Abstract
Background
The risk of ischemic heart disease (IHD) due to the impact of gonadotropin‐releasing hormone (GnRH) agonists among female patients with breast cancer remains a controversy.
Methods
Information from the Registry for Catastrophic Illness, the National Health Insurance Research Database (NHIRD), and the Death Registry Database in Taiwan were analyzed. Female patients with breast cancer were selected from the Registry for Catastrophic Illness from January 1, 2000, to December 31, 2018. All the breast cancer patients were followed until new‐onset IHD diagnosis, death, or December 31, 2018. A Kaplan–Meier survival curve was drawn to show the difference between patients treated with and without GnRH agonists. The Cox regression analysis was used to investigate the effects of GnRH agonists and the incidence of IHD.
Results
A total of 172,850 female patients with breast cancer were recognized with a mean age of 52.6 years. Among them, 6071(3.5%) had received GnRH agonist therapy. Kaplan–Meier survival curves showed a significant difference between patients with and without GnRH therapy (log‐rank p < 0.0001). Patients who received GnRH therapy had a significantly decreased risk of developing IHD than those without GnRH therapy (HR = 0.18; 95% CI = 0.14–0.23). After adjusting for age, treatment, and comorbidity, patients who received GnRH therapy still had a significantly lower risk of developing IHD (AHR = 0.5, 95% CI = 0.39–0.64).
Conclusion
The study showed that the use of GnRH agonists for breast cancer treatment was significantly associated with a reduced risk of IHD. Further research is required to investigate the possible protective effect of GnRH on IHD.
Keywords: breast neoplasms, cardiovascular diseases, gonadotropin‐releasing hormone, heart disease risk factors, myocardial ischemia
For female patients with breast cancer, the use of GnRH agonists was significantly associated with a reduced risk of IHD. After adjusting for age, treatment, and comorbidity, patients who received GnRH therapy had a significantly lower risk of developing IHD (AHR=0.5, 95% CI=0.39‐0.64). GnRH agonists were significantly associated with a lower risk of incident IHD in all the subgroups, except in those with CKD or COPD, respectively.
1. INTRODUCTION
Breast cancer is the most common cancer among females worldwide, accounting for 25.4% of total women's cancer, with more than two million newly diagnosed cases. 1 In Asia, female patients with breast cancer were younger compared with patients from Western countries. Luminal histology subtypes were also more predominate among patients in Western countries. 2 For patients with premenopausal or perimenopause endocrine positive breast cancer, gonadotropin‐releasing hormone (GnRH) agonists are increasingly administered in combination with tamoxifen 3 or cyclin‐dependent kinase 4/6 inhibitor 4 , 5 in the adjuvant or metastatic settings. GnRH agonists inhibit the pituitary GnRH receptors and suppress the downstream effects of follicle‐stimulating hormone (FSH) and luteinizing hormone (LH), resulting in decreased estrogen production in premenopausal ovaries. 6
Previous studies have shown diverse results regarding the effects of GnRH agonists on the cardiovascular system for hormone‐dependent cancer management. A previous animal study showed that GnRH agonists may be associated with atherosclerotic effects. 7 Several observational studies showed that GnRH agonists were related to increased cardiovascular disease risk in patients with prostate cancer. 8 , 9 , 10 However, a meta‐analysis of randomized trials reported no significant associations between GnRH agonists and the risk of cardiovascular disease. 11 Most evidence suggesting an association between GnRH agonists and cardiovascular disease for male patients with prostate cancer came from population‐based studies. 8 , 9 , 12 , 13 Several meta‐analyses of observational studies disclosed that GnRH agonists were related to an increased incidence of non‐fatal cardiovascular disease. 14 , 15 Whether or not GnRH agonists are associated with an excess risk of cardiovascular morbidity remains a highly controversial question. 11
To the best of our knowledge, limited literature addressing the associations between GnRH agonists and the risk of cardiovascular disease in patients with breast cancer is available. Therefore, this study intended to determine the relationship between GnRH agonists and the risk of IHD in female breast cancers.
2. METHODS
2.1. Data source
Data from the Registry for Catastrophic Illness, the National Health Insurance Research Database (NHIRD), and the Death Registry Database in Taiwan were analyzed. The NHIRD contains healthcare data of more than 99% of the population in Taiwan, including both inpatient and outpatient medical records. 16 , 17 The NHIRD contained patient information such as diagnosis, drug administration, and examinations. The Institutional Review Board of TCH certified this research (no. TCHIRB‐10709107‐W).
2.2. Study subjects
Female subjects 18 years and older with a diagnosis of breast cancer between January 1, 2000, and December 31, 2018, were identified from the Registry for Catastrophic Illness (ICD‐9‐CM and ICD‐10‐CM code for female breast cancer: 174 and C50.x1x, respectively). All the cancer diagnoses recorded in the Registry of Catastrophic Illness were confirmed by pathologists. 18 The Death Registry Database in Taiwan confirmed cases of death. Study subjects were followed until new‐onset IHD diagnosis, death, or December 31, 2018.
2.3. Outcome variables
The incidence of IHD was recognized from the NHIRD. It was defined as the occurrence of more than once in inpatient medical records or more than three times in outpatient medical records (ICD‐9‐CM code, 411–414 except 414.1x and ICD‐10‐CM code I20‐I25 except for I21, I25.3, and I25.4). 19
2.4. Main explanatory variable
Information regarding GnRH agonist prescriptions were gathered from the NHIRD. The total administered daily dose of GnRH agonists was calculated and expressed as the defined daily dose (DDD); 0.134 mg for leuprorelin and triptorelin, and 0.129 mg for goserelin, which was suggested by the Anatomical Therapeutic Chemical Classification/Defined Daily Doses (ATC/DDD) system. 20
2.5. Potential confounders
The potential confounders were age, socioeconomic status, breast cancer therapy, including lumpectomy and radiotherapy, and comorbidities. The socioeconomic status included income level and residence. Income level was categorized as low, intermediate, and high (≤19,200; 19,201 to <40,000; ≥40,000 New Taiwan Dollars [NTD]). Residence was categorized as urban, suburban, and rural. The comorbidities were recognized by the presence of disease diagnosis recorded by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) and ICD‐10‐CM code, including diabetes (ICD‐9‐CM:250, ICD‐10‐CM: E08‐E13), chronic kidney disease (ICD‐9‐CM: 585–586, ICD‐10‐CM: N18), hypertension (ICD‐9‐CM: 401–405, ICD‐10‐CM: I1), dyslipidemia (ICD‐9‐CM: 272.0–272.4, ICD‐10‐CM: E78.0‐E78.5), cerebrovascular disease (ICD‐9‐CM: 430–437, ICD‐10‐CM: G46.3‐G46.4, I60‐I66, I69), chronic obstructive pulmonary disease (ICD‐9‐CM: 491–492, 518.1–518.2, 770.2; ICD‐10‐CM: J41‐J44), and liver cirrhosis (ICD‐9‐CM: 491–492, 518.1–518.2, 770.2; ICD‐10‐CM: J41‐J44). Comorbidities were recognized only if the condition occurred more than once in an inpatient setting or more than three times in outpatient medical records. 21
3. STATISTICAL ANALYSIS
First, the demographic data of the study subjects were shown as continuous data with mean and standard deviation (SD) or categorical data with numbers and percentages. Patients with and without GnRH agonist treatment were compared using the two‐sample t‐test and Pearson χ 2 test. The incidence of IHD was calculated using events per 1000 person‐years. Kaplan–Meier survival curves were drawn to show the difference between patients treated with and without GnRH agonists. The Cox regression analysis was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Dose–response relations were also evaluated between GnRH agonist (as a continuous variable) and incident IHD. Death events were analyzed as competing risk events. 22 Stratified analyses were performed according to age and comorbidities in case interaction may exist. Sensitivity analysis was performed by excluding missing data of the stage of breast cancer and including cancer stage in multivariable Cox regression analysis. The data analyses were conducted using the SAS 9.4 software package (SAS Institute).
4. RESULTS
A total of 196,539 female patients with breast cancer were recognized from the Registry for Catastrophic Illness between January 1, 2000, and December 31, 2018. After excluding those with antecedent IHD (n = 22,687), younger than 18 years old (n = 15), and those with incomplete data (n = 987), there were 172,850 patients included in the analysis. Table 1 shows the baseline features of participants. The overall mean (SD) age was 52.6 (11.5) years, and 3.5% of the subjects received treatment with GnRH agonist. The mean (SD) of the DDDs for GnRH agonists was 41.5 (6.4) among patients receiving hormone treatment. Moreover, the mean (SD) follow‐up times were 4.98 (3.80) years in patients receiving GnRH agonists and 7.19 (5.63) years in those not receiving GnRH agonists. Compared with patients not receiving GnRH agonists, those receiving GnRH agonists were younger and more likely to receive lumpectomy and radiotherapy. Moreover, patients receiving GnRH agonists had a lower proportion of comorbidities. Patients received treatment without GnRH agonists were more likely to live in rural areas and have lower incomes.
TABLE 1.
Characteristics of female patients with breast cancer using GnRH agonists
| Characteristics | Total, n = 172,850 No. (%) of subjects | Treatment with GnRH agonists, n = 6017 | Treatment without GnRH agonists, n = 166,833 | p‐Value |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 52.56 ± 11.47 | 41.45 ± 6.42 | 52.96 ± 11.41 | <0.001 |
| 18–49 | 75,146 (43.47) | 5502 (91.44) | 69,644 (41.74) | <0.001 |
| ≥50 | 97,704 (56.53) | 515 (8.56) | 97,189 (58.26) | |
| Income level | ||||
| Low | 18,497 (10.70) | 316 (5.25) | 18,181 (10.90) | <0.001 |
| Intermediate | 65,788 (38.06) | 2348 (39.02) | 63,440 (38.03) | |
| High | 88,565 (51.24) | 3353 (55.73) | 85,212 (51.08) | |
| Urbanization | ||||
| Rural | 8942 (5.17) | 247 (4.11) | 8695 (5.21) | <0.001 |
| Suburban | 100,028 (57.87) | 3515 (58.42) | 96,513 (57.85) | |
| Urban | 63,880 (36.96) | 2255 (37.48) | 61,625 (36.94) | |
| Lumpectomy | ||||
| No | 46,731 (27.04) | 1243 (20.66) | 45,488 (27.27) | <0.001 |
| Yes | 126,119 (72.96) | 4774 (79.34) | 121,345 (72.73) | |
| Radiotherapy | ||||
| No | 151,702 (87.77) | 4718 (78.41) | 146,984 (88.10) | <0.001 |
| Yes | 21,148 (12.23) | 1299 (21.59) | 19,849 (11.90) | |
| Comorbidity | ||||
| Diabetes | 37,657 (21.79) | 460 (7.65) | 37,197 (22.30) | <0.001 |
| Chronic kidney disease | 7209 (4.17) | 71 (1.18) | 7138 (4.28) | <0.001 |
| Hypertension | 62,597 (36.21) | 710 (11.80) | 61,887 (37.10) | <0.001 |
| Dyslipidemia | 57,083 (33.02) | 759 (12.61) | 56,324 (33.76) | <0.001 |
| Cerebrovascular disease | 14,812 (8.57) | 115 (1.91) | 14,697 (8.81) | <0.001 |
| Chronic obstructive pulmonary disease | 17,260 (9.99) | 293 (4.87) | 16,967 (10.17) | <0.001 |
| Liver cirrhosis | 34,802 (20.13) | 746 (12.40) | 34,056 (20.41) | <0.001 |
| Outcomes | ||||
| New‐onset of ischemic heart disease | 12,605 (7.29) | 63 (1.05) | 12,542 (7.52) | <0.001 |
| Incidence of ischemic heart disease* | 10.24 | 2.10 | 10.46 | <0.001 |
| Follow‐up years, mean ± SD | 7.12 ± 5.59 | 4.98 ± 3.80 | 7.19 ± 5.63 | <0.001 |
Abbreviations: GnRH, gonadotropin‐releasing hormone; SD, standard deviation.
Events per 1000 person‐years.
During the study follow‐up period, 12,605 female patients with breast cancer had a new‐onset of IHD, including 63 (1.05%) patients receiving GnRH agonists and 12,542 (7.52%) patients not receiving GnRH agonists. The incidence rate of IHD per 1000 person‐years was 2.10 in patients receiving GnRH agonists and 10.46 in those not receiving GnRH agonists (p < 0.001). In addition, the time to incident IHD was significantly longer in patients receiving GnRH agonists than in those not receiving GnRH agonists (p < 0.001, log‐rank test; Figure 1).
FIGURE 1.
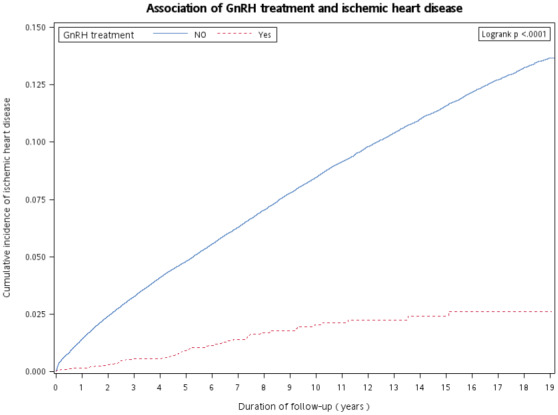
Kaplan–Meier curves for time to diagnosis of incident ischemic heart disease in patients receiving and not receiving GnRH agonists. GnRH, gonadotropin‐releasing hormone.
The univariable Cox proportional hazards model showed that female patients with breast cancer undergoing GnRH agonist therapy had a significantly decreased risk of incident IHD (HR: 0.18, 95% CI: 0.14–0.23). After adjusting for age, sex, and comorbidities, patients using GnRH agonist therapy still had a significantly lower risk of incident IHD (AHR: 0.50; 95% CI: 0.39–0.64) (Table 2). Patients with higher income levels had a lower risk of incident IHD. Other factors associated with decreased risk of incident IHD consisted of lumpectomy and radiotherapy. Moreover, risk factors of incident IHD consisted of age ≥ 50 years, diabetes, chronic kidney disease (CKD), hypertension, dyslipidemia, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), and liver cirrhosis. A significantly linear dose–response effect per DDD increase in GnRH agonists for incident IHD (AHR, 0.91; 95% CI <0.84–0.98; p = 0.011) was also noted. Figure 2 showed the results of stratified analysis. GnRH agonists were significantly associated with a lower risk of incident IHD in all the subgroups, except in those with CKD or COPD, respectively. Sensitivity analysis was performed after adjustment for the stage of breast cancer. Patients with missing data of stage were excluded from the analysis(n = 104,726). There were 68,124 participants included in multivariable Cox regression analysis. After adjusting for stage of breast cancer, the result showed that female patients with breast cancer undergoing GnRH agonist therapy had a significantly decreased risk of incident IHD (HR: 0.57, 95% CI: 0.38–0.84, p = 0.004) (Table S1).
TABLE 2.
Univariates and multivariate analyses for risk factors associated with ischemic heart disease among patients with breast cancer
| Characteristic | Number of patients | Incident IHD | Follow‐up person‐years | Incidence a | Univariate analysis HR (95% CI) | Multivariate analysis b AHR (95% CI) |
|---|---|---|---|---|---|---|
| Treatment with GnRH agonist | ||||||
| No | 166,833 | 12,542 | 1199529.27 | 10.46 | Ref | Ref |
| Yes | 6017 | 63 | 29964.66 | 2.10 | 0.18 (0.14–0.23)* | 0.50 (0.39–0.64)* |
| Age (years) | ||||||
| 18–49 | 75,146 | 2960 | 620705.96 | 4.77 | Ref | Ref |
| ≥50 | 97,704 | 9645 | 609672.96 | 15.82 | 2.96 (2.84–3.08)* | 1.43 (1.37–1.50)* |
| Income level | ||||||
| Low | 18,497 | 2012 | 107652.54 | 18.69 | Ref | Ref |
| Intermediate | 65,788 | 4598 | 453937.20 | 10.13 | 0.71 (0.67–0.75)* | 0.88 (0.83–0.92)* |
| High | 88,565 | 5995 | 668665.75 | 8.97 | 0.68 (0.65–0.72)* | 0.90 (0.85–0.94)* |
| Urbanization | ||||||
| Rural | 8942 | 846 | 61252.70 | 13.81 | Ref | Ref |
| Suburban | 100,028 | 7058 | 708198.24 | 9.97 | 0.76 (0.70–0.81)* | 0.87 (0.81–0.94)* |
| Urban | 63,880 | 4701 | 461213.60 | 10.19 | 0.79 (0.73–0.85)* | 0.93 (0.86–1.00)* |
| Lumpectomy | ||||||
| No | 46,731 | 5809 | 368240.28 | 15.78 | Ref | Ref |
| Yes | 126,119 | 6796 | 862653.96 | 7.88 | 0.53 (0.51–0.55)* | 0.61 (0.58–0.63)* |
| Radiotherapy | ||||||
| No | 151,702 | 11,685 | 1095288.44 | 10.67 | Ref | Ref |
| Yes | 21,148 | 920 | 134501.28 | 6.84 | 0.64 (0.60–0.68)* | 0.89 (0.84–0.96)* |
| Diabetes | ||||||
| No | 135,193 | 6796 | 939591.35 | 7.23 | Ref | Ref |
| Yes | 37,657 | 5809 | 289958.90 | 20.03 | 2.90 (2.80–3.00)* | 1.17 (1.12–1.21)* |
| Chronic kidney disease | ||||||
| No | 165,641 | 10,855 | 1176051.10 | 9.23 | Ref | Ref |
| Yes | 7209 | 1750 | 54355.86 | 32.20 | 3.53 (3.36–3.72)* | 1.55 (1.47–1.63)* |
| Hypertension | ||||||
| No | 110,253 | 2782 | 755233.05 | 3.68 | Ref | Ref |
| Yes | 62,597 | 9823 | 474485.26 | 20.70 | 5.91 (5.66–6.16)* | 3.19 (3.04–3.35)* |
| Dyslipidemia | ||||||
| No | 115,767 | 4557 | 765219.87 | 5.96 | Ref | Ref |
| Yes | 57,083 | 8048 | 464655.62 | 17.32 | 3.42 (3.30–3.55)* | 1.77 (1.70–1.85)* |
| Cerebrovascular disease | ||||||
| No | 158,038 | 9375 | 1118909.04 | 8.38 | Ref | Ref |
| Yes | 14,812 | 3230 | 110764.14 | 29.16 | 3.43 (3.30–3.57)* | 1.56 (1.49–1.63)* |
| Chronic obstructive pulmonary disease | ||||||
| No | 155,590 | 9759 | 1095353.60 | 8.91 | Ref | Ref |
| Yes | 17,260 | 2846 | 134628.00 | 21.14 | 2.47 (2.37–2.57)* | 1.57 (1.50–1.64)* |
| Liver cirrhosis | ||||||
| No | 13,8048 | 8534 | 949770.24 | 8.99 | Ref | Ref |
| Yes | 34,802 | 4071 | 280504.12 | 14.51 | 1.73 (1.67–1.80)* | 1.22 (1.17–1.26)* |
Abbreviations: AHR, adjusted hazard ratio; CI, confident interval; GnRH, gonadotropin‐releasing hormone; HR, hazard ratio; IHD, ischemic heart disease.
Events per 1000 person‐years.
Adjusted for: age, income level, urbanization, lumpectomy, radiotherapy, and comorbidities (diabetes, chronic kidney disease, hypertension, dyslipidemia, cerebrovascular disease, chronic obstructive pulmonary disease, and liver cirrhosis).
<0.001.
FIGURE 2.
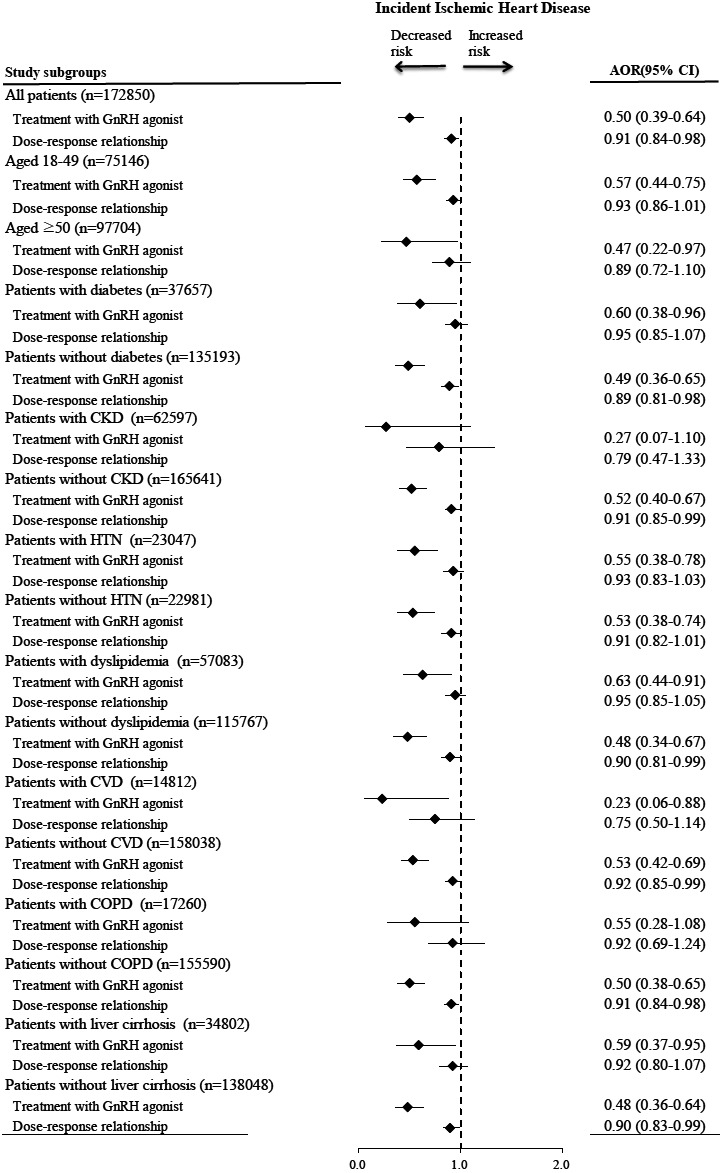
Stratified analysis for the associations of GnRH agonist with incident ischemic heart disease after adjusting for patient characteristics. Values greater than 1.0 indicate increased risk. AHR, adjusted hazard ratio; GnRH, gonadotropin‐releasing hormone.
5. DISCUSSION
This study found that female patients with breast cancer receiving GnRH agonists had a lower risk of developing IHD than patients not receiving GnRH agonists.
GnRH agonists bind to GnRH receptors in the pituitary gland, resulting in the secretion and initial surge of FSH and LH which stimulates the production of serum testosterone or estrogen. Subsequently, the negative feedback at the pituitary gland causes downregulation of GnRH receptors. On the contrary, no initial testosterone surge is found after administration of GnRH antagnosits. 14 The distinct impact of GnRH agonists in our study, and bilateral oophorectomy on IHD, might be partially explained by the fact that serum FSH and LH is sustainably inhibited after GnRH agonist administration but upregulated after bilateral oophorectomy. 23 Potential alternative mechanisms explaining the findings of our study were adipogenesis 24 and atherosclerosis. 25 Dysregulated fat deposits to the arterial wall cause atherosclerosis and IHD. 26 Peripheral blood mononuclear cells (PMN) and pro‐inflammatory T helper 1 lymphocytes both express GnRH receptors. The activation of these receptors is involved in the activation of PMNs, lymphocytes, and cytokine production, such as an increase in IFN‐γ, and decrease in IL‐4. 27 , 28 Different effects of GnRH‐I and GnRH‐II demonstrated that GnRH‐I enhanced proliferation of PMNs and IL‐2Rγ expression, while GnRH‐II attenuated proliferation of PMNs and IL‐2Rγ expression. 29
A large population study evaluating the side effects of bilateral oophorectomy‐induced menopause on premenopausal women before age 50 without hormone replacement therapy (HRT) demonstrated a statistically significant increased risk of multimorbidity including hyperlipidemia, and diabetes mellitus. The side effects of coronary artery disease became statistically significant only in adjusted analyses restricted to females receiving oophorectomy before the age of 45. 23 , 30 The deleterious effects of natural estrogen deprivation after menopause in the Study of Women's Health Across the Nation (SWAN) comprises of increased body and cardiovascular fat and alternations in body weight and waist circumference. 31 , 32 , 33 Association between lumpectomy and IHD risk was not yet investigated in previous studies. The procedure of lumpectomy may not be associated with pathogenesis of IHD. In this study, we tried to included detailed treatment procedure, including surgical procedure, radiotherapy, and medical treatment. The detailed surgical procedure was not available in our dataset. Further research is warranted to explore impact of lumpectomy on IHD risk. Previous studies had demonstrated that exposure of the heart to ionizing radiation during radiotherapy for breast cancer increases the subsequent rate of ischemic heart disease. 34 But the results of this study showed that radiotherapy appeared to be associated with lower risk of IHD. The detailed radiation therapy regimen including dose and area were not available in this dataset. Even radiotherapy for distal bone metastasis were included in analysis, which may lead to bias on IHD risks of radiotherapy.
This study enrolled a large number of patients with breast cancer and had a long follow duration from 2000 to 2018. The diagnoses of breast cancer were confirmed by pathology reports in the Registry for Catastrophic Illness, and the diagnoses of comorbidities were confirmed by medical reports to ensure the validity of this study. Additionally, socioeconomic status and treatment strategies were included as potential confounders. Our study has several limitations. First, similarly to other retrospective population studies, patients were not randomized to both treatment groups. Patients allocated to the GnRH treatment group had significantly higher income levels, urbanization, more lumpectomy, and radiotherapy. However, these patients were younger and had fewer comorbidities including diabetes mellitus and dyslipidemia. Nonetheless, multivariate analysis demonstrated treatment with GnRH agonists as an independent predictive factor associated with lower risk of IHD. The stratified analysis also showed that GnRH agonists were significantly associated with a lower risk of IHD in all subgroups of patients. Second, we used ICD codes to identify the diagnosis of IHD in the administrative database. Although patients with less frequent visits were less likely to be diagnosed with IHD, the frequency of visits ranged from once every month to every 3 months. Patients receiving GnRH agonists usually received treatment at a one‐month interval, which made the attribution of lower risk of IHD to lower frequency of visits less likely. The generalizability of this study to other regions requires further certification because most of the study subjects were Taiwanese.
Our study provides preliminary report for evaluating breast cancer treatment, considering the scarce literature currently available regarding the associations of GnRH agonists and the risk of IHD among women with breast cancer. In conclusion, our large population study is the first to report that treatment using GnRH agonists for patients with breast cancer was associated with a significantly reduced risk of IHD after adjusting for variable confounders. Furthermore, endocrine therapy for breast cancer treatment should weigh the benefits of disease‐specific survival against long‐term side effects of cardiovascular events. Patients receiving endocrine therapy should try to avoid risk factors of cardiovascular disease. Further research to delineate and confirm the causality and mechanisms is needed.
AUTHOR CONTRIBUTIONS
Yi‐Sheng Chou: Conceptualization (lead); writing – original draft (lead). Chun‐Chieh Wang: Investigation (equal). Li‐Fei Hsu: Data curation (equal); investigation (equal). Pei‐Hung Chuang: Data curation (equal); formal analysis (equal). Chi‐Feng Cheng: Investigation (equal). Nai‐Hsin Li: Conceptualization (equal); investigation (equal). Chu‐Chieh Chen: Supervision (equal); visualization (equal). Chien‐Liang Chen: Conceptualization (equal); supervision (equal). Yun‐Ju Lai: Funding acquisition (equal); investigation (equal); resources (equal); visualization (equal); writing – review and editing (equal). Yung‐Feng Yen: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal).
FUNDING INFORMATION
This research is supported by Taichung Veterans General Hospital of Puli branch, Grant Number: PL‐2021002.
CONFLICT OF INTEREST
The authors declare no competing interests.
Supporting information
Table S1
Chou Y‐S, Wang C‐C, Hsu L‐F, et al. Gonadotropin‐releasing hormone agonist treatment and ischemic heart disease among female patients with breast cancer: A cohort study. Cancer Med. 2023;12:5536‐5544. doi: 10.1002/cam4.5390
Contributor Information
Yun‐Ju Lai, Email: lailai841081@yahoo.com.tw.
Yung‐Feng Yen, Email: yfyen1@gmail.com.
DATA AVAILABILITY STATEMENT
The datasets of the current study are available from the Health and Welfare Data Science Center of Ministry of Health and Welfare in Taiwan.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Lin CH, Chuang PY, Chiang CJ, et al. Distinct clinicopathological features and prognosis of emerging young‐female breast cancer in an east Asian country: a nationwide cancer registry‐based study. Oncologist. 2014;19:583‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jonat W, Kaufmann M, Sauerbrei W, et al. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node‐positive breast cancer: the Zoladex early breast cancer research association study. J Clin Oncol. 2002;20:4628‐4635. [DOI] [PubMed] [Google Scholar]
- 4. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone‐receptor‐positive, advanced breast cancer (MONALEESA‐7): a randomised phase 3 trial. Lancet Oncol. 2018;19:904‐915. [DOI] [PubMed] [Google Scholar]
- 5. Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307‐316. [DOI] [PubMed] [Google Scholar]
- 6. Robertson JF, Blamey RW. The use of gonadotrophin‐releasing hormone (GnRH) agonists in early and advanced breast cancer in pre‐ and perimenopausal women. Eur J Cancer. 2003;39:861‐869. [DOI] [PubMed] [Google Scholar]
- 7. Hopmans SN, Duivenvoorden WC, Werstuck GH, Klotz L, Pinthus JH. GnRH antagonist associates with less adiposity and reduced characteristics of metabolic syndrome and atherosclerosis compared with orchiectomy and GnRH agonist in a preclinical mouse model. Urol Oncol. 2014;32:1126‐1134. [DOI] [PubMed] [Google Scholar]
- 8. Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448‐4456. [DOI] [PubMed] [Google Scholar]
- 9. Van Hemelrijck M, Garmo H, Holmberg L, et al. Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the population‐based PCBaSe Sweden. J Clin Oncol. 2010;28:3448‐3456. [DOI] [PubMed] [Google Scholar]
- 10. O'Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen‐deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33:1243‐1251. [DOI] [PubMed] [Google Scholar]
- 11. Nguyen PL, Je Y, Schutz FA, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta‐analysis of randomized trials. Jama. 2011;306:2359‐2366. [DOI] [PubMed] [Google Scholar]
- 12. Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493‐1500. [DOI] [PubMed] [Google Scholar]
- 13. Keating NL, O'Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu JR, Duncan MS, Morgans AK, et al. Cardiovascular effects of androgen deprivation therapy in prostate cancer: contemporary meta‐analyses. Arterioscler Thromb Vasc Biol. 2020;40:e55‐e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saylor PJ, Keating NL, Freedland SJ, Smith MR. Gonadotropin‐releasing hormone agonists and the risks of type 2 diabetes and cardiovascular disease in men with prostate cancer. Drugs. 2011;71:255‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906‐1914. [DOI] [PubMed] [Google Scholar]
- 17. Cheng TM. Taiwan's new national health insurance program: genesis and experience so far. Health Aff (Millwood). 2003;22:61‐76. [DOI] [PubMed] [Google Scholar]
- 18. Su VY, Yen YF, Pan SW, et al. Latent tuberculosis infection and the risk of subsequent cancer. Medicine (Baltimore). 2016;95:e2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tseng MF, Chou CL, Chung CH, et al. Association between heat stroke and ischemic heart disease: a national longitudinal cohort study in Taiwan. Eur J Intern Med. 2019;59:97‐103. [DOI] [PubMed] [Google Scholar]
- 20. METHODOLOGY WCCFDS . Guidelines for ATC Classification and DDD Assignment 2021. WHO Collaborating Centre for Drug Statistics Methodology. Accessed December 15, 2021. https://www.whocc.no/atc_ddd_index/. [Google Scholar]
- 21. Yen YF, Chung MS, Hu HY, et al. Association of pulmonary tuberculosis and ethambutol with incident depressive disorder: a nationwide, population‐based cohort study. J Clin Psychiatry. 2015;76:e505‐e511. [DOI] [PubMed] [Google Scholar]
- 22. Sico JJ, Chang CC, So‐Armah K, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84:1933‐1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okwuosa TM, Morgans A, Rhee JW, et al. Impact of hormonal therapies for treatment of hormone‐dependent cancers (breast and prostate) on the cardiovascular system: effects and modifications: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2021;14:e000082. [DOI] [PubMed] [Google Scholar]
- 24. Ferrara CM, Lynch NA, Nicklas BJ, Ryan AS, Berman DM. Differences in adipose tissue metabolism between postmenopausal and perimenopausal women. J Clin Endocrinol Metab. 2002;87:4166‐4170. [DOI] [PubMed] [Google Scholar]
- 25. El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton‐Tyrrell K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis. 2012;225:180‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17:332‐341. [DOI] [PubMed] [Google Scholar]
- 27. Chen HF, Jeung EB, Stephenson M, Leung PC. Human peripheral blood mononuclear cells express gonadotropin‐releasing hormone (GnRH), GnRH receptor, and interleukin‐2 receptor gamma‐chain messenger ribonucleic acids that are regulated by GnRH in vitro. J Clin Endocrinol Metab. 1999;84:743‐750. [DOI] [PubMed] [Google Scholar]
- 28. Dixit VD, Yang H, Udhayakumar V, Sridaran R. Gonadotropin‐releasing hormone alters the T helper cytokine balance in the pregnant rat. Biol Reprod. 2003;68:2215‐2221. [DOI] [PubMed] [Google Scholar]
- 29. Tanriverdi F, Gonzalez‐Martinez D, Hu Y, Kelestimur F, Bouloux PM. GnRH‐I and GnRH‐II have differential modulatory effects on human peripheral blood mononuclear cell proliferation and interleukin‐2 receptor gamma‐chain mRNA expression in healthy males. Clin Exp Immunol. 2005;142:103‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sehl ME, Ganz PA. Potential mechanisms of age acceleration caused by estrogen deprivation: do endocrine therapies carry the same risks? JNCI Cancer Spectr. 2018;2:pky035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thurston RC, Sowers MR, Sternfeld B, et al. Gains in body fat and vasomotor symptom reporting over the menopausal transition: the study of women's health across the nation. Am J Epidemiol. 2009;170:766‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gold EB, Crawford SL, Shelton JF, et al. Longitudinal analysis of changes in weight and waist circumference in relation to incident vasomotor symptoms: the study of women's health across the nation (SWAN). Menopause. 2017;24:9‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El Khoudary SR, Shields KJ, Janssen I, et al. Cardiovascular fat, menopause, and sex hormones in women: the SWAN cardiovascular fat ancillary study. J Clin Endocrinol Metab. 2015;100:3304‐3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987‐998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The datasets of the current study are available from the Health and Welfare Data Science Center of Ministry of Health and Welfare in Taiwan.


