Introduction
Eosinophilic fasciitis (EF) is a rare scleroderma-like syndrome considered to be an autoimmune disease.1,2 It was first described by Shulman3 in 1974, and is characterized by induration of the skin, peripheral eosinophilia, hypergammaglobulinemia, and elevated erythrocyte sedimentation rate. The disorder occurs equally in men and women, and average age of onset has been reported to be between 40 and 50 years, although it can appear from childhood to older ages.4
The etiology of EF remains unknown. Multiple triggers have been suggested, such as muscle trauma; drugs (statins, phenytoin, ramipril, subcutaneous heparin or trichloroethylene); infections (Borrelia burgdorferi, Mycoplasma arginini); hematologic disorders; solid neoplasms; autoimmune diseases; or physical factors, such as radiotherapy and burns.1
Clinically, EF is characterized by an abrupt onset of pain and swelling on the affected extremities, mainly with a symmetrical presentation. The edema is followed by the thickening of the skin, showing the following 2 characteristic signs of the disease: Peau d’orange appearance and the “groove sign,” which is a depression along the course of superficial veins, accentuated with the elevation of the limb. Other symptoms include joint contractures, morphea-like lesions, inflammatory polyarthritis, myalgia, weight loss, asthenia, and morning stiffness. Less frequently restrictive lung disease manifestations may be associated, and pleural and pericardial effusion, as well as renal involvement, can be present.1,5
We present the case of a patient who developed EF after receiving the first dose of vaccination against SARS-CoV-2 with a COVID-19 vaccine (Vaxzevria, AstraZeneca and Serum Institute of India).
Case report
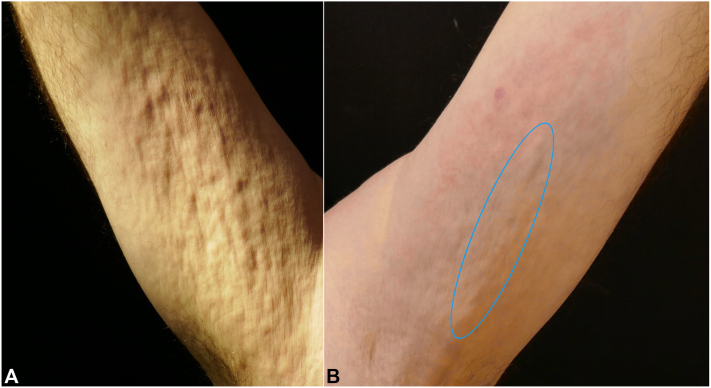
A 55-year-old White man with medical history of dyslipidemia presented with abrupt painful swelling of the trunk and 4 extremities, arthralgia, and limited joint mobility of the wrists. The patient did not have any allergies, and no previous trauma or strenuous exercise was reported. He did acknowledge having received the first dose of the ChAdOx1-S/nCoV-19 (recombinant) vaccine (Vaxzevria) 1 week before the beginning of the symptoms. He was initially asymptomatic, but 1 week later, the patient complained of skin induration, which started in the flanks and progressed over days to involve the proximal upper and lower portion of the limbs. Physical examination revealed the presence of mild erythema and skin induration in the affected areas. The groove sign and Peau d’orange aspect were observed in the patient’s forearms. (Fig 1).
Fig 1.
A,Peau d’orange appearance and (B) depression along the course of the superficial veins was seen, which was more evident during the elevation of the affected limb (blue oval).
Notable laboratory abnormalities included 2.6 × 109/L eosinophils (reference range, 0-0.7 × 109/L), 44 mm/h erythrocyte sedimentation rate (reference range, 1-20 mm/h), and 4 mg/dL C-reactive protein (reference range, 0-0.5 mg/dL). The eosinophil count of a blood test performed 2 weeks before was normal.
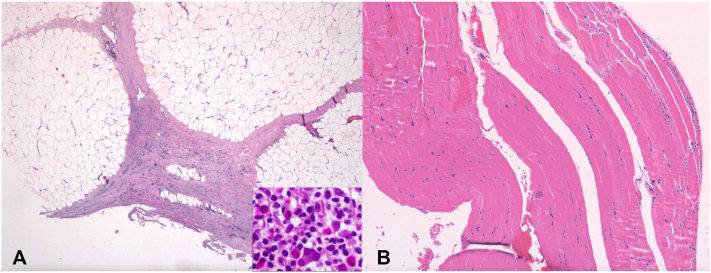
Biopsy and magnetic resonance imaging were performed. Skin biopsy results revealed mixed inflammatory infiltrate with abundant eosinophils affecting the reticular dermis, septal fat, and fascia (Fig 2). Magnetic resonance images showed thickening and edema of the superficial fascia of the forearms and the posterior compartments of the lower limbs. The patient received a diagnosis of EF, and treatment with 3 pulses of methylprednisolone was started. The skin lesions gradually improved, and the surface softened. However, clinical worsening during steroid-tapering required the addition of a weekly dose of 15 mg methotrexate. Nine months after the onset, the skin has completely recovered, and the patient remains asymptomatic with low doses of prednisone and methotrexate.
Fig 2.
Skin biopsy sample image showed mixed inflammatory infiltrate with abundant eosinophils affecting the reticular dermis and septal and perivesicular fascia. Numerous eosinophils are observed (inset). Hematoxylin-eosin stain; original magnification: A, ×20, ×100 (inset); B, ×40)
With the clinical suspicion of EF secondary to Vaxzevria, the decision was made to switch subsequent doses to the Pfizer-BioNTech COVID-19 vaccine, and the patient has not experienced any new symptoms or worsening of the disease.
Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
Discussion
EF is a rare scleroderma-like syndrome. The differential diagnosis of this entity includes localized scleroderma and systemic sclerosis, as well as other scleroderma-like disorders, such as nephrogenic systemic fibrosis, scleromyxedema, eosinophilia-myalgia, or graft-versus-host disease.2,4,6
The pathophysiology of EF is unknown. Some authors have proposed an aberrant immune response, supported by the presence of hypergammaglobulinemia in peripheral blood and IgG and C3 deposition in fascia in some patients.4 Infiltrating eosinophils in the fascia and their degranulation result in the release and tissue accumulation of cationic granule proteins with toxic and potentially fibrogenic properties.
The current evidence does not support the hypothesis that eosinophils play a protective or pathogenic role in COVID-19.5 Nevertheless, the role of eosinophils during Coronavirus disease vaccination has been demonstrated in several papers,7 which induced pulmonary eosinophilia in animal models after viral challenge, and some authors have advised on the possibility that SARS-CoV-2 vaccines can cause a similar vaccine-associated immunopathology.5
A few reports of EF induced by simvastatin have been published,8,9 making it important to highlight the growing evidence on the immunomodulatory properties of statins.10 Due to our patient’s dyslipidemia, he had received simvastatin in the past, but it was voluntarily interrupted 2 months before the onset of the EF. Considering the chronology of the symptoms, in this case, simvastatin does not seem to have triggered the condition, and its role in the development of the disease is unlikely. However, we cannot rule out the possibility that the previous statin use could have enhanced the effect of the COVID-19 vaccination.
Vaxzevria is a monovalent vaccine composed of a single recombinant, nonreplicative chimpanzee adenovirus vector encoding the SARS-CoV-2 glycoprotein S.11 After its administration, this protein is expressed locally by stimulating the formation of neutralizing IgG antibodies and the cellular immune response using T2 lymphocytes, the latter being responsible for the recruitment of eosinophils.
In the literature, there are already several reported cases of other eosinophilic-associated diseases following vaccination against COVID-19 (Supplementary Table I, available via Mendeley at https://data.mendeley.com/datasets/fdpwpc4h82).12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 A definitive relationship between these pathologies and vaccination has not been established; however, the increasing number of reported cases of vaccine-associated eosinophilic diseases suggests that there may be a true association, not yet specified, between COVID-19 vaccination and eosinophilia. Further investigation is needed to determine this relationship.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Pinal-Fernandez I., Selva-O’ Callaghan A., Grau J.M. Diagnosis and classification of eosinophilic fasciitis. Autoimmun Rev. 2014;13(4-5):379–382. doi: 10.1016/j.autrev.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Ihn H. Eosinophilic fasciitis: from pathophysiology to treatment. Allergol Int. 2019;68(4):437–439. doi: 10.1016/j.alit.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Shulman L.E. Diffuse fasciitis with eosinophilia: a new syndrome? Trans Assoc Am Physicians. 1975;88:70–86. [PubMed] [Google Scholar]
- 4.Bischoff L., Derk C.T. Eosinophilic fasciitis: demographics, disease pattern and response to treatment: report of 12 cases and review of the literature. Int J Dermatol. 2008;47(1):29–35. doi: 10.1111/j.1365-4632.2007.03544.x. [DOI] [PubMed] [Google Scholar]
- 5.Mertens J.S., Seyger M.M.B., Thurlings R.M., Radstake T.R.D.J., de Jong E.M.G.J. Morphea and eosinophilic fasciitis: an update. Am J Clin Dermatol. 2017;18(4):491–512. doi: 10.1007/s40257-017-0269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazori D.R., Femia A.N., Vleugels R.A. Eosinophilic fasciitis: an updated review on diagnosis and treatment. Curr Rheumatol Rep. 2017;19(12):74. doi: 10.1007/s11926-017-0700-6. [DOI] [PubMed] [Google Scholar]
- 7.Simon H.U., Karaulov A.V., Bachmann M.F. Strategies to prevent SARS-CoV-2-mediated eosinophilic disease in association with COVID-19 vaccination and infection. Int Arch Allergy Immunol. 2020;181(8):624–628. doi: 10.1159/000509368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choquet-Kastylevsky G., Kanitakis J., Dumas V., Descotes J., Faure M., Claudy A. Eosinophilic fasciitis and simvastatin. Arch Intern Med. 2001;161(11):1456–1457. doi: 10.1001/archinte.161.11.1456. [DOI] [PubMed] [Google Scholar]
- 9.Serrano-Grau P., Mascaró-Galy J.M., Iranzo P. Desarrollo de fascitis eosinofílica tras la ingesta de simvastatina. Eosinophilic fasciitis after taking simvastatin. Actas Dermosifiliogr. 2008;99(5):420–421. [PubMed] [Google Scholar]
- 10.Gurevich V.S., Shovman O. Slutzky L, Meroni PL, Shoenfeld Y. Statins and autoimmune diseases. Autoimmun Rev. 2005;4:123–129. doi: 10.1016/j.autrev.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 11.Marfe G., Perna S., Shukla A.K. Effectiveness of COVID-19 vaccines and their challenges (Review) Exp Ther Med. 2021;22(6):1407. doi: 10.3892/etm.2021.10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda M., Ishio N., Shoji T., Mori N., Matsumoto M., Shikama N. Eosinophilic myocarditis following Coronavirus Disease 2019 (COVID-19) vaccination. Circ J. 2022;86(6):1020. doi: 10.1253/circj.CJ-21-0935. [DOI] [PubMed] [Google Scholar]
- 13.Ameratunga R., Woon S.T., Sheppard M.N., et al. First identified case of fatal fulminant necrotizing eosinophilic myocarditis following the initial dose of the Pfizer-BioNTech mRNA COVID-19 Vaccine (BNT162b2, Comirnaty): an extremely rare idiosyncratic hypersensitivity reaction. J Clin Immunol. 2022;42(3):441–447. doi: 10.1007/s10875-021-01187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan-Chung C., Ong C.S., Chan L.L., Tan E.K. Eosinophilic granulomatosis with polyangiitis after COVID-19 vaccination. QJM. 2022;114(11):807–809. doi: 10.1093/qjmed/hcab273. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim H., Alkhatib A., Meysami A. Eosinophilic granulomatosis with polyangiitis diagnosed in an elderly female after the second dose of mRNA vaccine against COVID-19. Cureus. 2022;14(1):e21176. doi: 10.7759/cureus.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Montjoye L., Marot L., Baeck M. Eosinophilic cellulitis after BNT162b2 mRNA Covid-19 vaccine. J Eur Acad Dermatol Venereol. 2022;36(1):e26–e28. doi: 10.1111/jdv.17685. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y.H., Kim Y.C. Wells’ syndrome following Oxford-AstraZeneca COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2022;36(5):e328–e330. doi: 10.1111/jdv.17916. [DOI] [PubMed] [Google Scholar]
- 18.Cinotti E., Perrot J.L., Bruzziches F., et al. Eosinophilic dermatosis after AstraZeneca COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2022;36(3):e171–e172. doi: 10.1111/jdv.17806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaikati J., Ghanem A. El Bahtimi R, Helou J, Tomb R. Eosinophilic panniculitis: a new side effect of Sinopharm COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2022;36(5):e337–e339. doi: 10.1111/jdv.17920. [DOI] [PubMed] [Google Scholar]
- 20.Miqdadi A., Herrag M. Acute Eosinophilic pneumonia associated with the anti-COVID-19 vaccine AZD1222. Cureus. 2021;13(10):e18959. doi: 10.7759/cureus.18959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozturk A.B., Çağlayan B., Kapmaz M., et al. Hypersentivity reactions to COVID-19 vaccines: a case of Eosinophilic pneumonia following Sinovac/CoronaVac vaccination. Eur Ann Allergy Clin Immunol. 2023;55(1):41–45. doi: 10.23822/EurAnnACI.1764-1489.247. [DOI] [PubMed] [Google Scholar]
- 22.Barrio Piqueras M., Ezponda A., Felgueroso C., et al. Acute eosinophilic pneumonia following mRNA COVID-19 vaccination: A Case Report. Arch Bronconeumol. 2022;58:53–54. doi: 10.1016/j.arbres.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa E., Silva M., Sá Marques M., João D., Campainha S. Eosinophilic pneumonia associated to SARS-CoV-2 vaccine. Arch Bronconeumol. 2022;58:51–52. doi: 10.1016/j.arbres.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]