scholar]