Highlights
-
•
A strategic public health response to an emerging infectious disease pandemic.
-
•
Hospital swiftly repurposed into a tertiary dedicated COVID-19 centre in Brazil.
-
•
Retrospective cohort of 2492 patients during the first and second waves fot eh coronavirus disease 2019 pandemic.
-
•
Reduced in-hospital mortality during the second wave compared with the first wave.
-
•
Invasive mechanical ventilation showed the strongest association with risk of death.
Keywords: COVID-19, Public health, Mortality, Brazil
Abstract
Background
The first months of the coronavirus disease 2019 (COVID-19) pandemic demanded rapid re-organization of available local resources. This study evaluated the performance of a private hospital in the Brazilian state of Ceará that was swiftly repurposed into a public tertiary COVID-19 centre during the first wave of the COVID-19 pandemic, and how it improved in the second wave.
Methods
This retrospective cohort study included 2492 patients with COVID-19 at Hospital Estadual Leonardo da Vinci (HELV) during the first and second waves. Demographic, clinical and laboratory data were collected using a dedicated web platform (ResCOVID). A Poisson regression model was used to estimate factors associated with in-hospital mortality.
Results
Differences in demographics and clinical features were found between the two waves. There was reduced in-hospital mortality during the second wave (36.2%) in comparison with the first wave (48.8%). Invasive mechanical ventilation showed the strongest association with increased risk of death in both waves {first wave: relative risk (RR) 4.28 [95% confidence interval (CI) 2.86–6.41], P<0.001; second wave: RR 12.94 (95% CI 3.4–49.12), P<0.001}.
Conclusions
HELV was a pillar in the strategic public health plan to respond to COVID-19 in Ceará, helping to assist a group of moderate-to-severe cases and reduce the pressure on emergency and primary care facilities. Although mortality in intubated individuals remained high, there was an overall decrease in the in-hospital mortality rate in the second wave.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic was a clear threat to organized society. A few countries responded effectively, some countries were tragically engulfed by severe humanitarian challenges, and most countries displayed a wide range of suboptimal responses [1]. Understanding how organized societies and governments articulated their multi-faceted actions to mitigate the effects of such an incoming onslaught is critical for future contingency plans.
This article describes a microcosm of COVID-19 responses in the context of Hospital Estadual Leonardo da Vinci (HELV), a dedicated tertiary public hospital set up rapidly in Fortaleza, the state capital of Ceará, in Northeast Brazil. Brazil was one of the three countries most affected by the COVID-19 pandemic, with more than 34 million confirmed cases and 685,000 confirmed deaths by 16 September 2022 [2,3]. Ceará was one of the earliest and most affected Brazilian states, mainly because Fortaleza is an international hub to Europe and North America, and has the highest population density in the country.
The objectives of this study were: (1) to state the timing of concerted responses that led to the repurposing of a private medical centre into a tertiary hospital devoted to moderate and severe cases of COVID-19; (2) to evaluate its initial performance under the emergency context in the first wave; and (3) to establish how the initial response reached the improved level recorded in the second wave. As such, this study sought to explore in-hospital mortality during the first and second waves, and to find associations with epidemiological and clinical profiles, medical treatment, type of respiratory support, and the main complications of COVID-19.
Methods
This retrospective, single-centre cohort observational study of adult patients with confirmed severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection was performed at HELV, a dedicated, reference COVID-19 tertiary centre in Fortaleza, Brazil. Equipped with a multi-disciplinary team of medical doctors, respiratory physiotherapists, nurses and other health professionals, HELV had 291 hospital beds, of which 179 were in intensive care units (ICUs). The average rate of bed occupation was 87%. During the study period, over 3200 patients were admitted to HELV with suspected or confirmed COVID-19.
From 25 March to 4 July 2020 (first wave) and from 1 January to 13 April 2021 (second wave), all consecutively hospitalized adult patients with suspected or confirmed SARS-CoV-2 infection were followed until they left hospital (i.e. discharged home, transferred to another institution for continuation of care, or death in hospital). The inclusion criteria were: definite hospital outcome (discharged home or death in hospital), and confirmed infection by real-time reverse transcriptase polymerase chain reaction (RT-PCR) for SARS-CoV-2 by a certified laboratory, following standardized national and international protocols [4,5]. All patients transferred to other health institutions were excluded from the analysis of in-hospital mortality because it was not always possible to ascertain the hospital outcome in other institutions (i.e. whether discharged home or death in hospital). The primary outcome was in-hospital mortality. The following outcomes were also assessed: time from symptom onset to invasive mechanical ventilation; time from symptom onset to death; time from hospital admission to invasive mechanical ventilation; time from hospital admission to death; tracheal intubation rate; duration of invasive mechanical ventilation; hospital length of stay; and mortality among patients requiring invasive mechanical ventilation.
Patients received medical and multi-disciplinary treatment according to the HELV protocol, which changed over the study period based on local experience, new scientific evidence and recommendations from the Secretary of Health of the State of Ceará [6]. Mainly during the first wave, compassionate therapies, including prescription of medications under investigation for COVID-19, were administered at the discretion of the attending physician.
When necessary, endotracheal intubation and invasive mechanical ventilation were carried out, including administration and dosing of analgesic and sedative drugs throughout an ICU stay. During the second wave, non-invasive mechanical ventilation was implemented, including the use of a locally developed helmet for continuous positive airway pressure (CPAP) (Elmo respiratory support), utilized when appropriate [7]. Other hospitalization parameters were also recorded, such as need for oxygen supplementation, including non-invasive or invasive mechanical ventilation, at admission or over the course of hospital stay; ICU admission; and total length of hospital stay. Data were obtained from electronic medical records using a standardized web platform (ResCOVID) developed by Ceará Public Health School. Data collection at the time of hospital admission included anthropometric and demographic information, initial symptoms and vital signs, comorbidities, personal history, sequential organ failure assessment (SOFA) and quick SOFA scores, and laboratory findings. The following definitions were adopted when recording personal history: current smoker, an adult who has smoked 100 cigarettes in his/her lifetime and who currently smokes cigarettes; former smoker, an adult who has smoked 100 cigarettes in his/her lifetime but who had quit smoking at the time of the interview; alcohol abuse, a condition in which a person continues to drink despite recurrent social, interpersonal, health or legal problems as a result of their alcohol use; and former drinker, an adult who had not consumed alcohol in the last 12 months, but who did so previously [8], [9], [10].
The time from presentation, usually at a primary healthcare unit, until hospital admission was also recorded, and data related to oxygen support, mechanical ventilation, renal replacement therapy and use of specific interventions to treat SARS-CoV-2 infection were measured during the in-hospital follow-up period.
This research was approved by the Institutional Ethics Committee (No. 30423920.0.0000.5037; following Resolution No. 466/2012 of the National Health Council and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards) and registered in a public database (clinicaltrials.gov, NCT04649827). No identifiers were recorded to ensure anonymity. There was no contact with patients. Patient consent was waived due to the retrospective study design.
Statistical analyses
Statistical analysis was performed using SPSS Version 20 (IBM Corp., Armonk, NY, USA). Kolmogorov–Smirnov test was used to determine normality of the data distribution. Non-paired t-test, Mann–Whitney U-test, Fisher's exact test and Chi-squared test were used to assess differences between the two COVID-19 waves, and interaction between the primary outcome (COVID-19 in-hospital mortality in each wave) and other variables. Categorical variables have been presented as number and percentage, and continuous variables have been presented as median and interquartile range (IQR).
A robust Poisson regression model was used to analyse associations between patient characteristics and COVID-19 in-hospital mortality in each wave. Results have been reported as relative risk (RR) and 95% confidence interval (CI). All variables that were significant (P<0.05) on univariate analysis were used in the regression analysis. The regression model was also used to estimate associations between selected independent variables and in-hospital mortality. The selected independent variables were: wave; age; sex; presence of any comorbidities; obesity; diabetes; hypertension; coronary disease; asthma; chronic obstructive pulmonary disease; cancer; chronic kidney disease; neurological chronic disease; peripheral capillary oxygen saturation (SpO2); oxygen therapy; SpO2/fraction of inspired oxygen (FiO2) ratio at hospital admission; and quick SOFA score. In a subgroup of patients who had relevant data collected upon hospital admission, the SOFA score was also used to adjust the risk of in-hospital mortality.
Results
General information
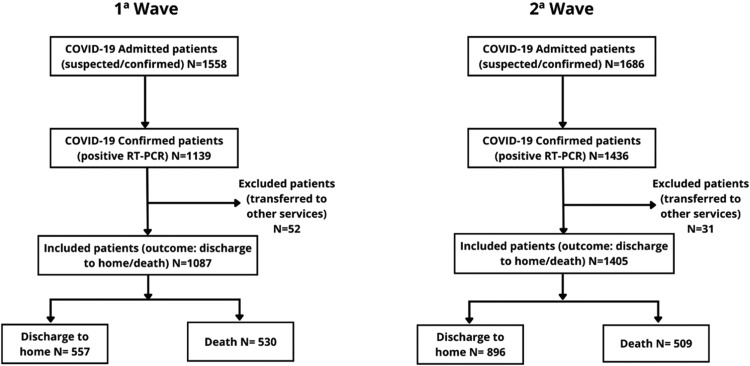
During the study period, 169,607 confirmed cases of COVID-19 (7522 deaths) and 306,765 confirmed cases (7002 deaths) were reported in the first and second waves, respectively, in the state of Ceará. After excluding patients without confirmed infection by real-time RT-PCR for SARS-CoV-2, as well as patients transferred to other health services, this study analysed data from 2492 (first wave: 1087 patients; second wave: 1405 patients) of 3244 patients admitted to HELV with suspected or confirmed COVID-19 during the study period (Figure 1). Importantly, the time from emergency or primary care facility presentation to HELV admission was significantly shorter in the second wave [median 2 (IQR 1–4) vs 3 (IQR 1–5) days; P<0.001] (Table 1), indicating an improvement in the referral system.
Figure 1.
Study flow chart.
Table 1.
Anthropometric and demographic characteristics of patients with coronavirus disease 2019 admitted to a reference hospital in Northeast Brazil during the first and second pandemic waves.
| First wave (n=1087) | Second wave (n=1405) | Total (n=2492) | P-value | |
|---|---|---|---|---|
| Anthropometrics/demographics (n=2492) | ||||
| Gender | ||||
| Male | 667 (61.4) | 799 (56.9) | 1466 (58.8) | 0.024 |
| Female | 420 (38.6) | 606 (43.1) | 1026 (41.2) | |
| Age, years | 64 (50–73) | 56 (44–67) | 59 (46–70) | <0.001 |
| Age group, years | ||||
| 0–19 | 0 (0) | 3 (0.2) | 3 (0.1) | <0.001 |
| 20–29 | 30 (2.8) | 66 (4.7) | 96 (3.9) | |
| 30–39 | 75 (6.9) | 179 (12.7) | 254 (10.2) | |
| 40–49 | 158 (14.5) | 260 (18.5) | 418 (16.8) | |
| 50–59 | 187 (17.2) | 319 (22.7) | 506 (20.3) | |
| 60–69 | 242 (22.3) | 300 (21.4) | 542 (21.7) | |
| 70–79 | 247 (22.7) | 207 (14.7) | 454 (18.2) | |
| >80 | 148 (13.6) | 71 (5.1) | 219 (8.8) | |
| Self-reported race (n=1814) | ||||
| Brown | 471 (66.2) | 696 (63.2) | 1167 (64.3) | NP |
| White | 176 (24.7) | 341 (30.9) | 517 (28.5) | |
| Black | 60 (8.4) | 61 (5.5) | 121 (6.7) | |
| Asian | 5 (0.7) | 4 (0.4) | 9 (0.5) | |
| Marital status (n=1849) | ||||
| Married/common law | 460 (61.6) | 679 (61.6) | 1139 (61.6) | NP |
| Single | 134 (18) | 259 (23.5) | 393 (21.3) | |
| Widowed | 106 (14.2) | 94 (8.5) | 200 (10.8) | |
| Separated/divorced | 46 (6.2) | 71 (6.4) | 117 (6.3) | |
| Origin (n=2492) | ||||
| Fortaleza | 504 (46.4) | 775 (55.2) | 1279 (51.3) | <0.001 |
| Countryside | 583 (53.6) | 630 (44.8) | 1213 (48.7) | |
| Human development index (neighbourhood) (n=2207) | ||||
| Very low/low | 496 (55) | 659 (50.5) | 1155 (52.3) | 0.110 |
| Medium | 7 (0.8) | 13 (1.0) | 20 (0.9) | |
| High/very high | 399 (44.2) | 633 (48.5) | 1032 (46.8) |
NP, not performed.
Statistical analysis was performed using t-test and Chi-squared test. Results are shown as number of cases (%) and median (interquartile range).
Clinical presentation
Patients admitted to HELV during the second wave were significantly younger [median 56 (IQR 44–67) vs 64 (IQR 50–73); P<0.001) than those admitted during the first wave. There was a higher proportion of females in the second wave (43.1 vs 38.6%; P=0.024) (Table 1). In addition, in the second wave, there was a lower proportion of patients with at least one comorbidity (70.8% vs 75.5%; P=0.009). Regarding the most common medical conditions, hypertension (49% vs 55.9%; P=0.001) and diabetes (28.9% vs 40.9%; P<0.001) were reported less frequently among patients in the second wave. In contrast, obesity (30.4% vs 23.4%; P<0.001) was found to be more prevalent during the second wave. Dyspnoea (84.4%), cough (64.6%) and fever (59.9%) were the most common symptoms at hospital admission in both waves. In the second wave, there was lower prevalence of dyspnoea (81.1% vs 88.8%; P<0.001), fever (57.3% vs 63.2%; P=0.003) and anosmia (6.8% vs 9.6%; P=0.013), but higher prevalence of headache (18.3% vs 11.9%; P<0.001) and sore throat (12.7% vs 6.9%; P<0.001). The overall median time from symptom onset to hospital admission was 11 days. Interestingly, this parameter increased by 1 day in the second wave [median 11 (IQR 9–14) days] compared with the first wave [median 10 (IQR 7–13) days; P<0.001]. Curiously, the time between emergency or primary care presentation and HELV admission reduced by 1 day during the second wave [median 2 (IQR 1–4) days] compared with the first wave [median 3 (IQR 1–5) days] (Table 2).
Table 2.
Clinical characteristics of patients with coronavirus disease 2019 admitted to a reference hospital in Northeast Brazil during the first and second pandemic waves.
| First wave (n=1087) | Second wave (n=1405) | Total (n=2492) | P-value | |
|---|---|---|---|---|
| Time from presentation at emergency department to hospital admission, days | 3 (1–5) | 2 (1–4) | 2 (1–4) | <0.001 |
| Personal history | ||||
| Current smoker | 57 (5.2) | 77 (5.5) | 134 (5.4) | 0.795 |
| Former smoker | 284 (26.1) | 283 (20.1) | 567 (22.8) | <0.001 |
| Alcohol abuse | 40 (3.7) | 63 (4.5) | 103 (4.1) | 0.317 |
| Former drinker | 48 (4.4) | 45 (3.2) | 93 (3.7) | 0.113 |
| Any comorbidities | 821 (75.5) | 995 (70.8) | 1816 (72.9) | 0.009 |
| Hypertension | 608 (55.9) | 689 (49) | 1297 (52) | 0.001 |
| Diabetes | 445 (40.9) | 406 (28.9) | 851 (34.1) | <0.001 |
| Obesity | 254 (23.4) | 427 (30.4) | 681 (27.3) | <0.001 |
| Coronary disease | 88 (8.1) | 48 (3.4) | 136 (5.5) | <0.001 |
| Neurological disease | 81 (7.5) | 32 (2.3) | 113 (4.5) | <0.001 |
| Chronic obstructive pulmonary disease | 34 (3.1) | 77 (5.5) | 111 (4.5) | <0.001 |
| Asthma | 32 (2.9) | 40 (2.8) | 72 (2.9) | 0.481 |
| Chronic kidney disease | 27 (2.5) | 18 (1.3) | 45 (1.8) | 0.025 |
| Rheumatological disease | 22 (2) | 18 (1.3) | 40 (1.6) | 0.143 |
| Cancer | 16 (1.5) | 0 (0) | 16 (0.6) | <0.001 |
| Number of comorbidities per patient | 2 (1–3) | 1 (0–2) | 1 (0–2) | <0.001 |
| Time from symptom onset to hospital admission, days | 10 (7–13) | 11 (9–14) | 11 (8–13) | <0.001 |
| Dyspnoea | 965 (88.8) | 1139 (81.1) | 2104 (84.4) | <0.001 |
| Cough | 721 (66.3) | 888 (63.2) | 1609 (64.6) | 0.106 |
| Fever | 687 (63.2) | 805 (57.3) | 1492 (59.9) | 0.003 |
| Myalgia | 268 (24.7) | 369 (26.3) | 637 (25.6) | 0.361 |
| Headache | 129 (11.9) | 257 (18.3) | 386 (15.5) | <0.001 |
| Asthenia | 117 (10.8) | 167 (11.9) | 284 (11.4) | 0.382 |
| Sore throat | 75 (6.9) | 178 (12.7) | 253 (10.2) | <0.001 |
| Anosmia | 104 (9.6) | 496 (6.8) | 200 (8) | 0.013 |
| Diarrhoea | 73 (6.7) | 119 (8.5) | 192 (7.7) | 0.103 |
| Coryza | 71 (6.5) | 100 (7.1) | 171 (6.9) | 0.566 |
| Ageusia | 64 (5.9) | 84 (6) | 148 (5.9) | 0.924 |
| Nausea | 36 (3.3) | 44 (3.1) | 80 (3.2) | 0.800 |
| Nasal congestion | 39 (3.6) | 40 (2.8) | 79 (3.2) | 0.295 |
| Fatigue | 37 (3.4) | 34 (2.4) | 71 (2.8) | 0.143 |
NP, not performed.
Statistical analysis was performed using t-test and Chi-squared test. Results are shown as number of cases (%) and median (interquartile range).
Among all admitted patients, 94.4% were already using supplementary oxygen at hospital admission; however, the proportion of patients needing high levels of oxygen support (oxygen masks with reservoir bags) was found to be significantly lower in the second wave (48.8% vs 68.6%; P<0.001). In addition, most clinical parameters, including arterial blood gas results, indicated lower severity of the disease at hospital admission among patients in the second wave (Table S1, see online supplementary material).
Initial laboratory parameters and medications utilized over the hospital stay are shown in Tables S2 and S3 (see online supplementary material). During the second wave, a smaller proportion of patients received ICU care (60.1 vs 71.8%; P<0.001), and among those, fewer underwent invasive mechanical ventilation (39.2% vs 48.2%; P<0.001), compared with the first wave (Table 3).
Table 3.
Main non-respiratory complications and outcomes of patients with coronavirus disease 2019 admitted to a reference hospital in Northeast Brazil during the first and second pandemic waves.
| First wave (n=1087) | Second wave (n=1405) | Total (n=2492) | P-value | |
|---|---|---|---|---|
| Non-respiratory complications | ||||
| Acute renal failure | 289 (26.6) | 272 (19.4) | 561 (22.5) | <0.001 |
| Septic shock | 169 (15.5) | 149 (10.6) | 318 (12.8) | <0.001 |
| Sepsis | 151 (13.9) | 108 (7.7) | 259 (10.4) | <0.001 |
| Cardiac arrhythmia | 85 (7.8) | 71 (5.1) | 156 (6.3) | 0.005 |
| Multiple organ failure | 76 (7) | 42 (3) | 118 (4.7) | <0.001 |
| Hypovolaemic shock | 46 (4.2) | 28 (2) | 74 (3) | <0.001 |
| Haemorrhage | 22 (2) | 49 (3.5) | 71 (2.8) | 0.029 |
| Thromboembolic complications | 28 (2.6) | 36 (2.6) | 64 (2.6) | 0.862 |
| Acute hepatic failure | 24 (2.2) | 13 (0.9) | 37 (1.5) | 0.009 |
| Neurological disorder | 20 (1.8) | 17 (1.2) | 37 (1.5) | 0.197 |
| Non-pharmacological supportive treatment | ||||
| Intensive care unit care | 780 (71.8) | 845 (60.1) | 1625 (65.2) | <0.001 |
| Oxygen therapy during hospital stay | ||||
| Low-flow nasal cannula | 470 (43.2) | 682 (48.5) | 1152 (46.2) | 0.008 |
| High-flow nasal cannula | 3 (0.3) | 12 (0.9) | 15 (0.6) | 0.064 |
| Mask oxygen | 630 (58) | 653 (46.5) | 1283 (51.5) | <0.001 |
| Non-IMV | 7 (0.6) | 251 (17.9) | 258 (10.4) | <0.001 |
| Helmet CPAP (Elmo) | 0 (0) | 128 (9.1) | 128 (5.1) | <0.001 |
| IMV | 524 (48.2) | 551 (39.2) | 1075 (43.1) | <0.001 |
| Tracheostomy | 46 (4.2) | 101 (7.2) | 147 (5.9) | 0.002 |
| Blood transfusion | 81 (7.5) | 116 (8.3) | 197 (7.9) | 0.460 |
| Haemodialysis | 253 (23.3) | 299 (21.3) | 552 (22.2) | 0.235 |
| Main outcomes | ||||
| Time from symptom onset to hospital admission, days | 10 (7–13) | 11 (9–14) | 11 (8 - 13) | <0.001 |
| Time from symptom onset to IMV, days | 10 (7–14) | 13 (9–16) | 11 (9–15) | <0.001 |
| Time from symptom onset to death, days | 19 (14–25) | 23 (17–30) | 21 (16–27) | <0.001 |
| Time from hospital admission to IMV, daysa | 2 (1–5) | 3 (1–5) | 2 (1–5) | 0.529 |
| Time from hospital admission to death, days | 9 (5–13) | 9 (7–17) | 10 (5–15) | <0.001 |
| Tracheal intubation ratea | 237 (29.4) | 254 (21.9) | 491 (23.7) | <0.001 |
| Duration of IMV, days | 8 (4–12) | 9 (5–15) | 9 (5–14) | 0.004 |
| Length of hospital stay, days | 7 (4–11) | 7 (4–13) | 7 (4–12) | 0.964 |
| Discharged home | 560 (51.5) | 897 (63.8) | 1457 (58.5) | <0.001 |
| In-hospital mortality | 530 (48.8) | 509 (36.2) | 1039 (41.7) | <0.001 |
| IMV mortality | 471 (89.5) | 242 (89) | 962 (89.2) | 0.753 |
CPAP, continuous positive airway pressure; IMV, invasive mechanical ventilation.
Included patients intubated after 24 h of hospital admission.
Statistical analysis was performed using Mann–Whitney U-test and Chi-squared test. Results are shown as number of cases (%) and median (interquartile range).
Although there was a significant relative reduction of 25.5% in the rate of tracheal intubation after hospital admission in the second wave (21.9% vs 29.4%; P<0.001), mortality associated with invasive mechanical ventilation remained high (89% vs 89.5%; P=0.753). In general, a decrease in the unadjusted in-hospital mortality rate was observed in the second wave compared with the first wave (36.2% vs 48.8%; P<0.001) (Table 3).
Factors associated with mortality
Differences between patients who died in hospital and those who were discharged home were compared in each wave. Using a multi-variate robust Poisson regression, the RR was estimated for factors found to be associated with in-hospital mortality on univariate analysis in the first and second waves, separately (Table 4). Younger age [30–39 years: RR 0.66 (95% CI 0.46–0.95); P=0.024] and sore throat [RR 0.84 (95% CI 0.7–1.0); P=0.049] were associated with decreased in-hospital mortality during the first wave, and headache [RR 0.87 (95% CI 0.79–0.96); P=0.004) was more likely to be associated with lower risk of death in the second wave. Previous neurological disease was associated with higher risk of death in the first wave alone [RR 1.16 (95% CI 1.01–1.33); P=0.035]. Acute renal failure was associated with increased risk of death in the second wave alone [RR 1.13 (95% CI 1.04–1.23); P=0.004]. The need for invasive mechanical ventilation showed the highest association with increased risk of in-hospital mortality in both waves [first wave: RR 4.28 (95% CI 2.86–6.41), P<0.001; second wave: RR 12.94 (95% CI 3.4–49.12), P<0.001]. Among 1075 patients who used invasive mechanical ventilation in the entire cohort, 962 (89.2%) died.
Table 4.
Multi-variate robust Poisson regression: relative risk (RR) for risk factors associated with in-hospital mortality during the first and second pandemic waves at a coronavirus disease 2019 reference hospital in Northeast Brazil.
| First wave |
Second wave |
|||
|---|---|---|---|---|
| Variables | RR (95% CI) | P-value | RR (95% CI) | P-value |
| Age group, years (reference group: <29 years) | ||||
| >80 | 1.18 (0.87–1.59) | 0.281 | 1.6 (1.07–2.38) | 0.021 |
| 70–79 | 1.15 (0.85–1.55) | 0.365 | 1.34 (0.95–1.91) | 0.172 |
| 60–69 | 1.02 (0.76–1.36) | 0.911 | 1.28 (0.90–1.81) | 0.172 |
| 50–59 | 1.00 (0.75–1.34) | 0.996 | 1.32 (0.93–1.87) | 0.124 |
| 40–49 | 0.93 (0.69–1.26) | 0.635 | 1.24 (0.87–1.76) | 0.243 |
| 30–39 | 0.66 (0.46–0.95) | 0.024 | 1.21 (0.85–1.74) | 0.294 |
| Initial symptoms | ||||
| Cough | 1.07 (0.99–1.15) | 0.091 | 1.00 (0.93–1.07) | 0.898 |
| Myalgia | 0.97 (0.89–1.06) | 0.525 | 1.00 (0.92–1.09) | 0.979 |
| Asthenia | 1.04 (0.90–1.20) | 0.611 | 0.93 (0.82–1.06) | 0.298 |
| Sore throat | 0.84 (0.7–1.0) | 0.049 | 1.04 (0.95–1.15) | 0.394 |
| Nasal congestion | 0.92 (0.68–1.22) | 0.553 | 0.97 (0.81–1.16) | 0.751 |
| Headache | 1.05 (0,90–1.22) | 0.562 | 0.87 (0.79–0.96) | 0.004 |
| Diarrhoea | 0.97 (0.83–1.14) | 0.748 | 1.06 (0.85–1.31) | 0.617 |
| Comorbidities | ||||
| Obesity | 1.00 (0.93–1.08) | 0.964 | 1.00 (0.93–1.06) | 0.943 |
| Chronic kidney disease | 0.99 (0.81–1.21) | 0.909 | 1.1 (0.81–1.51) | 0.54 |
| Neurological disease | 1.16 (1.01–1.33) | 0.035 | 1.12 (0.81–1.54) | 0.486 |
| Vital signs at hospital admission SpO2, % (reference group: >94) | ||||
| <90 | 1.02 (0.92–1.13) | 0.755 | 1.01 (0.92–1.12) | 0.786 |
| 90–94 | 1.06 (0.97–1.15) | 0.193 | 1.07 (0.99–1.15) | 0.104 |
| Respiratory rate >22 bpm | 1.03 (0.95–1.12) | 0.443 | 1.03 (0.96–1.11) | 0.368 |
| Heart rate >100 bpm | 1.1 (1.01–1.2) | 0.022 | 1.02 (0.96–1.08) | 0.529 |
| Glasgow coma scale score <15 | 1.34 (1.2–1.5) | <0,001 | 1.24 (1–1.53) | 0.046 |
| Oxygen therapy during hospital stay | ||||
| Low-flow nasal cannula | 0.66 (0.57–0.77) | <0,001 | 1.02 (0.9–1.15) | 0.768 |
| Mask oxygen | 1.21 (1.09–1.35) | <0,001 | 1.24 (1.02–1.51) | 0.03 |
| Invasive mechanical ventilation | 4.28 (2.86–6.41) | <0,001 | 12.94 (3.41–49.12) | <0,001 |
| Other supportive therapy | ||||
| Tracheostomy | 0.99 (0.88–1.11) | 0.82 | 0.99 (0.91–1.07) | 0.821 |
| Haemodialysis | 1.03 (0.94–1.12) | 0.55 | 0.99 (0.91–1.07) | 0.821 |
| Pharmacological treatment | ||||
| Albendazole | 0.91 (0.83–0.99) | 0.037 | 1.07 (0.98–1.18) | 0.129 |
| Opioid analgesic | 1.17 (1.07–1.28) | <0,001 | 1.13 (1.02–1.26) | 0.021 |
| Anxiolytic | 0.89 (0.81–0.98) | 0.023 | 0.91 (0.85–0.97) | 0.002 |
| Anticonvulsivant | 0.86 (0.67–1.12) | 0.259 | 1.09 (0.92–1.29) | 0.309 |
| Antidepressant | 0,82 (0.6–1.12) | 0.214 | 0.67 (0.46–0.99) | 0.042 |
| Prednisone | 0.81 (0.65–1.01) | 0.058 | 0.76 (0.65–0.89) | <0,001 |
| Diuretic | 1.11 (1.01–1.21) | 0.028 | 1.04 (0.97–1.12) | 0.269 |
| Vasoactive drug | 1.88 (1.46–2.42) | <0,001 | 3.3 (1.13–9.66) | 0.029 |
| Low-molecular-weight heparin | 0.94 (0.86–1.03) | 0.207 | 0.91 (0.78–1.06) | 0.222 |
| Unfractionated heparin | 1.02 (0.93–1.11) | 0.681 | 1.05 (0.91–1.22) | 0.478 |
| Azithromycin | 1.09 (0.98–1.2) | 0.114 | 1.02 (0.95–1.09) | 0.626 |
| Non-respiratory complications | ||||
| Acute renal failure | 0.95 (0.86–1.04) | 0.275 | 1.13 (1.04–1.23) | 0.004 |
| Thomboembolic complications | 1.11 (0.82–1.5) | 0.492 | 1.14 (1.03–1.26) | 0.677 |
| Sepsis | 0.99 (0.92–1.07) | 0.804 | 0.95 (0.87–1.04) | 0.236 |
| Septic shock | 0.98 (0.92–1.05) | 0.62 | 1.03 (0.96–1.09) | 0.437 |
| Hypovolaemic shock | 1.07 (0.95–1.21) | 0.29 | 0.95 (0.85–1.07) | 0.416 |
SpO2, peripheral capillary oxygen saturation; CI, confidence interval.
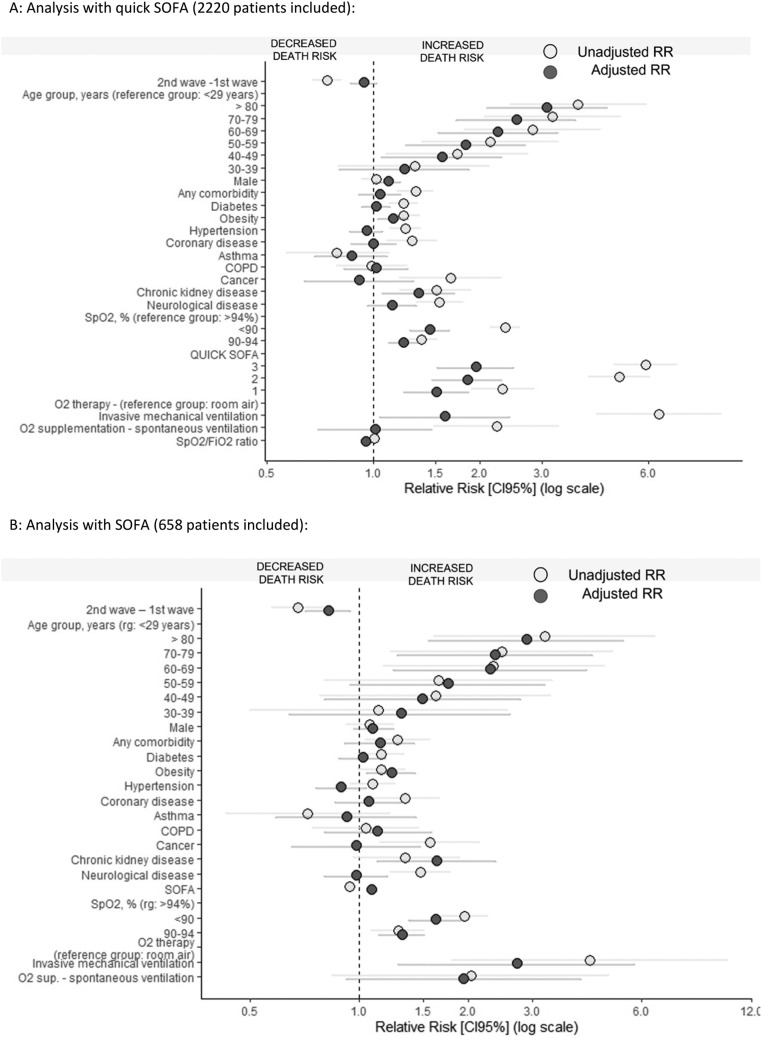
In another analysis, in which wave was treated as a covariate, the difference in the unadjusted risk of death between the waves did not remain after adjustment for age, sex, comorbidities, SpO2, SpO2/FiO2 ratio, oxygen supplementation and quick SOFA score at hospital admission [unadjusted RR 0.74 (95% CI 0.68–0.81), P<0.001; adjusted RR 0.94 (95% CI 0.86–1.02), P=0.164]. The risk of death increased with age, lower SpO2 and higher quick SOFA score, and was directly proportional to oxygen requirement (O2 cannula < O2 face mask < invasive mechanical ventilation) at hospital admission. After adjustments, male gender, obesity and chronic kidney disease were also associated with increased in-hospital mortality (Figure 2A). On the other hand, in a subgroup of 658 patients for whom the SOFA score at hospital admission was obtained, there was a significant decrease in the risk of death adjusted for SOFA score during the second wave [RR 0.82 (95% CI 0.71–0.94); P=0.01] (Figure 2B). More detailed data are provided in Tables S4 and S5 (see online supplementary material).
Figure 2.
Multi-variate robust Poisson regression: unadjusted and adjusted relative risk (RR) of factors associated with in-hospital death at a COVID-19 reference hospital in North-east Brazil. (A) Analysis with quick sequential organ failure assessment (SOFA) score (2220 patients included). (B) Analysis with SOFA score (658 patients included). CI, confidence interval; SpO2, peripheral capillary oxygen saturation; FiO2, fraction of inspired oxygen ratio.
Discussion
Since the early phases of the pandemic, Brazilian policies enacted to face COVID-19 have been primarily orchestrated by states and municipalities [11]. This created a mosaic of strategies from which health managers can extract valuable lessons gained in very recent, harrowing, circumstances. Taken by COVID-19 in a national vacuum of epidemiological surveillance, Ceará had very little time to organize a comprehensive defence plan before it was overwhelmed by rapidly increasing numbers of daily cases. Due to its elevated demographic density and its relatively new status as an international hub for flights to North America and Europe, Ceará was the third most-affected state in Brazil.
The defence plan elaborated by the state of Ceará in 2020 was multi-pronged and entailed a rapid intelligence re-assessment of its working resources; a major, gradual build-up of ICUs throughout the state; and the creation of a dedicated tertiary public hospital devoted entirely to patients with COVID-19 in Fortaleza, a city with approximately 2.7 million inhabitants. This new hospital, eventually known as HELV, faced a few foundational choices. Possible options at the time involved emergency de-novo construction in a public space, rapid modular construction from pre-adapted public spaces such as convention centres or football (soccer) fields (a strategy adopted by the municipality of Fortaleza for its primary care-medium complexity COVID-19 campaign hospital), or rapid repurposing of available private hospitals (the adopted choice).
In order to establish the outcome of repurposing a whole hospital to deal with the COVID-19 threat, this study undertook a retrospective analysis of time to access hospital services; epidemiological, clinical and laboratory data; and supportive treatments employed, and then looked for associations with in-hospital mortality. These analyses were performed for the complete sample, but particular emphasis was placed on the comparison of in-hospital mortality between the first and second waves. This was because it was assumed, based on public data, that the health system in Ceará was more efficient in dealing with COVID-19 during the second wave compared with the first wave, as suggested by a five-fold reduction in deaths in the second wave. This study aimed to determine how this improvement was reached.
Some studies have shown that the severity of illness of hospitalized patients with COVID-19 was very high, with mortality rates ranging up to 40% among patients requiring ICU admission during the early phase of the first wave [12]. The present study compared in-hospital mortality at HELV during the first wave in Ceará with the performance of established hospitals in Northeast Brazil or internationally. In Brazil, studies have described higher in-hospital mortality during the first wave compared with other countries, with reported rates of 59% among patients admitted to the ICU, 80% among those who were mechanically ventilated, and 86.8% considering Northeast Brazil specifically [13].
Data from several countries have shown differences in demographics and reduced mortality in the second wave compared with the first wave [14]. Nevertheless, specific studies on local responses to the COVID-19 burden on the health system are still lacking in the literature, particularly comparing the first and second waves.
The time from symptom onset to hospitalization at HELV represents a long period for a patient with COVID-19, given the rapid transition to potentially irreversible states that follows the beginning of the disease's hyperinflammatory phase around 8 days after symptom onset. The average delay from presentation at an emergency or primary care facility to admission to HELV was 2 days, due to the high rate of hospital occupancy during both waves. Such delays likely played a significant role in the increased severity of disease in patients enrolled at HELV.
Compared with patients admitted to HELV in the first wave, those admitted during the second wave were significantly younger, were more likely to be female, and displayed fewer comorbidities. Interestingly, patients from the second wave were less likely to have hypertension or diabetes, but more likely to be obese compared with patients from the first wave. Further, in the second wave, patients were less likely to present with dyspnoea and fever, and more likely to present with sore throat and headache, suggesting greater involvement of the upper airways than systemic compromise. Nevertheless, sore throat and headache were associated with decreased risk of mortality in the first and second waves, respectively. A large population-based analysis that included almost 69,000 patients found that the presentation of fever and cough was significantly associated with higher hospital admission and ICU admission, whereas sore throat was found to be protective against these outcomes [15]. Another retrospective cohort described headache as a frequent symptom in patients with COVID-19, and its presence was found to be an independent predictor of lower risk of mortality, suggesting it might be related to a different COVID-19 presentation in terms of severity and inflammatory response [16]. Consistent with this view, the present study found a significant decrease in the proportion of patients with severe hypoxaemia and requiring high levels of oxygen support in the second wave.
It is intuitive to expect that increases in health system efficiency during a pandemic caused by a novel disease will stem from improved medical therapies. While this may well be the case, at state level, health systems are immensely complex, and there are many opportunities for gains in less conspicuous areas that are not immediately evident. In general, better patient outcomes are due to better patient management, better initial patient conditions, or a combination of the two. Given the significant differences in health status between patients from the first and second waves, it is even appropriate to ask whether HELV faced the same disease in both waves. In order to clarify this, wave timing was treated as a covariate, and this showed that the differences in the unadjusted risk of death between the waves vanished after taking sex, comorbidities, SpO2, SpO2/FIO2 ratio, oxygen supplementation and quick SOFA score at hospital admission into account. Clearly, the disease must be the same, but the population substructure sampled by the virus in the second wave was clearly different. In all, the data suggest that, on average, patients from the second wave had a better health status than those from the first wave.
A better health referral system allied to younger, healthier patients may have contributed to better outcomes in the second wave. This, however, does not indicate that there were no changes in the quality of treatment at HELV in the second wave compared with the first wave. This study focused on assessing the initial performance of a repurposed hospital, and its evolution as a tertiary hospital dedicated to treatment of COVID-19. Therefore, a detailed assessment of treatment efficacies in the two COVID-19 waves is beyond the scope of this study. Nevertheless, it is possible to extract evidence for improved medical therapies in the second wave, especially on account of increased clinical experience, which was shown by a drastic reduction in the use of compassionate but ineffectual treatments proposed in the first wave, and the adoption of tested, effective therapies in the second wave. One particular area of high relevance was the overall improvement in oxygen therapy at HELV during the second wave (see below), which was associated with a smaller proportion of patients receiving ICU care; of those, an even smaller percentage required mechanical ventilation in the second wave compared with the first wave.
It is important to stress the role played by the evolution of oxygen therapy at HELV during the second wave. Current scientific evidence supports the use of high-flow nasal cannula oxygen for patients who are unresponsive to conventional oxygen therapy [17]. However, less than 1% of the enrolled patients used this therapy, and this may explain the higher rate of mechanically ventilated patients in this study, mainly during the first wave, and the very high mortality rate in this subset of patients. However, during the second wave, the use of non-invasive mechanical ventilation was implemented, including the use of helmet CPAP (Elmo) in a small selected group of patients. The reduction in the rate of tracheal intubation after 24 h of hospital admission in the second wave may also have played a role in the decrease in overall mortality in the second wave. At the same time, the use of invasive mechanical ventilation showed the strongest association with increased risk of death in both waves. Previous reports have demonstrated that nearly 30% of patients with COVID-19 required ICU admission due to pneumonia, and 20% developed acute respiratory distress syndrome while undergoing mechanical ventilation [18]. Corroborating the data from the present study, other studies showed that 41.8% of patients hospitalized due to COVID-19 developed acute respiratory failure, with a fatality rate of 52.4% [19]. Early endotracheal intubations were stimulated and performed in the first wave [20]. A systematic review comparing mortality outcomes between high-income countries and low- and middle-income countries (LMICs) showed significantly higher mortality rates among patients receiving invasive mechanical ventilation in LMICs [21]. The unknown disease and how to provide the correct treatment, in addition to the mismatch between demand and supply leading to a collapse of the healthcare system, could explain, in part, the high in-hospital mortality rates found in LMIC countries, including Brazil, and in the present study [22]. Unfortunately, information on adherence to best practices for use of invasive mechanical ventilation is not available for the present study. However, the extremely high mortality rate associated with the use of invasive mechanical ventilation in both waves shows the urgent need to adopt better management of ventilated patients.
From the point of view of the options available at the time (i.e. emergency de-novo construction in a public space, rapid modular construction from pre-adapted public spaces, or quick repurposing of available private hospitals), swift repurposing had evident advantages. First, although HELV was inoperative at the time, the hospital infrastructure was already in place, obviating the need to spend valuable time on construction, or incurring the risk of depending on new, untested, conditions, which, if they failed, could compromise ongoing patient care. The latter, a caveat of rapid modular construction from pre-adapted public spaces, was a frequent and recurrent problem associated with these solutions [23]. Perhaps even more to the point, the choice of repurposing a private hospital into a tertiary hospital proved to be a valuable asset in the overall response to COVID-19. In other circumstances, focusing on intermediate levels of care in the context of campaign hospitals set up in pre-adapted public spaces tied up valuable resources devoted to milder patient profiles, which did represent the most significant burden to public health.
Strengths and shortcomings
This study has several strengths. First, it is one of the largest studies to describe the characteristics and outcomes of patients with confirmed COVID-19 in Brazil, comparing the first and second waves. Second, the study was conducted exclusively in a dedicated tertiary hospital for patients with COVID-19 (not a field hospital), with a very high proportion of intensive care beds, which may have increased the level of diagnostic assertiveness. Finally, epidemiological, clinical and laboratory data were analysed, in addition to supportive therapies including medications and respiratory support, and their association with in-hospital mortality.
This study was limited because records on sociodemographic characteristics and laboratory test results before hospital admission displayed large amounts of missing data, effectively preventing their use. Missing data likely prevented the authors from identifying significant differences associated with the socio-economic features of the study cohort, such as annual incomes, or patients living in areas with very low human development indices, and thus these important parameters were not included in the regression model. However, it is quite possible that sociodemographic factors may have contributed to the high overall in-hospital mortality rate compared with other study cohorts [13]. Missing data also impacted the analysis of 83 patients who were transferred to other health services, and from whom no access to information about their hospital outcomes was available. Finally, as is well known, the retrospective observational design is susceptible to measurement and information bias.
Conclusions
Repurposing a private hospital to set up HELV as a tertiary centre dedicated to patients with COVID-19 within 2 weeks was a breakthrough in the overall strategy devised to face the threat of COVID-19 in the state of Ceará. In the critical days of the first wave, when the world was facing a largely unknown disease, HELV received over 1500 patients. Without HELV, these patients would have overwhelmed all other health facilities, while receiving less than ideal care, simply because available health institutions were never planned to accommodate the simultaneous demands of a large contingent of sick people. It may be argued that HELV was a major contributor to the avoidance of humanitarian disasters recorded in other regions in Ceará, although HELV did not attain the expected levels of performance regarding critically ill, intubated ICU patients. By receiving a large contingent of very ill individuals with protracted COVID-19, HELV contributed to reduce the pressure on emergency and primary care facilities, and provided a key referral site for these institutions.
Funding
This work was supported by Fundação cearense de apoio ao desenvolvimento científico e tecnológico and Secretaria de Saúde do estado do Ceará.
Author contributions
All authors contributed to conception, drafting and final approval of the manuscript.
Conflict of interest statement
None declared.
Acknowledgements
The authors wish to thank their colleagues from the ResCOVID Task Force (Escola de Saúde Pública do Estado do Ceará Paulo Marcelo Martins Rodrigues, Fortaleza, Brazil): Ana Naiara Alves Teixeira, Bruna Mara Machado Ribeiro, Daniel Germano Alcântara, Denise Coelho de Souza, Marcos Augusto de Paula Santos, Maria Iara Socorro Martins, Tayná Albuquerque Tabosa, Thalyta Gleyane Silva de Carvalho and Ticiane Freire Gomes.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2023.03.009.
Appendix. Supplementary materials
References
- 1.Organization for Economic Cooperation and Development . OECD; Paris: 2022. First lessons from government evaluations of COVID-19 responses: a synthesis. accessed 12 April 2022. [Google Scholar]; Available at: https://www.oecd.org/coronavirus/policy-responses/first-lessons-from-government-evaluations-of-covid-19-responses-asynthesis-483507d6/
- 2.Ponce D. The impact of coronavirus in Brazil: politics and the pandemic. Nat Rev Nephrol. 2020;16:1. doi: 10.1038/s41581-020-0327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Health emergency dashboard. Geneva: WHO. Available at: https://covid19.who.int/region/amro/country/br (accessed 23 June 2022).
- 4.Brasil. Agência Nacional de Vigilância Sanitária. Centro de Vigilância Sanitária . 2020. Portaria conjunta CVS/IAL-1. accessed 12 April 2022. [Google Scholar]; Available at: https://fehoesp360.org.br/noticia/6723/anvisa-divulga-portaria-para-habilitacao-de-laboratorios-ao-exame-de-covid-19
- 5.Centers for Disease Control and Prevention. CDC's diagnostic test for COVID-19 only and supplies. Atlanta, GA: CDC. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/virus-requests.html#print (accessed 12 April 2022).
- 6.Secretaria de Saúde do Estado do Ceará. COVID-19. Available at: https://www.saude.ce.gov.br/download/covid-19/(accessed 28 September 2021).
- 7.Tomaz BS, Gomes GC, Lino JA, Menezes DGA, Soares JB, Furtado V, et al. ELMO, a new helmet interface for CPAP to treat COVID-19-related acute hypoxemic respiratory failure outside the ICU: a feasibility study. J Bras Pneumol. 2022;48 doi: 10.36416/1806-3756/e20210349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. National Center for Health Statistics. Atlanta, GA: CDC. Available at: https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm (accessed 7 March 2023).
- 9.National Institute on Alcohol Abuse and Alcoholism. Alcohol use disorder: a comparison between DSM–IV and DSM–5. Available at: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-use-disorder-comparison-between-dsm (accessed 7 March 2023).
- 10.Global Health Observatory of the World Health Organization. Indicators index. Geneva: WHO. Available at: https://www.who.int/data/gho/data/indicators/indicators-index (accessed 7 March 2023).
- 11.Schaefer BM, Resende RC, de Sousa Fernandes Epitácio S, Aleixo MT. Government actions against the new coronavirus: evidence from the Brazilian states. Rev Admin Públ. 2020;54:1429–1445. [Google Scholar]
- 12.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 13.Ranzani OT, Bastos LSL, Gelli JGM, Marchesi JF, Baião F, Hamacher S, et al. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2020;9:407–418. doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan G, Yang Z, Lin Q, Zhao S, Yang L, He D. Decreased case fatality rate of COVID-19 in the second wave: a study in 53 countries or regions. Transbound Emerg Dis. 2021;68:213–215. doi: 10.1111/tbed.13819. [DOI] [PubMed] [Google Scholar]
- 15.Tong J, Wong A, Zhu D. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020;163:3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 16.Trigo J, García-Azorín D, Planchuelo-Gómez Á, Martínez-Pías E, Talavera B, Hernández-Pérez I, et al. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: a retrospective cohort study. J Headache Pain. 2020;21:94. doi: 10.1186/s10194-020-01165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robba C, Battaglini D, Ball L, Patroniti N, Loconte M, Brunetti I, et al. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir Physiol Neurobiol. 2020;279 doi: 10.1016/j.resp.2020.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khedr A, Al Hennawi H, Rauf I, Khan MK, Mushtaq HA, Lodhi HS, et al. Differential mortality with COVID-19 and invasive mechanical ventilation between high-income and low-and middle-income countries: a systematic review, meta-analysis, and meta-regression. Infez Med. 2022;30:51–58. doi: 10.53854/liim-3001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Núñez I, Soto-Mota A. Impact of healthcare strain on access to mechanical ventilation and mortality of hospitalized COVID-19 patients: a retrospective cohort study. Trans R Soc Trop Med Hyg. 2022 doi: 10.1093/trstmh/trac123. [DOI] [PubMed] [Google Scholar]
- 23.Jones JA, Siddiqui ZK, Callahan C, Leekha S, Smyth S, Preas MA, et al. Infection prevention considerations for a multi-mission convention center field hospital in Baltimore, Maryland, during COVID-10 pandemic. Disast Med Public Health Prep. 2021;16:1–21. doi: 10.1017/dmp.2021.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.