Abstract
TEL-JAK2 fusion proteins, which are a result of t(9;12)(p24;p13) translocations associated with human leukemia, activate Stat5 in vitro and in vivo and cause a myelo- and lymphoproliferative disease in a murine bone marrow transplant model. We report that Socs-1, a member of the SOCS family of endogenous inhibitors of JAKs and STATs, inhibits transformation of Ba/F3 cells by TEL-JAK2 but has no effect on Ba/F3 cells transformed by BCR-ABL, TEL-ABL, or TEL–platelet-derived growth factor receptor beta. TEL-JAK2, in addition to activating Stat5, associates with Shc and Grb2 and induces activation of Erk2, and expression of Socs-1 inhibits engagement of each of these signaling molecules. TEL-JAK2 kinase activity is inhibited by Socs-1, as assessed by in vitro kinase assays. In addition, Socs-1 induces proteasomal degradation of TEL-JAK2. Mutational analysis indicates that the SOCS box of Socs-1 is required for proteasomal degradation and for abrogation of growth of TEL-JAK2-transformed cells. Furthermore, murine bone marrow transplant assays demonstrate that expression of Socs-1 prolongs latency of TEL-JAK2-mediated disease in vivo. Collectively, these data indicate that Socs-1 inhibits TEL-JAK2 in vitro and in vivo through inhibition of kinase activity and induction of TEL-JAK2 protein degradation.
Numerous chromosomal translocations which result in constitutive activation of tyrosine kinases, including BCR-ABL, TEL–platelet-derived growth factor receptor beta (PDGFβR) TEL-TRKC, TEL-ABL, and TEL-JAK2, have been identified in patients with leukemia (6, 10, 11, 22, 33, 34, 39). Signaling pathways activated by the respective native kinases are also constitutively activated by the fusion proteins, including activation of STATs by BCR-ABL, TEL-PDGFβR, and TEL-JAK2 and activation of mitogen-activated protein kinase (MAPK) by BCR-ABL, TEL-JAK2, and TEL-TRKC (1, 19, 25, 40, 45). In addition, mechanisms exist by which these pathways are negatively regulated, such as dephosphorylation of Erk2 by MKP-3 or decreased activation of STATs through endogenous inhibitors in the SOCS (suppressors of cytokine signaling) family of proteins (7, 30, 31, 43). These endogenous negative regulatory loops may provide a means of inhibiting transformation by tyrosine kinase fusion proteins.
Three TEL-JAK2 fusion variants that are the consequence of t(9;12)(p24;p13) chromosomal translocations have been identified in patients with T-cell acute lymphoblastic leukemia (ALL), pre-B-cell ALL, and atypical chronic myelogenous leukemia (CML) (see Fig. 1) (22, 34). The translocations result in the fusion of the pointed domain (PNT) of TEL, which mediates oligomerization of the protein, to the JH1 kinase domain of JAK2. All fusion variants are localized to the cytoplasm of cells and transform the murine hematopoietic cell line Ba/F3 to factor-independent growth. Mutational analysis has demonstrated that transformation of hematopoietic cells by TEL-JAK2 in vitro and in vivo requires the PNT domain of TEL as well as the kinase activity of the JAK2 JH1 domain (15, 21, 40, 51).
FIG. 1.
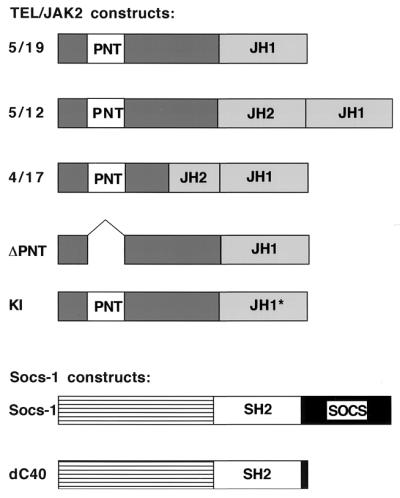
Schematic representation of TEL-JAK2 and Socs-1 constructs. Fusion variants involving TEL and JAK2 have been previously described (22, 35, 40). Briefly, the TEL-JAK2 variants result in the fusion of exon 5 of TEL to exon 19 of JAK2 (5/19), the exon 5 of TEL to exon 12 of JAK2 (5/12), and the exon 4 of TEL to exon 17 of JAK2 (4/17). These fusion variants have been identified in patients with T-cell ALL, atypical CML, and pre-B-cell ALL, respectively. The ΔPNT mutant contains a deletion of nucleotides 195 to 348 of the TEL gene, and the kinase-inactive (KI) mutant has mutations of two conserved amino acids (TRP→Gly and Glu→Ala) in the type VIII kinase motif (40, 51). Socs-1 and other Socs family members contain an unconserved amino-terminal region, a central SH2 domain, and a carboxy-terminal SOCS box (7, 31, 43). The dC40 mutant contains a deletion of the SOCS box.
Native JAKs are involved in regulation of both the STAT and MAPK pathways, and these pathways are potential targets of activation by TEL-JAK2. JAKs phosphorylate and activate STATs, resulting in dimerization of the STATs, translocation to the nucleus, and activation of transcription (18). JAKs can also interact with Shc and Grb2 and activate MAPK (3, 17, 18, 46). In addition, several reports indicate that activation of the MAPK pathway potentiates activation of STATs. For example, serine phosphorylation of STATs, in addition to tyrosine phosphorylation, is required for full activation (44, 47, 50). In addition, STATs can interact with MEK, and inhibition of MEK prevents full activation of Stat5 (5, 37, 38, 47). Stat5 is constitutively activated by each of the TEL-JAK2 fusion proteins, and by analogy with the native JAKs, activation of the MAPK pathway may also be important in TEL-JAK2-mediated transformation (1, 21, 22, 40).
In addition to transformation of hematopoietic cell lines, TEL-JAK2 transforms primary hematopoietic cells in both murine bone marrow transplant assays (40) and transgenic mice in which TEL-JAK2 expression is directed by the Eμ promoter (1). The bone marrow transplant assay demonstrates that the TEL-JAK2 fusions can cause both myeloproliferative and T-cell lymphoproliferative disease with a latency of 2 to 10 weeks. In addition, the kinase activity of JAK2 is absolutely required for transformation, as demonstrated by point mutants or TEL PNT deletion mutants that abrogate JAK2 kinase activity (40). Furthermore, transduction of primary hematopoietic cells by TEL-JAK2 does not induce disease in a Stat5-deficient background, and a constitutively active mutant of Stat5a is sufficient to induce myeloproliferative disease (41). Taken together, these data indicate that transformation mediated by TEL-JAK2 in vitro and in vivo is absolutely dependent on JAK2 kinase activation and subsequent activation of Stat5.
Members of the SOCS family of proteins were initially identified as target genes whose expression was induced by JAK-STAT signaling. SOCS proteins have subsequently been shown to be negative regulators of JAK- and STAT-mediated signal transduction (7, 27, 31, 43). SOCS family members have an amino-terminal nonconserved region, a central Src homology 2 (SH2) domain, and a carboxy-terminal conserved domain termed the SOCS box (Fig. 1) (14, 26, 29, 43). One member of this family, Socs-1 (also known as SSI-1 and Jab), associates with the JH1 domain of JAK2 via the Socs-1 SH2 domain and Y1007 of JAK2 (48). Socs-1 has been reported to impair tyrosine phosphorylation of JAK2 and inhibit both Stat3 and Stat5 activation (7, 31). Mutational analysis has shown that a Socs-1 mutant that lacks the SOCS box but includes the SH2 domain and the amino-terminal nonconserved region, retains the ability to inhibit Stat5 activation (48). Furthermore, the SOCS box recruits elongin B and elongin C to the Socs-1–JAK2 complex and targets the complex to the proteasome for degradation (49).
We were intrigued by the possibility that endogenous inhibitors of JAK2, such as Socs-1, might also inhibit transformation by TEL-JAK2. Here we report that expression of Socs-1 abrogates growth of TEL-JAK2-transformed Ba/F3 cells but does not affect Ba/F3 cells transformed by BCR-ABL, TEL-PDGFβR or TEL-ABL. Furthermore, Socs-1 inhibits activation of a spectrum of signal transduction pathways activated by TEL-JAK2, including Stat5 phosphorylation, association of Shc and Grb2, and Erk2 activation. In vitro kinase activity assays demonstrate a marked diminution of TEL-JAK2 kinase activity in the presence of Socs-1. In addition, Socs-1 promotes proteasomal degradation of TEL-JAK2 and requires the SOCS box for this activity. Deletion of the SOCS box severely impairs the ability of Socs-1 to abrogate growth of TEL-JAK2-transformed cells. Finally, murine bone marrow transplants indicate that expression of Socs-1 prolongs the latency of hematologic malignancy caused by TEL-JAK2. These data indicate that constitutively activated leukemogenic tyrosine kinase fusion proteins are subject to modulation by endogenous inhibitors and that inhibition of TEL-JAK2 by Socs-1 is mediated both by direct inhibition of kinase activity and by enhancing proteasomal degradation.
MATERIALS AND METHODS
Tissue culture.
Murine Ba/F3 cells were maintained in RPMI 1640 (BioWhittaker) with 10% fetal bovine serum (FBS) and 1 ng of recombinant interleukin-3 (IL-3; R&D Systems) per ml. Ba/F3 cells transformed by the fusion proteins were maintained in the absence of IL-3, and Ba/F3 cells expressing the ΔPNT and kinase-inactive mutants of TEL-JAK2 were maintained in IL-3) (1 ng/ml) and G418 (1 mg/ml) 293T cells were maintained in Dulbecco Modified Eagle medium (DMEM; Bio Whittaker) with 10% FBS. All cells were maintained in a 5% CO2 incubator at 37°C.
Transient transfections.
pcDNA3Myc-Socs-1 and pcDNA3Myc-dC40 were provided by A. Yoshimura (Institute of Life Science, Kurume University, Kurume, Japan). The TEL-JAK2 fusion variants were cloned into pcDNA3. 293T cells were transfected with SuperFect (Qiagen). Cells were analyzed 48 h after transfection.
Protein analysis.
Cells were lysed in 1% Triton X-100 buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, 10% glycerol, 1 mM sodium vanadate, 10 mM sodium fluoride, 10 mM EDTA, and protease inhibitor cocktail tablets (Complete Mini; Roche). Extracts were quantified by the Bradford colorimetric method. Immunoprecipitation and Western blotting were performed with a JAK2 polyclonal antibody (kindly provided by Andrew Ziemiecki, University of Berne, Berne, Switzerland), TEL polyclonal antibody (kindly provided by Peter Marynen, University of Leuven, Louvain, Belgium), Stat5b polyclonal antibody (Santa Cruz Biotechnology), Shc polyclonal antibody (immunoprecipitation; Transduction Laboratories), Shc monoclonal antibody (Western blotting; Transduction Laboratories), Erk2 polyclonal antibody (Santa Cruz), Grb2 polyclonal antibody (immunoprecipitation; Santa Cruz), Grb2 monoclonal antibody (Western blotting; Transduction Laboratories), glutathione S-transferase (GST) polyclonal antibody (Santa Cruz), 4G10 monoclonal antibody (Update Biotechnology, Inc.), and Myc monoclonal antibody (Santa Cruz). One milligram of lysate was used for immunoprecipitation at 4°C. Proteins were precipitated with protein A- or protein G-Sepharose, washed three times with 1% Triton X-100 lysis buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to a nylon membrane (Millipore). Membranes were blocked with 5% milk or 3% bovine serum albumin, incubated with primary antibody for 1 h, incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies for 30 min, and visualized by enhanced chemilumineseence. For far-Western analysis, GST or GST-Grb2 SH2 fusion protein (2 μg/ml), purified from Escherichia coli by affinity chromatography on glutathione-agarose, was hybridized to the membrane for 2 h and blotted with GST antibody (28, 32).
Retrovirus production and transduction into Ba/F3 cells and primary murine bone marrow.
The murine Socs-1 cDNA (kindly provided by T. Kishimoto, Osaka University Medical School Department of Medicine, Osaka, Japan) and murine dC40 (kindly provided by A. Yoshimura) were subcloned into the retroviral expression vector MSCV-puro for gene transfer into Ba/F3 cells. Constructs used for the primary bone marrow transplants consisted of Socs-1 expressed from the long terminal repeat and 5/19 expressed from the internal ribosomal entry site (IRES) of the MSCV retroviral vector (13, 23, 36). Retroviral stocks were generated by transient cotransfection of 293T cells with the appropriate MSCV constructs together with a packaging construct (pIK6.1MCV.ecopac.UTd; Cell Genesys Inc., Foster City, Calif.) providing sequences necessary for retrovirus production (8). At 48 h posttransfection, virus-containing supernatant was harvested, filtered (0.45-μm-pore-size filter), and stored at −70°C. Viral titer was determined by Southern blotting. Ba/F3 cells (105) expressing indicated fusion proteins were infected with retroviral supernatant containing MSCV–puro–Socs-1 with Polybrene (10 μg/ml). Forty-eight hours later, the cells were harvested, washed twice in phosphate-buffered saline (PBS), and selected in puromycin (2.5 μg/ml; Sigma). For infection of primary murine bone marrow, 6- to 8-week-old BALB/cBvJ mice (Jackson Laboratory) were primed with 5-fluorouracil (150 mg/kg; Sigma) administered intraperitoneally 6 days prior to harvest. Two days before transplantation, male donor mice were sacrificed, the femurs and tibias were removed, and the bone marrow was flushed with medium using sterile technique. The cells were incubated overnight in RPMI 1640 medium containing recombinant murine IL-3 (6 U/ml; Genzyme), recombinant murine stem cell factor (5 U/ml; Genzyme), recombinant murine IL-6 (10,000 U/ml; Peprotech), 20% FBS, and penicillin-streptomycin (100 U/ml; GIBCO BRL). Cells were infected with equivalent titer of virus-containing supernatant using Polybrene (6 μg/ml), spun at 1,000 × g for 90 min at 30°C, and placed in a 5% CO2 incubator at 37°C for 1 h, at which time fresh medium was added to the cells. The infection was repeated 24 h later. The cells were then harvested, washed in 1 × PBS, and injected (0.5 × 106 to 1 × 106 cells in 500 μl of 1 × PBS) into the tail vein of lethally irradiated (twice with 450 cGy each time) female syngeneic recipient mice (24). Statistical analysis was performed with the StatView program (Abacus Concepts, Inc.).
Kinase assay.
Ba/F3 cells were starved of IL-3; 293T cells were starved of FBS for 4 h prior to kinase assays, and indicated samples were stimulated with 10% FBS for 5 min. For MAPK assays, cells were treated with 1 μM okadaic acid for 15 min prior to cell lysis. Indicated samples were treated with the MEK inhibitor PD98059 (100 μM) or control dimethyl sulfoxide for 10 min prior to cell lysis. Cells were lysed in 1% Triton X-100 lysis buffer as indicated above. Erk2 was immunoprecipitated on protein A-Sepharose beads, and washed with kinase buffer (20 mM morpholinepropanesulfonic acid mM [MOPS; pH 7.2], 20 mM MgCl2, 5 mM EGTA, 30 mM β-glycerophosphate, 1 mM sodium vanadate, 1 mM dithiothreitol). Kinase assays were performed at 30°C for 30 min in a volume of 30 μl containing kinase buffer, 10 μM ATP, 9.0 μg of GST–Elk-1 substrate (New England BioLabs), and 2 μCi of [γ32-P]ATP (3,000 Ci/mmol). Products were resolved by SDS-PAGE and visualized by autoradiography. TEL-JAK2 kinase assays were performed with anti-TEL immunoprecipitates washed once with buffer consisting of 20 mM MOPS (pH 7.0) and 0.2 mM pervanadate and then washed a second time in kinase buffer consisting of 20 mM MOPS (pH 7.0), 5 mM MnCl2, and 0.2 mM pervanadate. Kinase assays were performed 30°C for 10 min in a volume of 30 μl containing kinase buffer, 5 μM ATP, 1 μg of GST-C′ Gab2 (purified from E. coli by affinity chromatography on glutathione-agarose), and 10 μCi of [γ32-P]ATP (3,000 Ci/mmol). Products were resolved by SDS-PAGE and visualized by autoradiography.
Phosphoamino acid analysis.
Protein extraction, proteolytic digestion, acid hydrolysis, and one-dimensional electrophoresis were performed as described previously (16).
Pulse-chase analysis.
Indicated cells were treated with 25 μM lactacystin (Calbiochem) for 30 min and maintained in the proteasome inhibitor throughout the pulse and chase. Cells were starved in methionine-free and cysteine-free DMEM (BioWhittaker) for 15 min, pulsed with medium containing 0.1 to 0.2 mCi of [35S]methionine and [35S]cysteine (ProMix; Amersham) for 15 min, and chased with DMEM for 2, 4, or 6 h. Equivalent amounts of extracts, as determined by the Bradford assay, were immunoprecipitated with TEL antibody and resolved by SDS-PAGE. The gel was treated with Amplify (Amersham), dried, and visualized by autoradiography.
RESULTS
Socs-1 abrogates growth of TEL-JAK2-transformed Ba/F3 cells but not cells transformed by BCR-ABL, TEL-ABL or TEL-PDGFβR.
Socs-1 has been reported to be an inhibitor of STATs, and we hypothesized that expression of Socs-1 might impair transformation mediated by one or more of the tyrosine kinase fusion proteins that activate Stat5, including TEL-JAK2, BCR-ABL, TEL-ABL, and TEL-PDGFβR. To test this hypothesis, Ba/F3 cells transformed to factor-independent growth with the tyrosine kinase fusion proteins were transduced with the MSCV–puro–Socs-1 or empty vector. Puromycin-resistant cells were obtained in all cells transduced with the empty vector. No puromycin-resistant clones were obtained in TEL-JAK2 cells transduced with MSCV–puro–Socs-1, whereas resistant clones were readily obtained in BCR-ABL, TEL-ABL, and TEL-PDGFβR cells transduced with MSCV–puro–Socs-1 (Fig. 1 and 2). In addition, no puromycin-resistant clones were obtained with IL-3-dependent Ba/F3 cells transduced with MSCV–puro–Socs-1. Northern and Western blot analysis confirmed expression of Socs-1 in puromycin-resistant cell lines, indicating that tyrosine kinase fusions were able to overcome inhibition by exogenous expression of Socs-1 (data not shown). These data indicate that Socs-1 expression in these experimental conditions completely inhibits transformation of cells mediated by TEL-JAK2 but not BCR-ABL, TEL-ABL, and TEL-PDGFβR.
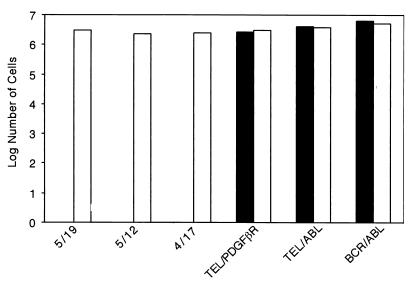
FIG. 2.
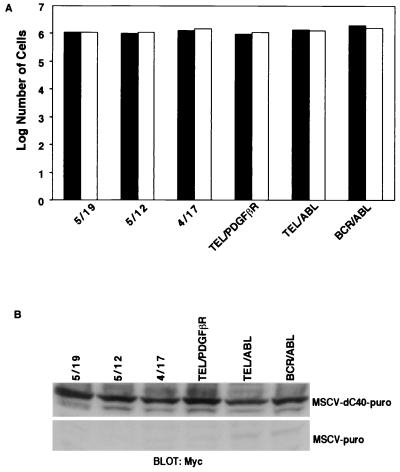
Expression of Socs-1 in Ba/F3 cells inhibits transformation by the TEL-JAK2 fusion variants. Ba/F3 cells (105) expressing indicated fusion proteins were maintained in the absence of IL-3. Cells were transduced with MSCV–puro–Socs-1 vector or empty vector. After 48 h, cells were selected for puromycin resistance. Cells were counted 4 days postselection. Puromycin-resistant cells were obtained with all Ba/F3 cell lines transduced with MSCV-puro (open bars). Puromycin-resistant cells were also obtained with BCR-ABL, TEL-ABL, and TEL-PDGFβR Ba/F3 cells transduced with MSCV–puro–Socs-1 but not the TEL-JAK2 fusion variants 5/19, 5/12, and 4/17 (solid bars). Similar results were obtained in replicate experiments.
Socs-1 associates with TEL-JAK2 and inhibits activation of downstream signaling targets.
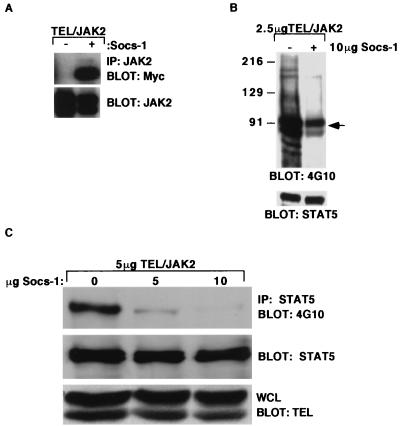
The SH2 domain of Socs-1 associates with Y1007 within the JH1 domain of JAK2 (48). To determine whether Socs-1 also associated with TEL-JAK2, TEL-JAK2 and Myc epitope-tagged Socs-1 (Myc–Socs-1) were coexpressed in 293T cells. Immunoprecipitation with anti-JAK2 antibody followed by Western blot analysis with anti-Myc antibody demonstrated association of TEL-JAK2 with Socs-1 (Fig. 3A). Coimmunoprecipitation with a Myc antibody followed by Western blotting with the anti-TEL antibody confirmed this interaction (data not shown). Western blot analysis of whole-cell lysates with antiphosphotyrosine antibody (4G10) demonstrated that expression of Socs-1 resulted in an overall decrease in tyrosine phosphorylation in the cell (Fig. 3B). Thus, Socs-1 abrogates growth of TEL-JAK2-transformed cells, associates with TEL-JAK2, and results in an overall decrease in cellular phosphotyrosine content.
FIG. 3.
Socs-1 associates with TEL-JAK2 and inhibits tyrosine phosphorylation of STAT5. (A) Socs-1 associates with TEL-JAK2. Extracts from 293T cells expressing the 5/19 variant of TEL-JAK2 (1 μg) in the absence or presence of Socs-1 (1 μg) were immunoprecipitated (IP) with a JAK2 antibody and blotted with an anti-Myc antibody to detect the Myc-tagged Socs-1 (top). The membrane was blotted with a JAK2 antibody to confirm precipitation of the TEL-JAK2 (bottom). (B) Socs-1 expression results in decreased overall tyrosine phosphorylation of 293T cells expressing TEL-JAK2. Whole-cell extracts from cells transfected with 2.5 μg of 5/19 variant of TEL-JAK2 in the absence or presence of 10 μg of Socs-1 were blotted with 4G10 (top) or a Stat5 antibody to confirm equal loading of the lysates (bottom). The arrow identifies TEL-JAK2, which is seen as a doublet due to an alternative start site for translation in TEL. The whole-cell extracts were blotted with a Myc antibody to confirm expression of Socs-1 and a TEL antibody to confirm expression of TEL-JAK2 (data not shown). (C) Socs-1 inhibits tyrosine phosphorylation of Stat5 in 293T cells expressing TEL-JAK2. Extracts from 293T cells expressing the 5/19 TEL-JAK2 variant and increasing amounts Socs-1 were immunoprecipitated with a Stat5 antibody and blotted with 4G10 (top) or a Stat5 antibody (middle). Whole-cell lysates (WCL) were blotted with an anti-TEL antibody to confirm equal expression of TEL-JAK2 (bottom).
We have previously reported that TEL-JAK2 tyrosine phosphorylates and activates Stat5 in Ba/F3 cells (40). We therefore tested whether Socs-1 impaired activation of Stat5 by TEL-JAK2. TEL-JAK2 was expressed in 293T cells in the presence of increasing concentrations of Socs-1. Immunoprecipitation of Stat5 followed by Western blotting with 4G10 antibody demonstrated a marked decrease in Stat5 phosphotyrosine content with increasing Socs-1 expression (Fig. 3C). These data indicate that Socs-1 impairs phosphorylation of Stat5 in cells expressing TEL-JAK2.
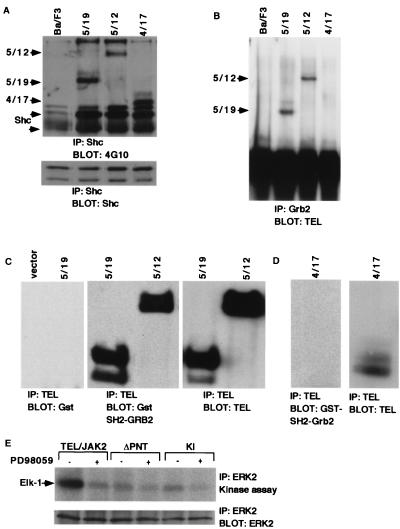
We next characterized other signaling molecules for association with, or activation by, TEL-JAK2. Shc was tyrosine phosphorylated in cells expressing each of the TEL-JAK2 variants (5/19, 4/17, and 5/12) and associated with all of the fusion variants, as demonstrated by immunoprecipitation of the respective Ba/F3 whole-cell lysates with anti-Shc antibody followed by Western blotting with 4G10 antibody (Fig. 4A). In contrast to the results with Shc, Grb2 immunoprecipitation from Ba/F3 cells expressing TEL-JAK2 fusion variants, followed by Western blotting with a TEL antibody, demonstrated that Grb2 associated with the 5/19 and 5/12 TEL-JAK2 variants but did not associate with the 4/17 variant (Fig. 4B). The direct association of Grb2 with the 5/19 and 5/12 variants, but not the 4/17 variant, was confirmed by far-Western analysis performed with the Grb2 SH2 domain (Fig. 4C and D). This observation suggested that Grb2 interacts with a phosphorylated tyrosine residue on TEL exon 5. Finally, we tested activation of Erk2 using in vitro kinase assays in which Erk2 was immunoprecipitated from Ba/F3 cells transformed by TEL-JAK2 and assayed for the ability to phosphorylate an exogenous GST–Elk-1 substrate (Fig. 4E). An increased level of Erk2 activation was observed in TEL-JAK2 cells compared with inactive TEL-JAK2 mutants that lacked the PNT domain or were kinase inactive. In addition, Erk2 activation in TEL-JAK2-transformed cells was impaired by the MEK inhibitor PD98059, suggesting that Erk2 was activated via a MEK-dependent mechanism. Collectively, these data support the hypothesis that, as for native JAK2, both the STAT and MAPK pathways are activated by TEL-JAK2.
FIG. 4.
TEL-JAK2 associates with Shc and Grb2 and activates Erk2. (A) Shc is tyrosine phosphorylated in Ba/F3 cells expressing the TEL-JAK2 fusion variants and associates with TEL-JAK2. Extracts from Ba/F3 cells expressing the TEL-JAK2 fusion variants were immunoprecipitated (IP) with an anti-Shc antibody and blotted with 4G10 (top). TEL-JAK2 is seen as a doublet due to an alternative start site for translation in TEL. The membrane was blotted with a Shc antibody to confirm equal precipitation of the protein (bottom). Control experiments showed no evidence of association of Shc with inactive TEL-JAK2 mutants that lacked the PNT domain or were kinase inactive (data not shown). (B) Grb2 associates with the 5/19 and 5/12 TEL-JAK2 fusion variants. Extracts from panel A were immunoprecipitated with a Grb2 antibody and blotted with a TEL antibody. Association of Grb2 with the 4/17 variant was not detected. Control experiments showed no evidence of association of Grb2 with inactive TEL-JAK2 mutants that lacked the PNT domain or were kinase inactive (data not shown). The membrane was blotted with a Grb2 antibody to confirm equal precipitation (data not shown). (C) The SH2 domain of Grb2 directly associates with the 5/19 and 5/12 TEL-JAK2 fusion variants. Extracts from 293T cells expressing empty vector or the 5/19 TEL-JAK2 fusion variant were immunoprecipitated with a TEL antibody. Far-Western analysis was performed with purified GST and blotted with a GST antibody (left). Extracts from 293T cells expressing the 5/19 and 5/12 TEL-JAK2 fusion variants were immunoprecipitated with a TEL antibody, and far-Western analysis was performed with GST–Grb2 SH2 (middle). The membrane was blotted with TEL antibody to confirm precipitation of 5/19 and 5/12 TEL-JAK2 (right). (D) The SH2 domain of Grb2 does not directly associate with the 4/17 fusion variant. Extracts from 293T cells expressing the 4/17 TEL-JAK2 fusion variant was immunoprecipitated with a TEL antibody. Far-Western analysis was performed with purified GST (data not shown) or GST-Grb2 SH2 and blotted with a GST antibody (left). The membrane was blotted with TEL antibody to confirm precipitation of 4/17 TEL-JAK2 (right). (E) Erk2 is activated in Ba/F3 cells expressing the TEL-JAK2 fusion variants. Ba/F3 cells expressing the TEL-JAK2 5/19 variant, ΔPNT mutant, or kinase-inactive (KI) mutant were starved of IL-3 for 4 h and treated with 100 μM PD98059 or dimethylsulfoxide for 10 min. Erk2 immunoprecipitated from these extracts was used for in vitro kinase assays using exogenous GST–Elk-1 as a substrate (top). Products were separated by SDS-PAGE followed by autoradiography. Parallel Erk2 Western blotting confirmed equal immunoprecipitation of the protein (bottom). Erk2 is also activated in cells expressing the 5/12 and 4/17 fusion variants (data not shown).
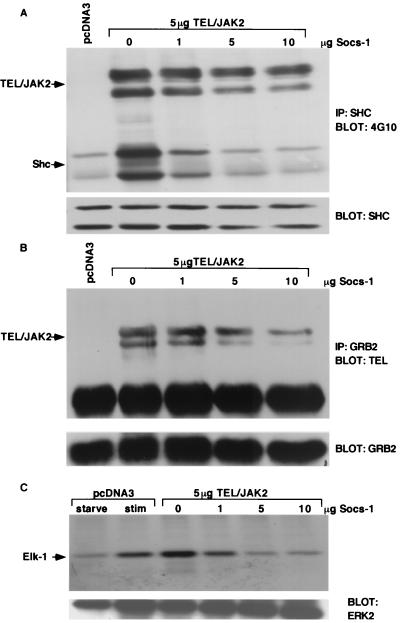
We then tested the effect of Socs-1 expression on the ability of TEL-JAK2 to tyrosine phosphorylate and associate with Shc, associate with Grb2, and activate Erk2. For each of these experiments, TEL-JAK2 was expressed in 293T cells in the presence of increasing amounts of Socs-1. Immunoprecipitation of Shc followed by Western blot analysis with 4G10 demonstrated that Socs-1 expression inhibited Shc tyrosine phosphorylation by TEL-JAK2 (Fig. 5A). There was no change in TEL-JAK2 tyrosine phosphorylation detected by 4G10 Western blotting (Fig. 6B), suggesting that the decrease in TEL-JAK2 tyrosine phosphorylation observed in Fig. 5A was attributable to a decrease in Shc association with TEL-JAK2 in the presence of increasing amounts of Socs-1 (Fig. 5A). Similar experiments demonstrated that Grb2 association with TEL-JAK2 was also markedly diminished by Socs-1 (Fig. 5B). Far-Western analysis confirmed that Socs-1 prevented direct association of Grb2 with TEL-JAK2 (data not shown). Finally, in vitro MAPK assays demonstrated that Socs-1 significantly reduced activation of Erk2 by TEL-JAK2 (Fig. 5C). These data indicate that in addition to inhibiting STAT5 activation, Socs-1 expression inhibits tyrosine phosphorylation of Shc, association of Grb2 with TEL-JAK2, and activation of Erk2.
FIG. 5.
Socs-1 inhibits association of Shc and Grb2 with TEL-JAK2 and activation of Erk2. (A) Socs-1 inhibits tyrosine phosphorylation of Shc and association with TEL-JAK2. Extracts from 293T cells expressing 5/19 TEL-JAK2 and increasing amounts of Myc–Socs-1 were immunoprecipitated (IP) with a Shc antibody and blotted with 4G10 (top) or a Shc antibody (bottom). TEL-JAK2 is seen as a doublet due to an alternative start site for translation in TEL. The whole-cell lysates were blotted with a Myc antibody to confirm expression of Socs-1 and a TEL antibody to confirm equal expression of TEL-JAK2 (data not shown; Fig. 6A). (B) Socs-1 inhibits association of GRB2 with TEL-JAK2. Extracts from panel A were immunoprecipitated with a Grb2 antibody and blotted with a TEL antibody (top) or a Grb2 antibody (bottom). (C) Socs-1 inhibits activation of Erk2 in cells expressing TEL-JAK2. 293T cells expressing equivalent amounts of 5/19 TEL-JAK2 and increasing amounts of Myc–Socs-1 were starved of FBS for 4 h and stimulated with 10% FBS for 5 min where indicated. Erk2 immunoprecipitated from these extracts was used for in vitro kinase assays using GST–Elk-1 as a substrate (top). Products were separated by SDS-PAGE followed by autoradiography. Parallel Erk2 Western blotting confirmed immunoprecipitation of the protein (bottom). The whole-cell lysates were blotted with a Myc antibody to confirm expression of Socs-1 and a TEL antibody to confirm equal expression of TEL-JAK2 (data not shown; Fig. 6A).
FIG. 6.
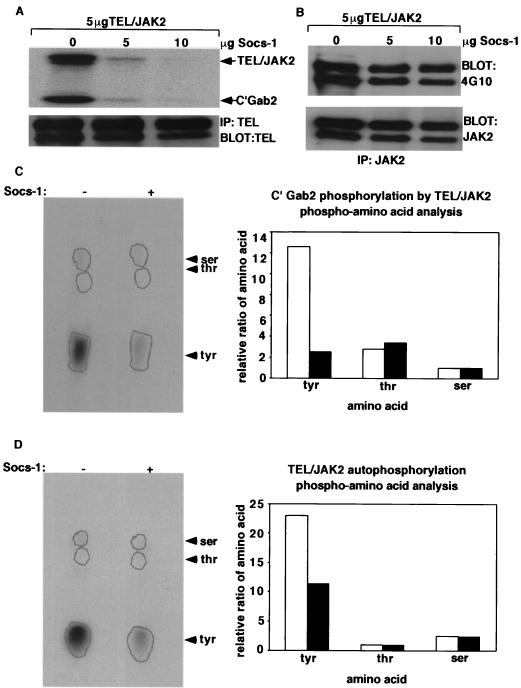
Socs-1 inhibits kinase activity of TEL-JAK2. (A) TEL-JAK2 in vitro kinase assay. 293T cells expressing 5/19 TEL-JAK2 and increasing amounts of Myc–Socs-1 were starved of FBS for 4 h. Extracts from these cells were immunoprecipitated (IP) with a TEL antibody and used for in vitro kinase assays using GST-C′Gab2 as a substrate. Products were separated by SDS-PAGE and visualized by autoradiography (top). Parallel TEL Western blotting confirmed equal immunoprecipitation of the protein (bottom). (B) 4G10 Western blot. 293T cells expressing 5/19 TEL-JAK2 and increasing amounts of Myc-Socs-1 were starved of FBS for 4 h. TEL-JAK2 was immunoprecipitated with the JAK2 antibody and blotted with 4G10 (top) or JAK2 antibody (bottom). The whole-cell lysates were blotted with a Myc antibody to confirm expression of Socs-1 and a TEL antibody to confirm equal expression of TEL-JAK2 (data not shown). (C) Phosphoamino acid analysis of C′Gab2. Phosphorylated GST-C′Gab2 substrate from panel was hydrolyzed to single amino acids and separated on a thin-layer chromatography plate (left). The plate was placed on a phosphorimager, and the ratio of phosphorylated amino acids was used to generate the graph (right). Open bars represent TEL-JAK2 samples, and solid bars represent Socs-1 and TEL-JAK2 samples. (D) Phosphoamino acid analysis of autophosphorylated TEL-JAK2. Analysis of the autophosphorylated TEL-JAK2 was performed as for panel C. Open bars represent TEL-JAK2 samples, and solid bars represent Socs-1 and TEL-JAK2 samples.
Socs-1 inhibits kinase activity and autophosphorylation of TEL-JAK2.
Since Socs-1 inhibits activation of all tested downstream effectors of TEL-JAK2, we hypothesized that Socs-1 directly inhibited TEL-JAK2 kinase activity. We therefore tested the effect of Socs-1 expression on TEL-JAK2 kinase activity by using in vitro kinase assays. TEL-JAK2 was immunoprecipitated from 293T cells expressing TEL-JAK2 with increasing amounts of Socs-1, followed by an in vitro kinase assay using the C terminus of Gab2 as a substrate. Immune complex kinase assays performed in the presence of [γ32-P]ATP demonstrated that phosphorylation of the substrate was inhibited in extracts from cells expressing Socs-1 (Fig. 6A). Phosphoamino acid analysis confirmed that the change in phosphorylation was due to a decrease in tyrosine phosphorylation rather than serine or threonine phosphorylation (Fig. 6C). Furthermore, kinase assays performed with extracts from cells expressing BCR-ABL indicated that Socs-1 did not inhibit the kinase activity of this fusion protein (data not shown). These data convincingly demonstrate that Socs-1 specifically inhibits the tyrosine kinase activity of TEL-JAK2.
Socs-1 expression also results in decreased autophosphorylation of native JAK2, and the in vitro kinase assays confirmed that autophosphorylation of TEL-JAK2 was inhibited by Socs-1 (Fig. 6A). Furthermore, phosphoamino acid analysis performed on the autophosphorylated bands indicate that tyrosine phosphorylation of TEL-JAK2 was decreased significantly by expression of Socs-1 (Fig. 6D). Of note, 4G10 Western blot analysis did not detect the decrease in tyrosine phosphorylation of TEL-JAK2 (Fig. 6B). Taken together, these data convincingly demonstrate that Socs-1 inhibits kinase activity of TEL-JAK2.
Socs-1 promotes proteasomal degradation of TEL-JAK2 and requires the Socs-1 SOCS box for this activity.
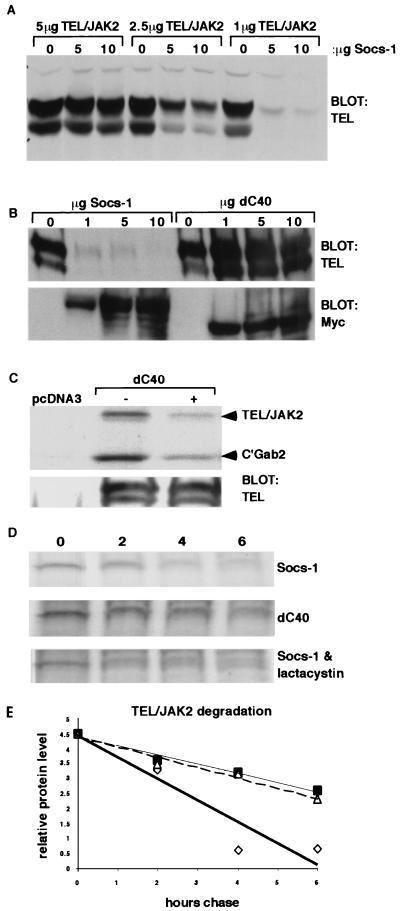
The conserved SOCS box of Socs-1 binds to elongin B and C and promotes proteasomal degradation of the protein (49). To determine if Socs-1 expression affected protein levels of TEL-JAK2, 293T cells were transfected with decreasing amounts of TEL-JAK2 DNA and increasing amounts of Myc–Socs-1 DNA. A marked decrease in TEL-JAK2 protein levels was observed with increased coexpression of Socs-1 (Fig. 7A). Analysis performed with a mutant of Socs-1 which lacks the SOCS box, dC40, indicated that coexpression of this mutant did not decrease the level of TEL-JAK2 protein (Fig. 1 and 7B). Since the dC40 mutant contains the SH2 domain, which mediates association of Socs-1 with native JAK2, we hypothesized that although the mutant did not affect TEL-JAK2 protein level, it would retain the ability to inhibit the kinase activity of TEL-JAK2. In vitro kinase assays performed with TEL-JAK2 immunoprecipitated from 293T cells expressing the fusion protein in the presence or absence of dC40 confirmed that the mutant inhibited TEL-JAK2 kinase activity (Fig. 7C). We next determined whether the change in TEL-JAK2 expression level was due to increased protein degradation by conducting pulse-chase experiments. Expression of Socs-1 promoted protein instability of TEL-JAK2, whereas expression of the dC40 mutant did not alter the protein levels of TEL-JAK2 (Fig. 7D and E). Cells were then treated with the proteasome inhibitor lactacystin to determine if decreased TEL-JAK2 protein levels were due to proteasomal degradation. Results indicated that the inhibitor promoted an increase in the stability of TEL/JAK2 in the presence of Socs-1 (Fig. 7D and E). Thus, Socs-1 promotes proteasomal degradation of TEL-JAK2, and the SOCS box of Socs-1 is required for this process. Furthermore, the observation that dC40 retains the ability to inhibit TEL-JAK2 kinase activity indicates that Socs-1 can inhibit the fusion protein by the independent mechanisms of kinase inhibition and acceleration of protein degradation.
FIG. 7.
The SOCS box is required for proteasomal degradation of TEL-JAK2. (A) Titration of 5/19 TEL-JAK2 and Socs-1. Whole-cell extracts from 293T cells expressing decreasing amounts of 5/19 TEL-JAK2 and increasing amounts of Socs-1 were blotted with a TEL antibody. Western blotting of whole-cell extracts with a Myc antibody confirmed expression of Myc–Socs-1 (data not shown). (B) SOCS box of Socs-1 is required for decreased levels of TEL-JAK2 protein. Whole-cell extracts from 293T cells transfected with 2.5 μg of 5/19 TEL-JAK2 and increasing amounts of Myc–Socs-1 or Myc-dC40, which results in deletion of the SOCS box, were blotted with a TEL antibody (top). Western blotting of whole-cell extracts with a Myc antibody confirmed expression of Myc–Socs-1 and Myc-dC40 (bottom). (C) TEL-JAK2 in vitro kinase assay. 293T cells expressing 5/19 TEL-JAK2 with or without dC40 were starved of FBS for 4 h. Extracts from these cells were immunoprecipitated with a TEL antibody and used for in vitro kinase assays using GST-C'Gab2 as a substrate. Products were separated by SDS-PAGE followed by autoradiography (top). Parallel TEL immunoprecipitation and Western blotting confirmed equal immunoprecipitation of the protein (bottom). (D) Decrease in TEL-JAK2 protein level is mediated by proteasomal degradation. 293T cells transfected with 2.5 μg of 5/19 TEL-JAK2 and 10 μg Socs-1 or 10 μg dC40 were pulsed with [35S]methionine and [35S]cysteine for 15 min and chased for 2, 4, or 6 h. Indicated samples were treated with 25 μM lactacystin throughout the pulse and chase. Equivalent amount of extracts, as determined by the Bradford assay, were immunoprecipitated with a TEL antibody, separated by SDS-PAGE, and visualized by autoradiography. (E) Images from the pulse-chase were placed on a densitometer for analysis, and numerical results were plotted on a graph. Open diamonds represent degradation of TEL-JAK2 in cells expressing Jab, open triangles represent degradation of TEL-JAK2 in cells expressing dC40, and solid squares represent degradation of TEL-JAK2 in cells expressing Jab and treated with 25 μM lactacystin.
The SOCS box is required for inhibition of TEL-JAK2-transformed Ba/F3 cells by Socs-1.
To determine whether the SOCS box was necessary for Socs-1 inhibition of TEL-JAK2 transformation, the TEL-JAK2 5/19, 5/12, and 4/17 cell lines were transduced with an MSCV-puro retroviral vector containing the murine dC40 cDNA (Fig. 1) or empty vector control. In contrast with results obtained with native Socs-1 (Fig. 2), expression of the dC40 Socs-1 mutant did not abrogate growth of TEL-JAK2-transformed cells (Fig. 8A). As expected, control experiments demonstrated that expression of the dC40 mutant also had no effect on TEL-PDGFβR-, TEL-ABL-, or BCR-ABL-transformed cell lines (Fig. 8A). Western blot analysis confirmed expression of the dC40 mutant (Fig. 8B).
FIG. 8.
Expression of dC40 does not inhibit transformation by the TEL-JAK2 fusion variants. Ba/F3 cells expressing indicated fusion proteins were maintained in the absence of IL-3. Cells (105) were transduced with the MSCV-puro vector containing the murine dC40 sequence or empty vector. After 48 h, cells were selected for puromycin resistance. Cells were counted 4 days postselection. Puromycin-resistant cells were obtained with all Ba/F3 cell lines transduced with MSCV-puro (open bars) and MSCV–puro–dC40 (solid bars). In particular, in comparison with Fig. 2, puromycin-resistant cells were obtained for all three of the TEL-JAK2 fusion variants. Similar results were obtained in replicate experiments.
Socs-1 causes an increase in latency of TEL-JAK2 disease in murine bone marrow transplants.
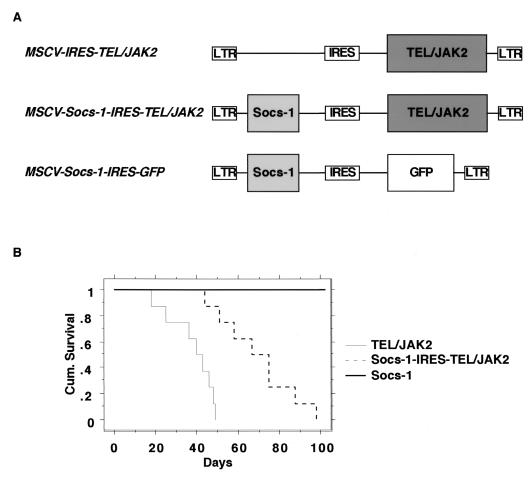
To confirm that Socs-1 impaired TEL-JAK2 transformation in vivo in primary hematopoietic progenitors, we performed murine bone marrow transplants with TEL-JAK2 in the presence or absence of Socs-1 (4, 20, 40). Lethally irradiated mice were reconstituted with bone marrow transduced with a bicistronic retrovirus expressing either TEL-JAK2 or TEL-JAK2 and Socs-1 (Fig. 9A). Histopathologic analysis indicated that both sets of mice presented with disease characteristic of the TEL-JAK2 murine bone marrow transplant, including a mixed myelo- and lymphoproliferation, elevated white blood cell counts, immature and mature myeloid cells in the peripheral blood, enlarged spleen, extramedullary hepatopoiesis, and hemorrhagic lungs (data not shown) (40). Southern blot confirmed integration of the provirus in the white blood cells, lymph nodes, liver, and spleen (data not shown). Coexpression of Socs-1 induced disease with significantly longer latency than TEL-JAK2 alone. As shown in the Kaplan-Meier plot, mice transplanted with TEL-JAK2 bone marrow died of disease within 18 to 49 days posttransplant, whereas mice transplanted with Socs-1–TEL–JAK2 bone marrow died of disease within 44 to 98 days posttransplant (P < 0.001) (Fig. 9B). Mice transplanted with bone marrow cells transduced with retrovirus expressing Socs-1 alone did not develop disease up to 1 year posttransplant. These results demonstrate that Socs-1 is a physiologic inhibitor of TEL-JAK2-mediated transformation in vivo.
FIG. 9.
Comparative survival analysis of TEL-JAK2 and Socs-1–TEL–JAK2 murine bone marrow transplant (Kaplan-Meier plot). (A) Representation of MSCV retroviral constructs used for bone marrow transplants. Socs-1 was expressed from the long terminal repeat (LTR), and 5/19 TEL-JAK2 was expressed from the IRES. GFP, green fluorescent protein. (B) Mice transplanted with bone marrow transduced by equivalent titers of retrovirus as assessed by Southern blotting. Mice transplanted with bone marrow transduced with 5/19 TEL-JAK2 (n = 8) died of disease at 18 to 49 days, whereas mice transplanted with bone marrow transduced with Socs-1–IRES–5/19 TEL-JAK2 (n = 8) died of disease at 44 to 98 days (P < 0.001). Mice transplanted with Socs-1 did not develop disease up to 1 year posttransplant (n = 4).
DISCUSSION
Socs-1, a member of the SOCS family of endogenous inhibitors of JAKs and STATs, inhibits the TEL-JAK2 fusion proteins. Expression of Socs-1 in Ba/F3 cells transformed by the TEL-JAK2 fusion variants inhibits IL-3-independent growth of these cells but does not inhibit growth of Ba/F3 cells transformed by TEL-PDGFβR, BCR-ABL, and TEL-ABL. This result is intriguing since although several tyrosine kinase fusions activate STATs, Socs-1 inhibits only TEL-JAK2 of the tyrosine kinase fusions tested. This suggests that TEL-JAK2-mediated transformation is dependent on STATs, whereas other tyrosine kinase fusions can bypass the requirement for STAT activation. This finding is consistent with murine bone marrow transplants conducted in Stat5a,b−/− background, which demonstrate that Stat5 is necessary for TEL-JAK2-mediated disease but is not required for BCR-ABL-mediated disease (41, 42).
Analysis of the mechanism by which Socs-1 inhibits TEL-JAK2 indicates that Socs-1 prevents activation of downstream targets. Since expression of Socs-1 in the Ba/F3 hematopoietic cell line induced rapid cell death, we analyzed the role of Socs-1 in TEL-JAK2 signaling by expressing the proteins in 293T cells. Results indicate that Socs-1 associates with TEL-JAK2 and causes an overall decrease in cellular phosphotyrosine content. Phosphorylation of Stat5, as well as other signaling molecules, is inhibited by Socs-1. For example, we demonstrated that Shc is phosphorylated by, and associates with, all of the TEL-JAK2 fusion variants in Ba/F3 cells. In addition, Grb2 directly associates with the 5/19 and 5/12 fusion variants, as demonstrated by coimmunoprecipitation and far-Western blotting, and Erk2 is activated in cells expressing all of the fusion variants. Although Socs-1 has not previously been reported to play a role in regulation of this pathway, it inhibits phosphorylation of Shc, association of Grb2 with TEL-JAK2, and activation of Erk2 in 293T cells expressing TEL-JAK2.
We hypothesized that the mechanism for this broad effect of Socs-1 on TEL-JAK2 signaling was due to inhibition of kinase activity of the fusion protein, an effect on the half-life of the fusion protein, or both. In vitro kinase assays performed with TEL-JAK2 indicate that expression of Socs-1 inhibits phosphorylation of an exogenous substrate by TEL-JAK2 as well as autophosphorylation of TEL-JAK2, and phosphoamino acid analysis of the in vitro-autophosphorylated TEL-JAK2 as well as the C′Gab2 substrate confirms that the decrease in phosphorylation is due to a decrease in tyrosine phosphorylation. Thus, expression of Socs-1 results in inhibition of TEL-JAK2 kinase activity, but in contrast, expression of Socs-1 has no effect on the kinase activity of BCR-ABL. Of note, we detected no change in TEL-JAK2 tyrosine phosphorylation in the presence of Socs-1 by 4G10 Western blotting. This disparity emphasizes the importance of definitive assays for kinase activation rather than use of phosphotyrosine Western blots as a surrogate for kinase activation.
Socs-1 also accelerates proteasome-mediated degradation of TEL-JAK2, in consonance with previous reports that Socs-1 recruits associated proteins to the proteasome through interaction with elongin B and elongin C (49). The SOCS box of Socs-1 is required for this function in that expression of the dC40 mutant, which lacks the SOCS box, does not result in a decrease of TEL-JAK2 protein level. Furthermore, deletion of the SOCS box abrogated the ability of Socs-1 to inhibit growth of a TEL-JAK2-transformed hematopoietic cell line, indicating a critical physiologic role for proteosomal degradation in this system. Although the SOCS box is required for efficient degradation, it is not required for inhibition of TEL-JAK2 kinase activity. These data are are consistent with known structure-function relationships in Socs-1. The SOCS box deletion mutant retains the Socs-1 SH2 domain, which mediates association of Socs-1 with Y1007 of JAK2, and inhibits activation of Stat5 by native JAK2 (48). Thus, the SOCS box of Socs-1 is required for proteasome-mediated degradation, but not kinase inhibition, of TEL-JAK2, indicating that Socs-1 can inhibit the fusion protein by dual mechanisms of kinase inhibition and protein degradation. However, of these two mechanisms, proteosomal degradation appears to play a more important role in Socs-1 inhibition of TEL-JAK2 activity in transformed Ba/F3 cells.
We confirmed the inhibitory effect of Socs-1 on TEL-JAK2 transformation of primary hematopoietic cells in vivo using the murine bone marrow transplant assay. For these experiments, the MSCV retroviral vector containing an IRES was used to allow for coexpression of Socs-1 and TEL-JAK2. Lethally irradiated mice were transplanted with syngeneic donor bone marrow transduced with either MSCV-IRES-TEL-JAK2 or MSCV–Socs-1–IRES–TEL–JAK2. Results indicate that Socs-1 is a potent inhibitor of TEL-JAK2-mediated disease and increases latency by 26 to 49 days. Additional experiments with SOCS box deletion mutants will be required to determine the relative contribution of Socs-1-mediated proteosomal degradation versus kinase inhibition of TEL-JAK2 in the murine bone marrow transplant assay.
Taken together, these data identify two separate mechanisms by which Socs-1 inhibits TEL-JAK2: (i) inhibition of kinase activity and (ii) proteasome-mediated degradation of TEL-JAK2. Socs-1 associates with TEL-JAK2 and inhibits kinase activity of the fusion protein, thereby preventing association with, and activation of, downstream targets including STAT5, Shc, Grb2, and Erk2. The SOCS box of Socs-1 is not required for inhibition of TEL-JAK2 kinase activity, as mutants lacking this domain still inhibit TEL-JAK2 kinase activity. However, the SOCS box is required for proteasomal degradation of TEL-JAK2. Deletion of the SOCS box abrogates proteosomal degradation of TEL-JAK2 as well as Socs-1-mediated inhibition of TEL-JAK2 in transformed Ba/F3 cells. Finally, we have extended these studies to demonstrate that Socs-1 inhibits TEL-JAK2 in primary hematopoietic cells in vivo in a murine bone marrow transplant model of TEL-JAK2 leukemia.
TEL-JAK2 provides a useful model for constitutive activation of the JAK-STAT signaling pathway and modulation of the pathway by endogenous inhibitors. SOCS family members may be therapeutically useful for treatment of certain hematologic malignances induced by tyrosine kinase fusions. In addition, they may be useful in novel or adjunctive treatment strategies for inhibition of the JAK-STAT pathway in hematologic malignancies known to involve constitutive activation of the pathway, including CML, ALL, acute myeloid leuukemia, and chronic lymphocytic leukemia (2, 9, 12).
ACKNOWLEDGMENTS
We acknowledge the invaluable assistance of D. Cain for murine bone marrow transplant experiments and K. Shannon (UCSF) for MAPK assays. We thank R. Van Etten and T. Golub for critical review of the manuscript, and we thank B. Neel and T. Roberts for valuable discussions and critical review of this work.
This work was supported in part by NIH grants POI DK50654 and POI CA66996 (D.G.G.), NIH grant CA82261 (D.W.S.), and the Leukemia Society of America (J.S.). D.G.G. is an Associate Investigator for Howard Hughes Medical Institute.
REFERENCES
- 1.Carron C, Cormier F, Janin A, Lacronique V, Giovannini M, Daniel M T, Bernard O, Ghysdael J. TEL-JAK2 transgenic mice develop T-cell leukemia. Blood. 2000;95:3891–3899. [PubMed] [Google Scholar]
- 2.Chai S K, Nichols G L, Rothman P. Constitutive activation of JAKs and STATs in BCR-Abl expressing cell lines and peripheral blood cells derived from leukemic patients. J Immunol. 1997;159:4720–4728. [PubMed] [Google Scholar]
- 3.Chauhan D, Kharbanda S M, Ogata T, Urashima M, Frank D, Malik N, Kufe D W, Anderson K C. Oncostatin M induces association of Grb2 with Janus kinase JAK2 in multiple myeloma cells. J Exp Med. 1995;182:1801–1806. doi: 10.1084/jem.182.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daley G Q, Van Etten R A, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 5.David M, Petricoin E, Benjamin C, Pine R, Weber M J, Larner A C. Requirement for MAP kinase (ERK2) activity in interferon alpha- and interferon beta-stimulated gene expression through STAT proteins. Science. 1995;269:1721–1723. doi: 10.1126/science.7569900. [DOI] [PubMed] [Google Scholar]
- 6.Eguchi M, Eguchi-Ishimae M, Tojo A, Morishita K, Suzuki K, Sato Y, Kudoh S, Tanaka K, Setoyama M, Nagamura F, Asano S, Kamada N. Fusion of ETV6 to neurotrophin-3 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25) Blood. 1999;93:1355–1363. [PubMed] [Google Scholar]
- 7.Endo T A, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 8.Finer M H, Dull T J, Qin L, Farson D, Roberts M R. kat: a high-efficiency retroviral transduction system for primary human T lymphocytes. Blood. 1994;83:43–50. [PubMed] [Google Scholar]
- 9.Frank D A, Mahajan S, Ritz J. B. lymphocytes from patients with chronic lymphocytic leukemia contain signal transducer and activator of transcription (STAT) 1 and STAT3 constitutively phosphorylated on serine residues. J Clin Investig. 1997;100:3140–3148. doi: 10.1172/JCI119869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golub T R, Barker G F, Lovett M, Gilliland D G. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994;77:307–316. doi: 10.1016/0092-8674(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 11.Golub T R, Goga A, Barker G, Afar D, McLaughlin J, Bohlander S, Rowley J, Witte O, Gilliland D G. Oligomerization of the ABL tyrosine kinase by the ETS protein TEL in human leukemia. Mol Cell Biol. 1996;16:4107–4116. doi: 10.1128/mcb.16.8.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouilleux-Gruart V, Gouilleux-Gruart F, Desaint C, Claisse J F, Capoid J C, Delobel J, Weber-Nordt R, Dusanter-Fourrt I, Dreyfus F, Groner B, Prin L. STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood. 1996;87:1692–1697. [PubMed] [Google Scholar]
- 13.Hawley R G, Lieu F H L, Fong A Z C, Hawley T S. Versatile retroviral vectors for potential use in mice by an interleukin 6 retrovirus. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]
- 14.Hilton D J, Richardson R T, Alexander W S, Viney E M, Willson T A, Sprigg N S, Starr R, Nicholson S E, Metcalf D, Nicola N A. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho J M, Beattie B K, Squire J A, Frank D A, Barber D L. Fusion of the ets transcription factor TEL to Jak2 results in constitutive Jak-Stat signaling. Blood. 1999;93:4354–5364. [PubMed] [Google Scholar]
- 16.Hunter T, Sefton B M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci USA. 1980;77:1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihle J N. Signaling by the cytokine receptor superfamily in normal and transformed hematpoietic cells. Adv Cancer Res. 1996;68:23–65. doi: 10.1016/s0065-230x(08)60351-6. [DOI] [PubMed] [Google Scholar]
- 18.Ihle J N, Kerr I M. Jaks and Stats in signaling by the cytokine receptor family. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 19.Ilaria R L J, Van Etten R A. P210 and P190(BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem. 1996;271:31704–31710. doi: 10.1074/jbc.271.49.31704. [DOI] [PubMed] [Google Scholar]
- 20.Johnson G R, Gonda T J, Metcalf D, Hariharan I K, Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989;8:441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacronique V, Boureux A, Monni R, Dumon S, Mauchauffe M, Mayeux P, Gouilleux F, Berger R, Gisselbrecht S, Ghysdael J, Bernard O A. Transforming properties of chimeric TEL-JAK2 proteins in Ba/F3 cells. Blood. 2000;95:2076–2083. [PubMed] [Google Scholar]
- 22.Lacronique V, Boureux A, Valle V D, Poirel H, Quang C T, Mauchauffe M, Berthou C, Lessard M, Berger R, Ghysdael J, Bernard O A. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 23.Lavau C, Szilvassy S J, Slany R, Cleary M L. Immortalization and leukemic transformation of a myelomonocytic precursor by retrovirally transduced HRX-ENL. EMBO J. 1997;16:4226–4237. doi: 10.1093/emboj/16.14.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Ilaria R L, Jr, Million R P, Daley G Q, Van Etten R A. The P190, P210, and p230 forms of the BCR/ABL oncogene induce a similar chronic myeloid lerkemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. 1999;189:1399–1412. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Schwaller J, Kutok J, Cain D, Aster J C, Williams I R, Gilliland D G. Signal transduction and transforming properties of the TEL/TRKC fusions associated with t(12;15)p(13;q25) in congenital fibrosarcoma and acute myelogenous leukemia. EMBO J. 2000;19:1827–1838. doi: 10.1093/emboj/19.8.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masuhara M, Sakamoto H, Matsumoto A, Suzuki R, Yasukawa H, Mitsui K, Wakioka T, Tanimura S, Sasaki A, Misawa H, Yokouchi M, Ohtsubo M, Yoshimura A. Cloning and characterization of novel CIS family genes. Biochem Biophys Res Commun. 1997;239:439–446. doi: 10.1006/bbrc.1997.7484. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto A, Masuhara M, Mitsui K, Yokouchi M, Ohtsubo M, Misawa H, Miyajima A, Yoshimura A. CIS, a cytokine inducible SH2 protein, is a target of the JAK-STAT5 pathway and modulates STAT5 activation. Blood. 1997;89:3148–3154. [PubMed] [Google Scholar]
- 28.Mayer B J, Jackson P K, Baltimore D. The noncatalytic src homology region 2 segment of abl tyrosine kinase binds to tyrosine-phosphorylated cellular proteins with high affinity. Proc Natl Acad Sci USA. 1991;88:627–631. doi: 10.1073/pnas.88.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minamoto S, Ikegame K, Ueno K, Narazaki M, Naka T, Yamamoto H, Matsumoto T, Saito H, Hosoe S, Kishimoto T. Cloning and functional analysis of new members of STAT induced STAT inhibitor (SSI) family: SSI-2 and SSI-3. Biochem Biophys Res Commun. 1997;237:79–83. doi: 10.1006/bbrc.1997.7080. [DOI] [PubMed] [Google Scholar]
- 30.Muda M, Boschert U, Dickinson R, Martinou J C, Martinou I, Camps M, Schlegel W, Arkinstall S. MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J Biol Chem. 1996;8:19–26. doi: 10.1074/jbc.271.8.4319. [DOI] [PubMed] [Google Scholar]
- 31.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 32.Okuda K, Golub T R, Gilliland D G, Griffin J D. p210BCR/ABL, p190BCR/ABL amd TEL/ABL activate similar signal transduction pathways in hematopoietic cell lines. Oncogene. 1996;13:1147–1152. [PubMed] [Google Scholar]
- 33.Papadopoulos P, Ridge S A, Boucher C A, Stocking C, Wiedemann L M. The novel activation of ABL by fusion to an ets-related gene, TEL. Cancer Res. 1995;55:34–38. [PubMed] [Google Scholar]
- 34.Peeters P, Raynaud S D, Cools J, Wlodarska I, Grosgeorge J, Philip P, Monpoux F, Van Rompaey L, Baens M, Van den Berghe H, Marynen P. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood. 1997;90:2535–2540. [PubMed] [Google Scholar]
- 35.Peeters P, Wlodarska I, Baens M, Criel A, Selleslag D, Hagemeijer A, Van den Berghe H, Marynen P. Fusion of ETV6 to MDS1/EVI1 as a result of t(3;12)(q26;p13) in myeloproliferative disorders. Cancer Res. 1997;57:564–569. [PubMed] [Google Scholar]
- 36.Persons D A, Allay J A, Allay E R, Smeyne R J, Ashmun R A, Sorrentino B P, Nienhuis A W. Retroviral-mediated transfer of the green fluorescent protein gene into murine hematopoietic cells facilitates scoring and selection of transduced progenitors in vitro and identification of genetically modified cells in vivo. Blood. 1997;90:1777–1786. [PubMed] [Google Scholar]
- 37.Pircher T J, Flores-Morales A, Mul A L, Saltiel A R, Norstedt G, Gustafsson J A, Haldosen L A. Mitogen-activated protein kinase kinase inhibition decreases growth hormone stimulated transcription mediated by STAT5. Mol Cell Endocrinol. 1997;133:169–176. doi: 10.1016/s0303-7207(97)00164-0. [DOI] [PubMed] [Google Scholar]
- 38.Pircher T J, Petersen H, Gustafsson J A, Haldosen L A. Extracellular signal-regulated kinase (ERK) interacts with signal transducer and activator of transcription (STAT) 5a. Mol Endocrinol. 1999;13:555–565. doi: 10.1210/mend.13.4.0263. [DOI] [PubMed] [Google Scholar]
- 39.Sawyers C L. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–1340. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- 40.Schwaller J, Frantsve J, Tomasson M, Aster J, Williams I, Van Rompey L, Marynen P, Van Etten R, Ilaria R, Gilliland D G. Transformation of hematopoietic cell lines to growth-factor independence and induction of a fatal myeloid and lymphoproliferative disease in mice by retrovirally transduced TEL/JAK2 fusion gene. EMBO J. 1998;17:5321–5333. doi: 10.1093/emboj/17.18.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwaller J, Parganas E, Wang D, Cain D, Aster J C, Williams I R, Lee C-K, Gerthner R, Kitamura T, Franstve J, Anastasiadou E, Loh M L, Levy D E, Ihle J, Gilliland D G. Stat5a/b is essential for the myelo- and lymphoproliferative disease induced by TEL/JAK2. Mol Cell. 2000;6:693–704. doi: 10.1016/s1097-2765(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 42.Sexl V, Piekorz R, Moriggl R, Rohrer J, Brown M P, Bunting K D, Rothammer K, Roussel M F, Ihle J N. Stat5a/b contribute to interleukin1-induced B-cell precursor expansion, but abl and bcr/abl-induced transformation are independent of Stat5. Blood. 2000;96:2277–2283. [PubMed] [Google Scholar]
- 43.Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, Hilton D J. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 44.Wen Z, Zhong Z, Darnell J E J. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 45.Wilbanks A M, Mahajan S, Frank D A, Druker B J, Gilliland D G, Carroll M. TEL/PDGFbetaR fusion protein activates STAT1 and STAT5: a common mechanism for transformation by tyrosine kinase fusion proteins. Exp Hematol. 2000;28:584–593. doi: 10.1016/s0301-472x(00)00138-7. [DOI] [PubMed] [Google Scholar]
- 46.Winston L A, Hunter T. JAK2, Ras, and Raf are required for activation of extracellular signal-regulated kinase/mitogen-activated protein kinase by growth hormone. J Biol Chem. 1995;270:30837–30840. doi: 10.1074/jbc.270.52.30837. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita H, Xu J, Erwin R A, Farrar W L, Kirken R A, Rui H. Differential control of the phosphorylation state of proline-juxtaposed serine residues Ser725 of Stat5a and Ser730 of Stat5b in prolactin-sensitive cells. J Biol Chem. 1998;273:30218–30224. doi: 10.1074/jbc.273.46.30218. [DOI] [PubMed] [Google Scholar]
- 48.Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle J N, Yoshimura A. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 1999;18:1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J G, Farley A, Nicholson S E, Willson T A, Zugaro L M, Simpson R J, Moritz R L, Cary D, Richardson R, Hausmann G, Kile B J, Kent S B, Alexander W S, Metcal D, Hilton D J, Nicola N A, Baca M. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci USA. 1999;96:2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Blenis J, Li H C, Schindler C, Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995;267:1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang H, Patel S V, He T, Sonsteby S K, Niu Z, Wojchowski D M. Inhibition of erythropoietin-induced mitogenesis by a kinase-deficient form of JAK2. J Biol Chem. 1994;269:21411–21414. [PubMed] [Google Scholar]