Abstract
On March 11, 2020, the World Health Organization declared the coronavirus disease 2019 (COVID-19), whose causative agent is the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a pandemic. This virus is predominantly transmitted via respiratory droplets and shed via sputum, saliva, urine, and stool. Wastewater-based epidemiology (WBE) has been able to monitor the circulation of viral pathogens in the population. This tool demands both in-lab and computational work to be meaningful for, among other purposes, the prediction of outbreaks. In this context, we present a systematic review that organizes and discusses laboratory procedures for SARS-CoV-2 RNA quantification from a wastewater matrix, along with modeling techniques applied to the development of WBE for COVID-19 surveillance. The goal of this review is to present the current panorama of WBE operational aspects as well as to identify current challenges related to it. Our review was conducted in a reproducible manner by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews. We identified a lack of standardization in wastewater analytical procedures. Regardless, the reverse transcription-quantitative polymerase chain reaction (RT-qPCR) approach was the most reported technique employed to detect and quantify viral RNA in wastewater samples. As a more convenient sample matrix, we suggest the solid portion of wastewater to be considered in future investigations due to its higher viral load compared to the liquid fraction. Regarding the epidemiological modeling, the data-driven approach was consistently used for the prediction of variables associated with outbreaks. Future efforts should also be directed toward the development of rapid, more economical, portable, and accurate detection devices.
Keywords: COVID-19, Wastewater-based epidemiology, SARS-CoV-2 detection, Systematic review, Wastewater, Epidemiological modeling
Graphical abstract
1. Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has caused a major pandemic where millions of people have been infected globally. It belongs to the Coronaviridae family and comprises spiked glycoproteins (S) on the surface of a spherical virion that varies from 60 to 140 nm in diameter and is surrounded by a lipid envelope (Zhu et al., 2020). Particularly, SARS-CoV-2 is the causative agent of the coronavirus disease 2019 (COVID-19), which is a life-threatening disease that represents a major threat to public health (Bar-Or et al., 2021; Flood et al., 2021; Li et al., 2020). SARS-CoV-2 is predominantly transmitted via respiratory droplets, which are generated during sneezing, breathing or coughing, and direct or indirect contact through different secretions (Tanhaei et al., 2021; van Doremalen et al., 2020). In this regard, this virus has not only been detected in sputum and saliva but its RNA has been also found in stools and urine, as well as in anal/rectal swabs (Cheung et al., 2020; Mesoraca et al., 2020; Peng et al., 2020). Rather than testing individuals, wastewater-based epidemiology (WBE) has been applied to detect viral pathogens in sewage shed from stool and urine, thus representing a viable alternative to estimate the infection prevalence in the community. WBE was theorized in 2001 (Jones-Lepp, 2001) with the original purpose of monitoring the use of illicit drugs at the community level (Claro et al., 2021). Recently, it has been successfully applied for the detection and monitoring of several viral pathogens in the population (e.g., poliovirus, enterovirus, norovirus, and hepatitis) (Barbosa et al., 2022; Hellmer et al., 2014; Medema et al., 2020; Nasseri et al., 2021; Robotto et al., 2022).
It has been proven that SARS-CoV-2 can be shed in feces after its replication in human intestine enterocytes (Ding and Liang, 2020; Haramoto et al., 2020; Lamers et al., 2020; Lescure et al., 2020), even when the patient had no gastrointestinal symptoms (Xiao et al., 2020a; Zuo et al., 2021). The shedding of this virus from stools can occur after becoming undetectable in the respiratory tract (Wu et al., 2020). Thereby, the SARS-CoV-2 shedding period was found to be longer in fecal than in upper respiratory samples, but its RNA is generally detected earlier in the latter (Zhang et al., 2021a). Additionally, it has been reported that SARS-CoV-2 RNA could be shed through respiratory and fecal routes before the infected individual exhibits symptoms (Buscarini et al., 2020; He et al., 2020; Zhang et al., 2021b). According to the above-mentioned insights, shedding in feces, sputum and saliva contributes to the SARS-CoV-2 load in wastewater (Markt et al., 2022). Interestingly, however, the analysis of wastewater performed through cell culture indicated that the SARS-CoV-2 particles were found non-infectious (Tiwari et al., 2022), bringing evidence to previous observations suggesting that SARS-CoV-2 is not potentially associated with a waterborne transmission risk in community wastewater influents (Rimoldi et al., 2020; Westhaus et al., 2021). The detection of SARS-CoV-2 in wastewater (or, interchangeably, sewage), even at low COVID-19 prevalence, makes sewage surveillance a sensitive tool to monitor its circulation in the population (Prakash, 2021). Quantifying a specific genome of an enteric virus in wastewater is an indirect, noninvasive form of assessing the current health status of the local population (Prevost et al., 2015). Moreover, wastewater surveillance enables both providing early notice of the SARS-CoV-2 (re)emergence in a population when applied routinely (Karthikeyan et al., 2021; Zhao et al., 2022), and supplementing clinical testing by assessing temporal and spatial trends, evaluating asymptomatic and symptomatic individuals, and observing the efficiency of public preventive strategies (Castiglioni et al., 2022; Gupta et al., 2020; Tomasino et al., 2021).
The development of analytical methods for WBE purposes, starting from sampling and viral detection to RNA quantification, emerges as an important research theme that has been approached by a considerable number of studies in the last two years (Carducci et al., 2020; Kabdasli and Tunay, 2021; Kitajima et al., 2020). In this context, a dearth of standardization in the sample analysis methodology was identified (Ahmed et al., 2021; Calderon-Franco et al., 2022; McMinn et al., 2021; Peinado et al., 2022), which has been characterized by the use of a myriad of methods to concentrate, extract, detect, and quantify SARS-CoV-2 RNA (de Sousa et al., 2022; Pillay et al., 2021; Xie et al., 2022). Furthermore, the normalization of quantitative information has not been addressed: for example, standard units to express viral loads in wastewater have not been established so far (Shah et al., 2022). Regarding the output of the laboratory analysis, the accurate estimation of viral genomic concentration in wastewater is an issue that must be addressed in future COVID-19 surveillance research since this variable has been used to estimate the number of COVID-19 cases when confronted with clinical testing data (Ahmed et al., 2020a; de Sousa et al., 2022; Pillay et al., 2021). Analytical accuracy is imperative for building the path toward understanding the infection dynamics through WBE by designing trustful correlations and mathematical models relating sewershed viral concentration and epidemiological clinical data. To address this issue, we elevated the need to systematize the available knowledge on the technology for wastewater analysis as well as the scientific effort to unravel and model COVID-19 infection dynamics through existing or developed WBE mathematical models.
The purpose of this systematic review is twofold: identify the reported methodology of techniques/procedures to quantify SARS-CoV-2 viral RNA in domestic wastewater, and the mathematical methods and models by which viral loads have been associated with epidemiological data. It should be noted that this integrated approach has not been considered by any previously published review on the field. Thus, this study may serve as a reference for upcoming research that requires detailed information on these subjects, thus readers interested in one or both operational aspects of WBE for COVID-19 can find this study relevant given the exposition of methods and findings from a total of 158 studies. This review is structured as follows. After this introduction, Section 2 brings a comprehensive description of the systematic search method and selection process for evidence-based publications discussing analytical methods and mathematical modeling for COVID-19 surveillance up to August 2022. Next, in Section 3, we report our findings from the selected literature regarding in-lab and computational works that have been performed. Section 4 critically discusses our findings in terms of current issues, gaps to fill, and promising alternatives to treat wastewater toward the refinement of WBE for COVID-19 surveillance. Finally, we finish with the main conclusions drawn from this analysis and directions for further research.
2. Methods
2.1. Search strategy
This systematic literature review (SLR) was conducted by following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) method (Page et al., 2021). The SLR is a reliable way to acquire a consistent overview of a specific research theme in an organized and replicable manner (Denyer and Tranfield, 2009; Tranfield et al., 2003). Before engaging in the systematic collection of studies, we conducted a non-structured search to identify regularly associated keywords and concepts about the subject. Keywords such as “COVID-19/SARS-CoV-2”, “wastewater”, “surveillance”, “methods”, “analysis”, “modeling”, and “correlation” were commonly used to identify records of peer-reviewed articles in the multidisciplinary literature. Next, we chose the following databases: ISI Web of Science (www.webofscience.com) and Scopus (www.scopus.com) given their relevance in the academic literature (Wang and Waltman, 2016), along with Engineering Village (Elsevier's Compendex) (www.engineeringvillage.com) due to its importance in the interdisciplinary engineering field (Cusker, 2013), and PubMed (MEDLINE) (www.pubmed.ncbi.nlm.nih.gov) for its reliability as a medical database for evidence-based studies such as systematic reviews (Falagas et al., 2008; Gusenbauer and Haddaway, 2020). As presented above, to address the current panorama of WBE for COVID-19 surveillance to a fuller extent, we considered a conjoint exploration of two pillars: laboratory procedures to quantify viral RNA in wastewater, and modeling (computational) methods to, among other goals, predict outbreaks in the community and city levels. The latter pillar is fed with input generated by the former. To fulfill this purpose, we performed two independent collections of records from the databases (one for viral RNA analysis and quantification and another for epidemiological modeling), with each one adopting suitable search strings as shown in Table 1 . We decided to approach the search in this manner due to the extensive number of studies returned upon conducting a single search for both subjects. Then, we were able to significantly reduce the initial number of records found in the databases and work with a reasonable sample of articles. Table 1 summarizes the keywords used as search strings and how they were combined to compose each search.
Table 1.
Search strings and Boolean operators used for each search.
| Boolean operator | Search strings | Category | |
|---|---|---|---|
| Analytical methods | COVID?19 OR SARS-CoV-2 OR coronavirus | Topic | |
| AND | wastewater OR ww or sewage | Topic | |
| AND | “SARS-CoV-2 RNA” OR RNA OR “ribonucleic acid” OR “nucleic acid” OR genet* | Topic | |
| AND | analy* OR method* OR procedure OR protocol OR techn* | All Fields | |
| AND | detect* OR concentrate* OR quantif* OR estimat* OR measur* | All Fields | |
| Mathematical modeling | COVID?19 OR SARS-CoV-2 OR coronavirus | Topic | |
| AND | wastewater OR ww OR “wastewater-based epidemiology” or WBE | Topic | |
| AND | surveill* OR monitor* OR track* | All Fields | |
| AND | predict* OR forecast* OR foreshadow* OR model* OR correlate* OR relation* | All Fields |
Note: “?” denotes a wildcard and was used due to different spellings of the term adopted in the literature. The “*” symbol allows variations of the search string. Quotation marks strictly limit the appearance of the word as it is input.
2.2. Selection of studies and filtering
The academic coverage and analysis of the COVID-19 pandemic and its consequences demand trustable data to mitigate the risk of misconceptions in matters of public health information and public policies (Davenport et al., 2020; Tagliabue et al., 2020). Accordingly, we favored the side of selecting records from trustworthy sources when pondering the trade-off that exists between considering a high-quality level of discussion and broadening the information basis, with the latter often associated with doubtful reliability (Tranfield et al., 2003). Thus, we decided to include only peer-reviewed, original articles, therefore excluding other types of studies and publication formats, such as reviews, short communications, technical reports, letters, notes, abstracts, and surveys. Any available but unpublished work was excluded as well. Studies published between January 2020 and August 2022 were included in the sample. The deduplication, screening, filtering, and application of inclusion criteria were performed in EndNote 20 to reduce the original sample of studies to a trustful and representative collection of knowledge in the field. The initial search using the terms expressed in Table 1 returned a total of 2400 articles, most of them unrelated to our subjects. Next, we engaged in the screening phase as described: collected records were primarily screened for their title only, and subsequently, for their abstracts and content in full. Throughout this phase, we considered ineligible any publication that addressed topics outside our focus, such as other types of viral pathogens in wastewater, COVID-19 diagnosis and treatment, other matrices such as soil, leachate and air, elimination of various pathogens in water, water quality, wastewater from aircraft and ships, drug detection, and biosensors, to name a few. About their content, we included publications that presented (1) clear and concise descriptions and/or comparisons of analytical methods, protocols, and technologies currently used for pre-treatment, concentration, extraction, and quantification of SARS-CoV-2 nucleic acid in a wastewater matrix, and/or (2) precise information on the characteristics of studied wastewater, study location, time range, application of statistical tests for correlating WBE variables, as well as any used or developed mathematical model toward exploring COVID-19 infection dynamics.
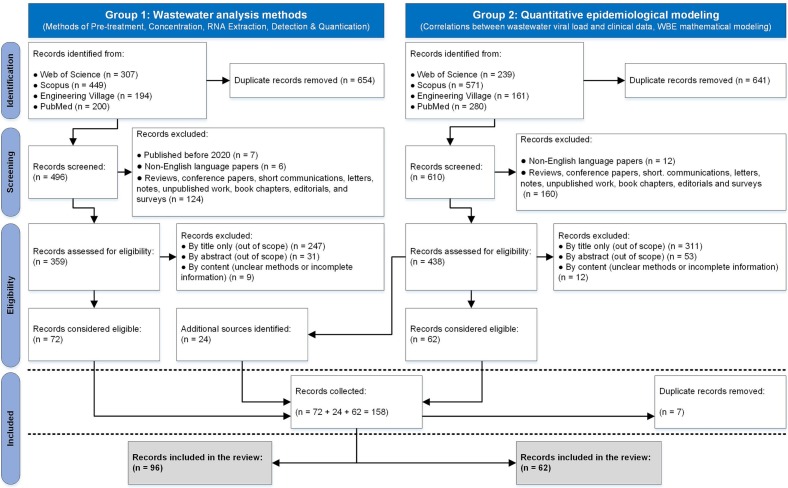
Regarding the filtering procedures, we first used EndNote 20 to detect and exclude duplicated records independently for each search, then we applied time range and language filters, followed by the last filter regarding the type of publication from an initial total of 1106 identified literature records. After this screening step, we assessed the remaining studies through the lens of the established inclusion and exclusion criteria, thus excluding 558 and 84 records by title-only and abstract, respectively; these studies were considered out of the scope of this review, and thus deemed ineligible. Besides, 21 studies were unclear about their methodological procedures or had not presented any type of wanted information, therefore excluded. Last, we combined the two groups into a single pool and ran a second deduplication, thus excluding another 7 studies. Finally, we finished with a list of 96 studies from the first group and 62 studies from the second group as presented in Fig. 1 , amounting to a final pool of 158 works.
Fig. 1.
Publication selection process: PRISMA-based flowchart for evidence-based research.
2.3. Data extraction
To properly organize the data extraction process, we used a MS Excel spreadsheet with designated columns to include the following information reported by the selected studies: study location, sample collection period, wastewater characteristics (wastewater treatment plant (WWTP) influent, sewage, or treated), sample pre-treatment, concentration/extraction methods, gene targets, quantification method/technology, initial sample volume processed, lowest and highest viral concentrations recovered in both solid and liquid phases, the estimated time offset (lag) between sample analysis and epidemiological reporting, statistical test to correlate wastewater viral load and clinical data and its result, and mathematical modeling strategy. Not all the studies were thorough in reporting this set of systematized categories, nevertheless, we reasoned that these categories were potentially discussed at some level in our sample of studies, and thus every publication on the selected portfolio should be able to contribute within the scope of this review.
3. Results
3.1. Scientific contribution of the selected studies to SARS-CoV-2 WBE
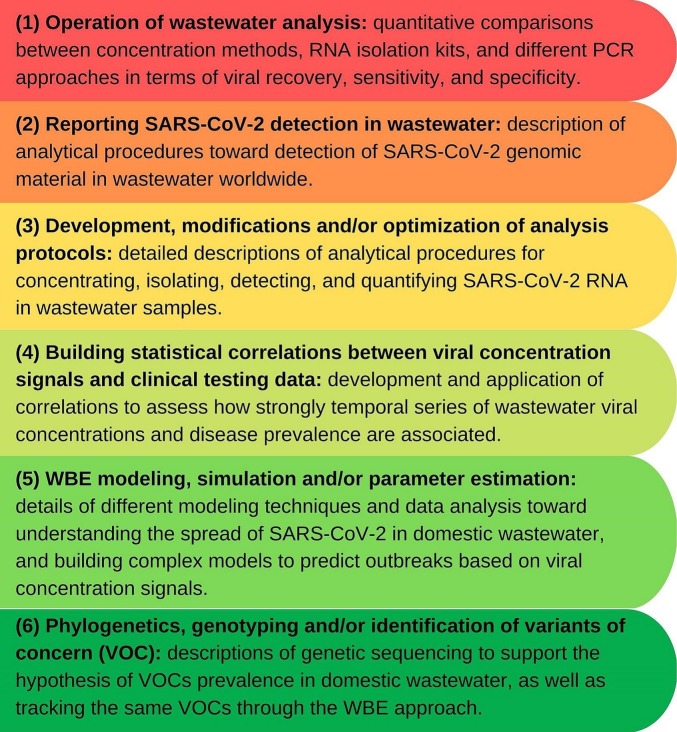
A meaningful result from the analysis of the reviewed publications was the identification of six main types of contributions in the WBE for COVID-19 surveillance field from 2020 to 2022, which are listed next and depicted in Fig. 2 : (1) quantitative comparison of concentration, extraction or quantification methods through parametric studies, (2) local reporting of SARS-CoV-2 detection in wastewater and the respective methodology, (3) development, adaptation and/or optimization of analysis protocols, (4) building correlations between viral concentration levels and clinical testing data, (5) mathematical modeling, simulation or parameter estimations for SARS-CoV-2 WBE, and (6) phylogenetics, genotyping and/or identification/quantification of variants of concern (VOC).
Fig. 2.
Types of contributions of the selected studies to SARS-CoV-2 WBE: findings from the analysis of the selected pool of publications.
3.2. Aspects of wastewater analysis for SARS-CoV-2 detection and quantification
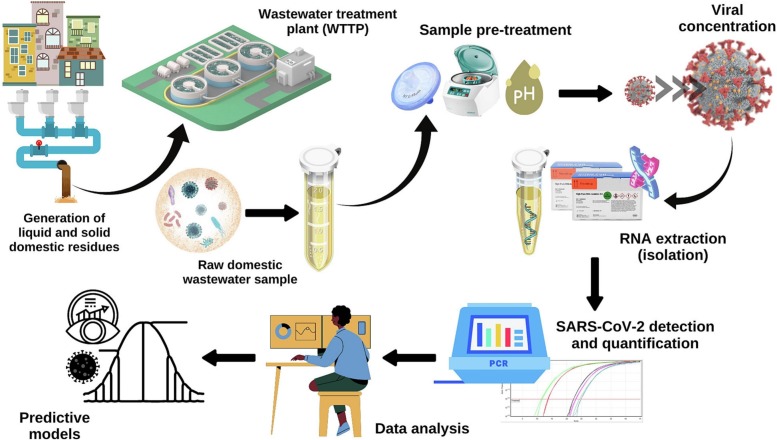
SARS-CoV-2 RNA can be found thermically stable in untreated wastewater at temperature values ranging from 4 to 37 °C (Ahmed et al., 2020c). This particularly wide range allows laboratory operations to reliably preserve and detect the virus, even having passed through sample collection and processing. The general methodology to generate a quantified viral concentration, in terms of cycle threshold (Ct) units or genomic concentration, from a wastewater sample, follows a sequential procedure of well-defined steps, namely sampling, pre-treatment, concentration, extraction (or isolation), and detection followed by quantification. This framework is depicted in Fig. 3 . We found that the operationalization of these steps is well diverse, containing different technologies and protocols that use a wide range of reagents (Kaya et al., 2022). A complete list of the procedures and technology used can be found in Table 2 . Next, we describe the general aspects of each step of the current paradigm of wastewater testing for SARS-CoV-2 WBE.
Fig. 3.
SARS-CoV-2 WBE: Overall framework for sample analysis and epidemiological modeling.
Table 2.
Description of analytical methods from selected literature and their contributions.
| Study | Location and sampling period | Wastewater type and sources | Sample pre-treatment | Concentration methods | RNA extraction Kit/protocol | Quantification method and gene targets | Analyzed initial sample volume (mL) | Type of contribution (Fig. 2) |
|---|---|---|---|---|---|---|---|---|
| Anderson-Coughlin et al. (2021) | USA August 2020–March 2021 |
Raw (sewage) | Filtration using a 0.22 μm polyethersulfone (PES) membrane | Centrifugal ultrafiltration | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 and N2 |
40 – 45 | 3 |
| Ahmed et al. (2020a) | Australia March 2020 – April 2020 |
Raw (influent) | (1) pH adjustment to 3.5/4 using HCl, (2) Centrifugation at 4750 g for 30 min | (1) Adsorption-elution with electronegative membranes, (2) Ultrafiltration | QIAgen RNeasy PowerWater Kit and QIAgen RNeasy PowerMicrobiome Kit |
RT-qPCR N |
100 – 200 | 5 |
| Ahmed et al. (2020c) | Australia NR |
Raw (influent) | (1) Acidification to pH 4 using 2 N HCl, (2) NR, (3) MgCl2 addition to a final concentration of 25 mM MgCl2, (4,5) Centrifugation at 4500 g for 10 min at 4 °C, (6) Centrifugation at 10000 g for 20 min at 4 °C, (7) Centrifugation at 10000 g for 1 h at 4 °C | (1,2,3) Adsorption-elution using electronegative membranes, (4,5) Ultrafiltration, (6) PEG precipitation, (7) Ultracentrifugation | QIAgen RNeasy PowerMicrobiome Kit | RT-qPCR NR |
50 | 1,5 |
| Ahmed et al. (2021a) | Bangladesh July 2020 – August 2020 |
Raw (sewage) | Centrifugation at 4500 g for 30 min, filtration using 0.22 μm filters | PEG precipitation | Favor Prep Viral Nucleic Acid Extraction Kit | RT-qPCR N and ORF1ab |
50 | 1,5 |
| Ahmed et al. (2021b) | NR | Raw (influent) | (1) Centrifugation at 4000 g for 30 min at 4 °C, (2) NR | (1) Concentrating pipette (InnovaPrep), (2) Adsorption-elution with electronegative membranes | QIAgen QIAamp® Viral RNA mini Kit and RNeasy PowerWater Kit | RT-qPCR N1 |
NR | 1 |
| Ahmed et al. (2021c) | Australia February 2020 – May 2020 |
Raw (influent) | NR | Adsorption-elution using electronegative membranes | QIAgen RNeasy PowerMicrobiome Kit | RT-qPCR N1, N2 and N3 |
100 – 200 | 2 |
| Ahmed et al. (2022b) | Australia June 2021 |
Raw (influent) | Centrifugation at 3000 g for 5 min | Concentration Pipette (InnovaPrep) | QIAgen QIAamp® Viral RNA mini Kit and QIAgen RNeasy PowerMicrobiome Kit (for the solid phase) | RT-qPCR and RT-dPCR N1 and N2 |
50 | 4,6 |
| Ai et al. (2021) | USA July 2020–January 2021 |
Raw (influent) | Centrifugation at 2500 g for 10 min at 4 °C, filtration using a 0.45 μM sterile filter unit | Sequential concentration using adsorption-elution with positively charged membranes, organic flocculation, and centrifugal ultrafiltration | QIAgen RNeasy PowerMicrobiome Kit | RT-ddPCR N1, N2 and E |
100 – 200 | 4,5,6 |
| Amereh et al. (2022) | Iran September 2020 – April 2021 |
Raw (influent) | Centrifugation at 4000 g for 10 min | PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N and ORF1ab |
50 | 4 |
| Amoah et al. (2021) | South Africa NR |
Raw (influent) | Heat inactivation 60 °C for 90 min | Centrifugal ultrafiltration | QIAgen QIAamp® Viral RNA mini Kit | RT-ddPCR and RT-LAMP E, N, ORF1ab, RdRP and S |
250 | 3 |
| Anneser et al. (2022) | USA March 2020 – March 2021 |
Raw (influent and sludge) | NR | (1) PEG precipitation, (2) Spectrophotometry | TRIzol-chloroform protocol and RNeasy PowerSoil Total RNA Kit | RT-qPCR N1, N2 and N3 |
NR | 4.5 |
| Arora et al. (2020) | India May 2020 – June 2020 |
Raw (influent) | Heat inactivation 60 °C for 90 min, filtration using a 0.45 μm membrane | PEG precipitation | Allplex 2019-nCoV Assay Kit | RT-PCR N, S, E, ORF1ab and RdRp |
50 | 2 |
| Bagutti et al. (2022) | Switzerland July 2021–December 2021 |
Raw (influent) | NR | NR | Maxwell® RSC Environ Wastewater TNA Kit | RT-qPCR N1, N2 and E |
40 | 4 |
| Baldovin et al. (2021) | Italy April 2020 – May 2020 |
Raw (influent) and treated (effluent: activated sludge, peractic acid and UV lamps) | Filtration using a 0.22 μm PES membrane | Ultrafiltration | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N and ORF1ab |
100 | 1,6 |
| Barbosa et al. (2022) | Brazil May 2020–October 2020 |
Raw (influent, sewage) | NR | Ultracentrifugation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 and N2 |
40 | 2,4,5 |
| Bar-Or et al. (2021a) | Israel August 2020–February 2021 |
Raw (influent) | Centrifugation at 4696 g for 5 min | Adsorption-elution with electronegative membranes | NucliSENS EasyMAG | RT-qPCR E |
25 | 2 |
| Bar-Or et al. (2021b) | Israel March 2020–April 2020 |
Raw (sewage) | NR | (1) PEG precipitation, (2) Skimmed milk flocculation, (3) Ultrafiltration | QIAgen RNeasy mini Kit and NucliSENS EasyMAG | RT-qPCR N and E |
250 – 1000 | 1,2,6 |
| Barril et al. (2021) | Argentina March 2020–October 2020 |
Raw (influent) | NR | A total of 11 different methods were evaluated. | Maxwell RSC 48 Extraction System | RT-qPCR N1 and N2 |
Varied from method to method | 2,4 |
| Barrios et al. (2021) | Argentina June 2020 – April 2021 |
Raw (influent) | Heat inactivation 60 °C for 90 min | PEG precipitation | TRIzol-chloroform protocol | RT-qPCR N1 |
200 | 4 |
| Barua et al. (2022) | USA June 2020 – November 2020 |
Raw (influent) | Heat inactivation 75 °C for 40 min | Electronegative filtration (HA) | QIAgen QIAamp® Viral RNA mini Kit and NucliSENS EasyMAG | RT-qPCR and RT-ddPCR N1 and N2 |
20 | 1 |
| Bertrand et al. (2021) | France April 2020–May 2020 |
Raw (influent after decantation) | NR | (1) Ultrafiltration, (2) PEG precipitation | Phenol-chloroform-isoamyl alcohol protocol | RT-PCR and RT-ddPCR E and RdRp |
50 | 1,2 |
| Bivins et al. (2022) | NR | Raw (influent, sewage) | NR | Centrifugal ultrafiltration | QIAgen QIAamp® Viral RNA mini Kit and AllPrep PowerViral DNA/RNA Kit | RT-ddPCR and RT-LAMP N2 and E |
NR | 1,3,4 |
| Boogaerts et al. (2021) | Belgium August 2020 – January 2021 |
Raw (influent) | (1) Centrifugation at 4600 g for 30 min at 4 °C, (2) Centrifugation at 4654 g for 30 min at 4 °C | (1) Ultracentrifugation, (2) PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit, RNeasy plus miniKit and QIAgen RNeasy PowerMicrobiome Kit |
RT-qPCr and RT-dPCR N1, N2, N3 and E |
20 – 90 | 1,4,5 |
| Boogaerts et al. (2022) | Belgium September 2020 – November 2021 |
Raw (influent) | Centrifugation at 4000 g for 30 min | Ultracentrifugation | Maxwell® RSC PureFood GMO and Authentication Kit | RT-qPCr and RT-dPCR N, S and E |
20 | 3,6 |
| Calderon-Franco et al. (2022) | Netherlands July 2020 – December 2020 |
Raw (influent) | Heat inactivation 65 °C for 30 min | (1) Adsorption-elution with electronegative membranes, (2) Polyethersulfone membranes, (3) Anion-exchange diethylaminethyl cellulose columns | Fast RNA Blue Kit, FAST RNA Kit and MagMax CORE Nucleic Acid Purification Kit | RT-qPCR S, N and ORF1ab |
50 – 550 | 1,3 |
| Canh et al. (2021) | Japan January 2021 – February 2021 |
Raw (influent) | Centrifugation at 3500 g for 15 min | (1) Ultrafiltration, (2) PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 |
100 | 1,2 |
| Carrillo-Reyes et al. (2021) | Mexico April 2020–July 2020 |
Raw (influent, sewage) and treated (secondary sludge, effluent) | Filtration using a 0.2 μm PES membrane | (1) Ultrafiltration, (2) Adsorption-elution with electronegative membranes | QIAgen RNeasy PowerMicrobiome Kit | RT-qPCR RdRp, S and E |
(1) 120, (2) 30 – 100 |
1,2 |
| Castiglioni et al. (2022) | March 2020–June 2020 | Raw (influent) | Under UV light for 30 min, Centrifugation at 4500 g for 30 min at 4 °C | PEG precipitation | QIAgen QIAamp® MinElute Virus Spin Kit | RT-PCR N1 and N3 |
45 | 1,2,5 |
| Chakraborty et al. (2021) | India September 2020 |
Raw (influent) and treated (primary sludge, effluent) | NR | Composite, Supernatant, Sediment and Syringe Filtration | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 and N2 |
250 | 1 |
| Chavarria-Miro et al. (2021) | Spain April 2020 – July 2020 |
Raw (influent) | NR | PEG precipitation | NucliSENS miniMAG | RT-qPCR N1, N2, RdRp, IP2 and IP4 |
800 | 5 |
| Claro et al. (2021) | Brazil June 2020 – April 2021 |
Raw (influent) | Centrifugation at 8000 g for 120 min at 4 °C | PEG precipitation | PureLink™ Viral RNA/DNA mini Kit | RT-qPCR N1 and N2 |
40 | 4 |
| D'Aoust et al. (2021) | Canada April 2020–June 2020 |
Raw (post-grid influence) and treated (primary clarified sludge) | Decantation and serially filtered through a 1.5 μm glass fiber filter followed by a 0.45 μm GF6 mixed cellulose-ester filter |
PEG precipitation | QIAgen RNeasy PowerMicrobiome Kit | RT-qPCR and RT-ddPCR N1 and N2 |
32 | 3,5 |
| de Freitas et al. (2022) | Brazil January 2021 – January 2022 |
Raw (influent) | NR | PEG precipitation | PureLink Viral RNA/DNA mini Kit | RT-qPCR N1 and N2 |
40 | 4.5 |
| de Sousa et al. (2022) | Brazil January 2021 – August 2021 |
Raw (influent) and treated (effluent) | pH adjustment to 3.5 using 1 M HCl, shaken at °4C for 30 min, Centrifugation at 2474 g for 30 min at 4 °C | PEG precipitation | MagMAX Viral/Pathogen Nucleic Acid Isolation Kit | RT-qPCR N1 and N2 |
50 | 5 |
| Dimitrakopoulos et al. (2022) | Greece November 2021–December 2021 |
Raw (influent) | NR | (1) PEG precipitation, (2) PEG precipitation with glycine, (3) Direct capture, (4) Adsorption-elution with electronegative membranes, (5) Ultrafiltration | Water DNA/RNA magnetic bead Kit, QIAgen RNeasy PowerMicrobiome Kit, AllPrepPowerViral DNA/RNA Kit and Manual EnviroWastewater TNA Kit | RT-qPCR and RT-ddPCR N1, N2 and N3 |
50 | 2,6 |
| Dumke et al. (2021) | Germany, NR |
Raw (influent) | Centrifugation at 3300 g for 30 min at 4 °C | (1) PEG precipitation, (2) Centrifugation with Vivaspin columns | QIAgen RNeasy kits (not specified what series) | RT-qPCR and RT-ddPCR S and E |
40 | 2 |
| Farkas et al. (2021) | NR | Raw (NR) | Centrifugation at 3000 g for 30 min at 4 °C or 1000 g for 10 min at 4 °C, pH adjustment of supernatant to 7–7.5 using 1 M NaOH | PEG precipitation | NucliSENS lysis buffer and NucliSENS miniMag extraction system | RT-qPCR N1 and N2 |
50 | 1,3 |
| Feng et al. (2021) | USA August 2020–January 2021 |
Raw (influent) | Filtration using 0.8 μm cellulose-ester filters | Bashing Bead Lysis | QIAgen RNeasy PowerMicrobiome Kit | RT-ddPCR N1 and N2 |
25 | 1 |
| Fernandez-Cassi et al. (2021) | Switzerland February 2020–April 2020 |
Raw (influent) | Filtered using 2 μm glass fiber filters | Centrifugal ultrafiltration | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 and N2 |
50 | 5 |
| Fitzgerald et al. (2021) | Scotland April 2020 – January 2021 |
Raw (influent) | Centrifugation at 4000 g for 30 min at 4 °C, filtration using a syringe filter | (1) Ultracentrifugation, (2) PEG precipitation, (3) skimmed milk flocculation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 and E |
20 – 40 | 1,2 |
| Flood et al. (2021) | USA March 2020 – September 2020 |
Raw (influent, sewage) | (1) Centrifugation at 2500 g for 5 min at 4 °C, (2) Centrifugation at 4654 g for 30 min at 4 °C, (3) Centrifugation at 4700 g for 45 min at 4 °C | (1,2) Ultrafiltration, (3) PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR, RT-ddPCR N1, N2 and E |
(1,2) 100, (3) NR | 1 |
| Fongaro et al. (2021) | Brazil October 2019–March 2020 |
Raw (sewage) | NR | PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1, S and RdRp |
25 | 4 |
| Fonseca et al. (2022) | Brazil March 2021 |
Raw (influent, river) | Heat inactivation 60 °C for 90 min, filtration using 1.2 μm pore size microfiber filters, Centrifugation at 4500 g for 30 min at 4 °C | (1) Ultrafiltration, (2) Adsorption-elution with electronegative membranes, (3) Aluminum hydroxide precipitation, (4) PEG precipitation | MagMax Viral/Pathogen II Kit and KingFisher Duo Purification System | RT-qPCR N1 and N2 |
40 | 4,6 |
| Galani et al. (2022) | Greece August 2020 – March 2021 |
Raw (influent) | Centrifugation at 4700 g for 30 min at 4 °C | (1) PEG precipitation, (2) centrifugal ultrafiltration | Water DNA/RNA Magnetic Bead Kit, QIAgen RNeasy Power Microbiome Kit and QIAgen RNeasy Serum/Plasma Advanced Kit | RT-qPCR N1 and N2 |
50 | 4,5 |
| Gerrity et al. (2021) | USA March 2020–May 2020 |
Raw (influent) | (1) NR, (2,3) Centrifugation at 3500 g for 15–30 min at 10 °C | (1) Hollow-fiber ultrafiltration, (2) Centrifugal Ultrafiltration, (3) PEG precipitation. | Purelink Viral RNA/DNA mini Kit | RT-qPCR N1, N2, E and ORF1a |
50 | 1,3 |
| Giraud-Billoud et al. (2021) | Argentina July 2020–November 2020 |
Raw (influent) | Heat inactivation 60 °C for 90 min | (1) PEG precipitation, (2) Polyaluminum chloride (PAC) flocculation | NucleoZOL | RT-qPCR N1 and N2 |
300 | 5,6 |
| Gonçalves et al. (2021) | Slovenia June 2020 |
Raw (sewage) | Filtration using a 0.70 μm glass fiber filter membrane | Ultracentrifugation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR E and RdRp |
100 | 1 |
| Gonzalez et al. (2020) | USA March 2020 – August 2020 |
Raw (influent) | (1) Centrifugation at 10000 g for 10 min, (2) NR | (1) Concentration Pipette (InnovaPrep), (2) Adsorption-elution using electronegative membranes | NucliSENS Easy Mag TNA Extraction Kit | RT-ddPCR N1, N2 and N3 |
125 | 2 |
| Gonzalez-Reyes et al. (2021) | Mexico June 2020–July 2020 |
Raw (influent, sewage) | Heat inactivation 60 °C for 90 min, filtration using a 0.2 μm membrane | PEG precipitation | TRIzol protocol | RT-qPCR N1, N2 and N3 |
150 | 2 |
| Haramoto et al. (2020) | Japan March 2020–May 2020 |
Raw (influent) and treated (activated sludge before chlorination) | NR | (1) Adsorption-elution with electronegative membranes, (2) Direct adsorption | QIAgen QIAamp® Viral RNA mini Kit and QIAgen RNeasy PowerMicrobiome Kit | RT-qPCR N1, N2, S and ORF1ab |
200 – 5000 | 2 |
| Hasan et al. (2021) | UAE May 2020–June 2020 |
Raw (influent) and treated (effluent) | (1,2) Heat inactivation 60 °C for 90 min, filtration using a 0.22 μm PES membrane | (1) Ultrafiltration, (2) PEG precipitation | ABIOpure Viral DNA/RNA Extraction Kit and TRIzol-chloroform protocol | RT-qPCR RdRp |
(1,2) 50 | 1,4 |
| Hasing et al. (2021) | Canada October 2020–December 2020 |
Raw (influent) | pH adjustment to 9.6–10 using 5 N NaOH, Centrifugation at 4500 g for 10 min | Ultrafiltration | MagMAX96 Viral RNA Isolation Kit and King Fisher Flex Purification System | RT-qPCR N2 and E |
100 | 3 |
| Hata et al. (2021) | Japan March 2020 – April 2020 |
Raw (influent) | NR | PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N2 and N3 |
80 | 2 |
| Hemalatha et al. (2021) | India July 2020 – August 2020 |
Raw (influent) | Gravity filtration with 1 mm thick blotting sheets to remove debris and larger particles followed by filtration using 0.2 μm filtration units | Centrifugal ultrafiltration | QIAamp® Viral RNA isolation Kit | RT-qPCR N, E and ORF1ab |
100 | 2,3 |
| Hoar et al. (2022) | USA April 2020 – February 2021 |
Raw (influent) | Heat inactivation 60 °C for 90 min, Centrifugation at 5000 g for 10 min at 4 °C, filtration using 0.22 μm acetate-cellulose membrane | PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 |
40 | 4.5 |
| Hokajarvi et al. (2021) | Finland April 2020 – May 2020 |
Raw (influent) | Centrifugation at 4654 g for 30 min | Ultrafiltration | Chemagic Viral300 DNA/RNA extraction Kit | RT-qPCR N2 and E |
60 | 2,3 |
| Huang et al. (2021) | Canada October 2020 – March 2021 |
Raw (influent) | NR | Ultrafiltration | QIAgen RNeasy PowerMicrobiome Kit | RT-qPCR N1, N2, N3 and E |
200 | 2 |
| Iglesias et al. (2021) | Argentina June 2020–September 2020 |
Raw (influent, surface water) | Heat inactivation 60 °C for 90 min | PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 and N2 |
250 | 2 |
| Jafferali et al. (2021) | Sweden and Italy May 2020–June 2020 |
Raw (influent) | (1) Centrifugation at 4600 g for 30 min at 4 °C, (2) Centrifugation at 1500 g for 15 min at 4 °C, (3) NR, (4) Centrifugation at 4600 g for 30 min at 4 °C | (1) Ultrafiltration, (2) Double Ultrafiltration, (3) Adsorption-elution with electronegative membranes, (4) Centrifugation combined with adsorption-extraction | TRIzol reagent and RNeasy PowerMicrobiome Kit | RT-qPCR N |
40 – 50 | 1 |
| Jmii et al. (2021) | Tunisia September 2020–October 2020 |
Raw (influent) | Coarse filtration and microfiltration, pH adjustment to 6 with aluminum hydroxide | Adsorption-elution with electronegative membranes | QIAgen RNeasy PowerMicrobiome Kit | RT-PCR N, E and RdRp |
100 | 1 |
| Johnson et al. (2021) | South Africa June 2020 |
Raw (influent) | Centrifugation at 3500 g for 20 min | NR | RNeasy PowerSoil Kit | RT-qPCR N1 and N2 |
50 – 100 | 2 |
| Juel et al. (2021) | USA October 2022 – March 2021 |
Raw (sewage) | NR | (1) Adsorption-elution with electronegative membranes, (2) Concentrating Pipette (InnovaPrep) | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 |
40 – 100 | 1,3 |
| Kevill et al. (2022) | Wales October 2020 – February 2021 |
Raw (influent) | Centrifugation at 15000 g for 10 min at 4 °C | (1) PEG precipitation, (2) Ammonium sulfate precipitation, (3) Concentration pipette (Innova Prep) | NucliSENS Lysis Buffer, NucliSENS Extraction Reagent Kit and King-Fisher 96 Flex System | RT-pPCR N1 |
200 | 1,3 |
| Kitamura et al. (2021) | Japan June 2020–August 2020 |
Raw (influent, sewage) | Centrifugation at 3000 rpm for 30 min | (1) Adsorption-elution with electronegative membranes, (2) PEG precipitation, (3) Ultrafiltration, (4) Solid precipitation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 and N2 |
400 | 1,3,4,5 |
| Koureas et al. (2021) | Greece October 2020–April 2021 |
Raw (influent) | NR | PEG precipitation | MagMAX™ Viral/Pathogen Nucleic Acid Isolation Kit | RT-PCR N, S and ORF1ab |
105 | 5 |
| Krivonakova et al. (2021) | Slovakia September 2020 – March 2021 |
Raw (influent) | Centrifugation at 4700 g for 30 min | Ultracentrifugation | Direct-zol RNA miniprep Kit | RT-qPCR E, RdRp and ORF1ab |
50 | 2.4 |
| Kuhn et al. (2022) | USA November 2020 – March 2021 |
Raw (sewage) | Filtration using a 70 μm mesh cell strainer | PEG precipitation | Bio-On-Magnetic-Beads platform | RT-qPCR N1 |
32 | 5 |
| Kumar et al. (2021) | India August 2020–September 2020 |
Raw (influent) | Centrifugation at 4000 g for 40 min, filtration using a 0.22 μm syringe filter | PEG precipitation | NucleoSpin® RNA Virus isolation Kit | RT-PCR N, S and ORF1ab |
30 | 2 |
| La Rosa et al. (2020) | Italy February 2020–April 2020 |
Raw (influent) | Heat inactivation 56 °C for 30 min | PEG-dextran two-phase separation | NucliSENS miniMAG | RT-qPCR ORF1ab, S and RdRp |
250 | 2,5 |
| Langan et al. (2022) | USA January 2021–March 2021 |
Raw (sewage) | Centrifugation at 4000 g for 20 min at 4 °C | Ultrafiltration | QIAgen PowerViral DNA/RNA Kit, Zymp EnvironWater RNA Extraction Kit and Monarch Total RNA miniprep Kit | RT-qPCR N1 and N2 |
200 | 1,5 |
| Lara-Jacobo et al. (2022) | Canada October 2020–April 2021 |
Raw (influent) | Adding 50 mL of acetone at 4 °C and stored overnight at −20 °C to precipitate proteins, Centrifugation at 3405 g for 15 min | Protein Precipitation and Digestion | QIAgen PowerMicrobiome Kit | RT-qPCR N1 |
40 | 1,3 |
| LaTurner et al. (2021) | USA October 2020 |
Raw (influent) | (1) NR, (2) Centrifugation at 4100 g for 10 min at 4 °C, (3) Centrifugation at 3000 g for 1 min at 4 °C, (4) Centrifugation at 7140 g for 15 min at 4 °C, (5) Centrifugation at 4100 g for 10 min at 4 °C | (1) Direct extraction, (2) HA filtration with bead beating, (3) HA filtration with elution, (4) PEG precipitation, (5) Ultrafiltration | Chemagic Prime Viral DNA/RNA 300 Kit H96 | RT-qPCR and RT-ddPCR N1 and N2 |
(1) 1, (2,3,5) 50 (4) 200 | 5 |
| Layton et al. (2022) | USA June 2020 – July 2020 |
Raw (sewage) | Centrifugation at 12000 g for 1 min | NR | MagMAX Viral Pathogen Kit | RT-ddPCR N1 and N2 |
30 – 40 | 4 |
| Li et al. (2022) | USA June 2020 – September 2021 |
Raw (influent) | Heat inactivation 60 °C for 60 min, Centrifugation at 3000 g for 15 min, and sequential filtration using 1.5, 0.8, and 0.45 μm sterile membrane filters | PEG precipitation | AllPrep PowerViral DNA/RNA Kit | RT-qPCR N1 and N2 |
NR | 2,4 |
| Maida et al. (2022) | Italy September 2021–July 2021 |
Raw (sewage) | NR | PEG-dextran two-phase separation | NucliSENS miniMAG | RT-qPCR NR |
NR | 5 |
| Mailepessov et al. (2022) | Singapore April 2020 |
Raw (sewage) | (1) Centrifugation at 4000 g for 30 min, (2) Centrifugation at 2000 g for 5 min | (1) PEG precipitation, (2) Ultrafiltration | Modified TRIzol-QIAgen protocol and QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR NR |
45 | 1,3 |
| Markt et al. (2022) | Liechtenstein Sept 2020 - March 2021 |
Raw (influent) | Centrifugation at 4500 g for 30 min | PEG precipitation | Monarch total RNA miniprep Kit | RT-qPCR N1 |
70 | 1 |
| Masachessi et al. (2022) | Argentina May 2020–August 2021 |
Raw (influent) | Centrifugation at 4750 g for 20 min at 4 °C | PEG precipitation | MagNa Pure 96 DNA and Viral NA Large Volume Kit | RT-qPCR N and E |
500 | 2 |
| McMahan et al. (2021) | USA May 2020 – August 2020 |
Raw (sewage) | Heat inactivation 60 °C for 30 min, Centrifugation at 6500 g for 10 min at 6 °C | PEG precipitation | TRIzol-chloroform protocol | RT-qPCR N |
225 | 5 |
| McMinn et al. (2021) | USA July 2020–October 2020 |
Raw (influent, primary treated) | Heat inactivation 121 °C for 60 min | (1) Ultrafiltration, (2) Concentration Pipette | QIAgen All Prep PowerViral Kit | RT-qPCR N |
2000 | 1 |
| Mlejnkova et al. (2020) | Czech Republic April 2020–June 2020 |
Raw (influent) | NR | Skimmed milk flocculation | NucliSENS miniMAG | RT-qPCR NR |
500 | 1 |
| Mondal et al. (2021) | USA October 2020 - Jan 2021 |
Raw (influent) | NR | Direct Capture | NR | RT-qPCR N1, N2 and E |
40 | 1 |
| Monteiro et al. (2022) | Portugal April 2020–December 2020 |
Raw (influent) | Hollow-fiber filtration | PEG precipitation | QIAgen QIAamp® Fast DNA Stool mini Kit | RT-qPCR N, E and RdRp |
1000 | 1 |
| Nagarkar et al. (2022) | USA May 2020 – November 2020 |
Raw (influent) | NR | Ultrafiltration | RNeasy PowerWater Kit | dd-PCR N1 and N2 |
225 | 4 |
| Nasseri et al. (2021) | Iran April 2020–May 2020 |
Raw (influent) and treated (effluent) | Decantation for 5 min, Centrifugation at 1500 g for 20 min at 4 °C, pH adjustment to 7–7.5 using HCl and NaOH | PEG-dextran two-phase separation | FastPure Viral RNA mini Kit | RT-PCR N and ORF1ab |
250 | 2 |
| Navarro et al. (2021) | Italy December 2020 – February 2021 |
Raw (influent) | Centrifugation at 4500 g for 30 min at 4 °C | Centrifugal ultrafiltration | Quick-RNA Fecal/Soil Microbe Microprep | RT-qPCR N1, N3 and S |
100 | 1 |
| Ni et al. (2021) | Australia March 2020–April 2020 |
Raw (influent) | Centrifugation at 9000 g for 20 min at 4 °C | Ultrafiltration | QIAgen RNeasy PowerMicrobiome Kit | RT-qPCR N1 and N2 |
50 | 3,6 |
| Nourbakhsh et al. (2022) | Canada September 2020–June 2021 |
Raw (influent) | (1,2,3) Centrifugation at 4000 g for 20 min at 4 °C | (1) Centrifugal ultrafiltration, (2) Zirconia-silica beads in a Bead Mill 24 Homogenizer, (3) Centrifugation | MagNA Pure 96 DNA, Viral NA Large Volume Kit and QIAgen RNeasy PowerMicrobiome Kit | RT-qPCR N1 and N2 |
15 – 30 | 5 |
| Novoa et al. (2022) | Spain May 2020–May 2021 |
Raw (influent, sewage) and treated (effluent) | Filtration using a 20–25 μm cellulose filter, pH adjustment to 6 | Adsorption-precipitation with AlCl3 | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1, N2 and E |
150 | 5,6 |
| O’Brien et al. (2021) | USA June 2020 |
Raw (sewage) | NR | Ultrafiltration | QIAgen All Prep PowerViral DNA/RNA KIT, Monarch RNA miniprep Kit and Zymo Quick RNA-Viral | RT-qPCR N2 |
250 | 5 |
| Parra-Guardado et al. (2022) | Canada NR |
Raw (influent) | Centrifugation at 5000 rpm for 5 min | NR | Direct Magnetic Bead Extraction | RT-qPCR NR |
50 | 1 |
| Peinado et al. (2022) | Spain February 2021 – June 2021 |
Raw (influent) | (1) Centrifugation at 4600 g for 30 min, pH adjustment to 6, (2) Centrifugation at 8000 g for 30 min at 4 °C, (3) Centrifugation at 4600 g for 30 min | (1) Adsorption-precipitation with aluminum hydroxyde, (2) PEG precipitation, (3) Ultrafiltration | NZY Viral RNA Isolation Kit | RT-qPCR N1 and N2 |
100 – 200 | 1 |
| Pellegrinelli et al. (2022) | Italy March 2019–December 2020 |
Raw (influent) | (1) Centrifugation at 4500 g for 30 min, (2) Centrifugation at 4500 g for 30 min at 4 °C, (3) Centrifugation at 1200 g for 30 min at 4 °C | (1) PEG-Dextran two-phase separation, (2) PEG precipitation chloroform purification, (3) PEG precipitation with chloroform purification | QIAgen QIAamp® MinElute Virus Spin Kit and NucliSENS EasyMAG | RT-PCR N1, N3 and ORF1ab |
(1,3) 250, (2) 80 | 2 |
| Perez-Cataluna et al. (2021) | NR | NR | (1) NR, (2) Centrifugation at 2500 g for 10 min at 4 °C | (1) Aluminum hydroxyde adsorption-precipitation, (2) PEG precipitation | NucleoSpin RNA Virus Kit | RT-qPCR N1, N2, E, IP2 AND IP4 |
200 | 1 |
| Petala et al. (2022) | Greece October 2020 – January 2021 |
Raw (influent) | pH adjustment to 4 using 2 M HCl, Centrifugation at 4000 g for 30 min | Adsorption-elution using electronegative membranes | Phenol-chloroform-based RNA extraction protocol | RT-PCR N2 and E |
200 | 5 |
| Philo et al. (2021) | USA March 2020–July 2020 |
Raw (influent after sedimentation) | NR | (1) Bag-mediated Filtration System (BMFS), (2) Skimmed milk flocculation, (3) PEG precipitation, (4) Ultrafiltration | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1, N2 and N3 |
(1) 100, (2) 500, (3) 1000 | 1 |
| Philo et al. (2022) | October 2020–March 2021 | Raw (influent after sedimentation) | NR | Skimmed milk flocculation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 and N2 |
50 | 1 |
| Pillay et al. (2021) | South Africa July 2020 – October 2020 |
Raw (influent) | Heat activation 60 °C for 90 min, Centrifugation at 3500 g for 10 min | Ultrafiltration | QIAgen QIAamp® Viral RNA mini Kit | dd-PCR N2 |
250 | 5 |
| Pino et al. (2021) | Colombia NR |
Raw (influent) | NR | (1) Flocculation with AlCl3, (2) PEG precipitation, (3) Flocculation with skimmed milk, (4) Ultrafiltration | EZNA Total RNA Kit | RT-qPCR N, E and RdRp |
200 | 2 |
| Prakash (2021) | India June 2020–July 2021 |
Raw (sewage) | (1) Centrifugation at 4700 g for 30 min, (2) Precentrigufation at 5000 rpm for 30 min, (3) NR | (1) Ultrafiltration, (2) PEG precipitation (3) PEG-dextran two-phase separation | QIAgen RNeasy PowerMicrobiome Kit | RT-qPCR N1, N2 and E |
200 – 550 | 1 |
| Qiu et al. (2022) | Canada May 2020 |
Raw (influent) | pH adjustment to 9.6–10 using 5 N NaOH, Centrifugation at 4500 g for 10 min, Removal of supernatant and pH readjustment to 7 | Centrifugal ultrafiltration | QIAgen RNeasy PowerMicrobiome Kit, MagMAX-96 Viral RNA Isolation Kit, MagMAX Viral/Pathogen Viral RNA mini Kit and ReliaPrep RNA miniprep System | RT-qPCR N1, N2, E and RdRp |
100 | 3 |
| Ramos-Mandujano et al. (2021) | Saudi Arabia June 2020 |
Raw (sewage) | NR | (1) Adsorption-elution with electronegative membranes, (2) Silica-coated magnetic nanoparticles | QIAamp® RNA mini Kit | RT-PCR N1 and N2 |
300 – 500 | 1,3 |
| Randazzo et al. (2020) | Spain March 2020 – April 2020 |
Raw (influent) and treated (secondary and tertiary effluents) | NR | Aluminum hydroxide adsorption-precipitation | NucleoSpin RNA virus Kit | RT-qPCR N1, N2 and N3 |
200 | 1 |
| Reynolds et al. (2022) | Ireland September 2020 – March 2021 |
Raw (influent) | Centrifugation at 3200 g for 5 min | Ultracentrifugation | QIAgen RNeasy PowerMicrobiome Kit | RT-qPCR and dd-PCR N1 |
200 – 225 | 2.6 |
| Robotto et al. (2022) | Italy July 2020–March 2021 |
Raw (influent) | NR | NR | Wastewater Large Volume Total Nucleic Acid Capture Kit AX9550 | RT-qPCR N1, N2 and E |
40 | 1,3 |
| Rocha et al. (2022) | USA July 2020–May 2021 |
Raw (influent) | 2.5 M MgCl2 was added at a ratio of 1:100 to a final concentration of 25 mM | Adsorption-elution with electronegative membranes | QIAgen PowerViral Kit | RT-qPCR N1 and N2 |
40 – 495 | 3,4,5 |
| Rodriguez Rasero et al. (2022) | Spain July 2020 – February 2021 |
Raw (sewage) | pH adjustment to 6 using 2 N HCl | AlCl3 precipitation | NucleoSpin RNA Virus Kit and QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N, E and IP4 |
200 | 5 |
| Roka et al. (2021) | Hungary June 2020 – October 2020 |
Raw (influent) | (1) NR, (2) Centrifugation at 4500 g for 30 min at 4 °C | (1) Skimmed milk flocculation, (2) Ultrafiltration | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N |
(1,2) 50 | 1,4 |
| Rondeau et al. (2021) | USA NR |
Raw (sewage) | Heat inactivation 60 °C for 1 h, filtering using 0.22 μm filter | Centrifugal ultrafiltration | Quick RNA miniprep Kit | RT-qPCR N1 |
40 | 3 |
| Rosiles-Gonzalez et al. (2021) | Mexico August 2020–January 2021 |
Treated (primary, biofilter and biological treatment) | (1) Filtration using a 0.45 μm cellulose-ester membrane, (2,3) Sequential filtration using 0.8, 0.65, 0.45 and 0.22 μm cellulose-ester membranes | (1) Adsorption-elution with electronegative membranes, (2) PEG precipitation, (3) Centrifugal filtration. | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 and N2 |
(1) 1.0 – 5.4, (2) 200 – 1000, (3) 0.6 – 1.3 | 1,3 |
| Sapula et al. (2021) | Australia NR |
Raw (influent) | (1) Centrifugation at 5000 g for 30 min at 4 °C, (2) adding MgCl2 to a final concentration of 25 mM | (1) PEG precipitation, (2) Adsorption-elution with electronegative membranes | TRIzol-phenol extraction, NucleoSpin RNA Virus Extraction Kit and RNeasy PowerWater Kit | RT-qPCR N1 and N2 |
100 | 1,3 |
| Saththasivam et al. (2021) | Qatar June 2020 – August 2020 |
Raw (influent) | Heat inactivation 56 °C for 30 min, Centrifugation 4500 g for 30 min at 4 °C | PEG precipitation | Quick RNA Viral Kits (Zymo) | RT-qPCR N1, N2 and RdRp |
200 | 2,5 |
| Scott et al. (2021) | USA August 2020 – December 2020 |
Raw (sewage) | NR | PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR and dd-PCR N1 and N2 |
200 | 1 |
| Sharma et al. (2021) | India May 2020 – May 2020 |
Raw (sewage) | Chloroform was added and mixed thoroughly using a magnetic stirrer for 30 min at 4 °C, Centrifugation at 3000 g for 20 min at 4 °C | PEG-dextran phase separation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR E and RdRp |
500 | 2 |
| Sherchan et al. (2020) | USA January 2020–April 2020 |
Raw (influent) and treated (secondary treatment, effluent) | (1) Centrifugation at 3000 g for 30 min, (2) NR | (1) Ultrafiltration, (2) Adsorption-elution with electronegative membranes | ZR Viral RNA Kit | RT-qPCR N1 and N2 |
100 – 1000 | 1 |
| Song et al. (2021) | USA April 2020 – June 2020 |
Raw (influent) | (1) Heat inactivation 60 °C for 90 min, Centrifugation at 4000 g for 30 min, filtration using 0.45 μm sterile membrane filter, (2) NR | (1) PEG precipitation, (2) Direct extraction method | QIAgen QIAamp® Viral RNA mini Kit and Zymo Quick-RNA Fecal/Soil Microbe Microprep Kit | RT-qPCR and ddPCR N1 and N2 |
50 | 1,2,6 |
| Tandukar et al. (2022) | Nepal July 2020–February 2021 |
Raw (influent, sewage) | NR | Electronegative membrane-vortex (EMV) | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1, N2 and E |
100 | 1,3 |
| Tanhaei et al. (2021) | Iran June 2020–July 2020 |
Raw (influent) and treated (effluent) | NR | Adsorption-elution with electronegative membranes | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N and ORF1ab |
200 | 2 |
| Tanimoto et al. (2022) | Japan February 2021 – October 2021 |
Raw (influent) | Centrifugation at 10000 g for 30 min | PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N |
40 | 4 |
| Thongpradit et al. (2022b) | Thailand January 2021–February 2021 |
Raw (sewage) | Centrifugation at 3000 g for 10 min at room temperature | Adsorption-elution using electronegative membranes | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N, S and ORF1ab |
100 – 400 | 2 |
| Tiwari et al. (2022) | Finland August 2020–May 2021 |
Raw (influent) | Centrifugation at 3000 g for 25 min | Ultrafiltration | Chemagic Viral300 DNA/RNA Extraction Kit | RT-qPCR E and N2 |
NR | 1 |
| Toledo et al. (2022) | USA Sept 2020 - Feb 2021 |
Raw (influent) | Centrifugation at 4600 g for 30 min at 4 °C | PEG precipitation | Promega Wastewater Large-Volume TNA Capture Kit | RT-qPCR and RT-ddPCR N1 and N2 |
45 | 1 |
| Tomasino et al. (2021) | Portugal May 2020–March 2021 |
Raw (influent) | pH adjustment to 3.5/4 using HCl, Heat inactivation 60 °C for 90 min | (1) NR, (2) Sequential centrifugations followed by PEG precipitation | QIAgen RNeasy Powersoil Total RNA, QIAgen RNeasy PowerMicrobiome Kit and IDEXX DNA/RNA Magnetic Bead Kit | RT-qPCR N1 and N2 |
(1) 10 – 80, (2) 35 | 3 |
| Torii et al. (2021) | Japan NR |
Raw (influent) | (1) Centrifugation at 3500 g for 15 min, (2) filtration using a through 0.45 μm cellulose-ester membrane, (3) Centrifugation at 3500 g for 5 min | (1) Ultracentrifugation, (2) EMV, (3) PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit and Acid guanidium thiocyanate-phenol-chloroform extraction using TRIzol protocol | RT-qPCR N1, N2 and N3 |
40 – 50 | 2 |
| Torii et al. (2022) | Japan July 2020–October 2020 |
Raw (influent) | (1,2) Centrifugation at 3500 g for 5 min, (3) Centrifugation at 4700 g for 30 min at 4 °C, (4) filtration using a 0.2 μm hydrophilic polytetrafluoroethylene membrane (Millipore), (5) NR | PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 and N2 |
41 | 1,2 |
| Trottier et al. (2020) | France May 2020–July 2020 |
Treated (effluent) | Centrifugation at 4500 g for 30 min at 4 °C, | Centrifugal filtration | NucleoSpin RNA Virus Kit | RT-qPCR N1, N2 and RLP27 |
50 | 3 |
| Trujillo et al. (2021) | NR | NR | Heat inactivation 60 °C for 60 min, filtration using a 0.22 μm filter | PEG precipitation | TRIzol-chloroform protocol | RT-qPCR N1 |
40 | 3 |
| Vallejo et al. (2022) | Spain March 2020 – May 2020 |
Raw (influent) | Centrifugation at 4000 g for 30 min, filtration using 0.22 μm membranes | Ultrafiltration | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N |
100 | 4.5 |
| Wehrendt et al. (2021) | Argentina April 2021–May 2021 |
NR | (1) Centrifugation at 12000 g for 1 h at 4 °C, (2) pH adjustment to 6–7 | (1) PEG precipitation, (2) Centrifugation with PAC | High Pure Viral Nucleic Acid Kit and Viral Nucleic Extraction Kit II | RT-qPCR N and ORF1 |
(1) 200, (2) 40 | 1,3,4 |
| Westhaus et al. (2021) | Germany April 2020 |
Raw (influent after sand trap) and treated (activated sludge) | Centrifugation at 4700 g for 30 min | Ultracentrifugation | NucleoSpin RNA virus Kit | RT-qPCR N, E and RdRp |
45 | 2 |
| Whitney et al. (2021) | USA NR |
Raw (influent) | NR | NR | 4S-column and 4S-Milk-of-Silica | RT-qPCR N1 |
40 | 1 |
| Wu et al. (2022) | USA January 2020 – Ma 2020 |
Raw (influent) | Heat inactivation 60 °C for 90 min, filtration using a 0.2 μm sterile membrane filter | PEG precipitation | TRIzol-chloroform protocol | RT-qPCR N1 and N2 |
40 | 4,5 |
| Xiao et al. (2022) | USA March 2020 – June 2020 |
Raw (influent) | Heat inactivation 60 °C for 1 h, filtration using a 0.2 μm vacuum-driven filter |
Centrifugal ultrafiltration | NR | RT-qPCR N1 and N2 |
15 | 5 |
| Xu et al. (2021) | Hong Kong June 2020 – September 2020 |
Raw (influent and sewage) | Heat inactivation 60 °C for 30 min, Centrifugation at 4750 g for 30 min | Ultrafiltration | TRIzol Plus RNA Purification Kit | RT-qPCR N |
50 – 90 | 1 |
| Yanac et al. (2022) | Raw (influent) and treated (primary sludge, secondary effluent, final effluent) | (1) Cheesecloth and low-protein binding 0.45 and 0.2 μm 47-mm Supor-200 membrane disc filters, (2) NR |
(1) Ultrafiltration, (2) Skimmed milk flocculation | QIAgen RNeasy PowerMicrobiome Kit and MagMAX Microbiome Kit | RT-qPCR N1 and N2 |
120 | 1,4,5 | |
| Yaniv et al. (2021) | Israel November 2020–March 2021 |
Raw (influent) | Shaken and mixed for 2 min manually and left standing 15 min to large particle settlement | Ultrafiltration | NucleoSpin RNA Extraction Kit | RT-qPCR N1, N2, N3 and N4 |
2000 – 5000 | 1,5 |
| Zhang et al. (2022) | Australia August 2020 – September 2020 |
Raw (influent) | NR | Adsorption-elution with electronegative membranes | QIAgen RNeasy PowerWater Kit | RT-qPCR N and E |
100 | 2 |
| Zhao et al. (2022) | USA September 2020 – August 2021 |
Raw (influent) | NR | PEG precipitation | QIAgen QIAamp® Viral RNA mini Kit | RT-ddPCR N1 and N2 |
NR | 4 |
| Zheng et al. (2022) | Hong Kong September 2020 – November 2020 |
Raw (influent and sewage) | Heat inactivation 60 °C for 30 min | (1) Ultracentrifugation, (2) PEG precipitation, (3) AlCl3 flocculation, (4) MgCl2 flocculation, (5) Ultracentrifugation 10 kDa, (6) Ultracentrifugation 30 kDa, (7) Membrane adsorption with AlCl3, (8) Adsorption-elution using electronegative membranes, (9) Combination of centrifugation and ultracentrifugation, (10) AlCl3 precipitation, (11) Membrane adsorption | QIAgen QIAamp® Viral RNA mini Kit and TRIzol Plus RNA Purification Kit | RT-qPCR N1 |
30 – 1000 | 1 |
| Zhu et al. (2022) | Japan August 2020 – February 2021 |
Raw (influent) | NR | Ultracentrifugation | QIAgen QIAamp® Viral RNA mini Kit | RT-qPCR N1 |
40 | 4,5 |
Note: NR stands for Not Reported.
3.2.1. Pre-treatment
Pre-treatment of the wastewater samples has been ignored as a step of the laboratory analysis process in previous review articles, even though several procedures preceding the concentration step were found in roughly 80 % of the studies in our pool. Nevertheless, the term “pre-treatment” was employed only in a few studies (Torii et al., 2021; Zhang et al., 2022). Common pre-treatment procedures involve viral inactivation, pre-centrifugation, pH adjustment, and filtration through a single or a sequence of membrane filters. Pre-treatment serves the purpose of removing coarse solid material (Jmii et al., 2021), separating fine solids, and further purifying against bacterial beings (Reynolds et al., 2022). For the inactivation, we found that it can be performed through thermal treatment (Calderon-Franco et al., 2022; McMinn et al., 2021), UV light (Castiglioni et al., 2022; Pellegrinelli et al., 2022), or chemically (Tomasino et al., 2021). Filtration was done at the micrometer level (maximum pore size of 2 μm), and pre-centrifugation was performed at a minimal value of 1500 g but not exceeding 6000 g for a minimum duration of 5 min and a maximum of 45 min. Adjustment of sample pH was done when required for the following concentration step by using negatively charged membranes or precipitation using polyethylene glycol (PEG) (Farkas et al., 2021; Hasing et al., 2021).
3.2.2. Concentration
Concentration methods should ideally fulfill some features, including but not limited to being sensitive, reproducible, simple from a technical point of view, economical, rapid, and provide high viral recoveries (Prakash, 2021). A single standardized method for SARS-CoV-2 concentration from sewage has not been reported (Wehrendt et al., 2021). However, several methods have been described in the literature for that purpose (Prakash, 2021). Following the criteria of Birnbaum et al. (2022), these methods can be classified into two categories: (i) size-based techniques, such as ultrafiltration (Dumke et al., 2021; Hasing et al., 2021), ultracentrifugation (Zheng et al., 2022), centrifugal ultrafiltration (Anderson-Coughlin et al., 2021), and adsorption-elution with electronegative membranes (Barril et al., 2021; Jmii et al., 2021), and (ii) entrapment in chemical precipitates, namely, PEG precipitation (Alexander et al., 2020; Farkas et al., 2021), aluminum flocculation (Pino et al., 2021; Salvo et al., 2021), or skimmed milk flocculation (Philo et al., 2021; Pino et al., 2021). Explaining the fundamentals of these concentration methods is beyond the scope of this review, nevertheless the literature is rich in guidelines for the application of these methods. Readers can refer to the studies of Kaya et al. (2022), Dumke et al. (2021), Barril et al. (2021), and Salvo et al. (2021) to understand in detail these concentration methods and how they have been compared quantitatively. Overall, these studies share the conclusion that PEG precipitation, aluminum flocculation, and ultrafiltration methods favor higher viral recovery rates during the concentration step.
3.2.3. Detection & quantification
The most frequently used method in WBE for SARS-CoV-2 RNA detection is polymerase chain reaction (PCR)-based quantification (Ni et al., 2021). In this regard, real-time reverse transcription–PCR (real-time RT-PCR) has been employed for identifying SARS-CoV-2 genetic targets (Ni et al., 2021; Thongpradit et al., 2022) and is still considered the gold standard method for the detection of SARS-CoV-2 (Ambrosi et al., 2021). Regarding its genomic targets, the nucleocapsid (N) or the envelope (E) protein genes, as well as the ORF1ab gene are the most often used RT-PCR targets, as presented in Table 2 (Corman et al., 2020; Kitajima et al., 2020). The Centers for Disease Control and Prevention (CDC) indicates the use of probes targeting several loci (N1 and N2) of the nucleocapsid via separate reactions (CDC, 2020). Particularly, N1 is commonly employed as an indicator for detecting SARS-CoV-2 in wastewater (Navarro et al., 2021). Different PCR procedures form a list that encompasses the reverse transcription loop-mediated isothermal amplification (RT-LAMP) (Amoah et al., 2021), the reverse transcription droplet digital PCR (RT-ddPCR) (Flood et al., 2021), the reverse transcription quantitative PCR (RT-qPCR) (Ahmed et al., 2020a) and its variations and improvements (La Rosa et al., 2020; Navarro et al., 2021). As can be concluded from Table 2, the RT-qPCR is the most often employed detection/quantification technology and was used in roughly 87 % of the studies in our pool.
3.3. Correlating clinical testing data to viral concentrations in wastewater
The correlation between SARS-CoV-2 viral concentration in sewage water and the number of COVID-19 cases is one of the major challenges of applying viral detection in sewage water to track the scale of SARS-CoV-2 spread in a community (Haque et al., 2021). According to Peccia et al. (2020), some studies have reported the successful correlation of viral RNA levels in wastewater and sludge with the number of reported COVID-19 cases. Such correlation is useful to predict the number of active cases in the population (Ahmed et al., 2020a; Hellmer et al., 2014; Li et al., 2021b; Saththasivam et al., 2021). Particularly, with this information, WBE models can translate viral concentrations in wastewater to the incidence of SARS-CoV-2 shedders within a community (Cao and Francis, 2021).
There are mainly two statistical-based approaches to evaluate these correlations, the estimation of Pearson's correlation coefficient (Forthofer et al., 2007), which is applied to evaluate the level of linear association between two normally distributed variables, and Spearman's rank correlation coefficient for non-normally distributed data prone to contain outliers (Schober et al., 2018). Both coefficients vary within the range from −1 to +1, where −1 indicates a perfect negative relationship between the variables, 0 indicates the inexistence of a linear relationship and +1 points to a strong positive linear association. We found rather high positive coefficients peaking at values of 0.947 (Galani et al., 2022), 0.95 (D'Aoust et al., 2021), and 0.96 (Layton et al., 2022), for instance, but we also collected moderate (Giraud-Billoud et al., 2021; Tandukar et al., 2022; Tomasino et al., 2021) and weak (Ahmed et al., 2020a) correlations when evaluated through these two statistical approaches depending on the nature of their data. Additionally, some studies in the literature have reported negative correlations (Wehrendt et al., 2021). Recently, a meta-analysis study conducted by Li et al. (2023) collected 133 correlation coefficients ranging from −0.38 to 0.99 for Pearson's or Spearman's coefficients; according to the authors, such a wide range of coefficient values is endorsed by several factors, including variations of the environmental conditions, epidemiological conditions, sampling design, air temperature, etc. This observation is consistent with the numbers found throughout our review process, which also showed a spacious range of values.
To maintain concentration levels meaningful and consistent, normalizing the viral concentration is of paramount importance due to the variability of viral levels in wastewater, which is caused by several factors (Li et al., 2023), such as wastewater flow rate, weather conditions, total suspended solids, and daily fecal discharge. This normalization has been reported in the literature to be addressed via various approaches, such as daily mass flux and/or the use of biomarkers (Qiu et al., 2022). One of these biomarkers, the Pepper Mild Mottle Virus (PMMoV), found in human fecal excreta (Rosario et al., 2009), has been used to normalize the SARS-CoV-2 signal (LaTurner et al., 2021; Qiu et al., 2022; Robotto et al., 2022), contributing to obtain strong correlations between the viral concentration level in wastewater and COVID-19 clinical cases (D'Aoust et al., 2021). When addressing the correlation between viral concentration in wastewater and COVID-19 cases it should be noted that viral RNA concentrations in wastewater can be considered a lagging indicator since the virus continues to be shed after the infected individuals have been recovered (McMahan et al., 2021). This lag time has been reported in several studies to range between 2 and 28 days (Zhao et al., 2022), but it lacks a well-accepted definition as discussed in the next paragraphs. Such variation in the lag times can be caused by multiple factors, including but not limited to, daily changes in population size, wastewater sampling methods, responses of the society to the pandemic, and variations in the time required for reporting case data (Medema et al., 2020; Peccia et al., 2020). For instance, some authors have reported that the duration of viral shedding in the stools can be extended up to 33 days after obtaining a negative nasopharyngeal swab (Gupta et al., 2020; Jones et al., 2020). From a symptom onset perspective, it has been suggested that fecal viral shedding can hold up to >20 days (Wolfel et al., 2020; Wu et al., 2020), with Miura et al. (2021) having estimated a value of 26 days. Although different lag time values have been proposed in several WBE studies, these works usually lack a definition for this term, which can be a potential source of confusion when comparing SARS-CoV-2 WBE studies. Zhao et al. (2022) consider the lag time as the temporal gap between the measured SARS-CoV-2 concentration peaks and the reported COVID-19 clinical testing cases peaks, while Omori et al. (2021) define this term as “the lag between the detection timing from wastewater and reporting by passive surveillance”; ideally, detection and reporting timing should be concurrent, however in practice that is not the case, especially in low and middle-income countries (Li et al., 2021d). Finally, lag times may also be influenced by SARS-CoV-2 incubation time and shedding duration (Zhao et al., 2022). For instance, Wu et al. (2022) explained that the lag time they reported (4 days) was consistent with the common incubation period from viral infection to symptom inception, which is considered to be between 4 and 5 days. Other studies reported lag periods similar to this value (Lara-Jacobo et al., 2022; Peccia et al., 2020; Xiao et al., 2022).
3.4. Modeling of WBE for COVID-19 surveillance
Modeling techniques for COVID-19 surveillance in wastewater comprise a rather wide spectrum, going from a plethora of regression techniques to the application of conservation principles and more elegant and contemporary data-driven methods. Comparing or ranking the results from each modeling approach is beyond the scope of this review and itself is a complex task, although several studies have presented comparisons between the performance of different predictive models as in Zhao et al. (2022), Aberi et al. (2021), and Li et al. (2021b). Table 3 brings a complete description of the methodological approaches reported in our pool of studies for modeling WBE for COVID-19 surveillance.
Table 3.
Summary of variables involved in SARS-CoV-2 WBE modeling and respective modeling techniques.
| Study | Location and sampling period | Lowest and highest conc. (solid phase) | Lowest and highest conc. (liquid phase) | Estimated lag period | Statistical correl. and coeff. valuea | Modeling technique/algorithmb | Type of contribution (Fig. 2) |
|---|---|---|---|---|---|---|---|
| Aberi et al. (2021) | Austria (Data collected from databases) |
NR | NR | 2–7 days | NR | Regression models applied to predicting the number of active cases: Linear (LR), Polynomial (PL), K-Nearest Neighbor (KNN), Multilayer Perceptron (MLP), Support Vector Regression (SVR), Generalized Additive Models (GAM), Decision Tree (DT) and Random Forest (RF) | 5 |
| Acosta et al. (2022) | Canada June 2020–May 2021 |
NR | NR | 4 weeks | Pearson's correlation (r = 0.70) |
NR | 4 |
| Ahmed et al. (2021c) | Australia February 2020–May 2020 |
NR | 1.35E2 – 1.2E4 gc/100 mL | NR | NR | NR | 2 |
| Ai et al. (2021) | USA July 2020–January 2021 |
NR | 1E2 – 1E5 gc/L | 5 days | Pearson correlation (r = 0.89) and Spearman's rank correlation (r = 0.88) | Polynomial models | 4,5,6 |
| Amereh et al. (2022) | Iran September 2020–April 2021 |
NR | 4E1 – 4.5E4 gc/L | NR | NR | Monte Carlo simulation to estimate disease prevalence, LR between estimated infected population and confirmed cases (R2 = 0.80, p < 0.001) | 4 |
| Anneser et al. (2022) | USA March 2020–March 2021 |
NR | NR | NR | Spearman's rank correlation (r = NR) |
LR (R2 = 0.80), GAM (R2 = 0.86), Poisson (R2 = 0.84) and negative binomial models (R2 = 0.15) | 4,5 |
| Bagutti et al. (2022) | Switzerland July 2021–December 2021 |
NR | 1E2 – 4.13E5 gc/L | 14 days | Spearman's rank correlation (r = 0.9395) |
NR | 4 |
| Barrios et al. (2021) | Argentina June 2020–April 2021 |
NR | 1E-1 – 1E3 gc/L | NR | Spearman rank correlation (r = 0.812) |
NR | 4 |
| Cao and Francis (2021) | USA April 2020–February 2021 |
NR | NR | NR | NR | Vector Autoregression (VAR) model | 5 |
| Claro et al. (2021) | Brazil June 2020–April 2021 |
NR | 2.7 – 7.7 log10 gc/L | 2 weeks | NR | Monte Carlo simulations to estimate COVID-19 prevalence for each sampling site | 4 |
| de Freitas et al. (2022) | Brazil January 2021–January 2022 |
NR | NR | NR | Spearman's rank correlation (r = 0.67) |
Monte Carlo statistical model to introduce uncertainty in the virus shedding | 4,5 |
| de Sousa et al. (2022) | Brazil January 2021–August 2021 |
NR | N1: 2.73 – 3.73 log10 gc/L; N2: 2.69 – 5.47 log10 gc/L | NR | NR | Prediction model for infected individuals published by Ahmed et al. (2020a) with Monte Carlo simulations to introduce uncertainties | 5 |
| Fernandez-Cassi et al. (2021) | Switzerland February 2020–April 2020 |
NR | NR | 5.5 days | NR | Incidence estimation by the Susceptible-Exposed-Infectious-Recovered (SEIR) model with Gamma distribution to represent virus shedding and time between infection and symptom onset | 5 |
| Fitzgerald et al. (2021) | Scotland April 20202 - January 2021 |
NR | NR | NR | Spearman's rank correlation (r = 0.91) |
Basic linear mixed model | 1,2 |
| Galani et al. (2022) | Greece August 2020–March 2021 |
NR | NR | 5–9 days | Pearson's correlation (r = 0.947) |
Distributed/fixed lag modeling, LR, and artificial neural networks (ANN) were utilized to build relationships between SARS-CoV-2 RNA load in wastewater and pandemic health indicators | 4,5 |
| Gonzalez et al. (2020) | USA March 2020–August 2020 |
NR | 1E1 – 1E4 gc/100 mL | NR | NR | NR | 2 |
| Hemalatha et al. (2021) | India July 2020–August 2020 |
NR | NR | NR | NR | Predictions models for infected individuals published by Ahmed et al. (2020a) and by Hellmer et al. (2014) | 2,3 |
| Hoar et al. (2022) | USA August 2020–April 2021 |
NR | NR | NR | Spearman's rank correlation (r = 0.81) |
LR (R2 = 0.65) | 4,5 |
| Jiang et al. (2022) | USA May 2020–December 2021 |
NR | NR | NR | NR | ANN (Best fit with R2 = 0.89) | 5 |
| Karthikeyan et al. (2021) | USA July 2020–October 2020 |
NR | NR | 3 weeks | Pearson's correlation (r = 0.84) |
Linear Regression model with Autoregressive model (ARIMA) | 3,4 |
| Koureas et al. (2021) | Greece October 2020–April 2021 |
NR | NR | NR | NR | LR (R2 = 0.9511) and RF (R2 = 0.9956) | 5 |
| Krivonakova et al. (2021) | Slovakia September 2020–March 2021 |
NR | NR | 2 weeks | NR | Regression models to calculate viral concentration: Simple Linear, Double Square Root, and Square Root-Y | 2,4 |
| Kuhn et al. (2022) | USA November 2020–March 2021 |
NR | 1.6E1 – 7.3E6 gc/L | 4–10 days | Pearson's correlation and Spearman rank correlation (r = NR) |
General Multivariate Linear Regression, multivariate Poisson (best accuracy obtained) and Negative Binomial models | 5 |
| Layton et al. (2022) | USA June 2020–July 2020 |
NR | 2.9 – 5.1 log10 gc/L | NR | Pearson's correlation (r = 0.96) |
Monte Carlo simulation to account for the uncertainty in the point estimates for each sampling event | 4 |
| Li et al. (2021b) | Australia Used data from seven papers |
NR | NR | NR | NR | Three types of data-driven models were applied to a multi-national WBE dataset: multiple linear regression (MLR), ANN and adaptive neuro-fuzzy inference system (ANFIS) to predict upcoming new cases | 5 |
| Li et al. (2022) | USA June 2020–September 2021 |
NR | 2.76E3 – 3.86E6 gc/L | 7 days | Spearman's rank correlation (r = 0.790) |
NR | 2,4 |
| Maida et al. (2022) | Italy September 2021–July 2021 |
NR | NR | NR | NR | A logistic regression model was calculated to evaluate the association between the active SARS-CoV-2 incidence rates and the probability of positive PCR results of wastewater samples | 5 |
| McMahan et al. (2021) | USA May 2020–August 2020 |
NR | 4.7E3 – 3.3E6 gc/L | NR | NR | SEIR model to predict the number of infected individuals based on the mass rate (gc/day) of SARS-CoV-2 RNA in wastewater | 5 |
| Nagarkar et al. (2022) | USA May 2020–November 2020 |
NR | 1E3 – 1E4 gc/L for N1 and N2 | NR | Pearson's correlation (r = 0.70) |
NR | 4 |
| Nourbakhsh et al. (2022) | Canada September 2020–June 2021 |
NR | NR | 3–20 days | NR | Viral transmission is simulated via a standard epidemiological SEIR-like model and the fate of SARS-CoV-2 in wastewater using an advection-dispersion-decay model | 5 |
| Omori et al. (2021) | USA April 2020 – June 2020 |
NR | ~10 – ~4E2 gc/mL | 8.4–11.6 days | NR | Data fitting using Poisson distribution | 5 |
| Peccia et al. (2020) | USA March 2020–June 2020 |
NR | 1.7E3 – 4.6E5 gc/mL | 6–8 days | NR | LRs were used to estimate the relationship between SARS-CoV-2 RNA copies per ml results for replicated RNA extractions of each daily sample. Estimation of primary sludge as a potential leading indicator was performed using a distributed lag measurement error time series model | 4,5 |
| Petala et al. (2022) | Greece October 2020–January 2021 |
NR | NR | NR | NR | Developed a set of parametric equations to estimate the evolution of global virus shedding rate in wastewater | 5 |
| Pillay et al. (2021) | South Africa July 2020–October 2020 |
NR | 0 – 7.12E5 gc/100 mL | NR | NR | Prediction model for infected individuals published by Ahmed et al. (2020a) | 5 |
| Proverbio et al. (2022) | Luxembourg NR (Data collected from databases) |
NR | NR | NR | NR | SEIR epidemiological model in combination with the extended Kalman filter (EKF) | 5 |
| Reynolds et al. (2022) | Ireland June 2020–August 2021 |
NR | NR | 0 days | Spearman's rank correlation (r = 0.500) |
NR | 2,6 |
| Rodriguez Rasero et al. (2022) | Spain July 2020 – February 2021 |
NR | NR | 6 days | NR | Data fitting using quasi-Poisson modeling | 5 |
| Roka et al. (2021) | Hungary June 2020–October 2020 |
NR | ~5E3 – ~1E6 gc/L | NR | NR | Data fitting using LR (Best fit value using a weighted average of viral load against daily new cases, R2 = 0.720 and p < 0.0001) | 1,4 |
| Saththasivam et al. (2021) | Qatar June 2020–August 2020 |
NR | 7.889E3 – 5.42E5 gc/L | NR | NR | Conservation principles to estimate the number of infected populations based on measuring RNA concentration | 2,5 |
| Scott et al. (2021) | USA August 2020–December 2020 |
NR | N1: 22.5 – 5.27E3 gc/100 mL; N2: 81.6 –3.91E4 gc/100 mL | NR | Spearman's rank correlation (r = 0.50) |
MLR, Simple Logistic Regression and Multiple Logistic Regression | 1,5 |
| Song et al. (2021) | USA April 2020 – June 2020 |
NR | ~8E0 – 9E5 gc/mL | NR | NR | NR | 1,2,6 |
| Tanimoto et al. (2022) | Japan February 2021–October 2021 |
1.5E7 – 2.0E8 gc/L | 3.1E7 – 5.5E8 gc/L | NR | NR Solid phase: r = 0.8482, Liquid phase: (r = 0.7803) |
LR | 4 |
| Vallejo et al. (2022) | Spain March 2020–May 2020 |
NR | 1E4 – 15E4 gc/mL | NR | NR | LR (R2 = 0.8515), GAM with a Cubic Regression Spline (R2 = 0.8767), locally estimated scatterplot smoothing (LOESS) Linear (R2 = 0.8685), LOESS Quadratic (R2 = 0.8833) | 4,5 |
| Wang et al. (2021) | USA NR |
NR | N1: 3.85E5 – 2.55E6 gc/L, N2: 3.79E5 – 2.15E6 gc/L | NR | Pearson's correlation (r = 0.94) |
Monte Carlo simulations to estimate the number of infected individuals | 4 |
| Wu et al. (2022) | USA January 2020–May 2020 |
NR | NR | 4–10 days | Pearson's correlation (r = NR) |
Wastewater data was modeled as a convolution of new clinical cases and used Markov Chain Monte Carlo (MCMC) simulation to quantify uncertainty in the shedding model | 4,5 |
| Wurtzer et al. (2022) | France March 2020–June 2021 |
NR | 0 – 1E6 gc/L | 3 days | Spearman's rank correlation (r = NR) |
LR | 4 |
| Xiao et al. (2022) | USA March 2020–June 2020 |
NR | NR | 6.4 days | NR | Approximate Bayesian computation for estimating delay distribution, convolution to estimate the transfer function model, and MCMC simulation to quantify uncertainty in transfer functions | 5 |
| Zhao et al. (2022) | USA Sept 2020 - August 2021 |
N1: 7.14E2 – 7.14E3 gc/L, N2: 8.02E2 –6.2E3 gc/L |
NR | 5 weeks | Pearson's correlation (N1: r = 0.62 and N2: r = 0.64) |
LR, ARIMA, Regression Model with Autoregressive Model with Seasonal Pattern (SARIMA) and (4) VAR | 4 |
| Zhu et al. (2022) | Japan August 2020–February 2021 |
NR | ~10 – ~70 gc/mL | NR | NR | Generalized linear model, ANN and RF to predict the cumulative number of cases | 4,5 |
Notes: NR stands for Not Reported;
The indicated value corresponds to the best fit obtained in the respective study.
The designated models aim to associate viral concentration signals from wastewater to clinical testing data.
4. Discussion
4.1. Issues and promising alternatives for SARS-CoV-2 analysis in wastewater
Quantifying low viral loads from non-clinical samples represents one of the major challenges of WBE (Calderon-Franco et al., 2022). When viral particles enter the sewage system, dilution occurs by the addition of other types of water (i.e., domestic sewage and stormwater combined or separated in the sewer), while concomitantly being exposed to a diverse range of chemical agents and physical conditions (Haramoto et al., 2020; Krivonakova et al., 2021). In this regard, a concentration step is required due to the low levels at which SARS-CoV-2 RNA is found in wastewater (Boogaerts et al., 2021; Peinado et al., 2022; Zheng et al., 2022). Several works have reported viral RNA detection in untreated wastewater (influent of the WWTP), being its concentration in the range of 102–105 copies per liter and the maximum exceeding 106 copies per liter (Kitajima et al., 2020). PEG precipitation represents a simple and low-cost alternative for viral concentration in wastewater (Flood et al., 2021). In PEG precipitation, the solvent is preferentially trapped, and proteins (e.g., virion) are sterically excluded from the solvent phase by PEG. This way, proteins can be concentrated and precipitated once their concentrations surpass the saturated solubility (Torii et al., 2022). Despite being used in many laboratories, this method suffers from losing approximately half of the viral fragments bound to solid matter (Perez-Cataluna et al., 2021). On the other hand, skimmed milk flocculation can be considered a promising approach for low-resource areas since extensive laboratory resources are not needed. Additionally, this method does not require consumables that are challenging to acquire, thus enabling the performance of uninterrupted surveillance (Philo et al., 2021). It is worth highlighting that some of these methods were developed for detecting non-enveloped enteric viruses (e.g., norovirus, adenovirus, and enterovirus), which have been the focus of most studies that investigate the existence of viruses in municipal wastewater and human excreta (Ahmed et al., 2020c; Flood et al., 2021). Additionally, SARS-CoV-2 concentration methods have been assessed using surrogate viruses to mimic SARS-CoV-2, since personnel with special training and a laboratory that fulfills Biosafety Level 3 are required for the culture of this virus. Examples of these surrogate viruses include Alphacoronavirus HCoV 229E, bovine respiratory syncytial virus, bovine coronavirus BCoV, porcine epidemic diarrhea virus, murine hepatitis virus, F-specific RNA phages, avian coronavirus of infectious bronchitis virus, mengovirus or Pseudomonas phage Phi6 (Ahmed et al., 2020c; Aquino de Carvalho et al., 2017; Balboa et al., 2020; Flood et al., 2021; Gendron et al., 2010; Hata et al., 2020; Kocamemi et al., 2020; La Rosa et al., 2020; LaTurner et al., 2021; Medema et al., 2020; Randazzo et al., 2020; Torii et al., 2022).
When it comes to the precise detection and viral quantification, multiplex PCR enables multiple target detection and/or quantification with a sensitivity comparable to that of singleplex PCR. Hence, multiplex PCR is a promising technology since it is more cost-effective and time-saving, reduces the required sample volume, and minimizes the variability due to pipetting. Nevertheless, the complexity of this assay requires optimization to prevent several undesired phenomena, such as primer-probe sets interaction (Navarro et al., 2021; Xiao et al., 2020b). On a different note, the implementation of RT-qPCR to detect SARS-CoV-2 in wastewater has some limitations, as is time-consuming (it could take 24 h), is highly susceptible to the presence of inhibitors, and sample contamination may occur, resulting in false negative results (Ahmed et al., 2022a). Following this problem, it is necessary to explore new, alternative approaches for the detection of SARS-CoV-2 RNA in wastewater. Apart from PCR-based approaches, other methods can also be employed for viral detection (Lara-Jacobo et al., 2022). For instance, metatranscriptomic sequencing also referred to as Next Generation Sequencing is an alternative for SARS-CoV-2 detection and quantification. This technology allows capturing the whole virus genome, which is of paramount importance due to the incidences of mutation events that increase the virulence, thus significantly improving the sensitivity (Boogaerts et al., 2022; Ni et al., 2021). However, the low SARS-CoV-2 RNA concentration in wastewater, along with the existence of nucleic acids from bacteria, other viruses, animal products, and humans, makes conventional metatranscriptomic sequencing an inappropriate technology for WBE applications at this developmental stage (Boogaerts et al., 2022; Ni et al., 2021). To surmount the low viral RNA concentration challenge, the ARTIC Network amplicon library (Nemudryi et al., 2020) and ATOPlex (Xiao et al., 2020b) have been developed. Moreover, RT-LAMP has also been used for SARS-CoV-2 detection (Wei et al., 2021). This method is based on the amplification of the nucleic acids under isothermal conditions, thus avoiding the need for thermal cyclers. Different RT-LAMP approaches can be distinguished, such as the colorimetric or visual RT-LAMP or the fluorescent RT-LAMP. The former enables the visual reading of the results, whereas the latter facilitates the detection of positive amplification by using a fluorescent dye (Amoah et al., 2021; Huang et al., 2020).
Prominent alternatives in the field may be the use of sensors based on electrochemical principles (Chaibun et al., 2021; Kumar et al., 2022; Ramanujam et al., 2021), which have been extensively studied in terms of specificity and selectivity for different types of nanomaterials. Another plausible approach is the use of magnetic devices where magnetic nanoparticles designed for the separation and detection of the viral pathogens in water samples are employed (Gómez-Pastora et al., 2014; Materón et al., 2021; Yue et al., 2020). These devices might be a promising detection technology as they could be simpler, more accurate, economic, rapid, and portable, allowing the measurements to be performed at the WWTPs by plant technicians.
Finally, it has been reported that viral RNA detection can be influenced by several factors, such as the method used for RNA concentration, or the prevalence of COVID-19 infections in the community (Haramoto et al., 2020; Ni et al., 2021). As an instance of the urgent need for analytical accuracy when performing wastewater processing for WBE, we found a decent number of studies that addressed the comparison of the measured viral recovery among distinct concentration and extraction procedures; we also found studies exploring the divergences in the detection and quantification of the viral loads using variations of the PCR approach (Ahmed et al., 2022b; Flood et al., 2021). These problematics elevate the magnitude of the issue. Standardization will be a natural consequence of addressing this problem.
4.2. SARS-CoV-2 epidemiological modeling
The common assumption when using WBE for COVID-19 surveillance is that the number of viral copies observed in the wastewater samples and the reported cases from clinical sources result from the real number of infections, which encapsulates symptomatic and asymptomatic cases (Schmitz et al., 2021; Xiao et al., 2022). Following this premise, wastewater-based epidemiological models have demonstrated to be a valuable tool for estimating the number of infected individuals within a population and identifying COVID-19 infection hotspots. WBE has been hampered by the difficulty of properly correlating viral RNA measurements in wastewater to the number of infections. Also, the real number of infected individuals is generally unknown due to the limitations of the current individual testing capacity systems, especially in low-income areas. To overcome these issues, the number of infected individuals has been linked to the viral concentration (gene copies per volume) and the mass rate of viral RNA in wastewater (gene copies per day) (McMahan et al., 2021). The latter is argued to be preferable over the former because of the serial dilutions of wastewater that might occur due to rainfall, for instance, which alters the viral concentration levels along the sewer network. Using the viral mass rates can be a promising approach when developing epidemiological models given that fluctuations in flow rates compensate for the changes in viral concentrations, leaving viral mass rates unaltered. Regarding the modeling techniques, a wide range of tools has been used as demonstrated in Table 3, which can be categorized into (1) regression techniques (Krivonakova et al., 2021; Peccia et al., 2020; Tomasino et al., 2021), (2) conservation principles (McMahan et al., 2021; Saththasivam et al., 2021), and (3) data-driven methods (Aberi et al., 2021; Li et al., 2021b; Pereira et al., 2020). Different regression approaches have been explored, to name a few: simple univariate and multivariate linear regression (Kuhn et al., 2022; Roka et al., 2021; Zhao et al., 2022), logistic regression (Scott et al., 2021), Autoregressive Integrated Moving Average (ARIMA) (Karthikeyan et al., 2021; Zhao et al., 2022), and the Vector Autoregression (VAR) model (Cao and Francis, 2021; Zhao et al., 2022). WBE can be applied for the back-calculation of infection prevalence. For that purpose, regression techniques are one of the most important tools in WBE modeling. However, these methods may lead to misleading inferences, since they are proposed for independent data with linear correlations, and the WBE data are time series data (Aberi et al., 2021; Cao and Francis, 2021). In the conservation principles category, an example is the application of the susceptible-exposed-infectious-recovered (SEIR) model, which has shown promising results to predict infection prevalence through a set of interconnected ordinary differential equations (Fernandez-Cassi et al., 2021; McMahan et al., 2021; Nourbakhsh et al., 2022; Proverbio et al., 2022). Furthermore, we found that data-driven methods have acquired considerable popularity given the number of studies that employed these approaches to address the complex task of building epidemiological models, with Artificial Neural Networks (ANN) (Galani et al., 2022; Jiang et al., 2022; Li et al., 2021b; Zhu et al., 2022), Adaptive Neuro-Fuzzy Inference System (ANFIS) (Li et al., 2021b), and the Generalized Additive Model (GAM) method (Aberi et al., 2021; Anneser et al., 2022; Vallejo et al., 2022), as examples of a larger group of techniques listed in Table 3. The common interest in using these approaches may have a root in the fact that epidemiological data are generated in large amounts with a daily frequency, and that data-driven models must be constantly fed and updated with new inputs for better prediction performance.
Ideally, WBE models should account for the changeability and uncertainty in their variables, specifically for the shedding quantities and secretion routes, such as feces, urine, and sputum (Tiwari et al., 2022). We found that the usual way to associate uncertainty appears to be through the Monte Carlo simulation, which was mostly used to associate uncertainty when estimating the infection prevalence (Amereh et al., 2022; de Sousa et al., 2022; Gonzalez-Reyes et al., 2021; Wang et al., 2021) and the shedding rates (de Freitas et al., 2022; Wu et al., 2022). Going further into modeling uncertainty, it should be noted that relevant variables are potentially able to create a certain degree of uncertainty. These variables are included as model variables or functions in different WBE models. Examples are: the number of active cases influencing viral counts in wastewater (persons) (Gonzalez-Reyes et al., 2021; Rodriguez Rasero et al., 2022), daily stool mass (gfeces.person−1) (Ahmed et al., 2020a; Amereh et al., 2022; Claro et al., 2021; Pillay et al., 2021), shedding rate of SARS-CoV-2 RNA (gene copies. g−1 feces or gene copies. g−1 feces.day−1) (Ahmed et al., 2020a; Claro et al., 2021; Kuhn et al., 2022; Li et al., 2021c; McMahan et al., 2021; Pillay et al., 2021; Schmitz et al., 2021), decay of SARS-CoV-2 RNA due to storage (time−1) (Kaya et al., 2022; Li et al., 2021c; Yanac et al., 2022) time-dependent RNA degradation (McMahan et al., 2021), the offset between the observed wastewater viral RNA concentration and the estimated patient viral load (Zhu et al., 2022), and RNA temperature-dependent half-life (h) (Ahmed et al., 2020b; McMahan et al., 2021). On the same note, from a clinical perspective, it is not established the influence of the severeness of the disease on the magnitude of daily shedding (genome copies per gram of stool), and this constitutes another major source of uncertainty in WBE modeling.
Apart from the aforementioned uncertainty sources, Pillay et al. (2021) reported that the variability of the WBE approach may be mainly caused by changes in the environmental conditions (e.g., the viral dilution and stability in water are influenced by rainfall events and temperature) and the unique features of WWTPs. They highlighted the major importance of accurate knowledge of the shedding pattern within the WWTP catchment. Additionally, these authors explained that the weight of stool that is daily produced per person, which is regionally dependent and may be impacted by several factors, influences the accurate estimation of the number of infected individuals. Furthermore, we found several factors that may influence the accuracy of the back-calculation of the infection prevalence, namely population size, bioindicators' stability (PMMoV), excretion rates, sampling method, and sample preparation. Additionally, several parameters, including the temperature, per-capita water, and average travel time in the sewer, represent critical variables that are needed for identifying infection hotspots when the WBE model is applied.
4.3. Current research gaps and future guidelines for SARS-CoV-2 WBE
Further clarifications on SARS-CoV-2 WBE that need to be addressed in the near future include the persistence of the virus in the wastewater, the effect of the shedding dynamics of the virus in feces, whether urban and rural wastewater systems exhibit significative differences in their characteristics, and how the normalization of viral levels in wastewater with regard to population size should be performed (Fitzgerald et al., 2021). Additionally, one must bear in mind that COVID-19 is unevenly distributed across population types so considering cross-city differences is of paramount importance. Hence, a ‘one size fits all’ approach should not be applied to disease surveillance (Kuhn et al., 2022). We suggest that public health information should not be predicted by wastewater analysis alone but by a combination of wastewater-derived information and other data sources. This is due to the fact that changes in factors such as local demographics along with the limitations of current clinical testing/reporting systems may affect the potential of domestic wastewater as a source of information for prediction tools (Xiao et al., 2022). Other factors that should be considered in WBE modeling are reported by Kuhn et al. (2022), including the shedding duration (i.e., how long an infected individual may shed viral particles through feces), and the relationship between the infection severity and the number of viral particles that are shed. At this point, it is not completely understood how these two variables may cause changes in the observed wastewater viral concentration.
We also suggest the utilization of the solid portion of the wastewater as an alternative matrix for the analysis. There is evidence that enveloped viruses feature a high inclination to bind to the surface of solids in wastewater in comparison to non-enveloped viruses (Ye et al., 2016). As it was previously mentioned, SARS-CoV-2 possesses a lipid outer envelope (Klein et al., 2020) whose hydrophobicity may promote greater viral binding to solids in the wastewater, thus affecting viral recovery (Ahmed et al., 2020a; Ahmed et al., 2020c; Anderson-Coughlin et al., 2021). It has been pointed out that the chain of wastewater analysis procedures should not only focus on the supernatant fraction but also on the solid portion of the wastewater (Westhaus et al., 2021; Yanac et al., 2022). Additionally, and from a WBE perspective, concentration levels from the solid portion have been correlated better with COVID-19 incidence numbers when compared to signals obtained from the liquid part of the wastewater (Tanimoto et al., 2022). For modeling purposes, normalization of the concentration levels from the solid portion of the wastewater can be performed through either total suspended solids measurements (Nourbakhsh et al., 2022) or using the concentration of PMMoV; however, comparability between the concentrations obtained from the solid and liquid phases through PMMoV normalization is still restricted (Kim et al., 2022). In this regard, different studies have reported the prevalence of viral particles in the solid phase obtained from domestic wastewater, as well as observations pointing to a significantly higher amount of viral RNA in the solid portion (Kim et al., 2022; Kumblathan et al., 2023). For instance, Kitamura et al. (2021) reported that a higher level of SARS-CoV-2 RNA, compared to PMMoV RNA, was contained in the solid fraction, whereas supernatant fractions comprised lower SARS-CoV-2 RNA levels. They reasoned that the different detection of SARS-CoV-2 RNA and PMMoV RNA in the solid and liquid fractions could result from the fact that PMMoV lacks an envelope, which is present in SARS-CoV-2. Similarly, Li et al. (2021a) found that SARS-CoV-2 RNA was considerably more abundant in the solid than in the liquid fraction. This observation was further endorsed by the studies of Ni et al. (2021) and Tomasino et al. (2021) in terms of viral recovery. The higher viral RNA concentration in the solid phase of wastewater, along with the more time-efficient processing of the solid fraction (Li et al., 2021a; Nourbakhsh et al., 2022), led us to suggest that wastewater solids may represent a more convenient sample matrix, thus being a promising approach to improve analytical accuracy in WBE for SARS-CoV-2.
As highlighted throughout the present study, WBE represents a valuable tool for predicting COVID-19 cases. To this end, WBE can be implemented via several statistical models with data gathered from wastewater (Ando et al., 2023; Anneser et al., 2022). However, establishing and standardizing protocols are still required so that worldwide conducted studies could be successfully compared (Amereh et al., 2022; Fitzgerald et al., 2021). In further words, the current lack of standardization is revealed by the wide range of sample initial volumes and concentration methods that have been reported by the different studies. Thereby, sample initial volumes range from 2 mL to 1 L; moreover, the extensive variety of concentration methods that have been used include size-based and entrapment in chemical precipitates techniques, such as conventional filtration, ultrafiltration, ultracentrifugation, centrifugation, filtration using negatively charged membranes, precipitation, and direct extraction, as well as their combinations. Furthermore, it is still unclear how different pre-treatment techniques may affect the detection performance through PCR methods. In this sense, the recognition of the pre-treatment step in the wastewater analysis process is crucial to further develop standard protocols for SARS-CoV-2 detection and quantification. Finally, quality controls, variable testing, and the optimization of the methodology are considerably lacking; however, they are required in order to provide analytical accuracy (Calderon-Franco et al., 2022).
5. Conclusions
SARS-CoV-2 will remain a constant threat to public health given the increasing infectibility of new VOCs. In this study, we reviewed the recent WBE research endeavor to mitigate the hefty burden of COVID-19 on the health systems around the globe. More specifically, this review collects and organizes the recent progress on the analytical methods reported between 2020 and 2022 to detect and quantify SARS-CoV-2 RNA from wastewater samples. We also review the methods by which SARS-CoV-2 wastewater-based epidemiological modeling has been approached to use the output of lab analysis for diverse purposes, such as predicting outbreaks in a community, estimation of active human shedders (or infected individuals), and shedding rates, to name a few. Correlating the amount of genomic material in wastewater with the number of COVID-19 cases within a community is a component of epidemiological modeling that has been tried through a wide range of mathematical methods, with data-driven models considered the most popular approach to address predictions of variables correlated to outbreaks within a certain time horizon, based on genomic viral material measurements in domestic wastewaters. We also highlight the promising opportunities to improve the accuracy and rapidness of viral detection using the solid portion of wastewater as an alternative testing matrix, and the design of novel sensors based on electrochemical or magnetic devices. However, as evidenced throughout this work, recent research has not focused on ways to standardize the analytical procedures for comparability between different locations. Implementing the WBE surveillance as a prediction tool for outbreaks and infection waves, which in turn would result in the mitigation of the COVID-19 burden, remains challenging. To promote the worldwide applicability of WBE surveillance, this lack of standardization should be managed along with the establishment of a testing framework that accounts for the different analytical sensitivities throughout the different steps of the analysis. Notably, this study contributes to future research as a reference guide for what has been proposed and worked so far to understand the dynamics of viral concentrations in wastewater. Since the effort to mitigate the effects of COVID-19 is a global one, future research must expand the scope of this review and consider the needs of low-income countries, whose health systems are often restricted and the implementation of the WBE surveillance strategy can thus become more arduous.
CRediT authorship contribution statement
Stefano Ciannella: Methodology, Formal analysis, Investigation, Writing – original draft, Visualization. Cristina González-Fernández: Writing – original draft, Writing – review & editing, Visualization. Jenifer Gomez-Pastora: Conceptualization, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Financial support from Texas Tech University is acknowledged. Dr. Cristina González-Fernández thanks the Spanish Ministry of Universities for the Margarita Salas postdoctoral fellowship (grants for the requalification of the Spanish university system for 2021-2023, University of Cantabria), funded by the European Union – NextGenerationEU.
Editor: Damià Barceló
Data availability
Data will be made available on request.
References
- Aberi P., Arabzadeh R., Insam H., Markt R., Mayr M., Kreuzinger N., et al. Quest for optimal regression models in SARS-CoV-2 wastewater based epidemiology. Int. J. Environ. Res. Public Health. 2021;18 doi: 10.3390/ijerph182010778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta N., Bautista M.A., Waddell B.J., McCalder J., Beaudet A.B., Man L., et al. Longitudinal SARS-CoV-2 RNA wastewater monitoring across a range of scales correlates with total and regional COVID-19 burden in a well-defined urban population. Water Res. 2022;220 doi: 10.1016/j.watres.2022.118611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed F., Islam M.A., Kumar M., Hossain M., Bhattacharya P., Islam M.T., et al. First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation Centre in Bangladesh: variation along the sewer network. Sci. Total Environ. 2021;776 doi: 10.1016/j.scitotenv.2021.145724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., et al. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., et al. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Simpson S.L., Bertsch P.M., Bibby K., Bivins A., Blackall L.L., et al. Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance. Sci. Total Environ. 2022;805 doi: 10.1016/j.scitotenv.2021.149877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Smith W.J.M., Metcalfe S., Jackson G., Choi P.M., Morrison M., et al. Comparison of RT-qPCR and RT-dPCR platforms for the trace detection of SARS-CoV-2 RNA in wastewater. ACS ES T Water. 2022;2:1871–1880. doi: 10.1021/acsestwater.1c00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai Y., Davis A., Jones D., Lemeshow S., Tu H., He F., et al. Wastewater SARS-CoV-2 monitoring as a community-level COVID-19 trend tracker and variants in Ohio, United States. Sci. Total Environ. 2021;801:149757. doi: 10.1016/j.scitotenv.2021.149757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M.R., Rootes C.L., van Vuren P.J., Stewart C.R. Concentration of infectious SARS-CoV-2 by polyethylene glycol precipitation. J. Virol. Methods. 2020;286 doi: 10.1016/j.jviromet.2020.113977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi C., Prezioso C., Checconi P., Scribano D., Sarshar M., Capannari M., et al. SARS-CoV-2: comparative analysis of different RNA extraction methods. J. Virol. Methods. 2021;287 doi: 10.1016/j.jviromet.2020.114008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amereh F., Jahangiri-Rad M., Mohseni-Bandpei A., Mohebbi S.R., Asadzadeh-Aghdaei H., Dabiri H., et al. Association of SARS-CoV-2 presence in sewage with public adherence to precautionary measures and reported COVID-19 prevalence in Tehran. Sci. Total Environ. 2022;812 doi: 10.1016/j.scitotenv.2021.152597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoah I.D., Mthethwa N.P., Pillay L., Deepnarain N., Pillay K., Awolusi O.O., et al. RT-LAMP: a cheaper, simpler and faster alternative for the detection of SARS-CoV-2 in wastewater. Food Environ Virol. 2021;13:447–456. doi: 10.1007/s12560-021-09489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson-Coughlin B.L., Shearer A.E.H., Omar A.N., Wommack K.E., Kniel K.E. Recovery of SARS-CoV-2 from wastewater using centrifugal ultrafiltration. Methods Protoc. 2021;4:9. doi: 10.3390/mps4020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando H., Murakami M., Ahmed W., Iwamoto R., Okabe S., Kitajima M. Wastewater-based prediction of COVID-19 cases using a highly sensitive SARS-CoV-2 RNA detection method combined with mathematical modeling. Environ. Int. 2023;173 doi: 10.1016/j.envint.2023.107743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneser E., Riseberg E., Brooks Y.M., Corlin L., Stringer C. Modeling the relationship between SARS-CoV-2 RNA in wastewater or sludge and COVID-19 cases in three New England regions. J. Water Health. 2022;20:816–828. doi: 10.2166/wh.2022.013. [DOI] [PubMed] [Google Scholar]
- Aquino de Carvalho N., Stachler E.N., Cimabue N., Bibby K. Evaluation of Phi6 persistence and suitability as an enveloped virus surrogate. Environ Sci Technol. 2017;51:8692–8700. doi: 10.1021/acs.est.7b01296. [DOI] [PubMed] [Google Scholar]
- Arora S., Nag A., Sethi J., Rajvanshi J., Saxena S., Shrivastava S.K., et al. Sewage surveillance for the presence of SARS-CoV-2 genome as a useful wastewater based epidemiology (WBE) tracking tool in India. Water Sci. Technol. 2020;82:2823–2836. doi: 10.2166/wst.2020.540. [DOI] [PubMed] [Google Scholar]
- Bagutti C., Alt Hug M., Heim P., Maurer Pekerman L., Ilg Hampe E., Hubner P., et al. Wastewater monitoring of SARS-CoV-2 shows high correlation with COVID-19 case numbers and allowed early detection of the first confirmed B.1.1.529 infection in Switzerland: Results of an observational surveillance study. Swiss Med. Wkly. 2022;152:w30202. doi: 10.4414/smw.2022.w30202. [DOI] [PubMed] [Google Scholar]
- Balboa S., Mauricio-Iglesias M., Rodriguez S., Martínez-Lamas L., Vasallo F.J., Regueiro B., et al. medRxiv; 2020. The Fate of SARS-CoV-2 in WWTPs Points out the Sludge Line as a Suitable Spot for Monitoring. 2020.05.25.20112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or I., Yaniv K., Shagan M., Ozer E., Weil M., Indenbaum V., et al. Regressing SARS-CoV-2 sewage measurements onto COVID-19 burden in the population: a proof-of-concept for quantitative environmental surveillance. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.561710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldovin T., Amoruso I., Fonzo M., Buja A., Baldo V., Cocchio S., et al. SARS-CoV-2 RNA detection and persistence in wastewater samples: An experimental network for COVID-19 environmental surveillance in Padua, Veneto Region (NE Italy) Sci. Total Environ. 2021;760:143329. doi: 10.1016/j.scitotenv.2020.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M.R.F., Garcia S.C., Bruni A.C., Machado F.S., de Oliveira R.X., Dropa M., et al. One-year surveillance of SARS-CoV-2 in wastewater from vulnerable urban communities in metropolitan Sao Paulo, Brazil. J Water Health. 2022;20:471–490. doi: 10.2166/wh.2022.210. [DOI] [PubMed] [Google Scholar]
- Barril P.A., Pianciola L.A., Mazzeo M., Ousset M.J., Jaureguiberry M.V., Alessandrello M., et al. Evaluation of viral concentration methods for SARS-CoV-2 recovery from wastewaters. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios M.E., Diaz S.M., Torres C., Costamagna D.M., Blanco Fernandez M.D., Mbayed V.A. Dynamics of SARS-CoV-2 in wastewater in three districts of the Buenos Aires metropolitan region, Argentina, throughout nine months of surveillance: A pilot study. Sci. Total Environ. 2021;800:149578. doi: 10.1016/j.scitotenv.2021.149578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua V.B., Juel M.A.I., Blackwood A.D., Clerkin T., Ciesielski M., Sorinolu A.J., et al. Tracking the temporal variation of COVID-19 surges through wastewater-based epidemiology during the peak of the pandemic: A six-month long study in Charlotte, North Carolina. Sci. Total Environ. 2022;814:152503. doi: 10.1016/j.scitotenv.2021.152503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand I., Challant J., Jeulin H., Hartard C., Mathieu L., Lopez S., et al. Epidemiological surveillance of SARS-CoV-2 by genome quantification in wastewater applied to a city in the northeast of France: Comparison of ultrafiltration- and protein precipitation-based methods. Int. J. Hyg. Environ. Health. 2021;233:113692. doi: 10.1016/j.ijheh.2021.113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum D.P., Vilardi K.J., Anderson C.L., Pinto A.J., Joshi N.S. Simple affinity-based method for concentrating viruses from wastewater using engineered curli fibers. ACS ES T Water. 2022;2:1836–1843. doi: 10.1021/acsestwater.1c00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., Lott M., Shaffer M., Wu Z., North D., Lipp E.K., et al. Building-level wastewater surveillance using tampon swabs and RT-LAMP for rapid SARS-CoV-2 RNA detection. Environ. Sci. Water Res. Tech. 2022;8:173–183. [Google Scholar]
- Boogaerts T., Jacobs L., De Roeck N., Van den Bogaert S., Aertgeerts B., Lahousse L., et al. An alternative approach for bioanalytical assay optimization for wastewater-based epidemiology of SARS-CoV-2. Sci. Total Environ. 2021;789 doi: 10.1016/j.scitotenv.2021.148043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogaerts T., Van den Bogaert S., Van Poelvoorde L.A.E., El Masri D., De Roeck N., Roosens N.H.C., et al. Optimization and application of a multiplex digital PCR assay for the detection of SARS-CoV-2 variants of concern in belgian influent wastewater. Viruses. 2022;14:17. doi: 10.3390/v14030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscarini E., Manfredi G., Brambilla G., Menozzi F., Londoni C., Alicante S., et al. GI symptoms as early signs of COVID-19 in hospitalised italian patients. Gut. 2020;69:1547–1548. doi: 10.1136/gutjnl-2020-321434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Franco D., Orschler L., Lackner S., Agrawal S., Weissbrodt D.G. Monitoring SARS-CoV-2 in sewage: toward sentinels with analytical accuracy. Sci. Total Environ. 2022;804 doi: 10.1016/j.scitotenv.2021.150244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canh V.D., Torii S., Yasui M., Kyuwa S., Katayama H. Capsid integrity RT-qPCR for the selective detection of intact SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;791:148342. doi: 10.1016/j.scitotenv.2021.148342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Francis R. On forecasting the community-level COVID-19 cases from the concentration of SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;786 doi: 10.1016/j.scitotenv.2021.147451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci A., Federigi I., Liu D., Thompson J.R., Verani M. Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Reyes J., Barragan-Trinidad M., Buitron G. Surveillance of SARS-CoV-2 in sewage and wastewater treatment plants in Mexico. J. Water Process Eng. 2021;40 doi: 10.1016/j.jwpe.2020.101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni S., Schiarea S., Pellegrinelli L., Primache V., Galli C., Bubba L., et al. SARS-CoV-2 RNA in urban wastewater samples to monitor the COVID-19 pandemic in Lombardy, Italy (March-june 2020) Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.150816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . CDC; Atlanta GA: 2020. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel Catalog # 2019-nCoVEUA-01 1000 Reactions. [Google Scholar]
- Chaibun T., Puenpa J., Ngamdee T., Boonapatcharoen N., Athamanolap P., O'Mullane A.P., et al. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nat. Commun. 2021;12:802. doi: 10.1038/s41467-021-21121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P., Pasupuleti M., Jai Shankar M.R., Bharat G.K., Krishnasamy S., Dasgupta S.C., et al. First surveillance of SARS-CoV-2 and organic tracers in community wastewater during post lockdown in Chennai, South India: Methods, occurrence and concurrence. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Miro G., Anfruns-Estrada E., Martinez-Velazquez A., Vazquez-Portero M., Guix S., Paraira M., et al. Time evolution of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in wastewater during the first pandemic wave of COVID-19 in the metropolitan area of Barcelona, Spain. Appl. Environ. Microbiol. 2021;87:1–9. doi: 10.1128/AEM.02750-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claro I.C.M., Cabral A.D., Augusto M.R., Duran A.F.A., Graciosa M.C.P., Fonseca F.L.A., et al. Long-term monitoring of SARS-COV-2 RNA in wastewater in Brazil: a more responsive and economical approach. Water Res. 2021;203 doi: 10.1016/j.watres.2021.117534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusker J. Elsevier compendex and Google scholar: a quantitative comparison of two resources for engineering research and an update to prior comparisons. J. Acad. Librariansh. 2013;39:241–243. [Google Scholar]
- D'Aoust P.M., Mercier E., Montpetit D., Jia J.J., Alexandrov I., Neault N., et al. Quantitative analysis of SARS-CoV-2 RNA from wastewater solids in communities with low COVID-19 incidence and prevalence. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport T.H., Godfrey A.B., Redman T.C. To fight pandemics, we need better data. MIT Sloan Manag. Rev. 2020;62:1–4. [Google Scholar]
- de Freitas Bueno R., Claro I.C.M., Augusto M.R., Duran A.F.A., Camillo L.M.B., Cabral A.D., et al. Wastewater-based epidemiology: a Brazilian SARS-COV-2 surveillance experience. J. Environ. Chem. Eng. 2022;10 doi: 10.1016/j.jece.2022.108298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa A.R.V., do Carmo Silva L., de Curcio J.S., da Silva H.D., Eduardo Anunciacao C., Maria Salem Izacc S., et al. "pySewage": a hybrid approach to predict the number of SARS-CoV-2-infected people from wastewater in Brazil. Environ Sci Pollut Res Int. 2022;29:67260–67269. doi: 10.1007/s11356-022-20609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer D., Tranfield D. The Sage Handbook of Organizational Research Methods. Sage Publications Ltd; Thousand Oaks, CA: 2009. Producing a systematic review; pp. 671–689. [Google Scholar]
- Dimitrakopoulos L., Kontou A., Strati A., Galani A., Kostakis M., Kapes V., et al. Evaluation of viral concentration and extraction methods for SARS-CoV-2 recovery from wastewater using droplet digital and quantitative RT-PCR. Case Stud. Chem. Environ. Eng. 2022:6. doi: 10.1016/j.cscee.2022.100224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Liang T.J. Is SARS-CoV-2 also an enteric pathogen with potential fecal-Oral Transmission? A COVID-19 virological and clinical review. Gastroenterology. 2020;159:53–61. doi: 10.1053/j.gastro.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumke R., de la Cruz Barron M., Oertel R., Helm B., Kallies R., Berendonk T.U., et al. Evaluation of two methods to concentrate SARS-CoV-2 from untreated wastewater. Pathogens. 2021;10:1–7. doi: 10.3390/pathogens10020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas M.E., Pitsouni E.I., Malietzis G.A., Pappas G. Comparison of PubMed, scopus, web of science, and Google scholar: strengths and weaknesses. FASEB J. 2008;22:338–342. doi: 10.1096/fj.07-9492LSF. [DOI] [PubMed] [Google Scholar]
- Farkas K., Hillary L.S., Thorpe J., Walker D.I., Lowther J.A., McDonald J.E., et al. Concentration and quantification of SARS-CoV-2 RNA in wastewater using polyethylene glycol-based concentration and qRT-PCR. Methods Protoc. 2021;4:9. doi: 10.3390/mps4010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S.C., Roguet A., McClary-Gutierrez J.S., Newton R.J., Kloczko N., Meiman J.G., et al. Evaluation of sampling, analysis, and normalization methods for SARS-CoV-2 concentrations in wastewater to assess COVID-19 burdens in Wisconsin communities. Acs Es&T Water. 2021;1:1955–1965. [Google Scholar]
- Fernandez-Cassi X., Scheidegger A., Banziger C., Cariti F., Tunas Corzon A., Ganesanandamoorthy P., et al. Wastewater monitoring outperforms case numbers as a tool to track COVID-19 incidence dynamics when test positivity rates are high. Water Res. 2021;200 doi: 10.1016/j.watres.2021.117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald S.F., Rossi G., Low A.S., McAteer S.P., O'Keefe B., Findlay D., et al. Site specific relationships between COVID-19 cases and SARS-CoV-2 viral load in wastewater treatment plant influent. Environ. Sci. Technol. 2021;55:15276–15286. doi: 10.1021/acs.est.1c05029. [DOI] [PubMed] [Google Scholar]
- Flood M.T., D'Souza N., Rose J.B., Aw T.G. Methods evaluation for rapid concentration and quantification of SARS-CoV-2 in raw wastewater using droplet digital and quantitative RT-PCR. Food Environ. Virol. 2021;13:303–315. doi: 10.1007/s12560-021-09488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fongaro G., Stoco P.H., Souza D.S.M., Grisard E.C., Magri M.E., Rogovski P., et al. The presence of SARS-CoV-2 RNA in human sewage in Santa Catarina, Brazil, November 2019. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca M.S., Machado B.A.S., CdA R., Hodel K.V.S., Almeida. E.D.S., de Andrade J.B. Evaluation of SARS-CoV-2 concentrations in wastewater and river water samples. Case Stud. Chem. Environ. Eng. 2022:6. doi: 10.1016/j.cscee.2022.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthofer R.N., Lee E.S., Hernandez M. Elsevier; 2007. Biostatistics. [Google Scholar]
- Galani A., Aalizadeh R., Kostakis M., Markou A., Alygizakis N., Lytras T., et al. SARS-CoV-2 wastewater surveillance data can predict hospitalizations and ICU admissions. Sci. Total Environ. 2022;804 doi: 10.1016/j.scitotenv.2021.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L., Verreault D., Veillette M., Moineau S., Duchaine C. Evaluation of filters for the sampling and quantification of RNA phage aerosols. Aerosol Sci. Technol. 2010;44:893–901. [Google Scholar]
- Gerrity D., Papp K., Stoker M., Sims A., Frehner W. Early-pandemic wastewater surveillance of SARS-CoV-2 in Southern Nevada: Methodology, occurrence, and incidence/prevalence considerations. Water Res. 2021;10 doi: 10.1016/j.wroa.2020.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud-Billoud M., Cuervo P., Altamirano J.C., Pizarro M., Aranibar J.N., Catapano A., et al. Monitoring of SARS-CoV-2 RNA in wastewater as an epidemiological surveillance tool in MendozaArgentina. Sci Total Environ. 2021;796 doi: 10.1016/j.scitotenv.2021.148887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Pastora J., Bringas E., Ortiz I. Recent progress and future challenges on the use of high performance magnetic nano-adsorbents in environmental applications. Chem. Eng. J. 2014;256:187–204. [Google Scholar]
- Gonçalves J., Koritnik T., Mioc V., Trkov M., Boljesic M., Berginc N., et al. Detection of SARS-CoV-2 RNA in hospital wastewater from a low COVID-19 disease prevalence area. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.143226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R., Curtis K., Bivins A., Bibby K., Weir M.H., Yetka K., et al. COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Res. 2020;186 doi: 10.1016/j.watres.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Reyes J.R., Hernandez-Flores M.L., Paredes-Zarco J.E., Tellez-Jurado A., Fayad-Meneses O., Carranza-Ramirez L. Detection of SARS-CoV-2 in wastewater northeast of Mexico City: strategy for monitoring and prevalence of COVID-19. Int. J. Environ. Res. Public Health. 2021;18:14. doi: 10.3390/ijerph18168547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Parker J., Smits S., Underwood J., Dolwani S. Persistent viral shedding of SARS-CoV-2 in faeces - a rapid review. Color. Dis. 2020;22:611–620. doi: 10.1111/codi.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusenbauer M., Haddaway N.R. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google scholar, PubMed, and 26 other resources. Res. Synth. Methods. 2020;11:181–217. doi: 10.1002/jrsm.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M.F.U., Bukhari S.S., Ejaz R., Zaman F.U., Sreejith K.R., Rashid N., et al. A novel RdRp-based colorimetric RT-LAMP assay for rapid and sensitive detection of SARS-CoV-2 in clinical and sewage samples from Pakistan. Virus Res. 2021;302 doi: 10.1016/j.virusres.2021.198484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.W., Ibrahim Y., Daou M., Kannout H., Jan N., Lopes A., et al. Detection and quantification of SARS-CoV-2 RNA in wastewater and treated effluents: Surveillance of COVID-19 epidemic in the United Arab Emirates. Sci. Total Environ. 2021;764 doi: 10.1016/j.scitotenv.2020.142929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasing M., Yu J.A., Qiu Y.Y., Maal-Bared R., Bhavanam S., Lee B., et al. Comparison of detecting and quantitating SARS-CoV-2 in wastewater using moderate-speed centrifuged solids versus an ultrafiltration method. Water. 2021;13:16. [Google Scholar]
- Hata A., Hara-Yamamura H., Meuchi Y., Imai S., Honda R. Detection of SARS-CoV-2 in wastewater in Japan during a COVID-19 outbreak. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Honda R., Hara-Yamamura H., Meuchi Y. medRxiv; 2020. Detection of SARS-CoV-2 in Wastewater in Japan by Multiple Molecular Assays-implication for Wastewater-based Epidemiology (WBE) 2020.06.09.20126417. [Google Scholar]
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Hellmer M., Paxeus N., Magnius L., Enache L., Arnholm B., Johansson A., et al. Detection of pathogenic viruses in sewage provided early warnings of hepatitis a virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80:6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemalatha M., Kiran U., Kuncha S.K., Kopperi H., Gokulan C.G., Mohan S.V., et al. Surveillance of SARS-CoV-2 spread using wastewater-based epidemiology: Comprehensive study. Sci. Total Environ. 2021;768 doi: 10.1016/j.scitotenv.2020.144704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoar C., Chauvin F., Clare A., McGibbon H., Castro E., Patinella S., et al. Monitoring SARS-CoV-2 in wastewater during New York City’s second wave of COVID-19: Sewershed-level trends and relationships to publicly available clinical testing data. Environ. Sci Water Res. Tech. 2022;8:1021–1035. [Google Scholar]
- Hokajarvi A.M., Rytkonen A., Tiwari A., Kauppinen A., Oikarinen S., Lehto K.M., et al. The detection and stability of the SARS-CoV-2 RNA biomarkers in wastewater influent in Helsinki, Finland. Sci. Total Environ. 2021;770 doi: 10.1016/j.scitotenv.2021.145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.N., Johnston L., Parra A., Sweeney C., Hayes E., Hansen L.T., et al. Detection of SARS-CoV-2 in wastewater in Halifax, Nova Scotia, Canada, using four RT-qPCR assays. Facets. 2021;6:959–965. [Google Scholar]
- Huang W.E., Lim B., Hsu C.C., Xiong D., Wu W., Yu Y., et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb. Biotechnol. 2020;13:950–961. doi: 10.1111/1751-7915.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias N.G., Gebhard L.G., Carballeda J.M., Aiello I., Recalde E., Terny G., et al. SARS-CoV-2 surveillance in untreated wastewater: Detection of viral RNA in a low-resource community in Buenos Aires, Argentina. Rev. Panam. Salud. Publica. 2021;45:e137. doi: 10.26633/RPSP.2021.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafferali M.H., Khatami K., Atasoy M., Birgersson M., Williams C., Cetecioglu Z. Benchmarking virus concentration methods for quantification of SARS-CoV-2 in raw wastewater. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G., Wu J., Weidhaas J., Li X., Chen Y., Mueller J., et al. Artificial neural network-based estimation of COVID-19 case numbers and effective reproduction rate using wastewater-based epidemiology. Water Res. 2022;218 doi: 10.1016/j.watres.2022.118451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jmii H., Gharbi-Khelifi H., Assaoudi R., Aouni M. Detection of SARS-CoV-2 in the sewerage system in Tunisia: a promising tool to confront COVID-19 pandemic. Future Virol. 2021;16:751–759. doi: 10.2217/fvl-2021-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Lepp C.G.D.T.L. American Chemical Society; 2001. Pharmaceuticals and Personal Care Products in the Environment: Scientific and Regulatory Issues. [Google Scholar]
- Johnson R., Muller C.J.F., Ghoor S., Louw J., Archer E., Surujlal-Naicker S., et al. Qualitative and quantitative detection of SARS-CoV-2 RNA in untreated wastewater in Western Cape Province, South Africa. S. Afr. Med. J. 2021;111:198–202. doi: 10.7196/SAMJ.2021.v111i3.15154. [DOI] [PubMed] [Google Scholar]
- Jones D.L., Baluja M.Q., Graham D.W., Corbishley A., McDonald J.E., Malham S.K., et al. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel M.A.I., Stark N., Nicolosi B., Lontai J., Lambirth K., Schlueter J., et al. Performance evaluation of virus concentration methods for implementing SARS-CoV-2 wastewater based epidemiology emphasizing quick data turnaround. Sci. Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabdasli I., Tunay O. Concentration techniques tailored for the detection of SARS-CoV-2 genetic material in domestic wastewater and treatment plant sludge: a review. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/j.jece.2021.106296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan S., Ronquillo N., Belda-Ferre P., Alvarado D., Javidi T., Longhurst C.A., et al. High-throughput wastewater SARS-CoV-2 detection enables forecasting of community infection dynamics in San Diego County. mSystems. 2021;6 doi: 10.1128/mSystems.00045-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya D., Niemeier D., Ahmed W., Kjellerup B.V. Evaluation of multiple analytical methods for SARS-CoV-2 surveillance in wastewater samples. Sci. Total Environ. 2022;808 doi: 10.1016/j.scitotenv.2021.152033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevill J.L., Pellett C., Farkas K., Brown M.R., Bassano I., Denise H., et al. A comparison of precipitation and filtration-based SARS-CoV-2 recovery methods and the influence of temperature, turbidity, and surfactant load in urban wastewater. Sci. Total Environ. 2022;808 doi: 10.1016/j.scitotenv.2021.151916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kennedy L.C., Wolfe M.K., Criddle C.S., Duong D.H., Topol A., et al. SARS-CoV-2 RNA is enriched by orders of magnitude in primary settled solids relative to liquid wastewater at publicly owned treatment works. Environ. Sci. (Camb) 2022;8:757–770. doi: 10.1039/d1ew00826a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., et al. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Sadamasu K., Muramatsu M., Yoshida H. Efficient detection of SARS-CoV-2 RNA in the solid fraction of wastewater. Sci. Total Environ. 2021;763 doi: 10.1016/j.scitotenv.2020.144587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Cortese M., Winter S.L., Wachsmuth-Melm M., Neufeldt C.J., Cerikan B., et al. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat. Commun. 2020;11:5885. doi: 10.1038/s41467-020-19619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocamemi B.A., Kurt H., Sait A., Sarac F., Saatci A.M., Pakdemirli B. medRxiv; 2020. SARS-CoV-2 Detection in Istanbul Wastewater Treatment Plant Sludges. 2020.05.12.20099358. [Google Scholar]
- Koureas M., Amoutzias G.D., Vontas A., Kyritsi M., Pinaka O., Papakonstantinou A., et al. Wastewater monitoring as a supplementary surveillance tool for capturing SARS-COV-2 community spread. A case study in two Greek municipalities. Environ. Res. 2021;200 doi: 10.1016/j.envres.2021.111749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivonakova N., Soltysova A., Tamas M., Takac Z., Krahulec J., Ficek A., et al. Mathematical modeling based on RT-qPCR analysis of SARS-CoV-2 in wastewater as a tool for epidemiology. Sci. Rep. 2021;11:19456. doi: 10.1038/s41598-021-98653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn K.G., Jarshaw J., Jeffries E., Adesigbin K., Maytubby P., Dundas N., et al. Predicting COVID-19 cases in diverse population groups using SARS-CoV-2 wastewater monitoring across Oklahoma City. Sci. Total Environ. 2022;812 doi: 10.1016/j.scitotenv.2021.151431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Joshi M., Patel A.K., Joshi C.G. Unravelling the early warning capability of wastewater surveillance for COVID-19: A temporal study on SARS-CoV-2 RNA detection and need for the escalation. Environ. Res. 2021;196 doi: 10.1016/j.envres.2021.110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Shetti N.P., Jagannath S., Aminabhavi T.M. Electrochemical sensors for the detection of SARS-CoV-2 virus. Chem. Eng. J. 2022;430 doi: 10.1016/j.cej.2021.132966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumblathan T., Liu Y.M., Qiu Y.Y., Pang L.L.Y., Hrudey S.E., Le X.C., et al. An efficient method to enhance recovery and detection of SARS-CoV-2 RNA in wastewater. J. Environ. Sci. 2023;130:139–148. doi: 10.1016/j.jes.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., et al. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan L.M., O’Brien M., Rundell Z.C., Back J.A., Ryan B.J., Chambliss C.K., et al. Comparative analysis of RNA-extraction approaches and associated influences on RT-qPCR of the SARS-CoV-2 RNA in a university residence hall and quarantine location. Acs Es&T Water. 2022;2:1929–1943. doi: 10.1021/acsestwater.1c00476. [DOI] [PubMed] [Google Scholar]
- Lara-Jacobo L.R., Islam G., Desaulniers J.P., Kirkwood A.E., Simmons D.B.D. Detection of SARS-CoV-2 proteins in wastewater samples by mass spectrometry. Environ. Sci. Technol. 2022;56:5062–5070. doi: 10.1021/acs.est.1c04705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaTurner Z.W., Zong D.M., Kalvapalle P., Gamas K.R., Terwilliger A., Crosby T., et al. Evaluating recovery, cost, and throughput of different concentration methods for SARS-CoV-2 wastewater-based epidemiology. Water Res. 2021;197 doi: 10.1016/j.watres.2021.117043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton B.A., Kaya D., Kelly C., Williamson K.J., Alegre D., Bachhuber S.M., et al. Evaluation of a wastewater-based epidemiological approach to estimate the prevalence of SARS-CoV-2 infections and the detection of viral variants in disparate Oregon communities at City and neighborhood scales. Environ. Health Perspect. 2022;130:67010. doi: 10.1289/EHP10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Di D.Y.W., Saingam P., Jeon M.K., Yan T. Fine-scale temporal dynamics of SARS-CoV-2 RNA abundance in wastewater during a COVID-19 lockdown. Water Res. 2021;197 doi: 10.1016/j.watres.2021.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Kulandaivelu J., Zhang S., Shi J., Sivakumar M., Mueller J., et al. Data-driven estimation of COVID-19 community prevalence through wastewater-based epidemiology. Sci. Total Environ. 2021;789 doi: 10.1016/j.scitotenv.2021.147947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Mazurowski L., Dewan A., Carine M., Haak L., Guarin T.C., et al. Longitudinal monitoring of SARS-CoV-2 in wastewater using viral genetic markers and the estimation of unconfirmed COVID-19 cases. Sci. Total Environ. 2022;817 doi: 10.1016/j.scitotenv.2022.152958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang S., Sherchan S., Orive G., Lertxundi U., Haramoto E., et al. Correlation between SARS-CoV-2 RNA concentration in wastewater and COVID-19 cases in community: a systematic review and meta-analysis. J. Hazard. Mater. 2023;441 doi: 10.1016/j.jhazmat.2022.129848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang S., Shi J., Luby S.P., Jiang G. Uncertainties in estimating SARS-CoV-2 prevalence by wastewater-based epidemiology. Chem. Eng. J. 2021;415 doi: 10.1016/j.cej.2021.129039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Jones C., Ejigu G.S., George N., Geller A.L., Chang G.C., et al. Countries with delayed COVID-19 introduction - characteristics, drivers, gaps, and opportunities. Global Health. 2021;17:28. doi: 10.1186/s12992-021-00678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maida C.M., Amodio E., Mazzucco W., La Rosa G., Lucentini L., Suffredini E., et al. Wastewater-based epidemiology for early warning of SARS-COV-2 circulation: A pilot study conducted in Sicily, Italy. Int. J. Hyg. Environ. Health. 2022;242 doi: 10.1016/j.ijheh.2022.113948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailepessov D., Arivalan S., Kong M., Griffiths J., Low S.L., Chen H., et al. Development of an efficient wastewater testing protocol for high-throughput country-wide SARS-CoV-2 monitoring. Sci. Total Environ. 2022;826 doi: 10.1016/j.scitotenv.2022.154024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markt R., Endler L., Amman F., Schedl A., Penz T., Buchel-Marxer M., et al. Detection and abundance of SARS-CoV-2 in wastewater in Liechtenstein, and the estimation of prevalence and impact of the B.1.1.7 variant. J. Water Health. 2022;20:114–125. doi: 10.2166/wh.2021.180. [DOI] [PubMed] [Google Scholar]
- Masachessi G., Castro G., Cachi A.M., Marinzalda M.L.A., Liendo M., Pisano M.B., et al. Wastewater based epidemiology as a silent sentinel of the trend of SARS-CoV-2 circulation in the community in central Argentina. Water Res. 2022;219 doi: 10.1016/j.watres.2022.118541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materón E.M., Miyazaki C.M., Carr O., Joshi N., Picciani P.H.S., Dalmaschio C.J., et al. Magnetic nanoparticles in biomedical applications: a review. Appl. Surf. Sci. Adv. 2021;6 [Google Scholar]
- McMahan C.S., Self S., Rennert L., Kalbaugh C., Kriebel D., Graves D., et al. COVID-19 wastewater epidemiology: a model to estimate infected populations. Lancet Planet Health. 2021;5:e874–e881. doi: 10.1016/S2542-5196(21)00230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn B.R., Korajkic A., Kelleher J., Herrmann M.P., Pemberton A.C., Ahmed W., et al. Development of a large volume concentration method for recovery of coronavirus from wastewater. Sci. Total Environ. 2021;774 doi: 10.1016/j.scitotenv.2021.145727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol.Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Mesoraca A., Margiotti K., Viola A., Cima A., Sparacino D., Giorlandino C. Evaluation of SARS-CoV-2 viral RNA in fecal samples. Virol. J. 2020;17:86. doi: 10.1186/s12985-020-01359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura F., Kitajima M., Omori R. Duration of SARS-CoV-2 viral shedding in faeces as a parameter for wastewater-based epidemiology: re-analysis of patient data using a shedding dynamics model. Sci. Total Environ. 2021;769 doi: 10.1016/j.scitotenv.2020.144549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlejnkova H., Sovova K., Vasickova P., Ocenaskova V., Jasikova L., Juranova E. Preliminary study of Sars-Cov-2 occurrence in wastewater in the Czech Republic. Int. J. Environ. Res. Public Health. 2020;17:1–9. doi: 10.3390/ijerph17155508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S., Feirer N., Brockman M., Preston M.A., Teter S.J., Ma D., et al. A direct capture method for purification and detection of viral nucleic acid enables epidemiological surveillance of SARS-CoV-2. Sci. Total Environ. 2021;795 doi: 10.1016/j.scitotenv.2021.148834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro S., Rente D., Cunha M.V., Gomes M.C., Marques T.A., Lourenco A.B., et al. A wastewater-based epidemiology tool for COVID-19 surveillance in Portugal. Sci. Total Environ. 2022;804 doi: 10.1016/j.scitotenv.2021.150264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkar M., Keely S.P., Jahne M., Wheaton E., Hart C., Smith B., et al. SARS-CoV-2 monitoring at three sewersheds of different scales and complexity demonstrates distinctive relationships between wastewater measurements and COVID-19 case data. Sci. Total Environ. 2022;816 doi: 10.1016/j.scitotenv.2021.151534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri S., Yavarian J., Baghani A.N., Azad T.M., Nejati A., Nabizadeh R., et al. The presence of SARS-CoV-2 in raw and treated wastewater in 3 cities of Iran: Tehran, Qom and anzali during coronavirus disease 2019 (COVID-19) outbreak. J. Environ. Health Sci. Eng. 2021;19:573–584. doi: 10.1007/s40201-021-00629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A., Gomez L., Sanseverino I., Niegowska M., Roka E., Pedraccini R., et al. SARS-CoV-2 detection in wastewater using multiplex quantitative PCR. Sci. Total Environ. 2021;797 doi: 10.1016/j.scitotenv.2021.148890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., et al. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep. Med. 2020;1 doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni G.F., Lu J., Maulani N., Tian W., Yang L., Harliwong I., et al. Novel multiplexed amplicon-based sequencing to quantify SARS-CoV-2 RNA from wastewater. Environ. Sci. Technol. Lett. 2021;8:683–690. doi: 10.1021/acs.estlett.1c00408. [DOI] [PubMed] [Google Scholar]
- Nourbakhsh S., Fazil A., Li M., Mangat C.S., Peterson S.W., Daigle J., et al. A wastewater-based epidemic model for SARS-CoV-2 with application to three Canadian cities. Epidemics. 2022;39 doi: 10.1016/j.epidem.2022.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa B., Rios-Castro R., Otero-Muras I., Gouveia S., Cabo A., Saco A., et al. Wastewater and marine bioindicators surveillance to anticipate COVID-19 prevalence and to explore SARS-CoV-2 diversity by next generation sequencing: One-year study. Sci. Total Environ. 2022;833 doi: 10.1016/j.scitotenv.2022.155140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien M., Rundell Z.C., Nemec M.D., Langan L.M., Back J.A., Lugo J.N. A comparison of four commercially available RNA extraction kits for wastewater surveillance of SARS-CoV-2 in a college population. Sci. Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori R., Miura F., Kitajima M. Age-dependent association between SARS-CoV-2 cases reported by passive surveillance and viral load in wastewater. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Guardado A.L., Sweeney C.L., Hayes E.K., Trueman B.F., Huang Y., Jamieson R.C., et al. Development of a rapid pre-concentration protocol and a magnetic beads-based RNA extraction method for SARS-CoV-2 detection in raw municipal wastewater. Environ. Sci. Water Res. Tech. 2022;8:47–61. [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., et al. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38:1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado B., Martinez-Garcia L., Martinez F., Nozal L., Sanchez M.B. Improved methods for the detection and quantification of SARS-CoV-2 RNA in wastewater. Sci. Rep. 2022;12:7201. doi: 10.1038/s41598-022-11187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrinelli L., Castiglioni S., Cocuzza C., Bertasi B., Primache V., Schiarea S., et al. Evaluation of pre-analytical and analytical methods for detecting SARS-CoV-2 in municipal wastewater samples in northern Italy. Water. 2022;14:12. [Google Scholar]
- Peng L., Liu J., Xu W., Luo Q., Chen D., Lei Z., et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J. Med. Virol. 2020;92:1676–1680. doi: 10.1002/jmv.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira I.G., Guerin J.M., Silva Júnior A.G., Garcia G.S., Piscitelli P., Miani A., et al. Forecasting Covid-19 dynamics in Brazil: a data driven approach. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17145115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Cataluna A., Cuevas-Ferrando E., Randazzo W., Falco I., Allende A., Sanchez G. Comparing analytical methods to detect SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petala M., Kostoglou M., Karapantsios T., Dovas C.I., Lytras T., Paraskevis D., et al. Relating SARS-CoV-2 shedding rate in wastewater to daily positive tests data: A consistent model based approach. Sci. Total Environ. 2022;807 doi: 10.1016/j.scitotenv.2021.150838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philo S.E., Keim E.K., Swanstrom R., Ong A.Q.W., Burnor E.A., Kossik A.L., et al. A comparison of SARS-CoV-2 wastewater concentration methods for environmental surveillance. Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.144215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philo S.E., Ong A.Q.W., Keim E.K., Swanstrom R., Kossik A.L., Zhou N.A., et al. Development and validation of the skimmed milk pellet extraction protocol for SARS-CoV-2 wastewater surveillance. Food Environ. Virol. 2022;14:355–363. doi: 10.1007/s12560-022-09512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay L., Amoah I.D., Deepnarain N., Pillay K., Awolusi O.O., Kumari S., et al. Monitoring changes in COVID-19 infection using wastewater-based epidemiology: a south african perspective. Sci. Total Environ. 2021;786 doi: 10.1016/j.scitotenv.2021.147273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino N.J., Rodriguez D.C., Cano L.C., Rodriguez A. Detection of SARS-CoV-2 in wastewater is influenced by sampling time, concentration method, and target analyzed. J. Water Health. 2021;19:775–784. doi: 10.2166/wh.2021.133. [DOI] [PubMed] [Google Scholar]
- Prakash C. Sewage analysis as a tool for environmental surveillance of SARS-CoV-2: experience from Delhi, India. Journal of Communicable Diseases. 2021;53:1–13. [Google Scholar]
- Prevost B., Lucas F.S., Goncalves A., Richard F., Moulin L., Wurtzer S. Large scale survey of enteric viruses in river and waste water underlines the health status of the local population. Environ. Int. 2015;79:42–50. doi: 10.1016/j.envint.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Proverbio D., Kemp F., Magni S., Ogorzaly L., Cauchie H.M., Goncalves J., et al. Model-based assessment of COVID-19 epidemic dynamics by wastewater analysis. Sci. Total Environ. 2022;827 doi: 10.1016/j.scitotenv.2022.154235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Yu J., Pabbaraju K., Lee B.E., Gao T., Ashbolt N.J., et al. Validating and optimizing the method for molecular detection and quantification of SARS-CoV-2 in wastewater. Sci. Total Environ. 2022;812 doi: 10.1016/j.scitotenv.2021.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanujam A., Almodovar S., Botte G.G. Ultra-fast electrochemical sensor for point-of-care COVID-19 diagnosis using non-invasive saliva sampling. Processes. 2021;9 [Google Scholar]
- Ramos-Mandujano G., Salunke R., Mfarrej S., Rachmadi A.T., Hala S., Xu J., et al. A robust, safe, and scalable magnetic nanoparticle workflow for RNA extraction of pathogens from clinical and wastewater samples. Glob. Chall. 2021;5 doi: 10.1002/gch2.202000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simon P., Allende A., Sanchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds L.J., Gonzalez G., Sala-Comorera L., Martin N.A., Byrne A., Fennema S., et al. SARS-CoV-2 variant trends in Ireland: wastewater-based epidemiology and clinical surveillance. Sci. Total Environ. 2022;838 doi: 10.1016/j.scitotenv.2022.155828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robotto A., Lembo D., Quaglino P., Brizio E., Polato D., Civra A., et al. Wastewater-based SARS-CoV-2 environmental monitoring for Piedmont, Italy. Environ Res. 2022;203:111901. doi: 10.1016/j.envres.2021.111901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A.Y., Verbyla M.E., Sant K.E., Mladenov N. Detection, quantification, and Simplified wastewater surveillance model of SARS-CoV-2 RNA in the Tijuana River. Environ. Res. 2022;203 doi: 10.1021/acsestwater.2c00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Rasero F.J., Moya Ruano L.A., Rasero Del Real P., Cuberos Gomez L., Lorusso N. Associations between SARS-CoV-2 RNA concentrations in wastewater and COVID-19 rates in days after sampling in small urban areas of Seville: a time series study. Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.150573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roka E., Khayer B., Kis Z., Kovacs L.B., Schuler E., Magyar N., et al. Ahead of the second wave: early warning for COVID-19 by wastewater surveillance in Hungary. Sci. Total Environ. 2021;786 doi: 10.1016/j.scitotenv.2021.147398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondeau N.C., Rose O.J., Ariyan L.A., Mailloux B.J., Miranda J.L. Accessible and validated processing of SARS-CoV-2 from wastewater. Microbiol. Resour. Announc. 2021;10 doi: 10.1128/MRA.00174-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario K., Symonds E.M., Sinigalliano C., Stewart J., Breitbart M. Pepper mild mottle virus as an indicator of fecal pollution. Appl. Environ. Microbiol. 2009;75:7261–7267. doi: 10.1128/AEM.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosiles-Gonzalez G., Carrillo-Jovel V.H., Alzate-Gaviria L., Betancourt W.Q., Gerba C.P., Moreno-Valenzuela O.A., et al. Environmental surveillance of SARS-CoV-2 RNA in wastewater and groundwater in Quintana Roo, Mexico. Food Environ. Virol. 2021;13:457–469. doi: 10.1007/s12560-021-09492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvo M., Moller A., Alvareda E., Gamazo P., Colina R., Victoria M. Evaluation of low-cost viral concentration methods in wastewaters: implications for SARS-CoV-2 pandemic surveillances. J. Virol. Methods. 2021;297 doi: 10.1016/j.jviromet.2021.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapula S.A., Whittall J.J., Pandopulos A.J., Gerber C., Venter H. An optimized and robust PEG precipitation method for detection of SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;785 doi: 10.1016/j.scitotenv.2021.147270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saththasivam J., El-Malah S.S., Gomez T.A., Jabbar K.A., Remanan R., Krishnankutty A.K., et al. COVID-19 (SARS-CoV-2) outbreak monitoring using wastewater-based epidemiology in Qatar. Sci. Total Environ. 2021;774 doi: 10.1016/j.scitotenv.2021.145608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz B.W., Innes G.K., Prasek S.M., Betancourt W.Q., Stark E.R., Foster A.R., et al. Enumerating asymptomatic COVID-19 cases and estimating SARS-CoV-2 fecal shedding rates via wastewater-based epidemiology. Sci. Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober P., Boer C., Schwarte L.A. Correlation coefficients: appropriate use and interpretation. Anesth. Analg. 2018;126:1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- Scott L.C., Aubee A., Babahaji L., Vigil K., Tims S., Aw T.G. Targeted wastewater surveillance of SARS-CoV-2 on a university campus for COVID-19 outbreak detection and mitigation. Environ. Res. 2021;200 doi: 10.1016/j.envres.2021.111374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S., Gwee S.X.W., Ng J.Q.X., Lau N., Koh J., Pang J. Wastewater surveillance to infer COVID-19 transmission: a systematic review. Sci. Total Environ. 2022;804 doi: 10.1016/j.scitotenv.2021.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D.K., Nalavade U.P., Kalgutkar K., Gupta N., Deshpande J.M. SARS-CoV-2 detection in sewage samples: Standardization of method & preliminary observations. Indian J. Med. Res. 2021;153:159–165. doi: 10.4103/ijmr.IJMR_3541_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., et al. First detection of SARS-CoV-2 RNA in wastewater in North America: A study in Louisiana, USA. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z.Q., Reinke R., Hoxsey M., Jackson J., Krikorian E., Melitas N., et al. Detection of SARS-CoV-2 in wastewater: Community variability, temporal dynamics, and genotype diversity. Acs Es&T Water. 2021;1:1816–1825. [Google Scholar]
- Tagliabue F., Galassi L., Mariani P. The, "Pandemic" of disinformation in COVID-19. SN Compr. Clin. Med. 2020;2:1287–1289. doi: 10.1007/s42399-020-00439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandukar S., Sthapit N., Thakali O., Malla B., Sherchan S.P., Shakya B.M., et al. Detection of SARS-CoV-2 RNA in wastewater, river water, and hospital wastewater of Nepal. Sci. Total Environ. 2022;824 doi: 10.1016/j.scitotenv.2022.153816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanhaei M., Mohebbi S.R., Hosseini S.M., Rafieepoor M., Kazemian S., Ghaemi A., et al. The first detection of SARS-CoV-2 RNA in the wastewater of Tehran, Iran. Environ Sci Pollut Res Int. 2021;28:38629–38636. doi: 10.1007/s11356-021-13393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto Y., Ito E., Miyamoto S., Mori A., Nomoto R., Nakanishi N., et al. SARS-CoV-2 RNA in Wastewater Was Highly Correlated With the Number of COVID-19 Cases During the Fourth and Fifth Pandemic Wave in Kobe City, Japan. Front Microbiol. 2022;13:892447. doi: 10.3389/fmicb.2022.892447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongpradit S., Prasongtanakij S., Srisala S., Chanprasertyothin S., Pasomsub E., Ongphiphadhanakul B. The detection of SARS-CoV2 antigen in wastewater using an automated chemiluminescence enzyme immunoassay. Int. J. Environ. Res. Public Health. 2022;19 doi: 10.3390/ijerph19137783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A., Lipponen A., Hokajarvi A.M., Luomala O., Sarekoski A., Rytkonen A., et al. Detection and quantification of SARS-CoV-2 RNA in wastewater influent in relation to reported COVID-19 incidence in Finland. Water Res. 2022;215 doi: 10.1016/j.watres.2022.118220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo D.M., Robbins A.A., Gallagher T.L., Hershberger K.C., Barney R.E., Salmela S.M., et al. Wastewater-based SARS-CoV-2 surveillance in Northern New England. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.02207-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasino M.P., Semedo M., Vieira E.M.P., Ferraz E., Rocha A., Carvalho M.F., et al. SARS-CoV-2 RNA detected in urban wastewater from Porto, Portugal: method optimization and continuous 25-week monitoring. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S., Furumai H., Katayama H. Applicability of polyethylene glycol precipitation followed by acid guanidinium thiocyanate-phenol-chloroform extraction for the detection of SARS-CoV-2 RNA from municipal wastewater. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.143067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S., Oishi W., Zhu Y., Thakali O., Malla B., Yu Z., et al. Comparison of five polyethylene glycol precipitation procedures for the RT-qPCR based recovery of murine hepatitis virus, bacteriophage phi6, and pepper mild mottle virus as a surrogate for SARS-CoV-2 from wastewater. Sci. Total Environ. 2022;807 doi: 10.1016/j.scitotenv.2021.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranfield D., Denyer D., Smart P. Towards a methodology for developing evidence-informed management knowledge by means of systematic review. Br. J. Manag. 2003;14:207–222. [Google Scholar]
- Trottier J., Darques R., Ait Mouheb N., Partiot E., Bakhache W., Deffieu M.S., et al. Post-lockdown detection of SARS-CoV-2 RNA in the wastewater of Montpellier, France. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo M., Cheung K., Gao A., Hoxie I., Kannoly S., Kubota N., et al. Protocol for safe, affordable, and reproducible isolation and quantitation of SARS-CoV-2 RNA from wastewater. PLoS One. 2021;16 doi: 10.1371/journal.pone.0257454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo J.A., Trigo-Tasende N., Rumbo-Feal S., Conde-Perez K., Lopez-Oriona A., Barbeito I., et al. Modeling the number of people infected with SARS-COV-2 from wastewater viral load in Northwest Spain. Sci. Total Environ. 2022;811 doi: 10.1016/j.scitotenv.2021.152334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Zarei-Baygi A., Sauceda C., Iskander S.M., Smith A.L. Long-term surveillance of wastewater SARS-CoV-2 in Los Angeles County. Environ. Sci. Water Res. Technol. 2021;7:2282–2294. [Google Scholar]
- Wang Q., Waltman L. Large-scale analysis of the accuracy of the journal classification systems of web of science and scopus. J. Informetrics. 2016;10:347–364. [Google Scholar]
- Wehrendt D.P., Masso M.G., Gonzales Machuca A., Vargas C.V., Barrios M.E., Campos J., et al. A rapid and simple protocol for concentration of SARS-CoV-2 from sewage. J. Virol. Methods. 2021;297 doi: 10.1016/j.jviromet.2021.114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Suryawanshi H., Djandji A., Kohl E., Morgan S., Hod E.A., et al. Field-deployable, rapid diagnostic testing of saliva for SARS-CoV-2. Sci. Rep. 2021;11:5448. doi: 10.1038/s41598-021-84792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhaus S., Weber F.A., Schiwy S., Linnemann V., Brinkmann M., Widera M., et al. Detection of SARS-CoV-2 in raw and treated wastewater in Germany - suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., et al. SARS-CoV-2 RNA concentrations in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. Sci. Total Environ. 2022;805 doi: 10.1016/j.scitotenv.2021.150121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney O.N., Kennedy L.C., Fan V.B., Hinkle A., Kantor R., Greenwald H., Sewage S., et al. Silica, and SARS-CoV-2 (4S): An economical kit-free method for direct capture of SARS-CoV-2 RNA from wastewater. Environ. Sci. Technol. 2021;55:4880–4888. doi: 10.1021/acs.est.0c08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao A., Wu F., Bushman M., Zhang J., Imakaev M., Chai P.R., et al. Metrics to relate COVID-19 wastewater data to clinical testing dynamics. Water Res. 2022;212 doi: 10.1016/j.watres.2022.118070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(1831–1833) doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Waldman P., Levert M., Cluzel N., Almayrac J.L., Charpentier C., et al. SARS-CoV-2 genome quantification in wastewaters at regional and city scale allows precise monitoring of the whole outbreaks dynamics and variants spreading in the population. Sci. Total Environ. 2022;810 doi: 10.1016/j.scitotenv.2021.152213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M., Liu X., Ji J., Li M., Li J., Yang L., et al. Multiple approaches for massively parallel sequencing of SARS-CoV-2 genomes directly from clinical samples. Genome Med. 2020;12:57. doi: 10.1186/s13073-020-00751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y.W., Challis J.K., Oloye F.F., Asadi M., Cantin J., Brinkmann M., et al. RNA in municipal wastewater reveals magnitudes of COVID-19 outbreaks across four waves driven by SARS-CoV-2 variants of concern. ACS Es&T Water. 2022;2:1852–1862. doi: 10.1021/acsestwater.1c00349. [DOI] [PubMed] [Google Scholar]
- Xu X., Zheng X., Li S., Lam N.S., Wang Y., Chu D.K.W., et al. The first case study of wastewater-based epidemiology of COVID-19 in Hong Kong. Sci. Total Environ. 2021;790 doi: 10.1016/j.scitotenv.2021.148000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanac K., Adegoke A., Wang L., Uyaguari M., Yuan Q. Detection of SARS-CoV-2 RNA throughout wastewater treatment plants and a modeling approach to understand COVID-19 infection dynamics in Winnipeg, Canada. Sci Total Environ. 2022;825:153906. doi: 10.1016/j.scitotenv.2022.153906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv K., Ozer E., Shagan M., Lakkakula S., Plotkin N., Bhandarkar N.S., et al. Direct RT-qPCR assay for SARS-CoV-2 variants of concern (Alpha, B.1.1.7 and Beta, B.1.351) detection and quantification in wastewater. Environ. Res. 2021;201 doi: 10.1016/j.envres.2021.111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Yue H., Shin J.M., Tegafaw T., Han H.S., Chae K.S., Chang Y.M., et al. Magnetic separation of nucleic acids from various biological samples using silica-coated iron oxide nanobeads. J. Nanopart. Res. 2020;22:366. [Google Scholar]
- Zhang D., Duran S.S.F., Lim W.Y.S., Tan C.K.I., Cheong W.C.D., Suwardi A., et al. SARS-CoV-2 in wastewater: from detection to evaluation. Mater. Today Adv. 2022;13 doi: 10.1016/j.mtadv.2022.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Gong Y., Meng F., Shi Y., Wang J., Mao P., et al. Comparative study on virus shedding patterns in nasopharyngeal and fecal specimens of COVID-19 patients. Sci. China Life Sci. 2021;64:486–488. doi: 10.1007/s11427-020-1783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Cen M., Hu M., Du L., Hu W., Kim J.J., et al. Prevalence and persistent shedding of fecal SARS-CoV-2 RNA in patients with COVID-19 infection: a systematic review and meta-analysis. Clin. Transl. Gastroenterol. 2021;12 doi: 10.14309/ctg.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Zou Y., Li Y., Miyani B., Spooner M., Gentry Z., et al. Five-week warning of COVID-19 peaks prior to the omicron surge in Detroit, Michigan using wastewater surveillance. Sci. Total Environ. 2022;844 doi: 10.1016/j.scitotenv.2022.157040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Deng Y., Xu X., Li S., Zhang Y., Ding J., et al. Comparison of virus concentration methods and RNA extraction methods for SARS-CoV-2 wastewater surveillance. Sci. Total Environ. 2022;824 doi: 10.1016/j.scitotenv.2022.153687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Oishi W., Maruo C., Bandara S., Lin M., Saito M., et al. COVID-19 case prediction via wastewater surveillance in a low-prevalence urban community: a modeling approach. J. Water Health. 2022;20:459–470. doi: 10.2166/wh.2022.183. [DOI] [PubMed] [Google Scholar]
- Zuo T., Liu Q., Zhang F., Lui G.C., Tso E.Y., Yeoh Y.K., et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70:276–284. doi: 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.