Abstract
Human toxoplasmosis is a global public health concern and a commercial vaccine is still lacking. The present in silico study was done to design a novel vaccine candidate using tachyzoite-specific SAG1-realted sequence (SRS) proteins. Overlapping B-cell and strictly-chosen human MHC-I binding epitopes were predicted and connected together using appropriate spacers. Moreover, a TLR4 agonist, human high mobility group box protein 1 (HMGB1), and His-tag were added to the N- and C-terminus of the vaccine sequence. The final vaccine had 442 residues and a molecular weight of 47.71 kDa. Physico-chemical evaluation showed a soluble, highly antigenic and non-allergen protein, with coils and helices as secondary structures. The vaccine 3D model was predicted by ITASSER server, subsequently refined and was shown to possess significant interactions with human TLR4. As well, potent stimulation of cellular and humoral immunity was demonstrated upon chimeric vaccine injection. Finally, the outputs showed that this vaccine model possesses top antigenicity, which could provoke significant cell-mediated immune profile including IFN-γ, and can be utilized towards prophylactic purposes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40203-023-00140-w.
Keywords: Toxoplasma gondii, Vaccinology, Multi-epitope vaccine, Tachyzoite, SRS proteins
Introduction
Toxoplasmosis, due to Toxoplasma gondii (T. gondii), is a widespread parasitic zoonosis, affecting warm-blooded animals such as humans (Dubey 2021). The life cycle involves oocyst-shedding felids as definitive hosts, causing environmental and food contamination (Almeria and Dubey 2021; Asghari et al. 2021a). Toxoplasma can be disseminated via congenital and transfusion-transmitted infections (by tachyzoites) and/or through transplants and uncooked cyst-contaminated meat (by bradyzoites) (Deng et al. 2021). Approximately, one-third of the global human population have anti-T. gondii antibodies due to previous parasite exposure (Ahmadpour et al. 2022). The most significant consequences of the infection are serious life-threatening disorders for the unborn, comprising mental retardation, blindness, hydrocephaly and fetal abortion as well as fulminant disease mostly characterized by encephalitis and pneumonitis in the immunocompromised patients (Austhof et al. 2021). On this basis, Toxoplasma infection entails substantial health and socioeconomic impact to the society (Hill et al. 2002).
Despite advances in studies evaluating chemical and herbal compounds against T. gondii infection, no broad-spectrum drug has been discovered to target both acute and chronic forms of the disease. At present, therapies are only active against the acute form of the infection (tachyzoites), with side-effects reported in treated patients (Dunay et al. 2018). Alternatively, vaccination seem to be an effective strategy for long-term disease control without emphasis on chemotherapy (Chu and Quan 2021). Similar to other intracellular pathogens, T. gondii elicits strong and protective CD4+ and CD8+ T-cell responses, dominated by interferon gamma (IFN-γ) upsurge (Dupont et al. 2012). The parasite surface is coated with multiple glycosylphosphatidylinositol-linked proteins with structural and biological similarity to the immunogenic surface antigen SAG1. It is believed that SAG1-related sequence (SRS) proteins mediate host cell attachment and play a major role in immune evasion. The accessibility of these proteins on the parasite surface makes them a favorable candidate for vaccine design. The SRS proteins are expressed in all developmental stages of T. gondii life cycle. For example, SAG1 (SRS29B), SRS1 (SRS29A), SAG2A, SRS2, SRS3 (SRS51), SAG3 (SRS57), SRS20A, SRS25, SRS52A and SRS67 are abundantly expressed in tachyzoite stage (Theisen and Boothroyd 2021).
Developing a vaccine candidate implicates a mutual interconnection among physical chemistry, cell biology and immunology scientific branches (Han and research 2015; Asghari et al. 2021b). Conventional vaccine design demands costly, time-consuming and toilsome wet lab experiments both in the pre-clinical and clinical stages, in order to consider disease conditions, vaccine formulation and human safety standards (Brisse et al. 2020). In recent decades, advances in biology and computer sciences, known as bioinformatics, have improved our knowledge on the rational vaccine design using immunogenic fragments of a given protein called epitopes (Parvizpour et al. 2020). This is facilitated through B- and T-cell-specific epitope prediction, forecasting residues with higher affinity to specific molecules (Major histocompatibility complex; MHC) or with higher chance of induction of cytokines (e.g., Interferon-γ, Interleukin-4), engineering vaccine candidates and in silico validation of the vaccine safety and efficacy, using comprehensive web-based tools, in a costly and time-saving manner (Soria-Guerra et al. 2015). “The amount and specificity of antigen necessary to activate T cells can be better controlled with an epitope-based vaccine and can give superior possibilities for producing either humoral or cellular (memory Helper T cells or memory Cytotoxic T cells)” (Aasim et al. 2022). The aim of the present in silico study was to model and construct a vaccine candidate against Toxoplasma infection using immunodominant epitopes inferred from a number of SRS proteins (SRS29B, SRS20A, SRS51, SRS52A and SRS57).
Methods
Protein sequence retrieval
The amino acid sequences of the examined tachyzoite-specific proteins were retrieved in FASTA format via the UniProt Knowledgebase, available at https://www.uniprot.org/, under accession numbers of S8FBM7 (SRS20A), A0A125YP09 (SRS29B), S7W107 (SRS51), A0A125YJA5 (SRS52A) and A0A125YY85 (SRS57) (research 2019) (UniProt Consortium 2019).
Prediction and screening of continuous B-cell epitopes
The immunodominant linear B-cell epitopes of the selected SRS proteins were predicted using various servers in a multi-method approach. For this aim, BepiPred v2.0 (https://services.healthtech.dtu.dk/service.php?BepiPred-2.0) (Jespersen et al. 2017), BCEPS (http://imbio.med.ucm.es/bceps/) (Ras-Carmona et al. 2021), ABCpred (http://crdd.osdd.net/raghava/abcpred/) (Saha and Raghava 2007) and SVMTriP (http://sysbio.unl.edu/SVMTriP/) (Yao et al. 2020) web servers were utilized and shared linear B-cell epitopes were determined. Subsequently, these epitopes were screened in terms of antigenicity, allergenicity and solubility using VaxiJen v2.0 (http://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html), AllergenFP v1.0 (https://ddg-pharmfac.net/AllergenFP/) and PepCalc (https://pepcalc.com/) servers, respectively. Of each protein, only one highly antigenic, soluble and non-allergenic epitope was chosen to be included in the final vaccine construct.
Prediction of human major histocompatibility complex (MHC)-binding epitopes
In order to predict human CD8+ T-cell epitopes from the tachyzoite-specific SRS proteins, the MHC-I binding prediction tool of the Immune Epitope Database (IEDB) server was used (http://tools.iedb.org/mhci/). The prediction method was IEDB recommended method 2020.09 (NetMHCpan EL 4.1) with selecting HLA allele reference set option. Finally, top ten 9-mer epitopes having lower percentile ranks were selected for further screening regarding antigenicity and allergenicity using VaxiJen v2.0 and AllerTOP v2.0 (https://www.ddg-pharmfac.net/AllerTOP/), respectively. Ultimately, two antigenic, non-allergenic epitopes having higher affinity to human MHC-I alleles were selected from each protein for vaccine construction.
Engineering and assemblage of the multimeric vaccine construct
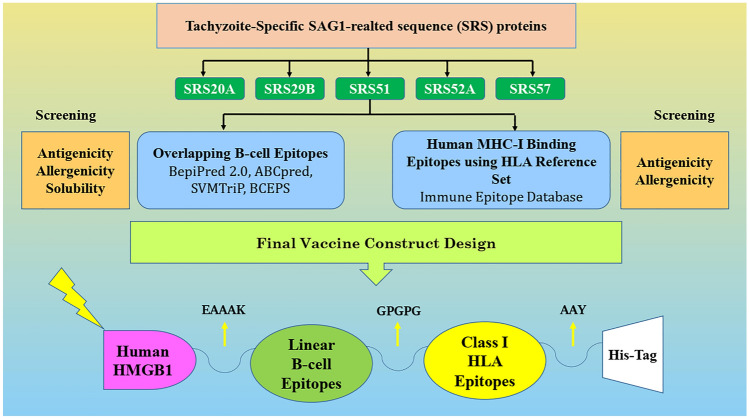
Based on the outputs of the immunoinformatics epitope analysis, the selected immunodominant regions of Toxoplasma SRS proteins were connected together using specific linkers, so that GPGPG was used for B-cell epitopes, while AAY was employed to connect T-cell epitopes. In this study, four vaccine adjuvants were evaluated, including Mycobacterial heparin-binding hemagglutinin (HBHA), human high mobility group box protein 1 (HMGB1), Human Interferon gamma (IFN-γ) and Brucella abortus Omp16. The adjuvant yielding higher vaccine antigenicity and solubility was human HMGB1, which was embedded in the N-terminal of the vaccine using EAAAK spacer. Moreover, a 6 × His tag was added to the C-terminal of the designed vaccine sequence for purification purposes.
Physico-chemical features of the vaccine construct
The basic physico-chemical properties of the designed vaccine construct was evaluated using ExPASy ProtParam web tool, available at https://web.expasy.org/protparam/, which predicts the molecular weight (MW), speculated isoelectric pH, extinction coefficient, estimated half-life, instability index, aliphatic index and the grand average of hydropathicity (GRAVY) score (Gasteiger et al. 2005).
Prediction of antigenicity, allergenicity and solubility profiles
The antigenicity of the vaccine model was assessed using VaxiJen v2.0 (Doytchinova and Flower 2007) and ANTIGENpro web tool (http://scratch.proteomics.ics.uci.edu/) (Cheng et al. 2005). Furthermore, the allergenicity was evaluated using two web servers, AllergenFP v1.0 and AllerTOP v2.0 (Dimitrov et al. 2014a, 2014b). The vaccine solubility profile was, also, predicted using Protein-Sol web server, available at https://protein-sol.manchester.ac.uk/ (Hebditch et al. 2017).
Prediction of post-translational modification (PTM) sites
Possible PTM sites such as N- and O-glycosylation as well as phosphorylation within the vaccine sequence were determined using NetNGlyc 1.0, NetOGlyc 4.0 and NetPhos 3.1 web tools of the DTU Health Tech. server, respectively (https://services.healthtech.dtu.dk/).
Prediction of the secondary and tertiary structures of the vaccine model
The secondary structure of the vaccine construct was predicted using NetSurfP-2.0 web tool, available at https://services.healthtech.dtu.dk/service.php?NetSurfP-2.0. This server predicts the surface accessibility, secondary structure, disorder, and phi/psi dihedral angles of amino acids in an amino acid sequence. A single model, using a combination of Convolutional and Bi-Directional Long-Short Term Memory Neural Networks, predicts all structural features together (Klausen et al. 2019). Also, the tertiary structure of the vaccine model was predicted using Iterative Threading ASSEmbly Refinement (ITASSER) server, which employs LOMETS multiple-threaded structural templates. The predicted model confidence is measured by C-score, being estimated on the basis of the significance of threading template alignments and the convergence parameters of the structure assembly simulations (Yang and Zhang 2015).
Refinement and validation of the three-dimensional (3D) model
GalaxyRefine web server was used to rehash and perform structural relaxations by packing and re-establishing side-chains. The server provides five refined models, among which one model should be selected for further analysis (https://galaxy.seoklab.org/cgi-bin/submit.cgi?type=REFINE) (Heo et al. 2013). Allowed (psi, ψ) and disallowed (phi, φ) dihedral angles of an amino acid residue could be visualized by creating.
Ramachandran plots, based on van der Waal radius of the side chains. Validation of the refined 3D model was accomplished using Ramachandran plot analysis, through PROCHECK tool of the SAVES 6.0 server (https://saves.mbi.ucla.edu/) (Laskowski et al. 2006).
Prediction of conformational B-cell epitopes
Conformational B-cell epitopes, critical regarding antigen–antibody interaction, were predicted by ElliPro tool of the IEDB web server, available at http://tools.iedb.org/ellipro/, with a substantial AUC score of 0.732 and default settings of 6 Å max-distance and 0.5-min score (Ponomarenko et al. 2008).
Vaccine protein disulfide engineering
The expectation of the cysteine bond formation in the designed model was determined by disulfide engineering tool of the Disulfide by Design 2 (DbD2) online server, available at http://cptweb.cpt.wayne.edu/DbD2/index.php. Disulfide bonds strengthen the total conformation and stability of the protein of interest. Those residues located at the highly-mobile region of the sequence will undergo cysteine mutation, and only those residues having < 2.5 kcal/mol energy and chi3 value between – 87 to + 97 can be qualified for disulfide engineering (Craig and Dombkowski 2013).
Interaction between the vaccine model and human TLR4
In order to assess the binding affinity between the multi-epitope vaccine construct and human TLR4 receptor (RCSB ID: 3FXI), the ClusPro 2.0 protein–protein docking server was employed using default settings (https://cluspro.bu.edu/login.php). The most populated docking pose with lowest energy score was chosen for visualization (Kozakov et al. 2017).
Immune simulation profile of the vaccine model
The immune profile of the injected vaccine model (without LPS) was in silico evaluated using C-ImmSim online server, available at https://150.146.2.1/C-IMMSIM/index.php. A position-specific scoring matrix (PSSM) is applied by machine-learning techniques to predict likely immune interactions. The output of this server is provided based on the immunostimulatory activities in anatomical regions, comprising bone marrow, thymus and lymph node. Default parameters such as random seed 12,345, simulation volume 10, simulation steps 100, time step of injection 1 were selected for this prediction (Rapin et al. 2011).
Results
Screening and selection of continuous B-cell epitopes
Linear B-cell epitopes of the selected tachyzoite-specific SRS proteins of T. gondii were predicted using a multi-method approach. The prediction threshold for BepiPred, BCEPS and ABCpred servers was 0.5%, 0.5% and 0.75%, respectively. Shared epitopes among servers’ outputs were selected and screened regarding antigenicity, allergenicity and solubility. Only one epitope was selected from each protein for the vaccine construction. Details of the B-cell epitope prediction are provided in Supplementary Tables 1–5.
Exploring top human MHC-I binding epitopes
Human MHC-I binding epitopes were predicted using the HLA reference set of the IEDB web server and top ten high rank (lower percentile) epitopes were screened regarding antigenicity and allergenicity. Of each protein, only two epitopes were qualified for the final vaccine construction, as shown in Supplementary Table 6.
Mounting the multi-epitope vaccine construct
The multi-epitope vaccine construct was designed using B-cell and human MHC-I binding epitopes connected together via specific linkers. In the following, four internal adjuvants were added to the N-terminal of vaccine sequence, including HBHA, human HMGB1, human IFN-γ and B. abortus Omp16 protein, and the antigenicity, allergenicity and solubility of the total vaccine candidate sequences was appraised along with a C-terminally located His-tag. The highest antigenicity and solubility belonged to the vaccine model with human HMGB1 adjuvant (Fig. 1).
Fig. 1.
Schematic representation of the chimeric vaccine design
Prediction of physico-chemical, solubility, antigenicity and allergenicity properties
The selected vaccine sequence had 442 amino acids in length, a MW of 47,712.18 Dalton and a theoretical pI of 5.40. Negatively-charged residues were higher in the sequence (74) than positively-charged residues (61). The estimated half-life of the protein was 30 h (mammalian reticulocytes), > 20 h (yeast) and > 10 h (E. coli). The protein was borderline stable, with an instability index of 39.89. The aliphatic index of the vaccine was 46.56 (relatively thermotolerant) and the calculated GRAVY score was -0.789, showing hydrophilic nature of the vaccine construct. Moreover, the estimated antigenicity of the selected vaccine was 0.9501 and 0.961929 by VaxiJen and ANTIGENpro servers, respectively. Also, it was non-allergenic in nature, as demonstrated by AllergenFP and AllerTOP servers. Finally, the calculated solubility of the vaccine model was 0.622, showing high solubility.
Prediction of PTM sites in the vaccine model
Based on the employed web servers, there were 6 O-Glycosylation, 2 N-Glycosylation and 34 phosphorylation sites (17 serine, 7 threonine and 10 tyrosine) in the final selected vaccine sequence.
Secondary structure prediction of the vaccine construct
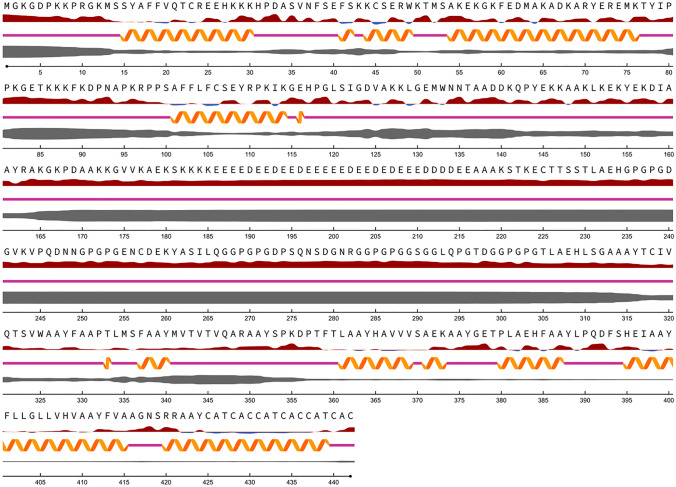
The output of the NetSurfP online tool demonstrated that residues from 165 to 317 are probably highly disordered and most of the vaccine construct is prevailed by random coils and helices, respectively. Also, exposed residues were plenty in comparison with those buried in the sequence (Fig. 2).
Fig. 2.
Secondary structure analysis of the multi-epitope vaccine, based on NetSurfP-2.0 web server; random coils and helices are prevalent in the vaccine sequence
3D homology modelling, refining and validation
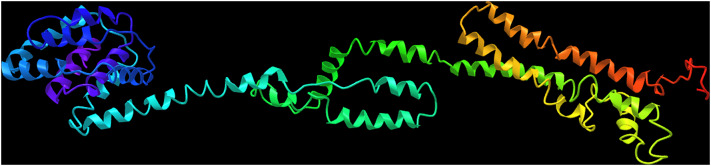
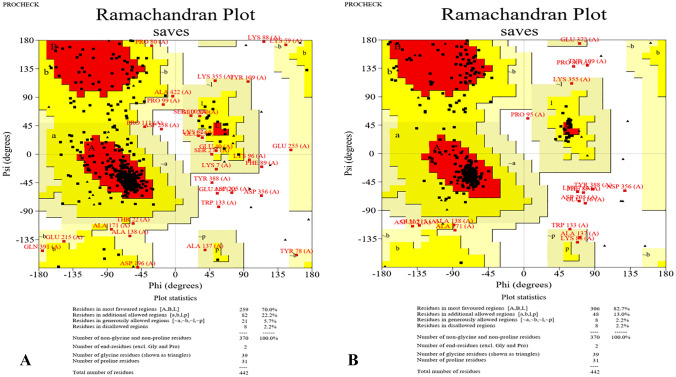
According to ITASSER server output, model number 2 possessed the highest C-score (-0.91); so, it was chosen for further rehashing and validation analyses (Fig. 3). This model was submitted to GalaxyRefine server for structural relaxations and among the five refined models, model number 3 was selected with qualifying parameters of GDT-HA: 0.9146, RMSD: 0.501, MolProbity: 2.350, Clash score: 17.8, Poor rotamers: 0.3 and Rama favored: 88.0. Ramachandran plot analysis, also, showed improvement in the structural quality of the refined model, in comparison with the crude model. In this regard, in the crude model, 259 (70%), 82 (22.2%), 21 (5.7%) and 8 (2.2%) of the residues were allocated to the most favored, additional allowed, generously allowed and disallowed regions, respectively. In the refined model, the residues were changed as 306 (82.7%), 48 (13%), 8 (2.2%) and 8 (2.2%), correspondingly (Fig. 4).
Fig. 3.
3D structure of the designed vaccine model, predicted by I-TASSER server
Fig. 4.
Validation of the refined model in comparison with crude model, using Ramachandran plot analysis by PROCHECK online tool. In the refined model, 306 (82.7%), 48 (13%), 8 (2.2%) and 8 (2.2%) residues were assigned to the most favored, additional allowed, generously allowed and disallowed regions, respectively
Prediction of conformational B-cell epitopes
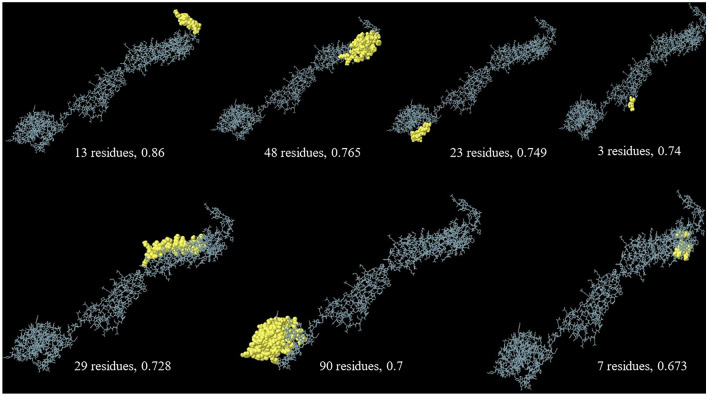
The output of the ElliPro online tool demonstrated that there are 7 conformational B-cell epitopes within the refined vaccine sequence, playing important role in antigen–antibody interaction. The length and score of each epitope is as follows: (a) 13 residues, 0.86; (b) 48 residues, 0.765; (c) 23 residues, 0.749; (d) 3 residues, 0.74; (e) 29 residues, 0.728; (f) 90 residues, 0.7; and (g) 7 residues, 0.673 (Fig. 5).
Fig. 5.
Predicted conformational B-cell epitopes of the chimeric vaccine by ElliPro tool of IEDB analysis Resource. The length of the epitopes and their scores are provided
Vaccine disulfide engineering
Disulfide engineering was performed using DbD2 web server and upon mutating residues to cysteine, only one pair of residues were qualified for disulfide bond formation (PRO92 – ALA138), with energy of 0.27 kcal/mol and chi3 value of + 96.87.
Protein–protein interaction between the vaccine model and human TLR4
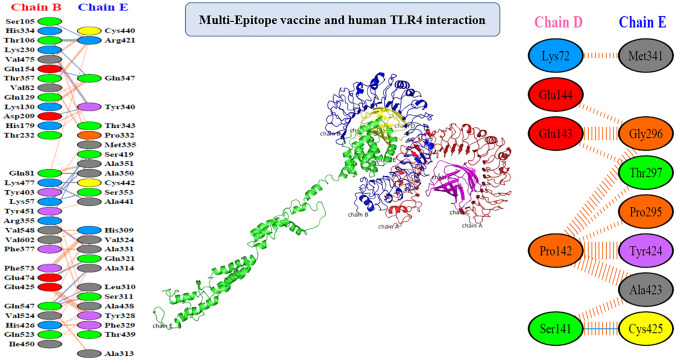
The ClusPro 2.0 server was employed for protein–protein docking and human TLR4 (PDB code: 3FXI) was used as the partner receptor for the designed vaccine construct. As results showed, the designed vaccine protein was associated with chains B (blue) and D (yellow) of the human TLR4. The details of the intermolecular interaction at the amino acid level are illustrated in Fig. 6.
Fig. 6.
The binding conformation of the chimeric vaccine and human TLR-4. The designed peptide in green possessed interactions with chains B (blue) and D (yellow) of the human TLR4. At the amino acid level, Blue line denotes hydrogen bond, red denotes salt bridge and orange striped lines denote non-bonded contacts. In cases which the non-bonded contacts are plentiful, the width of the striped line increases depending on the number of the non-bonded contacts
Simulation of the vaccine-specific immune responses
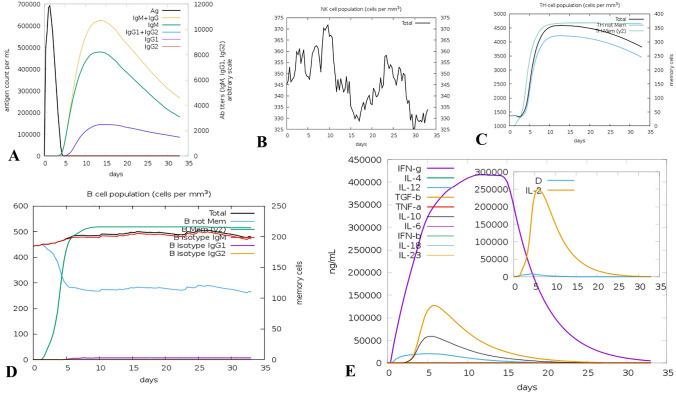
A noticeable increase in the generation of specific immune responses against T. gondii infection was observed in the outputs of the C-ImmSim web server. Specific antibody responses (IgM + IgG) reached beyond 600,000 upon vaccine introduction into the body and about day 5 post injection onwards the memory B-cell population reached a plateau of about 500 cells per mm3. There observed two upsurge peaks in the NK cell population, near days 8 and 23 post injection, and the IFN-γ level increased until reached a peak (over 400,000 ng/ml) about 5–10 days post injection. OF note, T-helper cell population reached a plateau almost about day 10 post injection (Fig. 7).
Fig. 7.
In silico immune simulation with the subunit vaccine. A Immunoglobulin production in response to antigen; B Natural killer (NK) cell population (cells per mm3); C Helper T-cell population (cells per mm3); D B-cell population (cells per mm3); and E level of cytokine production (ng/ml)
Discussion
A wide range of antigenic proteins has been discovered for T. gondii so far, and plenty of antigenic formulations along with different adjuvants have been tested as potential vaccine candidates (Rezaei et al. 2019). Since some of the Toxoplasma SRS proteins are present at the tachyzoite surface and are good immunogens (e.g., SAG1), they seem to be a good vaccine candidate (Wasmuth et al. 2012). The present study was aimed at designing and engineering a novel peptide-based vaccine based on the predicted B- and MHC-I binding epitopes derived from SRS proteins.
Since peptide-based vaccines are weak regarding immunogenicity, they must accompany with an adjuvant to enhance the quality of protein delivery and immunogenicity as well as to protect early vaccine degradation (Reed et al. 2013). In this sense, four internal adjuvants were evaluated in the final vaccine construct, among which the vaccine candidate with N-terminal human HMGB1 possessed highest antigenicity and solubility among others. This adjuvant is actually a conserved nuclear protein and danger-associated molecular pattern (DAMP) which binds to toll-like receptor (TLR) 2/4 or the receptor for advanced glycation and products (RAGE) and promotes dendritic cell maturation and T-cell activation (Talebi et al. 2017). The adjuvant was linked to the vaccine body using strict “EAAAK” linker, in order to prevent likely interactions between the adjuvant and other vaccine domains. Moreover, B-cell and MHC-I binding epitopes were connected together using “GPGPG” and “AAY” spacers, which induce humoral responses and play as proteasome cleavage site in mammalian cells, respectively (Asghari et al. 2021b; Mahdevar et al. 2021). Also, based on Livingstone et al. (2002), GPGPG linker can also act as inducers for helper lymphocytes in vivo (Livingston et al. 2002). In total, spacers facilitate the biological activity and antigen-presentation of peptide-based multi-epitope vaccines and prevent the establishment of neoepitopes or junctional epitopes (Meza et al. 2017). Also, a histidine tag sequence was embedded at the C-terminus of the vaccine construct for further purification purposes.
The final multi-epitope vaccine construct was stable (instability index: 39.89), had 442 amino acids in length with a relatively high molecular weight (47.71 kDa). Also, it was moderately thermotolerant (aliphatic index: 45.56) and highly hydrophilic (GRAVY: – 0.789). The speculated pI of the vaccine construct was 5.40, which is advantageous for isoelectric focusing and ion-exchange chromatography. Overall, estimation of such basic biochemical properties is beneficial for future protein extraction during wet experiments. In the next step, outputs of different web servers demonstrated that the peptide-based vaccine had no allergenic traits and it was highly antigenic in nature, based on VaxiJen sores of 1.0439 (crude sequence) and 0.9501 (with adjuvant and H6 tag). Asghari et al. (2021a, b) and Khodadadi et al. (2021), also, used VaxiJen v1.0 server and demonstrated a highly antigenic Toxoplasma multi-epitope vaccine candidate (Asghari et al. 2021b; Khodadadi et al. 2021). A relatively high protein solubility in E. coli was estimated for the vaccine protein, as shown by a scaled solubility of 0.622 in Protein-Sol web server. Hammed-Akanmu et al. (2022) performed a similar study using microneme 3 (MIC3), rhoptry 2 (ROP2) and dense granule antigen 7 (GRA7) and devised a 469-bp vaccine candidate using linear B-cell, cytotoxic T-lymphocyte (CTL) and helper T-lymphocyte (HTL) epitopes; the constructed protein was antigenic, soluble and stable, having a MW of about 51 kDa, acidic pI (5.46), aliphatic index of 77.08 and it was hydrophilic in nature (GRAVY: -0.439) (Hammed-Akanmu et al. 2022).
In the following, the vaccine sequence was subjected to NetSurfP web server for secondary structure analysis. It was shown that coils and helices were prevalent secondary structures within the protein sequence and most of the residues were exposed. The best-fit model predicted using ITASSER homology-modelling online tool (C-score: – 0.91) was further submitted to the GalaxyRefine server for rehashing purposes. The final refined high-quality 3D model, encompassing GDT-HA: 0.9146, RMSD: 0.501, MolProbity: 2.350, Clash score: 17.8, Poor rotamers: 0.3 and Rama favored: 88.0 was selected for validation and further analyses. The outputs of the PROCHECK web tool, i.e., Ramachandran plot analysis, showed advances in the quality of the refined model, in comparison with the crude vaccine protein. In other words, Ramachandran plot of the refined model illustrated that 82.7%, 13%, 2.2% and 2.2% of the residues were assigned to the additional allowed, generously allowed and disallowed regions, respectively.
Although, the cell-mediated immune responses are highly important for parasite clearance, B-cell induction, maturation and the production of neutralizing antibodies are, also, beneficial to combat the infection (Mévélec et al. 2020). Therefore, ElliPro tool was employed to predict potential conformational B-cell epitopes in the vaccine model. Previously, Foroutan-Rad et al. (2020) used ElliPro tool of IEDB for the prediction of discontinuous B-cell epitope prediction in Toxoplasma vaccine candidates (Foroutan et al. 2020). Based on our findings, seven non-linear B-cell epitopes were present, with 13, 48, 23, 3, 29, 90 and 7 residues in length with 0.86, 0.765, 0.749, 0.74, 0.728, 0.7 and 0.673 scores, respectively. Subsequently, a protein–protein molecular docking was virtually performed between the vaccine construct and human TLR4 using ClusPro 2.0 server, suggesting significant interactions between chains B and D of the TLR4 molecule with the vaccine construct. Based on the immune simulation outputs by the C-ImmSim web server, Single vaccine injection (without LPS) represented higher levels of Th1 responses, with a prominent upsurge in IFN-γ level, and significant titers of neutralizing antibodies (IgG + IgM). A determinative role has been suggested for IFN-γ, since this crucial cytokine can promote cytotoxic and helper lymphocytes along with natural killer cell populations; it also enhances B-cell class switching towards IgG2a isotype, which favors cell-mediated immunity. Notably, both B- and T-helper memory cells increased and reached a plateau 10 days post injection, which are extremely critical for an appropriate immune response during second antigenic exposure. A molecular docking using TLR2, TLR4, MHC-I and MHC-II molecules as well as in silico simulation was, also, performed by Hammed-Akanmu et al. (2022) using ClusPro and C-ImmSim servers, respectively; they showed substantial immunogenicity with good binding affinity for their vaccine construct (Hammed-Akanmu et al. 2022).
As a final word, rational vaccine design and in silico evaluation of a potential vaccine candidate demands utilization of stringent algorithms for immunodominant epitope prediction and screening using comprehensive immunoinformatics servers. In this study, high-ranked, antigenic and non-allergenic peptides were exploited from tachyzoite-specific SRS proteins of the ubiquitous T. gondii, a chimeric peptide-based vaccine was constructed and its 3D model was predicted. In the following, the safety, antigenicity, allergenicity of the designed protein, molecular affinity between the vaccine model and human TLR4 molecule as well as the elicited immune profile upon vaccine injection were all predicted using bioinformatics web servers. Nevertheless, such computer-aided studies meet some pitfalls such as lack of biochemical and safety assessment in laboratory animal models using different acute T. gondii strains. Thus, further interpretations of our findings demand subsequent in vitro and in vivo experiments.
Conclusion
In conclusion, a SRS-based multi-epitope vaccine construct was designed against toxoplasmosis using a set of bioinformatics web tools. Preliminary findings derived from current study revealed that the vaccine candidate possessed high antigenicity, good solubility and without allergenic properties, which could significantly bind with the human TLR4 and coherently induce humoral, and particularly, cellular immunity against Toxoplasma infection. However, the actual effectiveness of the proposed vaccine should be validated in future experimental studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors appreciate the staff of zoonotic diseases research center Ilam University of Medical Sciences, Ilam, Iran for their kind assistance in preparation of this manuscript.
Authors contributions
MS and NN conceived the study idea and protocol; HH, MCB and EG performed bioinformatics analyses, vaccine construction and subsequent validations; BNG and HM drafted the manuscript; HI performed docking analysis; MS and NN critically revised the work. All authors read and approved the final version.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and material
Data are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare none.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aasim SR, Patil CR, Kumar A, Sharma K. Identification of vaccine candidate against Omicron variant of SARS-CoV-2 using immunoinformatic approaches. Silic Pharmacol. 2022 doi: 10.1007/s40203-022-00128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadpour E, et al. Toxoplasma gondii Infection in marine animal species, as a potential source of food contamination: systematic review and meta-analysis. Acta Parasitol. 2022;67:592–605. doi: 10.1007/s11686-021-00507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeria S, Dubey JP. Foodborne transmission of Toxoplasma gondii infection in the last decade An overview. Res Vet Sci. 2021;135:371–385. doi: 10.1016/j.rvsc.2020.10.019. [DOI] [PubMed] [Google Scholar]
- Asghari A, et al. Development of a chimeric vaccine candidate based on Toxoplasma gondii major surface antigen 1 and apicoplast proteins using comprehensive immunoinformatics approaches. Eur J Pharm Sci. 2021;162:105837. doi: 10.1016/j.ejps.2021.105837. [DOI] [PubMed] [Google Scholar]
- Asghari A, Majidiani H, Nemati T, Fatollahzadeh M, Shams M, Naserifar R, Kordi BJBri, Toxoplasma gondii tyrosine-rich oocyst wall protein: a closer look through an in silico prism. BioMed Res Int. 2021;2021:1–13. doi: 10.1155/2021/1315618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austhof E, et al. Scoping review of toxoplasma postinfectious sequelae. Foodborne Pathog Dis. 2021;18:687–701. doi: 10.1089/fpd.2021.0015. [DOI] [PubMed] [Google Scholar]
- Brisse M, Vrba SM, Kirk N, Liang Y. Emerging concepts and technologies in vaccine development. Front Immunol. 2020;11:2578. doi: 10.3389/fimmu.2020.583077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Randall AZ, Sweredoski MJ, PJnar B. SCRATCH: a protein structure and structural feature prediction server. Nucl Acids Res. 2005;33:W72–W76. doi: 10.1093/nar/gki396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu KB, Quan FS. Advances in Toxoplasma gondii vaccines: current strategies and challenges for vaccine development. Vaccines. 2021;9:413. doi: 10.3390/vaccines9050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig DB, Dombkowski AA. Disulfide by Design 2.0: a web-based tool for disulfide engineering in proteins. BMC Bioinform. 2013;14:1–7. doi: 10.1186/1471-2105-14-S19-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, et al. Mathematical modelling of Toxoplasma gondii transmission: A systematic review. Food Waterborne Parasitol. 2021;22:e00102. doi: 10.1016/j.fawpar.2020.e00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov I, Bangov I, Flower DR, Doytchinova I. AllerTOP v. 2—a server for in silico prediction of allergens. J Mol Model. 2014;20:1–6. doi: 10.1007/s00894-014-2278-5. [DOI] [PubMed] [Google Scholar]
- Dimitrov I, Naneva L, Doytchinova I, Bangov I. AllergenFP: allergenicity prediction by descriptor fingerprints. Bioinformatics. 2014;30:846–851. doi: 10.1093/bioinformatics/btt619. [DOI] [PubMed] [Google Scholar]
- Doytchinova IA, Flower DR. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007;8:1–7. doi: 10.1186/1471-2105-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP. Outbreaks of clinical toxoplasmosis in humans: five decades of personal experience, perspectives and lessons learned. Parasit Vectors. 2021;14:1–12. doi: 10.1186/s13071-021-04769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunay IR, Gajurel K, Dhakal R, Liesenfeld O, Montoya, Treatment of toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin Microbiol Rev. 2018;31:e00057–e117. doi: 10.1128/CMR.00057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont CD, Christian DA, Hunter CA 2012 Immune response and immunopathology during toxoplasmosis. In: Seminars in immunopathology, Springer, pp 793–813 [DOI] [PMC free article] [PubMed]
- Foroutan M, Ghaffarifar F, Sharifi Z, Dalimi A. Vaccination with a novel multi-epitope ROP8 DNA vaccine against acute Toxoplasma gondii infection induces strong B and T cell responses in mice Comparative Immunology. Microbiol Infect Dis. 2020;69:101413. doi: 10.1016/j.cimid.2020.101413. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server The proteomics protocols handbook, pp. 571–607
- Hammed-Akanmu M, et al. Designing a multi-epitope vaccine against toxoplasma gondii: an immunoinformatics approach. Vaccines. 2022;10:1389. doi: 10.3390/vaccines10091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJC, Research (2015) Clinical vaccine development 4:46–53 [DOI] [PMC free article] [PubMed]
- Hebditch M, Carballo-Amador MA, Charonis S, Curtis R, Warwicker J. Protein–Sol: a web tool for predicting protein solubility from sequence. Bioinformatics. 2017;33:3098–3100. doi: 10.1093/bioinformatics/btx345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo L, Park H, Seok C. GalaxyRefine: Protein structure refinement driven by side-chain repacking. Nucl Acids Res. 2013;41:W384–W388. doi: 10.1093/nar/gkt458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D, Dubey JJCm, infection, Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8:634–640. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- Jespersen MC, Peters B, Nielsen M, Pjnar M. BepiPred-2.0: improving sequence-based B-cell epitope prediction using conformational epitopes. Nucl Acids Res. 2017;45:W24–W29. doi: 10.1093/nar/gkx346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodadadi M, Ghaffarifar F, Dalimi A, Ahmadpour E. Immunogenicity of in-silico designed multi-epitope DNA vaccine encoding SAG1, SAG3 and SAG5 of Toxoplasma gondii adjuvanted with CpG-ODN against acute toxoplasmosis in BALB/c mice. Acta Trop. 2021;216:105836. doi: 10.1016/j.actatropica.2021.105836. [DOI] [PubMed] [Google Scholar]
- Klausen MS, et al. NetSurfP-2.0: Improved prediction of protein structural features by integrated deep learning proteins: structure function. Bioinformatics. 2019;87:520–527. doi: 10.1002/prot.25674. [DOI] [PubMed] [Google Scholar]
- Kozakov D, et al. The ClusPro web server for protein–protein docking. Nat Protoc. 2017;12:255–278. doi: 10.1038/nprot.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski R, MacArthur M, Thornton J (2006) PROCHECK: validation of protein-structure coordinates
- Livingston B, Crimi C, Newman M, Higashimoto Y, Appella E, Sidney J, Sette A. A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J Immunol. 2002;168:5499–5506. doi: 10.4049/jimmunol.168.11.5499. [DOI] [PubMed] [Google Scholar]
- Mahdevar E, et al. Exploring the cancer-testis antigen BORIS to design a novel multi-epitope vaccine against breast cancer based on immunoinformatics approaches. J Biomol Struct Dyn. 2021;40(14):6363–6380. doi: 10.1080/07391102.2021.1883111. [DOI] [PubMed] [Google Scholar]
- Mévélec M-N, Lakhrif Z, Dimier-Poisson I. Key limitations and new insights into the Toxoplasma gondii parasite stage switching for future vaccine development in human, livestock, and cats. Front Cell Infect Microbiol. 2020 doi: 10.3389/fcimb.2020.607198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza B, Ascencio F, Sierra-Beltrán AP, Torres J, Angulo CJI. A novel design of a multi-antigenic, multistage and multi-epitope vaccine against Helicobacter pylori: an in silico approach. Infect Genet Evol. 2017;49:309–317. doi: 10.1016/j.meegid.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Parvizpour S, Pourseif MM, Razmara J, Rafi MA, Omidi Y. Epitope-based vaccine design: a comprehensive overview of bioinformatics approaches. Drug Discover Today. 2020;25:1034–1042. doi: 10.1016/j.drudis.2020.03.006. [DOI] [PubMed] [Google Scholar]
- Ponomarenko J, Bui HH, Li W, Fusseder N, Bourne PE, Sette A, Peters B. ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC Bioinform. 2008;9:1–8. doi: 10.1186/1471-2105-9-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin N, Lund O, Castiglione F. Immune system simulation online. Bioinformatics. 2011;27:2013–2014. doi: 10.1093/bioinformatics/btr335. [DOI] [PubMed] [Google Scholar]
- Ras-Carmona A, Pelaez-Prestel HF, Lafuente EM, Reche PAJC. BCEPS: A web server to predict linear B cell epitopes with enhanced immunogenicity and cross-reactivity. Cells. 2021;10:2744. doi: 10.3390/cells10102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- Rezaei F, Sarvi S, Sharif M, Hejazi SH, Sattar Pagheh A, Aghayan SA, Daryani A. A systematic review of Toxoplasma gondii antigens to find the best vaccine candidates for immunization. Microial Pathog. 2019;126:172–184. doi: 10.1016/j.micpath.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Saha S, Raghava GP. Prediction methods for B-cell epitopes. In: Flower DR, editor. Immunoinformatics. Springer: Humana Press; 2007. pp. 387–394. [Google Scholar]
- Soria-Guerra RE, Nieto-Gomez R, Govea-Alonso DO, Rosales-Mendoza SJJobi, An overview of bioinformatics tools for epitope prediction: implications on vaccine development. J Biomed Inform. 2015;53:405–414. doi: 10.1016/j.jbi.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Talebi S, Bolhassani A, Sadat SM, Vahabpour R, Agi E, Shahbazi SJB. Hp91 immunoadjuvant: An HMGB1-derived peptide for development of therapeutic HPV vaccines. Biomed Pharamcotherapy. 2017;85:148–154. doi: 10.1016/j.biopha.2016.11.115. [DOI] [PubMed] [Google Scholar]
- Theisen TC, Boothroyd JCJb, Transcriptional signatures of clonally derived Toxoplasma tachyzoites reveal novel insights into the expression of a family of surface proteins. PLoS ONE. 2021;17:e0262374. doi: 10.1371/journal.pone.0262374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniProt Consortium UniProt: a worldwide hub of protein knowledge. Nucl Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth JD, et al. Integrated bioinformatic and targeted deletion analyses of the SRS gene superfamily identify SRS29C as a negative regulator of Toxoplasma virulence. Mbbio. 2012;3:e00321–e1312. doi: 10.1128/mBio.00321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang Y. I-TASSER server: new development for protein structure and function predictions. Nucl Acids Res. 2015;43:W174–W181. doi: 10.1093/nar/gkv342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B, Zheng D, Liang S, Zhang C (2020) SVMTriP: a method to predict B-cell linear antigenic epitopes. Immunoinformatics 299–307 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author on reasonable request.