Abstract
While existing literature suggests an association between polycystic ovarian syndrome (PCOS) and endometrial cancer, the sparsity and inconsistency of current evidence indicates a lack of clarity regarding the exact strength of this association. It also remains uncertain whether the degree of risk of disease is affected by confounding factors, such as age and body mass index (BMI). The present meta-analysis is aimed to quantify the risk of endometrial cancer in female subjects with PCOS compared to those without PCOS. PubMed, MEDLINE, EMBASE, Scopus and Cochrane were searched from inception to October 31, 2022, to identify peer-reviewed case-control, cohort and cross-sectional studies that assessed the association between endometrial cancer and PCOS and contained original data. Two researchers independently extracted data and performed quality assessment using the Newcastle-Ottawa criteria. Pooled odds ratios (ORs) were calculated using the random-effect model and inverse variance. The degree of heterogeneity was assessed using I2 statistics. A total of 10 relevant studies were identified and included in the meta-analysis (comprising 12,248 female patients with PCOS and 54,120 controls). Females with PCOS had a significantly increased odds of developing endometrial cancer as compared to those without PCOS [OR, 4.07; 95% confidence interval (CI), 2.13-7.78; P<0.0001]. When postmenopausal subjects (age, >54 years) were excluded from the meta-analysis, the odds increased further (OR, 5.14; 95% CI, 3.22-8.21; P<0.00001). Patients with PCOS are up to 5 times more likely to develop endometrial cancer compared to those without PCOS. Larger, prospective studies that are well-controlled for confounding factors, such as BMI, are required.
Keywords: polycystic, polycystic ovarian syndrome, endometrial, premenopausal, postmenopausal, meta-analysis
Introduction
Polycystic ovarian syndrome (PCOS) is a common endocrine disorder affecting 4–12% of females of reproductive age (1,2). To overcome discrepancies, the 2003 Rotterdam consensus group recommended diagnosis when at least two of the following are present: Oligomenorrhea or amenorrhoea, hyperandrogenism and ultrasound appearance of polycystic ovaries, after exclusion of other causes of irregular menstrual cycles and hyperandrogenism (3).
Endometrial cancer is the most common gynaecological cancer type and the second most common female malignancy in developed countries, but only occurs in 4% of females under 40 years of age (4–6). The diagnosis of endometrial cancer is based on histology and is traditionally classified into two histological subtypes: Type I (mainly endometrioid adenocarcinomas) is most common, oestrogen-dependent and accounts for >75% of cases; Type II includes serous, clear cell and mixed cell histology endometrial cancers, and is less hormonally dependent (7–9).
An increased risk of endometrial cancer has been reported in females with PCOS (1,10), although the underlying mechanism remains elusive (10). However, there is evidence that endocrinologic and metabolic abnormalities observed in PCOS may have complex effects on the endometrium, such as increase in the expression of androgen and steroid receptors contributing to endometrial dysfunction (11). Chronic anovulation encountered in PCOS is a key factor in exposing the endometrium to prolonged unopposed oestrogen (12,13). Certain studies have identified overexpression of luteinising hormone and human chorionic gonadotrophin receptors in uterine adenocarcinoma cells, which has led to suggestions that increased secretion of luteinising hormone, a hallmark of PCOS, is also implicated in the development of endometrial cancer (12,14).
Important risk factors linked to increased risk of endometrial cancer (such as obesity, unopposed oestrogen in anovulatory females, insulin resistance, nulliparity and diabetes) are also known long-term complications of PCOS (7,12,15,16). Despite this, there is currently no effective or widely implemented screening program for individuals at increased risk of endometrial cancer in the UK.
Although the association between PCOS and endometrial cancer is acknowledged, the inconsistency of current evidence means that there remains a lack of clarity regarding the strength of this association and how it translates into quantifiable risk. Previous studies have found that subjects with PCOS have a significant risk of developing endometrial cancer (7,17,18), while others have concluded that evidence of an increased risk of endometrial cancer with PCOS was incomplete and contradictory (12,19), while no association between the two was also reported (20).
The present meta-analysis investigates the strength of the association between PCOS and endometrial cancer and whether confounding factors, such as age, have a role. The findings may influence the management of PCOS in clinical practice, highlighting the need for preventative strategies among high-risk groups.
Materials and methods
Meta-analysis guidelines
The ‘Preferred Reporting Items for Systematic Reviews and Meta-Analysis’ (PRISMA) and ‘Meta-analyses Of Observational Studies in Epidemiology’ (MOOSE) guidelines for the reporting of meta-analyses were adhered to (21,22). Appendices S1 and S2 detail the completed PRISMA and MOOSE checklists for the present study, respectively. Ethical approval and patient consent were not required for the present study, as the analysis was performed on data extracted from previously published articles. There was no patient or public involvement in this project and a core outcome set was not used. The literature was independently searched by two of the researchers (JEJ and DD), who assessed the eligibility and quality of the retrieved articles and extracted relevant data. Any disagreements were resolved by discussion.
Search strategy
A literature search was performed in the PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com/landing?status=grey), Medline (https://www.nlm.nih.gov/medline/index.html), Scopus (https://www.scopus.com/home.uri) and Cochrane (https://www.cochranelibrary.com/) electronic databases to identify relevant peer reviewed articles published from inception to October 31, 2022. The full search terms were provided in Appendix S3. There was no language restriction. However, all the retrieved articles were in English. In addition, the references of retrieved articles were hand-searched to identify any publications that may have been missed in the initial search process. There were no unpublished studies included in the meta-analysis.
Inclusion and exclusion criteria
Selection criteria were devised for the meta-analysis using the Population, Intervention, Comparison, Outcomes and Study (PICOS) criteria (23). The inclusion criteria were as follows: i) Participants: Females of any age with PCOS; ii) intervention: None; iii) comparison: Females of any age without PCOS; iv) outcome: Diagnosis of endometrial cancer; and v) study design: Retrospective or prospective, case-control, cohort studies, cross-sectional or randomised controlled studies. In addition to not meeting the above criteria, studies were also excluded on the basis of the following: i) Literature review articles, case reports and meta-analyses, editorials, commentaries or opinions, or conference abstracts; ii) studies that did not provide numerical data on the sample sizes of the participant and control groups; and iii) studies with incomplete data or without full-text articles available at all.
Data extraction
A total of two researchers (JEJ and DD) independently assessed the eligibility and quality of the retrieved articles and extracted relevant data. This process took place in two stages: Primary screening was conducted by the first reviewer, while a second reviewer performed a secondary screening of the search results. The use of secondary screening aimed to increase the reliability of the study selection process and further ensure that relevant studies were not overlooked. Any discrepancies were examined and discussed further and a consensus reached. Initial screening of the titles and abstracts was followed by evaluation of full-text papers independently. The full inclusion and exclusion criteria were applied to determine which studies would be included in the meta-analysis.
Relevant information from included papers was extracted into a pre-designed, piloted data extraction form. Data extracted from papers included the following: The first author, year of publication, study design, setting, sample size, study population demographics, diagnostic criteria, PCOS group, the comparison group, quality of data, risk of bias and the outcomes assessed. Each study was also commented on with regard to any strengths and limitations identified.
Data synthesis and assessment of heterogeneity
The odds ratios (ORs) and 95% confidence intervals (CIs) for the primary and secondary outcomes were calculated using Cochrane Revman 5 software (24). The random-effects model and inverse variance weighting were used for the meta-analysis. The Mantel-Haenszel method was used to account for confounding factors. The degree of heterogeneity was assessed using the I2 value, to measure the extent of inconsistency among results. The overall effect size was determined using χ2 statistics and P-values. It was agreed that an I2≥30% was likely to indicate moderate heterogeneity, whereas an I2>50% would suggest substantial heterogeneity. A χ2 test result of P<0.1 was considered to indicate statistically significant heterogeneity. This P-value was chosen, as opposed to a smaller value, to reduce the risk of Type II error. A Begg's funnel plot was used to compare and assess publication bias between studies.
Quality assessment
The methodological quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS) for case-control studies (25). This is a risk of bias assessment tool specifically designed for non-randomised studies, with adaptations for use with case-control, cohort and cross-sectional studies (25). Using this tool, studies were graded in three main areas: Selection of study groups, comparability of groups and ascertainment of either the exposure or outcome. Studies included for meta-analysis were given an overall NOS score depending on the number of stars they are awarded in each field.
Results
Eligible studies
As presented in Fig. 1, 24,265 potentially relevant articles were retrieved through electronic database searches. An additional 12 articles were identified by examining the references of retrieved articles. A total of 23,416 studies were initially excluded due to duplication. After title and abstract evaluation, a further 824 articles were excluded, as they either did not describe or investigate a relationship between PCOS and endometrial cancer, their study design did not meet the criteria for the present meta-analysis or they lacked a control group. The full texts of 37 papers were examined and a further 27 studies were excluded due to not meeting the inclusion criteria. Observational studies without a control group and studies that lacked data on endometrial cancer prevalence specifically among females with PCOS were excluded. Other meta-analyses were excluded from this study to avoid duplication of results. A consensus was reached at all stages of the article retrieval process. Secondary screening of the search results did not lead to the exclusion or inclusion of any additional articles.
Figure 1.
Flow diagram of the study selection process. PCOS, polycystic ovarian syndrome.
The characteristics of the 10 studies that met the inclusion criteria for the meta-analysis are described in Table I. A total of 66,368 females (12,248 with PCOS and 54,120 controls) were identified. Of those patients with PCOS, 100 had endometrial cancer; of those without PCOS, 1,215 had endometrial cancer. The majority of studies had a case-control design and endometrial cancer had been diagnosed histologically. PCOS was diagnosed using a range of methods across studies, including self-reporting, serum hormone levels, histology and clinical diagnosis. Only 2 studies were controlled for body mass index (BMI).
Table I.
Characteristics of the 10 studies included in the meta-analysis to assess the risk of endometrial cancer in females with PCOS.
| First author, year | Country | Study design | Characteristics of participants | Diagnostic criteria for PCOS | Diagnostic criteria for EC | Controlled for BMI? | Research findings | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| Escobedo, 1991 | USA | Case-control | 399 females with EC aged 20–54 years; 3,040 age-matched randomly selected controls | Self-reported to trained interviewers; criteria type not stated | Histologically confirmed; physician diagnosed. Infertility patients | N | OR for EC of 4.2 (95% CI, 1.7-10.4) for ‘ovarian factor’ infertility | (31) |
| Niwa, 2000 | Japan | Case-control | 134 females with EC aged 40–70 years (42 premenopausal and 92 postmenopausal); 376 controls | Clinical data; diagnosis based on the criteria of Goldzieher (39) | Histologically confirmed primary carcinoma; physician diagnosed | N | Higher frequency of EC in a group of females with PCOS aged <40 years | (30) |
| Wild, 2000 | UK | Cohort | 786 females with PCOS with a mean age of 56.7 years (range, 38–98 years); 1,060 age-matched controls | Clinical data from various hospital records; criteria type not stated | General practice records of diagnosis and self-reported in questionnaires | Y | OR for EC of 5.3 (95% CI, 1.5-18.6). OR adjusted for BMI was 6.1 (95% CI, 1.0-36.9) | (32) |
| Iatrakis, 2006 | Greece | Case-control | 81 females with EC with a mean age of 46.3 years (range, 43–48 years); 100 female controls aged 43–48 years | Self-reported; criteria type not stated | Histologically confirmed carcinoma; physician diagnosed | N | Higher frequency in females with PCOS aged <50 years | (33) |
| Pillay, 2006 | UK | Cross-sectional | 128 females with EC with an age range of 20–90 years; 83 age-matched controls | Presence of PCO morphology as a marker of PCOS | Histologically confirmed | N | PCO was more prevalent in females with EC aged <50 years | (15) |
| Zucchetto, 2009 | Italy | Case-control | 454 females with EC with a median age of 60 years (range, 18–79 years); 908 controls with a median age of 61 years (range, 19–79 years) | Self-reported to trained interviewers; criteria type not stated | Histologically confirmed <1 year before hospitalisation, no earlier diagnosis | N | PCOS not associated with overall EC risk (OR, 1.25; 95% CI, 0.72-2.16); an increased risk among premenopausal females (OR, 2.03; 95% CI, 0.76-5.38) | (34) |
| Fearnley, 2010 | Australia | Case-control | 156 females with EC aged 18–79 years; 398 age-matched controls. Analyses restricted to females aged <50 years | Self-reported; criteria type not stated | Histologically confirmed; physician diagnosed | Y | 4-fold significantly increased risk of EC in females with PCOS | (35) |
| Kilicdag, 2011 | Turkey | Case-control | 417 premenopausal females with a mean age of 34.9 years. 52 with EC; 365 controls | Clinical data; diagnosis based on the Rotterdam criteria (3) | Histologically confirmed | N | More abnormal histology, including EC, in females with PCOS (9.6 vs. 1.1%; OR, 9.6; 95% CI, 2.5-37) | (27) |
| Shen, 2015 | Taiwan | Cohort | 3,566 females with PCOS; 14,264 controls. Median age at enrolment was 27 years | Clinical data; diagnosis based on ICD-9-CM code 256.4 (40) | Histologically confirmed; physician diagnosed | N | HR for developing EC during follow-up period was 10 times greater for females with PCOS | (29) |
| Ding, 2018 | Taiwan | Cohort | 8,155 females with PCOS aged 15–49 years; 32,620 age-matched randomly selected controls | Clinical data; diagnosis based on ICD-9-CM code 256.4 (40) | Clinical records; diagnosis based on ICD-9-CM code 182 (40) | N | Significantly increased risk of EC in PCOS group | (28) |
CI, confidence interval; BMI, body mass index; EC, endometrial cancer; HR, hazards ratio; OR, odds ratio; PCO, polycystic ovary; PCOS, polycystic ovarian syndrome; Y, yes; N, no.
Methodological quality
The quality of evidence in the majority of studies was considered moderate, with scores ranging from 2–7. None of the included studies was deemed to be of low quality following assessment. Table SI, Table SII, Table III detail the results of the quality assessment, performed according to the study design using the NOS criteria.
Data analysis
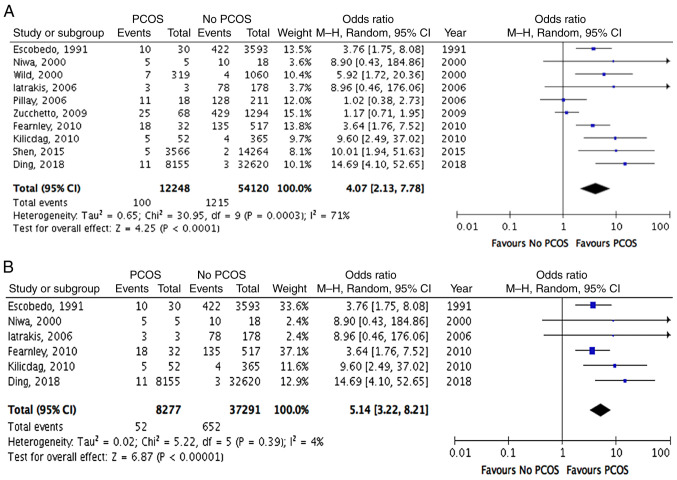
Heterogeneity was assessed using I2 and χ2 statistics. The studies were analysed using a random-effects model and a high degree of heterogeneity was identified (I2=71%; χ2, P=0.0003). As indicated in Fig. 2, the ORs of all 10 studies suggested an increased risk of endometrial cancer in females with PCOS.
Figure 2.
Forest plots of risk of endometrial cancer in females with PCOS. (A) Endometrial cancer in females with PCOS compared to controls. (B) Endometrial cancer in females aged ≤54 years with PCOS compared to controls. CI, confidence interval; M-H, Mantel-Haenszel; PCOS, polycystic ovarian syndrome; df, degrees of freedom.
Using the Mantel-Haenszel method, meta-analysis of the aggregated data also indicated an increased odds of endometrial cancer associated with PCOS. Specifically, the odds of developing endometrial cancer was 4 times higher among females with PCOS, when compared with those in the control group without PCOS (OR, 4.07; 95% CI, 2.13-7.78; P<0.0001; Fig. 2A). This increased odds was statistically significant. When adjusted to exclude postmenopausal patients aged >54 years, the odds ratio increased further (OR, 5.14; 95% CI, 3.22-8.21; P<0.00001; Fig. 2B), which suggested that the odds of developing endometrial cancer among premenopausal women with PCOS was five times greater than the odds among the control group of premenopausal women without PCOS. The risk for females with PCOS aged ≤54 years was also statistically significant. In light of the predicated 3% lifetime risk of developing endometrial cancer among the general population (26), the lifetime risk of endometrial cancer in patients with PCOS may be estimated to be as high as 12–15%.
Publication bias
Fig. 3 depicts the funnel plot analysis of publication bias among the included studies measuring the risk of endometrial cancer. The funnel plot exhibits asymmetry, with more studies containing ORs greater than the predicated overall OR of 4.07. The asymmetry of the funnel plot suggests that publication bias cannot be excluded and the risk of endometrial may have been overestimated.
Figure 3.
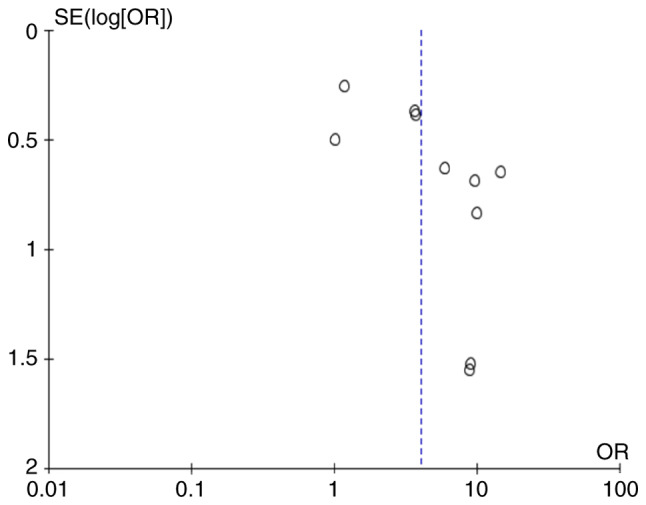
Funnel plot of studies of endometrial cancer in females with polycystic ovarian syndrome compared to controls. OR, odds ratio; SE, standard error.
Discussion
The present meta-analysis of 10 observational studies with 66,368 female subjects (12,248 with PCOS and 54,120 controls) confirmed that the odds of developing endometrial cancer among females with PCOS is greater than the odds among females without PCOS. Specifically, the results demonstrated 4 times greater odds of developing endometrial cancer in subjects with PCOS, compared to controls without PCOS (OR, 4.07; 95% CI, 2.13-7.78; P<0.0001). A subgroup analysis demonstrated an even greater odds in premenopausal females with PCOS (aged ≤54 years), compared to premenopausal controls without PCOS (OR, 5.14; 95% CI, 3.22-8.21; P<0.00001). The increased risk of endometrial cancer in females with PCOS was statistically significant in both groups.
The present meta-analysis provides clear statistical evidence to reinforce an unambiguous association between PCOS and endometrial cancer. Compared to previous meta-analyses, the present meta-analysis was enhanced by the inclusion of a greater number of studies with substantially larger sample sizes, as well as adherence to the PRISMA guidelines. As indicated in the funnel plot, although publication bias across studies cannot be excluded, none of the included studies was regarded as being of poor quality, as per the NOS criteria.
The main limitation of the present meta-analysis is the variation in the diagnostic method of PCOS across studies. Only Kilicdag et al (27) used the Rotterdam criteria to identify patients, whilst 4 studies used non-standard criteria (15,28–30) and the diagnostic criteria were unknown for the remaining 5 studies (31–35). Furthermore, the use of self-reporting of previous diagnosis or relevant clinical symptoms and signs to identify females with PCOS across 4 included studies (31,33–35) posed a risk of recall bias, subsequently impacting the reliability of their findings. Similarly, whilst the majority of included studies diagnosed endometrial cancer histologically, 1 study described using clinical data with ICD-9 coding for endometrial cancer (28), whilst another relied on data from clinical records and self-reporting (32). The validity of the endometrial cancer diagnoses in these studies is therefore uncertain. The use of self-reporting of clinical symptoms to identify females with PCOS, variation in endometrial cancer diagnostic criteria, and disparities in sample sizes across studies is likely to account for the considerable degree of heterogeneity present in the current study.
In addition, only 2 of the 10 studies took into account the significant confounding factor of obesity by controlling for BMI (32,35). Therefore, it remains unclear to what extent BMI contributes to endometrial cancer risk in females with PCOS. The potential for other confounding factors, such as diabetes, parity and the use of hormones, to transform the results of the present analysis, must also be considered. The high degree of heterogeneity in the present meta-analysis may also reflect the varying degrees to which confounding factors may have influenced the results within each included study. Finally, a proportion of relevant studies identified in the literature search were excluded, as they were non-comparative studies. As a result, the number of studies eligible for inclusion in the present meta-analysis was reduced.
To the best of our knowledge, the present study was the first systematic review and meta-analysis examining endometrial malignancy in females with PCOS since 2014 and it therefore provides up-to-date evidence to reinforce an unambiguous association between PCOS and endometrial cancer. The results of the present study agree with the findings of the preceding meta-analysis by Barry et al (18), which reported an overall effect size of PCOS on endometrial cancer risk of almost 3-fold (OR, 2.79; 95% CI, 1.31-5.95; P<0.008) based on 5 case-control studies. However, it is noted that the CIs of the ORs calculated for 3/5 studies analysed in that study crossed 1, suggesting no evidence of effect.
The present meta-analysis is also enhanced by the inclusion of a greater number of studies, with substantially large sample sizes. Specifically, the present analysis identified two studies published after 2014, which used larger sample sizes of 17,830 and 40,775 female subjects (28,29). Strikingly, these two studies suggested that females with PCOS have a 10 to 14-fold increased hazards ratio for developing endometrial cancer compared with the hazard ratios for controls without PCOS, with a 95% CI>1 (28,29). The evidence from these more recent studies alone suggests a 30–44% lifetime risk of developing endometrial cancer amongst females with PCOS within the studied demographics. The addition of more recent studies to the present meta-analysis therefore adds statistical power to the association between PCOS and endometrial cancer.
Considering more recent data, the analysis of premenopausal patients (those aged ≤54 years) substantiates and further strengthens the notion that the odds of developing endometrial cancer among females with PCOS compared to those without PCOS may be even greater within this subgroup, as suggested in a previous meta-analysis (18). These findings also agree with evidence suggesting that Type II endometrial cancer (the subtype less associated with PCOS) is more prevalent amongst older and postmenopausal females (9).
The OR of 4.0 (95% CI, 1.7-9.3) for the endometrial cancer risk amongst females with PCOS stated in one of the included studies (35) differs from the OR calculated in the present meta-analysis (OR, 3.64; 95% CI, 1.76-7.52) using the primary data available in the publication. When comparing these ORs, this difference was deemed unlikely to significantly impact or change the findings of the present meta-analysis. This discrepancy has also been identified and dismissed in a previous meta-analysis (7).
A proportion of relevant studies identified in the literature search were excluded from the present meta-analysis, as they were non-comparative studies. Although it was not possible to statistically determine the strength of the association between PCOS and endometrial cancer from the studies excluded from the meta-analysis, an association between these two variables was suggested across all of these studies (11,36–38). Similarly, Gottschau et al (36) described an almost 4-fold increased risk of endometrial cancer in females with PCOS.
Of note, across most included studies, only a small proportion of females diagnosed with PCOS went on to develop endometrial cancer. This suggests that while an association between both diagnoses is clear, the incidence of endometrial cancer in this group is still relatively low. Therefore, the use of large-scale screening programs to detect endometrial cancer in this subgroup is unlikely to be cost-effective for most healthcare systems.
In light of the evidence currently available, it may be concluded that the odds of having endometrial cancer were greater among females with PCOS, compared to the odds among those without PCOS. The results of the present meta-analysis add statistical strength to this notion and further contribute to the current evidence base. However, the present study has highlighted the sheer sparseness and defects in existing evidence. Consequently, the strength of current evidence continues to be diluted by the unclear impact of variation in PCOS diagnosis, selection bias and confounding risk factors (particularly high BMI) on the exact risk. More large-scale, prospective studies are required to further investigate the association between PCOS and endometrial cancer, while also considering potential confounding factors.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Authors' contributions
PIS and CT conceived the study. The design of the study, extraction and analysis of data and manuscript writing were conducted by JEJ and DD. Editing and revising of the manuscript was completed by PIS and CT. JEJ and DD have seen and confirm the authenticity of the raw data generated during the study. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Harris HR, Terry KL. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: A systematic review. Fertil Res Pract. 2016;2:14. doi: 10.1186/s40738-016-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okamura Y, Saito F, Takaishi K, Motohara T, Honda R, Ohba T, Katabuchi H. Polycystic ovary syndrome: Early diagnosis and intervention are necessary for fertility preservation in young women with endometrial cancer under 35 years of age. Repro Med Biol. 2016;16:67–71. doi: 10.1002/rmb2.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, Gabra H, Paraskevaidis E, Martin-Hirsch P, Tsilidis KK, Kyrgiou M. Risk factors for endometrial cancer: An umbrella review of the literature. Int J Cancer. 2019;145:1719–1730. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Gong TT, Liu FH, Jiang YT, Sun H, Ma XX, Zhao YH, Wu QJ. Global, regional and national burden of endometrial cancer, 1990–2017: Results from the gobal burden of disease study, 2017. Front Oncol. 2019;19:1440. doi: 10.3389/fonc.2019.01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balen A. Polycystic ovary syndrome and cancer. Hum Reprod Update. 2001;7:522–525. doi: 10.1093/humupd/7.6.522. [DOI] [PubMed] [Google Scholar]
- 7.Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum Reprod. 2012;27:1327–1331. doi: 10.1093/humrep/des042. [DOI] [PubMed] [Google Scholar]
- 8.Yang HP, Wentzensen N, Trabert B, Gierach GL, Felix AS, Gunter MJ, Hollenbeck A, Park Y, Sherman ME, Brinton LA. Endometrial cancer risk factors by 2 main histologic subtypes. Am J Epidemiol. 2013;177:142–151. doi: 10.1093/aje/kws200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinberg J, Albright B, Black J, Lu L, Passarelli R, Gysler S, Whicker M, Altwerger G, Menderes G, Hui P, et al. Ten-year comparison study of type 1 and 2 endometrial cancers: Risk factors and outcomes. Gynecol Obs Invest. 2019;84:290–297. doi: 10.1159/000493132. [DOI] [PubMed] [Google Scholar]
- 10.Navaratnarajah R, Pillay OC, Hardiman P. Polycystic ovary syndrome and endometrial cancer. Semin Reprod Med. 2008;26:62–71. doi: 10.1055/s-2007-992926. [DOI] [PubMed] [Google Scholar]
- 11.Park JC, Lim SY, Jang TK, Bae JG, Kim JI, Rhee JH. Endometrial histology and predictable clinical factors for endometrial disease in women with polycystic ovary syndrome. Clin Exp Reprod Med. 2011;38:42–46. doi: 10.5653/cerm.2011.38.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardiman P, Pillay OS, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361:1810–1812. doi: 10.1016/S0140-6736(03)13409-5. [DOI] [PubMed] [Google Scholar]
- 13.Gadducci A, Gargini A, Palla E, Fanucchi A, Genazzani AR. Polycystic ovary syndrome and gynaecological cancers: Is there a link? Gynecol Endo. 2005;20:200–208. doi: 10.1080/09513590400021201. [DOI] [PubMed] [Google Scholar]
- 14.Fanta M. Is polycystic ovary syndrome, a state of relative oestrogen excess, a real risk factor for oestrogen-dependant malignancies? Gynecol Endocrinol. 2013;29:145–147. doi: 10.3109/09513590.2012.730575. [DOI] [PubMed] [Google Scholar]
- 15.Pillay OC, Fong LFW, Crow JC, Benjamin E, Mould T, Atiomo W, Menon PA, Leonard AJ, Hardiman P. The association between polycystic ovaries and endometrial cancer. Hum Reprod. 2006;21:924–929. doi: 10.1093/humrep/dei420. [DOI] [PubMed] [Google Scholar]
- 16.Atiomo W, Khalid S, Parameshweran S, Houda M, Layfield R. Proteomic biomarkers for the diagnosis and risk stratification of polycystic ovary syndrome: A systemic review. BJOG. 2009;116:137–143. doi: 10.1111/j.1471-0528.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- 17.Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: A systematic review. Reprod Biomed Online. 2009;19:398–405. doi: 10.1016/S1472-6483(10)60175-7. [DOI] [PubMed] [Google Scholar]
- 18.Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2014;20:748–758. doi: 10.1093/humupd/dmu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holm NSL, Glintborg D, Andersen MS, Schledermann D, Ravn P. The prevalence of endometrial hyperplasia and endometrial cancer in women with polycystic ovary syndrome or hyperandrogenism. Acta Obstet Gynecol Scand. 2012;91:1173–1176. doi: 10.1111/j.1600-0412.2012.01458.x. [DOI] [PubMed] [Google Scholar]
- 20.Ho SP, Tan KT, Pang MW, Ho TH. Endometrial hyperplasia and the risk of endometrial carcinoma. Singapore Med J. 1997;38:11–15. [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The Prisma 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Version 5.4. The Cochrane Collaboration; 2020. Review Manager (RevMan) [Computer program] [Google Scholar]
- 25.Wells GA, Wells G, Shea B, Shea B, O'Connell D, Peterson J, Welch, Losos M, Tugwell P, Ga SW, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses [Google Scholar]
- 26.Smittenaar C, Petersen K, Stewart K, Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016;25:1147–1155. doi: 10.1038/bjc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilicdag EB, Haydardedeoglu B, Cok T, Parlakgumus AH, Simsek E, Bolat FA. Polycystic ovary syndrome and increased polyp numbers as risk factors for malignant transformation of endometrial polyps in premenopausal women. Int J Gynecol Obstet. 2011;112:200–203. doi: 10.1016/j.ijgo.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Ding DC, Chen W, Wang JH, Lin SZ. Association between polycystic ovarian syndrome and endometrial, ovarian, and breast cancer: A population-based cohort study in Taiwan. Medicine (Baltimore) 2018;97:e12608. doi: 10.1097/MD.0000000000012608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen CC, Yang AC, Hung JH, Hu LY, Tsai SJ. A nationwide population-based retrospective cohort study of the risk of uterine, ovarian and breast cancer in women with polycystic ovary syndrome. Oncologist. 2015;20:45–49. doi: 10.1634/theoncologist.2014-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niwa K, Imai A, Hashimoto M, Yokoyama Y, Mori H, Matsuda Y, Tamaya T. A case-control study of uterine endometrial cancer of pre- and post-menopausal women. Oncol Rep. 2000;7:89–93. [PubMed] [Google Scholar]
- 31.Escobedo LG, Lee NC, Peterson HB, Wingo PA. Infertility-associated endometrial cancer risk may be limited to specific subgroups of infertile women. Obstet Gynecol. 1991;77:124–128. [PubMed] [Google Scholar]
- 32.Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: Results of a 31 year follow-up study. Hum Fertil (Camb) 2000;3:101–105. doi: 10.1080/1464727002000198781. [DOI] [PubMed] [Google Scholar]
- 33.Iatrakis G, Tsionis C, Adonakis G, Stoikidou M, Anthouli-Anagnostopoulou F, Parava M, Vouxinou A, Georgopoulos NA, Kourounis G. Polycystic ovarian syndrome, insulin resistance and thickness of the endometrium. Eur J Obstet Gynecol Reprod Biol. 2006;127:218–221. doi: 10.1016/j.ejogrb.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Zucchetto A, Serraino D, Polesel J, Negri E, De Paoli A, Dal Maso L, Montella M, La Vecchia C, Franceschi S, Talamini R. Hormone-related factors and gynecological conditions in relation to endometrial cancer risk. Eur J Cancer Prev. 2009;18:316–321. doi: 10.1097/CEJ.0b013e328329d830. [DOI] [PubMed] [Google Scholar]
- 35.Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: An Australian case-control study. Cancer Causes Control. 2010;21:2303–2308. doi: 10.1007/s10552-010-9658-7. [DOI] [PubMed] [Google Scholar]
- 36.Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: A Danish cohort study. Gynecol Oncol. 2015;136:99–103. doi: 10.1016/j.ygyno.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Prakansamut N, Sirayapiwat P, Triratanachat S. The percentages of endometrial hyperplasia and endometrial cancer among polycystic ovary syndrome (PCOS) patients presenting with abnormal menstrual pattern. J Med Assoc Thai. 2014;97:159–164. [PubMed] [Google Scholar]
- 38.Indhavivadhana S, Rattanachaiyanont M, Wongwananuruk T, Techatraisak K, Rayasawath N, Dangrat C. Endometrial neoplasia in reproductive-aged Thai women with polycystic ovary syndrome. Int J Gynaecol Obstet. 2018;142:170–175. doi: 10.1002/ijgo.12522. [DOI] [PubMed] [Google Scholar]
- 39.Goldzieher JW. Polycystic ovarian disease. Fertil Steril. 1981;35:371–394. doi: 10.1016/S0015-0282(16)45429-4. [DOI] [PubMed] [Google Scholar]
- 40.CDC, corp-author. Health Statistics 2011. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/ICD9-CM/2011/ [ December 3; 2022 ]; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.