Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in late 2019, and is the infectious agent that caused the coronavirus disease 2019 (COVID-19) pandemic. Although respiratory and gastrointestinal manifestations of SARS-CoV-2 are well defined, the spectrum of neurological involvement is less defined. The classic type of Guillain–Barré syndrome (GBS) progresses over days to weeks and has a monophasic course. Areflexia/hyporeflexia and ascending and symmetrical paralysis are observed clinically in patients. It is an autoimmune process that typically leads to the destruction of myelin after infection. There have been numerous reports of adult patients with the coexistence of GBS disease and active COVID-19 illness, but this number is lacking for children. In this study, we present a literature review of the etiological correlation between SARS-CoV-2 and GBS and describe the cases of two pediatric patients with acute monophasic Guillain–Barré syndrome (GBS) during active COVID-19 infection.
Keywords: Child, Guillain–Barre syndrome, SARS-CoV-2, COVID-19, AMAN, Myasthenia gravis, Neurological complication, Autoimmune
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the infectious agent that emerged in late 2019 and caused the coronavirus disease 2019 (COVID-19) pandemic [1]. The manifestations of COVID-19 are often nonspecific, such as respiratory symptoms, fever, malaise, and fatigue, which can range from a mild cough to severe pneumonia, but most cases are asymptomatic in childhood [2,3]. However, neurological complications have been reported recently, such as dizziness, headache, febrile seizures, myalgia, encephalopathy, encephalitis, stroke, and acute peripheral nerve diseases [4,5]. Guillain–Barré syndrome (GBS) is the most common cause of acute flaccid paralysis in children, and acute inflammatory demyelinating polyradiculoneuropathy (AIDP) is the most common subtype [6]. GBS is an acute immune-mediated polyradiculoneuropathy, of which approximately 60% is associated with a previous respiratory or gastrointestinal tract infection [7,8]. Multiple central and peripheral neurological findings have been associated with SARS-CoV-2 in adults and children during the COVID-19 pandemic. However, very few studies have reported an association between COVID-19 and GBS in children. This article presents two cases of GBS associated with active SARS-CoV-2 infection. The first case is a pharyngeal–cervical–brachial variant of GBS associated with active COVID-19 infection, which has not been reported before in the literature.
2. Case reports
2.1. Case 1
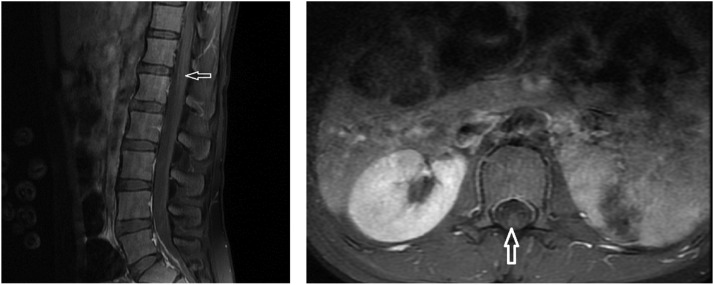
Our first case was a previously healthy 17-year-old girl who presented with complaints of weakness in walking, pain in the legs, and subfebrile temperature. There was no history of gastroenteritis or vaccination before the admission. A nasopharyngeal SARS-CoV-2 polymerase chain reaction (PCR) test result was positive. On physical examination, her vital signs were stable. She could not walk and sit. At the neurological examination the patient had clear consciousness with full awareness. She had bilateral ptosis. Facial weakness and speech–swallowing difficulties were present. There was also weakness in the neck and shoulder muscles. In the upper extremities, the proximal muscle strength was decreased to 2/5 and the distal muscle strength to 3/5, while the proximal muscle strength in the lower extremities was 3/5 and the distal muscle strength was 5/5. Deep tendon reflexes were significantly decreased in the bilateral upper extremity and were normoactive in the lower extremity. There were no signs of upper motor neuron disorder or meningeal irritation. Sensory examination was regular. There was a history of eating canned food 3 days before her presentation. Based on the history and clinical examination findings, GBS was considered in the preliminary diagnosis. However, myasthenic crisis and botulism were investigated in the differential diagnosis. Laboratory test results showed lymphopenia with a complete blood count of 0.27 × 103/µL (normal: 1–3.5). C-reactive protein level, erythrocyte sedimentation rate, creatinine kinase, liver function tests and electrolytes, serum vitamin B12 level, fibrinogen, and ferritin levels were all normal. A nasopharyngeal SARS-CoV-2 PCR test was positive. Cerebrospinal fluid (CSF) analysis revealed the following: protein, 98 mg/dL (normal: 15–45 mg/dL); glucose, 54 mg/dL (normal: 40–70 mg/dL); no cells were detected (albuminocytological dissociation). A cerebrospinal fluid (CSF) meningitis PCR panel did not detect antibodies for Cryptococcus neoformans/gattii, Streptococcus pneumonia, Streptococcus agalactiae, Neisseria meningitidis, Listeria monocytogenes, Haemophilus influenzae, Escherichia coli K1, herpes simplex virus 1, herpes simplex virus 2, herpes simplex virus 6, cytomegalovirus, human parechovirus, enterovirus. No growth was detected in the CSF culture. Anti-ganglioside panel analysis of CSF and serum (GM1, GQ1b, GD1b, GT1b, GD1a GM3, GM2) did not detect antibodies. Serum anti-acetylcholine and anti-musk receptor antibodies were negative. Test results for oligoclonal band antibodies in CSF were negative. Additional serological test results (ANA, anti-DNA, c-ANCA, p-ANCA, cytomegalovirus, Epstein–Barr virus, human immunodeficiency virus, Brucella agglutination) were negative. Tests of body fluid (stool and gastric juice) for botulinum toxin yielded negative results. Brain parenchyma was normal on magnetic resonance imaging (MRI), and faintly thin pial brightening was detected in cauda equina fibers and the conus medullaris level on spinal MRI (Fig. 1 ). In the electrophysiological study, we observed acute motor axonal involvement (there was normal motor conduction and low M response amplitude). F latency could not be obtained, and there was no pathological finding in needle electromyography (EMG) (Table 1 ). Based on the aforementioned clinical picture and investigations, we diagnosed the patient with a pharyngeal–cervical–brachial variant of GBS. The patient was treated with intravenous immunoglobulins (IVIG). Partial improvement of the motor findings was observed after treatment. The prognosis was not good. The patient's outpatient follow-up continues with a physical therapy rehabilitation program in the pediatric neurology unit.
Fig. 1.
Post-contrast pial brightness (left) in T1 sagittal section at conus medullaris level in spinal magnetic resonance imaging (MRI); pial contrast in conus medullaris/cauda equina in axial T1 section (right).
Table 1.
Motor nerve conduction and sensory nerve conduction studies.
| Motor nerve | Segment | Distal latency (ms) |
Amplitude (mV) |
NCV (m/s) |
F latency (ms) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 1 | Case 2 | Case 1 | Case 2 | Case 1 | Case 2 | ||
| Right median nerve | Wrist | 4.6 (RV ≤ 3.8) | NR | 3 (RV ≥ 4) | NR | 56 (RV≥49) | - | Absent | Absent |
| Elbow | NR | 3 | NR | Absent | Absent | ||||
| Right ulnar nerve | Wrist | 2.9 (RV≤ 3.8) | NR | 2 (RV ≥ 4) | NR | 50 (RV≥49) | - | Absent | Absent |
| Elbow | NR | 2 | NR | Absent | Absent | ||||
| Right peroneal nerve | Ankle | 4.1 (RV≤ 5.6) | NR | 1 (RV≥ 3) | NR | 43 (RV≥44) | - | Absent | Absent |
| Head of the fibula | NR | 1 (RV≥ 3) | NR | Absent | Absent | ||||
| Sensory nerve | Distal latency (ms) |
Amplitude (mV) |
NCV (m/s) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 1 | Case 2 | Case 1 | Case 2 | ||||
| Right median nerve | 1.7(RV≤ 3,5) | 2.6(RV≤ 3.5) | 37 (RV≥ 20) | 34 (RV≥ 20) | 50 (RV≥50) | 50 (RV≥50) | |||
| Right ulnar nerve | 2.1(RV≤ 3,1) | 2.6(RV≤ 3.1) | 39 (RV≥ 17) | 23 (RV≥ 17) | 57 (RV≥50) | 51 (RV≥50) | |||
| Left sural nerve | 4.2(RV≤ 4,4) | 3.3(RV≤ 4.4) | 8 (RV≥ 6) | 20 (RV≥ 6) | 40 (RV≥40) | 41 (RV≥40) | |||
NCV: nerve conduction velocity; NR: no response; -: not applicable; RV: reference value.
In our case, brainstem encephalitis, myasthenia gravis, and botulism were also considered in the differential diagnosis: encephalitis with preservation of consciousness, myasthenia gravis with negative detection of myasthenic antibodies, normal sequential nerve stimulation in the nerve conduction study, and negative detection of botulinum toxin from body fluids, respectively, were excluded.
2.2. Case 2
Our second case, a previously healthy 15-year-old male patient, was admitted with complaints of fever that had started 2 days earlier, coughing, difficulty walking, and pain in the legs. There was no vaccination history before the admission, but he had been in isolation at home for 6 days due to the diagnosis of COVID-19 infection. A nasopharyngeal SARS-CoV-2 PCR test result was positive. On physical examination, his vital signs were stable. There were no respiratory symptoms of COVID-19 disease. In the neurological examination, the patient's consciousness was clear and there was full awareness. The cranial nervous system examination was normal. There was no difficulty in swallowing. Sensory examination was normal. In the upper extremity, distal muscle strength was 2/5, and proximal muscle strength was 3–4/5. The lower extremity muscle strength was 1–2/5 in the proximal and distal parts, and bilateral deep tendon reflexes could not be obtained. There were no signs of upper motor neuron disorder or meningeal irritation. Laboratory tests were performed similarly to Case 1; complete blood count, C-reactive protein level, sedimentation, creatinine kinase, liver function tests and electrolytes, serum vitamin B12 level, fibrinogen, and ferritin levels were normal. A nasopharyngeal SARS-CoV-2 PCR test result was positive. In the CSF analysis, the following findings were reported: protein, 124 mg/dL (normal: 15–45 mg/dL); glucose, 66 mg/dL (normal: 40–70 mg/dL); no cells were detected (albuminocytological dissociation). No antibodies were detected in the CSF meningitis PCR panel. No growth was detected in the CSF culture. Autoimmune and infectious serological test results were negative. The GM1 antibody was significantly positive in the serum anti-ganglioside antibody panel analysis. No anti-ganglioside antibodies were detected in CSF analysis. There was no pathological finding in the brain and spinal MRI study. Considering GBS with the history and clinical examination findings, IVIG treatment was administered at a dose of 1 g/kg for 2 days. Acute motor axonal neuropathy was found, and F latency could not be obtained. In the early-stage needle EMG, no voluntary activity could be detected in any muscle group, and no signs of denervation were detected (Table 1). On the basis of all the results, the patient was considered to have AMAN, a GBS subtype. The prognosis was good. Before discharge, the patient started walking. Pediatric neurology follow-ups are carried out along with the physical therapy program.
3. Discussion
This study presents two cases of GBS developing during active COVID-19 infection, one with AMAN and the other with AMAN/FSB variant and an atypical course. COVID-19 remains a global epidemic, with over 510 million reported cases worldwide. As of April 2022, approximately 15,028,000 cases of COVID-19 had been reported in Turkey [9]. Mohammad Aladawi et al. described 109 patients with SARS-CoV-2-associated GBS, with a mean age of 56.07 years. In most of these patients, neurological symptoms appeared on average 12 days after SARS-CoV-2 infection, and classic sensorimotor GBS with acute demyelinating polyneuropathy were the most common GBS variants in these patient [10]. Another published literature review reported a relationship between GBS and COVID-19 infection in 35 pediatric cases [11]: Nasopharyngeal SARS-CoV-2 PCR test results were positive in 17 and negative in five patients. SARS-CoV-2 serological test results were positive in 12 patients. In our cases, a SARS-CoV-2 serological test of the CSF could not be performed. As far as we know, the most common variant in pediatric GBS cases associated with COVID-19 reported until May 2022 was AIDP. Together with our cases, AMAN was the second most frequently reported (Table 2 ). The most common presentation was increasing progressive weakness. The time from onset of COVID-19 symptoms to clinical signs of GBS ranged from 1 to 6 weeks. However, many pediatric case reports have described the association between parainfectious COVID-19 and GBS [12], [13], [14]. GBS has been reported to be associated with other forms of coronavirus in one child before the COVID-19 pandemic [15].
Table 2.
COVID-19-related GBS cases reported with our cases until May 2022.
| Gender | Age (years) | Serology test of SARS-CoV-2 | GBS variant | Treatment | ||||
|---|---|---|---|---|---|---|---|---|
| Male | 23 | 2–18 | Positive | 19 | AMAN | 7 | IVIG | 23 |
| Female | 14 | Negative | 5 | AIDP | 9 | Steroid | 4 | |
| Not done | 13 | MFS | 3 | Plasma exchange | 2 | |||
| FSB | 1 | Not mentioned | 13 | |||||
| Unexcitable | 1 | |||||||
| Not mentioned | 16 | |||||||
AMAN: acute motor axonal neuropathy; AIDP: acute inflammatory demyelinating polyneuropathy; IVIG: intravenous immunoglobulins; MFS: Miller–Fisher syndrome; GBS: Guillain–Barré syndrome; FSB: pharyngeal–cervical–brachial Variant.
GBS is an immune-mediated polyradiculoneuropathy known to develop after various types of infections. In the pathophysiological process, there is demyelination and axonal damage to peripheral nerves or nerve roots. The neurological findings of COVID-19 occur due to ACE-2 receptors in the nervous and skeletal system. Possible entry points to the nervous system are thought to be hematogenous spread, disruption of the blood–brain barrier, and direct transmission via cranial nerves [16]. Neurological symptoms in pediatric COVID-19 patients may present with heterogeneous central and peripheral nervous system manifestations. The World Health Organization's provisional case definition for the association of SARS-CoV-2 with neurological disease is when symptoms occur within 6 weeks of suspected acute infection, there is evidence of SARS-CoV-2 antibodies or viral RNA in any specimen, and no other possible etiology [17]. In addition, given that the COVID-19 condition can remain for a long time in the body, it is difficult to pinpoint the possible immune processes that occur after COVID-19 infection [18].
In this context, the term postinfectious describes GBS disease that develops after the SARS-CoV-2 infection has cleared, and parainfectious describes patients who develop GBS during active COVID-19 infection [19]. Our first case had a fever, lymphopenia, positive SARS-CoV-2 test result, and muscle weakness. Thus, there was a temporal relationship with a parainfectious profile, indicating a possible association between GBS and SARS-CoV-2 infection. Our second case had clinical features similar to the first case but without lymphopenia. In this respect, the picture is suggestive of a possible parainfectious immune mechanism. Based on our literature review, post-COVID-19 GBS and classic GBS appear similar in clinical presentation and outcome. However, unlike most case reports of GBS subtype, our cases were AMAN variants. Moreover, we report the first case of the pharyngeal–cervical–brachial variant of GBS associated with COVID-19. In the AMAN variant of GBS, anti-ganglioside antibodies are found to be positive in 86% of cases [20]. While serum anti-ganglioside GM1 antibody was positive in one of our patients, there were no anti-ganglioside antibodies in our other patient.
There are many possible ways in which COVID-19 affects the nervous system: first, a secondary effect is associated with the vascular and prothrombotic impact of viral infection on CNS or PNS vascularity (strokes, necrotizing leukoencephalopathy); secondly, the direct neurotropic or neuroinvasive effect of SARS-CoV-2 (e.g., anosmia, encephalopathy); third, a secondary effect of systemic inflammatory responses triggered by a viral infection; finally, there is an immune-mediated parainfectious or postinfectious autoimmune effect (e.g., GBS) in response to viral infection [16].
It is assumed that the overall lower-than-normal GBS cases reported since the start of the COVID-19 pandemic are linked to public health-related hygiene, isolation, and distancing measures implemented in many regions [21]. However, the presence of GBS symptoms in some patients in whom active SARS-CoV-2 infection persists, along with high albuminocytological dissociation rates in most reports, suggest a direct infectious condition and a postinfectious inflammatory process [22].
4. Conclusion
Analyzing antibodies against structural proteins and glycolipids in peripheral nerves in the etiology of GBS associated with COVID-19 will improve our understanding of the immunological cascade. On the other hand, detailed case definitions and epidemiological analyses are needed to determine the GBS subtype with electrophysiological studies.
Funding
No financial resources were used for this article.
Declaration of Competing Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.World Health Organization (WHO). WHO Director-General's opening remarks at the media briefing on COVID-19 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020
- 2.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 3.Castagnoli R., Votto M., Licari A., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 4.Needham E.J., Chou S.H.-Y., Coles A.J., et al. Neurological implications of COVID-19 infections. Neurocrit Care. 2020;32:667–671. doi: 10.1007/s12028-020-00978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asbury A.K. New concepts of Guillain-Barré syndrome. J Child Neurol. 2000;15:183–191. doi: 10.1177/088307380001500308. [DOI] [PubMed] [Google Scholar]
- 7.Cosi V., Versino M. Guillain-Barré syndrome. Neurol Sci. 2006;27:S47–S51. doi: 10.1007/s10072-006-0548-4. [DOI] [PubMed] [Google Scholar]
- 8.Ottaviani D., Boso F., Tranquillini E., et al. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. 2020;41:1351–1354. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO). Coronavirus disease (COVID-19) dashboard. 2022. https://covid19.who.int/
- 10.Aladawi M., Elfil M., Abu-Esheh B., et al. Guillain Barre syndrome as a complication of COVID-19: a systematic review. Can J Neurol Sci. 2022;49:38–48. doi: 10.1017/cjn.2021.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Jaberi M., Shihadat R., Masri A. Post SARS-CoV-2 Guillain-Barré syndrome in a child: case report and review of the literature. Childs Nerv Syst. 2022;38:2011–2016. doi: 10.1007/s00381-022-05536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis M., Bhumbra S., Felker M.V., et al. Guillain-Barré syndrome in a child with COVID-19 infection. Pediatrics. 2021;147 doi: 10.1542/peds.2020-015115. [DOI] [PubMed] [Google Scholar]
- 13.Akçay N., Menentoğlu M.E., Bektaş G., et al. Axonal Guillain-Barre syndrome associated with SARS-CoV-2 infection in a child. J Med Virol. 2021;93:5599–5602. doi: 10.1002/jmv.27018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanou S., Wardeh L., Govindarajan S., et al. Guillain-Barre syndrome (GBS) associated with COVID-19 infection that resolved without treatment in a child. BMJ Case Rep. 2022;15 doi: 10.1136/bcr-2021-245455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turgay C., Emine T., Ozlem K., et al. A rare cause of acute flaccid paralysis: human coronaviruses. J Pediatr Neurosci. 2015;10:280–281. doi: 10.4103/1817-1745.165716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stafstrom C.E., Jantzie L.L. COVID-19: neurological considerations in neonates and children. Children. 2020;7:133. doi: 10.3390/children7090133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellul M.A., Benjamin L., Singh B., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallett S., Allen A.J., Graziadio S., et al. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Med. 2020;18:346. doi: 10.1186/s12916-020-01810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padroni M., Mastrangelo V., Asioli G.M., et al. Guillain-Barrè syndrome following COVID-19: new infection, old complication? J Neurol. 2020;267:1877–1879. doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J., Zhang Y., Li R., et al. Anti-ganglioside antibodies in Guillain-Barre syndrome: a novel immunoblotting-panel assay. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.760889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keddie S., Pakpoor J., Mousele C., et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021;144:682–693. doi: 10.1093/brain/awaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filosto M., Cotti Piccinelli S., Gazzina S., et al. Guillain-Barré syndrome and COVID-19: an observational multicentre study from two Italian hotspot regions. J Neurol Neurosurg Psychiatry. 2021;92:751–756. doi: 10.1136/jnnp-2020-324837. [DOI] [PubMed] [Google Scholar]