Abstract
This review focuses on vaccine distribution and allocation in the context of the current COVID-19 pandemic. The implications discussed are in the areas of equity in vaccine distribution and allocation (at a national level as well as worldwide), vaccine hesitancy, game-theoretic modeling to guide decision-making and policy-making at a governmental level, distribution and allocation barriers (in particular in low-income countries), and operations research (OR) mathematical models to plan and execute vaccine distribution and allocation. To conduct this review, we adopt a novel methodology that consists of three phases. The first phase deploys a bibliometric analysis; the second phase concentrates on a network analysis; and the last phase proposes a refined literature review based on the results obtained by the previous two phases. The quantitative techniques utilized to conduct the first two phases allow describing the evolution of the research in this area and its potential ramifications in future. In conclusion, we underscore the significance of operations research (OR)/management science (MS) research in addressing numerous challenges and trade-offs connected to the current pandemic and its strategic impact in future research.
Keywords: COVID-19, Vaccine, Allocation, Distribution, Operations research
Introduction
The impact of the COVID-19 pandemic is not same for everyone: Vulnerable individuals, such as those economically disadvantaged or those with chronic health status, might be more at risk of suffering the consequences of the crisis induced by the pandemic [1]. This crisis is impacting multiple interconnected life domains, also interacting with pre-existing inequalities related to multiple aspects of life, such as gender, age, geography, and ethnicity [1].
Some considerations are inevitable when developing models for optimal vaccine allocation, making sure to address equity in allocation between countries as well as disparities in accessing healthcare services [2]. Immunization inequities are connected to important concepts such as ethics, social justice, and fairness. According to Boyce et al. [3], equity in immunization is achieved when all individuals are offered the same vaccines through the implementation of tailored services able to meet their needs. On the other hand, when immunization coverage between population groups faces barriers (not appropriately addressed through policies, dedicated structures, program or governance implementation), we witness avoidable differences in immunization coverage between population groups [4], WHO [5].
Previously, the Global Vaccine Action Plan (GVAP) set a strategic goal: to achieve 90% national immunization coverage in all countries and 80% in each district [6]. The vision behind this project referred to a world in which all individuals and communities could enjoy living free from vaccine-preventable diseases. In this context, equitable access to immunization is considered a fundamental component of the right to health. Although the forecasts pointed out the unlikelihood of achieving most of the goals and targets by 2020, an important progress has been made in many areas [7].
Part of the effort to strengthen equity in immunization coverage consists of ensuring availability and adequate effectiveness of immunization products in the communities affected by low coverage [3, 8]. To achieve this goal, strategic supply chain approaches can help to overcome the barriers to an equitable vaccine distribution, improving on strategic services such as vaccine stockouts, inadequate storage capacity, challenging terrain and road conditions, and non-functional cold chain equipment [8–14]. The main concern regarding traditional supply chain approaches is that they are centered on cost-effectiveness and efficiency. This means that their focus on streamlining distribution and storage might miss communities that are more marginalized, such as those living in rural or remote areas, and consequently fail to tackle the problem of equity in vaccine distribution.
The recent COVID-19 pandemic has hastened our need to understand the complexities around mass vaccination so as to develop efficient and equitable vaccine production and distribution plans. According to Mullard [15], manufacturers and supply chains all over the world are facing a task for which they are largely unready for the global distribution plan of the COVID-19 vaccines. Fulfilling the global demand of a successful vaccine implicates the fast production of an unprecedented number of doses [16]. The numbers of vaccine doses and the global infrastructure required to produce them depend on the type of vaccine: One big challenge in massive vaccine production relates to the necessity of rapidly scaling up manufacturing [17].
Possible insights for the COVID-19 vaccine prioritization can be derived from previous studies focused on vaccine allocation in the mid-pandemic stage, such as during the novel A/H1N1 influenza pandemic in the USA [18–21]. In the USA, the legacy from previous efforts to allocate vaccines for 2009 H1N1 pandemic influenza and Ebola virus disease allowed the Committee on Equitable Allocation of Vaccine for the Novel Coronavirus to develop a framework for equitable distribution of COVID-19 disease [16]. The allocation framework proposed is based on ethical and procedural principles, embedded in US social institutions on culture. The (1) ethical principles refer to maximum benefit, equal concern, and mitigation on health inequities,the (2) procedural principles must be characterized by fairness, transparency, and an evidence-based approach. At the same time, in August 2020, an interim framework for COVID-19 vaccine allocation and distribution in the USA was published by the Johns Hopkins University’s Center for Health Security [22]. The framework is grounded on three broad ethical values: (1) well-being and promoting the common good, (2) equal and fair treatment of individuals (3) promoting legitimacy, trust, and a sense of ownership in a pluralistic society.
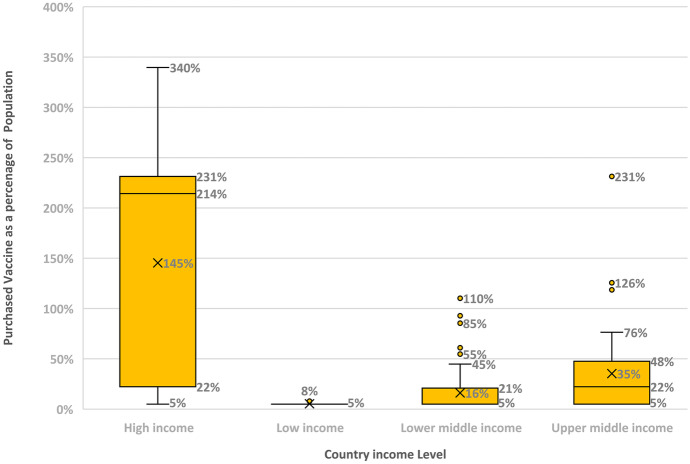
Several factors make equitable allocation more challenging for COVID-19 vaccines: (1) The unprecedented large scale need for vaccination that is estimated to be 11 billion [23] had resulted in competing needs for the vaccines and made it harder for poor countries to access it, see Fig. 1; (2) the urgency by which the vaccines have been developed, given the impact of the pandemic, which led to exceptional government approval procedures that have increased the rate of hesitancy; (3) the immense production and distribution challenges given the huge demand and complexity of the vaccine supply chains that can require more than 200 components [23],(4) the use of the new technology mRNA which aggravated the pressure on the vaccine supply chain given the scarcity of both materials (specially nucleotides, enzymes, and lipids) and labor [23], (5) given the combined effects of shortage and high demand, countries have engaged in shortage gaming [24] and the politics of embargo such as the restrictions on vaccine exports that were imposed by India and the European Union. The shortage gaming has resulted in high-income countries purchasing more than their population needs. The box and whisker plot in Fig. 1 provides information about the amount of purchased vaccines as a percentage of the population, depending on the countries’ level of income. For each level of income, each box shows the median (indicated with an “x”), the quartiles, with the first quartile on the top of the box, and the whiskers, that indicate the upper and lower extreme values. The individual dots show outliers. The median purchase of COVID-19 vaccines as a percentage of the population is 145% for high-income countries versus only 5% for low-income countries! For lower-income and upper-middle-income countries, the median purchase of COVID-19 vaccines is respectively 16% and the 35%. We note that we have ordered the x-categories in Fig. 1 so as to contrast high-income and low-income countries as well as low-income and high-middle-income countries; (6) the relatively large number of different vaccines (7 have been approved by the [25]) and their different characteristics (such as the required number of dozes and their efficacy) has introduced more complexity in planning for purchase and allocation of vaccines; (7) misinformation, politics, and social media platforms have contributed to the increase in vaccine hesitancy Pan American Health [26].
Fig. 1.
Average percentages of covered population by country income level
Despite the initiatives taken, evidence from the literature suggests that the problem of inequitable distribution is still present. For example, a recent review of COVID-19 vaccination plans in 50 states and Washington DC has found that most of the plans were created without consultation with a health and equity committee, despite the fact that COVID-19 diseases and mortality were more prevalent among racial and ethnic minority groups [27].
The goal of this paper is to address some of these COVID-19 challenges on a global scale, from the OR perspective. We chose to focus on COVID-19 given its urgency and the saliency of sociological issues such as equity and hesitancy. We do so by adopting a novel literature review approach that constitutes of three phases. In the first phase, we conduct a bibliometric analysis of the related literature. This is followed by a network analysis to identify the main literature themes. Finally, we carry a refined literature review based on the results obtained by the previous two phases. In the bibliometric analysis, we performed a topic analysis using the co-citation data. That resulted in six clusters and included historical influential papers that were covered in those clusters. When relevant, we have also referred to other influential OR-related studies on vaccine allocation that were not cited in our review dataset. The rest of the paper is organized as follows. We discuss our research methodology in Sect. 2. The three-phase literature review process is detailed in Sects. 3, 5 and 5.1, respectively. Finally, we provide suggestions for future research and our conclusions in Sects. 5.2 and 5.2.2, respectively.
Research Focus and Methodology
This review aims to consider different aspects connected to the problem of vaccine distribution and allocation in the context of the current COVID-19 pandemic.
The main topics covered will be as follows:
The problem of equity in COVID-19 vaccine distribution
Models to optimize COVID-19 vaccine allocation and distribution
Vaccine hesitancy and game theory in the context of the current COVID-19 pandemic
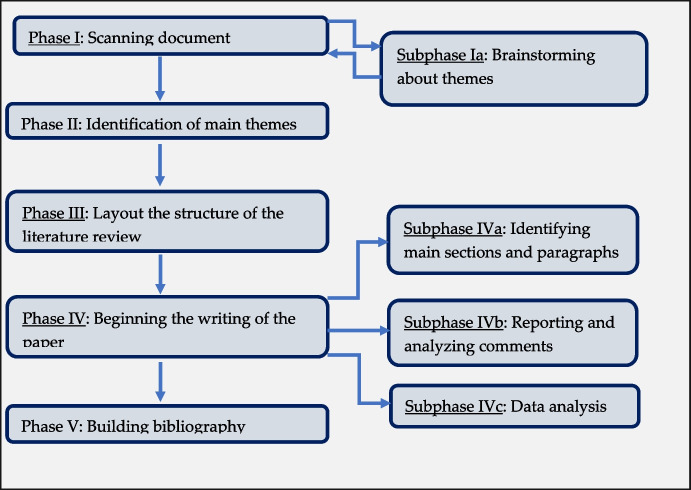
The whole approach adopted to conduct this review consisted of three main components: a bibliometric analysis, a network analysis, and a literature review. The literature review follows the five-step model proposed by Rowley and Slack [28] (Fig. 2). In its entirety, the work was divided in three main sections: a bibliometric analysis, a network analysis, and a further review, based on the results obtained by previous two analyses.
Fig. 2.
Literature review process, readapted from Rowley and Slack [28]
Bibliometric Analysis
We started by conducting a bibliometric analysis and then used the insights obtained by this analysis to further refine this work. We have used Web of Science (WoS), a trusted publisher-independent citation database, that includes almost 1.9 billion cited references from over 171 million records. The keywords identified for the bibliometric analysis are “Vaccine,” “COVID-19″ and “Distribution”. The search command used was (”Vaccine” AND”COVID-19″ AND “Distribution”). The search led to a total of 371 papers. Given that research on COVID-19 started in early 2020, our search period was from 2020 and 2021 (up to July 28, 2021). We then downloaded the full publications data in a RIS formatted file, containing all the bibliographic information about the literature articles identified and their references.
BibExcel, a bibliometric software [29], was used to conduct preliminary analysis of the publications and to generate a co-citation network file. Subsequently, the network file was input to Gephi, a network science software [30], to display and further analyze the co-citation data generated by BibExcel, resulting in different network maps and topic clusters of the co-citation network.
Published Literature Descriptive Analytics
In this section, we use BibExcel to report some descriptive statistics on the collected publications data.
Most Cited Authors and Papers
The first analysis performed was related to the most cited authors (top 10 authors). Table 1 shows the list of the top 10 authors cited and Table 2 displays the top 10 cited papers related to the most cited authors.
Table 1.
Most cited authors
| Citations | Author |
|---|---|
| 32 | Polack FP, 2020 |
| 28 | Huang CL, 2020 |
| 22 | Baden LR, 2021 |
| 20 | Zhu N, 2020 |
| 20 | Zhou F, 2020 |
| 19 | Wrapp D, 2020 |
| 19 | Li Q, 2020 |
| 18 | Jackson LA, 2020 |
| 18 | Wu F, 2020 |
| 18 | Voysey M, 2021 |
Table 2.
Most cited papers
| Citations | Paper |
|---|---|
| 32 | Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., … & Gruber, W. C. (2020). Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England Journal of Medicine |
| 28 | Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., … & Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet, 395(10,223), 497–506 |
| 22 | Baden, L. R., El Sahly, H. M., Essink, B., Kotloff, K., Frey, S., Novak, R., … & Zaks, T. (2021). Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New England Journal of Medicine, 384(5), 403–416 |
| 20 | Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., … & Tan, W. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New England journal of medicine |
| 20 | Zhou, F., Yu, T., Du, R., Fan, G., Liu, Y., Liu, Z., … & Cao, B. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet, 395(10,229), 1054–1062 |
| 19 | Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A., Hsieh, C. L., Abiona, O., … & McLellan, J. S. (2020). Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science, 367(6483), 1260–1263 |
| 19 | Li, Q., Guan, X., Wu, P., Wang, X., Zhou, L., Tong, Y., … & Feng, Z. (2020). Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England journal of medicine |
| 18 | Jackson, L. A., Anderson, E. J., Rouphael, N. G., Roberts, P. C., Makhene, M., Coler, R. N., … & Beigel, J. H. (2020). An mRNA vaccine against SARS-CoV-2—preliminary report. New England Journal of Medicine |
| 18 | Wu, F., Zhao, S., Yu, B., Chen, Y. M., Wang, W., Song, Z. G., … & Zhang, Y. Z. (2020). A new coronavirus associated with human respiratory disease in China. Nature, 579(7798), 265–269 |
| 18 | Voysey, M., Clemens, S. A. C., Madhi, S. A., Weckx, L. Y., Folegatti, P. M., Aley, P. K., … & Bijker, E. (2021). Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet, 397(10,269), 99–111 |
We note that the first and third most cited paper (Polack et al., 2020 and Baden et al., 2021) that received 54 citations are concerned with the COVID-19 vaccine safety. On the other hand, the second most cited paper, Huang et al. (2020), with 28 citations, was one of the first papers that outlined the key features of patients that tested positive for COVID-19. It is also noteworthy to indicate that the research included in these papers was also disseminated largely through mainstream news outlets, providing us with an excellent example of alignment between the agendas of scientific research and the public, as emancipated in the media and political arenas.
Most Cited Journals
Table 3 shows the most cited journals. It can be noticed that the journals Vaccines (13 articles) and Vaccine (9 articles) lead the list of the top 10 most cited journals. It is worthwhile noting that Vaccines is a relatively new journal (started in 2013 versus 1983 for Vaccine). Vaccines is an entirely open-access journal and that may explain its rank. It is published by the Multidisciplinary Digital Publishing Institute (MDPI), which was “once included on Beall’s list of potential, possible or probable predatory scholarly open-access publishers” and a recent analysis of its 53 journals that were included in the 2018 WoS Journal Citation Reports revealed concerning evidence that they meet certain predatory journal classification [31].
Table 3.
Most cited journals
| Journal name | Citations |
|---|---|
| Vaccines | 13 |
| Vaccine | 9 |
| International Journal of Environmental Research and Public Health | 7 |
| Journal of Medical Virology | 7 |
| Plos One | 7 |
| BMJ – British Medical Journal | 6 |
| Journal of Clinical Medicine | 5 |
| Journal of Medical Ethics | 5 |
| JAMA Network Open | 4 |
| Frontiers in Immunology | 4 |
| Health Affairs | 4 |
| Journal of Biomolecular Structure and Dynamics | 4 |
| Nature Communications | 4 |
| Scientific Reports | 4 |
We would also like to highlight that all the list journals fall within the following category(ies):
Immunology
Medicine, Research and Experimental
Environmental Sciences
Public, Environmental and Occupational Health
Virology
Multidisciplinary Sciences
Medicine, General and Internal
Ethics
Medical Ethics
Health Care Sciences and Services
Health Policy and Services
Biochemistry and Molecular Biology
Biophysics
This is understandable given the nascency of the topic. As to the journals that fall in WoS category of “Operations Research and Management Science” (ORMS), we found only 3 articles in this category, Bertsimas et al. [95], Martonosi et al. [32], and Tavana et al. [33]. One goal of our review is to bring to the forefront the major relevant ORMS issues and facilitate research in this timely and impactful area of research.
Most Used Keywords
The last section of the descriptive analysis is dedicated to the most commonly used keywords. In conducting this analysis, we combined the words that are synonyms and are directly related (such as COVID-19, SARS-CoV-2, Coronavirus, Virus, and Viral) into one single field. Table 4 summarizes the results. As expected, the first top two most commonly used keywords refer to COVID-19 (and synonyms) and vaccine (and its synonyms). We also note that keywords that are related to our review focus are ranked as third (equity), fifth (distribution and allocation), six (modeling and analysis), and tenth (hesitancy).
Table 4.
Top 10 most used keywords
| Frequency | Keyword(s) |
|---|---|
| 352 | COVID-19, SARS-CoV-2, Coronavirus, Virus, Viral |
| 198 | Vaccine, Vaccines, Vaccination, Immunization, Immunity |
| 63 | Public, Social, Policy, Justice, Equity |
| 55 | Health, Healthcare |
| 30 | Distribution, Allocation, Supply |
| 24 | Model, Analysis |
| 20 | Pandemic |
| 18 | Disease |
| 17 | Ethics |
| 16 | Hesitancy |
Network Analysis
To conduct a network analysis, software Gephi was used. Gephi utilizes a plethora of filtering techniques and tools that enhance the flexibility and performance of this software in handling large volumes and various types of data.
Prior to using Gephi, we use BibExcel to generate a network file that includes the relevant information that we would like to visualize and analyze in Gephi.
Co-citation Analysis
One of the important features of co-citation analysis lies in its ability to uncover the intellectual composition of a certain field [34] and to clarify the evolution of research over time [35]. The ultimate goal of a co-citation is to reveal emergent patterns and decomposing the co-citation network into clusters. The decomposition of this network further enhances the mapping and visualizing of both the structure and dynamics of the subfields. In brief, while the main purpose of the citation analysis is to identify the major publications in a specific field, the main goal of the co-citation analysis is to reveal the knowledge groups in that field, the general relationship between them, and how they evolve over time [35].
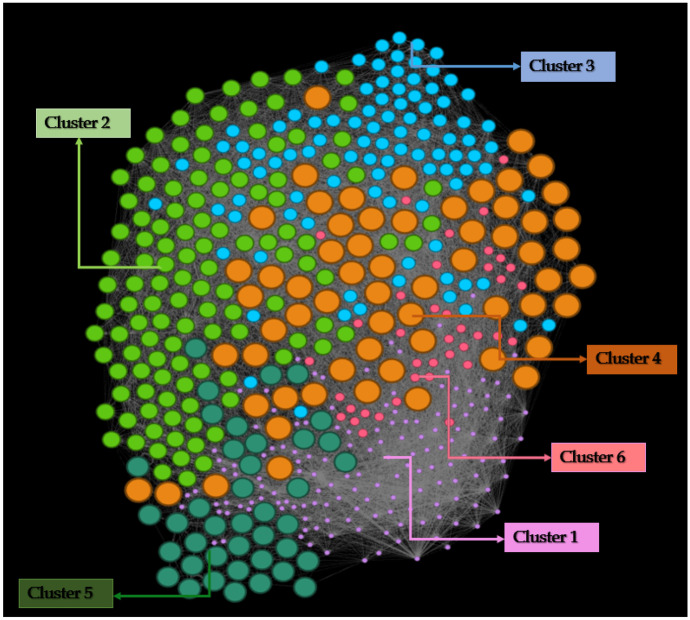
The co-citation network data was generated from BibExcel and input into Gephi for network analysis and visualization. The network graph is weighted and undirected. It has 501 nodes and 14,340 edges. The density of the links has been measured using the concept of Modularity through the Louvain algorithm [36] whereby the modularity index varies between − 1 and + 1. The application of modularity generated 6 clusters (Fig. 3), with a modularity index calculated as 0.336.
Fig. 3.
Co-citation network refined with the algorithm ForceAtlas2
To create a network of co-cited papers with improved visibility, we used the ForceAtlas 2 algorithm [37]. It is a force directed layout which simulates a physical system with the aim to spatialize a network. Similarly to charge particles, nodes repulse each other, whereas edges attract their nodes like springs [37]. Ultimately, the corpus of forces acting on the network converges in a balanced state. It is found to be faster than comparable algorithms and scales well with the number of nodes [37].
We analyzed the research areas for each cluster. For this purpose, we identified the top 10 publications of each cluster, based on the co-citation PageRank [38]. The results are shown in Table 5. PageRank [39] represents an index that identifies the importance score of a network node which in this case is an article.
Table 5.
Top 10 ranked papers in each cluster
| Cluster 1 | PageRank | Cluster 2 | PageRank | Cluster 3 | PageRank |
|---|---|---|---|---|---|
| Zhu et al. (2020) | 0.0345 | F. Zhou et al. (2020) | 0.0320 | Zhu et al. (2020) | 0.0940 |
| Zhu, Li, et al. (2020) | 0.0335 | Walls et al. (2020) | 0.0097 | Zhou et al. (2020) | 0.0350 |
| Zhou et al. (2020) | 0.0320 | Wrapp et al. (2020) | 0.0043 | Wu et al. (2020) | 0.0098 |
| WHO (2020) | 0.0078 | Li et al. (2020) | 0.0016 | Yuan et al. (2020) | 0.0090 |
| Yancy (2020) | 0.0064 | Huang et al. (2020) | 0.0014 | Yan et al. (2020) | 0.0080 |
| Voysey et al. (2021) | 0.0060 | Lauer et al. (2020) | 0.0014 | Zaki et al. (2012) | 0.0071 |
| Zhang et al. (2021) | 0.0057 | Lan et al. (2020) | 0.0013 | D. Wang et al. (2020) | 0.0062 |
| Xia et al. (2020) | 0.0051 | Guan et al. (2020) | 0.0012 | Zheng et al. (2020) | 0.0061 |
| Xia et al. (2021) | 0.0050 | Kermack and McKendrick (1927) | 0.0009 | Y. Zhou et al. (2020) | 0.0047 |
| Walsh et al. (2020) | 0.0047 | Linton et al. (2020) | 0.0008 | A. Wu et al. (2020) | 0.0046 |
| Cluster 4 | PageRank | Cluster 5 | PageRank | Cluster 6 | PageRank |
| Z. Wu and McGoogan (2020) | 0.0068 | Wortham (2020) | 0.0043 | F. Zhou et al. (2020) | 0.0320 |
| J. Zhang et al. (2020) | 0.0059 | Williams and Dawson (2020) | 0.0022 | Jinyong Zhang et al. (2020) | 0.0087 |
| Tay et al. (2020) | 0.0024 | Wiersinga et al. (2020) | 0.0016 | C. Zhang et al. (2019) | 0.0061 |
| N. Tang et al. (2020) | 0.0023 | W. Wang et al. (2020) | 0.0013 | Zellweger et al. (2020) | 0.0042 |
| Williamson et al. (2020) | 0.0021 | Persad et al. (2020) | 0.0013 | Song et al. (2019) | 0.0032 |
| X. Tang et al. (2020) | 0.0021 | So and Woo (2020) | 0.0011 | H. Zhang et al. (2020) | 0.0032 |
| Ou et al. (2020) | 0.0018 | Puri et al. (2020) | 0.0010 | van Riel and de Wit (2020) | 0.0025 |
| Seow et al. (2020) | 0.0018 | McClung et al. (2020) | 0.0009 | Shin et al. (2020) | 0.0014 |
| Shu and McCauley (2017) | 0.0016 | Larson et al. (2014) | 0.0007 | Smith et al. (2020) | 0.0013 |
| Shen et al. (2020) | 0.0015 | Nat. Acad. of Sci. Eng. (2020) | 0.0007 | Pardi et al. (2018) | 0.0010 |
The examination of the most important papers allows us to identify the main research themes, as synthetized in Table 6. Cluster 1 is primarily populated by studies that look at the development process of vaccines for COVID-19, analyzing the vaccine candidates that have been proposed during the first year of the pandemic. Cluster 2 focuses more on the biological characteristics of the COVID-19 virus and of the disease that results from contracting the infection. Cluster 3 appears to be more expanded in terms of the main research areas, spanning between the biology of the SARS-CoV-2, the implications of the infection for the cardiovascular and respiratory system, and the exploration of possible drugs for treatment. Cluster 4 focuses mainly on the treatments for the disease cause by the SARS-CoV-2 infection. Cluster 5 embraces the issues connected to how to prioritize vaccine for COVID-19 and how to ensure equity and fairness in its distribution. It also addresses the problem of vaccine hesitancy. Lastly, cluster 6 mainly revolves around the next-generation vaccine platforms for COVID-19, the mRNA vaccines.
Table 6.
Cluster classification with main areas of research
| Cluster and label | Main research areas |
|---|---|
|
Cluster 1 Vaccine development for SARS-Cov-2 virus and vaccine candidates |
Development of COVID-19 vaccines Landscape of COVID-19 vaccines up to December 2020 Identification and characterization of a new coronavirus (2019-nCoV) Impact of COVID-19 infection in underrepresented minorities, who contract the infection more frequently and suffer from a higher mortality rate |
|
Cluster 2 Clinical characteristics of the SARS-Cov-2 virus and of the disease resulting from it |
Characteristics of the SARS-Cov-2 (incubation period, infectiousness, biological characteristics of the virus) Clinical characteristics of individuals infected with SARS-Cov-2 and of the disease resulting from the virus |
|
Cluster 3 SARS-Cov-2 virus, respiratory system and drugs for treatment |
Biological characteristics of the novel SARS-CoV-2 virus Association between the novel SARS-CoV-2 virus, cardiovascular system and respiratory diseases Identification of candidate repurposable drugs targeting the new SARS-CoV-2 |
|
Cluster 4 Treatments for COVID-19 |
Progression and evolution of the disease resulting from SARS-CoV-2 infection Overview of therapeutic interventions targeting viral infection and/or immunoregulation |
|
Cluster 5 Vaccine prioritization and equitable allocation; vaccine hesitancy |
COVID-19 vaccine prioritization, fairness, and equity in vaccine allocation Issues related to global access to COVID-19 vaccines Vaccine hesitancy towards vaccines and vaccination. Vaccine hesitancy and social media |
|
Cluster 6 mRNA vaccines and next-generation vaccine platforms |
COVID-19 vaccine development, next-generation vaccine platforms (technologies underlying vaccine development) for COVID-19 Technological advancements with mRNA vaccines |
Further Classification of the Literature
It can be observed in Table 6 that the first four clusters include studies of the medical aspects of handling COVID-19, from vaccine development to clinical characteristics of the disease to symptoms and medical treatment. Cluster 5 constitutes the most frequently studied non-medical subject in dealing with COVID-19 which encompass studying COVID-19 vaccine prioritization, fairness and equity in vaccine allocation, issues related to global access to COVID-19 vaccines, and vaccine hesitancy towards vaccines and vaccination. We think that the amount of scholarly work concentrating on the above topics provides insights into the significance of this area of knowledge. In this section, we focus on each of the mentioned topics in more details and review the literature around them.
To further enrich our review, we use the results obtained by (1) the bibliometric analysis and in particular on the most used keywords, and (2) the results obtained by the network analysis, more specifically focusing on cluster 5. To run the new search in WoS, two new keywords have been added the previous ones: “Allocation” and “Equit*”. Additional advanced searches have been conducted using the following combinations:
”Vaccine” AND”COVID-19″ AND”Allocation”
”Vaccine” AND”COVID-19″ AND “Equit*” AND “Distribution”
”Vaccine” AND”COVID-19″ AND “Equit*” AND “Allocation”
The combination “Vaccine” AND “COVID-19″ AND “Allocation” resulted in 160 papers. When we added the word “Equit*” into the previous 2 strings, we obtained 60 results for the combination”Vaccine” AND”COVID-19″ AND “Equit*” AND “Distribution” and 30 results for the combination”Vaccine” AND”COVID-19″ AND “Equit*” AND “Allocation”.
The results were refined by removing duplicates, considering as a category only scientific articles published in peer-reviewed journals. The time period of the publications considered covers 2020 and 2021 (up to July 28, 2021). Articles considered non relevant and duplicates have been subsequently removed.
Within each database, a further search was conducted to identify the most relevant articles that could address the research questions and create subsections for the review.
Equity in Vaccine Distribution and Allocation
There is no doubt about the negative impact of the COVID-19 pandemic on the inequalities that already exist between populations and communities in terms of healthcare services. Access to vaccines can be defined as “equitable” when all individuals are offered the same vaccines through the implementation of tailored services able to meet their needs [3]. On the other hand, when immunization coverage between population groups is obstructed by barriers (not appropriately addressed through policies, dedicated structures, program or governance implementation), we witness avoidable differences in immunization coverage between population groups. This is the very essence of inequity in immunization [4],WHO Regional Office for Europe [43].
Equitable distribution of the vaccine globally, ensuring transparency, and taking into account the socioeconomic variables are highly complex tasks. Critics have been moved to the approach of distributing the vaccine to nations in need in proportion to their population, as this might not consider the specific needs of various populations and consider only the proportionality factor of the population size [44]. Bolcato et al. [44] analyzed the relationship between the principle of equity and its deployment in the context of vaccine distribution in the current pandemic. According to the authors, there is no uniformity in the way of perceiving and interpreting equity globally, depending on the reference values characterizing the context: Transparency is a necessary component of the process, and it must be ensured along a continuum of interventions going from parameter identification to implementation of appropriate plans. The time-sensitive nature of implementing equity measures reprimands appropriate prioritization to reduce the risk of contracting COVID-19 for the largest number of individuals in a specific population at a given time [44].
Aborode et al. [45] study the problem of equity of vaccine distribution in Africa. The authors advocate for equal access and sustainability of the COVID-19 vaccines in African countries, independently from their developmental or economic status. An African-based framework dedicated to streamline the distribution process of COVID-19 vaccines and technologies to Africa is seen as urgent, especially considering the occurrence of the new strains of the virus and the reduction of the supplies from the COVAX facility (a program to finance vaccine acquisition guaranteeing equal access to purchase) linked to the waves in other countries [45]. The authors call on developed nations to facilitate the transition towards a shared-test-of-humanity to replace the current neoliberal “donor” and investment opportunity model so as to support the production of vaccines in Africa. All-inclusive principles for effective and equal access to COVID-19 vaccines should derive from a joint effort of African agencies, institutions, and stakeholders.
Other authors also support the idea of the primary role of high-income countries in assisting low-income countries, not only allowing equitable access to vaccines at a reasonable cost, but also implementing flexible policies on intellectual property and facilitating the access to latest technology and research on potential treatments [46]. This point of view assumes an even higher relevance considering that a sustainable healthcare and a universal access to it are paramount to the global health security [46] propose some strategies that help to reduce the global inequities in health, which include knowledge and technology transfer, mobilizing human rights obligations to pursue a more equitable access to vaccines, an equity focus in the International Health Regulations (IHR) [47], prioritization of COVID-19 prevention and vaccine rollout plans (including tackling vaccine hesitancy), and finally the adoption of community-based behavioral programs to contrast the infection at the community level.
Brown et al. [48] observe the necessity to improve vaccine allocation and educational outreach with respect to higher levels of vulnerability such as marginalized communities. The authors investigated the association between community vulnerability and COVID-19 vaccination in the USA and found a significant association between lower vaccination rates and increased measures of community vulnerability.
Vaccination equity is also connected to geographical distribution of vaccine delivery locations. Geospatial analysis can facilitate the transition towards a social justice approach addressing geographical barriers, such as travel time barriers. To maximize its effectiveness, it should be associated with transparent and culturally safe information to contrast vaccine hesitancy [49].
Ismail et al. [50] proposed an Equity Matrix as a comprehensive validated tool to contrast the inequities connected to the inequitable allocation of COVID-19 vaccines. This tool that was initially developed to support equitable allocation of COVID-19 vaccine in the context of limited supplies was later intended to act as a roadmap and provide evidence-based guidance to navigate the complex spectrum of health inequities to direct decisions and interventions. While receiving consistently increasing attention at a conceptual level, equity appears not to be explored systematically or transparently. The authors recommend the application of the matrix during the development of the recommendations to inform different types of interventions (including but not limited to vaccine distribution). It is worth mentioning that no scientific consensus has been reached about how to take up effective action plans that reduce inequities.
Finally, we have the challenges connected to the current COVID-19 inequities in global healthcare supply chains. Some authors propose the adoption of evidence-based health supply chain interventions that make the distribution supplies, vaccines, and services more resilient and equitable [51]. This point of view is particularly relevant for lower-income and middle-income countries (LMICs) that during the COVID-19 pandemic have experienced an exacerbation of the already existing power imbalance over global supply chains. These evident power disparities implicate higher challenges in accessing resources, a pattern already observed in previous pandemics such as the Ebola epidemic in West Africa [51].
Vaccine prioritization is not only a question of science, but also a question of ethics. It is prominent to consider inequities and disparities when developing models for optimal vaccine allocation, addressing the problem of equity in allocation between countries as well as disparities in access to healthcare [2]. Ensuring equity and efficiency in vaccine coverage is a challenging task as equity and efficiency are conflicting goals. Before distributing a vaccine, decisions must be taken by governments or public health organization about how to allocate the available vaccines. Differently from other resource allocation problems, vaccine allocation has an important ethical relevance, complicated by the fact that equity and efficiency are frequently conflicting objectives [52]. The main focus of the ORMS community is to support the decision-making process, rather than solving the ethical issues.
The criteria governing a specific allocation rule, under a limited stockpile of vaccine, can be classified in two categories: effectiveness and fairness. While effectiveness focuses on how effectively a certain rule leads a system to a certain outcome, fairness focuses on how effectively the allocation rule can implement some fairness principles [53]. Fairness could be more challenging when it involves a collective of decision makers Griffin [54], such as the case with COVID-19 global vaccine allocation, Unlike the problem of effectiveness in allocating limited stockpiles of vaccine, the problem of fairness has been poorly investigated.
The strategies suggested by researchers in this area can be summarized in four categories: equal treatment of individuals, equally, favoring the worst-off, maximizing total benefits, and promoting and rewarding social usefulness. A carefully planned trade-off between efficiency and equity seems unavoidable: Previous studies have noticed how an allocation policy prioritizing efficiency would sacrifice fairness, and vice versa [53, 55].
Mathematical Models for Vaccine Allocation
Vaccine Allocation and Epidemiological Models
The allocation of limited vaccines to control an infectious disease requires policy-makers to formulate goals that address health benefits at both the individual level and population level. Different approaches have been explored to address the problem of vaccine allocation in the past, such as the studies that considered vaccination for seasonal influenza.
Prior to the current COVID-19 pandemic, some researchers have proposed age-structured compartmental models [56, 57] with numerical simulations [19, 58], to examine different scenarios about vaccine allocation and evaluate the theoretical implementation of different vaccination strategies. Compartmental models are mathematical models which play an important role in understanding the mechanisms that contribute to the spread of disease and informing possible control strategies [59]. One of the advantages of these mathematical models is their capacity to identify behaviors that lack clarity in experimental data [59].
Compartmental models are so called because the population under examination is divided into mutually exclusive groups (compartments), with regard to the disease stage. Models are also based on assumptions about the nature and time rate of transfer from one compartment to another [59]. The independent variable in the compartment models is the time t. Assuming that the size of each compartment (defined by the number of members in it) is a differentiable function of time [59], the models are initially described by differential equations with the transfer rate between compartments expressed in mathematical terms as derivatives of the sizes of each compartment with respect to the independent variable t. In each compartment, it is assumed that individuals mix uniformly and randomly. These models are called deterministic, because the prediction of these models is determined entirely by their initial conditions, the set of underlying equations, and the input parameter values. In a deterministic model, the number of newly infected individuals, for a given number of susceptible and infectious individuals, remains the same [60].
Since the early phases of the pandemic, several authors have developed models grounded on traditional mathematical epidemiology addressing evolution and control of COVID-19 disease [61–67]. Soon after the introduction of vaccines, different compartmental models have been proposed: The main aim has been to investigate the effects of the immunization on the spread of the disease together with analyzing different vaccine optimal allocation strategies [68–71]. Meehan et al. [72] adopt an age-structured mathematical model to optimize dose-specific allocation of COVID-19 vaccine. The authors identify two main strategies for vaccination: those aimed at protecting against initial infection and those aimed at reducing the severity of symptoms. The author’s results suggest prioritizing individuals between 30 and 59 years of age to minimize transmission, due to their high contact rates and higher risk of transmission. In order to reduce mortality and morbidity, however, their model suggests targeting those 60 + year old, due to higher probability to experience severe disease. Bubar et al. [2] developed a mathematical model to prioritize COVID-19 vaccine doses comparing five age-stratified prioritization strategies. The authors used an age-stratified SEIR model (susceptible, exposed, infectious, recovered), embedding in their model an age-dependent contact matric, susceptibility to infection and infection fatality rate (IFR). By running 1 year of simulations of infection dynamics, the authors estimated the cumulative incidence of COVID-19 infections, mortality rate, and years of life lost (YLL). The authors found that in order to minimize deaths across countries, vaccines should be prioritized for individuals aged 60 years and older. Interestingly, to tackle the problem of inequities caused by the pandemic, the authors propose to pair individual serological testing with vaccination among the populations that have been mostly affected, redirecting doses to seronegative individuals to improve marginal impact of each dose. Shim [73] adopts an age-structured model to analyze the spread of COVID-19 in South Korea, with the aim to determine optimal vaccine allocation strategies to minimize infections, deaths, and years of life lost (YLL). Assuming a vaccine efficacy of 70% and a supply to immunize 50% of the population, to minimize incidence, the model suggests to prioritize adults aged 20–49 years. To minimize mortality, the model suggests strategies that prioritize adults aged 50 years and older, across efficacy values. Optimal strategies to minimize YYL involve vaccinating adults aged 40–69 years [73]. Similar results have been obtained by Foy et al. [74], who studied vaccine allocation strategies in India using an age-structured SEIR model. The authors’ results recommend prioritizing adults aged 60 years and older to minimize deaths. Conversely, to reduce symptomatic infections, the authors suggest to prioritize individuals aged 20–40 years old. Chen et al. [75] developed an age-structured SAPHIRE model using data from the New York City, with the aim to study the best allocation strategies to allocated COVID-19 vaccines under a limited supply. By differentiating between static and dynamic policies, the authors’ result shows that (a) in case of static policies, the older groups should be prioritized to reduce deaths while to prevent infections, it is recommended to prioritize younger groups,(b) in case of dynamic policies, it is recommended to immunize the older groups at early days and then proceed with younger groups. Matrajt et al. [76, 77] developed an age-stratified mathematical model paired with optimization algorithms to determine four main outcomes under different scenarios: deaths, symptomatic infections, non-ICU, and ICU hospitalizations. In case of a vaccine with effectiveness ≥ 50%, it would be necessary to immunize a high percentage of the population to contrast the spread of the virus. Prioritizing older age groups at higher risk is recommended when using a vaccine with low effectiveness, regardless of the coverage. On the other hand, when using a vaccine with high effectiveness, the model proposed by the authors suggests to allocate vaccines to younger age groups with higher probability to transmit the infection [76, 77]. Babus et al. [78] developed a model to identify what groups should be prioritized for vaccination based on age and occupation. To minimize the cost of infections plus economic losses, the authors’ model suggests to optimize vaccine allocation prioritizing age-based mortality more than the exposure risk caused by occupations [78].
One-Dose Vs. Two-Dose Strategies
The debate about what would be the usage of the available COVID-19 vaccines is ongoing, especially in the context of novel and more contagious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants in various countries [61, 79–81]. Research evidence shows that there is not a definitive answer to this problem [76, 77]. Matrajt et al. [76, 77] explored the use of a single-dose vaccination campaign and mixed vaccination campaigns adopting a mathematical, parametrized with data on COVID-19 vaccine efficacy, and pairing it with optimization algorithms. According to the authors, the level of single-dose efficacy is the most important factor influencing the optimal use of resources. The results of Matrajt et al. [76, 77] suggest that vaccination campaigns that are able to optimally distribute highly efficacious single-dose vaccines, in combination with stringent social distancing interventions, can prevent deaths more effectively than a two-dose vaccination campaign prioritizing subpopulations at high risk of COVID-19 severe disease and death. In addition, the level of transmission plays an important role in the authors’ model. In case of a well-controlled transmission with rigid non-pharmaceutical interventions in place, both the high risk and high transmission groups will be targeted by the optimal allocation strategy. Conversely, with a moderate or high level of transmission, individuals at high risk of disease and death should be targeted first [76, 77]. Tuite et al. [82] developed a decision analytic cohort model to study alternative dose allocation strategies to increase benefits from constrained COVID-19 vaccine supply. In line with Matrajt et al. [76, 77], this model shows that vaccinating more individuals with the first dose would significantly lower the expected number of COVID-19 cases, even if this choice was made at the expense of deferring the second dose. Moghadas et al. [83] used an agent-based model of COVID-19 transmission to compare 2 vaccination strategies: administering vaccines 3–4 weeks apart against adopting a delayed second dose (DSD) strategy. The authors found that a delay of 9 weeks for the Moderna vaccine, compared to the recommended 4-week interval between the 2 doses, could maximize vaccination program effectiveness and prevent at least an additional 17.3% infections, 0.69% hospitalizations, and 0.34% deaths per 10,000 population. A 9-week second dose delay for the Pfizer–BioNTech vaccine, compared to the recommended 3-week interval between the 2 doses, can prevent additional 0.60% hospitalizations and 0.32% deaths per 10,000 population. The DSD strategy of the Pfizer–BioNTech vaccine did not lead to any advantage in minimizing infections, unless the efficacy of the first dose did not decrease over time.
Mathematical Programming
Another approach for vaccine allocation consists of formulating the problem as a mixed-integer or linear programming problem. In the past, researchers have utilized this methodology with the objective to minimize the number or cost of vaccines enforcing the constraint of a reproductive ratio R0 below 1 [84–86]. Buhat et al. [87] attempted to determine optimal and equitable allocation of COVID-19 vaccines in the Philippines, aiming to minimize deaths while prioritizing the most vulnerable categories. The authors used a linear programming model for every city or province in the country, considering only one brand of vaccine. Interestingly, the authors’ methodology is not based on a compartmental model, with the interpretation of the objective function value seen as the estimated additional COVID-19 deaths after the rollout of vaccines. According to the results, between 60 and 70% of the population should be vaccinated for a community with limited supplies. Critical factors influencing the vaccine allocation strategy are the total vaccine supply, vaccine effectiveness, vaccine cost, and projected deaths. Cabanilla et al. [88] proposed a model to optimize the vaccination sites at the municipal level in San Juan, a town in the Philippines, to bring vaccines closer to those who are in need. The model aims to address a minimization problem connected to a facility location problem, formulated using three main factors: location of the sites, population density, and the number of COVID-19 cases per residential area. Çakır et al. [89] focused on locating mobile vaccination clinics in three cities in Turkey. To analyze the multi-facility location problem for the vaccination sites, the authors used Lagrange relaxation method and a modified saving heuristic algorithm. The authors combined fuzzy multi-criteria decision-making (MDMC) methods and a multi-facility location problem. Using real-world data from the district of Chennai (India), Emu et al. [90] proposed a clustering-based solution to identify optimal distribution centers and a framework to optimize vaccine distribution taking into account priority (age, exposure, vulnerability, etc.) and distance. The model, which is based on a constraint satisfaction programming framework, can efficiently distribute the available vaccines adjusting different parameters (such as the distribution of population, total population, total number of available vaccines, the number and capacity of distribution centers). Leithaeuser et al. [91] formulated a model to optimally select vaccination sites in Germany within a given set (such as university hospitals). The authors considered different patient-to-facility and doctor-to-facility assignments as well as various constraints connected to the number of vaccines per site or maximum travel. Using data from Quezon City, Minoza et al. [92] formulated a multi-objective linear programming model applied to an age-stratified and quarantine-stratified compartmental model to optimize equitable vaccine distribution. The authors performed 10 simulations, under different scenarios, on the distribution of 500,000 vaccines. According to the results, prioritizing mobile workforce reduces infections by 21.14%, the best result among all the scenarios,prioritizing elderly, however, is associated with the highest protection (439%). Yin and Büyüktahtakın [93] adopted a multi-stage stochastic programming approach, extending the deterministic epidemic-logistics model of Büyüktahtakın et al. [94], with equity and fairness considerations in the context of resource allocation to control an epidemic. The authors’ objective is to overcome some limitations posed by compartmental models in epidemiology, as the disease transmission rate can be uncertain and subjected to changes over time and space in different scenarios [93]. Therefore, the decision to adopt a stochastic model is to better represent the uncertainty that characterize the transmission dynamics of a disease. The authors defined two new measures to address equity: infection equity and capacity equity. The model is tested to control the Ebola virus disease (EVD) in different African countries. As pointed out by the authors, the model shows versatility to be applicable to control other infectious diseases, including COVID-19. Bertsimas et al. [95] proposed a novel data-driven prescriptive approach to resolve the problem of COVID-19 vaccine distribution in a scenario of limited vaccine capacity. The authors utilized the DELPHI epidemiological model to generate a bilinear nonconvex optimization model aiming to optimize the sites for vaccine distribution and vaccine allocation. For this purpose, the DELPHI model was first extended to variation named DELPHI-V capable of (1) capturing the effects of vaccination and (2) disaggregating the effects of COVID-19 on mortality across risk classes. The final optimization model, named DEPLHI-V-OPT, resolves the problem formulated as a bilinear optimization model using a customized algorithm based on coordinate descent. According to the authors, when using real-world data, the model performs significantly better than benchmark solutions, increasing “the effectiveness of the vaccination campaign by 20%, saving an extra 4000 lives over a three-month period” [95], page. 18). Rastegar et al. [96] have investigated equitable influenza vaccine distribution during the COVID-19 pandemic. They developed an inventory-location mixed-integer linear programming model and applied it to a case study in Iran. The vaccine distribution is prioritized according to the population’s critical need (such as first responders, pregnant women, and elderly). Roy et al. [97] have proposed a time-varying linear optimization-based approach for vaccine allocation amid pandemic. The authors’ model incorporates economic and epidemiological aspects with the purpose to provide recommendations on how to distribute the vaccine across different areas on the New York State. According to the authors, the model proposed is well adaptable to meet the specific epidemiological and economic policy requirements of zones. For their purpose, the authors have adapted the susceptible-exposed-infected-recovered-death (SEIRD) model, implementing a spatial version of it in an agent-based simulation environment that considers human mobility.
Vaccine Hesitancy and OR models
Vaccines represent the most cost-effective health investment to contrast the devastating outcomes inflicted by infectious diseases on humankind for centuries. Immunization is estimated to prevent between 2 and 3 million deaths every year from diseases such as tetanus, measles, diphtheria, or pertussis [25]. Although immunization is considered an unquestionable human right and a key component of primary healthcare, far too many individuals worldwide lack sufficient access to vaccines. In some countries, progress in vaccination coverage has stalled or even reversed, with tangible threats to the achievement obtained so far [25]. The lack of trust that characterize vaccine hesitancy represents one of the several negative consequences of a phenomenon defined as “post-truth” or “post-factual” society [98], where the term “post-truth” is defined by the Oxford Dictionaries as “relating to or denoting circumstances in which objective facts are less influential in shaping public opinion than appeals to emotion and personal belief” (Dictionaries [99]. Anti-vaccine sentiments attracted the media’s attention during each new outbreak of vaccine-preventable disease [100]. The consequences related to vaccine refusal sentiments have led the WHO to constitute a special work group, specifically dedicated to studying this problem: the SAGE Working Group on Vaccine Hesitancy [101]. According to them, “vaccine hesitancy” is a complex and context-specific phenomenon, characterized by the delay in accepting or refusing to get vaccinated despite the availability of vaccine services. It can be seen as a continuum, ranging from complete acceptance to complete refusal. The group of individuals who are vaccine-hesitant are heterogeneous in the middle of this continuum [43, 101]. The SAGE Working Group on Vaccine Hesitancy has established a 3C model to describe the determinants that characterize this phenomenon: (1) complacency, (2) convenience, and (3) confidence. Complacency is defined by a low perceived risk of vaccine-preventable diseases; convenience refers to physical accessibility to vaccines, affordability, quality of the vaccination service (real and/or perceived), and quality of the delivery of the context-specific vaccination services; confidence is a concept that relates to trust in the effectiveness and safety of vaccines, in the reasons identified by policy-makers about the necessity of vaccines and in the system delivering them [101]. Subsequently, a new framework to understand this phenomenon was developed from the research efforts in high-income countries, named the 5C model of the drivers of vaccine hesitancy. According to this model, there are five main individual-person level factors that determine vaccine hesitancy: confidence, compliancy, convenience (or constraints), risk calculation, and collective responsibility [102, 103].
Vaccine hesitancy is the result of biased information processing or ill-informed decision-making, given the fact that the risks seen in being immunized are overestimated. For this reason, it has been frequently studied as a decision-making task, where the risks of being unvaccinated and contract the diseases are compared with the risks of getting vaccinated [104]. In the past, different studies applied the framework of game theory to analyze individuals’ behavior when facing the problem of vaccination, for example conceiving this dilemma as the free-rider problem [105–111]. In the context of COVID-19, the topics of vaccine acceptance and hesitancy show a high degree of complexity. The emergence of the new SARS-CoV-2 variants might add further complexity to the scenario [112]: The introduction of new vaccine should then be accompanied by appropriate communication strategies [113]. Below, we summarize the main studies that emerged from our literature review concerning the application of game theory to address vaccine hesitancy in the context of the current COVID-19 pandemic.
Piraveenan et al. [114] argue that the most effective analytical strategy to model the vaccination uptake might result from combining game-theoretic modeling, social network analysis, and agent-based modeling techniques used to simulate the dynamic disease. According to the authors, to model vaccination decision-making, two components need to be distinct: (1) the decision-making process by individuals to get the vaccine,(2) the decision-making process by governments and policy-makers about how to manage the vaccines (for example what vaccines to choose and how to prioritize them). The decision-making process in both sides is influenced by multiple factors. Non-cooperative game theory represents the ideal route to study individual behavior regarding vaccine acceptance, whereas cooperative game theory is the ideal strategy to analyze and inform decisions regarding vaccine prioritization at a governmental level [114].
Using data from social networks is also considered a viable strategy to tackle the problem of vaccine hesitancy. A study adopted text feature modeling based on evolutionary computation and topic modeling served as a basis to identify vaccine hesitancy using two datasets: the flu vaccine dataset and UK COVID-19 vaccine tweets [115]. The authors found that in the first dataset the proposed approach outperformed the standard system in place.
Game theory was used by Stoddard et al. [116] to study the relationship between noncompliance and rationality on one side, and measures in place to contrast the COVID-19 on the other side. More specifically, the authors analyzed the scenarios in which individuals perceive a benefit from being noncompliant. They demonstrated that under a wide set of conditions, noncompliance is a Nash equilibrium: Using an SEIR model, it was found that noncompliance represents a severe obstacle to contain the spread of the COVID-19 pandemic. A minimum of 80% compliance is required even in case of completely effective interventions, if the goal is to achieve complete suppression.
Guo and Cao [117] applied a two-stage decision framework for vaccine hesitancy to investigate (1) the reasons behind delaying the decision-making (such as receiving or refusing a vaccine), and (2) the interventions that the government can adopt to increase vaccination demand. Therefore, the proposed framework characterizes individuals’ decisions and their equilibrium behavior in two stages. In the first stage, both the reference point formation and the contradictory nature of the information resulting from it contribute to the delay in making decisions. In the second stage, more information becomes accessible, which leads to the update of the map of reference points. This creates psychological utility which, in turn, will contribute to the final decision to refuse or accept the vaccine. The concept of reference points, introduced by Dubé et al. [118], allows for an opportunity to increase vaccination uptake using specific government interventions, rather than considering them as the main cause of the rejection.
Future Research
Guided mainly by our network analysis of the literature, in this section, we propose future research directions.
Vaccine Hesitancy
The problem of vaccine hesitancy, identified by the WHO as a major threat to global health, requires thinking about new strategies that would take into consideration internet and in particular social media platforms, whose popularity has increased globally. Future work dedicated to social network analysis to tackle anti-vaccination sentiments can support further development of new strategies to promote and increase evidence-based literacy. Interventions promoting vaccination delivering factual information might fail and produce the opposite effect: Public health communication strategies about vaccines might not only be ineffective, but they may increase misperceptions and reduce vaccination intention [119, 120]. A possible explanation might relate to the theory of psychological reactance, which explains why individuals resist persuasion. Game theory might be of help to better understand what new and more effective strategies can be adopted to successfully contrast this phenomenon.
In addition, a relatively unexplored research avenue relates to studying the impact of eHealth literacy on behavioral attitudes and health outcomes. eHealth literacy is defined as a metaliteracy composed by six main competencies: (1) traditional literacy and numeracy, (2) health literacy, (3) computer literacy, (4) science literacy, (5) media literacy, and (6) information literacy [121]. Future studies could investigate (1) the impact of increased eHealth literacy on vaccination hesitancy, and (2) the role of eHealth literacy within the context of social networks.
Vaccine Manufacturing Barriers and Equity
Future research directions include analyzing the possible justifications for different distributions of COVID-19 vaccines, highlighting the normative arguments in different contexts (for example national, regional, or population-based), with an important role played by ethicists [40]. The gap in securing vaccine supplies for high-income and upper-middle countries on one side and low-income countries on the other side still remains a prime research venue. More research is necessary to help reduce this gap. Prospective research avenues include understanding how to insure higher transparency about manufacturer agreements and costs of research and development [41], or how to increase the efficacy of the global collaboration to promote a more equitable distribution of the vaccines across the world, so that the rest of the world is facing uncertainty.
Another important area of focus for future research includes new developments in accurate and rapid methods aimed at detecting unknown respiratory pathogens. With specific regard to the SARS-CoV-2, several authors stress the necessity of clarifying essential aspects such as etiology, epidemiology, biology, pathogenesis, or pathological immune response. These aspects play a central role in successful vaccine development. The development of next-generation vaccine platforms, initially introduced for potential use in cancer therapy, has been fast-tracked by COVID-19. Future research might clarify how to use some of the platform technologies to combat seasonal or new strains of coronaviruses. The existence of some conservation between the coronaviruses is one of the important reasons why to continue research and product development to tackle any new version of coronavirus that might emerge in the future [42]. The next generation of ‘‘plug and play’’ vaccines utilizing mRNA, viral vector, or protein subunit technology paves the way for the development of new products which can play a primary role in reducing the impact of infectious diseases. With no doubt, a legacy of the COVID-19 pandemic refers to possibility of developing the new vaccines at a pace that before was not imaginable. Several types of barriers will need to be addressed to ensure global demand needs, such as legal barriers (including intellectual property transfer), lack of physical infrastructure, technical expertise, and supply chain capacity [122]. Future efforts should be made to identify viable strategies for the liberalization of intellectual property rules around the patent-protected technologies, for example adopting a Trade-Related Aspects of Intellectual Property Rights (TRIPS) waiver, which would help to make these technologies available at a global scale [123]. However, legal barriers encounter production barriers as well as technical barriers referring to technology transfer and regulatory capacity, for example the great imbalance represented by concentration of vaccine production knowledge in high-income countries (HIC) and the scarcity of qualified personnel in LMICs [122–124]. Progresses in information sharing of manufacturing know-how might bring broader positive effects across the industry: Sharing in the pandemic can be the catalyst for an “industry-wide move to a high-information, high-innovation state of manufacturing,” tackling more aggressively and directly the free-rider dilemma [124].
To summarize, future research efforts directed aimed at reducing inequities in vaccine distribution should address the following: (1) how to plan and coordinate the redistribution of vaccine surplus in HICs; (2) strategies to boost production where this is already possible thanks to the infrastructures; (3) improving the export of single vaccine components in countries lacking manufacturing capacity despite the presence of the so-called finish and fill capabilities. The African continent is one clear example: Due to incomplete COVID-19 manufacturing chains, the strategy is to use the active ingredient produced abroad and subsequently shipped to the finish and fill facilities; (4) how to facilitate technology transfer; (5) how to facilitate transfer of intellectual property rights; (6) strategies to support local manufacturers with programs to purchase vaccines locally; (7) implementing collaborative programs to ameliorate global biopharmaceutical regulatory capacity [123] and (8) effective ways of coordinating the global vaccine supply chain taking into account disease spread patterns and possible export barriers.
LMICs can also be supported by adopting and improving evidence-based health supply chain interventions. We have learnt some important lessons from the past at a global level, such as the success in achieving universal access to HIV treatment. Organizations such as Clinton Health Access Initiative (CHAI) and Global Fund to Fight AIDS, Tuberculosis and Malaria (Global Fund) identified some barriers for the effective distribution of antiretrovirals (ARVs) to LMICs. These barriers, which involved complex supply chains and elevated costs of procurement as barriers for effective distribution of ARVs to LMICs, have been successfully addressed with strategies that included pooled procurement arrangements, third-party price negotiation, and differential pricing [125]. In addition, they supported suppliers in targeting the best strategies to reduce manufacturing costs: This extensive combined effort resulted in LMIC capacity to access generic ARVs at lower prices [125]. Similarly, barriers have been identified and overcome at a national and subnational level. This approach can be a viable option to contrast the inequities in COVID-19 vaccination supply. To this aim, supply chains can be analyzed with an equity lens (for example reconsidering global regulations, policies, and context-specific strategies to optimize equity in healthcare delivery), and an implementation research approach can be adopted to find and test new solutions whose efficacy will be beneficial not only for COVID-19 but also for future threats. Many commentators advocate a more equitable distribution of COVID-19 vaccines as this would contribute to contain the pandemic sooner. Stronger efforts should be directed in this direction, as vaccine development is the first step. More research is needed at all levels to understand the best trade-offs, nationally as well as globally so that all individuals are vaccinated in a timely manner. A combined effort is expected to incorporate four essential dimensions in ensuring global access to COVID-19 vaccines: affordability, accessibility, trust, and efficiency in their utilization [122].
OR Models
Various models for vaccine prioritization have been already formulated. However, it is important to highlight the fact that vaccine prioritization and ethics are two concepts that cannot be separated, if we want to successfully resolve the problems connected to inequalities and disparities. Therefore, we think that more efforts should be devoted to include more elements when developing an age-stratified model, such as disparities in accessing healthcare, equity in allocation, or vaccine hesitancy. One possible equitable strategy hypothesized by Bubar et al. [2] consists in pairing serological testing with vaccination in the hardest-hit populations.
The research area of vaccine distribution is a fast-growing field, whose expansion has been certainly boosted by the COVID-19 pandemic. One promising direction for future research might be represented by further refining the integration of epidemiological models, such as the SEIR model, into optimization models that are combined with simulations, heuristics, or scenario analysis. For example, an extension of the SEIR model, widely adopted, is represented by the DELPHI model. As explained by Bertsimas et al. [95], the novelty of this model compared to the other COVID-19 forecasting models lies in its capacity to capture three elements of the pandemic: under-detection (cases remained undetected), governmental and societal response (such as non-pharmacological strategies to contrast the spread of the virus), and declining mortality rates. The utilization of the epidemiological model DELPHI can be further adopted and tested in light of a possible third dose of COVID-19 vaccine.
Given the complexity of the variables in place, we can hypothesize various improvements of the models proposed so far. For example, part of the objectives could be represented by (1) trying to prioritize allocation among patients with comorbidities and categories of workers at a higher risk, (2) trying to capture the interactions among individuals according to the population density of urban areas and according to the age of individuals, (3) capturing the effect of a possible second or third dose of vaccination and comparing the effect of different vaccine types.
The current pandemic is forcing us to somehow rethink OR models formulated for resource allocation, incorporating the elements of fairness and equity which have been largely ignored in the past. The efforts made in the past to formulate strategies for resource allocation led to models on epidemic control that, for the most part, analyzed the optimal solution without considering fairness. In our view, this is an important lesson learnt. Interestingly, the concept of fairness in resource allocation has been addressed with other applications, such as food allocation [126], kidney allocation [127], or healthcare resource allocation decision-making [128].
Conclusions
Achieving global COVID-19 vaccine coverage represents an unprecedented challenge and a test: It necessitates collaboration on a global scale and at multiple levels. On both a national and global scale, the expected efforts should address not only technical aspects such as investments, research and development, production, affordability, distribution, and allocation, but also mutual support and solidarity.
The fact that the topic spans across different disciplines may create a barrier for ORMS researchers to contribute to answering questions in this field. In an effort to induce the ORMS community to work on finding solutions to these challenges, in this paper we have reviewed the literature on COVID-19 vaccine allocation and distribution. We have conducted a bibliometric analysis of the literature that reveals five major research areas: vaccine development, clinical properties of the virus, complications relating to the respiratory system, vaccine allocation, and next generation of vaccines. We then focused on further synthesis of the research area on vaccine allocation as it is the most relevant to the ORMS community. To this end, we discussed the literature on equity in vaccine allocation and distribution and vaccine hesitancy and the use of OR models in this area. Finally, we propose research directions in each of those areas.
The challenge of fairness and equity remains a priority: Future efforts in the development of mathematical models to optimize distribution and allocation of COVID-19 vaccines should evaluate how to enforce constraints to tackle the problem of equity and to contrast uncertainties. These aspects are essential, to make sure that progresses in vaccine production and effectiveness can be sustained by consequential progresses in managing vaccine shortages.
While bibliometric analysis provides tools to aid in analyzing and synthesizing the literature, it has its limitations. One such limitation is that the analysis depends on the database used. This database could be limited in that it includes only works that meet certain standard requirements determined by the owner. The database could also be limited in that it is updated periodically and may not include all recent publications that could be significant when the topic of study is timely such as the COVID-19 pandemic. This is indeed an incredibly fast-growing field of study; for example, we have found that the dataset almost doubled in size (from 371 to 736 publications) in a 5-month period (from July 29, 2022 to December 2022). Another extension to the bibliometric analysis is to consider the robustness of our findings when using other algorithms for visualization or for ranking publications’ influence. For example, it will be interesting to test more recent centrality approached to the co-citation network such as the works of Everett and Borgatti [129], Vogiatzis et al. [130], and Angriman et al. [131].
Data availability
The authors confirm that there is no supplementary data or material associated with this work.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blundell R, Costa Dias M, Joyce R, Xu X. COVID-19 and inequalities. Fisc Stud. 2020;41(2):291–319. doi: 10.1111/1475-5890.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bubar KM, Reinholt K, Kissler SM, Lipsitch M, Cobey S, Grad YH, Larremore DB. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021;371(6532):916–921. doi: 10.1126/science.abe6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce T, Gudorf A, de Kat C, Muscat M, Butler R, Habersaat KB. Towards equity in immunisation Eurosurveillance. 2019;24(2):1800204. doi: 10.2807/1560-7917.ES.2019.24.2.1800204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sodha SV, Dietz V. Strengthening routine immunization systems to improve global vaccination coverage. Br Med Bull. 2015;113(1):5–14. doi: 10.1093/bmb/ldv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Europe (2014) European vaccine action plan 2015–2020 (2014). https://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/publications/2014/european-vaccine-action-plan-20152020-2014
- 6.World Health Organization . Global vaccine action plan 2011–2020. Geneva: World Health Organization; 2013. p. 2013. [Google Scholar]
- 7.MacDonald N, Mohsni E, Al-Mazrou Y, Andrus JK, Arora N, Elden S, Madrid M-Y, Martin R, Mustafa AM, Rees H. Global vaccine action plan lessons learned I: Recommendations for the next decade. Vaccine. 2020;38(33):5364–5371. doi: 10.1016/j.vaccine.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks A, Habimana D, Huckerby G. Making the leap into the next generation: a commentary on how Gavi, the Vaccine Alliance is supporting countries’ supply chain transformations in 2016–2020. Vaccine. 2017;35(17):2110–2114. doi: 10.1016/j.vaccine.2016.12.072. [DOI] [PubMed] [Google Scholar]
- 9.Favin M, Steinglass R, Fields R, Banerjee K, Sawhney M. Why children are not vaccinated: a review of the grey literature. Int Health. 2012;4(4):229–238. doi: 10.1016/j.inhe.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Mutua MK, Kimani-Murage E, Ettarh RR. Childhood vaccination in informal urban settlements in Nairobi, Kenya: who gets vaccinated? BMC Public Health. 2011;11(1):1–11. doi: 10.1186/1471-2458-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogbuanu IU, Li AJ, Anya BM, Tamadji M, Chirwa G, Chiwaya KW, Djalal ME-H, Cheikh D, Machekanyanga Z, Okeibunor J. Can vaccination coverage be improved by reducing missed opportunities for vaccination? Findings from assessments in Chad and Malawi using the new WHO methodology. PLoS ONE. 2019;14(1):e0210648. doi: 10.1371/journal.pone.0210648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose FD, Brooks BM, Rizzo AA. Virtual reality in brain damage rehabilitation. Cyberpsychol Behav. 2005;8(3):241–262. doi: 10.1089/cpb.2005.8.241. [DOI] [PubMed] [Google Scholar]
- 13.Sridhar S, Maleq N, Guillermet E, Colombini A, Gessner BD. A systematic literature review of missed opportunities for immunization in low-and middle-income countries. Vaccine. 2014;32(51):6870–6879. doi: 10.1016/j.vaccine.2014.10.063. [DOI] [PubMed] [Google Scholar]
- 14.Zameer M, Phillips-White N, Folorunso O, Belt R, Setayesh H, Asghar N, Chandio A (2020) Promoting equity in immunization coverage through supply chain design in Pakistan version 1; peer review: 1 approved, 3 approved with reservations, 1 not approved. Gates Open Res 4(31). 10.12688/gatesopenres.13121.1 [DOI] [PMC free article] [PubMed]
- 15.Mullard A (2020) How COVID vaccines are being divvied up around the world. Nature [DOI] [PubMed]
- 16.National Academies of Sciences, Engineering, and M (2020) Framework for equitable allocation of COVID-19 vaccine [PubMed]
- 17.Khamsi R. If a coronavirus vaccine arrives, can the world make enough. Nature. 2020;580(7805):578–580. doi: 10.1038/d41586-020-01063-8. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Morales R, Castillo-Chavez C. A note on the use of influenza vaccination strategies when supply is limited. Math Biosci Eng. 2011;8(1):171. doi: 10.3934/mbe.2011.8.171. [DOI] [PubMed] [Google Scholar]
- 19.Medlock J, Galvani AP. Optimizing influenza vaccine distribution. Science. 2009;325(5948):1705–1708. doi: 10.1126/science.1175570. [DOI] [PubMed] [Google Scholar]
- 20.Shim E. Prioritization of delayed vaccination for pandemic influenza. Mathematical Biosciences and Engineering: MBE. 2011;8(1):95. doi: 10.3934/mbe.2011.8.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shim E, Meyers LA, Galvani AP. Optimal H1N1 vaccination strategies based on self-interest versus group interest. BMC Public Health. 2011;11(1):1–17. doi: 10.1186/1471-2458-11-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toner E, Barnill A, Krubiner C, Bernstein J, Privor-Dumm L, Watson M (2020) Interim framework for COVID-19 vaccine allocation and distribution in the United States. Baltimore: Johns Hopkins Center for Health Security
- 23.Irwin A, Nkengasong J. What it will take to vaccinate the world against COVID-19. Nature. 2021;592(7853):176–178. doi: 10.1038/d41586-021-00727-3. [DOI] [PubMed] [Google Scholar]
- 24.Lee HL, Padmanabhan V, Whang S. Information distortion in a supply chain: the bullwhip effect. Manage Sci. 1997;43(4):546–558. doi: 10.1287/mnsc.43.4.546. [DOI] [Google Scholar]
- 25.World Health Organization (2021) Vaccines and Immunization. https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1
- 26.Pan American Health Organization (2021) Misinformation fueling vaccine hesitancy, PAHO Director says. https://www.paho.org/en/news/21-4-2021-misinformation-fueling-vaccine-hesitancy-paho-director-says
- 27.Hardeman A, Wong T, Denson JL, Postelnicu R, Rojas JC. Evaluation of health equity in COVID-19 vaccine distribution plans in the United States. JAMA Netw Open. 2021;4(7):e2115653–e2115653. doi: 10.1001/jamanetworkopen.2021.15653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowley J, Slack F (2004) Conducting a literature review. Management Research News
- 29.Persson O, Danell R, Schneider JW (2009) How to use Bibexcel for various types of bibliometric analysis. Celebrating Scholarly Communication Studies: A Festschrift for Olle Persson at His 60th Birthday 5:9–24
- 30.Bastian M, Heymann S, Jacomy M (2009) Gephi: an open source software for exploring and manipulating networks. Third International AAAI Conference on Weblogs and Social Media
- 31.Oviedo-García M (2021) Journal citation reports and the definition of a predatory journal: the case of the Multidisciplinary Digital Publishing Institute (MDPI). Res Eval
- 32.Martonosi SE, Behzad B, Cummings K. Pricing the COVID-19 vaccine: a mathematical approach. Omega. 2021;103:102451. doi: 10.1016/j.omega.2021.102451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavana M, Govindan K, Nasr AK, Heidary MS, Mina H (2021) A mathematical programming approach for equitable COVID-19 vaccine distribution in developing countries. Ann Oper Res 1–34 [DOI] [PMC free article] [PubMed]
- 34.Chen C, Ibekwe-SanJuan F, Hou J. The structure and dynamics of cocitation clusters: a multiple-perspective cocitation analysis. J Am Soc Inform Sci Technol. 2010;61(7):1386–1409. doi: 10.1002/asi.21309. [DOI] [Google Scholar]
- 35.Pilkington A, Meredith J. The evolution of the intellectual structure of operations management—1980–2006: a citation/co-citation analysis. J Oper Manag. 2009;27(3):185–202. doi: 10.1016/j.jom.2008.08.001. [DOI] [Google Scholar]
- 36.Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech: Theory Exp. 2008;2008(10):P10008. doi: 10.1088/1742-5468/2008/10/P10008. [DOI] [Google Scholar]
- 37.Jacomy M, Venturini T, Heymann S, Bastian M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS ONE. 2014;9(6):e98679. doi: 10.1371/journal.pone.0098679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra D, Gunasekaran A, Papadopoulos T, Childe SJ. Big Data and supply chain management: a review and bibliometric analysis. Ann Oper Res. 2018;270(1):313–336. doi: 10.1007/s10479-016-2236-y. [DOI] [Google Scholar]
- 39.Brin S, Page L. The anatomy of a large-scale hypertextual web search engine. Computer Networks and ISDN Systems. 1998;30(1–7):107–117. doi: 10.1016/S0169-7552(98)00110-X. [DOI] [Google Scholar]
- 40.Williams JH, Dawson A. Prioritising access to pandemic influenza vaccine: a review of the ethics literature. BMC Med Ethics. 2020;21:1–8. doi: 10.1186/s12910-020-00477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.So AD, Woo J (2020) Reserving coronavirus disease 2019 vaccines for global access: cross sectional analysis. BMJ 371 [DOI] [PMC free article] [PubMed]
- 42.Shin MD, Shukla S, Chung YH, Beiss V, Chan SK, Ortega-Rivera OA, Wirth DM, Chen A, Sack M, Pokorski JK. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020;15(8):646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization (2014) Report of The SAGE Working Group on Vaccine Hesitancy. https://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf
- 44.Bolcato M, Rodriguez D, Feola A, Di Mizio G, Bonsignore A, Ciliberti R, Tettamanti C, Trabucco Aurilio M, Aprile A. COVID-19 pandemic and equal access to vaccines. Vaccines. 2021;9(6):538. doi: 10.3390/vaccines9060538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aborode AT, Olofinsao OA, Osmond E, Batubo AP, Fayemiro O, Sherifdeen O, Muraina L, Obadawo B, Ahmad S, Fajemisin EA (2021) Equal access of COVID‐19 vaccine distribution in Africa: challenges and way forward. J Med Virol [DOI] [PMC free article] [PubMed]
- 46.Šehović AB, Govender K (2021) Addressing COVID-19 vulnerabilities: how do we achieve global health security in an inequitable world. Global Pub Health 1–11 [DOI] [PubMed]
- 47.World Health Organization (2008) International health regulations (2005). World Health Organization.
- 48.Brown CC, Young SG, Pro GC (2021) COVID-19 vaccination rates vary by community vulnerability: a county-level analysis. Vaccine [DOI] [PMC free article] [PubMed]
- 49.Whitehead J, Scott N, Carr PA, Lawrenson R. Will access to COVID-19 vaccine in Aotearoa be equitable for priority populations? The New Zealand Medical Journal (Online) 2021;134(1535):25–34. [PubMed] [Google Scholar]
- 50.Ismail SJ, Tunis MC, Zhao L, Quach C. Navigating inequities: a roadmap out of the pandemic. BMJ Glob Health. 2021;6(1):e004087. doi: 10.1136/bmjgh-2020-004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frisch MF, Scott KW, Binagwaho A (2021) An implementation research approach to re-orient health supply chains toward an equity agenda in the COVID-19 era. Ann Global Health 87(1). [DOI] [PMC free article] [PubMed]
- 52.Duijzer LE, van Jaarsveld W, Dekker R. Literature review: the vaccine supply chain. Eur J Oper Res. 2018;268(1):174–192. doi: 10.1016/j.ejor.2018.01.015. [DOI] [Google Scholar]
- 53.Yi M, Marathe A. Fairness versus efficiency of vaccine allocation strategies. Value in Health. 2015;18(2):278–283. doi: 10.1016/j.jval.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffin J. Some problems of fairness. Ethics. 1985;96(1):100–118. doi: 10.1086/292722. [DOI] [Google Scholar]
- 55.Kaplan EH, Merson MH. Allocating HIV-prevention resources: balancing efficiency and equity. Am J Public Health. 2002;92(12):1905–1907. doi: 10.2105/AJPH.92.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mylius SD, Hagenaars TJ, Lugnér AK, Wallinga J. Optimal allocation of pandemic influenza vaccine depends on age, risk and timing. Vaccine. 2008;26(29–30):3742–3749. doi: 10.1016/j.vaccine.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 57.Tuite AR, Fisman DN, Kwong JC, Greer AL. Optimal pandemic influenza vaccine allocation strategies for the Canadian population. PLoS ONE. 2010;5(5):e10520. doi: 10.1371/journal.pone.0010520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matrajt L, Longini IM., Jr Optimizing vaccine allocation at different points in time during an epidemic. PLoS ONE. 2010;5(11):e13767. doi: 10.1371/journal.pone.0013767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brauer F (2008) Compartmental models in epidemiology. In Mathematical epidemiology (pp. 19–79). Springer
- 60.Chang H-J, Chuang J-H, Chern T-C, Stein M, Coker R, Wang D-W, Hsu T (2014) A comparison between a deterministic, compartmental model and an individual based-stochastic model for simulating the transmission dynamics of pandemic influenza. 2014 4th International Conference On Simulation And Modeling Methodologies, Technol Appl (SIMULTECH), 586–594
- 61.Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, Pearson CAB, Russell TW, Tully DC, Washburne AD (2021) Estimated transmissibility and impact of SARS-CoV-2 lineage B. 1.1. 7 in England Sci 372(6538) [DOI] [PMC free article] [PubMed]
- 62.Della Rossa F, Salzano D, Di Meglio A, De Lellis F, Coraggio M, Calabrese C, Guarino A, Cardona-Rivera R, De Lellis P, Liuzza D. A network model of Italy shows that intermittent regional strategies can alleviate the COVID-19 epidemic. Nat Commun. 2020;11(1):1–9. doi: 10.1038/s41467-020-18827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dolbeault J, Turinici G. Heterogeneous social interactions and the COVID-19 lockdown outcome in a multi-group SEIR model. Mathematical Modelling of Natural Phenomena. 2020;15:36. doi: 10.1051/mmnp/2020025. [DOI] [Google Scholar]
- 64.Gatto M, Bertuzzo E, Mari L, Miccoli S, Carraro L, Casagrandi R, Rinaldo A. Spread and dynamics of the COVID-19 epidemic in Italy: effects of emergency containment measures. Proc Natl Acad Sci. 2020;117(19):10484–10491. doi: 10.1073/pnas.2004978117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giordano G, Blanchini F, Bruno R, Colaneri P, Di Filippo A, Di Matteo A, Colaneri M. Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy. Nat Med. 2020;26(6):855–860. doi: 10.1038/s41591-020-0883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kucharski AJ, Russell TW, Diamond C, Liu Y, Edmunds J, Funk S, Eggo RM (2020) Centre for Mathematical Modelling of Infectious Diseases COVID-19 working group. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis 20(5):553–558 [DOI] [PMC free article] [PubMed]
- 67.Ngonghala CN, Iboi E, Eikenberry S, Scotch M, MacIntyre CR, Bonds MH, Gumel AB. Mathematical assessment of the impact of non-pharmaceutical interventions on curtailing the 2019 novel Coronavirus. Math Biosci. 2020;325:108364. doi: 10.1016/j.mbs.2020.108364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buckner JH, Chowell G, Springborn MR (2020) Optimal dynamic prioritization of scarce COVID-19 vaccines. Med Rxiv [DOI] [PMC free article] [PubMed]
- 69.Choi W, Shim E. Optimal strategies for vaccination and social distancing in a game-theoretic epidemiologic model. J Theor Biol. 2020;505:110422. doi: 10.1016/j.jtbi.2020.110422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng J, Tang S, Shu H. Joint impacts of media, vaccination and treatment on an epidemic Filippov model with application to COVID-19. J Theor Biol. 2021;523:110698. doi: 10.1016/j.jtbi.2021.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mukandavire Z, Nyabadza F, Malunguza NJ, Cuadros DF, Shiri T, Musuka G. Quantifying early COVID-19 outbreak transmission in South Africa and exploring vaccine efficacy scenarios. PLoS ONE. 2020;15(7):e0236003. doi: 10.1371/journal.pone.0236003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meehan MT, Cocks DG, Caldwell JM, Trauer JM, Adekunle AI, Ragonnet RR, McBryde ES (2020) Age-targeted dose allocation can halve COVID-19 vaccine requirements. Med Rxiv
- 73.Shim E. Optimal allocation of the limited COVID-19 vaccine supply in South Korea. J Clin Med. 2021;10(4):591. doi: 10.3390/jcm10040591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foy BH, Wahl B, Mehta K, Shet A, Menon GI, Britto C. Comparing COVID-19 vaccine allocation strategies in India: a mathematical modelling study. Int J Infect Dis. 2021;103:431–438. doi: 10.1016/j.ijid.2020.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen X, Li M, Simchi-Levi D, Zhao T (2020) Allocation of COVID-19 vaccines under limited supply. Available at SSRN 3678986
- 76.Matrajt L, Eaton J, Leung T, Brown ER (2021) Vaccine optimization for COVID-19: who to vaccinate first? Sci Adv 7(6):eabf1374. 10.1126/sciadv.abf1374 [DOI] [PMC free article] [PubMed]
- 77.Matrajt L, Eaton J, Leung T, Dimitrov D, Schiffer JT, Swan DA, Janes H. Optimizing vaccine allocation for COVID-19 vaccines shows the potential role of single-dose vaccination. Nat Commun. 2021;12(1):1–18. doi: 10.1038/s41467-021-23761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Babus A, Das S, Lee S (2020) The optimal allocation of Covid-19 vaccines. MedRxiv [DOI] [PMC free article] [PubMed]
- 79.Mahase E (2021) Covid-19: What new variants are emerging and how are they being investigated? British Medical Journal Publishing Group [DOI] [PubMed]
- 80.van Oosterhout C, Hall N, Ly H, Tyler KM. COVID-19 evolution during the pandemic–Implications of new SARS-CoV-2 variants on disease control and public health policies. Taylor & Francis; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L, Hinsley WR, Laydon DJ, Dabrera G, O’Toole Á (2021) Transmission of SARS-CoV-2 lineage B. 1.1. 7 in England: insights from linking epidemiological and genetic data. MedRxiv, 2012–2020
- 82.Tuite AR, Zhu L, Fisman DN, Salomon JA (2021) Alternative dose allocation strategies to increase benefits from constrained COVID-19 vaccine supply. Annals of Internal Medicine [DOI] [PMC free article] [PubMed]
- 83.Moghadas SM, Vilches TN, Zhang K, Nourbakhsh S, Sah P, Fitzpatrick MC, Galvani AP. Evaluation of COVID-19 vaccination strategies with a delayed second dose. PLoS Biol. 2021;19(4):e3001211. doi: 10.1371/journal.pbio.3001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Becker NG, Starczak DN. Optimal vaccination strategies for a community of households. Math Biosci. 1997;139(2):117–132. doi: 10.1016/S0025-5564(96)00139-3. [DOI] [PubMed] [Google Scholar]
- 85.Enayati S, Özaltın OY. Optimal influenza vaccine distribution with equity. Eur J Oper Res. 2020;283(2):714–725. doi: 10.1016/j.ejor.2019.11.025. [DOI] [Google Scholar]
- 86.Tanner MW, Sattenspiel L, Ntaimo L. Finding optimal vaccination strategies under parameter uncertainty using stochastic programming. Math Biosci. 2008;215(2):144–151. doi: 10.1016/j.mbs.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 87.Buhat CAH, Lutero DSM, Olave YH, Quindala KM, Recreo MGP, Talabis DASJ, Torres MC, Tubay JM, Rabajante JF. Using constrained optimization for the allocation of COVID-19 vaccines in the Philippines. Appl Health Econ Health Policy. 2021;19(5):699–708. doi: 10.1007/s40258-021-00667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cabanilla KIM, Enriquez EAT, Mendoza R, Mendoza VMP (2021) Optimal selection of COVID-19 vaccination sites at the municipal level. MedRxiv [DOI] [PMC free article] [PubMed]
- 89.Çakır E, Taş MA, Ulukan Z (2021) Spherical bipolar fuzzy weighted multi-facility location modeling for mobile COVID-19 vaccination clinics. Journal of Intelligent & Fuzzy Systems, Preprint 1–14
- 90.Emu M, Chandrasekaran D, Mago V, Choudhury S (2021) Validating optimal COVID-19 vaccine distribution models. International Conference on Computational Science 352–366
- 91.Leithaeuser N, Schneider J, Johann S, Krumke S, Streicher M, Schmidt E, Scholz S (2020) Quantifying Covid19-vaccine location strategies for Germany. MedRxiv [DOI] [PMC free article] [PubMed]
- 92.Minoza JMA, Bongolan VP, Rayo JF (2021) COVID-19 agent-based model with multi-objective optimization for vaccine distribution. ArXiv Preprint 10.48550/arXiv.2101.11400
- 93.Yin X, Büyüktahtakın İE (2021) A multi-stage stochastic programming approach to epidemic resource allocation with equity considerations. Health Care Manag Sci 1–26 [DOI] [PMC free article] [PubMed]
- 94.Büyüktahtakın İE, des-Bordes E, Kıbış EY (2018) A new epidemics–logistics model: insights into controlling the Ebola virus disease in West Africa. Eur J Oper Res 265(3):1046–1063
- 95.Bertsimas D, Digalakis V, Jacquillat A, Li ML, Previero A (2021) Where to locate COVID-19 mass vaccination facilities? Nav Res Logist 1–34. 10.1002/nav.22007 [DOI] [PMC free article] [PubMed]
- 96.Rastegar M, Tavana M, Meraj A, Mina H. An inventory-location optimization model for equitable influenza vaccine distribution in developing countries during the COVID-19 pandemic. Vaccine. 2021;39(3):495–504. doi: 10.1016/j.vaccine.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roy S, Dutta R, Ghosh P. Optimal time-varying vaccine allocation amid pandemics with uncertain immunity ratios. IEEE Access. 2021;9:15110–15121. doi: 10.1109/ACCESS.2021.3053268. [DOI] [Google Scholar]
- 98.Adel Ali K, Pastore Celentano L. Addressing vaccine hesitancy in the “post-truth” era. Eurohealth. 2017;23(4):16–20. [Google Scholar]
- 99.Dictionaries Oxford (2016) Post-truth. https://languages.oup.com/word-of-the-year/2016/
- 100.Dubé E, Gagnon D, Ouakki M, Bettinger JA, Guay M, Halperin S, Wilson K, Graham J, Witteman HO, MacDonald S. Understanding vaccine hesitancy in Canada: results of a consultation study by the Canadian Immunization Research Network. PLoS ONE. 2016;11(6):e0156118. doi: 10.1371/journal.pone.0156118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 102.Betsch C, Schmid P, Heinemeier D, Korn L, Holtmann C, Böhm R. Beyond confidence: development of a measure assessing the 5C psychological antecedents of vaccination. PLoS ONE. 2018;13(12):e0208601. doi: 10.1371/journal.pone.0208601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wiysonge CS, Ndwandwe D, Ryan J, Jaca A, Batouré O, Anya B-PM, Cooper S (2021) Vaccine hesitancy in the era of COVID-19: could lessons from the past help in divining the future? Human Vaccines & Immunotherapeutics 1–3 [DOI] [PMC free article] [PubMed]
- 104.Böhm R, Betsch C, Korn L. Selfish-rational non-vaccination: experimental evidence from an interactive vaccination game. J Econ Behav Organ. 2016;131:183–195. doi: 10.1016/j.jebo.2015.11.008. [DOI] [Google Scholar]
- 105.Bauch CT. Imitation dynamics predict vaccinating behaviour. Proceedings of the Royal Society B: Biological Sciences. 2005;272(1573):1669–1675. doi: 10.1098/rspb.2005.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bauch CT, Bhattacharyya S, Ball RF. Rapid emergence of free-riding behavior in new pediatric immunization programs. PLoS ONE. 2010;5(9):e12594. doi: 10.1371/journal.pone.0012594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bauch CT, Earn DJD. Vaccination and the theory of games. Proc Natl Acad Sci. 2004;101(36):13391–13394. doi: 10.1073/pnas.0403823101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fu F, Rosenbloom DI, Wang L, Nowak MA. Imitation dynamics of vaccination behaviour on social networks. Proceedings of the Royal Society B: Biological Sciences. 2011;278(1702):42–49. doi: 10.1098/rspb.2010.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Galvani AP, Reluga TC, Chapman GB. Long-standing influenza vaccination policy is in accord with individual self-interest but not with the utilitarian optimum. Proc Natl Acad Sci. 2007;104(13):5692–5697. doi: 10.1073/pnas.0606774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Perisic A, Bauch CT. Social contact networks and disease eradicability under voluntary vaccination. PLoS Comput Biol. 2009;5(2):e1000280. doi: 10.1371/journal.pcbi.1000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vardavas R, Breban R, Blower S. Can influenza epidemics be prevented by voluntary vaccination? PLoS Comput Biol. 2007;3(5):e85. doi: 10.1371/journal.pcbi.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abdool Karim SS, de Oliveira T. New SARS-CoV-2 variants—clinical, public health, and vaccine implications. N Engl J Med. 2021;384(19):1866–1868. doi: 10.1056/NEJMc2100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Machingaidze S, Wiysonge CS (2021) Understanding COVID-19 vaccine hesitancy. Nat Med 1–2 [DOI] [PubMed]
- 114.Piraveenan M, Sawleshwarkar S, Walsh M, Zablotska I, Bhattacharyya S, Farooqui HH, Bhatnagar T, Karan A, Murhekar M, Zodpey S. Optimal governance and implementation of vaccination programmes to contain the COVID-19 pandemic. Royal Society Open Science. 2021;8(6):210429. doi: 10.1098/rsos.210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krishnan GS, Kamath SS, Sugumaran V (2021) Predicting vaccine hesitancy and vaccine sentiment using topic modeling and evolutionary optimization. International Conference on Applications of Natural Language to Information Systems, 255–263
- 116.Stoddard M, Van Egeren D, Johnson KE, Rao S, Furgeson J, White DE, Nolan RP, Hochberg N, Chakravarty A. Individually optimal choices can be collectively disastrous in COVID-19 disease control. BMC Public Health. 2021;21(1):1–12. doi: 10.1186/s12889-021-10829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guo F, Cao E. Can reference points explain vaccine hesitancy? A new perspective on their formation and updating. Omega. 2021;99:102179. doi: 10.1016/j.omega.2019.102179. [DOI] [Google Scholar]
- 118.Dubé E, Gagnon D, MacDonald NE. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33(34):4191–4203. doi: 10.1016/j.vaccine.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 119.Bloom BR, Marcuse E, Mnookin S (2014) Addressing vaccine hesitancy. Am Assoc Adv Sci [DOI] [PubMed]
- 120.Nyhan B, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133(4):e835–e842. doi: 10.1542/peds.2013-2365. [DOI] [PubMed] [Google Scholar]
- 121.Griebel L, Enwald H, Gilstad H, Pohl A-L, Moreland J, Sedlmayr M. eHealth literacy research—Quo vadis? Inform Health Soc Care. 2018;43(4):427–442. doi: 10.1080/17538157.2017.1364247. [DOI] [PubMed] [Google Scholar]
- 122.Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, Jit M (2021) Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. The Lancet [DOI] [PMC free article] [PubMed]
- 123.Asundi A, O’Leary C, Bhadelia N. Global COVID-19 vaccine inequity: the scope, the impact, and the challenges. Cell Host Microbe. 2021;29(7):1036–1039. doi: 10.1016/j.chom.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Price WN, Rai AK, Minssen T. Knowledge transfer for large-scale vaccine manufacturing. Science. 2020;369(6506):912–914. doi: 10.1126/science.abc9588. [DOI] [PubMed] [Google Scholar]
- 125.Waning B, Kaplan W, King AC, Lawrence DA, Leufkens HG, Fox MP. Global strategies to reduce the price of antiretroviral medicines: evidence from transactional databases. Bull World Health Organ. 2009;87:520–528. doi: 10.2471/BLT.08.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Orgut IS, Ivy J, Uzsoy R, Wilson JR. Modeling for the equitable and effective distribution of donated food under capacity constraints. IIE Trans. 2016;48(3):252–266. doi: 10.1080/0740817X.2015.1063792. [DOI] [Google Scholar]
- 127.Davis AE, Mehrotra S, Friedewald JJ, Daskin MS, Skaro AI, Abecassis MM, Ladner DP. Improving geographic equity in kidney transplantation using alternative kidney sharing and optimization modeling. Med Decis Making. 2015;35(6):797–807. doi: 10.1177/0272989X14557696. [DOI] [PubMed] [Google Scholar]
- 128.Lane H, Sarkies M, Martin J, Haines T. Equity in healthcare resource allocation decision making: a systematic review. Soc Sci Med. 2017;175:11–27. doi: 10.1016/j.socscimed.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 129.Everett MG, Borgatti SP. The centrality of groups and classes. The Journal of Mathematical Sociology. 1999;23(3):181–201. doi: 10.1080/0022250X.1999.9990219. [DOI] [Google Scholar]
- 130.Vogiatzis C, Veremyev A, Pasiliao EL, Pardalos PM. An integer programming approach for finding the most and the least central cliques. Optimization Letters. 2015;9(4):615–633. doi: 10.1007/s11590-014-0782-2. [DOI] [Google Scholar]
- 131.Angriman E, van der Grinten A, Bojchevski A, Zügner D, Günnemann S, Meyerhenke H. Group centrality maximization for large-scale graphs. Proceedings of the Twenty-Second Workshop on Algorithm Engineering and Experiments (ALENEX) 2020;2020:56–69. doi: 10.1137/1.9781611976007.5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that there is no supplementary data or material associated with this work.