Abstract
Introduction
We explored whether visceral fat accumulation mediates the development of hepatic steatosis in individuals living with overweight and obesity.
Methods
This cross-sectional study enrolled 769 outpatients with overweight and obesity aged 18–65 years. The controlled attenuation parameter (CAP) was used to quantify the degree of hepatic steatosis. Visceral fat accumulation, represented by the visceral fat area (VFA), was measured using magnetic resonance imaging. The associations of body mass index (BMI), VFA, and CAP with each other were assessed by univariate analysis, multivariate linear regression, and mediation analysis, respectively.
Results
Compared with women, male subjects had higher BMI, VFA, and CAP levels. In both sex, CAP was positively correlated with BMI and VFA by the univariate analysis. After adjusting for demographic and serum characteristics, the linear correlation coefficients between BMI and CAP were 1.738 (95% confidence interval (CI): 1.100, 2.377), 1.524 (95% CI: 0.798, 2.249), and 2.650 (95% CI: 1.292, 4.009) in all subjects, females, and males, respectively, while those between VFA and CAP were 0.190 (95% CI: 0.133, 0.247), 0.184 (95% CI: 0.117, 0.252), and 0.194 (95% CI: 0.086, 0.301). Mediation analysis showed that visceral fat accumulation contributed to 51.37%, 53.85%, and 26.51% of obesity-induced hepatic steatosis in the total, female, and male subjects, respectively.
Conclusion
Visceral fat accumulation partially mediates obesity-induced hepatic steatosis in individuals with overweight and obesity, especially in women. More focus on visceral fat reduction is needed in individuals with obesity.
Keywords: Hepatic steatosis, Controlled attenuation parameter, Visceral fat area, Mediation analysis, Obesity
Introduction
Hepatic steatosis is the marked accumulation of hepatic fat. Nonalcoholic fatty liver disease (NAFLD) is defined as the presence of ≥5% hepatic steatosis in the absence of secondary causes of hepatic fat accumulation. Over the past few decades, the prevalence of excess hepatic fat has increased gradually. Currently, the pooled overall global prevalence of NAFLD was estimated to be 25.24% [1]; it is reported to reach even 55% among people with diabetes [2]. Except for few important NAFLD genetically associated fatty liver [2], most of the NAFLD caused by an unhealthy lifestyle was thought to be benign and related to metabolic syndrome and insulin resistance [3]. However, many studies have linked hepatic steatosis to obesity [4, 5, 6], and its prevalence rate reaches up to 80% in extremely obese individuals [5]. There is evidence that obesity increases the risk of developing liver diseases, particularly NAFLD [6], cirrhosis, and liver cancer [7].
Liver biopsy remains the “gold standard” for diagnosing NAFLD. However, its invasiveness, poor reproducibility, and potential complications make it unsuitable for widespread clinical application. Controlled attenuation parameter (CAP) is calculated from transient elastography, which is an easy, rapid, noninvasive method for assessing the degree of hepatic steatosis [8, 9].
As it is well-known, obesity diagnosed by the body mass index (BMI), either metabolically healthy obesity or metabolically unhealthy obesity [10, 11], is accompanied by the accumulation of visceral adipose tissue (VAT). One way to assess the accumulation of VAT is to measure the visceral fat area (VFA) by magnetic resonance imaging (MRI) or computed tomography [12, 13]. Recently, Choi et al. [13] showed that the VFA quantified by computed tomography was significantly correlated with the degree of hepatic steatosis measured by MRI in a small sample of 95 subjects. Moreover, it has now been well established that lower body fat mass, independently of visceral fat mass, contributes to fat accumulation in the liver [14].
Although numerous studies have explored the relationships among obesity, VAT, and NAFLD [4, 5, 6, 15, 16, 17], previous studies have not addressed whether VAT accumulation contributes to the association between obesity and hepatic steatosis. Therefore, we hypothesized that a BMI → visceral adipose accumulation → hepatic steatosis pathway is present in individuals with overweight and obesity. In this study, we examined the association with multiple linear regression and mediation analyses.
Materials and Methods
Subjects
From April 2020 to February 2022, a total of 769 outpatients with overweight and obesity from the obesity clinic in the Endocrinology and Metabolism Department of Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine were consecutively observed in this cross-sectional study. All subjects signed written informed consent and the subjects' anonymity was maintained. The Ethics Committee of Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine approved the study protocol in accordance with the Helsinki Declaration.
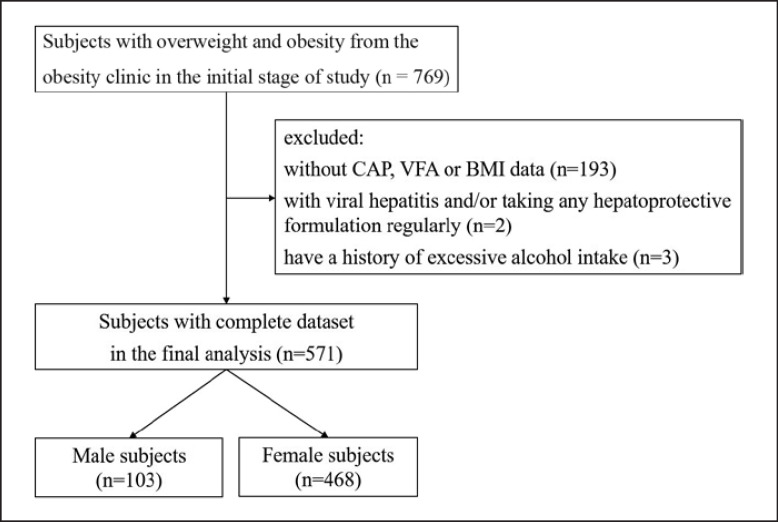
These subjects were excluded: those without CAP, VFA, or BMI data; those with a history of chronic hepatitis B and/or chronic hepatitis C; those with a history of excessive alcohol intake (alcohol intake >30 g daily in males and >20 g daily in females [18]); those who take any hepatoprotective formulation regularly, such as polyene phosphatidylcholine and bicyclol; severe chronic diseases, such as chronic obstructive pulmonary disease, cancer, cardiovascular disease, rheumatic arthritis, type 1 diabetes et al. Finally, 571 subjects were included in the analysis. The flow chart of enrolled subjects is showed in Figure 1.
Fig. 1.
Flow chart of the enrolled subjects.
Anthropometric and Laboratory Assessments
All subjects were interviewed by specialized physicians to obtain their medical history, medication history, as well as alcohol consumption. Then, anthropometric measurement was done including blood pressure, height, weight, waist circumference (WC), and hip circumference (HC) while only in light clothes and barefoot. Blood pressure was measured twice by a trained physician with a mercury sphygmomanometer after the subject had rested for at least 10 min, and the average was calculated and used for statistical analyses. BMI was calculated as weight divided by height squared (kg/m2). Overweight was defined as BMI 24–27.9 kg/m2, and obesity was defined as BMI ≥28 kg/m2 based on the definitions proposed by the Chinese Working Group on Obesity [19].
The blood sample was collected after overnight fasting from an antecubital vein in all subjects. General serum biochemical and routine blood examination were performed including: fasting plasma glucose (FPG), fasting plasma insulin (FINS), glycosylated hemoglobin A1c (HbA1c), alanine aminotransferase (ALT), aspartate aminotransferase, γ-glutamyl-transferase, alkaline phosphatase, blood urea nitrogen, serum creatinine (SCr), serum uric acid (SUA), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), free fatty acids (FFAs), white blood cell count, and C-reactive protein (CRP). Insulin sensitivity was estimated by homeostasis model assessment for insulin resistance (HOMA-IR) based on FPG and FINS measurements as follows: HOMA-IR = FINS (mU/L) × FPG (mmol/L)/22.5 [20].
Visceral Fat Area
VFA was quantified using the Achieva 3.0T MRI system (Philips Healthcare, Eindhoven, The Netherlands). All subjects were examined in the supine position by a medically trained technician to select one 10-mm slice at the L3 level with good contrast for the VFA measurements using the sliceOmatic 5.0 software (TomoVision, Magog, Canada). As previously reported, the software calculates the areas of different tissues and expresses the measurements in cm2 [21].
Controlled Attenuation Parameter
The CAP, in dB/m was captured by using the FibroScan® (Echosens, Paris, France) equipped with X- or XL-probes to assess hepatic steatosis. Details of this examination have been described in previous publications [22]. Briefly, all subjects were asked to fast at least 6 h before the examination and then placed in the supine position with their right arm maximally abducted. Measurements were performed by scanning the right liver lobe through an intercostal space. The goal was to capture ten successful acquisitions from each subject. Trained operators carried out the transient elastography, and they were blinded to all data and subject diagnoses. The final CAP score (dB/m) was the median of individual measurements. The quartile spacing of CAP is less than 40 dB/m.
Statistical Analysis
Data analysis was performed using SPSS 25.0 statistical software (SPSS Inc). The quantitative data were expressed as means ± standard deviation or as medians (interquartile range). The independent-samples t-test was used to compare normally distributed data. The χ2 test was used to compare categorical variables. The Mann-Whitney U test was used to compare non-normally distributed continuous variables. Associations among CAP, VFA, and BMI were computed by bivariate correlation analyses. Moreover, we used univariate analysis to screen for significant risk factors. Variables with p < 0.2 in univariate analysis were selected and entered into the final regression model. Finally, we evaluated the direct effects of BMI, VFA, and CAP, while controlling for other factors using multiple linear regression analysis.
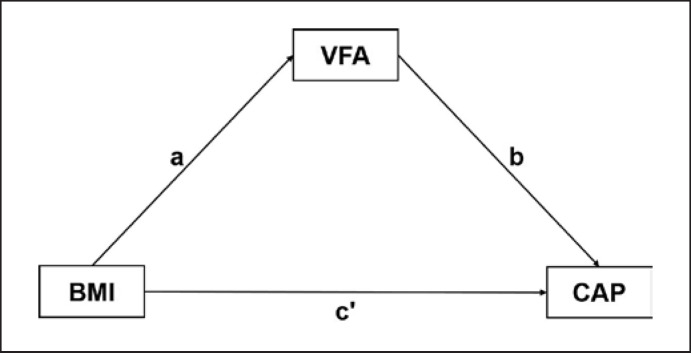
We constructed a causal pathway model: BMI→VFA→CAP (shown in Fig. 2) and conducted the mediating effect analysis using Process v3.4.1 by Andrew F. Hayes. In the mediation model, BMI was an independent variable, CAP was our concerned outcome, and VFA was the potential mediator. The “total effect” consisted of a “direct effect” of BMI on CAP (not mediated by VFA), and an “indirect effect” was completely or partly mediated by VFA. When the total effect, direct effect, and indirect effect were all significant (p(a), p(b), and p (c') < 0.05), VFA was thought to play a partial mediating effect on the relationship between BMI and CAP. A 2-sided p < 0.05 was considered statistically significant.
Fig. 2.
Mediation model for the association between BMI and CAP with VFA as a mediator. Pathway a and pathway b represented the indirect effect. Pathway c' represented the direct effect. BMI, body mass index; VAF, visceral fat area; CAP, controlled attenuation parameter.
Results
Characteristics of the Enrolled Patients
A total of 571 subjects with overweight/obesity were involved in this study, including 103 (18%) males and 468 (82%) females. Table 1 showed the characteristics between male and female subjects. Compared with the female subjects, the male ones had higher BMI, WC, HC, SBP, DBP, FPG, FINS, FCP, HbA1c, HOMA-IR, TG, ALT, AST, SCr, SUA, VFA, and CAP but lower HDL-C (all p < 0.05, Table 1). Age, WBC, CRP, TC, LDL, and FFA did not differ between sex subgroups (all p > 0.05, Table 1).
Table 1.
Comparison of characteristics between male and female subjects
| Variables | Male (n = 103) | Female (n = 468) | p value |
|---|---|---|---|
| Age, years | 32.4±8.1 | 32.0±7.3 | 0.626 |
| SBP, mm Hg | 144.6±18.0 | 131.1±16.1 | <0.001 |
| DBP, mm Hg | 91.9±13.0 | 85.5±11.1 | <0.001 |
| BMI, kg/m2 | 39.7±6.5 | 36.8±6.0 | <0.001 |
| WC, cm | 123.2±14.9 | 111.2±13.9 | <0.001 |
| HC, cm | 117.9±12.0 | 114.4±11.2 | 0.005 |
| WBCC, X109 | 9.2±9.1 | 8.2±3.5 | 0.084 |
| CRP, mg/L* | 4.1 (2.2, 7.0) | 4.6 (2.2, 7.8) | 0.249 |
| FPG, mmol/L | 6.9±2.9 | 6.3±2.5 | 0.049 |
| FINS, pU/mL* | 119.6 (61.1,201.3) | 85.0 (49.1,85.0) | <0.001 |
| FCP, ng/mL* | 4.8 (3.8, 6.0) | 3.8 (3.1, 4.9) | <0.001 |
| HbA1c, % | 6.5±1.5 | 6.2±1.5 | 0.039 |
| HOMA-IR* | 10.6 (6.3, 14.7) | 6.7 (4.7, 11.4) | <0.001 |
| TC, mmol/L | 5.3±1.1 | 5.3±1.1 | 0.800 |
| TG, mmol/L | 3.2±4.3 | 2.1±3.3 | 0.012 |
| HDL-C, mmol/L | 1.1±0.3 | 1.2±0.3 | <0.001 |
| LDL-C, mmol/L | 3.2±0.6 | 3.3±0.8 | 0.238 |
| ALT, U/L* | 63.5 (38, 98) | 37 (23, 37) | <0.001 |
| AST, U/L* | 32 (24, 52) | 23 (18, 23) | <0.001 |
| γ-GT, U/L* | 53 (41,74) | 33 (21,33) | <0.001 |
| ALP, U/L* | 80.5 (68, 95) | 74 (63, 74) | 0.003 |
| BUN, mmol/L | 5.3±1.3 | 4.8±1.2 | <0.001 |
| SCr, μmol/L | 78.5±13.4 | 58.8±9.8 | <0.001 |
| SUA, μmol/L | 476.6±95.9 | 391.4±81.8 | <0.001 |
| FFA | 620.9±214.9 | 635.24±219.6 | 0.567 |
| VFA | 240.7±76.8 | 161.7±63.9 | <0.001 |
| CAP, dB/m* | 367 (347, 392) | 341 (306, 371) | <0.001 |
| Smoking history, n (%) | 51 (49.5) | 73 (15.8) | <0.001 |
| Oral antidiabetic agents, n (%) | 19 (18.4) | 61 (13) | 0.152 |
| Insulin, n (%)# | 5 (4.9) | 10 (2.1) | 0.115 |
| Diuretics, n (%)# | 0 (0) | 3 (0.6) | 0.550 |
| Calcium channel blockers, n (%) | 15 (14.6) | 28 (6.0) | 0.003 |
| ACEI/ARB, n (%) | 12 (11.7) | 33 (6.6) | 0.08 |
| β-Blockers, n (%)# | 4 (3.9) | 11 (2.4) | 0.278 |
| Statins, n (%)# | 0 (0) | 5 (1.1) | 0.368 |
| Fibrates, n (%)# | 1 (1.0) | 6 (1.3) | 0.631 |
Values are expressed as the mean ± SD or median with interquartile range, unless otherwise indicated. SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; WC, waist circumference; HC, hip circumference; WBCC, white blood cell count; CRP, C-reactive protein; FBG, fasting plasma glucose; FINS, fasting insulin; FCP, fasting C-peptide; HbA1c, glycosylated hemoglobin A1c; HOMA-IR, homeostasis model assessment-insulin resistance; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; y-GT, y-glutamyl-transferase; ALP, alkaline phosphatase; BUN, blood urea nitrogen; SCr, serum creatinine; SUA, serum uric acid; FFA, free fatty acid; VFA, visceral fat area; CAP, controlled attenuation parameter; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers.
The Mann-Whitney U-test was applied.
Fisher's exact probability test was applied.
Correlation Analysis of BMI and VFA with CAP
The bivariate correlation analyses showed that CAP was closely associated with BMI, WC, HC, waist-to-hip ratio (WHR), FINS, HOMA-IR, and VFA in male subjects and was closely associated with most variables such as BMI, WC, HC, WHR, SBP, DBP, ALT, AST, SUA, TG, HDL-C, LDL-C, FPG, FINS, HbA1c, HOMA-IR, and VFA in females and all subjects (all p < 0.05, Table 2). Using the same bivariate correlation analysis, we also assessed the correlation of VFA and BMI and other parameters and found similar results. VFA was closely associated with age, BMI, WC, HC, WHR, SBP, DBP, FPG, FINS, HbA1c, HOMA-IR, and CAP in male subjects and was closely associated with all variables except TC and LDL-C in female and all subjects (all p < 0.05, Table 2).
Table 2.
Univariate analyses examining the associations of CAP and VFA with anthropometries and clinical parameters
| Male |
Female |
All |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAP |
VFA |
CAP |
VFA |
CAP |
VFA |
|||||||
| r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | |
| Age | 0.068 | 0.498 | 0.324 | 0.001 | –0.004 | 0.933 | 0.249 | <0.001 | –0.002 | 0.954 | 0.249 | <0.001 |
| BMI | 0.393 | <0.001 | 0.484 | <0.001 | 0.336 | <0.001 | 0.532 | <0.001 | 0.370 | <0.001 | 0.541 | <0.001 |
| WC | 0.372 | <0.001 | 0.512 | <0.001 | 0.350 | <0.001 | 0.525 | <0.001 | 0.396 | <0.001 | 0.577 | <0.001 |
| HC | 0.337 | <0.001 | 0.379 | <0.001 | 0.239 | <0.001 | 0.370 | <0.001 | 0.278 | <0.001 | 0.379 | <0.001 |
| SBP | 0.112 | 0.261 | 0.219 | 0.026 | 0.298 | <0.001 | 0.368 | <0.001 | 0.323 | <0.001 | 0.487 | <0.001 |
| DBP | 0.023 | 0.817 | 0.262 | 0.008 | 0.232 | <0.001 | 0.239 | <0.001 | 0.309 | <0.001 | 0.426 | <0.001 |
| ALT | 0.099 | 0.322 | 0.164 | 0.099 | 0.335 | <0.001 | 0.270 | <0.001 | 0.225 | <0.001 | 0.300 | <0.001 |
| AST | 0.073 | 0.463 | 0.184 | 0.065 | 0.310 | <0.001 | 0.271 | <0.001 | 0.334 | <0.001 | 0.322 | <0.001 |
| SCr | 0.188 | 0.057 | –0.017 | 0.868 | –0.040 | 0.388 | –0.144 | 0.014 | 0.308 | <0.001 | 0.319 | <0.001 |
| SUA | 0.045 | 0.650 | 0.055 | 0.584 | 0.288 | <0.001 | 0.150 | 0.001 | 0.111 | 0.008 | 0.136 | 0.001 |
| TC | 0.132 | 0.183 | 0.046 | 0.645 | 0.067 | 0.151 | 0.029 | 0.528 | 0.317 | <0.001 | 0.248 | <0.001 |
| TG | 0.095 | 0.338 | 0.064 | 0.521 | 0.226 | <0.001 | 0.213 | <0.001 | 0.058 | 0.171 | 0.011 | 0.787 |
| HDL-C | –0.115 | 0.249 | 0.043 | 0.665 | –0.150 | 0.001 | –0.179 | <0.001 | 0.233 | <0.001 | 0.254 | <0.001 |
| LDL-C | 0.144 | 0.146 | 0.083 | 0.402 | 0.097 | 0.036 | 0.051 | 0.275 | –0.182 | <0.001 | –0.203 | <0.001 |
| FBG | 0.027 | 0.786 | 0.275 | 0.005 | 0.270 | <0.001 | 0.328 | <0.001 | 0.082 | 0.051 | 0.030 | 0.479 |
| FINS | 0.315 | 0.001 | 0.451 | <0.001 | 0.321 | <0.001 | 0.340 | <0.001 | 0.242 | <0.001 | 0.337 | <0.001 |
| HOMA-IR | 0.245 | 0.013 | 0.474 | <0.001 | 0.284 | <0.001 | 0.363 | <0.001 | 0.343 | <0.001 | 0.384 | <0.001 |
| HbA1c | 0.035 | 0.725 | 0.202 | 0.042 | 0.284 | <0.001 | 0.363 | <0.001 | 0.361 | <0.001 | 0.435 | <0.001 |
| VFA | 0.382 | <0.001 | – | – | 0.351 | <0.001 | – | – | 0.259 | <0.001 | 0.366 | <0.001 |
| CAP | – | – | 0.382 | <0.001 | – | – | 0.429 | <0.001 | 0.463 | <0.001 | – | - |
BMI, body mass index; WC, waist circumference; HC, hip circumference; WHR, waist-to-hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; AST aspartate aminotransferase; ALT, alanine aminotransferase; SCr, serum creatinine; SUA, serum uric acid; TG, triglycerides; TC, total cholesterol; HDL, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FBG, fasting plasma glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment-insulin resistance; HbA1c, glycosylated hemoglobin A1c; VFA, visceral fat area; CAP, controlled attenuation parameter.
To further investigate the correlation between BMI, VFA, and CAP, the multiple linear regression analysis was conducted. We found that both BMI and VFA were risk factors for CAP with the crude model, and the association remained significant even after adjusting for age and sex (in all subjects), SBP, DBP, ALT, SCr, SUA, TG, HDL-C, LDL-C, HbA1c, and HOMA-IR. Meantime, it was also found that BMI was a risk factor for VFA with the crude model, adjusted for age and sex (in all subjects), and further adjusted for SBP, DBP, ALT, SCr, SUA, TG, HDL-C, LDL-C, HbA1c, and HOMA-IR (all p < 0.05, Table 3).
Table 3.
Multiple linear regression examining the association of BMI and VFA with CAP
| Male |
Female |
Total |
||||
|---|---|---|---|---|---|---|
| beta (95% CI) | p values | beta (95% CI) | p values | beta (95% CI) | p values | |
| BMI→CAP | ||||||
| Model 1 | 2.363 (1.250–3.477) | <0.001 | 2.634 (1.979–3.289) | <0.001 | 2.777 (2.210–3.343) | <0.001 |
| Model 2 | 2.383 (1.237–3.529) | <0.001 | 2.708 (2.045–3.371) | <0.001 | 2.646 (2.068–3.223) | <0.001 |
| Model 3 | 2.650 (1.292–4.009) | <0.001 | 1.524 (0.798–2.249) | <0.001 | 1.738 (1.100–2.377) | <0.001 |
| VFA→CAP | ||||||
| Model 1 | 0.180 (0.085–0.275) | <0.001 | 0.220 (0.152–0.289) | <0.001 | 0.278 (0.231–0.324) | <0.001 |
| Model 2 | 0.213 (0.114–0.311) | <0.001 | 0.315 (0.254–0.376) | <0.001 | 0.290 (0.238–0.343) | <0.001 |
| Model 3 | 0.194 (0.086–0.301) | <0.001 | 0.184 (0.117–0.252) | <0.001 | 0.190 (0.133–0.247) | <0.001 |
| BMI^VFA | ||||||
| Model 1 | 5.744 (3.694–7.793) | <0.001 | 6.110 (5.314–6.906) | <0.001 | 6.725 (5.924–7.525) | <0.001 |
| Model 2 | 6.871 (5.035–8.706) | <0.001 | 6.704 (5.969–7.439) | <0.001 | 6.725 (6.037–7.413) | <0.001 |
| Model 3 | 6.673 (4.342–9.005) | <0.001 | 5.700 (4.862–6.538) | <0.001 | 5.983 (5.915–6.770) | <0.001 |
Model 1: crude model. Model 2: adjusted for age and gender (adjusted only for all subjects). Model 3: further adjusted for SBP, DBP, ALT, SCr, SUA, TG, HDL-C, LDL-C, HbA1c, and HOMA-IR. BMI, body mass index; VFA, visceral fat area; CAP, controlled attenuation parameter; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; AST, aspartate aminotransferase; SCr, creatinine; SUA, serum uric acid; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin A1c; HOMA-IR, homeostasis model assessment-insulin resistance; ALT, alanine aminotransferase.
Direct and Indirect Effects of BMI on Hepatic Steatosis with VFA as a Mediator
The path analysis results are detailed in Table 4 and illustrated in Figure 2. In the mediational model, pathway (c') represents the direct effect, whereas pathways (a) and (b) together represent the indirect effect. BMI was directly associated with CAP (p (c') < 0.05, Table 4). Further, a potential causal effect between BMI and CAP mediated by VFA was observed (both p (a) and p (b) < 0.05, Table 4), which implied that VFA may partially mediate the relationship between obesity and hepatic steatosis degree. The analysis also showed that VFA contributed to 51.37%, 53.85%, and 26.51% of the indirect effects of obesity-induced hepatic steatosis in the total, female, and male subjects, respectively.
Table 4.
Direct and indirect effects of BMI on CAP with VFA as a mediator
| Direct effect |
Indirect effect |
Total effect |
VFA mediated% |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate1 | p (c') | Estimate2 | p (a) | Estimate3 | p (b) | Estimate4 | p (c) | ||
| Total | 0.181 | <0.001 | 0.569 | <0.001 | 0.339 | <0.001 | 0.374 | <0.001 | 51.37% |
| Male | 0.484 | <0.001 | 0.212 | 0.043 | 0.284 | 0.007 | 0.386 | <0.001 | 26.51% |
| Female | 0.159 | 0.002 | 0.573 | <0.001 | 0.323 | <0.001 | 0.344 | <0.001 | 53.85% |
BMI, body mass index; VFA, visceral fat area; CAP, controlled attenuation parameter.
Estimate represents the direct effect of BMI on CAP.
Estimate represents the indirect effect of BMI on VFA.
Estimate represents the indirect effect of VFA on CAP.
Estimate represents the total effect of BMI on CAP.
Discussion
In this study, we found that visceral fat accumulation played an intermediary role in obesity-induced hepatic steatosis. More interestingly, this mediating effect accounts for a larger proportion of hepatic steatosis in females with overweight and obesity (53.85%) than in males (26.51%).
Numerous studies have revealed that hepatic steatosis or NAFLD is strongly associated with obesity. In a study of 1,000 patients with severe obesity, more than 95% had NAFLD [23]. Some studies have also reported that CAP values are significantly correlated with BMI, especially in patients with NAFLD [24, 25]. Consistent with previous findings, we also found a positive correlation between the degree of hepatic steatosis (quantified by the CAP score) and BMI. It has been suggested that obesity and chronic low-grade systemic inflammation play important roles in the progression from hepatic steatosis to fibrosis [26].
BMI is an indicator of total body size and is commonly used as a measure of total adiposity, although it cannot differentiate subcutaneous fat from visceral fat and muscle [27]. Nobarani et al. [28] verified that BMI was significantly associated with VFA in healthy subjects and type 2 diabetics, which was consistent with our study. Pasanta [29] also showed that visceral fat remained associated with BMI after adjusting for sex and age. In a study of male C57BL/6J mice, epididymal VAT removal decreased the weight gain induced by a high-fat diet and prevented obesity-induced insulin resistance and hyperinsulinemia [30].
A Korean study revealed that patients with NAFLD had a higher VFA, albeit after adjusting for sex [31]. Lee et al. [32] found that VFA was significantly correlated with hepatic steatosis measured by CAP, independent of BMI and sex. Nobarani et al. [28] showed that both BMI and VFA were important determinants of liver steatosis and that the VAT area was associated with the severity of hepatic steatosis, independent of anthropometric measures of obesity. Our results concur with these findings. Data from animal models have indicated that the high lipolytic rate of visceral fat generates large amounts of FFAs that are delivered to the liver, causing hepatic steatosis [33]. There was no difference in FFA between males and females in our study which can be possibly explained that measuring arterial plasma FFA concentrations does not reflect hepatic FFA delivery in humans with visceral obesity [34]. An animal study showed that the reduction in visceral fat caused by metformin suppresses extracellular matrix remodeling in white adipose tissue and inhibits obesity-induced inflammation [35]. Shimizu et al. [36] also suggested that an SGLT2 inhibitor improves NAFLD mainly by reducing the quantity of visceral fat and that VAT removal increased circulating adiponectin, an important insulin-sensitizing adipokine, whereas it decreased circulating interleukin-6, a proinflammatory adipokine [30]. Both increased VAT and its dysfunction contribute to the onset and development of obesity-related metabolic disorders [37]. Previous human and animal studies have indicated that NAFLD is closely associated with VAT inflammation and elevated circulating inflammatory factors, such as inflammatory adipokines and lipids [38, 39].
We also used mediation analysis to evaluate the interactions among BMI, VFA, and CAP in individuals with overweight and obesity. This showed that visceral fat accumulation contributed to 51.37% of obesity-induced hepatic steatosis in all subjects. This is consistent with the notion that visceral fat accumulation plays an important role in the development of NAFLD [40]. More remarkably, the analysis by sex showed that this affected a greater proportion of females (53.85%) than males (26.51%), although the average VFA and BMI were both higher in males. This indicates that VAT is a stronger risk factor in females, while the higher CAP scores in males is probably due to their greater average deposit of visceral fat. Similarly, a recent study found a causal effect of VAT in hypertension and type 2 diabetes, and the odds ratios were significantly higher in women after accounting for the difference in the average mass of visceral fat between females and males [41]. The mechanism of this sex difference is unclear. However, this phenomenon seems to explain why the WC cutoff for diagnosing abdominal obesity is lower in females than in males [42].
Some limitations remain in this study. First, causal relationships cannot be determined among BMI, VAT, and the degree of hepatic steatosis in a cross-sectional study. Second, because the participants were recruited from obesity outpatient clinics, the findings need to be verified in other populations. Third, VFA reflects only the volume of VAT and does not reflect the metabolic activity of VAT. Finally, gluteofemoral fat mass and leg fat mass were not measured in our study, and the sample size of male patients included in the study was relatively small. Further research in a larger cohort is needed to confirm the results.
To our knowledge, this is the first study to elucidate the influence of visceral adipose accumulation on the degree of hepatic steatosis among individuals with overweight and obesity. We also stratified our data by sex and found differences in the effects of VFA on hepatic steatosis between males and females. Finally, CAP can quantify the degree of hepatic steatosis, unlike previous qualitative studies of hepatic steatosis.
Conclusion
VAT accumulation partially mediates obesity-induced hepatic steatosis, especially in females. Weight loss, especially targeting reducing visceral fat, may contribute to prevent and treat hepatic steatosis.
Statement of Ethics
This study protocol was approved by the Ethics Committee of Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, approval number 2019-KY-025(K), in compliance with the Declaration of Helsinki. All participants provided their written informed consent to participate in this study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was supported by grants from the Clinical Research Plan of SHDC (SHDC2020CR1017B), the National Key Research and Development Project of China (2016YFA0502003), the Shanghai Key Clinical Center for Metabolic Disease (2017ZZ01013), the Shanghai Municipal Key Clinical Specialty and Shanghai Science and Technology Commission (21Y11910900), and the Hainan Provincial Natural Science Foundation of China (number 822MS207).
Author Contributions
Fengjing Liu drafted the manuscript. Fengjing Liu, Si Chen, and Xiao Li performed the statistical analysis. Si Chen, Junfeng Han, and Yinfang Tu drafted the figure and legend. Xiao Li and Yunfeng Xiao collected ultrasound and MRI data. Shaobo Li, Junfeng Han, Yinfang Tu, and Haoyong Yu collected clinical data. Yuqian Bao, Wenkun Bai, and Haoyong Yu designed the outline of the topic and helped in revising the manuscript. All the authors contributed to the article and approved the submitted version.
Data Availability Statement
Due to the privacy of participants, the study data cannot be available for public access. Further inquiries can be directed to the corresponding author on reasonable request approved by the Institutional Review Board of Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.
Acknowledgments
The authors would like to thank study participants for their contribution. Authors and participants gave consent for data to be shared.
Funding Statement
This study was supported by grants from the Clinical Research Plan of SHDC (SHDC2020CR1017B), the National Key Research and Development Project of China (2016YFA0502003), the Shanghai Key Clinical Center for Metabolic Disease (2017ZZ01013), the Shanghai Municipal Key Clinical Specialty and Shanghai Science and Technology Commission (21Y11910900), and the Hainan Provincial Natural Science Foundation of China (number 822MS207).
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016 Jul;64((1)):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022 Apr;10((4)):284–296. doi: 10.1016/S2213-8587(22)00003-1. [DOI] [PubMed] [Google Scholar]
- 3.Reginato E, Pippi R, Aiello C, Sbroma Tomaro E, Ranucci C, Buratta L, et al. Effect of short term intensive lifestyle intervention on hepatic steatosis Indexes in adults with obesity and/or type 2 diabetes. J Clin Med. 2019 Jun 14;8((6)):851. doi: 10.3390/jcm8060851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346((16)):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 5.Spagnuolo R, Montalcini T, De Bonis D, Ferro Y, Cosco C, Mazza E, et al. Weight gain and liver steatosis in patients with inflammatory bowel diseases. Nutrients. 2019 Feb 1;11((2)):303. doi: 10.3390/nu11020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camilleri M, Malhi H, Acosta A. Gastrointestinal complications of obesity. Gastroenterology. 2017;152((7)):1656–1670. doi: 10.1053/j.gastro.2016.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchesini G, Moscatiello S, Di Domizio S, Forlani G. Obesity-associated liver disease. J Clin Endocrinol Metab. 2008;93((11 Suppl 1)):S74–80. doi: 10.1210/jc.2008-1399. [DOI] [PubMed] [Google Scholar]
- 8.Lomonaco R, Godinez Leiva E, Bril F, Shrestha S, Mansour L, Budd J, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care. 2021 Feb;44((2)):399–406. doi: 10.2337/dc20-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eilenberg M, Munda P, Stift J, Langer FB, Prager G, Trauner M, et al. Accuracy of non-invasive liver stiffness measurement and steatosis quantification in patients with severe and morbid obesity. Hepatobiliary Surg Nutr. 2021 Oct;10((5)):610–622. doi: 10.21037/hbsn-20-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefan N, Häring HU, Schulze MB. Metabolically healthy obesity: the low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2018 Mar;6((3)):249–258. doi: 10.1016/S2213-8587(17)30292-9. [DOI] [PubMed] [Google Scholar]
- 11.Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019 Oct 1;129((10)):3978–3989. doi: 10.1172/JCI129186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato F, Tamura Y, Watada H, Kumashiro N, Igarashi Y, Uchino H, et al. Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J Clin Endocrinol Metab. 2007 Aug;92((8)):3326–3329. doi: 10.1210/jc.2006-2384. [DOI] [PubMed] [Google Scholar]
- 13.Choi MH, Choi JI, Park MY, Rha SE, Oh SN, Jung SE, et al. Validation of intimate correlation between visceral fat and hepatic steatosis: quantitative measurement techniques using CT for area of fat and MR for hepatic steatosis. Clin Nutr. 2018 Feb;37((1)):214–222. doi: 10.1016/j.clnu.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020 Jul;8((7)):616–627. doi: 10.1016/S2213-8587(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Wu Z, Liu J, Lv Y, Li W. Metabolic syndrome and its components reduce coronary collateralization in chronic total occlusion: an observational study. Cardiovasc Diabetol. 2021;20((1)):104. doi: 10.1186/s12933-021-01297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang JW, Yang CY, Wu HY, Liu KL, Su CT, Wu CK, et al. Metabolic syndrome and abdominal fat are associated with inflammation, but not with clinical outcomes, in peritoneal dialysis patients. Cardiovasc Diabetol. 2013 Jun 8;12((1)):86. doi: 10.1186/1475-2840-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu CP, Ali H, Rachakonda VP, Oczypok EA, DeLany JP, Kershaw EE. The ZJU index is a powerful surrogate marker for NAFLD in severely obese North American women. PLoS One. 2019 Nov 26;14((11)):e0224942. doi: 10.1371/journal.pone.0224942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell GC, Chitturi S, Lau GK, Sollano JD. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22((6)):775–777. doi: 10.1111/j.1440-1746.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28((7)):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 19852004 Dec;97((6)):2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 21.Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36((1)):13–20. doi: 10.1016/j.clinre.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Zhou BF. Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases--report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. Biomed Environ Sci. 2002;15((3)):245–252. [PubMed] [Google Scholar]
- 23.Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11((1)):137–141. doi: 10.1016/j.soard.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Tasneem AA, Luck NH, Majid Z. Factors predicting non-alcoholic steatohepatitis (NASH) and advanced fibrosis in patients with non-alcoholic fatty liver disease (NAFLD) Trop Doct. 2018;48((2)):107–112. doi: 10.1177/0049475517742261. [DOI] [PubMed] [Google Scholar]
- 25.Patel PJ, Hossain F, Horsfall LU, Banh X, Hayward KL, Williams S, et al. Controlled attenuation parameter in NAFLD identifies risk of suboptimal glycaemic and metabolic control. J Diabetes Complications. 2018 Aug;32((8)):799–804. doi: 10.1016/j.jdiacomp.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Varghese Z, Ruan XZ. The molecular pathogenic role of inflammatory stress in dysregulation of lipid homeostasis and hepatic steatosis. Genes Dis. 2014;1((1)):106–112. doi: 10.1016/j.gendis.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millar SR, Perry IJ, Phillips CM. Assessing cardiometabolic risk in middle-aged adults using body mass index and waist-height ratio: are two indices better than one? A cross-sectional study. Diabetol Metab Syndr. 2015 Sep 7;7((1)):73. doi: 10.1186/s13098-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nobarani S, Alaei-Shahmiri F, Aghili R, Malek M, Poustchi H, Lahouti M, et al. Visceral adipose tissue and non-alcoholic fatty liver disease in patients with type 2 diabetes. Dig Dis Sci. 2022 Apr;67((4)):1389–1398. doi: 10.1007/s10620-021-06953-z. [DOI] [PubMed] [Google Scholar]
- 29.Pasanta D, Htun KT, Pan J, Tungjai M, Kaewjaeng S, Chancharunee S, et al. Waist circumference and BMI are strongly correlated with MRI-derived fat compartments in young adults. Life. 2021 Jul 1;11((7)):643. doi: 10.3390/life11070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franczyk MP, He M, Yoshino J. Removal of epididymal visceral adipose tissue prevents obesity-induced multi-organ insulin resistance in male mice. J Endocr Soc. 2021 Feb 20;5((5)):bvab024. doi: 10.1210/jendso/bvab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu SJ, Kim W, Kim D, Yoon JH, Lee K, Kim JH, et al. Visceral obesity predicts significant fibrosis in patients with nonalcoholic fatty liver disease. Medicine. 2015 Dec;94((48)):e2159. doi: 10.1097/MD.0000000000002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HW, Kim KJ, Jung KS, Chon YE, Huh JH, Park KH, et al. The relationship between visceral obesity and hepatic steatosis measured by controlled attenuation parameter. PLoS One. 2017 Oct 27;12((10)):e0187066. doi: 10.1371/journal.pone.0187066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371((12)):1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004 Jun;113((11)):1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, Massey S, Story D, Li L. Metformin: an old drug with new applications. Int J Mol Sci. 2018 Sep 21;19((10)):2863. doi: 10.3390/ijms19102863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019 Feb;21((2)):285–292. doi: 10.1111/dom.13520. [DOI] [PubMed] [Google Scholar]
- 37.Kovačević S, Brkljačić J, Vojnović Milutinović D, Gligorovska L, Bursać B, Elaković I, et al. Fructose induces visceral adipose tissue inflammation and insulin resistance even without development of obesity in adult female but not in male rats. Front Nutr. 2021 Nov 11;8:749328. doi: 10.3389/fnut.2021.749328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulder P, Morrison MC, Wielinga PY, van Duyvenvoorde W, Kooistra T, Kleemann R. Surgical removal of inflamed epididymal white adipose tissue attenuates the development of non-alcoholic steatohepatitis in obesity. Int J Obes. 2016;40((4)):675–684. doi: 10.1038/ijo.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith U. Abdominal obesity: a marker of ectopic fat accumulation. J Clin Invest. 2015;125((5)):1790–1792. doi: 10.1172/JCI81507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koda M, Kawakami M, Murawaki Y, Senda M. The impact of visceral fat in nonalcoholic fatty liver disease: cross-sectional and longitudinal studies. J Gastroenterol. 2007;42((11)):897–903. doi: 10.1007/s00535-007-2107-z. [DOI] [PubMed] [Google Scholar]
- 41.Karlsson T, Rask-Andersen M, Pan G, Hoglund J, Wadelius C, Ek WE. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat Med. 2019;25((9)):1390–1395. doi: 10.1038/s41591-019-0563-7. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organisation . Obesity: preventing and managing the global epidemic. Geneva: World Health Organisation; 1997. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the privacy of participants, the study data cannot be available for public access. Further inquiries can be directed to the corresponding author on reasonable request approved by the Institutional Review Board of Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.