Abstract
Background
Parents of children treated for cancer may experience mental health difficulties, such as depression and anxiety. There is a lack of evidence‐based psychological interventions for parents, with psychological support needs unmet. An internet‐administered, guided, low‐intensity cognitive behavioral therapy‐based (LICBT) self‐help intervention may provide a solution.
Methods
The feasibility and acceptability of such an intervention was examined using a single‐arm feasibility trial (ENGAGE). Primary objectives examined: (1) estimates of recruitment and retention rates; (2) feasibility and acceptability of data collection instruments and procedures; and (3) intervention feasibility and acceptability. Clinical outcomes were collected at baseline, post‐treatment (12 weeks), and follow‐up (6 months).
Results
The following progression criteria were met: sample size was exceeded within 5 months, with 11.0% enrolled of total population invited, study dropout rate was 24.0%, intervention dropout was 23.6%, missing data remained at ≤10% per measure, and no substantial negative consequences related to participation were reported. Intervention adherence was slightly lower than progression criteria (47.9%).
Conclusion
Findings suggest an internet‐administered, guided, LICBT self‐help intervention may represent a feasible and acceptable solution for parents of children treated for cancer. With minor study protocol and intervention modifications, progression to a pilot randomized controlled trial (RCT) and subsequent superiority RCT is warranted.
Keywords: anxiety, cancer, cognitive behavioral therapy, depression, internet‐based intervention, parents
Short abstract
A single‐arm feasibility trial showed overall acceptability and feasibility of an internet‐administered, guided, low‐intensity cognitive behavioral therapy intervention for parents of childhood cancer survivors, and progression criteria were met for recruitment, retention, missing data, and harms, indicating methods, study procedures, and the overall the trial and intervention was feasible and acceptable. However, completion of assessments at each timepoint and intervention adherence were under progression criteria, meaning some modifications to the study protocol intervention are required before commencing a pilot randomized controlled trial.
1. INTRODUCTION
Advances in cancer treatment have resulted in increased childhood cancer survival rates worldwide. 1 , 2 Parents are the primary source of support for children with cancer, with many actively involved in care years after treatment completion. 3 While treatment completion is an important milestone, it is also a period of vulnerability for parents. 4 , 5 Psychological difficulties such as anxiety (19.7% to 43.4%), 6 , 7 and depression (14.4% to 43.4%) 6 , 7 are reported. Parents also report post‐traumatic stress symptoms 8 , 9 and 19.1% of mothers and 7.8% of fathers report at least partial post‐traumatic stress disorder (PTSD) 5 years after treatment. 10 Further, parents face socioeconomic impacts 11 and restrictions on daily life activities. 12 However, parents' psychological needs are unmet 13 and barriers to seeking support include lack of time, guilt, and putting the child's needs first. 14 , 15
Solutions to increase access to psychological interventions are being implemented globally, 16 for example low‐intensity cognitive behavioral therapy (LICBT). 17 LICBT is delivered through self‐help interventions (e.g., print or digital format), including internet‐administered CBT (iCBT) 18 rather than by traditional psychologists. Guided iCBT (supported by a trained professional) is associated with higher effect sizes than unguided interventions 19 and show equivalent overall effects to traditional face‐to‐face interventions. 20 An internet‐administered, guided, LICBT based self‐help intervention may also address barriers to seeking support given increased privacy and flexibility. 21 In previous research, we have shown that an iCBT self‐help intervention decreases symptoms of anxiety, depression, and post‐traumatic stress in parents of children on cancer treatment. 22 , 23 Recent research has also demonstrated a video‐conference‐based internet‐administered intervention to be effective for parents of children living with a life‐threatening illness (including cancer). 24 However, to the best of our knowledge, the only existing internet‐administered intervention for parents of children who have completed treatment, with published results, is an online group‐based, intervention delivered in real time via videoconferencing by psychologists. 25 , 26 As such, there is currently no internet‐administered, LICBT based self‐help intervention available for parents of children who have completed cancer treatment.
A program of phase I (development) research, following the Medical Research Council complex interventions framework 27 , 28 informed development of the internet‐administered LICBT intervention EJDeR. 10 , 29 , 30 , 31 , 32 , 33 , 34 Following phase II (feasibility) 27 , 28 we conducted the current study, the single‐arm feasibility trial ENGAGE. Primary objectives examined methodological, procedural, and clinical uncertainties 35 to prepare for the design and conduct of a future pilot RCT and subsequent superiority RCT. Information was gathered on: (1) estimates of recruitment and retention rates; (2) feasibility and acceptability of data collection instruments and procedures; and (3) feasibility and acceptability of the intervention. An embedded mixed‐method process evaluation examined the feasibility of collecting weekly assessments and semi‐structured interviews at baseline and post‐treatment explored: (1) self‐reported psychological concerns, healthcare utilization, and productivity losses; (2) treatment expectations; (3) intervention acceptability; and (4) perceived impact of the intervention on difficulties and mechanisms of change. Findings from semi‐structured interviews at baseline and post‐treatment to inform the embedded process evaluation will be reported elsewhere.
2. MATERIALS AND METHODS
The study protocol is published 36 and registered, with results following the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement extension for randomized pilot and feasibility trials. 37
2.1. Study design
A single‐arm feasibility trial of a guided, internet‐administered LICBT‐based intervention (EJDeR), with data collected at baseline, post‐treatment (12 weeks), and follow‐up (6 months) with an embedded mixed‐methods process evaluation. EJDeR is delivered via the U‐CARE‐portal (Portal), a web‐based platform, designed to deliver internet‐administered interventions and support the execution of study procedures.
2.2. Participants
Eligible participants were: (1) parent of a child diagnosed with childhood cancer (0–18 years) who completed treatment 3 months to 5 years previously (timespan informed by our previous longitudinal research that has identified this as a time period of vulnerability for parents) 9 , 10 , 29 , 30 ; (2) resident in Sweden; (3) able to read and understand Swedish; (4) able to access e‐mail, internet, and Bank‐ID (a Swedish citizen authentication system); and (5) self‐reporting a need for psychological support related to the child's cancer. Exclusion criteria were: (1) a self‐reported or clinician assessed (with the Mini‐International Neuropsychiatric Interview, M.I.N.I., version 7.0.0) 38 severe and enduring mental health difficulty (e.g., PTSD) and/or misuse of alcohol, street drugs, or prescription medication; (2) acute suicidality; and (3) ongoing psychological treatment respectively.
2.3. Recruitment
2.3.1. Postal study invitations
Personal identification numbers of children who had completed treatment 3 months to 5 years previously were provided by the Swedish Childhood Cancer Registry (CCR), and linked to parents' names and addresses via NAVET, a population registry from the Swedish Tax Agency. The first recruitment block was pre‐selected with parents of children who had ended treatment near to 5 years previously. The following four blocks were randomly selected by a member of the Portal team, independent to the research team, using a computer‐generated simple randomization procedure. Postal study invitation packs, sent to parents' home addresses, included a: (1) study invitation letter; (2) study information sheet and link to a secure website on the Portal (information in text and video format); (3) paper reply‐slip to register interest in participation; (4) paper‐based opt‐out form and reasons for non‐participation questionnaire; and (5) freepost envelope. Parents could register interest in participation and request more study information via the Portal, post, telephone or e‐mail. ENGAGE included an embedded recruitment RCT, investigating the effect of personalized versus non‐personalized study invitations on recruitment and retention 39 with results reported separately. 40
2.3.2. Online advertisements
Advertisements were placed on social media sites, websites, and newsletters of 12 cancer organizations and interest groups.
2.3.3. Opt‐out and reminders
Parents invited via the post could opt‐out of ENGAGE via the Portal, post, telephone, or e‐mail. Up to five reminder telephone contact attempts were made if parents did not respond within 4 weeks of invitation. Telephone numbers were identified using internet search engines. Contact attempts were documented in paper‐based case report forms (CRFs). If a telephone number was not identified, a postal study invitation reminder letter was sent.
2.3.4. Reasons for non‐participation
Parents opting out of ENGAGE were asked to complete a reason for non‐participation questionnaire including a closed, multiple choice question and an open question for other reason(s). 41 Reasons for non‐participation were collected to enable the identification of potential modifiable barriers to participation (e.g., treatment preferences, interest in internet‐administered self‐help, burden of trial procedures).
2.4. Consent, eligibility, and baseline
Parents provided consent via the Portal. Parents who registered interest in participation but did not provide consent, or opt out, within 2 weeks, were contacted to confirm interest in participation (maximum five reminders via telephone, SMS or e‐mail).
Parents providing consent were contacted to organize a telephone eligibility interview with a licensed psychologist. Interviews included: (1) questions concerning eligibility criteria; with those eligible completing specific modules of the M.I.N.I., and; (2) questions concerning parent and child sociodemographic and clinical characteristics (Table 1).
TABLE 1.
Overview of measures taken at respective assessment time‐point
| Variable | Measure | Time‐point | Mode of administration | ||||
|---|---|---|---|---|---|---|---|
| Eligibility interview | Baseline | Post‐treatment | Weekly process evaluation | Follow‐up | |||
| Child age, legal gender, cancer diagnosis, date of first diagnosis, date of end of treatment (where available), type of treatment | Childhood Cancer Registry | Swedish Childhood Cancer Registry | |||||
| Eligibility (inclusion and exclusion) criteria; parent sociodemographic and clinical characteristics (age, gender, relationship status, highest level of education, employment status, number and ages of children, current housing situation, region of birth, previous psychological treatment, physical health problem, previous traumatic/difficult life events and internet usage); child sociodemographic and clinical characteristics (age, gender, cancer diagnosis, time since end of treatment, type of treatment, cancer recurrence) | Structured questions | ✓ | Telephone | ||||
| Psychiatric (mood and anxiety) disorders, drug and alcohol misuse, suicidality | M.I.N.I. | ✓ | ✓ | ✓ | Telephone | ||
| PTSS | PCL‐5 | ✓ | ✓ | ✓ | ✓ |
Portal/Telephone Weekly process evaluation: Portal only |
|
| PTSS | PCL‐C | ✓ | ✓ | ✓ | ✓ |
Portal/Telephone Weekly process evaluation: Portal only |
|
| Depression | PHQ‐9 | ✓ | ✓ | ✓ | ✓ |
Portal/Telephone Weekly process evaluation: Portal only |
|
| Anxiety | GAD‐7 | ✓ | ✓ | ✓ | Portal/Telephone | ||
| Fear of recurrence | FRHC | ✓ | ✓ | ✓ | Portal/Telephone | ||
| Fear of serious health condition | FRHC | ✓ | ✓ | ✓ | Portal/Telephone | ||
| Psychological inflexibility and experiential avoidance | AAQ‐6 | ✓ | ✓ | ✓ | ✓ |
Portal/Telephone Weekly process evaluation: Portal only |
|
| Depressed inactivity | BADS | ✓ | ✓ | ✓ | ✓ |
Portal/Telephone Weekly process evaluation: Portal only |
|
| Fatigue | FSS | ✓ | ✓ | ✓ | Portal/Telephone | ||
| Quality of life | EQ‐5D | ✓ | ✓ | ✓ | Portal/Telephone | ||
| Self‐compassion | SCS‐SF | ✓ | ✓ | ✓ | Portal/Telephone | ||
| Health economics | TIC‐P | ✓ | ✓ | Portal/Telephone | |||
Abbreviations: AAQ‐6, Acceptance and Action Questionnaire; BADS, Behavioral Activation for Depression Scale; EQ‐5D, EuroQol 5‐dimension questionnaire; FRHC, Fear of recurrence and serious health condition (structured questions); FSS, Fatigue Severity Scale; GAD‐7, Generalized Anxiety Disorder 7‐item scale; M.I.N.I., Mini‐International Neuropsychiatric Interview version 7.0.0; PCL‐5, Post‐traumatic Stress Disorder Checklist for DSM‐5; PCL‐C, adapted version of Post‐traumatic Stress Disorder Checklist‐Civilian version; PHQ‐9, Patient Health Questionnaire; PTSS, Post‐traumatic stress symptoms; SCS‐SF, Self‐Compassion Scale‐Short Form; TIC‐P, Treatment Inventory of Costs in Patients with psychiatric disorders.
Eligible participants were enrolled and invited to an optional semi‐structured telephone interview with a licensed psychologist to explore concerns, needs, healthcare utilization, and productivity loss, alongside expectations on the trial and intervention. Participants gained access to the Portal assessment at baseline (Table 1) and were required to complete the assessment within 28 days. Participants who had not completed within 14 days were reminded up to five times (telephone, SMS, or e‐mail). Upon completion of the Portal assessment at baseline, participants gained access to EJDeR and were allocated to an e‐therapist.
2.5. Intervention
The EJDeR protocol is published following the Template for Intervention Description and Replication (TIDieR) checklist. 34 , 42 The first version of the intervention used a multi‐strand approach utilizing several CBT techniques, including third‐wave CBT (e.g., mindfulness and compassion focused therapy), delivered over 10 modules. 32 , 36 Following public and professional involvement 34 the number of CBT techniques were minimized to reduce complexity and length 34 and a LICBT approach was adopted.
EJDeR is a guided internet‐administered LICBT intervention delivered over 12 weeks on the Portal and includes text, illustrations, film, audio files, in‐module exercises, and homework exercises. EJDeR includes two LICBT techniques: behavioral activation (BA) for depression, and worry management (WM) for generalized anxiety disorder (GAD). 34 It consists of four modules: (1) introduction and psychoeducation; (2) BA; (3) WM, and; (4) relapse prevention (Figure S1). After completing the first module and an initial assessment session with an e‐therapist, participants work with BA or WM, dependent on their main difficulty. After completion of BA or WM, parents may use the remaining LICBT technique. All participants gain access to the relapse prevention module.
E‐therapist guidance is provided via an initial assessment session (video‐conferencing or telephone, ≈45 min); weekly support via written messages via the Portal (≈20–30 min/week), and a mid‐intervention booster session (video‐conferencing or telephone, ≈30–45 min) following structured protocols. 43 , 44 , 45 E‐therapists also provided at‐need written messages to participants if requested. A 2‐day training program with two experts in LICBT, and weekly group clinical supervision via video‐conferencing with a Swedish licensed psychologist with expertise in iCBT were provided.
2.6. Outcomes
Feasibility outcomes are informed by the CONSORT 2010 statement extension for randomized pilot and feasibility trials, 35 , 37 and relate to methodological uncertainties (e.g. estimates of recruitment and retention rates, reasons for non‐participation and study drop‐out), procedural uncertainties (e.g. feasibility and acceptability of data collection instruments and procedures, including percentages completing assessments and numbers of missing items), and clinical uncertainties (e.g. intervention feasibility and acceptability, including participants' adherence to the intervention and impressions and experiences of working with the intervention). All feasibility outcomes are shown in Table 2 alongside progression criteria. 46 Some feasibility outcomes 36 were revised to improve clarity and reflect protocol modifications (Table S1). Intervention acceptability is further explored in the embedded process evaluation (reported elsewhere). Progression criteria were informed by the researchers' previous experience, our previous longitudinal research with the population 6 and relevant literature on recruitment, 47 , 48 attrition, 49 adherence, 50 , 51 and missing data. 52
TABLE 2.
Overview of feasibility outcomes, methods of evaluation, and progression criteria
| Outcome | Evaluation | Progression criteria to controlled trial a |
|---|---|---|
| Recruitment and eligibility | Number identified via postal study invitations (Swedish Childhood Cancer Registry and the Swedish Tax Agency [NAVET]) and/or via Online advertisements via cancer organizations and interest groups | No criteria set |
| Percentage consented to participate, assessed for eligibility, fulfilling eligibility criteria, and enrolled (of total number invited) | ≥9% enrolled of total participant population invited (e.g., included of total participant population invited) | |
| Reasons for ineligibility | No criteria set | |
| Ambiguities regarding eligibility criteria including diagnostic uncertainties in M.I.N.I. | No criteria set | |
| Reasons for non‐participation | No criteria set | |
| Data collection |
Percentage completing assessments M.I.N.I. (eligibility interview, post‐treatment, and follow‐up) Semi‐structured interview (baseline and post‐treatment) Portal assessment (baseline, post‐treatment, and follow‐up) Weekly Portal assessment |
≥70% answering all questions at all assessments |
|
Numbers of missing items M.I.N.I. (eligibility interview, post‐treatment, and follow‐up) Portal assessment (baseline, post‐treatment, and follow‐up) Weekly Portal assessment |
≤10% per measure | |
| Attrition |
Rate of study dropout Rate of intervention dropout |
≤30% ≤30% |
| Resources needed to complete the study and the intervention |
Length of time required for: Participants to work through the intervention Participants to complete the initial assessment session and mid‐intervention booster session with e‐therapist Participants to complete the eligibility interview, M.I.N.I., semi‐structured interview, Portal assessment at each time‐point E‐therapists to deliver the intervention |
No criteria set |
|
Number of: Internal and external study personnel Reminder contacts needed during recruitment Reminder contacts needed to complete Portal assessment at each time‐point Contacts needed to arrange eligibility interview, M.I.N.I. and semi‐structured interview over the telephone at each time‐point |
No criteria set | |
| Participants' adherence to intervention |
Number of: Participants adhering to the minimum treatment dose (MTD) Opened modules Completed LICBT modules started with Completed initial assessment sessions Completed mid‐intervention booster sessions Completed homework sheets |
≥50% adhering to MTD, i.e., attending the initial assessment session, completing the introduction and psychoeducation module and one LICBT treatment module (i.e. behavioral activation or worry management) and attending the mid‐intervention booster session. |
| Participants' use of the intervention |
Number of: Participant logins Participant written messages E‐therapist written messages |
No criteria set |
| E‐therapists' adherence to intervention | Content of initial assessment session, mid‐intervention booster session, and written messages via the Portal | No criteria set |
| Participants' acceptability of the intervention and data collection |
Reasons for low adherence and dropout from study and intervention Number of risk assessments Impressions and experiences of working with the intervention (including positive and negative consequences) and of completing assessments and interviews b |
No criteria set No criteria set <1 participant reporting substantial negative consequences related to participation in the study and/or intervention |
Abbreviations: LICBT, low intensity cognitive behavioral therapy; M.I.N.I., Mini‐International Neuropsychiatric Interview version 7.0.0.
If one or more criteria are not met revisions should be considered before proceeding to a controlled trial.
Outcome is to be reported in separate publications.
The post‐treatment time‐point was set at 12 weeks, immediately after the EJDeR intervention had finished. A 6‐month follow‐up time‐point was selected to examine the feasibility of longer‐term data collection.
Sociodemographic data on parents and children, specific modules of the M.I.N.I. assessing current and past psychiatric disorders and suicidality, and psychological and health economic measures are reported in Table 1, alongside data collection time‐point and mode of administration. A random 10% sample of M.I.N.I.s were coded by a member of the research team, with inter‐rater reliability calculated as satisfactory (α = 0.92). 53
Semi‐structured interviews were conducted at baseline and post‐treatment with licensed psychologists (data reported elsewhere).
2.7. Sample size
Following recommendations for feasibility trial sample sizes the target sample size was 50. 54
2.8. Double data entry
Paper‐based CRFs were used for data collected outside the Portal, with data independently entered onto a Microsoft® Access database by two research assistants, exported into Microsoft® Excel spreadsheets, with accuracy checked using Microsoft® Spreadsheet.
2.9. Reminders
A prompt (SMS and/or e‐mail) was sent when it was time to complete Portal assessments with automatic reminders (SMS and/or e‐mail) sent if not completed within 1 week. Participants who did not complete Portal assessments within 2 weeks, were offered to complete over the telephone, with up to six reminder attempts made via telephone, SMS, or email. Informed by evidence suggesting study newsletters can improve retention 55 a newsletter was sent via the Portal 6 weeks before post‐treatment and follow‐up.
2.10. Participant adherence
The minimum treatment dose (MTD) (i.e., full intervention adherence) was defined as: (1) attendance of the initial assessment session; (2) completion of the introduction and psychoeducation module; (3) completion of one LICBT module (BA or WM); and (4) attendance of the mid‐intervention booster session.
2.11. E‐therapist adherence
A 15% random sample of initial assessment and mid‐intervention booster sessions and written messages via the Portal from e‐therapists were marked for adherence, with each item within the structured support protocols marked as absent/present.
2.12. Statistical methods
Feasibility outcomes relating to recruitment and eligibility, data collection, attrition, resources needed to complete the study and the intervention, participants' adherence to the intervention, participants' use of the intervention, e‐therapists' adherence to the intervention, and participants' sociodemographic characteristics are reported using descriptive statistics. Numbers and percentages (and 95% CIs where appropriate) are reported for categorical variables, means and SDs for continuous variables. Numbers and percentages of participants meeting criteria for each M.I.N.I. diagnosis is reported at each time‐point. Means and SDs for continuous variables and numbers and percentages for categorical variables are reported for all outcomes at each time‐point. Mean change scores (with 95% CIs) are reported for Portal assessments of psychological outcomes at each time‐point, to describe the study sample.
2.13. Risk and safety procedures
Participants scoring >0 on PHQ‐9 (depression) question 9 (suicidal ideation), or a total score >20 (severe depression) were risk assessed by a licensed psychologist within one working day. If needed, participants were directed to appropriate support and excluded.
2.14. Public involvement
A Parent Research Partner (PRP) group was established consisting of four parents with lived experience of being a parent of a child treated for cancer (two fathers and two mothers, aged between 45 and 54 years of age). The PRP group was involved in optimizing the acceptability of EJDeR e.g., relevancy, ease of understanding, content, language, and structure. 34 The group was also consulted on the development of participant invitation letters. 39 , 40
3. RESULTS
Data supporting feasibility objectives pertaining to recruitment and eligibility, data collection, attrition, and resources needed to complete the study and intervention are available in Zenodo. 56
3.1. Recruitment and eligibility
Participant flow is summarized in an adapted CONSORT diagram (Figure 1). Recruitment took place over 5 months (03‐07‐2020 and 30‐11‐2020). Of 509 study invitations sent via CCR and NAVET, 60 consented (11.8%, 95%CI, [9.1–14.9]); 57 were assessed for eligibility (11.2%, 95%CI, [8.6–14.3]); and 56 fulfilled eligibility criteria and were enrolled (11.0%, 95%CI, [8.4–14.1]) exceeding progression criteria of ≥9% enrolled of total potential participant population invited. An additional 21 consented from other recruitment strategies (online advertisements and parents invited by the CCR passing the invitation to their partner), 19 were assessed for and fulfilled eligibility, and enrolled. Nine parents were excluded prior to consent and one was excluded during the eligibility interview (acute suicidality). In total, 75 participants were enrolled, exceeding sample size expectations (Figure 1).
FIGURE 1.
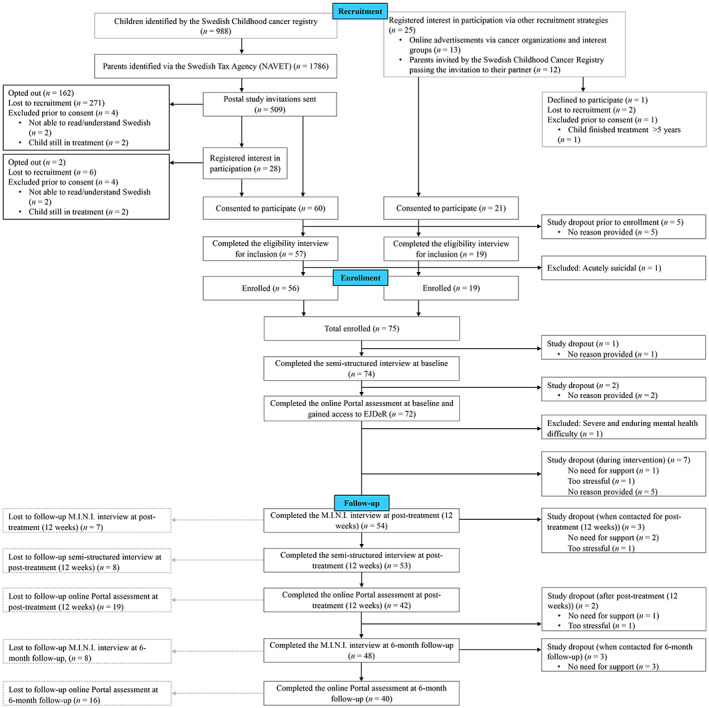
Study flow of participants in the ENGAGE feasibility trial. Solid black lines denote participant flow through the study, including study drop outs i.e., those who discontinued the study. Dashed gray lines represent participants that were lost to follow‐up during assessments at post‐treatment (12 weeks) and follow‐up (6 months) respectively, but had not dropped out of the study.
Ambiguities regarding eligibility arose in six cases. In three cases, parents met criteria for PTSD according to the M.I.N.I. but were included as symptoms were mild. In one case a parent met criteria for Alcohol Use Disorder, and was included due to being in early remission. One was attending a psychological support group; study inclusion was delayed until the group ended. One reported their child had recently relapsed, however, as treatment had not started, the parent was included.
Out of 509 parents identified via the CCR and NAVET, 164 (32.2%) opted out, and 137 provided a response to the multiple‐choice question regarding reasons for non‐participation. Not experiencing any need for psychological support (93/137, 67.9%) was most commonly reported (Table S2). Full results concerning opt‐out rates and reasons for non‐participation have been reported separately. 41
3.2. Sociodemographic and clinical characteristics
Baseline sociodemographic and clinical characteristics for participants (N = 75) are summarized in Table 3.
TABLE 3.
Baseline sociodemographic and clinical self‐report characteristics for participants (N = 75)
| Sociodemographic and clinical characteristics | n (%) |
|---|---|
| Age (years) | |
| Mean (SD) range | 42.8 (7.1) 26–62 |
| Gender | |
| Female | 48 (64.0) |
| Male | 27 (36.0) |
| Relationship status | |
| Partner | 63 (84.0) |
| Single | 12 (16.0) |
| If partner, cohabiting | |
| Yes | 62 (98.4) |
| No | 1 (1.6) |
| Highest level of education | |
| Lower secondary | 1 (1.3) |
| Upper secondary | 15 (20.0) |
| Post‐secondary non‐tertiary | 3 (4.0) |
| Tertiary | 54 (72.0) |
| PhD | 2 (2.7) |
| Employment status | |
| Employed | 66 (88.0) |
| Unemployed | 9 (12.0) |
| Number of children | |
| Median (range) | 2.0 (1–5) |
| Age of children | |
| Mean (SD) range | 12.0 (7.1) 0.5–37 |
| Housing situation | |
| Rental | 8 (10.7) |
| Apartment ownership | 17 (22.7) |
| House ownership | 47 (62.7) |
| Other | 3 (4.0) |
| Region of birth | |
| Nordic countries | 63 (84.0) |
| Asia | 6 (8.0) |
| Europe (excluding. Nordic countries) | 5 (6.7) |
| Africa | 1 (1.3) |
| Previous psychological treatment | |
| Yes | 40 (53.3) |
| No | 35 (46.7) |
| Physical health problems | |
| Yes | 24 (32.0) |
| No | 51 (68.0) |
| Type of physical health problem a | |
| Diseases of the musculoskeletal system and connective tissue | 9 (12.0) |
| Endocrine, nutritional and metabolic diseases | 5 (6.7) |
| Diseases of the genitourinary system | 3 (4.0) |
| Diseases of the circulatory system | 2 (2.7) |
| Diseases of the digestive system | 2 (2.7) |
| Diseases of the nervous system | 2 (2.7) |
| Diseases of the respiratory system | 2 (2.7) |
| Diseases of the skin and subcutaneous tissue | 1 (1.3) |
| Neoplasm | 1 (1.3) |
| Other cannot classify | 3 (4.0) |
| Previous traumatic/difficult life event | |
| Yes | 60 (80.0) |
| No | 15 (20.0) |
| Type pf previous traumatic/difficult life event a | |
| Child's cancer disease | 34 (45.3) |
| Death in family and miscarriage | 21 (28.0) |
| Severe disease/illness own/family/friends | 18 (24.0) |
| Divorce or separation | 12 (16.0) |
| Exposure to violence or sexual abuse | 6 (8.0) |
| Suicide/suicide attempt among family/friends | 4 (5.3) |
| War/terrorist attacks | 3 (4.0) |
| Other traumatic experiences | 13 (17.3) |
Note: Data are number (%) unless stated otherwise. Percentages may not always total 100 due to rounding. Nordic countries represented in the study sample include Denmark, Finland, Norway, and Sweden.
Multiple responses possible.
Participants' internet usage is reported in Table S10. According to participant self‐report data, children treated for cancer were predominantly male (n = 38, 54.3%), had been diagnosed with Leukemia (n = 32, 45.7%) and treated with chemotherapy (n = 55, 78.6%). The children's mean age at the time of the eligibility interview was 10.6 years (SD 5.2, range, 2–24). Baseline sociodemographic and clinical characteristics for children are provided in Table S10.
3.3. Data collection
Data collection (baseline, post‐treatment, and follow‐up) took place between 24‐07‐2020 and 04‐10‐2021. Percentage completing assessments at each time‐point are reported in Table 4, and progression criteria of 70% of participants answering all questions at all assessments was not met. Completion rates of weekly Portal assessments decreased from 65.7% (week one) to 38.9% (week 11) (Table S3).
TABLE 4.
Number and percentages of participants completing assessments of the total study sample (N = 75) and the study sample at baseline, post‐treatment, and follow‐up
| Assessment | Completed assessment | |||||||
|---|---|---|---|---|---|---|---|---|
| Total study sample a | Study sample at time‐point b | |||||||
| N | n | % | 95% CI | N | n | % | 95% CI | |
| Baseline | ||||||||
| Semi‐structured interview | 75 | 74 | 98.7 | 92.8, 100.0 | 75 | 74 | 98.7 | 92.8, 100.0 |
| Portal assessment | 75 | 72 | 96.0 | 88.8, 99.2 | 74 | 72 | 97.3 | 90.6, 99.7 |
| Post‐treatment | ||||||||
| M.I.N.I | 75 | 54 | 72.0 | 60.4, 81.8 | 64 | 54 | 84.4 | 73.1, 92.2 |
| Semi‐structured interview | 75 | 53 | 71.0 | 59.0, 80.6 | 64 | 53 | 82.8 | 71.3, 91.1 |
| Portal assessment | 75 | 42 | 56.0 | 44.1, 67.5 | 64 | 42 | 65.6 | 52.7, 77.1 |
| Follow‐up | ||||||||
| M.I.N.I | 75 | 48 | 64.0 | 52.1, 74.8 | 59 | 48 | 81.4 | 69.1, 90.3 |
| Portal assessment | 75 | 40 | 53.3 | 41.5, 65.0 | 59 | 40 | 67.8 | 54.4, 79.4 |
Total sample defined as all participants enrolled into the ENGAGE feasibility trial.
Study sample at time‐point defined as the total number of participants remaining in the ENGAGE feasibility trial (i.e., had not dropped out of, or been excluded from, the study at each time‐point).
Missing data ranged from 0.01%–4.1% items missing per measure, bettering progression criteria (≤10%). Missing data from the M.I.N.I. is reported in Table S4 and missing data for measures included in Portal assessments are reported in Table S5. Missing items for measures included in weekly Portal assessments are provided in Table S6.
3.4. Attrition
In total, 18/75 (24.0% [95%CI, 14.9–35.3]) of participants enrolled into the study dropped out of the study, bettering progression criteria (≤30%). In total, 17/72 (23.6% [95%CI, 14.4–35.1]) of participants gaining access to EJDeR, dropped out of EJDeR, bettering progression criteria (≤30%).
3.5. Resources needed to complete the study and the intervention
Length of time for participants to work through EJDeR and complete assessments at each time‐point are provided in Table S7. The number of reminder contacts needed during recruitment and for participants to complete Portal assessments are reported in Table S8. The number of contacts needed to arrange interviews at each time‐point are reported in Table S9.
Seventy‐two participants gained access to EJDeR and 71 were allocated to an e‐therapist (one dropped out before allocation). Psychology program students (n = 10) supported 27 participants (mean = 2.7, range, 1–7) and spent a mean of 76.9 h (SD 29.7, range, 22.3–109.8) delivering EJDeR, attending training, supervision, and administration, equating to a mean of 2.9 h per participant each week (SD 1.3, range, 0.9–4.6). Due to students not having adequate time to support participant caseloads, the majority were supported by a CBT‐therapist internal to the research team (n = 32), a licensed psychologist in the research team (n = 5), and a licensed psychologist external to the research team (n = 7). The clinical supervisor worked for 155 h, including training, supervision, and administration.
Difficulties recruiting research personnel was identified as a challenge. 57 The research team included the principal investigator, a researcher, a PhD student/e‐therapist, a research assistant, and an e‐therapist/research assistant. External study personnel included licensed psychologists (n = 7) and e‐therapists (n = 10). Paper‐based CRFs for study data were considered time and resource intensive, as was coordinating external study personnel.
3.6. Participants' adherence to intervention
Seventy‐two participants gained access to EJDeR. One was excluded shortly after access (severe and enduring mental health difficulty) and 34/71 (47.9%) adhered to the MTD, nearly meeting progression criteria of 50%. The mean number of modules opened was 2.3 (SD 0.9, range, 1–4), parents completed a mean of 1.7 modules (SD 1.3, range, 0–4), and a mean of 2.7 homework sheets (SD 2.8, range, 0–11). Initial assessment sessions were attended by 61/71 (85.9%) and mid‐intervention booster sessions were attended by 44/71 (62.0%).
Visual inspection of data indicated differences in adherence rates by first LICBT module started and by gender. A post hoc descriptive analysis was performed. In total, 54/71 (76.1%) started a LICBT module, with 26 starting with BA and 28 with WM. In total, 20/26 (76.9%) starting with BA, and 14/28 (50.0%) starting with WM adhered to the MTD.
Of the 71 participants, 25 were fathers, and 46 were mothers. For fathers: 8/25 (32.0%) started with BA and 7/8 (87.5%) adhered to the MTD; 12/25 (48.0%) started with WM, and 5/12 (41.7%) adhered to the MTD. For mothers, 18/46 (39.1%) started working with BA and 13/18 (72.2%) adhered to the MTD; 16/46 (34.8%) started with WM and 9/16 (56.3%) adhered to the MTD.
3.7. Participants' use of the intervention
A mean of 20 participant logins were made (SD 14.9, range, 1–72). A mean of 8.5 participant written messages were sent to e‐therapists (SD 7.6, range, 0–33), and a mean of 28.8 e‐therapist written messages (SD 16.3, range, 0–74) were sent to participants.
3.8. E‐therapists' adherence to intervention
Adherence rates were 90.5% for initial assessment sessions, 85.2% for mid‐intervention booster sessions, and 87.5% for written communication between participants and e‐therapists.
3.9. Participants' acceptability of the intervention and data collection
Reasons for study dropout are reported in Figure 1. Nineteen risk assessments were conducted and two resulted in study exclusion. No participant reported substantial negative consequences related to study and/or intervention. A structured question asking participants whether the intervention was helpful was omitted by researcher error and it was therefore not possible to assess whether ≥70% of participants using the intervention reported it as helpful (Table 2).
3.10. Psychological and health economics outcomes
M.I.N.I. data at baseline, post‐treatment, and follow‐up are provided in Table S12. The mean and SD of outcomes at baseline, post‐treatment, and follow‐up, with 95% CIs, are reported in Table 5, alongside observed changes from baseline to post‐treatment and from baseline to follow‐up (with 95% CI). From baseline to follow‐up depressive symptoms decreased by an average of 3.1 PHQ‐9 points. From baseline to follow‐up anxiety symptoms decreased by an average of 2.9 GAD‐7 points. Descriptive data from the Treatment Inventory of Costs in Patients with psychiatric disorders (TIC‐P) are reported in Table S14. However, due to a large amount of missing data on the TIC‐P it is difficult to interpret this data in a meaningful way.
TABLE 5.
Treatment outcomes at baseline, post‐treatment, and follow‐up with change scores
| Outcome measures | Baseline | Post‐treatment | Follow‐up | Change from baseline to Post‐treatment | Change from baseline to follow‐up | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | M | SD | 95% CI a | n | M | SD | 95% CI a | n | M | SD | 95% CI a | n | M | SD | n | M | SD | |
| PCL‐5 | 72 | 16.0 | 12.2 | 10.5, 14.6 | 42 | 9.0 | 8.7 | 7.2, 11.1 | 40 | 5.2 | 4.8 | 3.9, 6.2 | 42 | −5.4 | 6.3 | 40 | −8.2 | 8.8 |
| PCL‐C | 72 | 31.5 | 10.9 | 9.4, 13.0 | 42 | 25.1 | 8.4 | 6.9, 10.7 | 40 | 22.0 | 4.7 | 3.9, 6.0 | 42 | −4.9 | 6.8 | 40 | −7.3 | 7.9 |
| PHQ‐9 | 72 | 6.6 | 5.0 | 4.3, 6.0 | 42 | 3.8 | 2.9 | 2.4, 3.7 | 40 | 2.8 | 2.1 | 1.7, 2.7 | 42 | −2.0 | 3.1 | 40 | −3.1 | 4.7 |
| GAD‐7 | 72 | 6.1 | 4.7 | 4.0, 5.6 | 42 | 3.4 | 2.9 | 2.4, 3.7 | 40 | 2.6 | 2.8 | 2.3, 5.6 | 42 | −2.5 | 3.9 | 40 | −2.9 | 4.1 |
| FRHC | 72 | 6.5 | 1.9 | 1.6, 2.3 | 42 | 5.5 | 1.5 | 1.2, 1.9 | 40 | 5.0 | 1.7 | 1.4, 2.2 | 42 | −0.8 | 1.5 | 40 | −1.1 | 1.5 |
| AAQ‐6 | 72 | 16.2 | 8.1 | 7.0, 9.7 | 41 | 12.8 | 6.8 | 5.6, 8.7 | 40 | 12.4 | 6.0 | 4.9, 7.7 | 41 | −2.0 | 4.4 | 40 | −2.4 | 5.1 |
| BADS | 72 | 96.1 | 25.9 | 22.3, 31.0 | 42 | 113.1 | 16.3 | 13.4, 20.8 | 40 | 118.8 | 15.2 | 12.5, 19.5 | 42 | 11.5 | 17.3 | 40 | 16.6 | 22.5 |
| FSS | 72 | 34.2 | 13.7 | 11.8, 16.4 | 42 | 28.0 | 10.9 | 9.0, 13.9 | 40 | 26.2 | 12.3 | 10.1, 15.8 | 42 | −3.8 | 7.9 | 40 | −7.0 | 13.6 |
| EQ‐5D | 72 | 8.1 | 2.3 | 2.0, 2.8 | 42 | 7.3 | 1.8 | 1.5, 2.3 | 40 | 6.6 | 1.5 | 1.2, 1.9 | 42 | −0.5 | 1.6 | 40 | −1.0 | 1.6 |
| EQ‐5D‐VAS | 72 | 66.0 | 17.2 | 14.8,20.6 | 42 | 74.1 | 11.2 | 9.2,14,3 | 40 | 77.4 | 11.7 | 9.6,15.0 | 42 | 6.5 | 11.6 | 40 | 10.4 | 12.3 |
| SCS‐SF | 72 | 37.8 | 5.1 | 4.4, 6.1 | 42 | 37.6 | 7.1 | 5.8, 9.1 | 40 | 37.5 | 6.3 | 5.2, 8.1 | 42 | −0.1 | 6.8 | 40 | −0.6 | 6.2 |
Abbreviations: AAQ‐6, Acceptance and Action Questionnaire; BADS, Behavioral Activation for Depression Scale; EQ‐5D, EuroQol 5‐dimension questionnaire; EQ‐5D VAS, EuroQol 5‐dimension questionnaire visual analogue scale; FSS, Fatigue Severity Scale; FRHC, Fear of recurrence and serious health condition (structured questions); GAD‐7, Generalized Anxiety Disorder 7‐item scale; PCL‐5, Post‐traumatic Stress Disorder Checklist for DSM‐5; PCL‐C, adapted version of Post‐traumatic Stress Disorder Checklist‐Civilian version; PHQ‐9, Patient Health Questionnaire; PTSS, Post‐traumatic stress symptoms; SCS‐SF, Self‐Compassion Scale‐Short Form.
95% CIs around SD.
4. DISCUSSION
The ENGAGE feasibility trial demonstrated it is possible to recruit and retain parents of children treated for cancer into a single‐arm feasibility trial of an internet administered, guided, LICBT based, self‐help intervention. In summary: (1) 12.0% of invited parents consented and 11.0% of invited parents were enrolled, exceeding progression criteria of ≥9%; (2) 24.0% dropped out of the study, and 23.6% dropped out of the intervention, bettering progression criteria of ≤30%; (3) missing items per questionnaire ranged from 0.01% to 3.9%, remaining under ≤10% for all measures, bettering progression criteria; (4) percentage of participants completing assessments ranged from 65.6% to 98.7%, bettering progression criteria of ≥70% for M.I.N.I interviews at all time‐points and Portal assessments at baseline, and marginally under progression criteria of ≥70% for Portal assessments at post‐treatment and follow‐up; (5) intervention adherence was 47.9%, marginally under progression criteria of ≥50%; and (6) no participant reported a substantial negative consequence related to the study and/or intervention, meeting progression criteria. This study was not designed to detect differences in parental depression or anxiety at follow‐up, however reductions in depressive and anxiety symptoms were observed via visual inspection.
4.1. Strengths and limitations
To the best of our knowledge, ENGAGE is first trial worldwide designed to test the feasibility of an internet administered, guided, LICBT based, self‐help intervention for parents of children treated for cancer. Robust methods examined a range of feasibility objectives, alongside a priori specified progression criteria. The intervention protocol is published in accordance with TIDieR guidelines 34 and reporting of methods and results are transparent and complete in accordance with calls for better reporting of feasibility studies. 58 A novel recruitment strategy was adopted with participants identified via the CCR, meaning invited participants are a nationally representative sample of parents of children treated for cancer. We also successfully adopted an opt‐out recruitment strategy and explored reasons for non‐participation, 41 which will inform recruitment strategies used in the future pilot RCT. Use of retention strategies, including telephone reminders 59 and use of a study newsletters 55 may also have minimized study drop out. Finally, involvement of the PRP group resulted in valuable feedback on intervention content and informed intervention changes, as well as improving study procedures, in line with previous research on the benefits of public involvement in research. 60 , 61
The study also has limitations. E‐therapists adherence to the intervention was examined by only one licensed clinical psychologist with adherence marked as absent/present. Future studies should develop an intervention adherence checking tool, examining both adherence and quality. 62 Participants' adherence to the intervention e.g., the MTD, was defined a priori by the research team and determined by engagement with and use of EJDeR (e.g., module completion). This definition fails to consider activities participants may have engaged in outside of the Portal. 63 Progression criteria were informed by previous experience and relevant literature. While partly informed by our previous longitudinal research with the population 6 other literature used to inform the progression criteria include a range of psychological interventions with unique methodological, procedural, and clinical uncertainties. Indeed, lack of clarity on how to set progression criteria has been identified as a challenge in the literature. 64 A 6‐month follow‐up time‐point was selected to examine the feasibility of longer‐term data collection. However, the study could have been strengthened by examining the feasibility of longer‐term follow‐up data collection e.g., 9–18 months post‐treatment. The majority of participants were female and may limit the generalizability of findings. Further, the majority of participants (78.7%) had an education level higher than upper secondary school, compared to 44% in the general Swedish population 65 potentially further limiting generalizability. Our sample size was informed by recommendations primarily used for pilot RCTs 54 and literature on informing sample sizes for single‐arm feasibility studies is lacking. 66 There is a possibility study objectives could have been investigated with fewer participants. However, we examined the feasibility and acceptability of an internet‐administered intervention, which could be considered technically complex (e.g., including a range of technical elements such as a tab‐based interview view, film, audio files, in‐module exercises, online homework exercises, written messages via the Portal, and video‐conferencing), with a number of intervention components (e.g., four intervention modules and e‐therapist guidance). Literature suggests feasibility studies of interventions that are technically complex and include a number of components, may require a larger sample size than interventions with minimal complexity. 66
4.2. Interpretation and implications for future research
While we successfully recruited our target sample size with an enrolment rate of 11.0%, confidence intervals ranged from 8.4% to 14.1% and in a future pilot RCT we will continue to identify participants via additional sources such as cancer organizations and interest groups. Further, we targeted parents of children treated for cancer with a self‐reported need for psychological support. Lack of recognition of one's psychological difficulties and lack of acknowledgement for the need of support, are commonly identified barriers to seeking help. 67 Consequently, we may have failed to reach parents experiencing psychological difficulties who do not recognize or acknowledge a need for psychological support. Future research may look to identify methods to widen participation in the population and overcome potential barriers to help‐seeking, such as improving mental health literacy. 67 However, it is important to note that of the 509 parents invited via the CCR, only 20% (n = 101) may be anticipated to experience at least mild symptoms of depression and/or anxiety. 6 In depression trials utilizing recruitment strategies where study invitation letters are sent to patients identified via medical records with experience of depression, a recruitment rate of 12% may be anticipated. 48 , 49 , 51 Given study invitations were sent to all parents identified via the CCR, rather than to parents with a known history of depression and/or anxiety, our enrolment rate of 11.0% may be considered as high. Despite overall recruitment success, we will strive for further improvements in the future pilot RCT, for example the use of personalized study invitation letters which resulted in improvement in recruitment rates, however small, in our embedded recruitment RCT 39 with results reported separately. 40 Future research may adopt similar strategies, including registry‐based recruitment 36 ; an opt‐out recruitment strategy, 41 and the use of personalized study invitation letters 40 to optimize recruitment.
Our study dropout rate of 24.0% bettered progression criteria of ≤30%. Confidence intervals ranged from 14.9% to 35.3% and we aim to minimize study dropout in the forthcoming future pilot RCT by continuing to use retention strategies, including telephone reminders 59 and study newsletters. 55 In addition, assessment completion rates varied, with higher completion rates for the M.I.N.I. at each time‐point (84.4% at post‐treatment and 81.4% at follow‐up), in comparison to Portal assessment completion (65.6% at post‐treatment and 67.8% at follow‐up). Completion rates of weekly Portal assessments, to inform the process evaluation, were particularly low (decreasing over time from 65.7% to 38.9%). Difficulties with assessment completion are common. 68 Less than satisfactory Portal assessment completion suggests in the future pilot RCT, we should minimize the number of online assessments used and seek to collect data over the telephone. For example, we will collect process evaluation data at three time‐points during the intervention over the telephone, rather than weekly via the Portal.
Our intervention adherence rate of 47.9% was slightly lower than progression criteria (≥50%) and there was no evidence of harm. Results suggest the intervention may be feasible and acceptable for the population and are in line with other research suggesting internet‐administered delivery mechanisms are acceptable to parents of children on cancer treatment 22 , 23 and parents of children previously treated for cancer. 25 , 26 Benefits of internet‐administered delivery may relate to flexibility of use and perceptions of privacy, 21 overcoming common barriers to accessing support in the population such as guilt and putting the needs of the child before parents' own needs. 14 , 15 However, results also suggest a need to adapt the intervention to improve feasibility and acceptability before progressing to the future pilot RCT. While adherence to BA was high, adherence to WM was poor, especially for fathers. Challenges regarding adherence to internet‐administered interventions are common 69 , 70 and uptake within routine healthcare settings, 71 including Sweden, 72 is poor. Intervention acceptability is further explored in the embedded process evaluation, reported elsewhere, and will be used to adapt the intervention. However, adherence rates indicate a need to improve the acceptability of the intervention and there may be a need to improve the gender‐sensitivity of EJDeR, especially the WM module for fathers.
Recruitment of experienced research personnel was challenging 57 delaying study set‐up. The use of paper‐based CRFs was time consuming and coordinating interviews with external personnel was resource intensive. The use of the TIC‐P (health economic outcome) was not feasible given the large amount of missing data. The Adult Service Use Schedule (AD‐SUS) developed from instruments used in similar trials 73 will be used in the future pilot RCT.
Psychology program student e‐therapists did not have time to support caseloads and more experienced licensed psychologists and a CBT‐therapist supported the majority of participants. Further, psychology program student e‐therapists spent a mean time of 2.9 h per participant, per week, which is more therapist time than reported in other studies on guided internet‐administered CBT interventions. 74 , 75 , 76 This finding may be explained by psychology program students only supporting a mean of 2.7 parents. Consequently, they may not have gained the opportunity to develop competence in using the support protocol and a clear understanding of the intervention structure and content, or how to use the Portal. Results indicate e‐therapist training and supervision should be improved (e.g. increase length of time for training, include role‐play, and revise training material) in future research to facilitate working with the intervention more efficiently. 77 Additionally, recruiting part‐time employed e‐therapists could facilitate increased caseloads, potentially leading to increased efficiency.
In summary, the following modifications to the study protocol and EJDeR are warranted before commencing a pilot RCT: (1) collection of outcome assessment data via telephone; (2) reducing the number of measures; (3) adaptation of the intervention to improve the feasibility and acceptability of EJDeR; (4) recruitment of a trial coordinator; (5) recruitment of part‐time employed e‐therapists to increase caseloads and decrease time spent on each participant; (6) use of electronic CRFs to facilitate data collection and entry; and (7) training of research team members to collect research data over the telephone.
5. CONCLUSIONS
Using robust methods, including a priori specified progression criteria, the use of novel recruitment strategies 34 , 40 , 41 and evidence‐based retention stragies, 55 , 59 our findings indicate methods, study procedures, and the intervention are feasible and acceptable and progression to a pilot RCT to prepare for the design and conduct of a future superiority RCT is warranted. The EJDeR intervention represents a promising and novel solution, delivered with minimal therapist guidance to meet parents' current unmet need for psychological support.
AUTHOR CONTRIBUTIONS
Ella Thiblin: Data curation (equal); formal analysis (equal); investigation (equal); validation (equal); visualization (equal); writing – original draft (equal). Joanne Woodford: Formal analysis (equal); methodology (supporting); project administration (supporting); supervision (supporting); writing – original draft (equal). Christina Reuther: Data curation (equal); formal analysis (equal); investigation (equal); validation (equal); visualization (equal); writing – review and editing (supporting). Johan Lundgren: Supervision (supporting); writing – review and editing (supporting). Nina Lutvica: Data curation (supporting); investigation (equal); validation (supporting); writing – review and editing (supporting). Louise von Essen: Conceptualization (lead); funding acquisition (lead); methodology (lead); project administration (lead); resources (lead); supervision (lead); writing – review and editing (lead).
FUNDING INFORMATION
This work is supported by the Swedish Research Council (grant number 521‐2014‐3337/E0333701, 2018‐02578, and 2021‐00868), the Swedish Cancer Society (grant number 15 0673 and 17 0709), the Swedish Childhood Cancer Foundation (grant number PR2017‐0005), and funding via the Swedish Research Council to U‐CARE, a Strategic Research environment (Dnr 2009‐1093). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICTS OF INTEREST
Declaration of interest: none.
ETHICS APPROVAL STATEMENT
The ENGAGE feasibility trial was approved by the Regional Ethical Review Board in Uppsala, Sweden (Dnr: 2017/527) and was conducted in accordance with the Helsinki Declaration, ensuring the welfare and rights of all participants, and Good Clinical Practice (GCP) guidelines. Ethical amendment was obtained from Swedish Ethical Review Authority August 07, 2019, ref: 2019‐03083.
PATIENT CONSENT STATEMENT
Not applicable.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
TRIAL REGISTRATION
ISRCTN 57233429.
Supporting information
Data S1
ACKNOWLEDGMENTS
We wish to thank Ian Horne (Portal team member) for performing all data extractions on the Portal. We thank data coordinator Agnes von Essen for assistance with data entry, data processing, and data organization. We also thank the e‐therapists and clinical psychologists who facilitated intervention delivery and data collection. The authors are also thankful to Professor Paul Farrand for sharing his expertise and time to work with the research team to develop the EJDeR intervention. Finally, we are especially grateful to the four members of our Parent Research Partner Group.
Thiblin E, Woodford J, Reuther C, Lundgren J, Lutvica N, von Essen L. Internet‐administered, low‐intensity cognitive behavioral therapy for parents of children treated for cancer: A feasibility trial (ENGAGE). Cancer Med. 2023;12:6225‐6243. doi: 10.1002/cam4.5377
Ella Thiblin and Joanne Woodford joint first authorship.
DATA AVAILABILITY STATEMENT
Data supporting feasibility objectives pertaining to recruitment and eligibility, data collection, attrition, and resources needed to complete the study and intervention are available in Zenodo at https://doi.org/10.5281/zenodo.6325611. Data stored in Zenodo supports Figure 1, Table 3 and Tables S2, S3, S7–S9. Access to the data stored in Zenodo is available upon written request from the corresponding author. Due to the nature of this research, participants of this study did not agree for their clinical data to be shared publicly, so supporting clinical data is not available and further ethical approval would be needed in order to share this data.
REFERENCES
- 1. Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Girardi F, Atun R. Global childhood cancer survival estimates and priority‐setting: a simulation‐based analysis. Lancet Oncol. 2019;20(7):972‐983. doi: 10.1016/S1470-2045(19)30273-6 [DOI] [PubMed] [Google Scholar]
- 2. Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999–2007: results of EUROCARE‐5–a population‐based study. Lancet Oncol. 2014;15(1):35‐47. doi: 10.1016/S1470-2045(13)70548-5 [DOI] [PubMed] [Google Scholar]
- 3. Vetsch J, Rueegg CS, Mader L, et al. Follow‐up care of young childhood cancer survivors: attendance and parental involvement. Support Care Cancer. 2016;24(7):3127‐3138. doi: 10.1007/s00520-016-3121-6 [DOI] [PubMed] [Google Scholar]
- 4. Conway Keller M, King C, Hart L, et al. The end of cancer treatment experience for children, adolescents, and their parents: a systematic review of the literature. J Psychosoc Oncol. 2020;38(5):573‐591. doi: 10.1080/07347332.2020.1769795 [DOI] [PubMed] [Google Scholar]
- 5. Wakefield CE, McLoone JK, Butow P, Lenthen K, Cohn RJ. Parental adjustment to the completion of their child's cancer treatment: parental adjustment to treatment completion. Pediatr Blood Cancer. 2011;56(4):524‐531. doi: 10.1002/pbc.22725 [DOI] [PubMed] [Google Scholar]
- 6. Wikman A, Mattsson E, von Essen L, Hovén E. Prevalence and predictors of symptoms of anxiety and depression, and comorbid symptoms of distress in parents of childhood cancer survivors and bereaved parents five years after end of treatment or a child's death. Acta Oncol. 2018;57(7):950‐957. doi: 10.1080/0284186X.2018.1445286 [DOI] [PubMed] [Google Scholar]
- 7. Fardell JE, Wakefield CE, De Abreu LR, et al. Long‐term health‐related quality of life in young childhood cancer survivors and their parents. Pediatr Blood Cancer. 2021;68(12):e29398. doi: 10.1002/pbc.29398 [DOI] [PubMed] [Google Scholar]
- 8. van Warmerdam J, Zabih V, Kurdyak P, Sutradhar R, Nathan PC, Gupta S. Prevalence of anxiety, depression, and posttraumatic stress disorder in parents of children with cancer: a meta‐analysis. Pediatr Blood Cancer. 2019;66(6):e27677. doi: 10.1002/pbc.27677 [DOI] [PubMed] [Google Scholar]
- 9. Ljungman L, Cernvall M, Grönqvist H, Ljótsson B, Ljungman G, von Essen L. Long‐term positive and negative psychological late effects for parents of childhood cancer survivors: a systematic review. PLoS One. 2014;9(7):e103340. doi: 10.1371/journal.pone.0103340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ljungman L, Hovén E, Ljungman G, Cernvall M, von Essen L. Does time heal all wounds? A longitudinal study of the development of posttraumatic stress symptoms in parents of survivors of childhood cancer and bereaved parents. Psycho‐Oncol. 2015;24(12):1792‐1798. doi: 10.1002/pon.3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Öhman M, Woodford J, von Essen L. Socioeconomic consequences of parenting a child with cancer for fathers and mothers in Sweden: a population‐based difference‐in‐difference study. Int J Cancer. 2020;148(10):2535‐2541. doi: 10.1002/ijc.33444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hovén E, Grönqvist H, Pöder U, von Essen L, Lindahl NA. Impact of a child's cancer disease on parents' everyday life: a longitudinal study from Sweden. Acta Oncol. 2017;56(1):93‐100. doi: 10.1080/0284186X.2016.1250945 [DOI] [PubMed] [Google Scholar]
- 13. Kukkola L, Hovén E, Cernvall M, von Essen L, Grönqvist H. Perceptions of support among Swedish parents of children after end of successful cancer treatment: a prospective, longitudinal study. Acta Oncol. 2017;56(12):1705‐1711. doi: 10.1080/0284186X.2017.1374554 [DOI] [PubMed] [Google Scholar]
- 14. Hocking MC, Kazak AE, Schneider S, Barkman D, Barakat LP, Deatrick JA. Parent perspectives on family‐based psychosocial interventions in pediatric cancer: a mixed‐methods approach. Support Care Cancer. 2014;22(5):1287‐1294. doi: 10.1007/s00520-013-2083-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kearney JA, Salley CG, Muriel AC. Standards of psychosocial care for parents of children with cancer: standards of psychosocial care for parents of children with cancer. Pediatr Blood Cancer. 2015;62(S5):S632‐S683. doi: 10.1002/pbc.25761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel V, Saxena S, Lund C, et al. The lancet commission on global mental health and sustainable development. Lancet. 2018;392(10157):1553‐1598. doi: 10.1016/S0140-6736(18)31612-X [DOI] [PubMed] [Google Scholar]
- 17. Farrand PA, ed. Low‐Intensity CBT Skills and Interventions: A Practitioner's Manual. SAGE Publications Ltd; 2020. [Google Scholar]
- 18. Andersson G, Carlbring P. Internet‐assisted cognitive behavioral therapy. Psychiatr Clin North Am. 2017;40(4):689‐700. doi: 10.1016/j.psc.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 19. Karyotaki E, Efthimiou O, Miguel C, et al. Internet‐based cognitive behavioral therapy for depression: a systematic review and individual patient data network meta‐analysis. JAMA Psychiatry. 2021;78(4):361‐371. doi: 10.1001/jamapsychiatry.2020.4364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carlbring P, Andersson G, Cuijpers P, Riper H, Hedman‐Lagerlöf E. Internet‐based vs. face‐to‐face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta‐analysis. Cogn Behav Ther. 2018;47(1):1‐18. doi: 10.1080/16506073.2017.1401115 [DOI] [PubMed] [Google Scholar]
- 21. Knowles SE, Toms G, Sanders C, et al. Qualitative meta‐synthesis of user experience of computerised therapy for depression and anxiety. PLoS One. 2014;9(1):e84323. doi: 10.1371/journal.pone.0084323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cernvall M, Carlbring P, Ljungman L, Ljungman G, von Essen L. Internet‐based guided self‐help for parents of children on cancer treatment: a randomized controlled trial. Psycho‐Oncol. 2015;24(9):1152‐1158. doi: 10.1002/pon.3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cernvall M, Carlbring P, Wikman A, Ljungman L, Ljungman G, von Essen L. Twelve‐month follow‐up of a randomized controlled trial of internet‐based guided self‐help for parents of children on cancer treatment. J Med Internet Res. 2017;19(7):e273. doi: 10.2196/jmir.6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muscara F, McCarthy MC, Rayner M, et al. Effect of a videoconference‐based online group intervention for traumatic stress in parents of children with life‐threatening illness: a randomized clinical trial. JAMA Netw Open. 2020;3(7):e208507. doi: 10.1001/jamanetworkopen.2020.8507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wakefield CE, Sansom‐Daly UM, McGill BC, et al. Acceptability and feasibility of an e‐mental health intervention for parents of childhood cancer survivors: “Cascade”. Support Care Cancer. 2016;24(6):2685‐2694. doi: 10.1007/s00520-016-3077-6 [DOI] [PubMed] [Google Scholar]
- 26. Wakefield CE, Sansom‐Daly UM, McGill BC, et al. Providing psychological support to parents of childhood cancer survivors: “Cascade” intervention trial results and lessons for the future. Cancer. 2021;13(22):5597. doi: 10.3390/cancers13225597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;374:n2061. doi: 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carlsson T, Kukkola L, Ljungman L, Hovén E, von Essen L. Psychological distress in parents of children treated for cancer: an explorative study. PLoS One. 2019;14(6):e0218860. doi: 10.1371/journal.pone.0218860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ljungman L, Boger M, Ander M, et al. Impressions that last: particularly negative and positive experiences reported by parents five years after the end of a child's successful cancer treatment or death. PLoS One. 2016;11(6):e0157076. doi: 10.1371/journal.pone.0157076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ljungman L, Cernvall M, Ghaderi A, Ljungman G, von Essen L, Ljótsson B. An open trial of individualized face‐to‐face cognitive behavior therapy for psychological distress in parents of children after end of treatment for childhood cancer including a cognitive behavioral conceptualization. PeerJ. 2018;6:e4570. doi: 10.7717/peerj.4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wikman A, Kukkola L, Börjesson H, et al. Development of an internet‐administered cognitive behavior therapy program (ENGAGE) for parents of children previously treated for cancer: participatory action research approach. J Med Internet Res. 2018;20(4):e133. doi: 10.2196/jmir.9457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woodford J, Wikman A, Einhorn K, et al. Attitudes and preferences toward a hypothetical trial of an internet‐administered psychological intervention for parents of children treated for cancer: web‐based survey. JMIR Ment Health. 2018;5(4):e10085. doi: 10.2196/10085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woodford J, Farrand P, Hagström J, Hedenmalm L, von Essen L. Internet‐administered cognitive behavioral therapy for common mental health difficulties in parents of children treated for cancer: intervention development and description study. JMIR Form Res. 2021;5(7):e22709. doi: 10.2196/22709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eldridge SM, Lancaster GA, Campbell MJ, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS One. 2016;11(3):e0150205. doi: 10.1371/journal.pone.0150205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woodford J, Wikman A, Cernvall M, et al. Study protocol for a feasibility study of an internet‐administered, guided, CBT‐based, self‐help intervention (ENGAGE) for parents of children previously treated for cancer. BMJ Open. 2018;8(6):e023708. doi: 10.1136/bmjopen-2018-023708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. doi: 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini‐international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry. 1998;59(Suppl 20):22‐57. [PubMed] [Google Scholar]
- 39. Woodford J, Norbäck K, Hagström J, et al. Study within a trial (SWAT) protocol. Investigating the effect of personalised versus non‐personalised study invitations on recruitment: an embedded randomised controlled recruitment trial. Contemp Clin Trials Commun. 2020;18(100572):100572. doi: 10.1016/j.conctc.2020.100572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thiblin E, Woodford J, Öhman M, von Essen L. The effect of personalised versus non‐personalised study invitations on recruitment within the ENGAGE feasibility trial: an embedded randomised controlled recruitment trial. BMC Med Res Methodol. 2022;22(1):65. doi: 10.1186/s12874-022-01553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hagström J, Woodford J, von Essen A, Lähteenmäki P, von Essen L. Opt‐out rates and reasons for non‐participation in a single‐arm feasibility study of a guided internet‐administered CBT‐based intervention for parents of children treated for cancer: a nested cross‐sectional survey. BMJ Open. 2022;12(4):e056758. doi: 10.1136/bmjopen-2021-056758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 43. Farrand P, Woodford J, Small F, Mullan E. Behavioural activation self‐help to improve depression in people living with dementia: the PROMOTE treatment protocol. N Z J Psychol. 2017;46(2):51‐62. [Google Scholar]
- 44. Hadjistavropoulos HD, Schneider LH, Klassen K, Dear BF, Titov N. Development and evaluation of a scale assessing therapist fidelity to guidelines for delivering therapist‐assisted internet‐delivered cognitive behaviour therapy. Cogn Behav Ther. 2018;47(6):447‐461. doi: 10.1080/16506073.2018.1457079 [DOI] [PubMed] [Google Scholar]
- 45. Hadjistavropoulos HD, Gullickson KM, Schneider LH, Dear BF, Titov N. Development of the internet‐delivered cognitive behaviour therapy undesirable therapist behaviours scale (ICBT‐UTBS). Internet Interv. 2019;18:100255. doi: 10.1016/j.invent.2019.100255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mbuagbaw L, Kosa SD, Lawson DO, et al. The reporting of progression criteria in protocols of pilot trials designed to assess the feasibility of main trials is insufficient: a meta‐epidemiological study. Pilot Feasibility Stud. 2019;5(1):120. doi: 10.1186/s40814-019-0500-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Richards DA, Hill JJ, Gask L, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ. 2013;347:f4913. doi: 10.1136/bmj.f4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Richards DA, Ekers D, McMillan D, et al. Cost and outcome of behavioural activation versus cognitive behavioural therapy for depression (COBRA): a randomised, controlled, non‐inferiority trial. Lancet. 2016;388:871‐880. doi: 10.1016/S0140-6736(16)31140-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Richards D, Richardson T. Computer‐based psychological treatments for depression: a systematic review and meta‐analysis. Clin Psychol Rev. 2012;32(4):329‐342. doi: 10.1016/j.cpr.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 50. van Ballegooijen W, Cuijpers P, van Straten A, et al. Adherence to internet‐based and face‐to‐face cognitive behavioural therapy for depression: a meta‐analysis. PloS One. 2014;9(7):e100674. doi: 10.1371/journal.pone.0100674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sugg HVR, Richards DA, Frost J. Morita therapy for depression (Morita trial): a pilot randomised controlled trial. BMJ Open. 2018;8(8):e021605. doi: 10.1136/bmjopen-2018-021605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 53. Hayes AF, Krippendorff K. Answering the call for a standard reliability measure for coding data. Commun Methods Meas. 2007;1(1):77‐89. doi: 10.1080/19312450709336664 [DOI] [Google Scholar]
- 54. Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol. 2012;65(3):301‐308. doi: 10.1016/j.jclinepi.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 55. Mitchell N, Hewitt CE, Lenaghan E, et al. Prior notification of trial participants by newsletter increased response rates: a randomized controlled trial. J Clin Epidemiol. 2012;65(12):1348‐1352. doi: 10.1016/j.jclinepi.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 56. Thiblin E, Woodford J, Reuther C, et al. Dataset for manuscript internet‐administered, guided, low‐intensity cognitive behavioural therapy for depression and/or generalized anxiety in parents of children treated for cancer: a single arm feasibility trial (ENGAGE). Zenodo March. 2022. Accessed March 22, 2022;3. doi: 10.5281/zenodo.6325611 [DOI] [Google Scholar]
- 57. Woodford J, Karlsson M, Hagström J, et al. Conducting digital health care research: document analysis of challenges experienced during intervention development and feasibility study setup of an internet‐administered intervention for parents of children treated for cancer. JMIR Form Res. 2021;5(10):e26266. doi: 10.2196/26266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morgan B, Hejdenberg J, Kuleszewicz K, Armstrong D, Ziebland S. Are some feasibility studies more feasible than others? A review of the outcomes of feasibility studies on the ISRCTN registry. Pilot Feasibility Stud. 2021;7(1):195. doi: 10.1186/s40814-021-00931-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gillies K, Kearney A, Keenan C, et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev. 2021;3(4):MR000032. doi: 10.1002/14651858.MR000032.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brett J, Staniszewska S, Mockford C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. 2014;17(5):637‐650. doi: 10.1111/j.1369-7625.2012.00795.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tomlinson J, Medlinskiene K, Cheong VL, Khan S, Fylan B. Patient and public involvement in designing and conducting doctoral research: the whys and the hows. Res Involv Engagem. 2019;5:23. doi: 10.1186/s40900-019-0155-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Walton H, Spector A, Williamson M, Tombor I, Michie S. Developing quality fidelity and engagement measures for complex health interventions. Br J Health Psychol. 2020;25(1):39‐60. doi: 10.1111/bjhp.12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lenhard F, Mitsell K, Jolstedt M, et al. The internet intervention patient adherence scale for guided internet‐delivered behavioral interventions: development and psychometric evaluation. J Med Internet Res. 2019;21(10):e13602. doi: 10.2196/13602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Avery KNL, Williamson PR, Gamble C, et al. Informing efficient randomised controlled trials: exploration of challenges in developing progression criteria for internal pilot studies. BMJ Open. 2017;7:e013537. doi: 10.1136/bmjopen-2016-013537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Utbildningsnivån i Sverige . Statistiska Centralbyrån. Updated June 24, 2021. https://www.scb.se/hitta‐statistik/sverige‐i‐siffror/utbildning‐jobb‐och‐pengar/utbildningsnivan‐i‐sverige/. Accessed March 16, 2022.
- 66. Beets MW, von Klinggraeff L, Weaver RG, Armstrong B, Burkart S. Small studies, big decisions: the role of pilot/feasibility studies in incremental science and premature scale‐up of behavioral interventions. Pilot Feasibility Stud. 2021;7(1):173. doi: 10.1186/s40814-021-00909-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Waumans R, Muntingh A, Draisma S, Huijbregts K, van Balkom A, Batelaan N. Barriers and facilitators for treatment‐seeking in adults with a depressive or anxiety disorder in a Western‐European health care setting: a qualitative study. BMC Psychiatry. 2022;22(1):165. doi: 10.1186/s12888-022-03806-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Walters SJ, dos Anjos B, Henriques‐Cadby I, Bortolami O, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open. 2017;7(3):e015276. doi: 10.1136/bmjopen-2016-015276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bendig E, Bauereiss N, Schmitt A, Albus P, Baumeister H. ACTonDiabetes‐a guided psychological internet intervention based on acceptance and commitment therapy (ACT) for adults living with type 1 or 2 diabetes: results of a randomised controlled feasibility trial. BMJ Open. 2021;11(7):e049238. doi: 10.1136/bmjopen-2021-049238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Melville KM, Casey LM, Kavanagh DJ. Dropout from internet‐based treatment for psychological disorders. Br J Clin Psychol. 2010;49(Pt 4):455‐471. doi: 10.1348/014466509X472138 [DOI] [PubMed] [Google Scholar]
- 71. Fletcher S, Clarke J, Sanatkar S, et al. Recruiting to a randomized controlled trial of a web‐based program for people with type 2 diabetes and depression: lessons learned at the intersection of e‐mental health and primary care. J Med Internet Res. 2019;21(5):e12793. doi: 10.2196/12793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brantnell A, Woodford J, Baraldi E, van Achterberg T, von Essen L. Views of implementers and nonimplementers of internet‐administered cognitive behavioral therapy for depression and anxiety: survey of primary care decision makers in Sweden. J Med Internet Res. 2020;22(8):e1803. doi: 10.2196/18033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Byford S, Leese M, Knapp M, et al. Comparison of alternative methods of collection of service use data for the economic evaluation of health care interventions. Health Econ. 2007;5:531‐536. doi: 10.1002/hec.1175 [DOI] [PubMed] [Google Scholar]
- 74. Thase ME, McCrone P, Barrett MS, et al. Improving cost‐effectiveness and access to cognitive behavior therapy for depression: providing remote‐ready, computer‐assisted psychotherapy in times of crisis and beyond. Psychother Psychosom. 2020;89(5):307‐313. doi: 10.1159/000508143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wright JH, Owen J, Eells TD, et al. Effect of computer‐assisted cognitive behavior therapy vs usual care on depression among adults in primary care: a randomized clinical trial. JAMA Netw Open. 2022;5(2):e2146716. doi: 10.1001/jamanetworkopen.2021.46716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Clark DM, Wild J, Warnock‐Parkes E, et al. More than doubling the clinical benefit of each hour of therapist time: a randomised controlled trial of internet cognitive therapy for social anxiety disorder [published online ahead of print, 2022 Jul 15]. Psychol Med. 2022;1‐11. doi: 10.1017/S0033291722002008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thew GR. IAPT and the internet: the current and future role of therapist‐guided internet interventions within routine care settings. Cogn Behav Ther. 2020;13:e4. doi: 10.1017/S1754470X20000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
Data supporting feasibility objectives pertaining to recruitment and eligibility, data collection, attrition, and resources needed to complete the study and intervention are available in Zenodo at https://doi.org/10.5281/zenodo.6325611. Data stored in Zenodo supports Figure 1, Table 3 and Tables S2, S3, S7–S9. Access to the data stored in Zenodo is available upon written request from the corresponding author. Due to the nature of this research, participants of this study did not agree for their clinical data to be shared publicly, so supporting clinical data is not available and further ethical approval would be needed in order to share this data.


