Abstract
Introduction
Laparoscopic sleeve gastrectomy (LSG) for morbidly obese patients often results in remission of type 2 diabetes (T2DM), but diabetes relapses in some of those patients. The frequency of T2DM relapse in Asians and the factors involved have not been adequately investigated.
Methods
The J-SMART study was conducted on 322 Japanese subjects with body mass index (BMI) ≥32 kg/m<sup>2</sup> who underwent LSG at 10 accredited centers in Japan between 2011 and 2014. Of these, 82 T2DM subjects with diabetes in complete or partial remission at 1 year after LSG and followed postoperatively for 5 years were included in the subgroup analysis and classified into two groups: diabetes remission-maintained and diabetes relapse.
Results
The mean age of all included subjects was 49.2 years, median BMI was 41.5 kg/m<sup>2</sup>, and median HbA1c was 6.7%. Compared with the diabetes remission-maintained group, the diabetes relapse group at 5 years after LSG had significantly higher preoperative HbA1c, number of antidiabetic medications, and high-density lipoprotein cholesterol level; and lower BMI and homeostasis model assessment-beta cell function (HOMA-β). As many as 83.0% of the subjects were able to achieve HbA1c <7% at 5 years after LSG, but 26.8% of the subjects had diabetes relapse. Preoperative HbA1c significantly contributed to diabetes relapse (odds ratio 1.54, p = 0.049). In addition, the diabetes relapse group tended to have lower percentage total weight loss (%TWL) at 1 year after LSG and higher percentage weight regain (%WR) from postoperative nadir weight, compared with the diabetes remission-maintained group. The hazard ratio for diabetes relapse was 3.14-fold higher in subjects with %TWL ≥20% and %WR ≥25%, and 5.46-fold higher in those with %TWL <20% and %WR ≥25%, compared with %TWL ≥20% and %WR <25%.
Conclusion
While LSG provides a high remission rate for T2DM, relapse is not uncommon. Preoperative HbA1c, poor weight loss, and excess weight regain after LSG contribute to diabetes relapse, suggesting the importance of treatment strategies focusing on these factors.
Keywords: Obesity, Laparoscopic sleeve gastrectomy, Diabetes relapse, Weight regain
Introduction
Bariatric surgery is becoming the most effective long-term treatment for severe obesity. Among the various bariatric surgical techniques, laparoscopic sleeve gastrectomy (LSG) is one of the most frequently performed procedures worldwide, due to the effective weight loss, simple procedure, and few complications [1]. The number of LSG is increasing rapidly not only in the USA but also in Japan [2, 3] − a total of 3,669 bariatric surgeries were performed in Japan from 2000 to 2019, with LSG being the most common procedure (n = 2866, 78.1%). In 2019, bariatric surgery was performed in 757 patients in Japan, 704 (93.0%) of whom underwent LSG.
Although LSG has slightly lower efficacy in weight loss and remission of complications including diabetes compared with Roux-en-Y gastric bypass (RYGB), the outcomes are favorable [4, 5]. The American Society for Metabolic and Bariatric Surgery (ASMBS) also recommends LSG as the primary bariatric surgery or as the first part of a staged approach in high-risk patients [6]. However, relapse of diabetes in some patients after bariatric surgeries including LSG has been reported in recent years [7, 8]. Furthermore, patients with inadequate initial weight loss or with late weight regain are found to be at higher risk of type 2 diabetes (T2DM) relapse [7, 9].
The Japanese Survey of Morbid and Treatment Resistant Obesity (J-SMART) study of morbidly obese Japanese patients reported a reliable remission rate of T2DM after LSG [10]. However, there are few reports on the relapse of diabetes after LSG in Asians including Japanese, and the association with weight regain is not clear. In addition, given the racial difference in pathogenesis of diabetes [11], it is important to examine in detail the long-term outcome of diabetes after LSG in Asians. The purpose of this study was to investigate factors associated with relapse of T2DM after morbidly obese Japanese subjects have undergone LSG, using the J-SMART database.
Methods
This study was a subgroup analysis of the J-SMART study [10], which retrospectively examined the effects of bariatric surgery on weight loss and remission of diabetes in representative centers performing bariatric surgery in Japan. The study had a retrospective and observational design. The J-SMART study included 322 Japanese subjects who underwent LSG at 10 bariatric surgery facilities accredited by the Japanese Society for the Treatment of Obesity (JSTO) between January 2011 and December 2014. The subjects were followed for 5 years after LSG. Body weight and body mass index (BMI) regained were measured, and percentage weight regain (%WR) was assessed by evaluating the nadir weight after LSG and the weight until the last follow-up.
The JSTO guidelines adopted the indications for bariatric surgery as follows: primary obesity between the ages of 18 and 65 years, inadequate response to medical treatment, and one of the following conditions: (1) BMI of 35 kg/m2 or higher for the purpose of weight loss (weight loss surgery), (2) BMI of 32 kg/m2 or higher for the purpose of treating comorbidities (including diabetes, hypertension, dyslipidemia, liver dysfunction, and sleep apnea syndrome). In the present subgroup analysis, 210 subjects with T2DM who underwent LSG based on the above guidelines were selected from the J-SMART database. Of these subjects, those whose diabetes was in initial remission at 1 year after LSG and who completed follow-up without dropout until 5 years postoperatively were assigned to the diabetes remission-maintained or relapse group, and factors associated with diabetes relapse were evaluated.
From the subjects' records, the following data were extracted: sex; age; and physical and clinical parameters such as height, body weight, visceral fat area (VFA), subcutaneous fat area (SFA), blood pressure, glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), fasting serum connecting peptide immunoreactivity (CPR) for calculating CPR index, and fasting immunoreactive insulin (IRI) for calculating homeostasis model assessment (HOMA)-beta cell function (HOMA-β) and HOMA-insulin resistance (HOMA-IR). The CPR index was calculated by the following formula: CPR index = 100 × fasting CPR (ng/mL)/FPG (mg/dL) [12, 13]. HOMA-β was calculated by the following formula: HOMA-β = (fasting IRI [μIU/mL] × 360)/(FPG [mg/dL] − 63) [14, 15]. HOMA-IR was calculated as follows: HOMA-IR = (fasting IRI [μIU/mL] × FPG [mg/dL])/405 [14, 15]. Other data extracted were lipid level markers, serum creatinine, estimated glomerular filtration rate calculated by the estimation formula for Japanese [16], urine albumin-to-creatinine ratio, history of insulin use, number of medications, and apnea-hypopnea index. Subjects with apnea-hypopnea index ≥5 and sleep-related symptoms of obstructive sleep apnea syndrome (OSAS) were diagnosed as having OSAS. VFA and SFA were measured by computed tomography at the level of the umbilicus with the subject in supine position.
Mental disorders, mental retardation, developmental disorders, and binge eating were diagnosed by skilled psychiatrists in doubtful cases, according to the criteria in Diagnostic and Statistical Manual of Mental Disorders 4th or 5th edition, or International Statistical Classification of Diseases and Related Health Problems 10th Revision. The Wechsler Adult Intelligence Scale 3rd edition (WAIS-III) that assessed full intelligence quotient (IQ), verbal IQ, and performance IQ was administered by a trained psychiatrist or psychologist as an intelligence test to estimate the postoperative outcome. We previously reported that childhood onset obesity was associated with weight regain (WR) after LSG [17]. Based on that study, subjects were also asked whether they were diagnosed with obesity based on diagnostic criteria during annual school physical examinations in childhood [18, 19]. Those diagnosed with obesity up to the age of 13 years were classified as childhood onset obesity.
Diabetes was diagnosed according to the diagnostic criteria of the Japan Diabetes Society [20]; hypertension according to the Japanese Society of Hypertension [21]; dyslipidemia according to the Japan Atherosclerosis Society [22]; and OSAS according to the Japanese Respiratory Society [20]. The ABCD score has been reported as a score that predicts improvement in T2DM, and is appropriate for the Japanese population [21, 22, 23]. Complete remission (CR) and partial remission of T2DM after LSG were defined as HbA1c less than 6.0% and 6.5%, respectively, without using antidiabetic drugs, and improvement was defined as HbA1c less than 7.0% with or without medication [24, 25].
At 5 years after LSG, maintenance of diabetes remission or diabetes relapse was assessed. Subjects who achieved initial remission, i.e., HbA1c <6.5% without antidiabetic medication after 1 year of LSG, and maintained it until 5 years after LSG, were classified in the remission-maintained group. Subjects who achieved initial remission at 1 year of LSG and then relapsed up to 5 years postoperatively, i.e., HbA1c ≥6.5% or used antidiabetic drugs after initial remission, were classified in the relapse group. Weight regain after bariatric surgery was evaluated using three major indices: (1) regaining more than 25% of lost weight from the nadir weight [26, 27], (2) regaining more than 10 kg from the nadir weight [28, 29, 30], and (3) regaining more than 5 BMI points from the BMI at nadir weight [26, 31].
Statistical Analysis
Results are expressed as mean ± standard deviation or median (interquartile range), or percentage. Normal distribution was tested using the Shapiro-Wilk test. For comparisons between two groups, Student's t test was used to analyze parametric data and Mann-Whitney U test for nonparametric data. Fisher's exact test was used to detect significant differences between proportions and categorical variables. Cochran's Q test was used to compare nominal measures for three or more groups. The changes in BMI, percentage total weight loss, and HbA1c over the 5-year period after LSG were analyzed by repeated measures ANOVA followed by Bonferroni multiple comparison test to evaluate the overall change (main effect p value) and differences between the diabetes remission-maintained and diabetes relapse groups (interaction effect p value). Logistic regression model and Cox proportional hazards regression analysis were used to identify preoperative or postoperative factors predicting diabetes relapse. Results were expressed as odds ratios and hazard ratios with 95% confidence interval. These analyses were performed using SPSS software version 26 (Armonk, NY: IBM Corp). A p value <0.05 was considered significant.
Results
Baseline Data of All Subjects and Comparison between Remission-Maintained and Relapse Groups at 5 Years Post-LSG
Baseline clinical data from 210 subjects included in the study showed that 159 (79.1%) had CR or partial remission of diabetes at 1 year after LSG; of the 159 subjects, 82 who completed follow-up without dropout up to 5 years after LSG were eligible for analysis. Of these, 60 (73.2%) were assigned to the remission-maintained group and 22 (26.8%) to the relapse group. Note that up to 5 years after LSG surgery, no subject in the remission-maintained group relapsed diabetes, and no subject in the relapse group had a remission after relapse.
Table 1 shows the baseline demographic and clinical data of all subjects included in the subgroup analysis, and comparison of the remission-maintained group and the relapse group at 5 years after LSG. The mean age of all included subjects was 49.2 ± 10.8 years; median BMI was 41.5 (interquartile range 37.6–46.8) kg/m2; and median HbA1c was 6.7 (6.2–7.8)%. The median duration of diabetes was 5.0 (2.0–9.0) years, and 20.5% of subjects were on insulin. They also had hypertriglyceridemia and hypertension, and approximately 70% of the subjects had OSAS. The mean VFA/SFA ratio was 0.53 ± 0.24, indicating visceral fat accumulation type obesity.
Table 1.
Demographic and clinical data of all subjects at baseline, and comparison of diabetes remission-maintained and relapse groups at 5 years after LSG
| Baseline All enrolled subjects (n = 210) |
5 years after LSG |
|||||
|---|---|---|---|---|---|---|
| remission-maintained group (n = 60) | relapse group (n = 22) | remission-maintained group versus relapse group; p value | ||||
| Gender (male/female) | 94/116 | 25/35 | 9/13 | 0.999 | ||
| Age, years | 49.2±10.8 | 50±9.5 | 50.9±12.7 | 0.745 | ||
| Body weight, kg | 118.5±28.9 | 118.9±22.9 | 106.5±22.7 | 0.032 | ||
| BMI, kg/m2 | 41.5 (37.6–46.8) | 41.2 (38.8–47.9) | 39.6 (34.2–44.3) | 0.029 | ||
| Subjects with BMI ≥35, % | 89.5 | 98.3 | 68.2 | <0.001 | ||
| HbAlc, % | 6.7 (6.2–7.8) | 6.9 (6.2–7.3) | 7.9 (6.6–9.6) | 0.020 | ||
| FPG, mg/dL | 128.0 (109.8–155.3) | 113.0(103.8–135.0) | 137.0(116.8–195.0) | 0.005 | ||
| Serum CPR, ng/mL | 3.4 (2.7–4.6) | 2.9 (2.8–5.1) | 3.1 (3.0–3.3) | 0.189 | ||
| CPR Index | 2.7(1.9–37) | 3.1 (2.6–3.9) | 3.3 (2.9–4.1) | 0.193 | ||
| HOMA-IR | 37(1.6–7.5) | 4.4 (3.1–7.1) | 5.1 (2.9–7.8) | 0.984 | ||
| HOMA-β, % | 96.0(57.0–164.9) | 93.9 (69.3–174.0) | 57.4 (35.0–126.4) | 0.019 | ||
| Duration of diabetes, years | 5.0 (2.0–9.0) | 6.6±6.2 | 5.7±4 | 0.552 | ||
| Number of diabetes medications, n | 1 (0–2) | 0(0–1) | 2 (2–3) | 0.004 | ||
| Insulin use, % | 20.5 | 8.3 | 13.6 | 0.437 | ||
| ABCD score | 6 (5–8) | 6 (5–7) | 6 (4–7) | 0.418 | ||
| TC, mg/dL | 199.0±42.9 | 196.6±38.2 | 202.9±34.5 | 0.523 | ||
| TG, mg/dL | 193.5 (119.0–276.3) | 138(104.5–218.0) | 147(108.5–196.5) | 0.911 | ||
| HDL-C, mg/dL | 43 (38.0–53.3) | 44 (41.0–51.0) | 50 (47.0–57.0) | 0.026 | ||
| Number of antidyslipidemic medications, n | 0(0–1) | 0(0–1) | 1 (0–1) | 0.181 | ||
| Serum creatinine, mg/dL | 0.68 (0.56–0.78) | 0.65 (0.55–0.80) | 0.66 (0.57–0.73) | 0.663 | ||
| eGFR, mL/mm/1.73 m2 | 75.1 ±23.1 | 74.9±21.2 | 80.7±30.1 | 0.371 | ||
| UACR, mg/gCr | 9.3 (4.0–57.4) | 6.4 (4.7–14.3) | 7.8 (4.6–42.7) | 0.942 | ||
| Uric acid, mg/dL | 6.2±1.7 | 6.3±1.7 | 6.2±1.5 | 0.710 | ||
| VFA, cm2 | 229 (189.5–308.7) | 264.2 (203.7–303.0) | 194(174.7–276.8) | 0.082 | ||
| SFA, cm2 | 493.9 (403.5–584.6) | 511 (438.4–662.5) | 404.9(317.5–536.6) | 0.102 | ||
| VFA/SFA ratio | 0.53±0.24 | 0.54±0.24 | 0.53±0.17 | 0.942 | ||
| SBP, mm Hg | 140.1 ±19.9 | 139.7±15.7 | 140.9±21.7 | 0.828 | ||
| DBP, mm Hg | 87.1 ±16.3 | 85.9±10 | 90.7±28.1 | 0.382 | ||
| Number of antihypertensive medications, n | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.738 | ||
| Subjects with OSAS, % | 71.6 | 70.7 | 73.7 | 1.000 | ||
| Childhood onset obesity, % | 37.0 | 40.0 | 28.6 | 0.436 | ||
| Mental disorder, % | 25.0 | 27.1 | 19.0 | 0.566 | ||
| Mental retardation and developmental disorders, % | 2.7 | 1.8 | 5.0 | 0.465 | ||
| Binge eating, % | 21.1 | 17.9 | 30.0 | 0.338 | ||
| Full IQ (WAIS-III) | 95.6±19.1 | 97.6±19.8 | 99.2±18.7 | 0.876 | ||
| Verbal IQ (WAIS-III) | 96.2±17.9 | 96.7±18.0 | 97.0±17.7 | 0.972 | ||
| Performance IQ (WAIS-III) | 96.7±19.2 | 97.6±21.4 | 102.4±17.2 | 0.656 | ||
Data are presented as mean ± SD or median (Interquartile range) or percentage. Student's f test was used to analyze parametric data and Mann-Whitney U test for nonparametric data. Fisher's exact test was used to detect significant differences between proportions and categorical variables. A p value <0.05 was considered significant. LSG, laparoscopic sleeve gastrectomy; BMI, body mass Index; HbA1c, glycosylated hemoglobin; FPG, fasting plasma glucose; CPR, connecting peptide immunoreactivity; FIOMA-IR, homeostasis model assessment-insulin resistance; HOMA-(3, homeostasis model assessment for beta cell function; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-creatinine ratio; VFA, visceral fat area; SFA, subcutaneous fat area; SBP, systolic blood pressure; DBP, diastolic blood pressure; OSAS, obstructive sleep apnea syndrome; IQ, Intelligence quotient; WAIS-III, Wechsler Adult Intelligence Scale 3rd edition.
Compared with the remission-maintained group, the relapse group at 5 years after LSG had significantly higher HbA1c, FPG, number of antidiabetic drugs, and high-density lipoprotein cholesterol (HDL-C); and lower BMI, frequency of BMI ≥35 kg/m2, and HOMA-β. Childhood onset obesity, mental disorders, mental retardation and developmental disorders, binge eating, full IQ, verbal IQ, and performance IQ did not differ between the remission-maintained and relapse groups.
Trend of Clinical Parameters from the First Visit to 5 Years Post-LSG
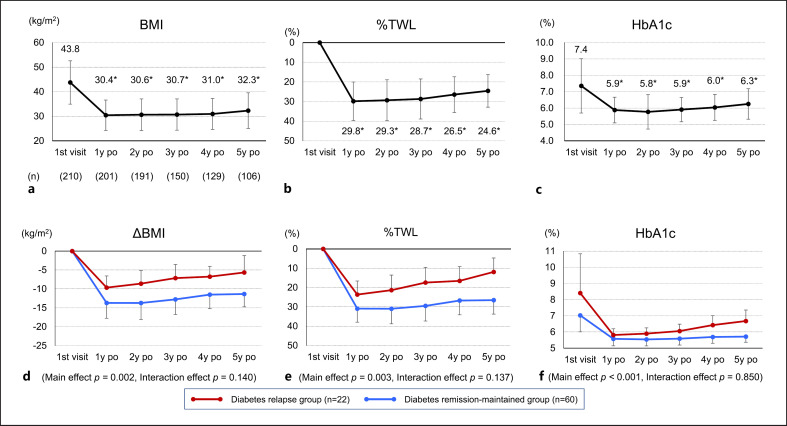
Figure 1a–c shows the trend of BMI, %TWL, and HbA1c in all included subjects (n = 210) from the first visit to 5 years after LSG. The mean postoperative observation period was 3.7 ± 1.3 years. Following LSG, subjects showed significant decreases in BMI, %TWL, and HbA1c compared with the first visit at all the time periods from 1 to 5 years after LSG.
Fig. 1.
Time courses of BMI, %TWL, and HbA1c from the first visit to 5 years after LSG for all included subjects who underwent LSG (n = 210) (Fig. a–c) and changes in ΔBMI, %TWL, and HbA1c in remission-maintained and relapse groups (Fig. d–f). Data are presented as means ± SD. *p < 0.05 versus 1st visit. Repeated measures ANOVA followed by Bonferroni multiple comparison test was used to evaluate the overall change (main effect p value) and differences between the two groups (interaction effect p value). LSG, laparoscopic sleeve gastrectomy; BMI, body mass index; %TWL, percentage total weight loss; po, postoperation.
Figure 1d–f shows the changes in ΔBMI, %TWL, and HbA1c from the first visit to 5 years after LSG in the remission-maintained and relapse groups. Overall, ΔBMI, %TWL, and HbA1c all showed significant changes from the first visit to 5 years after LSG (main effect). On the other hand, in the comparison between, no differences between the remission-maintained and relapse groups were found in the course of changes up to 5 years after LSG (interaction effect).
Percentage of Diabetes Remission and Relapse from 1 to 5 Years Post-LSG
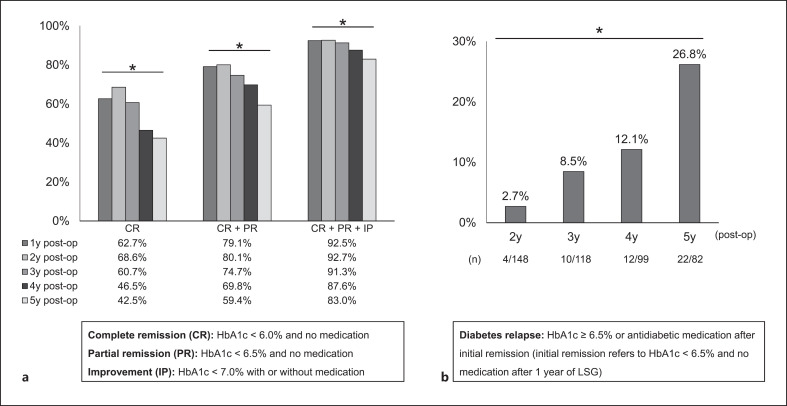
Figure 2a shows the trend of the percentage of diabetes CR, CR + PR, and CR + PR + IP from 1 to 5 years after LSG. At 1 year after LSG, CR was 62.7%, CR + PR was 79.1%, and CR + PR + IP was 92.5%. From 2 to 5 years after LSG, the percentage of CR, CR + PR, and CR + PR + IP all decreased over time. However, 83.0% of the subjects were able to achieve HbA1c <7% (IP) at 5 years after LSG.
Fig. 2.
Percentage of subjects with diabetes CR, CR + PR, CR + PR + IP (Fig. a), and relapse (Fig. b) during 5 years after LSG. *p < 0.05, Cochran's Q test. LSG, laparoscopic sleeve gastrectomy; CR, complete remission; PR, partial remission; IP, improvement; LSG, laparoscopic sleeve gastrectomy; y, years; post-op, postoperation.
Figure 2b shows the percentage of subjects who had diabetes relapse (percentage of subjects who once achieved HbA1c below 6.5% without antidiabetic medication after LSG, and subsequently deviated from this criterion). The percentage of diabetes relapse increased over time after LSG, with a significant increase at 5 years compared to 2 years after LSG.
Odds Ratios for Diabetes Relapse at 5 Years Post-LSG of Each Clinical Parameters Analyzed by Logistic Regression Models
Table 2 shows the odds ratios for diabetes relapse at 5 years after LSG for the clinical parameters analyzed by the logistic regression model. In this analysis, BMI, preoperative HbA1c, and HOMA-β, which are important indices related to diabetes control and found to be significantly different between the remission-maintained and relapse groups (Table 1), together with gender and age were entered into the models. Preoperative HbA1c was identified as an independent contributor to diabetes relapse (odds ratio 1.54, p = 0.049).
Table 2.
Odds ratios with 95% CI for diabetes relapse at 5 years after LSG for the clinical parameter analyzed by the logistic regression model
| OR | 95% CI | p value | |
|---|---|---|---|
| Male (1), female (0) | 1.02 | 0.30–3.50 | 0.980 |
| Age, years | 1.01 | 0.95–1.08 | 0.662 |
| BMI | 0.94 | 0.86–1.03 | 0.175 |
| Preoperative HbA1c, % | 1.54 | 1.00–2.37 | 0.049 |
| HOMA-β, % | 1.00 | 0.99–1.01 | 0.382 |
A p value <0.05 was considered significant. LSG, laparoscopic sleeve gastrectomy; OR, odds ratio; CI, confidence interval; BMI, body mass index, HOMA-β, homeostasis model assessment for beta cell function. Model: r2 = 0.229, p = 0.043.
Comparison of Changes in Clinical Parameters from the First Visit to 1 Year after LSG and Weight Gain Indicators from Nadir to the Most Recent Follow-Up
Table 3 shows comparisons between the remission-maintained and relapse groups of the changes in clinical parameters from the first visit to 1 year after LSG and weight gain indicators from nadir to the most recent follow-up. Compared with the remission-maintained group, the relapse group had lower changes in body weight, BMI, and triglycerides (TG); and lower %TWL at 1 year after LSG. In the diabetes relapse group, %WR of total weight loss from the nadir weight tended to be higher (p = 0.053), and the percentage of subjects who regained 25% or more weight from the nadir was significantly higher (p = 0.004).
Table 3.
Comparison of changes in clinical parameters from the first visit to 1 year after LSG, and regained BW, BMI, and %WR in the remission-maintained and relapse groups at 5 years after LSG
| Remission-maintained group (n = 60) | Relapse group (n = 22) | p value | |
|---|---|---|---|
| Change from the first visit to 1 year after LSG | |||
| ΔBW (kg) | –37.2[–44.4– (–29.2)] | –24.1[–33.3– (–18.9)] | <0.001 |
| ΔBMI, kg/m2 | –13.7±4.1 | –9.7±3.1 | <0.001 |
| %TWL, % | 30.9±7.0 | 23.6±5.9 | <0.001 |
| ΔHbA1c, % | –1.2[–2.5–(–0.7)] | –1.3[–4.0– (–0.6)] | 0.115 |
| ΔTC, md/dL | –1.7±40.9 | 3.7±48.3 | 0.644 |
| ΔTG, md/dL | –81.0[–154.5– (–30.5)] | –8.0 (–120.0–2.0) | 0.012 |
| ΔHDL-C, md/dL | 15.6±13.6 | 12.1±14.5 | 0.344 |
| ΔeGFR, mL/min/1.73 m2 | 1.3±12.4 | –2.1±20.1 | 0.397 |
| ΔVFA, cm2 | –126.8[–183.6– (–85.6)] | –125.8[–281.9– (–66.7)] | 0.754 |
| ΔSFA, cm2 | –145.1[–284.4– (–84.4)] | –92.0[–162.5– (–36.3)] | 0.247 |
| ΔSBP, mm Hg | 5.7±18.9 | 3.3±22.8 | 0.669 |
| ΔDBP, mm Hg | 3.6±12.3 | 1.1±17.2 | 0.518 |
| Indicators of postoperative weight regain at the most recent follow-upa | |||
| Regained BW from nadir weight, kg | 4.9 (2.4–8.3) | 5.8 (2.9–10.4) | 0.540 |
| Subjects with ≥10 kg weight regain from nadir, % | 15.2 | 31.6 | 0.176 |
| Regained BMI from the BMI at nadir weight, kg/m2 | 1.9 (0.9–3.1) | 2.0 (1.0–4.1) | 0.574 |
| Subjects with BMI regain ≥5 points from nadir, % | 10.9 | 21.1 | 0.430 |
| %WR of total weight loss from nadir weight, % | 14.3 (6.4–22.2) | 28.6 (9.2–51.7) | 0.053 |
| Subjects with %WR ≥25% from nadir, % | 15.2 | 52.6 | 0.004 |
LSG, laparoscopic sleeve gastrectomy; BW, body weight; BMI, body mass index; %TWL, percentage total weight loss; HbA1c, glycosylated hemoglobin; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; VFA, visceral fat area; SFA, subcutaneous fat area; SBP, systolic blood pressure; DBP, diastolic blood pressure; %WR, percentage weight regain. %WR was calculated using the following formula: (current weight – nadir weight)/(presurgery weight – nadir weight) × 100. Data are presented as mean ± SD or median (interquartile range) or percentage. Student's t test was used to analyze parametric data and Mann-Whitney U test for nonparametric data. Fisher's exact test was used to detect significant differences between proportions and categorical variables. A p value < 0.05 was considered significant.
Calculated using the change from nadir weight after LSG to the most recent weight.
Cox Proportional Hazard model for the Association of with Diabetes Relapse at 5 Years Post-LSG
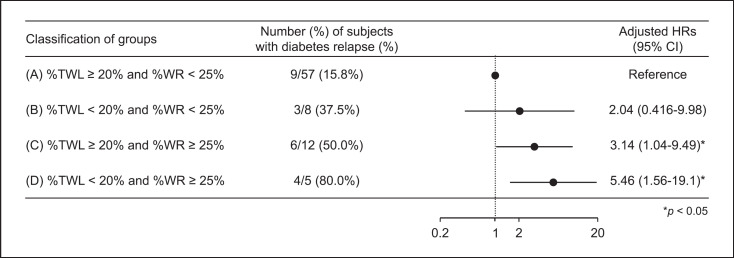
Figure 3 shows the Cox proportional hazard model for the association of postoperative weight parameters with diabetes relapse at 5 years after LSG. The original J-SMART study of morbidly obese Japanese subjects required 20.8% weight loss for determining CR of T2DM [10]. On the other hand, there is no clear evidence regarding the definition of postoperative WR that contributes to diabetes relapse after bariatric surgery in Japanese subjects. Generally, WR of 25% or more of the maximum weight loss is considered clinically meaningful [32, 33], and in Table 3, the percentage of subjects with %WR greater than 25% was significantly higher in the relapse group. Therefore, two criteria were used: %TWL of 20% or more at 1 year after LSG and %WR of less than 25% after LSG. The subjects were divided into four groups: (A) %TWL ≥20% and %WR <25%, (B) %TWL <20% and %WR <25%, (C) %TWL ≥20% and %WR ≥25%, and (D) %TWL <20% and %WR ≥25%. The hazard ratio for diabetes relapse compared to group A was not significantly different in group B, but increased significantly by 3.14-fold in group C and 5.46-fold in group D.
Fig. 3.
Cox proportional hazard model for the association of postoperative weight parameters with diabetes relapse at 5 years after LSG. HR, hazard ratio; %TWL, percentage total weight loss; %WR, percent age weight regain; CI, confidence interval. HRs were adjusted for age and gender. Percentage WR was calculated using the following formula: (current weight − nadir weight)/(presurgery weight − nadir weight) × 100. *p < 0.05. %TWL was calculated using the weight at 1 year after LSG, and %WR was calculated using the change from nadir weight after LSG to the most recent weight.
Discussion
The current study showed that preoperative HbA1c, poor postoperative weight loss, and postoperative WR contributed to T2DM relapse in Japanese subjects with severe obesity who were indicated for bariatric surgery. While bariatric surgery has been reported in many papers to induce remission of T2DM, it has become clear in recent years that relapse of diabetes is not uncommon during the long-term follow-up. The relapse rate after diabetes is remitted at one point is 32% after sleeve gastrectomy or RYGB [34]. Even after RYGB, a large clinical study found that 35% of the patients relapsed 5 years after surgery [35]. In this study which included only LSG, the diabetes relapse rate at 5 years after surgery was 26.8%, showing a similar trend as in previous reports [7, 36]. On the other hand, diabetes improved in 83.0% of all subjects at 5 years after LSG in this study. In a previous report comparing bariatric surgery with intensive medical therapy [37], only 21% of the subjects achieved an HbA1c of 7% or less with medical therapy during a 5-year observation period. In other words, although diabetes relapses in a portion of subjects after bariatric surgery, there is no doubt that this modality is superior to medical therapy for the control of diabetes.
Known independent baseline predictors of postoperative diabetes relapse include the number of preoperative antidiabetic medications, duration of preoperative T2DM, using sleeve gastrectomy as opposed to RYGB, and poor initial weight loss or postoperative WR [7, 34]. In the present study, the relapse group also had a larger number of antidiabetic medications, lower %TWL at 1 year after LSG, and higher WR after LSG, consistent with previous studies. On the other hand, since the present study included only subjects who underwent LSG, the impact of different techniques on diabetes relapse was not studied. In Japan, because RYGB is not covered by insurance, LSG which is covered by insurance is very often chosen [3, 38]. Although RYGB has been reported to be associated with less diabetes relapses than LSG [39], there are few studies on this aspect in Japanese subjects. It is very important to compare the difference in diabetes relapse rates between LSG and other procedures including RYGB.
A recent study in Asian subjects has reported that the ABCD score is also useful in predicting diabetes relapse [40]. The ABCD score that predicts remission of T2DM after bariatric surgery consists of the following factors: age (A), BMI (B), serum CPR (C), and duration of diabetes (D). Each factor is easy to calculate, and the effect of LSG on diabetes remission in Japanese subjects correlates well with the ABCD score [41, 42, 43]. In addition, impaired pancreatic beta-cell function, characterized by decreased serum CPR, correlates well with the duration of long-term diabetes [44]. In this sense, the ABCD score may be useful as an indicator of diabetes remission or relapse after bariatric surgery. The results of our study, however, showed no difference in ABCD scores between the remission-maintained and relapse groups. Whether the ABCD score is useful for detecting diabetes relapse after bariatric surgery needs to be studied in detail by accumulating more subjects indicated for bariatric surgery in the future. Other baseline data in this study included significantly higher HDL-C in the relapse group (Table 1). In general, HDL-C often decreases with increasing TG and low-density lipoprotein cholesterol levels. HDL-C is also known to increase with exercise. However, there was no difference in TG levels (Table 1) and exercise habits between two groups (data not shown). The direct cause of why HDL-C was higher in the relapse group is unknown.
In bariatric surgery, weight in kilogram, BMI, and percentage of presurgery weight are often used as indicators of postoperative weight loss. WR is often expressed as weight in kilogram, BMI, percentage of presurgery weight, percentage of nadir weight, or percentage of maximum weight loss [33]. In Table 3 of the current study, we also evaluated postoperative weight loss and WR using representative indices. The group with postoperative diabetes relapse had lower percentage of presurgery weight, i.e., %TWL, and higher percentage of maximum weight loss after LSG, i.e., %WR, at 1 year after LSG. However, many clinically meaningful criteria for WR have been evaluated in the context of gastric bypass [32, 45, 46]. Our study was confined to LSG, and the criteria for WR after LSG have not been studied in detail. Therefore, it is important to establish a definition of WR after LSG. Essentially, receiver-operating characteristic curves should be generated to obtain the cutoff value for %WR that contributes to diabetes relapse, and the hazard ratio that contributes to diabetes relapse should be evaluated. However, we did not obtain receiver-operating characteristic curves with sufficient sensitivity and specificity from the data in the present study. This may be due to inadequate number of subjects and follow-up duration. Additional analysis with larger sample size and longer observation period is needed. Few studies have combined a measure of postoperative weight loss with postoperative WR to obtain hazard ratios for diabetes relapse. In this study, postoperative %TWL less than 20% and %WR greater than or equal to 25% had a 5.46-fold hazard ratio for diabetes relapse. This means that not only adequate postoperative weight loss but also minimal WR is important.
Psychiatric disorders are known to be common in morbidly obese patients who are indicated for bariatric surgery [47], and there is a report of psychological factors influencing compliance with postoperative diet therapy [48]. In another study, patients with poor postoperative weight loss frequently had mood disorders and mental retardation/developmental disorders, and snacking and eating out habits were often observed [49]. We have also reported previously that childhood onset obesity, mental retardation and developmental disorders, and binge eating contribute to postoperative WR [17]. The present study revealed that postoperative WR contributed to diabetes relapse, but the frequencies of childhood onset obesity, psychiatric disorders, mental retardation and developmental disorders, binge eating, and IQ were not different between the remission-maintained and relapse groups (Table 1). One reason why childhood onset obesity contributes to WR, but not to diabetes relapse after LSG, may be due to the characteristics of “metabolically healthy” obesity in subjects with childhood onset obesity, such as relatively low preoperative HbA1c, preserved insulin secretion capacity, and low VFA fat area [17]. There are very few studies on the association between psychosocial factors and diabetes relapse after bariatric surgery, and further detailed studies are warranted.
This study has several limitations. First, the study had a retrospective design. However, there are difficulties in conducting prospective study on subjects undergoing bariatric surgery followed over a long period of time, and a feasible option is to analyze existing data retrospectively to search for factors that contribute to diabetes relapse. On the other hand, subjects who do not have diabetes before surgery or whose diabetes has not remitted will be excluded from the analysis, making it important to conduct a retrospective study that includes a large number of subjects followed for a long period. Furthermore, because this study included only subjects who underwent LSG, the results are not necessarily generalizable to patients undergoing other bariatric procedures including RYGB. However, since LSG occupies approximately 90% of bariatric surgeries in Japan and the number of LSG procedures and research on LSG are increasing worldwide [50, 51], the present study has important clinical relevance. Another weakness that must be noted is that this study included only Japanese subjects, and racial differences were not considered. One other limitation is the short follow-up period, which may affect the postoperative diabetes relapse rate.
Throughout this study, we examined the rate of diabetes relapse and the factors that contribute to relapse. Although diabetes relapsed at a certain rate after LSG, HbA1c in the relapse group at 5 years after LSG remained lower than that before the surgery. This result implies that even when diabetes relapses after bariatric surgery, LSG is not a failure because the procedure certainly contributes to lower the risk of cardiovascular events. Recently, evidence is accumulating not only for risk reduction of cardiovascular events and mortality [52, 53, 54] but also for amelioration of renal failure progression [55, 56] and reduction of cancer risk [57]. There is no doubt that bariatric surgery should be performed appropriately in patients with indications for the procedure.
Conclusion
While LSG provides a high remission rate for T2DM, diabetes relapse is not uncommon. Preoperative HbA1c, poor weight loss, and excess WR after LSG were found to contribute to diabetes relapse, suggesting that treatment strategies targeting these factors are important. It is desirable to increase the number of subjects who are eligible for LSG, to calculate a unique Japanese %WR cutoff for diabetes relapse, and to reevaluate the results over a longer follow-up period.
Statement of Ethics
This study was performed in accordance with the ethical standards of the Institutional and Japanese Research Committees and the ethical standards of the 1975 Declaration of Helsinki. Opt-out informed consent protocol was used in this study on the website without written consent. This consent procedure was reviewed and approved by the Ethics Committee of Toho University Sakura Medical Center (approval number S19074).
Conflict of Interest Statement
IT received lecture fee from Takeda Pharmaceutical Co., Ltd. and Novartis Pharma Ltd.; and honorarium from Takeda Pharmaceutical Co., Ltd., ONO PHARMACEUTICAL CO., LTD., and Bayer Yakunin, Ltd. TN received honorarium from Chugai Pharmaceutical Co., Ltd., TAIHO PHARMACEUTICAL CO., LTD., Medtronic, Inc., Johnson & Johnson, Inc., Olympus Corporation, TERUMO CORPORATION, Sumitomo Bakelite Co., Ltd., Takeda Pharmaceutical Co., Ltd., Merck & Co., MC MEDICAL, INC., DAIICHI SANKYO Co., Ltd., Bayer Yakuhin, Ltd., Nippon Boehringer lngelheim Co., Ltd., and Eli Lilly Japan K.K.; research grant from Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (C) (#17K10575), Chugai Pharmaceutical Co., Ltd., TAIHO PHARMACEUTICAL CO., LTD., and Medtronic, Inc. YM received honorarium from Otsuka Pharmaceutical Co., Ltd. and COVIDIEN JAPAN INC. MT received lecture fee from Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., and ONO PHARMACEUTICAL CO., LTD. KY received grant from Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., MSD K.K., Pfizer Japan Inc., Novo Nordisk Pharma Ltd., Taisho Pharmaceutical Co., Ltd., Kao Corporation, ONO PHARMACEUTICAL CO., LTD., Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., DAIICHI SANKYO Co., Ltd., Teijin Pharma Limited, Shionogi Co., Ltd., Astellas Pharma Inc., Kowa Company, Ltd., and Bayer Yakuhin, Ltd.; consulting fees from Kowa Company, Ltd., Mitsubishi Tanabe Pharma Corporation, Novartis Pharma K.K., Novo Nordisk Pharma Ltd., AstraZeneca K.K., Pfizer Japan Inc., and Bayer Yakuhin, Ltd.; honorarium from Kowa Company, Ltd., MSD K.K., Astellas Pharma Inc., Mitsubishi Tanabe Pharma Corporation, Amgen K.K., Takeda pharmaceutical Co., Ltd., Sanofi K.K., ONO PHARMACEUTICAL CO., LTD., AstraZeneca K.K., Daiichi Sankyo Company Limited, Novartis Pharma K.K., Sumitomo Dainippon Pharma Co., Ltd., Kyowa Kirin Co., Ltd., Pfizer Japan Inc., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., Taisho Pharmaceutical Co., Ltd., and Janssen Pharmaceutical K.K. KK received lecture fee from ETHICON, COVIDIEN JAPAN INC. YI received honorarium from MSD K.K., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., ONO PHARMACEUTICAL CO., LTD., Sanofi K.K., and Mitsubishi Tanabe Pharma Corporation. YS received research grant from Medtronic, Inc., Johnson & Johnson, Inc., Nikkiso Co., Ltd., Sunny Health Co., Ltd., and Daiwa Securities Health Foundation.
Funding Sources
J-SMART was supported by a grant for research on intractable diseases from the Ministry of Health, Labour and Welfare of Japan (H28-nanji-ippan-014).
Author Contributions
Atsuhito Saiki and Takashi Yamaguchi designed the original concept. Yasuhiro Watanabe wrote the initial draft of the manuscript. Atsuhito Saiki and Ichiro Tatsuno reviewed and edited the manuscript. Daiji Nagayama, Sho Tanaka, Akira Sasaki, Takeshi Naitoh, Hisahiro Matsubara, Koutaro Yokote, Shinichi Okazumi, Satoshi Ugi, Hiroshi Yamamoto, Masayuki Ohta, Yasushi Ishigaki, Kazunori Kasama, Yosuke Seki, Motoyoshi Tsujino, Kohji Shirai, Yasuhiro Miyazaki, and Takayuki Masaki contributed to data collection and interpretation, and critically reviewed and approved the final version of the manuscript. Atsuhito Saiki agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgment
The authors wish to thank Aki Hagiwara for the collection and assembly of data.
Funding Statement
J-SMART was supported by a grant for research on intractable diseases from the Ministry of Health, Labour and Welfare of Japan (H28-nanji-ippan-014).
References
- 1.Angrisani L, Santonicola A, Iovino P, Ramos A, Shikora S, Kow L. Bariatric surgery survey 2018 similarities and disparities among the 5 IFSO chapters. Obes Surg. 2021 May;31((5)):1937–1948. doi: 10.1007/s11695-020-05207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018 Mar;14((3)):259–263. doi: 10.1016/j.soard.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Ohta M, Kasama K, Sasaki A, Naitoh T, Seki Y, Inamine S, et al. Current status of laparoscopic bariatric/metabolic surgery in Japan the sixth nationwide survey by the Japan Consortium of Obesity and Metabolic Surgery. Asian J Endosc Surg. 2021 Apr;14((2)):170–177. doi: 10.1111/ases.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McTigue KM, Wellman R, Nauman E, Anau J, Coley RY, Odor A, et al. Comparing the 5-year diabetes outcomes of sleeve gastrectomy and gastric bypass the national patient-centered clinical research network (PCORNet) bariatric study. JAMA Surg. 2020 May 1;155((5)):e200087. doi: 10.1001/jamasurg.2020.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofsø D, Fatima F, Borgeraas H, Birkeland KI, Gulseth HL, Hertel JK, et al. Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg) a single-centre, triple-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2019 Dec;7((12)):912–924. doi: 10.1016/S2213-8587(19)30344-4. [DOI] [PubMed] [Google Scholar]
- 6.Ali M, El Chaar M, Ghiassi S, Rogers AM. American Society for Metabolic and Bariatric Surgery Clinic; al Issues Committee. American Society for Metabolic and Bariatric Surgery updated position statement on sleeve gastrectomy as a bariatric procedure. Surg Obes Relat Dis. 2017 Oct;13((10)):1652–1657. doi: 10.1016/j.soard.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Aminian A, Vidal J, Salminen P, Still CD, Nor Hanipah Z, Sharma G, et al. Late relapse of diabetes after bariatric surgery not rare, but not a failure. Diabetes Care. 2020 Mar;43((3)):534–540. doi: 10.2337/dc19-1057. [DOI] [PubMed] [Google Scholar]
- 8.Conte C, Lapeyre-Mestre M, Hanaire H, Ritz P. Diabetes remission and relapse after bariatric surgery a nationwide population-based study. Obes Surg. 2020 Dec;30((12)):4810–4820. doi: 10.1007/s11695-020-04924-3. [DOI] [PubMed] [Google Scholar]
- 9.Pessoa BM, Browning MG, Mazzini GS, Wolfe L, Kaplan A, Khoraki J, et al. Factors mediating type 2 diabetes remission and relapse after gastric bypass surgery. J Am Coll Surg. 2020 Jan;230((1)):7–16. doi: 10.1016/j.jamcollsurg.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Saiki A, Yamaguchi T, Tanaka S, Sasaki A, Naitoh T, Seto Y, et al. Background characteristics and postoperative outcomes of insufficient weight loss after laparoscopic sleeve gastrectomy in Japanese patients. Ann Gastroenterol Surg. 2019 Nov;3((6)):638–647. doi: 10.1002/ags3.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma RCW, Chan JCN. Type 2 diabetes in East Asians similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013 Apr;1281((1)):64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funakoshi S, Fujimoto S, Hamasaki A, Fujiwara H, Fujita Y, Ikeda K, et al. Utility of indices using C-peptide levels for indication of insulin therapy to achieve good glycemic control in Japanese patients with type 2 diabetes. J Diabetes Investig. 2011 Aug 2;2((4)):297–303. doi: 10.1111/j.2040-1124.2010.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albareda M, Rigla M, Rodríguez-Espinosa J, Caballero A, Chico A, Cabezas R, et al. Influence of exogenous insulin on C-peptide levels in subjects with type 2 diabetes. Diabetes Res Clin Pract. 2005 Jun;68((3)):202–206. doi: 10.1016/j.diabres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Ahuja V, Kadowaki T, Evans RW, Kadota A, Okamura T, El Khoudary SR, et al. Comparison of HOMA-IR HOMA-β% and disposition index between US white men and Japanese men in Japan the ERA JUMP study. Diabetologia. 2015 Feb;58((2)):265–271. doi: 10.1007/s00125-014-3414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28((7)):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009 Jun;53((6)):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe Y, Yamaguchi T, Tanaka S, Sasaki A, Naitoh T, Matsubara H, et al. Characteristics of childhood onset and post-puberty onset obesity and weight regain after laparoscopic sleeve gastrectomy in Japanese subjects a subgroup analysis of J-SMART. Obes Facts. 2022 May 9;15((4)):498–507. doi: 10.1159/000524941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubo T. Common approach to childhood obesity in Japan. J Pediatr Endocrinol Metab. 2014 Jul;27((7–8)):581–592. doi: 10.1515/jpem-2014-0047. [DOI] [PubMed] [Google Scholar]
- 19.Isojima T, Yokoya S. The value of anthropometric indices for childhood obesity in Japan. Ann Hum Biol. 2019 Jun;46((4)):293–297. doi: 10.1080/03014460.2019.1643404. [DOI] [PubMed] [Google Scholar]
- 20.Akashiba T, Inoue Y, Uchimura N, Ohi M, Kasai T, Kawana F, et al. Sleep apnea syndrome (SAS) clinical practice guidelines 2020. Respir Investig. 2022 Jan;60((1)):3–32. doi: 10.1016/j.resinv.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SKH, Chen SC, et al. Predicting success of metabolic surgery age, body mass index, C-peptide, and duration score. Surg Obes Relat Dis. 2013 May-Jun;9((3)):379–384. doi: 10.1016/j.soard.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Lee WJ, Almulaifi A, Tsou JJ, Ser KH, Lee YC, Chen SC. Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus predicting the success by ABCD score. Surg Obes Relat Dis. 2015 Sep-Oct;11((5)):991–996. doi: 10.1016/j.soard.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Lee MH, Lee WJ, Chong K, Chen JC, Ser KH, Lee YC, et al. Predictors of long-term diabetes remission after metabolic surgery. J Gastrointest Surg. 2015 Jun;19((6)):1015–1021. doi: 10.1007/s11605-015-2808-1. [DOI] [PubMed] [Google Scholar]
- 24.Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015 May-Jun;11((3)):489–506. doi: 10.1016/j.soard.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association 6. Glycemic targets standards of medical care in diabetes-2019. Diabetes Care. 2019 Jan;42((Suppl 1)):S61–S70. doi: 10.2337/dc19-S006. [DOI] [PubMed] [Google Scholar]
- 26.Lauti M, Lemanu D, Zeng ISL, Su'a B, Hill AG, MacCormick AD. Definition determines weight regain outcomes after sleeve gastrectomy. Surg Obes Relat Dis. 2017 Jul;13((7)):1123–1129. doi: 10.1016/j.soard.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 27.Liu SYW, Wong SKH, Lam CCH, Yung MY, Kong APS, Ng EKW. Long-term results on weight loss and diabetes remission after laparoscopic sleeve gastrectomy for A morbidly obese Chinese population. Obes Surg. 2015 Oct;25((10)):1901–1908. doi: 10.1007/s11695-015-1628-4. [DOI] [PubMed] [Google Scholar]
- 28.Abdallah E, El Nakeeb A, Youssef T, Abdallah H, Ellatif MA, Lotfy A, et al. Impact of extent of antral resection on surgical outcomes of sleeve gastrectomy for morbid obesity (a prospective randomized study) Obes Surg. 2014 Oct;24((10)):1587–1594. doi: 10.1007/s11695-014-1242-x. [DOI] [PubMed] [Google Scholar]
- 29.Braghetto I, Csendes A, Lanzarini E, Papapietro K, Cárcamo C, Molina JC. Is laparoscopic sleeve gastrectomy an acceptable primary bariatric procedure in obese patients? Early and 5-year postoperative results. Surg Laparosc Endosc Percutan Tech. 2012 Dec;22((6)):479–486. doi: 10.1097/SLE.0b013e318262dc29. [DOI] [PubMed] [Google Scholar]
- 30.Casella G, Soricelli E, Giannotti D, Collalti M, Maselli R, Genco A, et al. Long-term results after laparoscopic sleeve gastrectomy in a large monocentric series. Surg Obes Relat Dis. 2016 May;12((4)):757–762. doi: 10.1016/j.soard.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Brethauer SA, Aminian A, Romero-Talamás H, Batayyah E, Mackey J, Kennedy L, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013 Oct;258((4)):628–636. doi: 10.1097/SLA.0b013e3182a5034b. discussion 636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper TC, Simmons EB, Webb K, Burns JL, Kushner RF. Trends in weight regain following roux-en-Y gastric bypass (RYGB) bariatric surgery. Obes Surg. 2015 Aug;25((8)):1474–1481. doi: 10.1007/s11695-014-1560-z. [DOI] [PubMed] [Google Scholar]
- 33.King WC, Hinerman AS, Belle SH, Wahed AS, Courcoulas AP. Comparison of the performance of common measures of weight regain after bariatric surgery for association with clinical outcomes. JAMA. 2018;320((15)):1560–1569. doi: 10.1001/jama.2018.14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Debédat J, Sokolovska N, Coupaye M, Panunzi S, Chakaroun R, Genser L, et al. Long-term relapse of type 2 diabetes after roux-en-Y gastric bypass prediction and clinical relevance. Diabetes Care. 2018 Oct;41((10)):2086–2095. doi: 10.2337/dc18-0567. [DOI] [PubMed] [Google Scholar]
- 35.Arterburn DE, Bogart A, Sherwood NE, Sidney S, Coleman KJ, Haneuse S, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013 Jan;23((1)):93–102. doi: 10.1007/s11695-012-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jans A, Szabo E, Näslund I, Ottosson J, Näslund E, Stenberg E. Factors affecting relapse of type 2 diabetes after bariatric surgery in Sweden 2007-2015 a registry-based cohort study. Surg Obes Relat Dis. 2022 Mar;18((3)):305–312. doi: 10.1016/j.soard.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes 5-year outcomes. N Engl J Med. 2017 Feb 16;376((7)):641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oshiro T, Kasama K, Nabekura T, Sato Y, Kitahara T, Matsunaga R, et al. Current status and issues associated with bariatric and metabolic surgeries in Japan. Obes Surg. 2021 Jan;31((1)):343–349. doi: 10.1007/s11695-020-05056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayoz C, Hermann T, Raptis DA, Brönnimann A, Peterli R, Zuber M. Comparison of metabolic outcomes in patients undergoing laparoscopic roux-en-Y gastric bypass versus sleeve gastrectomy a systematic review and meta-analysis of randomised controlled trials. Swiss Med Wkly. 2018;148:w14633. doi: 10.57187/smw.2018.14633. [DOI] [PubMed] [Google Scholar]
- 40.Yang PJ, Su YH, Shen SC, Lee PC, Lin MT, Lee WJ, et al. Predictors of diabetes relapse after metabolic surgery in Asia. Surg Obes Relat Dis. 2022 Apr;18((4)):454–461. doi: 10.1016/j.soard.2021.11.018. [DOI] [PubMed] [Google Scholar]
- 41.Lee MH, Almalki OM, Lee WJ, Chen SC, Chen JC, Wu CC. Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus long-term result and recurrence of diabetes. Obes Surg. 2020 Oct;30((10)):3669–3674. doi: 10.1007/s11695-020-04737-4. [DOI] [PubMed] [Google Scholar]
- 42.Umemura A, Sasaki A, Nitta H, Nikai H, Baba S, Takahara T, et al. Prognostic factors and a new preliminary scoring system for remission of type 2 diabetes mellitus after laparoscopic sleeve gastrectomy. Surg Today. 2020 Sep;50((9)):1056–1064. doi: 10.1007/s00595-020-01990-z. [DOI] [PubMed] [Google Scholar]
- 43.Haruta H, Kasama K, Ohta M, Sasaki A, Yamamoto H, Miyazaki Y, et al. Long-term outcomes of bariatric and metabolic surgery in Japan results of a multi-institutional survey. Obes Surg. 2017 Mar;27((3)):754–762. doi: 10.1007/s11695-016-2361-3. [DOI] [PubMed] [Google Scholar]
- 44.Leighton E, Sainsbury CA, Jones GC. A practical review of C-peptide testing in diabetes. Diabetes Ther. 2017 Jun;8((3)):475–487. doi: 10.1007/s13300-017-0265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanos BR, Saules KK, Schuh LM, Sogg S. Predictors of lowest weight and long-term weight regain among roux-en-Y gastric bypass patients. Obes Surg. 2015 Aug;25((8)):1364–1370. doi: 10.1007/s11695-014-1536-z. [DOI] [PubMed] [Google Scholar]
- 46.Roslin M, Damani T, Oren J, Andrews R, Yatco E, Shah P. Abnormal glucose tolerance testing following gastric bypass demonstrates reactive hypoglycemia. Surg Endosc. 2011 Jun;25((6)):1926–1932. doi: 10.1007/s00464-010-1489-9. [DOI] [PubMed] [Google Scholar]
- 47.Dawes AJ, Maggard-Gibbons M, Maher AR, Booth MJ, Miake-Lye I, Beroes JM, et al. Mental Health conditions among patients seeking and undergoing bariatric surgery a meta-analysis. Jama. 2016 Jan 12;315((2)):150–163. doi: 10.1001/jama.2015.18118. [DOI] [PubMed] [Google Scholar]
- 48.Bergh I, Lundin Kvalem I, Risstad H, Sniehotta FF. Preoperative predictors of adherence to dietary and physical activity recommendations and weight loss one year after surgery. Surg Obes Relat Dis. 2016 May;12((4)):910–918. doi: 10.1016/j.soard.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Saiki A, Kanai R, Nakamura S, Tanaka S, Oka R, Watanabe Y, et al. Impact of mental Health background and nutrition intake on medium-term weight loss in Japanese patients undergoing laparoscopic sleeve gastrectomy. Obes Facts. 2020;13((4)):371–383. doi: 10.1159/000509342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dang JT, Shelton J, Mocanu V, Sun W, Birch DW, Karmali S, et al. Trends and outcomes of laparoscopic sleeve gastrectomy between 2015 and 2018 in the USA and Canada. Obes Surg. 2021 Feb;31((2)):675–681. doi: 10.1007/s11695-020-04939-w. [DOI] [PubMed] [Google Scholar]
- 51.Barqawi A, Abushamma FA, Akkawi M, Al-Jabi SW, Shahwan MJ, Jairoun AA, et al. Global trends in research related to sleeve gastrectomy a bibliometric and visualized study. World J Gastrointest Surg. 2021 Nov 27;13((11)):1509–1522. doi: 10.4240/wjgs.v13.i11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doumouras AG, Wong JA, Paterson JM, Lee Y, Sivapathasundaram B, Tarride JE, et al. Bariatric surgery and cardiovascular outcomes in patients with obesity and cardiovascular disease: a population-based retrospective cohort study. Circulation. 2021 Apr 13;143((15)):1468–1480. doi: 10.1161/CIRCULATIONAHA.120.052386. [DOI] [PubMed] [Google Scholar]
- 53.Singh P, Subramanian A, Adderley N, Gokhale K, Singhal R, Bellary S, et al. Impact of bariatric surgery on cardiovascular outcomes and mortality a population-based cohort study. Br J Surg. 2020 Mar;107((4)):432–442. doi: 10.1002/bjs.11433. [DOI] [PubMed] [Google Scholar]
- 54.Blanco DG, Funes DR, Giambartolomei G, Lo Menzo E, Szomstein S, Rosenthal RJ. Laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass in cardiovascular risk reduction a match control study. Surg Obes Relat Dis. 2019 Jan;15((1)):14–20. doi: 10.1016/j.soard.2018.09.488. [DOI] [PubMed] [Google Scholar]
- 55.Docherty NG, le Roux CW. Bariatric surgery for the treatment of chronic kidney disease in obesity and type 2 diabetes mellitus. Nat Rev Nephrol. 2020 Dec;16((12)):709–720. doi: 10.1038/s41581-020-0323-4. [DOI] [PubMed] [Google Scholar]
- 56.Cohen RV, Pereira TV, Aboud CM, Petry TBZ, Lopes Correa JL, Schiavon CA, et al. Effect of gastric bypass vs best medical treatment on early-stage chronic kidney disease in patients with type 2 diabetes and obesity a randomized clinical trial. JAMA Surg. 2020 Aug 1;155((8)):e200420. doi: 10.1001/jamasurg.2020.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Courcoulas AP. Bariatric surgery and cancer risk. Jama. 2022 Jun 3;327((24)):2400–2402. doi: 10.1001/jama.2022.9166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.