Background
Nirmatrelvir/ritonavir, a newly authorized drug for the treatment of COVID-19, is a strong CYP3A4 inhibitor that can interact with many drugs, such as tacrolimus, reducing its metabolism. The reported case is a renal transplant patient who experienced a strong increase in tacrolimus blood concentration (up to 112ng/mL, more than ten times above the target range) when treated with both drugs at the same time, which caused a neurologic condition that required hospital admission for its control and treatment. The resolution of the symptoms was rapid, but the elevation of tacrolimus concentration remained for several days, even after discontinuing both drugs.
This case shows the importance of developing a protocol for managing this interaction to guarantee the efficacy and safety of treatment with nirmatrelvir/ritonavir in transplant patients.
Over the past few months, new drugs have been approved for treating COVID-19 infection. Nirmatrelvir/ritonavir, approved in Spain in March 2022 for treating mild COVID-19 infections in high-risk patients, has demonstrated an 89% reduction in the risk of progression to hospitalization and death when treatment is initiated within the first few days of symptom onset [1]. The presence of ritonavir in this drug causes a rapid and strong inhibition of cytochrome P450 (CYP3A4), which represents the main pathway for the metabolism of many drugs. This interaction can become especially relevant in transplant patients treated with tacrolimus, the main drug used in post-transplant immunosuppressive therapy, because this enzyme metabolizes it in the liver and intestine [2]. The interaction can cause elevations in tacrolimus blood levels leading to adverse effects such as nephrotoxicity or neurotoxicity.
We present the case of a kidney transplant patient who suffered a large elevation of tacrolimus blood levels due to interaction with nirmatrelvir/ritonavir.
Case Description
The case was a female patient of 83 years, transplanted in October 2010 for polycystic renal disease. Her immunosuppressive treatment consisted of 5 mg daily of extended-release tacrolimus (Advagraf), 500 mg of mycophenolate mofetil every 12 hours, and 5 mg daily of prednisone. The patient started with mild symptoms (cough, odynophagia, and dyspnea), and after a positive result in the COVID-19 antigen test, she contacted the nephrology service of our hospital. Being a patient at high risk of progression to severe disease, she was prescribed nirmatrelvir/ritonavir (2 tablets every 12 hours [dose adjusted to renal function] for 5 days). After consultation with the pharmacy service, immunosuppressive treatment was adjusted, maintaining prednisone and mycophenolate. As for tacrolimus, the patient was informed about interrupting its administration during antiviral treatment and reintroducing it progressively according to the blood concentrations once treatment was completed to avoid possible adverse effects. Before starting treatment, an analytical control was performed, presenting a minimum tacrolimus blood concentration of 7.9ng/mL and a serum creatinine concentration of 1.31 mg/dL.
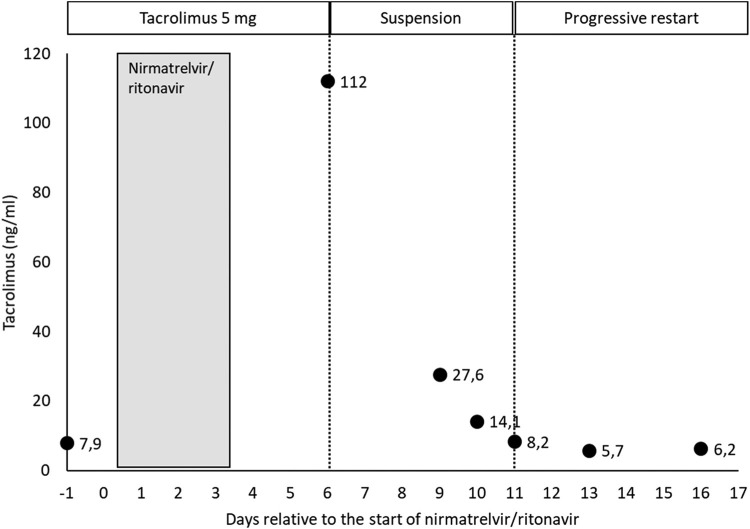
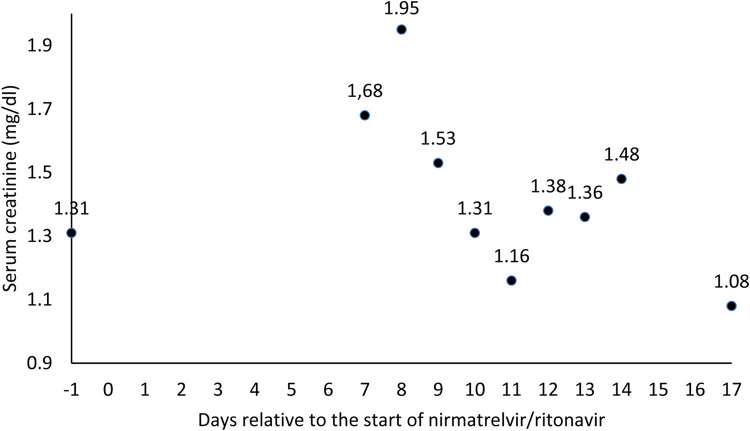
The patient did not properly understand the medical order and continued to take 5 mg of tacrolimus at the same time as nirmatrelvir/ritonavir for 3 days (Fig 1 ). At the next medical review, on day +6 relative to the initiation of nirmatrelvir/ritonavir treatment, a tacrolimus blood concentration of 112ng/mL was determined, more than ten times above the reference range (5-10ng/mL), which leads to an immediate suspension of the immunosuppressant administration. The following day (day +7), the patient went to the emergency room due to neurologic manifestations such as impaired level of consciousness, amnesia, tremors, decreased intake, and mucocutaneous dryness. Therefore, she was admitted to the hospital to control the neurologic symptoms and closely monitor tacrolimus concentrations. Analytically, a progressive worsening of renal function was observed, reaching a serum creatinine concentration peak of 1.95 mg/dL (Fig 2 ), although diuresis did not decrease at any time.
Fig 1.
Evolution of tacrolimus blood concentration and its dosing regimen.
Fig 2.
Evolution of serum creatinine.
During admission, tacrolimus remained suspended, and the patient was treated with 1500 mL of sodium chloride 0.9% saline solution daily. They experienced rapid improvement of the neurologic symptoms and decreased serum creatinine to baseline values without the need for other drugs. Tacrolimus blood concentration decrease was slower due to the persistence of CYP3A4 inhibition by ritonavir over time, even after treatment had been terminated [3]. Tacrolimus intake was reintroduced on day +11 at reduced doses (1 mg), when blood concentrations were back in range (8.2ng/mL), and was progressively increased; it grew to 2 mg the following day and 2.5 mg on day +14. After ten days of hospital admission, the patient was discharged on day +17 due to the resolution of her clinical picture, with a daily tacrolimus dose of 3 mg daily. During the 20 days described in this case, a coefficient of variation in tacrolimus concentrations of 138% was observed. She did not present dyspnea or other symptoms associated with COVID-19.
Discussion
The commercialization of new drugs to treat SARS-CoV-2 changes this infection's conception and therapeutic management, offering alternatives with proven efficacy. The exclusion of kidney transplant patients from many clinical trials increases the uncertainty regarding the efficacy and safety of these drugs in patients with this condition, but this should not be a contraindication for their use because there are studies demonstrating benefits when used appropriately [4,5].
There is little published evidence regarding the management of tacrolimus interaction with nirmatrelvir/ritonavir. Most studies [6], [7], [8] propose a similar plan of action: discontinue the immunosuppressant before starting treatment with nirmatrelvir/ritonavir, and restart it at reduced doses, according to tacrolimus blood concentrations. Dose adjustment of the immunosuppressant during and after antiviral treatment should always be individualized according to the needs of each patient, and patients should be subjected to closer monitoring of tacrolimus blood concentrations than usual.
The reported case shows the strong elevation of tacrolimus blood concentrations caused by the interaction with nirmatrelvir/ritonavir and its persistence over time, as well as the clinical manifestations that may develop, exposing the need to establish a standardized protocol for the management of transplanted patients prescribed these drugs.
The information flow between health care personnel and patients is also crucial in cases such as the one described in this study, as this impacts the patient's understanding of modifications to their treatment.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. New Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marzolini C, Kuritzkes DR, Marra F, Boyle A, Gibbons S, Flexner C, et al. Recommendations for the management of drug–drug interactions between the COVID -19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications. Clin Pharmacol Ther. 2022;112:1191–1200. doi: 10.1002/cpt.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzenmaier S, Markert C, Riedel KD, Burhenne J, Haefeli WE, Mikus G. Determining the time course of CYP3A inhibition by potent reversible and irreversible CYP3A inhibitors using a limited sampling strategy. Clin Pharmacol Ther. 2011;90:666–673. doi: 10.1038/clpt.2011.164. [DOI] [PubMed] [Google Scholar]
- 4.Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiremath S, McGuinty M, Argyropoulos C, Brimble KS, Brown P, Chagla Z, et al. Prescribing nirmatrelvir/ritonavir (Paxlovid) for COVID-19 in advanced chronic kidney disease. Clin J Am Soc Nephrol. 2022;17:1247–1250. doi: 10.2215/CJN.05270522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemaitre F, Grégoire M, Monchaud C, Bouchet S, Saint-Salvi B, Polard E, SFPT Therapeutic Drug Monitoring and Treatment Personalization group (STP-PT) of the French Society of Pharmacology and Therapeutics (SFPT) French Pharmacovigilance Network (CRPV) ANRS-MIE AC-43 Clinical Pharmacology Committee, joint working group. SFPT Therapeutic Drug Monitoring and Treatment Personalization group (STP-PT) of the French Society of Pharmacology and Therapeutics (SFPT) French Pharmacovigilance Network (CRPV) ANRS-MIE AC-43 Clinical Pharmacology Committee, joint working group Management of drug-drug interactions with nirmatrelvir/ritonavir in patients treated for Covid-19: Guidelines from the French Society of Pharmacology and Therapeutics (SFPT) Therapie. 2022;77:509–521. doi: 10.1016/j.therap.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange NW, Salerno DM, Jennings DL, Choe J, Hedvat J, Kovac D, et al. Nirmatrelvir/ritonavir use: managing clinically significant drug-drug interactions with transplant immunosuppressants. Am J Transplant. 2022;22:1925–1926. doi: 10.1111/ajt.16955. [DOI] [PubMed] [Google Scholar]
- 8.Salerno DM, Jennings DL, Lange NW, Kovac D, Shertel T, Chen JK, et al. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients. Am J Transplant. 2022;22:2083–2088. doi: 10.1111/ajt.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]