Abstract
The present study aimed to explore the final diagnosis of pulmonary nodules with an initial non-diagnostic result on electromagnetic navigation bronchoscopy (ENB) biopsy and the predictive factors for a non-diagnostic result. A total of 198 nodules from 194 patients that were suspected to be malignant tumors were included in the present study. The initial biopsy pathology results were divided into two groups: The diagnostic group and the non-diagnostic group. The diagnostic group was defined as a successful initial biopsy to obtain a diagnosis, including malignant and benign diagnoses. The non-diagnostic group was defined as a non-specific benign diagnosis, normal lung tissue or an unsuccessful biopsy. Among the 198 nodules, 139 (70.2%) were in the diagnostic group and 59 (29.8%) were in the non-diagnostic group. Predictive factors for a non-diagnostic biopsy included nodule size ≤1.5 cm [odds ratio (OR), 2.05; 95% confidence interval (CI), 1.03-4.09], non-solid nodules (OR, 2.71; 95% CI, 1.33-5.64) and nodules in the left lung (OR, 2.50; 95% CI, 1.27-4.92). Of the 59 non-diagnostic biopsies, 46 were finally confirmed to be malignant by surgery. Notably, non-diagnostic biopsies with non-solid nodules (OR, 7.64; 95% CI, 3.11-18.76) were more likely to be malignant. In conclusion, the predictive factors for a non-diagnostic biopsy were nodule size ≤1.5 cm and non-solid nodules. It was not rare for patients to finally be diagnosed with a malignancy in the non-diagnostic group. Therefore, care should be taken when the results of an ENB are non-diagnostic to prevent misdiagnosis.
Keywords: pulmonary nodule, electromagnetic navigation bronchoscopy, biopsy, lung cancer
Introduction
Lung cancer is one of the most deadly tumors, and it is associated with a high incidence and mortality rate worldwide. Lung cancer-associated deaths account for 18.7% of all deaths caused by malignant tumors worldwide (1,2). Currently, lung cancer is typically divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) according to pathological type. In a recent study of statistics in the USA, NSCLC accounted for 82% of all patients with lung cancer, SCLC accounted for 14%, and the pathological histology of the remaining 3% of patients with lung cancer was unclear (3). The early diagnosis and appropriate treatment of lung cancer are critical for improving the survival rate of patients (4,5). Unfortunately, most patients with lung cancer often delay medical attention in the early stages as the symptoms are not obvious, resulting in numerous patients reaching a progressive stage at the time of diagnosis. Only 30% of NSCLC cases are typically diagnosed at stage I (6). The overall 5-year survival rate for stage I is 65%, but this rate decreases to 5% for stage IV (6). Therefore, the early diagnosis of lung cancer is extremely important.
In the early diagnosis of lung cancer, performing pathology examinations after biopsy is an important method to identify the nature of lung lesions. These biopsy methods include CT-guided percutaneous lung puncture biopsy, bronchoscopic biopsy, endobronchial ultrasound-guided transbronchial needle aspiration, and electromagnetic navigation bronchoscopy (ENB) biopsy (7,8). Electromagnetic navigation bronchoscopy is a diagnostic method that has been widely used in lung nodule biopsies in recent years. ENB uses electromagnetic positioning, combined with high-resolution spiral CT data for the reconstruction of lung structure, and chooses the best channel to extract the biopsy (9). Previous studies have suggested that ENB biopsy has a malignant detection rate of 50–85% (10–14). The majority of previous studies on ENB have assessed a small number of cases, have used inconsistent practices (e.g., differences in the study design and follow-up) and have shown marked differences in the accuracy of lung nodule biopsy (12,14,15).
Notably, most investigations have focused on the diagnostic yield, sensitivity and negative predictive value of ENB, and only a limited number of studies have analyzed the final diagnoses of non-diagnostic biopsies (16,17). The final diagnoses of indeterminate diagnoses are often incomplete, which may result in a delay in treatment. The present study aimed to explore the actual final diagnosis of pulmonary lesions with an initial non-diagnostic result on ENB biopsy and aimed to identify the predictive factors for a non-diagnostic result.
Materials and methods
Patients and grouping
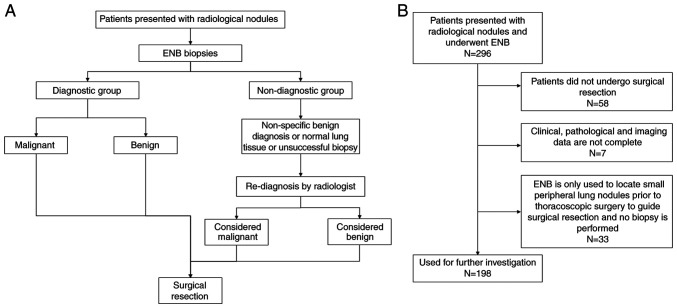
The present study retrospectively analyzed 296 patients who underwent ENB biopsy at The First Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China) between February 2017 and September 2019. The diagnostic procedure is shown in Fig. 1A. The eligibility criteria were as follows: i) Patients were diagnosed with pulmonary nodules that were suspected to be malignant tumors; ii) patients underwent ENB; and iii) patients who eventually underwent surgical resection. The exclusion criteria were as follows: i) Patients who did not undergo surgical resection; ii) inoperative localization of pulmonary nodule resection using the ENB technique; and iii) patients with incomplete clinicopathological data (Fig. 1B). Finally, a total of 198 nodules from 194 patients that were suspected to be malignant tumors were included in the present study.
Figure 1.
Flowchart of the present study. (A) Diagnostic procedure. (B) Inclusion and exclusion criteria. A total of 198 samples were obtained from 194 patients. ENB, electromagnetic navigation bronchoscopy.
The initial pathology was obtained by ENB biopsy, and the results of the ENB biopsy were divided into a diagnostic group and a non-diagnostic group. The diagnostic group contained cases with biopsy results of adenocarcinoma, squamous cell carcinoma, other types of primary lung malignant tumors and metastatic tumors, and other malignant tumors, or of benign lung diseases, such as inflammatory nodules, fungal nodules and benign tumors, such as hamartoma and sclerosed hemangioma. The non-diagnostic group contained cases in which a clear diagnosis of malignancy or benignity was not obtained after performing ENB. This usually refers to cases in which the lesion was not successfully punctured and the pathology was reported as normal lung tissue, no bright malignant cells were seen, the specimen volume was too small to be produced and cases where no specific diagnoses have been made. In cases where the initial biopsy of ENB was non-diagnostic, the nodules were evaluated again using imaging and a portion of the patients will undergo surgical resection of malignant tumors or benign tumors, such as hamartoma. In addition, some patients had multiple nodules, and at their request, the ipsilateral nodule together with the lesion considered malignant was removed. The present study retrospectively reviewed cases of surgical resection following ENB biopsy. All ENB biopsy procedures were performed by the same experienced endoscopist, and the lung biopsy slides were reviewed by two experienced thoracic pathologists to obtain a pathological diagnosis.
Composition and standard procedure of ENB
ENB system (V Super Dimension version 7; Medtronic) components mainly include: i) Electromagnetic positioning plate that can generate a low-frequency uniform electromagnetic field. The plate is generally required to be placed under the mattress of the examination bed (>50 cm from the examined individual) so that the patient's chest is in the electromagnetic field. ii) Navigation probe, which is fixed to the tip of a bendable catheter (diameter, 1 mm; length, 8 mm) and can be rotated 360°. In the electromagnetic field, the orientation of the probe can be obtained by the positioning system and transmitted to the computer in real-time when X, Y and Z axis and tilt and rotation are carried out in the body of the patient. iii) Extended operation channel, which can be placed into the relevant operating instruments by the navigation system to guide the target area for operation. iv) ENB system hosts and monitors, through receiving and processing magnetic navigation signals, actual organ images under the bronchoscope are displayed and virtual navigation 3D tracheal images are presented through the computer platform, to monitor and guide the position and direction of the probe.
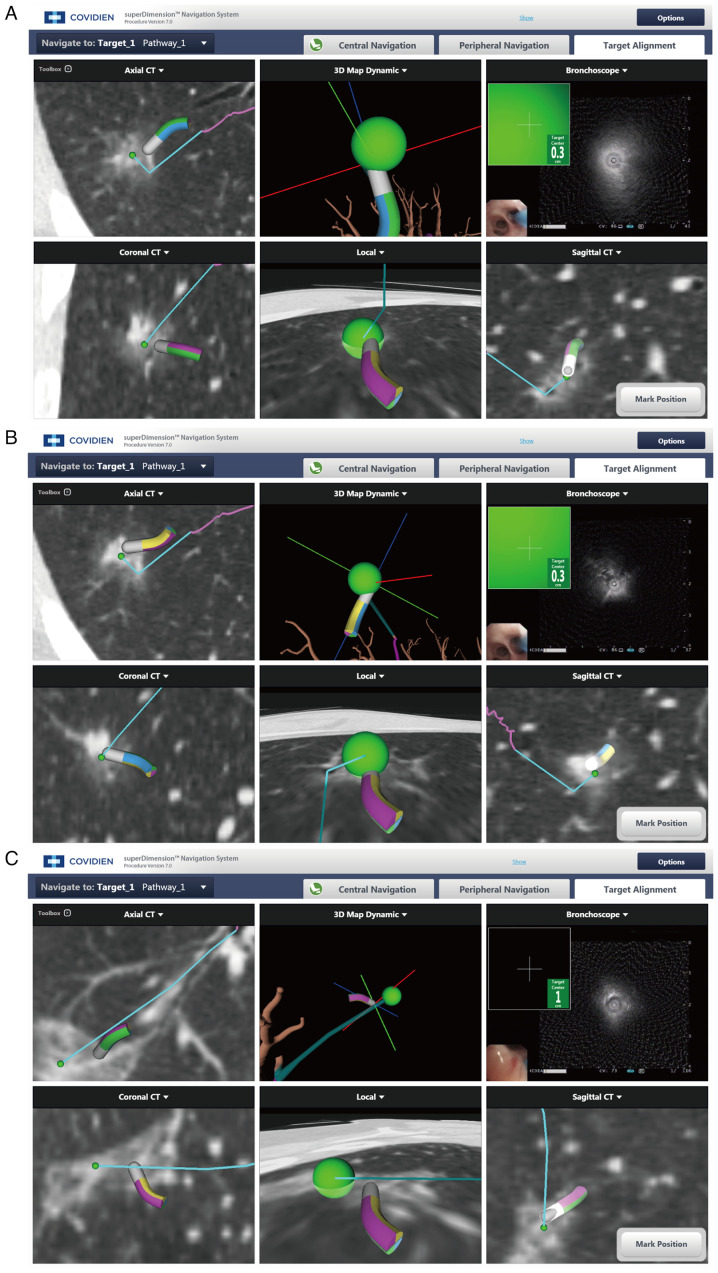
The ENB standard operation consists of two main parts: i) Preoperative path planning, a virtual bronchial tree image is generated by 3D reconstruction of the original CT image data using ENB software, the target lesion markers are found on the image, 5–7 anatomical markers are selected, and a navigation path to the target lesion is generated; and ii) intraoperative endotracheal navigation, after the patient is anesthetized, the physician operates the electronic bronchoscope and places the navigation probe through the working channel. The virtual image is matched with the actual image by confirming the selected markers on the virtual image with the actual position of the in vivo probe. After successful matching, the target lesion is reached via the preoperative planning path. The navigation ends with exit of the localization probe, then the needle aspiration and biopsy are performed via the extended channel (Fig. 2).
Figure 2.
Images from ENB operation. ENB biopsies of a (A) 1.9-cm diameter ground glass lesion, (B) 0.8-cm diameter mixed ground glass lesion and (C) 2.3-cm diameter mixed ground glass. ENB, electromagnetic navigation bronchoscopy.
Data collection
The clinical, pathological and imaging data of the included patients were collected through the hospital's electronic medical record system. The variables, including age, sex and history of prior malignancy, were extracted from the patients' records. The radiological variables analyzed for each patient included the nodule size, nodule type, distance between the pleura and the nodule, and nodule position.
Statistical analysis
Categorical variables were assessed using the χ2 test and Fisher's exact test. When continuous variables obeyed normal distribution, such as age, the data were analyzed using the independent sample t-test (unpaired parametric Student's t-test). Nodule size, CT value and distance from pleura were analyzed using the non-parametric Mann-Whitney U test. Odds ratio (OR) with a 95% confidence interval (CI) was determined using binary logistic regression. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis of all data was performed using SPSS software (version 26.0; IBM Corp.).
Results
Distribution of pathological diagnostic results
Among the 198 nodules, there were 139 cases in the diagnostic group and 59 (29.8%) cases in the non-diagnostic group. A total of 165 cases were diagnosed as malignant tumors by surgical pathology and 33 cases as benign diseases. In the diagnostic group, there were 119 cases of malignant tumors and 20 cases of benign diseases. In the non-diagnostic group, there were 46 cases of malignant tumors and 13 cases of benign diseases, according to surgical pathology (Table I).
Table I.
Distribution of pathological diagnostic results of study subjects.
| Category | Diagnostic group (n=139) | Non-diagnostic group (n=59) | Total (n=198) | P-value |
|---|---|---|---|---|
| Malignancy | 119 | 46 | 165 | 0.45 |
| Adenocarcinoma | 112 | 42 | ||
| Squamous carcinoma | 4 | 1 | ||
| Metastasis | 1 | 0 | ||
| Other types | 2 | 3 | ||
| Benign | 20 | 13 | 33 | 0.06 |
| Inflammatory nodules | 10 | 6 | ||
| Hamartoma | 1 | 2 | ||
| Fungal nodules | 3 | 2 | ||
| Sclerosed hemangioma | 1 | 2 | ||
| Other types | 5 | 1 |
Diagnostic versus non-diagnostic group
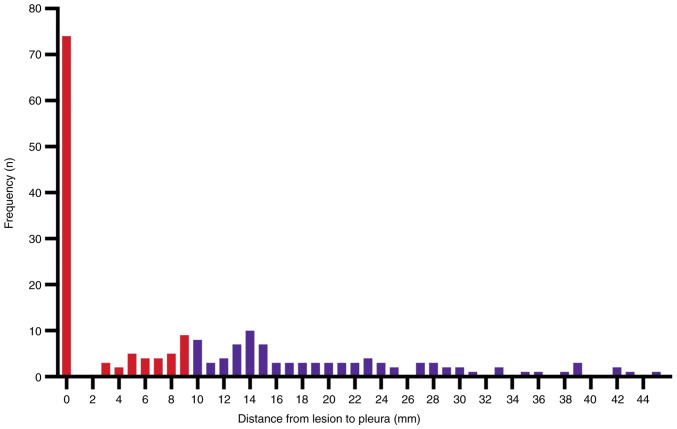
The median lesion size was 1.7 and 1.4 cm for patients in the diagnostic and non-diagnostic groups, respectively. Comparing the clinical and imaging data of the patients, there was no significant difference regarding sex, age, previous history of malignancy and bronchus signs (Table II). There was a statistically significant difference between the groups regarding nodule size (P<0.01), CT value (P<0.01) and location of the nodules (P=0.04). Compared with subjects in the diagnostic group, there were more non-solid nodules in the non-diagnostic group. There were also significant differences in whether the nodules were in contact with the pleura (P=0.02). Lesions were ≥10 mm from the pleura in 46.5% (92/198) of all cases; and were on the pleura in 37.4% of all cases (Fig. 3).
Table II.
Clinicopathologic characteristics of the study population.
| Category | Diagnostic group (n=139) | Non-diagnostic group (n=59) | P-value |
|---|---|---|---|
| Sex | 0.44 | ||
| Male, n (%) | 72 (36.4) | 27 (13.6) | |
| Female, n (%) | 67 (33.8) | 32 (16.2) | |
| Mean age + SD, years | 61.09±10.87 | 60.22±9.20 | 0.59 |
| History of malignancy | 0.50 | ||
| Yes, n (%) | 8 (4.0) | 4 (2.0) | |
| No, n (%) | 131 (66.2) | 55 (27.8) | |
| Pathology results | 0.19 | ||
| Benign, n (%) | 20 (10.1) | 13 (6.6) | |
| Malignancy, n (%) | 119 (60.1) | 46 (23.2) | |
| Median nodule size, cm (IQS) | 1.7 (1.2) | 1.4 (1.0) | <0.01 |
| Nodule type | <0.01 | ||
| Solid, n (%) | 66 (33.3) | 14 (7.0) | |
| Pure GGO, n (%) | 7 (3.5) | 11 (5.6) | |
| Mixed GGO, n (%) | 66 (33.3) | 34 (17.2) | |
| Median CT value, Hu (IQS) | −120 (−418) | −270 (−432) | <0.01 |
| Median distance from pleura, cm (IQS) | 0.80 (1.60) | 1.00 (1.70) | 0.12 |
| Pleural contact | 0.02 | ||
| Yes, n (%) | 57 (28.8) | 14 (7.1) | |
| No, n (%) | 82 (41.4) | 45 (22.7) | |
| Bronchus sign | 0.18 | ||
| Yes, n (%) | 7 (3.5) | 6 (3.0) | |
| No, n (%) | 132 (66.7) | 53 (26.8) | |
| Nodule position | 0.04 | ||
| Upper left lung, n (%) | 25 (12.6) | 20 (10.1) | |
| Lower left lung, n (%) | 17 (8.6) | 10 (5.1) | |
| Upper right lung, n (%) | 55 (27.8) | 14 (7.1) | |
| Middle lung, n (%) | 18 (9.1) | 4 (2.0) | |
| Right lower lung, n (%) | 24 (12.1) | 11 (5.6) |
GGO, ground-glass opacity; IQS, interquartile spacing.
Figure 3.
Lesion location. The graph shows the distance from lung lesion to pleura in 198 lesions. Lesions were <10 mm from the pleura (red bars) or ≥10 mm from the pleura (blue bars).
Predictive factor analysis
To explore the non-diagnostic predictors of ENB biopsy results, a comparison of clinicopathological characteristics between the two groups was performed using logistic regression. Following univariate analysis, variables yielding P<0.05 were analyzed using multivariate logistic regression analysis. The multivariate analyses demonstrated that nodule size (P=0.04; OR, 2.05; 95% CI, 1.03-4.09), nodule type (P=0.01; OR, 2.71; 95% CI, 1.33-5.64) and nodule position (P=0.008; OR, 2.50; 95% CI, 1.27-4.92) were independent non-diagnostic risk factors for ENB biopsy (Fig. 4).
Figure 4.
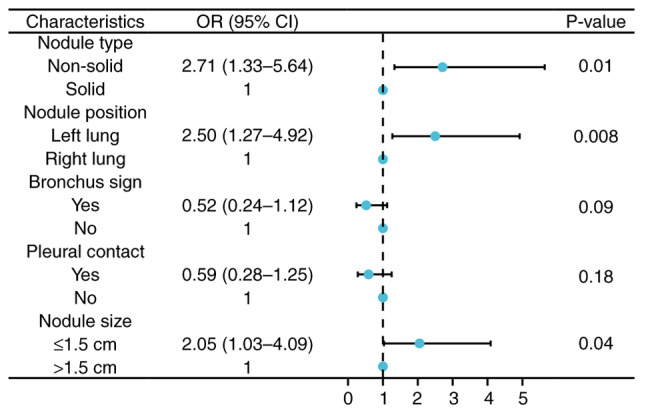
Multivariate regression analysis of non-diagnostic-related risk factors for electromagnetic navigation bronchoscopy biopsy. CI, confidence interval; OR, odds ratio.
Subgroup analysis of the non-diagnostic group
In the non-diagnostic group, the final pathological results for 46 cases were malignant tumors, and the final pathological results of the remaining 13 cases were benign; 78.0% of non-diagnosed cases were found to be malignant by surgery. The present study compared and analyzed the data of the two groups and the results are presented in Table III. Among them, there was a statistically significant difference in the nodule type and CT value (both P<0.05).
Table III.
Non-diagnostic subgroup analysis.
| Category | Malignant (n=46) | Benign (n=13) | P-value |
|---|---|---|---|
| Sex | 0.22 | ||
| Male, n | 19 | 8 | |
| Female, n | 27 | 5 | |
| Mean age + SD, years | 61.28±7.33 | 56.46±7.88 | 0.10 |
| History of malignancy | 0.99 | ||
| Yes, n | 3 | 1 | |
| No, n | 43 | 12 | |
| Median nodule size, cm (IQS) | 1.4 (1.0) | 1.3 (0.7) | 0.18 |
| Nodule type | <0.01 | ||
| Solid, n | 5 | 9 | |
| Pure GGO, n | 10 | 1 | |
| Mixed GGO, n | 31 | 3 | |
| Median CT value, Hu (IQS) | −395.00 (−397.50) | −12.00 (−282.00) | <0.01 |
| Median distance from pleura, cm (IQS) | 1.00 (1.63) | 1.10 (1.20) | 0.61 |
| Pleural contact | 0.99 | ||
| Yes, n | 11 | 3 | |
| No, n | 35 | 10 | |
| Bronchus sign | 0.63 | ||
| Yes, n | 11 | 3 | |
| No, n | 35 | 10 | |
| Nodule position | 0.40 | ||
| Upper left lung, n | 16 | 4 | |
| Lower left lung, n | 7 | 3 | |
| Upper right lung, n | 13 | 1 | |
| Middle lung, n | 3 | 1 | |
| Right lower lung, n | 7 | 4 |
GGO, ground-glass opacity; IQS, interquartile spacing.
In addition, a multivariate regression model was used to explore the independent risk factors for the initial non-diagnosed cases that received a final surgical diagnosis of malignant tumors. Among them, non-solid nodules (P<0.05; OR, 7.64; 95% CI, 3.11-18.76) was an independent risk factor for malignant tumors in the initial non-diagnostic group (data not shown).
Discussion
With the promotion of lung cancer screening, the detection rate of lung nodules has increased, and the mortality rate of patients with lung cancer has continued to decline (18,19). In the age of precision medicine, it is necessary to formulate precise strategies for every nodule detected in each patient, either by observation and follow-up, direct surgical resection or biopsy to determine the pathology. In particular, individuals at a high risk of lung cancer need to be treated with caution and different strategies need to be adopted for different nodules to maximize efficacy (20). Previous studies have reported an 88% diagnostic yield for large central lesions using bronchoscopy for tissue sampling, but a significant decrease in diagnostic efficacy has been identified for small peripheral lung lesions, especially small nodular lesions below the segmental plane. In one study, bronchoscopy had a diagnostic yield of only 14% for peripheral pulmonary lesions <2 cm, and it has been shown that for peripheral pulmonary lesions, the diagnosis rate of navigation bronchoscopy is higher than that of non-navigated bronchoscopy (21). Navigation bronchoscopy is split into virtual navigation and electromagnetic navigation (22). Among them, ENB has been shown to have better specificity than virtual navigation bronchoscopy (22). ENB was first applied to the human body in the early 21st century, and it has been widely used in the diagnosis and treatment of lung cancer by a number of centers. Previous studies have reported that the overall accuracy, sensitivity and specificity of ENB biopsy is acceptable (14,23); however, these studies are limited by sample size, diagnostic definitions, alternative definitions and heterogeneity between groups. The results have large volatility and poor reliability. In 2014, a systematic review and analysis on ENB biopsy reported that although the overall accuracy of ENB in diagnosing malignant tumors is considered acceptable, the negative predictive value of ENB for malignant tumors is only 52.1% (95% CI, 43.5-60.6) (16); in different research reports, the negative predictive value fluctuates between 25.0 and 89.5% (10,13,21), but the histological benign diagnosis obtained by an ENB biopsy is not enough to exclude cancer (16). The prospective multi-center NAVIGATE study included >1,000 cases to initially evaluate the safety and accuracy of ENB. The pneumothorax rate published by NAVIGATE was 3.1% and the accuracy of the ENB initial biopsy was 72.9% (24). Even if the samples obtained show chronic inflammation or granulomatous inflammation, the negative predictive value of ENB is still not ideal.
The present study included ~200 biopsy cases and the final pathology was obtained by lung surgery. The results revealed that nodules sized ≤1.5 cm, non-solid nodules and nodules in the left lung were independent non-diagnostic risk factors for ENB biopsy.
It is generally known that nodule size is one of the most important factors in the success of ENB biopsy, and the well-known NAVIGATE study reported that nodule size ≥2 cm was a significant univariate predictor of diagnostic yield (24). Non-solid nodules contain a large number of ground glass nodules and are relatively small in diameter (25). In addition, most do not have bronchial signs, are relatively difficult to obtain and pose a great diagnostic challenge to pathologists (23). Notably, the present study revealed that the presence of nodules in the left lung was also a predictor of initial non-diagnosis by ENB biopsy; to the best of our knowledge, this has not been reported previously. The present study hypothesized that this may be related to the movement of pulmonary nodules due to respiratory motion of the left, as well as the right, lung during ENB. It has previously been reported that the change from full inspiration during a chest CT scan to tidal volume breathing during a bronchoscopy may significantly affect the diagnostic rate of ENB (26). The present study has the limitation that it was a single-center retrospective study; however, further studies to explore the factors that influence the success of ENB biopsy will be conducted.
Non-solid nodules were independent risk factors for malignant tumors in the ENB non-diagnostic group. The accuracy of the overall initial biopsy pathology and final pathological diagnosis in the present study was not high, because the overall diameter of the lung nodules included in the present study was relatively small. The median nodule size of the 198 cases was 1.6 cm, and the interquartile range was 1.1 cm, including 90 cases with nodules ≤1.5 cm in diameter (45.6% of the total cases). Among them, there were 27 patients with nodules ≤1.0 cm in diameter, including more ground glass nodules. Usually, if the nodule is in contact with the pleura, the diagnostic rate of bronchoscopy becomes worse because there are fewer bronchial signs; however, in the present study, there was no significant statistical difference between the two groups in terms of the bronchial signs. The possible reasons why there was no statistical difference are as follows: First, the bronchoscopic transparenchymal nodule access technique has been well established at our center, which is also known as the tunneling technique; during this technique, a hole is made in the bronchial wall and a tunnel is created to reach the nodule through a working channel in the lung parenchyma, allowing theoretical ‘whole lung access’ to the nodule without relying on the natural bronchial lumen. Moreover, in the present study, the diameter of the lesion in pleural contact was relatively large, which may cause a bias in the study.
In the non-diagnostic group, 78.0% of the cases were finally confirmed as malignant tumors by surgery, which indicates that clinicians should be cautious in making decisions when the initial ENB biopsy fails to obtain a clear diagnosis. Options to improve diagnosis may include combining ENB biopsy with PET-CT, another ENB biopsy or another biopsy method, in order to prevent delays in diagnosis and treatment, especially for patients with ground glass lesions.
In general, ENB biopsy is a safe and effective technical method; however, it is more difficult to apply if the nodules have a diameter of <1.5 cm or if the nodules have fewer solid components. In addition, it is necessary to combine multiple diagnostic methods to ensure an accurate diagnosis. The present study explored the related factors of ENB biopsy failure and inaccurate diagnosis. In addition, the final outcome of the included cases was obtained through surgery to obtain the pathological gold standard. Compared with imaging follow-up, the data quality was more reliable. In addition, in the present study, more difficult explorations of ENB biopsy of peripheral lung nodules were conducted and sampling biopsy of smaller nodules and more ground glass lesions was performed. Notably, the present study has certain limitations. This study is a single-center retrospective study, and there is inevitably a selection bias, which limits the applicability of the conclusions to a certain extent. In the future, a multicenter, prospective study will be conducted to explore the diagnosis of ENB biopsy further.
In conclusion, the predictive factors for a non-diagnostic ENB biopsy were nodule size ≤1.5 cm and non-solid nodules. It was not rare for patients to finally be diagnosed with a malignancy in the non-diagnostic group. Therefore, care should be taken when the results of ENB biopsy are non-diagnostic to prevent misdiagnosis.
Acknowledgements
Not applicable.
Funding Statement
This work was supported by the Zhejiang Province Major Science and Technology Special Program Project (grant no. 2020C03058) and the Zhejiang Province Lung Tumor Diagnosis and Treatment Technology Research Center (grant no. JBZX-202007).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
WY, HM, BY and JH developed the concept for the study. ZA, PX, LY and GY contributed to the data collection. WY, WL and HM conducted the statistical analysis. WY and HM contributed to the production of the manuscript. All authors contributed to the article, and read and approved the final manuscript. WY and HM confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present study was approved by the Medical Ethics Committee and Institutional Review Board of The First Affiliated Hospital, Zhejiang University (approval no. 2022-764). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with The 1964 Declaration of Helsinki, and its later amendments or comparable ethical standards. Written informed consent for participation was obtained from all patients.
Patient consent for publication
Written informed consent for publication was obtained from all patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: Good or bad news from the 2018 global cancer statistics? Cancer Commun (Lond) 2019;39:22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, Kramer J, Siegel RL. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409–436. doi: 10.3322/caac.21731. [DOI] [PubMed] [Google Scholar]
- 4.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, et al. The iaslc lung cancer staging project: Proposals for revision of the tnm stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Oudkerk M, Liu S, Heuvelmans MA, Walter JE, Field JK. Lung cancer LDCT screening and mortality reduction-evidence, pitfalls and future perspectives. Nat Rev Clin Oncol. 2021;18:135–151. doi: 10.1038/s41571-020-00432-6. [DOI] [PubMed] [Google Scholar]
- 6.Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallières E, Groome P, Kennedy C, Krasnik M, Peake M, Shemanski L, et al. The IASLC lung cancer staging project: External validation of the revision of the Tnm stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2017;12:1109–1121. doi: 10.1016/j.jtho.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Borelli C, Vergara D, Simeone A, Pazienza L, Castorani G, Graziano P, Micco CD, Quarato CMI, Sperandeo M. CT-guided transthoracic biopsy of pulmonary lesions: Diagnostic versus nondiagnostic results. Diagnostics (Basel) 2022;12:359. doi: 10.3390/diagnostics12020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Criner GJ, Eberhardt R, Fernandez-Bussy S, Gompelmann D, Maldonado F, Patel N, Shah PL, Slebos DJ, Valipour A, Wahidi MM, et al. Interventional bronchoscopy. Am J Respir Crit Care Med. 2020;202:29–50. doi: 10.1164/rccm.201907-1292SO. [DOI] [PubMed] [Google Scholar]
- 9.Mehta AC, Hood KL, Schwarz Y, Solomon SB. The evolutional history of electromagnetic navigation bronchoscopy: State of the art. Chest. 2018;154:935–947. doi: 10.1016/j.chest.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Folch EE, Labarca G, Ospina-Delgado D, Kheir F, Majid A, Khandhar SJ, Mehta HJ, Jantz MA, Fernandez-Bussy S. Sensitivity and safety of electromagnetic navigation bronchoscopy for lung cancer diagnosis: Systematic review and meta-analysis. Chest. 2020;158:1753–1769. doi: 10.1016/j.chest.2020.05.534. [DOI] [PubMed] [Google Scholar]
- 11.Cheng SL, Chu CM. Electromagnetic navigation bronchoscopy: The initial experience in Hong Kong. J Thorac Dis. 2019;11:1697–1704. doi: 10.21037/jtd.2018.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho HJ, Roknuggaman M, Han WS, Kang SK, Kang MW. Electromagnetic navigation bronchoscopy-Chungnam national university hospital experience. J Thorac Dis. 2018;10((Suppl 6)):S717–S724. doi: 10.21037/jtd.2018.03.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen FD, Degn KB, Rasmussen TR. Electromagnetic navigation bronchoscopy for lung nodule evaluation. Patient selection, diagnostic variables and safety. Clin Respir J. 2020;14:557–563. doi: 10.1111/crj.13168. [DOI] [PubMed] [Google Scholar]
- 14.Patrucco F, Gavelli F, Daverio M, Antonini C, Boldorini R, Casadio C, Balbo PE. Electromagnetic navigation bronchoscopy: Where are we now? Five years of a single-center experience. Lung. 2018;196:721–727. doi: 10.1007/s00408-018-0161-3. [DOI] [PubMed] [Google Scholar]
- 15.Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117:1049–1054. doi: 10.1378/chest.117.4.1049. [DOI] [PubMed] [Google Scholar]
- 16.Gex G, Pralong JA, Combescure C, Seijo L, Rochat T, Soccal PM. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: A systematic review and meta-analysis. Respiration. 2014;87:165–176. doi: 10.1159/000355710. [DOI] [PubMed] [Google Scholar]
- 17.Taton O, Bondue B, Gevenois PA, Remmelink M, Leduc D. Diagnostic yield of combined pulmonary cryobiopsies and electromagnetic navigation in small pulmonary nodules. Pulm Med. 2018;2018:6032974. doi: 10.1155/2018/6032974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, Lammers JWJ, Weenink C, Yousaf-Khan U, Horeweg N, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. New Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Han W, Zhang W, Xue F, Wang Y, Hu Y, Wang L, Zhou C, Huang Y, Zhao S, et al. Mortality outcomes of low-dose computed tomography screening for lung cancer in urban China: A decision analysis and implications for practice. Chin J Cancer. 2017;36:57. doi: 10.1186/s40880-017-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh S, Mehta AC, Abuquyyas S, Raju S, Farver C. Primary lung neoplasms presenting as multiple synchronous lung nodules. Eur Respir Rev. 2020;29:190142. doi: 10.1183/16000617.0142-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuire AL, Myers R, Grant K, Lam S, Yee J. The diagnostic accuracy and sensitivity for malignancy of radial-endobronchial ultrasound and electromagnetic navigation bronchoscopy for sampling of peripheral pulmonary lesions: Systematic review and meta-analysis. J Bronchology Interv Pulmonol. 2020;27:106–121. doi: 10.1097/LBR.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 22.Jiang S, Xie F, Mao X, Ma H, Sun J. The value of navigation bronchoscopy in the diagnosis of peripheral pulmonary lesions: A meta-analysis. Thorac Cancer. 2020;11:1191–1201. doi: 10.1111/1759-7714.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishiwata T, Gregor A, Inage T, Yasufuku K. Bronchoscopic navigation and tissue diagnosis. Gen Thorac Cardiovasc Surg. 2019;68:672–678. doi: 10.1007/s11748-019-01241-0. [DOI] [PubMed] [Google Scholar]
- 24.Folch EE, Pritchett MA, Nead MA, Bowling MR, Murgu SD, Krimsky WS, Murillo BA, LeMense GP, Minnich DJ, Bansal S, et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: One-year results of the prospective, multicenter navigate study. J Thorac Oncol. 2019;14:445–458. doi: 10.1016/j.jtho.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Mazzone PJ, Lam L. Evaluating the patient with a pulmonary nodule: A review. Jama. 2022;327:264–273. doi: 10.1001/jama.2021.24287. [DOI] [PubMed] [Google Scholar]
- 26.Chen A, Pastis N, Furukawa B, Silvestri GA. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy. Chest. 2015;147:1275–1281. doi: 10.1378/chest.14-1425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.