Abstract
Cost-effective, and accessible vaccines are needed for mass immunization to control the ongoing coronavirus disease 2019 (COVID-19), especially in low- and middle-income countries (LMIC). A plant-based vaccine is an attractive technology platform since the recombinant proteins can be easily produced at large scale and low cost. For the recombinant subunit-based vaccines, effective adjuvants are crucial to enhance the magnitude and breadth of immune responses elicited by the vaccine. In this study, we report a preclinical evaluation of the immunogenicity, efficacy and safety of a recombinant plant-based SARS-CoV-2 RBD vaccine formulated with 3M-052 (TLR7/8 agonist)-Alum adjuvant. This vaccine formulation, named Baiya SARS-CoV-2 Vax 2, induced significant levels of RBD-specific IgG and neutralizing antibody responses in mice. A viral challenge study using humanized K18-hACE2 mice has shown that animals vaccinated with two doses of Baiya SARS-CoV-2 Vax 2 established immune protection against SARS-CoV-2. A study in nonhuman primates (cynomolgus monkeys) indicated that immunization with two doses of Baiya SARS-CoV-2 Vax 2 was safe, well tolerated, and induced neutralizing antibodies against the prototype virus and other viral variants (Alpha, Beta, Gamma, Delta, and Omicron subvariants). The toxicity of Baiya SARS-CoV-2 Vax 2 was further investigated in Jcl:SD rats, which demonstrated that a single dose and repeated doses of Baiya SARS-CoV-2 Vax 2 were well tolerated and no mortality or unanticipated findings were observed. Overall, these preclinical findings support further clinical development of Baiya SARS-CoV-2 Vax 2.
Keywords: COVID-19, SARS-CoV-2, Plant-produced subunit vaccine, Receptor binding domain, Nicotiana benthamiana, Neutralizing antibody
1. Introduction
The recently emerged coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, China, and is responsible for the global Coronavirus disease 2019 (COVID-19) pandemic. Currently, numerous vaccine candidates against SARS-CoV-2 are in development, and some of them have been approved worldwide, using a wide range of platforms, including live attenuated, viral vectored, DNA/RNA-based, recombinant protein-based, and inactivated vaccines [1], [2]. The recent emergence of new SARS-CoV-2 variants poses a major challenge for the control of COVID-19 with currently licensed vaccines. SARS-CoV-2 variants contribute to re-infections and immune escape, demonstrating that the current vaccines are less than optimal for these new variants. Therefore, safe and effective vaccines are needed to improve the magnitude, duration, and breadth of the immune response against the circulating and future SARS-CoV-2 variants in order to control the COVID-19 pandemic.
Towards the development of a cost-effective vaccine, we have utilized a plant expression system for SARS-CoV-2 vaccine development because of its low cost and high scalability, among other advantages [3], [4], [5]. Previously, we developed a first generation COVID-19 subunit vaccine named, Baiya SARS-CoV-2 Vax 1 based on plant-based technology to produce the RBD protein of SARS-CoV-2. In brief, the gene encoding the RBD protein from the SARS-CoV-2 prototype strain was expressed in Nicotiana benthamiana plants and purified. The purified proteins were mixed with excipients and aluminum salts (Alum) and tested in mice and cynomolgus monkeys. The results showed that the Baiya SARS-CoV-2 Vax 1 induced a robust neutralizing antibody response and drastically reduced the mortality and severity of symptoms associated with COVID-19. In addition, the vaccine was safe and well-tolerated in preclinical studies [6], [7], [8]. This vaccine is currently in clinical trial Phase I (NCT04953078).
Adjuvants play a major role in protein-based subunit vaccine effectiveness by enhancing the immunogenicity and efficacy. Several adjuvants are either being tested in clinical trials or are approved for use in humans [9]. Alum have been used as an adjuvant in several currently available licensed vaccines to enhance the immune response to various antigens [10]. Newer classes of adjuvants, which include oil-in-water emulsions or molecules activated via., Toll-like receptors (TLR) have been developed in the past decade, such as AS03 (oil-in-water emulsion) and CpG1018 (TLR-9 agonist), and these adjuvants are now components of approved COVID-19 vaccines [11], [12].
More recently, small-molecule TLR agonists have been extensively evaluated as potential adjuvants for human use [13]. TLRs expressed on or in the cells of the innate immune system are the targets for adjuvants to elicit a robust adaptive immune response, leading to long-lasting protection. 3M-052, a synthetic next-generation TLR-7/8 agonist bearing a C18 lipid moiety, is designed to be released slowly from the administration site by forming a depot to reduce systemic drug levels and cytokines such as tumor necrosis factor-alpha [14]. In addition, 3M-052 is very potent, with doses of 5 µg and less being effective in a number of animal species. 3M-052 has been well studied as an adjuvant component in the development of several vaccine candidates, including vaccines against H1N1 [14], leishmaniasis [15], and HIV [16]. The Access to Advanced Health Institute (AAHI) has formulated 3M-052 using a phospholipid adjuvant with alum [17]. Recently, an HIV vaccine formulated with 3 M052-Alum adjuvant was shown to elicit high and durable antibody responses in non-human primates, and it is currently being evaluated for use in clinical trials [16]. In addition, 3M-052-Alum has also been applied in different experimental COVID-19 vaccines [18], [19]. Moreover, TLR agonists are also currently being investigated for use in cancer therapies [20], [21]. Based on these positive results of 3M-052, it strongly supports the testing and feasibility of the 3M-052-Alum combination as an adjuvant for our plant-derived SARS-CoV-2 vaccine.
In the present study, we describe the preclinical evaluation of our second-generation vaccine candidate, Baiya SARS-CoV-2 Vax 2 (recombinant plant-based SARS-CoV-2 RBD vaccine formulated with 3M-052 (TLR7/8 agonist)-Alum adjuvant. We found that the vaccine is safe, and elicits neutralizing antibody responses in mice and nonhuman primates, and protects against viral challenge in K18-hACE mice. There was no evidence of adverse events, unexpected findings, or systemic toxicity in any of these studies. Therefore, these study results support further development of this subunit vaccine formulation in clinical trials.
2. Materials and methods
2.1. Vaccine and adjuvants
The codon-optimized gene sequences of the RBD of SARS-CoV-2 were fused to the Fc region of human IgG, and the fusion protein was efficiently produced in Nicotiana benthamiana [6]. The purified RBD-Fc protein is formulated in the required doses with Alum and 3M-052-AF as adjuvants and sucrose and glycine as excipients to produce the final vaccines.
For the present study, 3M-052-alum adjuvant containing 3M-052 in aqueous form (3M-052-AF) and an aqueous suspension of aluminum hydroxide (Alhydrogel) were procured from AAHI, USA. Vaccine excipients sucrose (catalog: 1008929029, CAS number: 57–50-1) were purchased from Merck, Germany, and glycine pharma grade (catalog: 141340, CAS number: 56–40-6) from Panreac Quimica SLU, Spain.
2.2. Animal ethics statement and the OECD-GLP compliance
The immunogenicity studies using ICR mice (Protocol No. PN21-05) and the challenge study using K18–hACE2 mice (Protocol No. PN21-04) were performed following the Institutional Animal Care and Use Committee (IACUC) protocol approved by the Biosafety Review Committee at the Armed Forces Research Institute of Medical Sciences (AFRIMS), Thailand. The animal experiments were conducted in accordance with Thai laws, the Animal Welfare Act, and all applicable U.S. Department of Agriculture, Office of Laboratory Animal Welfare and U.S. Department of Defense guidelines.
The experiments with cynomolgus monkeys were approved by the IACUC of the National Primate Research Center of Thailand-Chulalongkorn University (NPRCT-CU) (Protocol review no. 2175008).
The single-dose (Protocol no. NU-TS640202-04) and repeated-dose toxicity (Protocol no. NU-TS640203-04) studies involving Jcl:SD rats were approved by the Naresuan University Animal Care and Use Committee (NUACUC), Naresuan University; Thailand. Single- and repeated-dose toxicology studies in Jcl:SD rats and safety pharmacology studies in cynomolgus monkeys were conducted in accordance with the OECD-GLP principles (G42/2021, G43/2021, and GLP-21-01, respectively). The protocol for the toxicology studies in Jcl:SD rats was designed in accordance with WHO Technical Report Series No. 927, the WHO guidelines on nonclinical evaluation of vaccines (2005)-Part; Toxicity Assessment and ICH guideline M3 (R2), and the protocol for the safety pharmacology studies in cynomolgus monkeys followed ICH S7A: Safety Pharmacology Studies for Human Pharmaceuticals (ICH, 2000) published by the European Medicines Agency.
All animal facilities in this study; AFRIMS, Naresuan University and NPRCT-CU (AAALAC International Accredited: 1752), are AAALAC International Accredited.
All the experiments strictly adhered to the principles stated in the “Guide for the Care and Use of Laboratory Animals [22]”.
2.3. Evaluation of Baiya SARS-CoV-2 Vax 2 immunogenicity in mice
Female ICR mice (6 weeks old) were purchased from AFRIMS colony (Thailand) and housed in microisolator cages in a certified biosafety level 2 (BSL-2) facility. Fifteen mice were randomly divided into three groups (n = 5/group). The groups of mice were intramuscularly injected via., the quadriceps muscle with prime-boost immunizations of 5, or 10 µg of Baiya SARS-CoV-2 Vax 2 or 3M-052-Alum adjuvant as a control (3M-052-AF 1 µg + Aluminum Hydroxide; Al content, 0.05 mg) at a three-week interval (days 0 and 21). The mice were bled on days 0, 14, and 35 to determine RBD-specific antibodies and neutralizing titers. CD4+ and CD8+ T cell responses were quantified for the intracellular cytokine staining (ICS) assay. Briefly, 106 of isolated splenocytes in a volume of 100 μl were stimulated with peptide pools corresponding to Wuhan-RBD at a concentration of 4 μg/ml/peptide in a 96-well plate. The plate was incubated for 2 h at 37 °C. After the incubation, cytokine secretions were then blocked with Golgi Plug (BFA), and further incubation was performed for 12–18 h at 37 °C. Cells were labeled with murine antibodies as follows: anti-mouse CD4-PerCp, CD8-APC-Cy7 and CD3-FITC. After fixation with Cytofix/Cytoperm, cells were incubated with anti-mouse IFN-g PE-Cy7. All stained samples were acquired using a FACS Calibur flow cytometer.
2.4. SARS-CoV-2 viral challenge and efficacy study in K18-hACE2 mice
Female K18-hACE2 mice (6–10 weeks of age) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA; stock No. 034860) and maintained in microisolator cages at the BSL-2 facility prior to the SARS-CoV-2 challenge or at the BSL-3 facility after the challenge. Thirty-six K18-hACE2 mice were randomly assigned to three groups (n = 12/group). The groups of mice were intramuscularly administered with two doses of 5 µg or 10 µg Baiya SARS-CoV-2 Vax 2, or 3M-052-AF-Alum (3M-052-AF 1 µg + Aluminum Hydroxide; Al content, 0.05 mg) at a three-week interval (days 0 and 21) via., the quadriceps muscle. The mice were bled on days 0, 14, 21, and 35 prior to the challenge. On day 35, the mice were intranasally inoculated with 2 × 104 PFU of SARS-CoV-2 virus (50 µl), Wuhan lineage, isolate hCoV-19/Hong Kong/VM20001061/2020 (stock titer of 2 × 104 PFU/ml). The mice were observed daily for clinical signs of disease, including changes in body weight, inappetence, and behaviors, and were humanely euthanized when they met a euthanasia criterion or at the end point (Days 39 (n = 6/group) and 41 (n = 6/group) by qualified technicians using CO2 inhalation, in accordance with institutional and AVMA guidelines. Blood and tissues were collected to determine virus titers in different tissues and for histopathology. The SARS-CoV-2 viral loads in serum and tissue samples were measured using quantitative RT–PCR; histopathological analysis of major organs and in situ hybridization of tissues were performed following previously described methods [7].
2.5. Evaluation of Baiya SARS-CoV-2 Vax 2 immunogenicity and safety pharmacology in cynomolgus macaques
Cynomolgus monkeys (n = 5/group; juvenile/subadult) were administered 0.5 mL of 1, 10, or 100 μg Baiya SARS-CoV-2 Vax 2 with the 3M-052-AF-alum adjuvant formulation (5 μg 3M-052-AF, 0.5 mg aluminum containing excipients) or the adjuvant formulation alone as a control via intramuscular injection into the quadriceps muscle on days 0 and 21. Skin irritation at the injection site was evaluated by Draize’s test on days 0 and 21. The grading scales for local reactions followed the OECD Test guideline 404: 0, None/absent; 1, very slight; 2, slight; 3, moderate; 4, severe.
Body weight, body temperature, cardiovascular system (CVS), central nervous system (CNS), and respiratory system (RS) endpoints were monitored at days 0 (pre-dose) 14 and 35. CNS safety pharmacology parameters included behavior and physical conditions, paresis, posture, visual field, auditory response, and the pinch test. CVS endpoints included systolic pressure, diastolic pressure, mean arterial pressure, heart rate, and an electrocardiogram. RS endpoints were SpO2, respiratory rate, and lung sound. Blood samples were collected for analysis of biochemical, hematological parameters and immunogenicity at day 0 (pre-dose), 14 and 35.
2.6. Immunological analysis for mice and monkey sera samples
ELISA was performed as previously described [7] with minor modifications for mouse and monkey sera. The induction of SARS-CoV-2-specific antibody responses was evaluated using the SARS–CoV-2 RBD-His tag protein (GenScript, USA) produced from Sf9 insect cells as the capture antigen. After preparation of the plates, and incubation with sera samples from the studies, the level of IgG was visualized by goat anti-monkey IgG HRP (Abcam, UK) with TMB stabilized substrate used for colorimetric development. The absorbance was measured at 450 nm. The endpoint titer was determined by a previously described method [23].
The microneutralization (MN) assay was performed to measure the neutralizing titers of the sera in a certified BSL-3 facility at the Department of Microbiology, Faculty of Science, Mahidol University, Thailand, as described previously with some modifications [6]. The sera collected from the ICR and the K18-hACE2 mice were tested against the ancestral (Wuhan) strain. For the monkey sera, neutralization antibody titers were determined against ancestral (Wuhan), Alpha, Beta, and Delta strains as previously described [6], [24].
The neutralizing activity of sera isolated from SARS-CoV-2-immunized monkeys against pseudoviruses using the firefly luciferase reporter displaying full length spike protein of various SARS-CoV-2 variants including Alpha, Beta, Gamma, Delta and Omicron was determined in a spike-pseudovirus neutralization assay using methods as described previously [25]. This test was performed at the Virology and Cell Technology Laboratory, National Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Technology Development Agency, Thailand.
2.7. Nonclinical toxicology study
2.7.1. Single-dose toxicity study in Jcl:SD rats
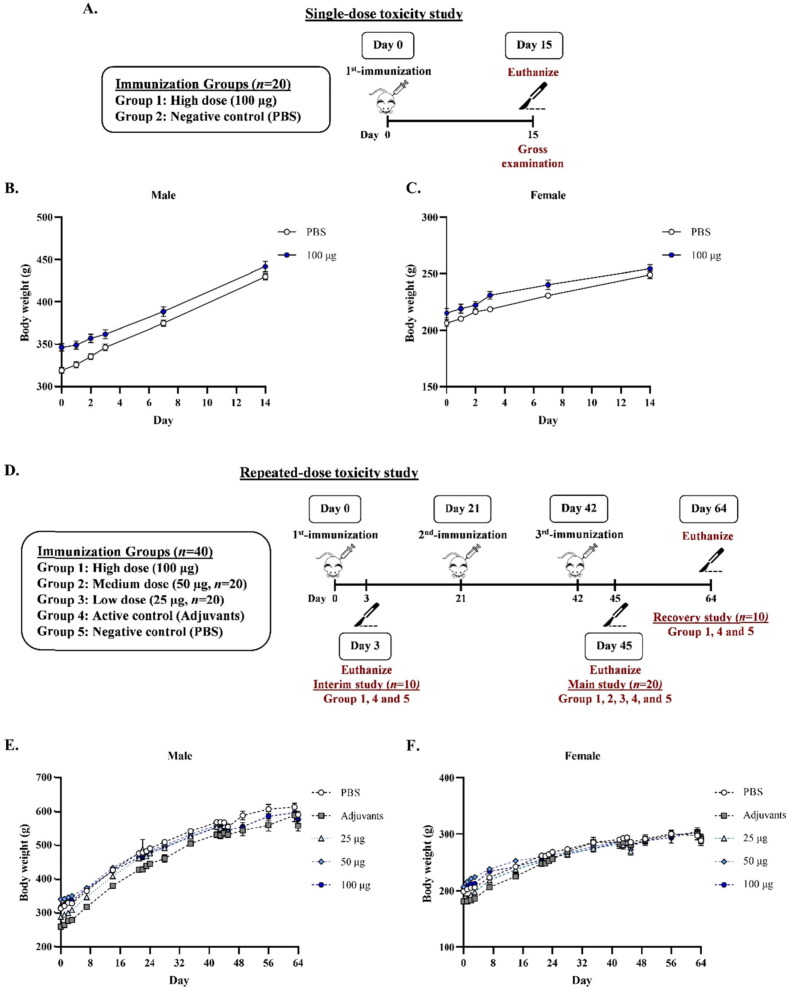
A total of 40 rats (20 males and 20 females) were randomly divided into two groups, each containing an equal number of males and females. Animals were assigned to the inactive control group (phosphate-buffered saline; PBS) and the high dose group of Baiya SARS-CoV-2 Vax 2 (100 µg) with adjuvant (3M-052-AF 5 µg + Aluminum Hydroxide; Al content, 0.5 mg). The rats were injected intramuscularly with 0.1 mL of the test item on day 0. The animals were sacrificed after 15 days of immunization (day 15). Rats were observed for 24, 48, and 72 h after dose administration and daily until day 14 for clinical signs such as coat and eye observation, respiration, pain expression, somatic activity, behavior, tremor, convulsion, salivation, diarrhea, lethargy, and coma. Body temperature was measured after test administration for 6, 24, and 48 h. Body weight measurements were taken on days 1, 2, 7, and 14. The vital organs (heart, liver, kidney, lungs, spleen, digestive tract, lymph nodes, and reproductive organs) were grossly examined.
2.7.2. Repeat-dose toxicity study in Jcl:SD rats
A total of 160 rats (80 males and 80 females) were randomly divided into different groups, each containing an equal number of males and females. Animals were assigned to the inactive control group (PBS), active control group (adjuvants), and the three Baiya SARS-CoV-2 vaccine groups at low (25 µg), medium (50 µg), and high (100 µg) doses. The inactive control group was administered PBS alone, and the active control group was administered PBS with 3M-052-AF-Alum to ensure that there were no adverse effects associated with the adjuvants or excipients. In this study, 20 animals per sex (n = 40/group) were assigned to the inactive control group, active control group, and high-dose group and evaluated for three sub-studies, interim (n = 10/group), main (n = 20/group), and recovery studies (n = 10/group). For low and medium dose groups, 10 animals per sex (n = 20/group) were assigned for the main study. In the interim study, animals were injected on day 0 and euthanized on day 3. For main and recovery studies, animals were injected on day 0, 21, and 42 (3-week interval). Animals were euthanized on day 45 (3 days after the third immunization) for the main study, whereas animals in the recovery group were euthanized on day 64 (22 days after the third immunization).
Mortality, morbidity, and clinical signs were monitored before immunization (pre-dose), twice during the first week of administration, and once a week thereafter. Body weight was monitored before immunization (pre-dose), daily during the first of each administration, and weekly thereafter. Rectal temperature was measured after each immunization. Urinalysis, hematology, and blood chemistry were analyzed by the urine analyzer Dirui H10 (Dirui industrial Co., ltd., China) with a urine test strip, the complete blood count analyzer (Vetscan® HM5 Hematology Analyzer, Abaxis, USA), and the blood chemistry analyzer (Vetscan® VS2 Chemistry Analyzer, Abaxis, USA), respectively.
For the gross examination, the external surface of the body, the injection site and all internal organs in the abdomen were grossly examined. The selected organs were weighed at the time of scheduled necropsy, and organ weights were converted to relative organ weights based on the organ-to-body weight percent. Histopathological examination was performed on selected organs that were harvested from all the animals. For histopathological analysis, tissues were microscopically examined following the European RITA and American NACAD working group guidelines. The sample slides were captured by Olympus BX53 and DP26. In some tissue or organ slides, the whole slide scan was used by NanoZoomer Hamamatsu with the image scope program. The lesion or findings were reported as semi-quantitative following the International Harmonization of Nomenclature and Diagnostic Criteria (INHAND).
2.8. Statistical analysis
The results of the immunogenicity study in mice, the challenge study in hACE2 mice, and the safety pharmacology study in monkeys were statistically analyzed with GraphPad Prism 9.0 (GraphPad Software Inc., USA). The differences were considered statistically significant at p value < 0.05 (*: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001).
For the immunogenicity in mice and monkeys, and challenge studies, the antigen-specific total IgG titer, MN50 titer, and PVNT50 results were analyzed by two-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. The percent weight change and viral load in the challenged mice were compared with those in the control mice, and the differences were statistically analyzed by two-way ANOVA and Dunnett’s multiple comparisons test. The IFN-γ ELISpot in monkeys was statistically analyzed by non-parametric Kruskal-Wallis test and Dunn’s multiple comparisons test. The hematological and blood chemical parameters and cardiovascular and respiratory system observations in monkeys were compared with the values on Day 0, and differences were analyzed by Dunnett’s multiple comparisons test. In the toxicity studies, statistical differences were analyzed by comparison t-tests, which were used to compare the inactive control (PBS) group and treatment groups and were divided into three sub-studies (interim, main, and recovery studies).
3. Results
3.1. Baiya SARS-CoV-2 Vax 2 induced antibody response in mice
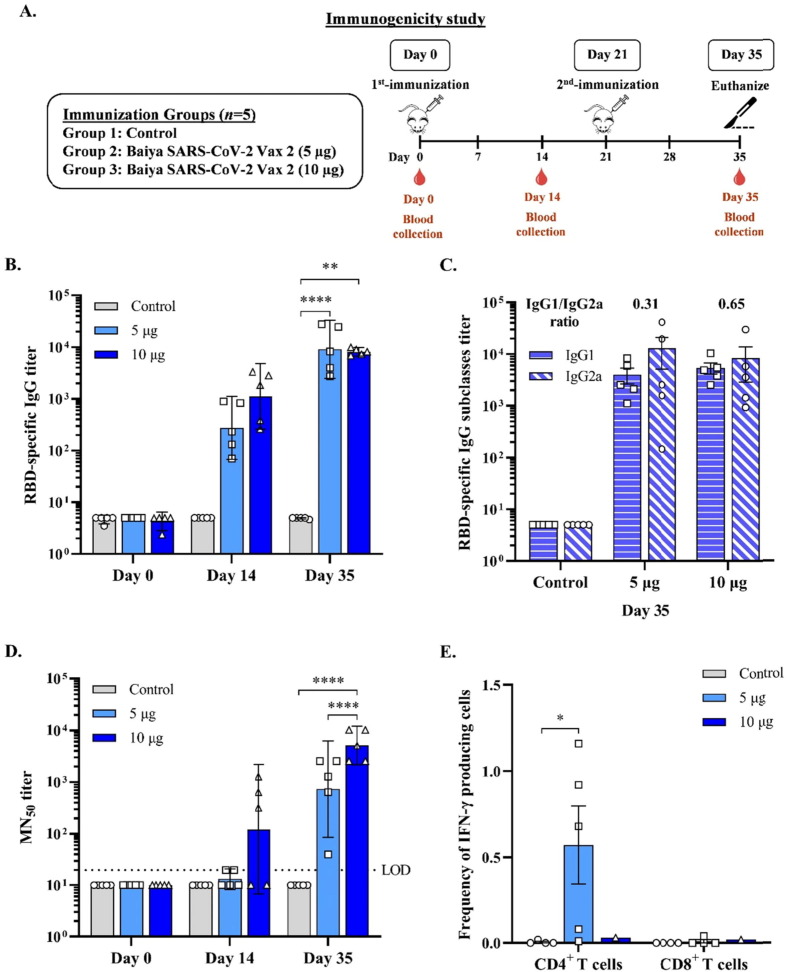
To investigate the immunogenicity of the Baiya SARS-CoV-2 vaccine, ICR mice were immunized twice and serum samples were collected for determination of antibody titers to the SARS-CoV-2 virus. Mice were intramuscularly administered with two different dosages (5 μg or 10 μg) of Baiya SARS-CoV-2 vax 2. As shown in Fig. 1 A, after two immunizations at day 0 and 21, the vaccine elicited effective immune responses. After second immunization on days 35, 5 µg-dose vaccine group (GMT = 9,102, 95 % CI: 2,482–33,381) induced the highest total IgG titer compared to the group with 10 µg dose (GMT = 8,174, 95 % CI: 6,786–9,846) (Fig. 1B).
Fig. 1.
(A) Schematic representation showing the timeline of vaccine immunizations and blood collection in ICR mice. Mice were divided into 3 groups (n = 5): the control and Baiya SARS-CoV-2 Vax 2 (dose 5, 10 µg of RBD-Fc adjuvanted with 1 µg of 3 M-052-AF plus 50 µg Al content of alum) groups. The mice were intramuscularly injected twice on day 0 and 21, and blood was collected on day 0, 14, and 35,14 days after each immunization. (B) RBD-specific total IgG determined by ELISA (C) RBD-specific IgG subtype responses assessed by ELISA (D) 50 % neutralizing antibody (MN50) titer against SARS-CoV-2 clinical isolate as determined in a live virus microneutralization assay. Dotted lines indicate the assay limit of quantitation. The data are presented as geometric mean titers with 95 % confidence intervals. (E) Baiya SARS-CoV-2 Vax 2-specific T cell responses were measured by flow cytometry with intracellular cytokine straining of splenocytes harvested from mice immunized with the vaccine. Frequency of IFN-γ producing CD4+, and IFN-γ producing CD8+ T cells after stimulation with Wuhan-RBD peptide pool for 12 to 18 h. The data are presented as mean ± SEM. Two-way ANOVA, Tukey test, was used (*: p < 0.05, **: p < 0.01, ****: p < 0.0001). Values smaller than the limit of detection (LOD) are plotted as 0.5*LOD.
IgG subtypes (IgG1 and IgG2a) were determined by ELISA, and the IgG1/IgG2a ratio was also calculated. A ratio of 0.5 or less indicates a T-helper type I (Th1)-biased response. A ratio of 2.0 or more indicates a Th2-biased response. Ratios between 0.5 and 2.0 indicate a mixed or balanced response [26]. As demonstrated in Fig. 1C, the vaccine induced a Th-1 biased-response (IgG1/IgG2a ratio = 0.31) at a 5 µg vaccine dose and a mixed Th1-Th2 response (IgG1/IgG2a ratio = 0.65) at a 10 µg vaccine dose.
3.1.1. Baiya SARS-CoV-2 Vax 2 induces neutralizing antibodies and T cell responses in mice
Mouse sera from all immunized groups (days 0, 14, and 35) were then assayed in vitro for the presence of neutralizing antibodies to SARS-CoV-2. The microneutralization assay was performed in a 96-well ELISA plate using live SARS-CoV-2 virus and Vero E6 cells. The 10 µg dose group (GMT = 121, 95 % CI: 7–2,189) induced detectable neutralizing antibodies on day 14. On day 35, the 10 µg dose group (GMT = 5,120, 95 % CI: 2,165–12,107) induced a high MN50 titer compared to the 5 µg dose group (GMT = 735, 95 % CI: 86–6,267) (Fig. 1D).
In vitro stimulation of splenocytes with peptide pools for RBD showed that splenic CD4+ T cells from animals receiving a 5 µg vaccine dose produced significantly high levels of IFN-γ (Th1 cytokine), whereas those receiving a10 µg vaccine dose produced negligible IFN-γ. We did not observe production of IL-5 (Th2 cytokine). Cytokine responses of splenic CD8 + T cells were also not detected (Fig. 1E).
3.2. Baiya SARS-CoV-2 Vax 2 effectively protected mice from SARS-CoV-2 challenge
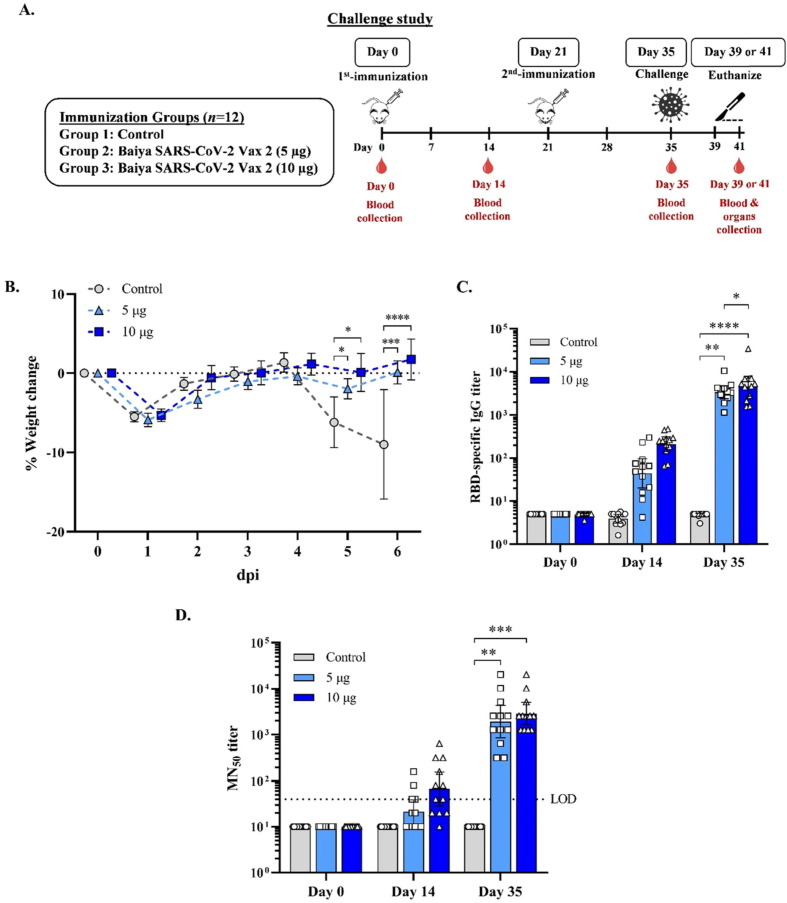
The protective efficacy of the Baiya SARS-CoV-2 Vax 2 vaccine was evaluated in K18-hACE2 mice. Animals were challenged with SARS-CoV–2 (Wuhan strain) via the intranasal route. Half of the mice (n = 6) in each group were euthanized on the fourth day (day 39), and the other half were euthanized on the sixth day after challenge (day 41) (Fig. 2 A). No animal mortality was observed. However, two animals in the control group were euthanized on day 40 due to reduced body weight and clinical signs of anorexia, increased respiration, movement with moderate stimulation, lethargy, and slightly rough coat. Two additional control animals were euthanized on day 41 because of the severity of clinical signs. These included decreased appetite and lethargy, signs of slow walking with moderate stimulation, and lack of grooming. In contrast, immunized animals showed no signs of clinical illness. The body weight of mice on day 36 (one day after challenge) showed a slight decrease in all groups compared with the body weight on day 35. The decrease in body weight was accompanied by a decreased appetite. From day 37, the body weight of Baiya SARS-CoV-2 Vax 2-treated mice increased steadily until day 41, whereas the body weight of control animals decreased on day 39 due to the onset of disease symptoms associated with the observed severe clinical signs (Fig. 2B).
Fig. 2.
(A) Vaccination. blood collection, and challenge regimen in K18-hACE2 mice challenge study. Mice were divided into 3 groups (n = 12): the control and Baiya SARS-CoV-2 Vax 2 (dose 5, 10 µg of RBD-Fc adjuvanted with 1 µg of 3 M-052-AF plus 50 µg Al content of alum) groups. The mice were intramuscularly injected on day 0 and 21, and blood was collected on day 0, 14, and 35. On day 35, the immunized mice were intranasally infected with SARS-CoV-2. Half number of mice in each group (n = 6) were humanely euthanized on four days after SARS-CoV-2 challenge and the other half were euthanized on 6 days after SARS-CoV-2 challenge. The organs were collected 4 or 6 days post-infection. (B) The percent body weight change of challenged mice after infection. The data are presented as mean ± SEM. Mixed-effects analysis, Tukey test, was used (*: p < 0.05, ***: p < 0.001, ****: p < 0.0001). (C) RBD-specific total IgG determined by ELISA and (D) 50 % neutralizing antibody (MN50) titer against SARS-CoV-2 clinical isolate as determined in a live virus microneutralization assay. Dotted lines indicate the assay limit of quantitation. The data are presented as geometric mean titers with 95 % confidence intervals. Two-way ANOVA, Tukey test, was used (*: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001). (D) Values smaller than the limit of detection (LOD) are plotted as 0.5*LOD.
Like the ICR mice, the K18-hACE2 transgenic mice that received 5 and 10 ug of the vaccine developed strong antibody responses. As shown in Fig. 2C and D, fourteen days before challenge, immunized animals receiving 5 and 10 µg of the vaccine generated high RBD-specific IgG (GMT = 3,340, 95 %CI: 2,377–4,695 and 4,563, 95 % CI: 2,683–7,763) and neutralizing antibody titers (GMT = 1,918, 95 % CI: 856–4,297and 2,874 95 %CI: 1,644–5,021).
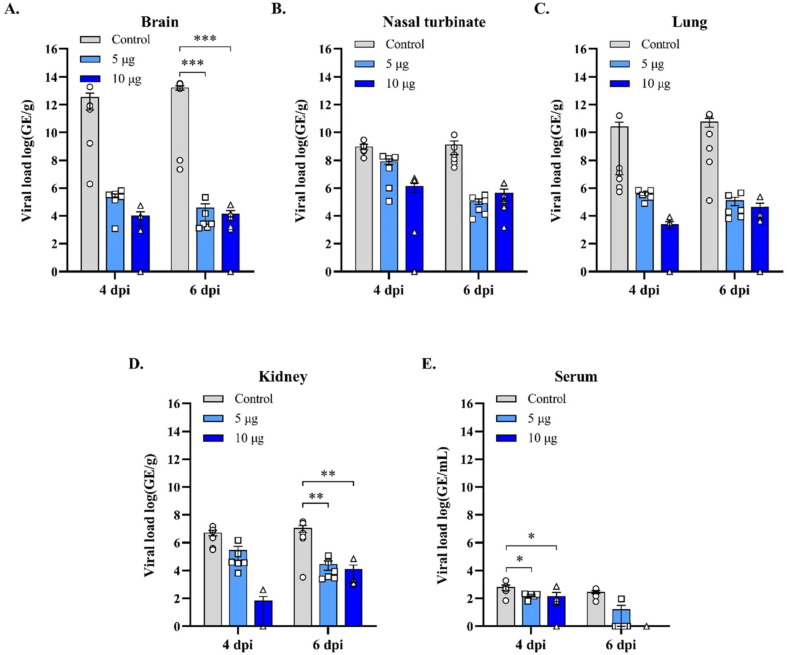
To extend the analysis of the protective immunity of Baiya SARS-CoV-2 vax 2, the viral load in different tissue compartments was compared with that of control animals. The immunized animals exhibited a significant decrease in viral replication compared with control animals, as indicated by the reduction in viral load in the blood, brain, and kidneys, with an overall reduction in the nasal turbinates and lungs, which was confirmed by in-situ hybridization studies in the lungs, nasal turbinates, and olfactory bulb, which showed a reduction in viral load. At day 41 (6 days post challenge), both the 5 and 10 μg dose groups significantly reduced viral genomic equivalents in the brain (p < 0.001) and kidney (p < 0.01) compared to control (Fig. 3 ).
Fig. 3.
Viral load in the mice tissues collected from the Baiya SARS-CoV-2 Vax 2 vaccinated (Dose 5, 10 µg) and control group on 4 and 6 dpi (n = 6) determined by qRT-PCR., (A) brain, (B) nasal turbinate, (C) lung, (D) kidney, and (E) serum. The data are expressed as the mean ± SEM. Two-way ANOVA, Dunnett test, was used to compare the control group (**: p < 0.01, ***: p < 0.001).
Histopathology results showed that administration of either 5 or 10 μg Baiya SARS-CoV-2 Vax 2 provided significant protection against the tissue and organ damage observed in the lungs and brain resulting from the SARS-CoV-2 challenge. Minimal peribronchiolar/perivascular inflammation was seen in 3 out of 12 and 2 out of 12 animals in the 5 or 10 μg Baiya SARS-CoV-2 Vax 2 groups, respectively. There were no histopathological observations in animals administered with Baiya SARS-CoV-2 Vax 2 (Supplementary figure S1).
3.3. Immunogenicity and safety pharmacology of Baiya SARS-CoV-2 Vax 2 in cynomolgus macaques
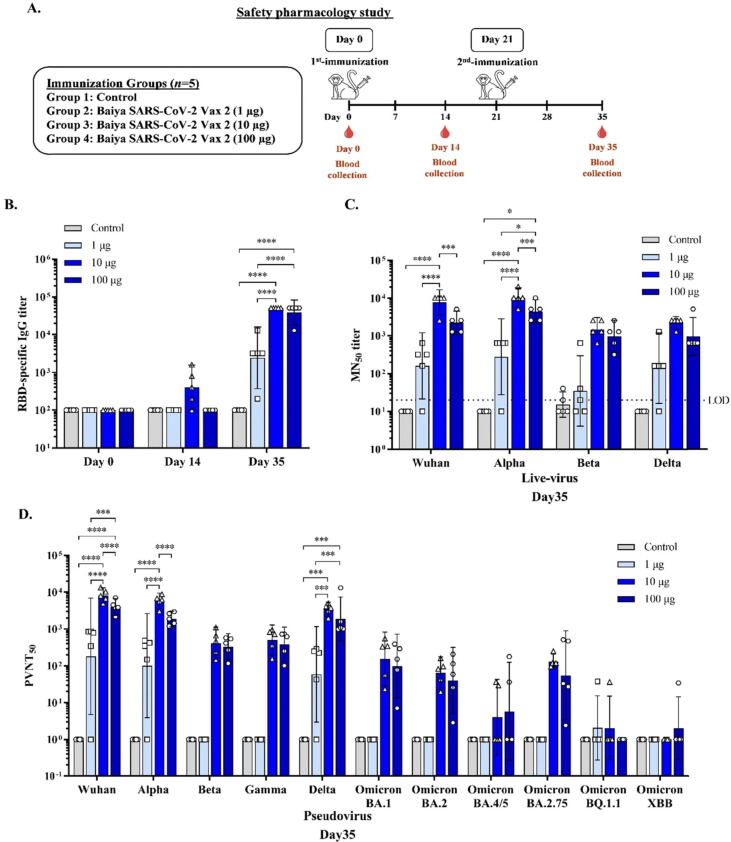
After the immunogenicity and efficacy in mice were done, the safety and immunogenicity of Baiya SARS-CoV-2 Vax 2 were evaluated further in cynomolgus macaques. Animals were immunized intramuscularly with two doses of 1, 10 and 100 μg of the vaccine (Fig. 4 A). There were no significant effects on body weight or body temperature (Supplementary table S1 and S2). Adverse effects at the injection site were minimal including slight erythema in some monkeys on day 0 and 2 after injection which resolved by the next day. There were no edema or allergenic effects in the immunized animals (Supplementary table S3). Blood chemistry results and complete blood counts from samples collected from control and vaccine treated monkeys on days 0, 14, and 35 were within the normal reference range (Supplementary table S4 and S5).
Fig. 4.
(A) Experimental design for the immunogenicity and safety pharmacology study in cynomolgus monkeys. Monkeys were divided into 4 groups (n = 5): the control and Baiya SARS-CoV-2 Vax 2 (dose 1, 10, and 100 µg of RBD-Fc adjuvanted with 5 µg of 3 M-052-AF plus 0.5 mg Al content of alum) groups. The monkeys were intramuscularly injected on Days 0 and 21, and blood was collected on day 0, 14, and 35 to assess the humoral immune response (B) RBD-specific total IgG (C) 50 % microneutralizing (MN50) titer, Values smaller than the limit of detection (LOD) are plotted as 0.5*LOD and (D) 50 % pseudovirus neutralizing titer (PVNT50) in the sera collected immunized monkeys were measured by ELISA. The data are presented as geometric mean titers with 95 % confidence intervals. The data are presented as mean ± SD. Two-way ANOVA, Tukey test, was used (*: p < 0.05, ***: p < 0.001, ****: p < 0.0001).
The CVS, RS, and CNS endpoints were evaluated on days 0, 14 and 35 in the monkeys in the control group, and only the highest dose (100 μg) of Baiya SARS-CoV-2 Vax 2. Baiya SARS-CoV-2 Vax 2 did not elicit abnormal CNS symptoms as determined by behavioral and physical conditions, including paresis, posture, visual field, auditory response, pinch test, and body temperature. Similarly, no abnormal CVS signs based on the endpoints of systolic pressure, diastolic pressure, mean arterial pressure, heart rate, and the electrocardiogram were detected. Based on the endpoints of SpO2, respiratory rate, and lung sounds. Baiya SARS CoV-2 Vax 2 did not elicit any abnormal signs in the RS (Supplementary tables S6, S7 and S8).
Immunogenicity results in nonhuman primates showed that the Baiya SARS CoV-2 Vax 2 at either 1, 10 or 100 μg can induce RBD-specific IgG antibodies. After the second dose of Baiya SARS CoV-2 Vax 2, RBD-specific IgG antibody titer in both 10 (GMT = 51,200, 95 %CI: 51,200–51,200) and 100 μg dose (GMT = 38,802, 95 % CI: 17,970–83,787) groups increased significantly (p < 0.0001) compared to the 1 μg-dose (GMT = 2,425, 95 % CI: 368–15,983) and control group (GMT = 100, 95 % CI: 100–100). There was no significant difference in RBD-specific IgG antibody response between 10 and 100 μg dose groups (Fig. 4B).
A single dose of 1, 10, or 100 μg of Baiya SARS-CoV-2 Vax 2 injection did not significantly increase neutralizing antibody titers against the SARS-CoV-2 Wuhan strain. However, after the second dose of Baiya SARS-CoV-2 Vax 2, markedly increased neutralizing antibody levels were observed in the 10 μg dose group (GMT = 7,760, 95 % CI: 3,594–16,757), which were significantly higher than the 100 μg dose group (GMT = 2,229, 95 % CI: 1,085–4,579) and a 1 μg dose group (GMT = 160, 95 %CI: 21–1,204). High cross-neutralizing activity in sera was observed in animals receiving 10 ug of vaccine. Compared with the Wuhan strain, the MN50 titer of total virus against Alpha was increased 1.3 folds and the MN50 titer against Beta and Delta were reduced 5.3 folds and 3.5 folds respectively in 10 μg dose group, whereas in the 100 μg dose group, the MN50 titer against Alpha was increased 2 folds and the MN50 titer against both Beta and Delta was reduced by 2.3 folds compared with the Wuhan strain (Fig. 4C).
The pseudovirus neutralization assay was also performed and extended to the SARS-2 Omicron variants. Consistent with the neutralizing activity of live virus, neutralizing antibodies to pseudovirus were higher in the 10 and 100 μg dose groups. Overall, 10 μg showed a similar neutralizing effect compared with the 100 μg dose group. A marginal 19.8- and 11.5-folds decrease in neutralizing antibody titer against the Beta and Gamma strains, respectively, was observed in the 10 μg dose group compared with the Wuhan strain. Compared with Beta and Gamma, the neutralizing antibodies induced by the vaccine effectively neutralized the Delta variant. Against the recently emerged Omicron sub-variants 4/5 and 2.75, 2020.8- and 685.8-folds and 43.9- and 34.2-folds decrease in neutralizing activity was observed in the 10 and 100 μg dose groups, respectively (Fig. 4D).
3.4. Toxicity of Baiya SARS-CoV-2 Vax 2 in rats
The toxicity of the Baiya SARS-CoV-2 Vax 2 was evaluated in single-dose and repeated-dose toxicity study in Jcl:SD rats (Table 1 ). In the single dose study, PBS or the vaccine at 100 μg was administered intramuscularly and the rats were euthanized on day 15 (Fig. 5 A). No deaths, morbidity, or significant changes in clinical signs, body temperature or food and water intake were observed during the study. No significant changes in body weight were observed in either males or females (Fig. 5B and C). Gross necropsy changes were observed in the submandibular lymph nodes and thymus, but these occurred in both the vaccine and control groups and were therefore not considered to be due to the test article.
Table 1.
Summary of single- and repeated-dose toxicity studies in Jcl:SD rats.
|
Group 1 High dose (100 µg) |
Group 2 Medium dose (50 µg) |
Group 3 Low dose (25 µg) |
Group 4 Active control (Adjuvant) | Group 5 Negative control (PBS) | |
|---|---|---|---|---|---|
| Single dose |
n = 10 (M/F) Immunization: day 0 Euthanized: day 15 |
NA | NA | NA |
n = 10 (M/F) Immunization: day 0 Euthanized: day 15 |
| Mortality | None observed | NA | NA | NA | None observed |
| Clinical sign observation | None | NA | NA | NA | None |
| Body temperature | No significant changes | NA | NA | NA | No changes |
| Body weights | No significant changes | NA | NA | NA | No changes |
| Food consumption | No vaccine-related effect | NA | NA | NA | No changes |
| Water consumption | No vaccine-related effect | NA | NA | NA | No changes |
| Gross examination | No vaccine-related macroscopic findings | NA | NA | NA | No changes |
| Repeated dose |
n = 40 (M/F) Immunization: days 0, 21, 42 Interim analysis n = 10 (M/F) Euthanized: day 3 Main Study n = 20 (M/F) Euthanized: day 45 Recovery Study n = 10 (M/F) Euthanized: day 64 |
Main Study n = 20 (M/F) Immunization: days 0, 21, 42 Euthanized: day 45 |
Main Study n = 20 (M/F) Immunization: days 0, 21, 42 Euthanized: day 45 |
n = 40 (M/F) Immunization: days 0, 21, 42 Interim analysis n = 10 (M/F) Euthanized: day 3 Main Study n = 20 (M/F) Euthanized: day 45 Recovery Study n = 10 (M/F) Euthanized: day 64 |
n = 40 (M/F) Immunization: days 0, 21, 42 Interim analysis n = 10 (M/F) Euthanized: day 3 Main Study n = 20 (M/F) Euthanized: day 45 Recovery Study n = 10 (M/F) Euthanized: day 64 |
| Mortality | None observed | None observed | None observed | None observed | None observed |
| Clinical sign observation | No significant changes | No significant changes | None | None | None |
| Local tolerance |
Erythema formation After first immunization: 2/40 (day 0–2)After second immunization: 8/30 (day 21–23) Edema formation After second immunization: 5/30 (day 21–24) |
Erythema formation After first immunization: 1/20 (day 0–3) |
Erythema formation After first immunization: 6/20 (day 0–2)After second immunization: 7/20 (day 21–24) Edema formationAfter first immunization: 1/20 (day 0–1) |
Erythema formation After second immunization: 2/30 (day 21–23) Edema formation After second immunization: 1/30 (day 23) |
Erythema formation After first immunization: 2/40 rats (day 2–3)After second immunization: 3/30 rats (day 23) Edema formation After first immunization: 1/40 rat (day 3) |
| Body temperature | No vaccine-related effect | No vaccine-related effect | No vaccine-related effect | No adjuvant-related effect | No changes |
| Body weights | No vaccine-related effect | No vaccine-related effect | No vaccine-related effect | No adjuvant-related effect | No changes |
| Food consumption | No vaccine-related effect | No vaccine-related effect | No vaccine-related effect | No adjuvant-related effect | No changes |
| Water consumption | No vaccine-related effect | No vaccine-related effect | No vaccine-related effect | No adjuvant-related effect | No changes |
M: Male, F: Female; NA: Not Applicable.
Fig. 5.
(A) Single-dose toxicity study design in Jcl:SD rats. Rats were divided into 2 groups (n = 10): the negative control (PBS) and high dose (100 µg) of Baiya SARS-CoV-2 Vax 2 groups. The rats were intramuscularly injected on day 0. On day 15, rats were euthanized and gross examined. The change in body weight of (B) male and (C) female rats was measured throughout the study. (D) Schematic representation of repeated-dose toxicity study in Jcl:SD rats. Rats were divided into 6 groups (n = 20–40), i.e., low, medium, and high doses (25, 50, and 100 µg) of Baiya SARS-CoV-2 Vax 2, active control (adjuvants), and negative control (PBS) groups. The rats were intramuscularly immunized on day 0, 21, and 42 (21 days interval). On day 3, 10 rats of high dose, active and negative controls groups were euthanized and gross examined as an interim study. After the third immunization on day 45, 20 rats of all groups were euthanized as a main study. Then, 22 days after the 3rd-immunization (day 64), the rest 10 rats of high dose, active and negative controls groups were euthanized as a recovery study. The change of body weight of (E) male and (F) female rats were measured throughout the study. The data are presented as mean ± SEM.
A repeated-dose toxicity study was performed with three different doses of vaccine (25, 50 and 100 μg) with adjuvant (3M-052-AF 5 µg + Aluminum Hydroxide; Al content, 0.5 mg) administered to rats on days 0, 21, and 42 (Fig. 5D). No deaths, clinical signs or significant effects on body temperature, feed and water intake or rectal temperature changes were observed in association with the administration of Baiya SARS-CoV-2 Vax 2. Body weight of rats was slightly decreased 24 h after dosing, but thereafter weight was normal and increased (Fig. 5E and F). Local tolerance tests revealed signs of erythema and edema formation at 24, 48, and 72 h after each administration, but these were not considered to be related to the test article because they also occurred in animals in the control group. Notably, no rats exhibited erythema and edema after the third immunization (Supplementary table S9). Gross examination of the organs showed significant changes in lymph nodes and thymus with corresponding histopathological findings in the low-dose and medium-dose vaccine groups. However, these changes were also noted in some animals in the control groups in all sexes, which recovered almost completely later on day 64. In the recovery studies, the urine parameters of all rats were not significantly different (Supplementary table S10). As shown in supplementary table S11 and S12, hematology and clinical chemistry analysis revealed statistically significant differences in a few parameters tested, and most values had returned to pretest ranges by the end of the recovery period. For some parameters, significant differences between groups were small and inconsistent. At the end of the recovery phase, no vaccine-related weight changes were detected in spleen, liver, kidney, thymus or lungs at the end of recovery phase (Supplementary table S13). Histopathology analysis found minimal hemorrhage in tissues, but without hemosiderin pigments, vasculitis or other responses. Red blood cells were found in the submandibular lymph node and thymus gland around the cortical or paracortical area and cortex, respectively. Focal minimal changes or changes within normal limits were observed in the remaining tissues. Histopathological examination at the injection sites showed signs of inflammation both inside and outside the skeletal muscle in both the active control and treatment groups, and the severity and type of inflammation were similar in the groups In the recovery phase, animals in all groups showed partial or complete recovery of these findings (Supplementary table S14).
4. Discussion
The development of safe, affordable vaccines that are highly immunogenic and provide broad and durable protection against circulating SARS-CoV-2 variants are a vital tool to protect against severe illness and death. There is a need to develop a vaccine expression system that has the potential to produce affordable vaccines, especially for LMIC.
Several studies have evaluated RBD based vaccine designs for COVID-19 vaccine development and tested their efficacy in animal models [27], [28], [29], [30], [31]. In this study, we have utilized plant expression system for RBD-based subunit vaccine development due to its inherent advantages including low-cost, flexibility and high scalability. Extensive studies provided a solid foundation for using plants to produce a safe and viable vaccine [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]. In addition, the recent approval by Health Canada of the plant-based Covifenz vaccine with virus-like particles (VLPs) COVID −19 developed by Medicago Inc (Québec), following the positive outcome of phase III clinical trials, has provided great promise for the future acceptance and deployment of plant-based vaccines globally. Here, we systematically assessed the immunogenicity, efficacy, safety and toxicity of the plant-derived Baiya SARS-CoV-2 Vax 2 vaccine using two adjuvants including 3M-052 and Alum in three different animal models (mice, non-human primate and rats).
Our results showed that two different doses (5 μg and 10 μg) of Baiya SARS-CoV-2 Vax 2 rapidly generated anti-RBD antibodies in mice after immunization, whereas the neutralization antibody response was significantly increased only in the 10 μg dose group. In line with previous reports, neutralizing antibodies were observed in our study after administration of two doses of the vaccine [42], [43]. Furthermore, we demonstrate that our vaccine elicits sufficient antibody titers to protect against the lethal SARS-CoV-2 challenge.
In the K18-hACE2 mouse model, vaccination with Baiya SARS-CoV-2 Vax 2 induced protective immunity and reduced viral load and pathogenesis. In addition, the vaccinated mice exhibited no morbidity, clinical signs, or weight loss and had lower viral load and fewer histopathological findings suggestive of SARS–CoV-2 infection, suggesting that the vaccine induces immunity in the mice. Histopathological changes associated with SARS-CoV-2 infection, including findings in the lungs and brain, were reduced or absent in animals vaccinated with Baiya SARS-CoV-2 Vax 2. Therefore, Baiya SARS-CoV-2 Vax 2 is effective in preventing the signs and symptoms of COVID-19, including mortality in a mouse model of COVID-19. Compared with Baiya SARS-CoV-2 Vax 1 (RBD antigen with alum as adjuvant), Baiya SARS-CoV-2 Vax 2 elicited a significantly higher anti-RBD neutralization titer in both mice and monkeys [7], demonstrating that the combination 3M-052- AF -alum elicits the immune response in animals better than alum alone. In addition to the immunogenic RBD protein, the adjuvant 3M-052 also contributes to the generation of the humoral and cell-mediated immune response. Adjuvants based on TLR have been shown to induce high levels of neutralizing antibodies against different viruses [44], [45], [46]. A recent study reported that adjuvant combination alum-3M-052 enhanced neutralizing antibody responses and protection in mice compared to alum alone [18]. Consistent with our study, a vaccine based on a tandem-repeat dimeric RBD protein with alum adjuvant, ZF2001, was previously reported to induce neutralizing antibodies in both mice and nonhuman primates following the two doses of intramuscular injection of the vaccine, 21 days apart. The vaccine protects hACE2 mice and macaques after lethal SARS-CoV-2 challenge [35].
In the monkey study, the safety pharmacology endpoints demonstrated no abnormal signs in all three vital organ (CVS, CNS and RS) systems. The results of the live virus and pseudovirus assays correlated, and the vaccine demonstrated significant neutralizing immunity against SARS-CoV-2 variants, including the recently identified Omicron subvariants. Although the protective efficacy of our vaccine has not been studied in nonhuman primates, the significant induction of neutralizing antibodies in the mouse model suggests that vaccination with Baiya SARS-CoV-2 Vax 2 may confer protection. Collectively, the mouse and monkey results demonstrated that Baiya SARS-CoV-2 Vax 2 elicits an enhanced immune response. This supports previous findings that RBD based vaccines are highly immunogenic, induce robust antibody responses, and are protective against SARS-CoV-2 challenge in animal models [47], [48]. Of note, a toxicity study with a single dose of 100 μg Baiya SARS-CoV-2 Vax 2 was well tolerated by Jcl:SD rats and demonstrated no mortality or unexpected findings. Similarly, results of the repeat dose toxicity study have shown no mortality, clinical observations, changes in body weight or body temperature, hematology, blood chemistry or urinalysis associated with the administration of Baiya SARS-CoV-2 Vax 2. Although it is informative to compare the immune response induced by the plant-derived RBD vaccine with other RBD vaccine candidates, caution is warranted because different groups used different neutralization assays, small number of animals/group and variability between individual animals, assay variability can yield different results, making comparison difficult [18]. The pre-clinical data presented here are in line with previous studies on the efficacy, safety and toxicity of Baiya SARS-CoV-2 Vax 1 in animal models reported by our group [7]. The magnitude of the immune response and protective efficacy of this vaccine remain to be tested in humans.
In summary, we have developed a plant-based SARS-CoV-2 subunit vaccine adjuvanted with 3M-052 alum. This low-cost production platform could be easily adapted for local production in LMIC to meet the global demand for SARS-CoV-2 vaccine. Our study demonstrated that Baiya SARS-CoV-2 Vax 2 was well tolerated, safe, non-toxic and immunogenic in mice, non-human primates and rats. These preclinical findings provide important justification for further development of Baiya SARS-CoV-2 Vax 2 for clinical use (ClinicalTrials.gov Identifier: NCT05197712).
Author Contributions
ST and WP conceived the project. BS and NK performed the plant experiments and prepared the vaccine formulations. PS and SP performed the mice immunization and challenge experiments. PT and WPr performed the toxicity experiments. SM performed the safety pharmacology experiments in cynomolgus monkeys. SM and AT performed the virus neutralization assay. KS and AJ performed the pseudovirus neutralization assay. MT and CBF led preparation of the adjuvant formulation and advised on its use. BS drafted and revised the manuscript. All the authors analyzed the data and approved the submitted version.
Funding
This research was funded by the National Vaccine Institute, Thailand and Baiya Phytopharm Co., ltd. Thailand.
Disclaimer
The materials were reviewed by the Walter Reed Army Institute of Research. There is no objection to the presentation and/or publication of these materials. The opinions or assertions contained herein are solely those of the authors and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense. This research was conducted under an approved animal use protocol in an AAALACi accredited facility in compliance with the Animal Welfare Act and other federal statutes and regulations related to animals and experiments involving animal, and this research adhered to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ST and WP from Chulalongkorn University are founders/shareholders of Baiya Phytopharm Co., Ltd. Thailand. CBF from the Access to Advanced Health Institute is an inventor on patents and applications claiming rights in formulations of TLR 7/8 agonists such as 3M-052 and methods for using said formulations (e.g. WO/2017/200852A1).
Acknowledgments
We appreciate the technical assistance provided by the technicians and staff at the experimental animal facility during the study. The author (NK) would like to thank The Second Century Fund (C2F), Chulalongkorn University for the doctoral fellowship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.03.027.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
All the data supporting the findings of this study are available within the paper and are also available from the corresponding author upon request.
References
- 1.Malla A., Shanmugaraj B., Ramalingam S. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Emerging Zoonotic Respiratory Pathogen in Humans. J Pure Appl Microbiol. 2020;14:931–936. [Google Scholar]
- 2.Lee J.K., Shin O.S. Coronavirus disease 2019 (COVID-19) vaccine platforms: how novel platforms can prepare us for future pandemics: a narrative review. J Yeungnam Med Sci. 2022;39:89–97. doi: 10.12701/jyms.2021.01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobato Gómez M, Huang X, Alvarez D, He W, Baysal C, Zhu C, et al. Contributions of the international plant science community to the fight against human infectious diseases – part 1: epidemic and pandemic diseases. Plant Biotechnol J. 2021;19:1901–1920. doi: 10.1111/pbi.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shanmugaraj B., Bulaon CJ I., Phoolcharoen W. Plant Molecular Farming: A Viable Platform for Recombinant Biopharmaceutical. Production. Plants. 2020;9:842. doi: 10.3390/plants9070842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward B.J., Gobeil P., Séguin A., Atkins J., Boulay I., Charbonneau P.-Y., et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat Med. 2021;27:1071–1078. doi: 10.1038/s41591-021-01370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siriwattananon K, Manopwisedjaroen S, Shanmugaraj B, Rattanapisit K, Phumiamorn S, Sapsutthipas S, et al. Plant-Produced Receptor-Binding Domain of SARS-CoV-2 Elicits Potent Neutralizing Responses in Mice and Non-human Primates. Front Plant Sci. 2021:12.682953. doi: 10.3389/fpls.2021.682953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanmugaraj B., Khorattanakulchai N., Panapitakkul C., Malla A., Im-erbsin R., Inthawong M., et al. Preclinical evaluation of a plant-derived SARS-CoV-2 subunit vaccine: Protective efficacy, immunogenicity, safety, and toxicity. Vaccine. 2022;40:4440–4452. doi: 10.1016/j.vaccine.2022.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shanmugaraj B., Khorattanakulchai N., Paungpin W., Akkhawattanangkul Y., Manopwisedjaroen S., Thitithanyanont A., et al. Immunogenicity and Efficacy of Recombinant Subunit SARS-CoV-2 Vaccine Candidate in the Syrian Hamster Model. Biotechnol Rep. 2022:e00779. doi: 10.1016/j.btre.2022.e00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed S.G., Orr M.T., Fox C.B. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 10.Petrovsky N, Aguilar JC. Vaccine adjuvants: Current state and future trends. 2004; 82: 488-96. [DOI] [PubMed]
- 11.Sridhar S., Joaquin A., Bonaparte M.I., Bueso A., Chabanon A.L., Chen A., et al. Safety and immunogenicity of an AS03-adjuvanted SARS-CoV-2 recombinant protein vaccine (CoV2 preS dTM) in healthy adults: interim findings from a phase 2, randomised, dose-finding, multicentre study. Lancet Infect Dis. 2022;22:636–648. doi: 10.1016/S1473-3099(21)00764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh S.M., Liu M.C., Chen Y.H., Lee W.S., Hwang S.J., Cheng S.H., et al. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir Med. 2021;9:1396–1406. doi: 10.1016/S2213-2600(21)00402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luchner M., Reinke S., Milicic A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics. 2021:13. doi: 10.3390/pharmaceutics13020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smirnov D., Schmidt J.J., Capecchi J.T., Wightman P.D. Vaccine adjuvant activity of 3M–052: An imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine. 2011;29:5434–5442. doi: 10.1016/j.vaccine.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q., Barry M.A., Seid C.A., Hudspeth E.M., McAtee C.P., Heffernan M.J. 3M–052 as an adjuvant for a PLGA microparticle-based Leishmania donovani recombinant protein vaccine. J Biomed Mater Res B Appl Biomater. 2018;106:1587–1594. doi: 10.1002/jbm.b.33965. [DOI] [PubMed] [Google Scholar]
- 16.Kasturi S.P., Rasheed M.A.U., Havenar-Daughton C., Pham M., Legere T., Sher Z.J., et al. 3M–052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope-specific plasma cells and humoral immunity in nonhuman primates. Sci Immunol. 2020:5. doi: 10.1126/sciimmunol.abb1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox C.B., Orr M.T., Van Hoeven N., Parker S.C., Mikasa T.J., Phan T., et al. Adsorption of a synthetic TLR7/8 ligand to aluminum oxyhydroxide for enhanced vaccine adjuvant activity: A formulation approach. J Control Release. 2016;244:98–107. doi: 10.1016/j.jconrel.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Routhu N.K., Cheedarla N., Bollimpelli V.S., Gangadhara S., Edara V.V., Lai L., et al. SARS-CoV-2 RBD trimer protein adjuvanted with Alum-3M-052 protects from SARS-CoV-2 infection and immune pathology in the lung. Nat Commun. 2021;12:3587. doi: 10.1038/s41467-021-23942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pino M., Abid T., Pereira Ribeiro S., Edara V.V., Floyd K., Smith J.C., et al. A yeast expressed RBD-based SARS-CoV-2 vaccine formulated with 3M–052-alum adjuvant promotes protective efficacy in non-human primates. Sci Immunol. 2021:6. doi: 10.1126/sciimmunol.abh3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Ge X, Liu Y, Li H, Zhang Z. The Role of Toll-like Receptor Agonists and Their Nanomedicines for Tumor Immunotherapy. Pharmaceutics. 2022;14:1228. doi: 10.3390/pharmaceutics14061228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H. TLR Agonists for Cancer Immunotherapy: Tipping the Balance between the Immune Stimulatory and Inhibitory Effects. Front Immunol. 2014;5:83. doi: 10.3389/fimmu.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Council N.R. Eighth Edition. The National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 23.Frey A., Canzio J.D., Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods. 1998;221:35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 24.Shanmugaraj B, Rattanapisit K, Manopwisedjaroen S, Thitithanyanont A, Phoolcharoen W. Monoclonal Antibodies B38 and H4 Produced in Nicotiana benthamiana Neutralize SARS-CoV-2 in vitro. Front Plant Sci. 2020:11. doi: 10.3389/fpls.2020.589995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khorattanakulchai N, Manopwisedjaroen S, Rattanapisit K, Panapitakkul C, Kemthong T, Suttisan N, et al. Receptor binding domain proteins of SARS-CoV-2 variants produced in Nicotiana benthamiana elicit neutralizing antibodies against variants of concern. J Med Virol. 2022;94:4265–4276. doi: 10.1002/jmv.27881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang K, Whalen BJ, Tirabassi RS, Selin LK, Levchenko TS, Torchilin VP, et al. A DNA Vaccine Prime Followed by a Liposome-Encapsulated Protein Boost Confers Enhanced Mucosal Immune Responses and Protection. J Immunol. 2008;180:6159–6167. doi: 10.4049/jimmunol.180.9.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law J.L.M., Logan M., Joyce M.A., Landi A., Hockman D., Crawford K., et al. SARS-COV-2 recombinant Receptor-Binding-Domain (RBD) induces neutralizing antibodies against variant strains of SARS-CoV-2 and SARS-CoV-1. Vaccine. 2021;39:5769–5779. doi: 10.1016/j.vaccine.2021.08.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J., Wang W., Chen Z., Lu S., Yang F., Bi Z., et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020;586:572–577. doi: 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]
- 29.Boulton S., Poutou J., Martin N.T., Azad T., Singaravelu R., Crupi M.J.F., et al. Single-dose replicating poxvirus vector-based RBD vaccine drives robust humoral and T cell immune response against SARS-CoV-2 infection. Mol Ther: J Am Soc Gene Ther. 2022;30:1885–1896. doi: 10.1016/j.ymthe.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan H.X., Juno J.A., Lee W.S., Barber-Axthelm I., Kelly H.G., Wragg K.M., et al. Immunogenicity of prime-boost protein subunit vaccine strategies against SARS-CoV-2 in mice and macaques. Nat Commun. 2021;12:1403. doi: 10.1038/s41467-021-21665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W.H., Wei J., Kundu R.T., Adhikari R., Liu Z., Lee J., et al. Genetic modification to design a stable yeast-expressed recombinant SARS-CoV-2 receptor binding domain as a COVID-19 vaccine candidate. Biochim Biophys Acta. 2021;1865 doi: 10.1016/j.bbagen.2021.129893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanmugaraj B, Siriwattananon K, Malla A, Phoolcharoen W. Potential for Developing Plant-Derived Candidate Vaccines and Biologics against Emerging Coronavirus Infections. Pathogens. 2021;10:1051. doi: 10.3390/pathogens10081051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tottey S., Shoji Y., Jones R.M., Chichester J.A., Green B.J., Musiychuk K., et al. Plant-Produced Subunit Vaccine Candidates against Yellow Fever Induce Virus Neutralizing Antibodies and Confer Protection against Viral Challenge in Animal Models. Am J Trop Med Hyg. 2018;98:420–431. doi: 10.4269/ajtmh.16-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rattanapisit K., Shanmugaraj B., Manopwisedjaroen S., Purwono P.B., Siriwattananon K., Khorattanakulchai N., et al. Rapid production of SARS-CoV-2 receptor binding domain (RBD) and spike specific monoclonal antibody CR3022 in Nicotiana benthamiana. Sci Rep. 2020;10:17698. doi: 10.1038/s41598-020-74904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hager K.J., Pérez Marc G., Gobeil P., Diaz R.S., Heizer G., Llapur C., et al. Efficacy and Safety of a Recombinant Plant-Based Adjuvanted Covid-19 Vaccine. N Engl J Med. 2022;386:2084–2096. doi: 10.1056/NEJMoa2201300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pillet S., Couillard J., Trépanier S., Poulin J.F., Yassine-Diab B., Guy B., et al. Immunogenicity and safety of a quadrivalent plant-derived virus like particle influenza vaccine candidate-Two randomized Phase II clinical trials in 18 to 49 and ≥50 years old adults. PLoS One. 2019;14:e0216533. doi: 10.1371/journal.pone.0216533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurokawa N., Robinson M.K., Bernard C., Kawaguchi Y., Koujin Y., Koen A., et al. Safety and immunogenicity of a plant-derived rotavirus-like particle vaccine in adults, toddlers and infants. Vaccine. 2021;39:5513–5523. doi: 10.1016/j.vaccine.2021.08.052. [DOI] [PubMed] [Google Scholar]
- 38.Landry N., Ward B.J., Trépanier S., Montomoli E., Dargis M., Lapini G., et al. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS One. 2010;5:e15559. doi: 10.1371/journal.pone.0015559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanmugaraj B., Khorattanakulchai N., Phoolcharoen W. In: Biomedical Innovations to Combat COVID-19. Rosales-Mendoza S., Comas-Garcia M., Gonzalez-Ortega O., editors. Academic Press; 2022. Chapter 12 - SARS-CoV-2 vaccines: current trends and prospects of developing plant-derived vaccines; pp. 213–229. [Google Scholar]
- 40.Chan H.-T., Xiao Y., Weldon W.C., Oberste S.M., Chumakov K., Daniell H. Cold chain and virus-free chloroplast-made booster vaccine to confer immunity against different poliovirus serotypes. Plant Biotechnol J. 2016;14:2190–2200. doi: 10.1111/pbi.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B., Shanmugaraj B., Daniell H. Expression and functional evaluation of biopharmaceuticals made in plant chloroplasts. Curr Opin Chem Biol. 2017;38:17–23. doi: 10.1016/j.cbpa.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science (New York, NY) 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abhyankar M.M., Mann B.J., Sturek J.M., Brovero S., Moreau G.B., Sengar A., et al. Development of COVID-19 vaccine using a dual Toll-like receptor ligand liposome adjuvant. npj Vaccines. 2021;6:137. doi: 10.1038/s41541-021-00399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saunders K.O., Lee E., Parks R., Martinez D.R., Li D., Chen H., et al. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature. 2021;594:553–559. doi: 10.1038/s41586-021-03594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petitdemange C., Kasturi S.P., Kozlowski P.A., Nabi R., Quarnstrom C.F., Reddy P.B.J., et al. Vaccine induction of antibodies and tissue-resident CD8+ T cells enhances protection against mucosal SHIV-infection in young macaques. JCI Insight. 2019;4 doi: 10.1172/jci.insight.126047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z., Xu W., Xia S., Gu C., Wang X., Wang Q., et al. RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response. Signal Transduct Target Ther. 2020;5:282. doi: 10.1038/s41392-020-00402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan X., Shi J., Hu X., Wu Y., Zeng L., Yao Y., et al. RBD-homodimer, a COVID-19 subunit vaccine candidate, elicits immunogenicity and protection in rodents and nonhuman primates. Cell Discovery. 2021;7:82. doi: 10.1038/s41421-021-00320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the findings of this study are available within the paper and are also available from the corresponding author upon request.