Abstract
PURPOSE OF REVIEW:
This article discusses the clinical, neuroimaging, and biomarker profiles of sporadic atypical Alzheimer disease (AD) variants, including early-onset AD, posterior cortical atrophy, logopenic variant primary progressive aphasia, dysexecutive variant and behavioral variant AD, and corticobasal syndrome.
RECENT FINDINGS:
Significant advances are being made in the recognition and characterization of the syndromically diverse AD variants. These variants are identified by the predominant cognitive and clinical features: early-onset amnestic syndrome, aphasia, visuospatial impairments, dysexecutive and behavioral disturbance, or motor symptoms. Although understanding of regional susceptibility to disease remains in its infancy, visualizing amyloid and tau pathology in vivo and CSF examination of amyloid-β and tau proteins are particularly useful in atypical AD, which can be otherwise prone to misdiagnosis. Large-scale research efforts, such as LEADS (the Longitudinal Early-Onset Alzheimer Disease Study), are currently ongoing and will continue to shed light on our understanding of these diverse presentations.
SUMMARY:
Understanding the clinical, neuroimaging, and biomarker profiles of the heterogeneous group of atypical AD syndromes improves diagnostic accuracy in patients who are at increased risk of misdiagnosis. Earlier accurate identification facilitates access to important interventions, social services and disability assistance, and crucial patient and family education.
INTRODUCTION
Alzheimer disease (AD) is a gradually progressive neurodegenerative disorder characterized by the progressive deposition of amyloid plaques and neurofibrillary tangles in the cortical brain tissue. AD typically starts in the seventh or eighth decade of life. However, approximately 3% of the 5.8 million Americans with AD developed symptoms at age 65 or younger.1 These individuals are classified as having early-onset AD, also known as young-onset AD. In Europe, the incidence of dementia between the ages of 45 and 64 has been reported by one group as 12 cases per 100,000 persons per year and by another as seven cases per 100,000 persons per year. The prevalence is 54 per 100,000 in those 30 to 44 years of age and 98 per 100,000 in those 45 to 64 years of age.2-4
Individuals with young-onset cognitive impairment, especially those with rarer AD variants, often face a significant delay in diagnosis. The atypical nonamnestic phenotypes are also often misdiagnosed, which results in further setbacks in access to AD treatments and to social and financial support services. This article reviews the sporadic atypical Alzheimer disease presentations and discusses how recent advances in imaging and fluid biomarkers can be used to efficiently arrive at the correct diagnosis and guide treatment. For an in-depth review of genetically predetermined early-onset AD due to autosomal dominant mutations or Down syndrome, refer to the article “Genetics of Alzheimer Disease” by Suman Jayadev, MD,5 in this issue of Continuum.
AMNESTIC VARIANT EARLY-ONSET ALZHEIMER DISEASE
Amnestic variant early-onset AD is the most common atypical AD variant. It represents the vast majority of the cases diagnosed before age 65. Clinically, patients with amnestic early-onset AD present similarly to their late-onset AD counterparts with early progressive memory loss. However, compared to late-onset AD, patients with sporadic early-onset AD show more rapid clinical decline6,7 and greater impairment in attention, language, and visuospatial and executive functions.8-14 Aside from the mental status part of the examination, the neurologic examination in amnestic early-onset AD typically is normal. Occasionally patients may have pathologic reflexes such as grasp, root, and suck reflexes, but these typically manifest later in the disease course.
Neuropsychologically, the cognitive profile is predominated by memory impairment, specifically poor learning over repeated trials and rapid forgetting of material over short and long delays (eg, as assessed on the Rey Auditory Verbal Learning Test15 or the Wechsler Memory Scale 4th Edition [WMS-IV] Logical Memory and Visual Reproduction).16 Impairments in word generation (eg, as assessed on the Boston Naming Test,17 category fluency18), visuoconstruction (eg, as assessed on the Wechsler Adult Intelligence Scale 4th Edition [WAIS-IV] Block Design19 or the Rey-Osterrieth Complex Figure Test),18 and aspects of executive functioning (eg, as assessed on the Trail Making Test Part B)18 are typically present also. Although neuropsychological profiles tend to be similar to those found in late-onset AD, the rate of decline on testing tends to be much more rapid in early-onset AD (CASE 2-1).6,7
CASE 2-1.
A 56-year-old woman with 16 years of education presented with 6 months of short-term memory loss with preserved remote memory, as reported by her spouse. The patient repeated questions within minutes and forgot conversations within hours. She was frequently unsure of the date and sometimes the month and had attentional difficulty. She had no reported issues with language, navigation, vision, motor functioning, or behavior. She was independent in activities of daily living and instrumental activities of daily living and was working as a bank manager. She reported mild anxiety and depression, confirmed by her spouse.
On examination, she frequently repeated herself and relied on her spouse for much of her recent personal history. Her Mini-Mental State Examination (MMSE)20 score was 23/30, losing points for cube drawing, delayed recall, and serial 7s. Neurologic examination was normal. Neuropsychological testing revealed prominent impairments in learning and rapid forgetting over delays. Visuospatial construction was mildly impaired. She made errors on the Trail Making Test Part B. Language was generally within normal limits.
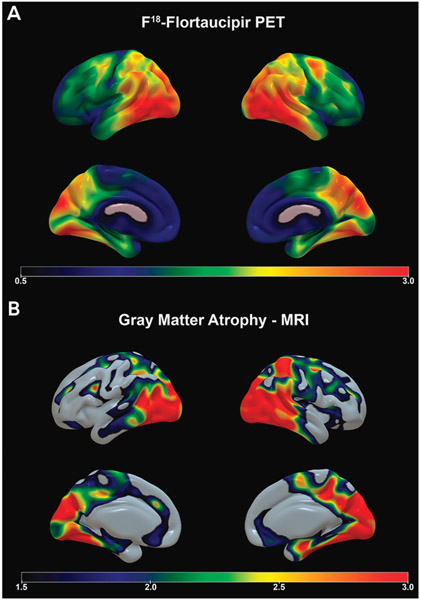
She met criteria for multidomain amnestic mild cognitive impairment, suspected to be secondary to early-onset Alzheimer disease (AD). MRI of her brain revealed bilateral medial temporal/hippocampal, parietal, and, to a lesser extent, frontal atrophy (FIGURE 2-1). Amyloid positron emission tomography (PET) revealed abnormally elevated amyloid throughout the brain consistent with AD. Flortaucipir F 18 PET (tau PET), which was part of an experimental protocol, showed intense tau ligand uptake in the medial and lateral temporal cortices and the parietal cortex, with milder uptake in the frontal lobes (figure 2-1).
The clinician counseled the family on establishing contingency plans for the future. Two years later, she experienced further decline in memory, language, executive functioning, and visuospatial abilities. She became lost several times, ceased driving, and stopped working. Her spouse took over the finances as she was forgetting to pay bills. She was more irritable, anxious, and apathetic. She became suspicious about neighbors stealing from them despite no evidence that this was occurring. She was started on donepezil with initial benefit.
COMMENT
On presentation, this patient met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for mild neurocognitive disorder (ie, mild cognitive impairment). Given her presentation, early onset, and pattern of impairments, sufficient concern existed for possible AD. Amyloid imaging of this patient assisted the clinician in directing therapeutic decisions and counseling.
The diagnostic criteria used for the amnestic variant of early-onset AD are the same as those used for late-onset AD. It must be noted that the National Institute on Aging-Alzheimer’s Association criteria for mild cognitive impairment and dementia have recognized the diagnostic value of disease biomarkers with proven sensitivity, specificity, and pathologic validity for detecting AD changes in the brain (molecular neuroimaging) or the CSF (abnormal levels of amyloid-β [Aβ] and tau proteins).21,22 Such disease-specific biomarkers are particularly useful when assessing atypical AD presentations, including early-onset AD.
Just like patients with late-onset AD, patients with early-onset AD must receive a structural neuroimaging examination (preferably an MRI study) to rule out potentially treatable causes of cognitive decline, such as normal pressure hydrocephalus, tumor, central nervous system infection, and subdural hematoma, as well as to determine the presence and extent of vascular pathology that can contribute to the cognitive syndrome.23,24 A pattern of medial and lateral temporal and parietal atrophy, although nonspecific, can be suggestive of AD etiology. Early involvement of the frontal lobe is also more typical of early-onset AD than late-onset AD (FIGURE 2-1). It should also be noted that early-onset AD can be associated with relative hippocampal sparing25 and less pronounced medial temporal lobe involvement.26,27
FIGURE 2-1.
Imaging of the patient in CASE 2-1. Amyloid imaging showed diffuse cortical and subcortical signal consistent with Alzheimer disease (not shown). A, Flortaucipir F 18 positron emission tomography (PET) shows intense tau ligand uptake in the medial and lateral temporal and parietal cortices and milder uptake in the frontal lobes. Flortaucipir F 18 PET images show the standardized uptake value ratio (SUVR) of tau, where warmer colors (yellow, red, and orange) indicate more tau uptake. B, Sagittal volumetric MRI presented in three-dimensional space shows bilateral medial temporal/hippocampal, parietal, and, to a lesser extent, frontal atrophy (bottom panel); warmer colors (yellow, red, and orange) indicate areas of greater gray matter atrophy.
Dementia onset at a young age has disproportionately devastating consequences for patients and their families. Thus, molecular neuroimaging visualizing amyloid and tau pathology in vivo or a CSF examination determining the levels of the Aβ and tau proteins is particularly useful when evaluating middle-aged individuals with cognitive decline suggestive of AD. The Appropriate Use Criteria for Amyloid PET identify patients with early-onset AD as one of the three categories of patients that would benefit the most from amyloid PET imaging.28,29
Although the pathologic substrate of early-onset AD and late-onset AD is the same, notable differences exist in the severity of neurofibrillary tangle deposition as well as in the neurodegenerative changes observed with neuroimaging.30 Early-onset AD is associated with greater baseline cortical atrophy and hypometabolism30,31 and more severe AD pathology (particularly neurofibrillary tangles and synaptic loss) than late-onset AD30,32,33 (FIGURE 2-1). These changes can be readily observed using tau or the recently developed synaptic vesicle protein 2A (SV2A) PET imaging34; however, the latter is currently only available in research settings. Recent developments in plasma Aβ and tau protein measurements are actively being developed as a minimally invasive and inexpensive diagnostic biomarker approach with significant diagnostic accuracy (CASE 2-1).35-37 The application of these biomarkers in early-onset AD and atypical variants is still in relative infancy, but large-scale studies are currently under way. For more information on the diagnostic accuracy of biomarkers, refer to the article “Fluid Biomarkers in Dementia Diagnosis” by Suzanne E. Schindler, MD, PhD,38 in this issue of Continuum.
The greater disease severity of early-onset AD indicates a more aggressive disease course that is already manifested in the young age at onset. The reason for this accelerated disease progression is not yet fully understood, but genetics is thought to play a major role. It might be that early-onset AD harbors major genetic risk variants that have not yet been discovered in major genome-wide association studies as these studies typically exclude atypical and early-onset disease variants. Large-scale research efforts focused on early-onset AD are under way and, it is hoped, will shed light on this attribute.
VISUOSPATIAL VARIANT ALZHEIMER DISEASE—POSTERIOR CORTICAL ATROPHY
The most up-to-date diagnostic criteria for posterior cortical atrophy (PCA) were published by Crutch and colleagues.39 Patients with PCA present with difficulties finding objects even when they are in plain sight, problems with driving (eg, veering out of the lane, accidents caused by not seeing objects to the side), difficulty navigating uneven surfaces such as going up and down stairs, loss of dexterity, and difficulties in putting objects together or dressing. Faced with a constellation of such presenting symptoms, inexperienced physicians often consider psychiatric abnormalities or frank malingering or may misdiagnose these patients with primary ocular abnormalities, sending them down the path of inappropriate treatments and even invasive procedures such as cataract surgeries.
The neurologic examination is a critical part of the evaluation of patients with PCA. The cardinal symptoms of the dorsal variant PCA are Balint syndrome (simultanagnosia, optic ataxia, and oculomotor apraxia) or Gerstmann syndrome (left/right disorientation, finger agnosia, dyscalculia, and dysgraphia).39,40 The dorsal variant can also manifest with various degrees of limb, constructional, or dressing apraxia.39,40 The ventral variant is characterized by apperceptive prosopagnosia and sometimes alexia with letter-by-letter reading.39 In the rare caudal variant, visual field defects and diminished visual acuity may also be observed.39-41 TABLE 2-1 presents a comparison of PCA variants. Other characteristic features of PCA are preserved insight, especially in the early stages of the disorder, and relative sparing of other cognitive domains.39 Some patients may manifest myoclonus, dystonia, or extrapyramidal signs suggestive of other neuropathologic entities, such as corticobasal degeneration or dementia with Lewy bodies.42
TABLE 2-1.
Posterior Cortical Atrophy Variants and Their Clinical Features
| Dorsal variant posterior cortical atrophy |
| ◆ Balint syndrome |
| ◇ Simultanagnosia |
| ◇ Optic ataxia |
| ◇ Oculomotor apraxia |
| ◆ Gerstmann syndrome |
| ◇ Left/right disorientation |
| ◇ Finger agnosia |
| ◇ Dyscalculia |
| ◇ Dysgraphia |
| ◆ Limb apraxia |
| ◆ Constructional apraxia |
| ◆ Dressing apraxia |
| Ventral variant posterior cortical atrophy |
| ◆ Apperceptive prosopagnosia |
| ◆ Alexia |
| ◇ Letter-by-letter reading |
| Caudal variant posterior cortical atrophy |
| ◆ Visual field defects |
| ◆ Diminished visual acuity |
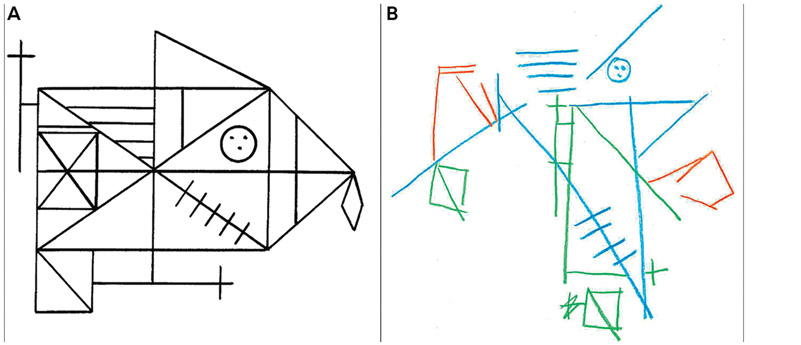
Neuropsychologically, patients with PCA present with striking impairments in visuospatial perception and construction that cut across cognitive domains, including attention, reasoning, and memory. Profound constructional apraxia is often demonstrated by an “exploded” complex figure copy with an absent integration of figural components (FIGURE 2-2) and severe impairment on visuospatial tasks (eg, as demonstrated on the WAIS-IV Block Design, Picture Completion, and Visual Puzzles). Writing in a straight line is difficult for patients with PCA, and ability to read might diminish because of poor spatial tracking. Visuospatial memory is typically impaired secondary to visuospatial impairments, whereas verbal memory may be relatively spared. Language impairments, when present, are similar to a logopenic syndrome (anomia, dysfluency, poor sentence repetition).43
FIGURE 2-2.
The original Rey-Osterrieth Complex Figure (A) is shown next to the “exploded” copy pattern (B) that can be seen in posterior cortical atrophy. Despite the accurate representation of some of the individual components, the overall concept is missing and integration of the various components is completely absent.
Ninety-six percent of PCA cases are due to AD.44 CT or MRI of the brain in PCA reveals atrophy corresponding to the observed clinical syndrome. The classic structural imaging finding is posterior-predominant atrophy with involvement of the visual associative cortices and, in the caudal variant, also the primary visual cortex. Additionally, Balint syndrome is associated with bilateral atrophy of the superior parietal lobule, and Gerstmann syndrome is accompanied by atrophy of the angular gyrus of the left hemisphere; dressing and constructional apraxia follow neurodegenerative changes localizing to the right lateral parietal lobe. The pattern of tau distribution observed on tau PET closely corresponds to the atrophy pattern, whereas amyloid is diffusely distributed through the brain (CASE 2-2). Fludeoxyglucose (FDG)-PET findings consist of posterior-predominant hypometabolism largely matching the tau distribution and the brain atrophy.
CASE 2-2.
A 56-year-old woman with 16 years of education presented with vision and navigation difficulty and memory loss that began 2 years earlier and had progressively worsened. Initially, she had difficulty with depth perception, occasionally resulting in falls (such as when going down stairs) and not seeing objects that were in front of her. An ophthalmologic evaluation was normal. Within the first year after symptom onset, she became lost going to familiar places (eg, the doctor’s office). Once, she drifted while driving and hit a guardrail. She and her family reported she misreached for objects and knocked them over, wrote on an upward slant, and had difficulty reading. Her instrumental and basic activities of daily living were generally intact at the time of the evaluation except for her driving difficulties and needing occasional assistance with dressing. Behaviorally, she was irritable, mildly anxious, and depressed.
On examination, she demonstrated simultanagnosia (seeing only three of nine objects presented together), optic ataxia (inaccurate visually guided reaching), left/right confusion, dysgraphia, and acalculia. She was oriented except for date. She had trouble reading because of poor tracking. Her Mini-Mental State Examination (MMSE) score was 22/30, with points lost on orientation, attention, writing, and overlapping figures. The remainder of her neurologic examination was normal. Neuropsychological testing revealed severe visuospatial perception and construction impairments, including constructional apraxia with an “exploded” complex figure (FIGURE 2-2). Her visual memory was severely impaired, and her verbal memory was mildly impaired. Executive functioning was mildly impaired, with particular difficulty on calculations. She was anomic.
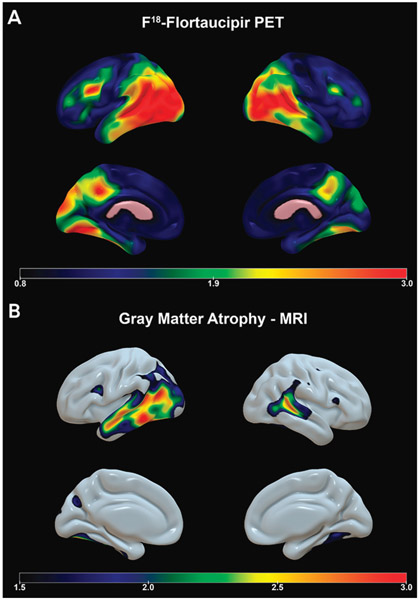
MRI revealed profound temporo-parieto-occipital atrophy (FIGURE 2-3). She was diagnosed with posterior cortical atrophy due to Alzheimer disease. Amyloid positron emission tomography (PET) was positive for Alzheimer disease pathology. Tau PET revealed intense tau ligand uptake in visual associative parietooccipital and temporooccipital cortices as well as the primary visual areas, milder bilateral involvement of the frontal lobes, and relative sparing of the primary motor and sensory cortices (FIGURE 2-3).
Eventually, she could not coordinate her fingers to play the piano and began falling frequently. Her memory and executive functioning continued to decline, whereas her language abilities (except for anomia) remained relatively preserved.
COMMENT
This patient demonstrates a classic posterior cortical atrophy pattern with involvement of the posterior brain regions, including the primary and secondary visual areas. The functional impairment in posterior cortical atrophy is significant as patients are unable to successfully navigate and interact with their environment. Discussions about driving are critical because of the risk of getting lost, lane drifting, and minor to major accidents because of poor judgment of distance and spatial awareness.
LANGUAGE VARIANT ALZHEIMER DISEASE—LOGOPENIC VARIANT PRIMARY PROGRESSIVE APHASIA
The primary progressive aphasias are a group of disorders consisting of logopenic variant primary progressive aphasia (PPA), semantic dementia, and nonfluent/agrammatic variant PPA.45 Of logopenic variant PPA cases, 86% to 90% are due to AD, compared to only 11% to 16% of cases of semantic dementia and 15% to 20% of cases of nonfluent/agrammatic variant PPA.44,46
The primary symptoms of patients with logopenic variant PPA are word-finding difficulties, circumlocution, and mispronouncing words (phonemic paraphasias). Many patients also report memory impairment. The elementary neurologic examination in logopenic PPA is ordinarily within normal limits.
Neuropsychologically, patients with logopenic variant PPA show prominent difficulties with word-generation (confrontation naming [as measured on the Boston Naming Test], verbal fluency [animals], and letter fluency), working memory (sentence repetition, digit span), and phonemic paraphasias. Speech during picture description (eg, the cookie theft picture) is relatively fluent, but filler words and circumlocutions are frequent. Phonemic errors (eg, blant for plant) are common. Comprehension is reduced; however, single-word and semantic knowledge (eg, as measured on the Pyramids and Palm Trees Test47), grammatical structure, and motor speech abilities are all spared, which distinguishes logopenic variant PPA from semantic dementia and nonfluent/agrammatic variant PPA. The profile is more mixed in other cognitive domains. Some individuals may not show any cognitive impairment other than language. Others demonstrate more pronounced cognitive impairment in verbally mediated memory tasks (eg, as measured on the Rey Auditory Verbal Learning Task or WAIS-IV Logical Memory), and still others demonstrate more widespread or global cognitive impairment.48
As with PCA, the amyloid distribution in logopenic variant PPA is diffuse. MRI, tau PET, and FDG-PET show left greater than right atrophy, tau deposition, and hypometabolism involving the lateral temporal and temporoparietal cortices (CASE 2-3). The most salient neurodegenerative changes are seen in the left inferior parietal lobule; the left superior, middle, and inferior temporal gyri; and the perisylvian cortical regions.49
CASE 2-3.
A 55-year-old man with 20 years of education presented with difficulties in language and memory over the previous 18 months. His coworkers first noticed he had difficulty following directions and performing his duties, which he and his spouse attributed to stress. However, the difficulties progressed, and he was terminated after a poor evaluation. Additional problems included difficulty with speech articulation, word retrieval, and comprehension and nonsensical written expressions. He frequently asked people to repeat themselves, misunderstood forms and other documents (eg, for taxes and disability), and did not finish his sentences. He forgot conversations. He was independent in activities of daily living but needed assistance with managing finances and reminders for medications and appointments. He stopped using the telephone because of his language issues. Behaviorally, his family was concerned about mild depression.
On examination, his speech was characterized by fillers (eg, um, you know), poor word retrieval, circumlocutions, and phonemic and semantic paraphasias (helichopper for helicopter, living tree for living room). The remainder of his neurologic examination was normal. His Mini-Mental State Examination (MMSE) score was 17/30, with points lost for orientation, registration, attention, repetition, memory, and following commands. Instructions and questions needed frequent repetition and rewording because of his poor comprehension and retention.
On neuropsychological evaluation, the most pronounced impairment was in language, particularly confrontation naming, with a score of 29/60 on the Boston Naming Test. He was able to repeat only very short sentences. He had difficulty reading longer and irregular words. Grammar and semantic knowledge were spared. Verbal memory demonstrated poor learning and retention. Digit span was severely impaired. He was perseverative on a problem-solving task and had poor cognitive flexibility (as measured on the Trail Making Test Part B). His visuospatial abilities were relatively spared.
MRI demonstrated more prominent left compared to right posterior perisylvian atrophy extending into the lateral temporal lobe commensurate with tau PET showing prominent tau binding bilaterally in the same area with mild involvement of the posterior medial frontal gyrus and the posterior cingulate/precuneus region more significant on the left (FIGURE 2-4).
COMMENT
Early prominent language impairment defines logopenic variant primary progressive aphasia and is the principal cause of impairment in daily living in patients with this condition. Anomia, poor word generation, and sentence repetition are the defining features but are often accompanied by poor reading of irregular words and severely impaired digit span. This patient had a fairly advanced syndrome at initial presentation, with less than 2 years’ duration. Logopenic variant primary progressive aphasia can be quite heterogeneous in syndrome presentation and duration, with some individuals showing only mild impairment despite lengthy disease duration.48,50
BEHAVIORAL VARIANT/DYSEXECUTIVE VARIANT ALZHEIMER DISEASE
The dysexecutive and behavioral variants of AD are relatively rare and seen in approximately 2% of AD cases.42 Among those diagnosed clinically with frontotemporal dementia (FTD), 7% to 20% are found to have AD pathology.51-55
No consensus criteria for dysexecutive variant AD and behavioral variant AD have been established to date. However, Townley and colleagues56 offered preliminary criteria for a dysexecutive syndrome due to AD. The dysexecutive variant of AD is characterized by a prominent dysexecutive syndrome and relatively milder impairment in other cognitive functions. Early features include difficulty with multitasking, planning, organizing, and project execution. In contrast to behavioral variant AD, dysexecutive variant AD is not typically associated with profound personality changes, although apathy is fairly common (CASE 2-4).
CASE 2-4.
A 57-year-old man with 12 years of education presented for a second opinion about a diagnosis of functional neurologic disorder. He appeared less concerned about his cognitive changes than his partner was. His partner described 3 years of progressive cognitive issues that resulted in the patient losing his job. He was unable to follow conversations or his own train of thought. He became concrete and rigid in his thinking, resulting in poor judgment and decision making. He was distractible, and his organizational skills declined. In the past year, he had struggled to find words and was more forgetful. His partner described a lack of empathy and mild compulsive behaviors. Apathy, anxiety, irritability, and mild depression were prominent. He was not disinhibited or emotionally or behaviorally dysregulated.
He was oriented except for date. He was unable to perform the Luria hand sequences or simple calculations. He repeated only short sentences. He demonstrated some features of logopenic aphasia (circumlocutions, lack of specificity, and deficient word retrieval). His Mini-Mental State Examination (MMSE) score was 20/30, with points lost on orientation, calculations, memory (registration and recall), sentence repetition, and following commands. The remainder of his neurologic examination was normal.
On neuropsychological evaluation, he demonstrated multidomain cognitive impairment, most prominent in working memory and executive functioning (cognitive flexibility, inhibition, problem solving), with less severe impairments in language and memory (worse learning than retention). Visuospatial perception was intact.
MRI demonstrated pronounced atrophy of the medial and lateral frontal lobes bilaterally with relative medial temporal lobe sparing. Tau binding revealed an Alzheimer disease–like pattern except for milder medial temporal lobe involvement (FIGURE 2-5). Amyloid positron emission tomography (PET) was diffusely positive.
At follow-up evaluation 18 months later, he was unable to engage with neuropsychological testing because of severe abulia.
COMMENT
Many patients with dysexecutive variant AD are initially misdiagnosed as having a primary psychiatric or functional neurologic disorder. As a result, these patients often have significant delay in diagnosis and access to needed services, with resulting undue stress on families who do not understand why their loved one is struggling. Although this patient presented with components of logopenic aphasia, this was not the initial symptom, nor was it the main reason for his functional impairment. Therefore, it did not meet criteria for primary progressive aphasia.
Behavioral variant AD closely resembles behavioral variant FTD (bvFTD).42 The typical presentation is cognitive decline closely followed by the personality changes characteristic of FTD, such as disinhibition, lack of empathy, disregard of social and societal norms, apathy, obsessive-compulsive behaviors, and occasionally also hyperorality. Patients with behavioral variant AD can also show other neuropsychiatric symptoms, such as delusions and hallucinations, which is rarely the case in patients with bvFTD (CASE 2-5).
CASE 2-5.
An 80-year-old man with 16 years of education presented 3 years post–symptom onset for a second opinion about a diagnosis of behavioral variant frontotemporal dementia (bvFTD). His spouse initially noticed he had trouble with judgment. He became obsessed with mail scams and spent between $30,000 and $70,000. His spouse took control of the mail, but the scammers began calling the house. The patient developed other obsessions, including use of adult coloring books, and would spend his entire day engaged in that activity. Within a year after symptom onset, his driving became erratic, and the family sold the car. He developed disinhibition (eg, inappropriate behavior with strangers), hoarding (eg, stealing napkins and silverware), an addiction to coffee and artificial sweetener, and obsessive eating habits (eating only sandwiches, bananas, and cereal). He was irritable and lacked empathy. He developed a delusion that his toy robotic dog was real and became upset when the dog would not eat. He could use his cell phone, but all other instrumental activities of daily living were impaired.
The patient had a normal neurologic examination except for brisk reflexes, with lower extremities worse than upper extremities. Sensory examination was inconsistent. His Montreal Cognitive Assessment (MoCA57) score was 3/30. Neuropsychological testing revealed global cognitive impairment (many tasks having to be discontinued), with relatively sparing of visuospatial skills. He was diagnosed with major neurocognitive disorder (according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5]) caused by frontotemporal dementia (FTD) versus Alzheimer disease (AD).
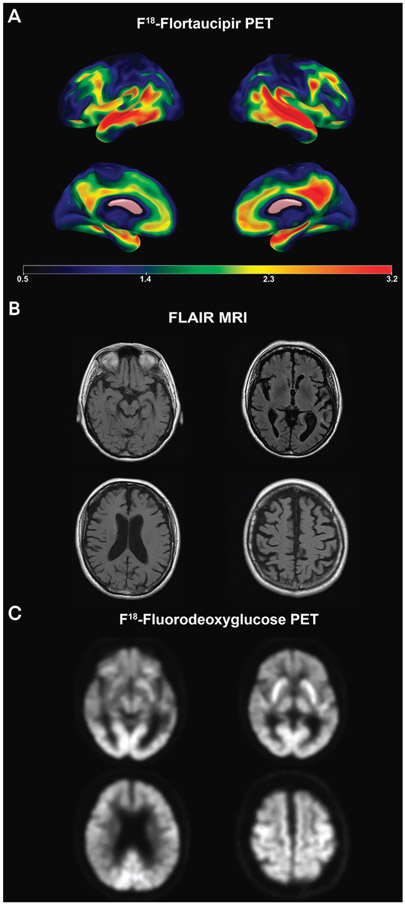
MRI of his brain showed significant global atrophy, inclusive of frontal lobe atrophy with mild to moderate hippocampal involvement. Fludeoxyglucose positron emission tomography (FDG-PET) revealed frontal, anterior and middle temporal, and bilateral parietal hypometabolism, with relative preservation of the posterior cingulate area. Tau PET showed significant medial and lateral frontal involvement in addition to the classic lateral temporoparietal and medial temporal involvement seen in typical AD (FIGURE 2-6). Amyloid PET was consistent with AD, leading to a diagnosis of behavioral variant AD over bvFTD, which was eventually pathologically confirmed.
COMMENT
This patient was originally diagnosed with bvFTD because of the clear behavioral and personality changes that were the initial and most predominant impairing symptom. However, amyloid PET and later postmortem examination of the brain confirmed that his pathology was AD. Early financial intervention by family members was also important given the patient’s poor judgment and obsessions surrounding finances. It is important for care partners to put a power of attorney in place to limit the patient’s access to spending money and ensure proper health care decisions are made.
Both dysexecutive variant AD and behavioral variant AD can have a normal neurologic examination except for findings on mental and behavioral status examination. Some patients show prominent frontal release signs (such as palmomental, grasp, snout, rooting, and sucking reflexes), poor learning of alternating sequences (Luria hand sequences), and perseverations.
The neuropsychological profiles of dysexecutive variant AD and behavioral variant AD are similar in that executive functioning (inhibition, set shifting, problem solving) is disproportionately affected. However, dysexecutive variant AD tends to be more cognitively impaired in the early stages than behavioral variant AD, which is more behaviorally impaired. In dysexecutive variant AD, working memory (eg, as measured by digit span backward) is particularly affected. Given that executive functioning and working memory are foundational cognitive skills that support other cognitive abilities, it is common to see a multidomain presentation (eg, additional impairment of memory [especially learning and retrieval] or language [logopenic pattern]), but performance is relatively worse on tests of executive functioning.56 It is also not uncommon for patients with dysexecutive variant AD to be so impaired that even a partial neuropsychological evaluation is not possible. Bedside cognitive screening tools can assist in making a determination about the possibility of a full neuropsychological evaluation.
As with all other atypical AD variants, amyloid deposition in dysexecutive variant AD and behavioral variant AD is diffuse. Structural imaging can reveal more prominent frontal atrophy (CASE 2-4, CASE 2-5), which can be asymmetric.58 The medial temporal regions can be relatively preserved.56,59 FDG-PET shows frontal and parietal hypometabolism in about half of cases.60 Some researchers have reported that in behavioral variant AD the hypometabolism localizes more to the medial frontal61 or the dorsolateral frontal regions than the orbitofrontal regions, as is seen in bvFTD.55 Compared to amnestic AD, behavioral variant AD shows greater frontoinsular hypometabolism but similar temporoparietal hypometabolism.62 The most hypometabolic regions in dysexecutive variant AD are the middle temporal, inferior temporal, and angular gyri.46 In frontal variant AD, tau PET classically reveals prominent tau tracer retention in the frontal lobes in addition to the classic medial, inferior and lateral temporal, and lateral parietal distribution.63
MOTOR VARIANT ALZHEIMER DISEASE—CORTICOBASAL SYNDROME DUE TO ALZHEIMER DISEASE
Although corticobasal syndrome (CBS) is most commonly linked with corticobasal degeneration (CBD), a 4-repeat tauopathy, neuropathologic studies have reported that 15% to 54% of cases are due to AD.52,64-66 Distinguishing CBS-CBD from CBS-AD is not straightforward because of significant overlap in clinical presentation. Biomarker assessments can thus be invaluable in the setting of CBS.
A detailed neurologic examination is critical in suspected CBS as the diagnostic criteria for CBS rely prominently on neurologic findings. The current diagnostic criteria for CBS include three major criteria (akinetic-rigid syndrome, limb apraxia, and cognitive impairment) and focal or segmental myoclonus, limb dystonia, alien limb phenomenon, cortical sensory loss, and dyscalculia as minor criteria.67
A 2016 analysis of 45 patients with CBS with CSF assessments of Aβ and tau protein levels revealed that the presence of myoclonus and Gerstmann syndrome is more frequent in CBS-AD.68 A 2011 literature review of another 43 cases reported longer disease duration, younger age at onset, hemisensory neglect, memory impairment, visuospatial difficulties, dressing apraxia, and myoclonus (but not Gerstmann syndrome) to be more frequently associated with CBS-AD than with CBS-CBD. No difference was seen in the frequency of aphasia, limb apraxia, alien limb phenomenon, pyramidal motor signs, parkinsonism, tremor, dystonia, dysarthria, dysphagia, postural instability, gait problems, or frontal release signs. Extraocular disturbances and rigidity were more frequent in CBS-CBD.69
Neuropsychologically, although cognitive impairment is a core criterion for diagnosis, the cognitive domains affected can be variable. Impairments are most common on tests of speech and language, specifically apraxia of speech (evaluated by speech-language pathology) and anomia (eg, as shown by naming impairment on the Boston Naming Test). Visuospatial deficits, especially construction (eg, as measured by the WAIS-IV Block Design and Rey-Osterrieth Complex Figure Test), often feature prominently. Finally, impaired executive functioning (set shifting, problem solving), perseveration, and difficulty with sequences may be present. Primary memory deficit (ie, frank amnesia) is typically not a prominent feature of CBS, although deficits in visuoconstruction and executive functioning can hinder encoding/learning.
Given the substantial overlap of clinical findings between CBS-AD and CBS-CBD, molecular imaging or CSF biomarkers could prove critical to establishing AD as the causative etiology antemortem.44 Diffuse brain amyloidosis and cortical tau deposition that is especially prominent in the hemisphere contralateral to the affected limb, often without sparing of the sensorimotor cortex,44 or low CSF amyloid-β 42 combined with high total tau and phosphorylated tau are highly suggestive of CBS-AD. The atrophy pattern seen on structural neuroimaging is that of frontoparietal neurodegeneration, more severe contralaterally to the affected limb (CASE 2-6). The lateral temporal lobe could also be involved.66,69 Such a diffuse atrophy pattern has been postulated to be somewhat suggestive of underlying AD pathology in CBS. Patients with CBS-AD often present with asymmetric posterior hypometabolism or hypoperfusion of the lateral temporal and lateral and medial parietal lobes and the posterior cingulate region.70
CASE 2-6.
A 57-year-old man presented for evaluation after approximately 2 years of cognitive decline that had recently impacted his ability to do his job operating heavy machinery. His spouse felt forgetting events was the first symptom, but his problems had progressed. His symptoms included confusion about directions (eg, going the wrong way to get to the barn on their property, getting lost in the grocery store), dressing apraxia (eg, putting buttons through the wrong holes, putting on his jacket upside down), illegible writing (eg, he could not sign his name), poor decision making, and word-finding difficulties. His activities of daily living were intact except that he occasionally needed help cutting up food. He needed assistance for all instrumental activities of daily living. He had developed irritability and social withdrawal, but he and his spouse denied hallucinations, delusions, or significant behavioral or personality change.
Examination showed reduced speech, myoclonus in both upper extremities, mild right-sided limb apraxia and rigidity, dyscalculia, mild left-sided pronator drift, positive Babinski sign on the left, and left-sided extinction to simultaneous tactile and visual stimuli. His Montreal Cognitive Assessment (MoCA) score was 6/30. Neuropsychological testing revealed global cognitive impairment with relatively more severe executive dysfunction and visuospatial impairments. He was unable to perform the Trail Making Test Part A or Part B and could not copy even simple geometric designs. Although spontaneous memory retrieval was extremely low, his recognition memory was near perfect, suggesting storage was spared, whereas executive aspects of memory were impaired.
He was diagnosed with mild to moderate stage dementia (DSM-5 Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5] major neurocognitive disorder), concerning for corticobasal syndrome (CBS), of unknown etiology. His brain MRI showed left greater than right frontoparietal atrophy with left-sided atrophy of the putamen and caudate head, suggestive of corticobasal degeneration (FIGURE 2-7). CSF amyloid-β 42, total tau, and phosphorylated tau levels were suggestive of Alzheimer disease (AD). He was diagnosed with CBS due to AD.
Just over a year after the initial evaluation, he developed convulsive seizures and myoclonus; cried more often; and did not recognize his home, spouse, or children.
COMMENT
In this case, the clinical presentation of the patient was important in identifying CBS but could not distinguish whether the disease process was AD or corticobasal degeneration (CBD). CSF biomarker studies were instrumental in providing an accurate diagnosis for this patient and his family.
DIAGNOSTIC CHALLENGES
Within atypical AD, overlapping symptoms can make distinguishing between phenotypes challenging. Both logopenic variant and dysexecutive variant AD present with prominent impairments in working memory. The language profile of both logopenic variant AD and CBS can be very similar. Both PCA and CBS involve visuospatial impairments and limb apraxia because of overlapping parietal involvement. Differentiation can be assisted by identifying the most prominent symptoms and the timeline of onset.
Perhaps even more challenging is differentiating atypical AD variants from other disorders. Misdiagnosis is not uncommon and particularly likely in the context of younger onset and more severe cognitive impairment. For example, Townley and colleagues56 found that 32 out of 39 individuals with dysexecutive variant AD were misdiagnosed, commonly as having a functional disorder. Similar to typical AD, atypical AD variants are often accompanied by neuropsychiatric symptoms, such as irritability, apathy, anxiety, depression, and anosognosia, which can lead to a primary psychiatric diagnosis rather than a neurologic diagnosis. This may be particularly common in frontal variant AD. Also, when patients have more subtle cognitive and motor symptoms, they can be misinterpreted as a stress reaction, especially if situational stressors or grief are present.
Variant-specific considerations are also necessary to avoid misdiagnosis. Although the majority of logopenic presentations have underlying AD pathology, a higher likelihood of misdiagnosis as another PPA syndrome exists, particularly nonfluent/agrammatic variant PPA, which has some shared symptoms but is typically a tauopathy. When language disturbances are subtle, such as early on in the disease course, they can be misidentified as stress or normal age-related cognitive changes. With frontal variant AD, symptom overlap with bvFTD can lead to misdiagnosis. Cognitive symptoms appearing before behavioral symptoms and prominent apathy rather than disinhibition suggest behavioral variant AD over bvFTD. Additionally, hallucinations and delusions are more common in behavioral variant AD than in bvFTD.54,71 In the motor variant (CBS-AD), the chance of misdiagnosis as CBS-CBD, FTD, or Parkinson disease and related disorders is higher. Prominent executive dysfunction, apraxia of speech or nonfluent aphasia, and gaze palsy increases the likelihood of a non-AD pathology, whereas an amnestic profile, impaired visuospatial abilities, and logopenic-type language impairments suggest AD pathology.66,72,73
TREATMENT AND MANAGEMENT
As is well known, no cure for AD has been discovered, and treatment largely consists of symptom management. Strategies for symptom management range from pharmacologic intervention for memory and cognitive impairment to behavioral and environmental modifications to reduce neuropsychiatric symptoms and support maximal independence in functioning.
Pharmacologic Management
The pharmacologic management of patients with early-onset or atypical AD is similar to that of patients with late-onset AD. Initial therapy with an acetylcholinesterase inhibitor (donepezil, galantamine, or rivastigmine) is recommended, followed by the addition of the N-methyl-d-aspartate (NMDA) receptor agonist memantine. Until recently, these were the only classes of medications approved by the US Food and Drug Administration (FDA) for the treatment of AD. However, in June 2021, the FDA issued an accelerated approval of aducanumab, a monoclonal antibody targeting both soluble and insoluble forms of Aβ. Aducanumab was FDA approved broadly for patients with AD, lacking guidance regarding the appropriate use of this therapeutic agent. An expert panel recently provided appropriate use recommendations74 for patient eligibility, safety monitoring, and the engagement of both patient and family when deciding whether to initiate treatment. The latter stems from the fact that aducanumab can cause serious adverse events, such as brain edema and brain hemorrhage, which were seen in 20% of APOE ε4 noncarriers and 40% of APOE ε4 carriers in the pivotal trials. The panel recommended that the drug can be offered to patients 50 to 85 years of age, which includes a subset of patients with early-onset AD. The panel also recommended that sporadic atypical AD variants can be offered aducanumab if all other eligibility criteria are met. However, the Centers for Medicare & Medicaid Services issued a decision to cover FDA-approved monoclonal antibody therapy solely under coverage with evidence development. This requires new randomized placebo-controlled therapeutic trials testing the efficacy of aducanumab showing clinical benefit to be developed before the CMS ruling in favor of clinical coverage of aducanumab.75,76
The first-line treatment for behavioral and neuropsychiatric symptoms of AD is nonpharmacologic behavioral and environmental management strategies. For example, a quiet familiar environment with labels on doors and sufficient lighting in all rooms is important to reduce disorientation, whereas aggressive behavior should always be addressed with positive and clear language to reassure and distract the patient.
Depressive symptoms can be treated with selective serotonin uptake inhibitors (SSRIs), given their low risk for anticholinergic effects. SSRIs may also ease anxiety and irritability. The SSRI citalopram may be useful for agitation. Agitation, psychosis, and disruptive behaviors may require a neuroleptic. The newer atypical antipsychotic medications (quetiapine, risperidone, olanzapine) are often used in low doses with careful titration. These antipsychotic agents, however, carry an FDA boxed warning because of an association with increased mortality in older patients and those with dementia. Traditional neuroleptics are more likely to produce extrapyramidal symptoms. These older antipsychotics also carry an increased risk of death.77 Thus, judicious use of antipsychotics with frequent reassessment of the therapeutic need is appropriate.
Safety and Planning Considerations
The atypical AD variants pose even greater safety and day-to-day caregiving challenges than typical AD. As with other dementias, it is important to establish a durable power of attorney early on. Consultation with an attorney familiar with elder law is recommended. Even early in the disease, more complex activities of daily living, such as managing finances, can be affected (eg, in frontal variant AD, in which increased spending can put the patient or family in financial hardship). Typically, driving is one of the earliest affected abilities, but impairment that jeopardizes safe driving can be different depending on the phenotype. For example, patients with visuospatial disturbances (PCA or CBS-AD) may have greater difficulty with navigation, perceiving the entirety of the driving environment, and keeping safe distances from other cars on the road. Patients with behavioral variant AD may be prone to riskier decision making, impulsivity, inattention, and poor emotion regulation. Therefore, driving evaluations are often warranted. If it is early in the disease process, neuropsychological reevaluation of the patient may be warranted to assess progression and assist with identifying new areas of needed support inside and outside the home. In all cases, it is important to be cautious about prematurely limiting autonomy if the patient is still able to function in a particular capacity. Occupational therapy home assessments can be helpful in identifying spared and impaired functioning. Care partner supervision of activities is another way to identify areas of intact functioning and areas where more support is needed.
Nonpharmacologic Management
Balancing independence with support can be difficult. Early establishment of routines and habits can reduce cognitive load and assist in extending independence. Routines and habits can be supported by other environmental modifications, such as using unique phone alarms for different reminders (eg, medication, appointments, orienting to the time of day), having several large calendars throughout the house with the date and the day’s activities clearly identified to support orientation, a to-do list to refer to, and written instructions for tasks. Finally, the best evidence for brain health, cognitive functioning, and prolonged independence is to ensure the patient is engaged in safe physical, social, and cognitive activities on a routine basis.
Although the focus is appropriately on the patient in terms of clinical care and safety, medical appointments can also be an opportunity to ensure that care partners, family members, and others involved in care are supported. Providing recommendations for educational resources, counseling, and support groups in the area can go a long way. Care partners should be encouraged to connect with their local Alzheimer’s Association chapter (alz.org) and local county or state services available for older adults and those with cognitive and physical disabilities. A high risk of social isolation exists for patients, families, and care partners, and connecting with these services can reduce that risk.
At-home or hospital-based occupational therapy, physical therapy, and speech and language pathology services are also frequently indicated. Physical therapy is particularly helpful with some of the movement issues that arise, especially in CBS (eg, balance and gait issues). Occupational therapists and speech and language pathologists can assist in identifying strategies to compensate for cognitive impairments, and speech and language pathologists can assist in identifying communication strategies or augmented communication devices (especially in logopenic variant AD). Occupational therapy assessments can help identify safety issues in the home and suggest modifications, such as to assist someone with significant visual impairment (eg, in PCA). In fact, individuals with PCA are often eligible to register as legally blind or severely sight impaired to receive these very specific types of services. Modifications in the home for visual changes are often necessary to keep the individual safe, especially to prevent falls (eg, using high-contrast coloring on the stairs; color-coding cupboards; getting rid of clutter, rugs, and other tripping hazards).
CONCLUSION
Young-onset (ie, early-onset AD) and atypical AD presentations are heterogeneous and understudied AD variants. Until recently, nonfamilial early-onset AD and the atypical AD presentations were only investigated in small single-site studies. The syndromic diversity in early-onset AD and especially the atypical variants provides a rich platform to improve our understanding of brain region vulnerability and the associated factors driving these atypical presentations as well as the clinical patterns of progression of these variants. The utility of CSF, peripheral blood, and PET biomarkers of Aβ and tau have likewise not been systematically studied in this population. Large-scale research efforts, such as LEADS (the Longitudinal Early-Onset AD Study) are currently ongoing and will help fill these gaps. The goals of LEADS are to define the clinical, imaging, and fluid biomarker characteristics of early-onset AD; develop sensitive cognitive and biomarker measures for future clinical and research use; and establish a trial-ready network.78 The study is still recruiting and aims to enroll and follow 400 Aβ-positive and 200 Aβ-negative cognitively impaired individuals that meet National Institute on Aging-Alzheimer’s Association criteria for mild cognitive impairment or AD dementia, as well as 100 age-matched controls. Additional study and referral information can be found at leads-study.org. Additional efforts are needed to raise awareness of early-onset and atypical AD variants and to educate medical practitioners more broadly about atypical AD to avoid delayed diagnosis/ misdiagnosis and streamline access to social services, health care, and insurance coverage as well as to assist with disability and patient and family education in a timely fashion.
FIGURE 2-3.
Imaging of the patient in CASE 2-2. Amyloid imaging showed diffuse cortical and subcortical signal consistent with Alzheimer disease (not shown). A, Flortaucipir F 18 positron emission tomography (PET) shows intense tau ligand uptake in the visual associative parietooccipital and temporooccipital cortices as well as the primary visual areas, milder bilateral involvement of the frontal lobes, and relative sparing of the primary motor and sensory cortices. Flortaucipir F 18 PET images show the standardized uptake value ratio (SUVR) of tau, where warmer colors (yellow, red, and orange) indicate more tau uptake. B, Sagittal volumetric MRI presented in three-dimensional space shows profound temporoparietooccipital atrophy (bottom panel); warmer colors (yellow, red, and orange) indicate areas of greater gray matter atrophy.
FIGURE 2-4.
Imaging of the patient in CASE 2-3. Amyloid imaging showed diffuse cortical and subcortical signal consistent with Alzheimer disease (not shown). A, Flortaucipir F 18 positron emission tomography (PET) shows prominent tau binding in the left greater than right posterior temporoparietal area, with milder involvement of the posterior medial frontal gyrus and the posterior cingulate/precuneus region. Flortaucipir F 18 PET images show the standardized uptake value ratio (SUVR) of tau, where warmer colors (yellow, red, and orange) indicate more tau uptake. B, Sagittal volumetric MRI presented in three-dimensional space shows less extensive left greater than right posterior perisylvian atrophy extending into the lateral temporal lobe; warmer colors (yellow, red, and orange) indicate areas of greater gray matter atrophy.
FIGURE 2-5.
Imaging of the patient in CASE 2-4. Amyloid imaging showed diffuse cortical and subcortical signal consistent with Alzheimer disease (not shown). A, Flortaucipir F 18 positron emission tomography (PET) shows a classic Alzheimer disease–like pattern aside for milder medial temporal lobe involvement (top panel). Flortaucipir F 18 PET images show the standardized uptake value ratio (SUVR) of tau, where warmer colors (yellow, red, and orange) indicate more tau uptake. B, Sagittal volumetric MRI presented in three-dimensional space shows more pronounced atrophy of the medial and lateral frontal lobes bilaterally with relative medial temporal lobe sparing; warmer colors (yellow, red, and orange) indicate areas of greater gray matter atrophy.
FIGURE 2-6.
Imaging of the patient in CASE 2-5. Amyloid imaging showed diffuse cortical and subcortical signal consistent with Alzheimer disease (not shown). A, Flortaucipir F 18 positron emission tomography (PET) showed significant medial and lateral frontal involvement in addition to the classic lateral temporoparietal and medial temporal involvement seen in typical Alzheimer disease. Flortaucipir F 18 PET images show the standardized uptake value ratio (SUVR) of tau, where warmer colors (yellow, red, and orange) indicate more tau uptake. B, Axial fluid-attenuated inversion recovery (FLAIR) MRI shows significant global and frontal lobe atrophy, mild to moderate hippocampal involvement, and minimal white matter changes. C, Axial fludeoxyglucose (FDG)-PET shows frontal, anterior, and middle temporal and bilateral parietal hypometabolism with relative preservation of the posterior cingulate area, which is atypical for Alzheimer disease.
FIGURE 2-7.
Imaging of the patient in CASE 2-6. Axial fluid-attenuated inversion recovery (FLAIR) MRI sequences show global left greater than right frontoparietal atrophy without sparing of the sensorimotor cortex and moderate bilateral hippocampal atrophy.
KEY POINTS.
Approximately 3% of the 5.8 million Americans with Alzheimer disease (AD) developed symptoms at age 65 or younger.
Misdiagnosis results in setbacks in access to AD treatments and social and financial support services.
Amnestic variant early-onset AD is the most common atypical AD variant. Compared to late-onset AD, patients with sporadic early-onset AD show more rapid clinical decline and greater impairment in attention, language, visuospatial, and executive functions.
Disease-specific biomarkers are particularly useful when assessing atypical AD presentations, including early-onset AD.
Early-onset AD is associated with greater baseline cortical atrophy and hypometabolism and more severe AD pathology (particularly neurofibrillary tangles and synaptic loss) than late-onset AD.
Patients with posterior cortical atrophy present with striking impairments in visuospatial perception and construction that cut across cognitive domains, including attention, reasoning, and memory.
Ninety-six percent of cases of posterior cortical atrophy are due to AD.
The classic structural imaging finding in posterior cortical atrophy is posterior-predominant atrophy with involvement of the visual associative cortices and, in the caudal variant, also the primary visual cortex.
The functional impairment in posterior cortical atrophy is significant as patients are unable to successfully navigate and interact with their environment.
Of logopenic variant primary progressive aphasia cases, 86% to 90% cases are due to AD, compared to only 11% to 16% of semantic dementia cases and 15% to 20% of primary progressive nonfluent aphasia cases. The primary symptoms of patients with logopenic variant primary progressive aphasia are word-finding difficulties, circumlocution, and mispronouncing words (phonemic paraphasias).
In logopenic variant primary progressive aphasia, MRI, tau positron emission tomography (PET), and fludeoxyglucose PET show left greater than right atrophy, tau deposition, and hypometabolism involving the lateral temporal and temporoparietal cortices.
Early prominent language impairment defines logopenic variant primary progressive aphasia and is the principal cause of impairment in daily living. Anomia, poor word generation, and sentence repetition are the defining features.
Early features of dysexecutive variant AD include difficulty with multitasking, planning, organizing, and project execution.
Many patients with dysexecutive variant AD are initially misdiagnosed as having a primary psychiatric or functional neurologic disorder.
Behavioral variant AD closely resembles behavioral variant frontotemporal dementia.
Patients with behavioral variant AD are commonly diagnosed with behavioral variant frontotemporal dementia because of the early pervasive behavioral and personality changes.
Given that executive functioning and working memory are foundational cognitive skills that support other cognitive abilities, it is common to see a multidomain presentation in dysexecutive variant AD.
Of cases of corticobasal syndrome, 15% to 54% are due to AD.
A detailed neurologic examination is critical in suspected corticobasal syndrome.
Although cognitive impairment is a core criterion for the diagnosis of corticobasal syndrome, the cognitive domains affected can be variable.
Given the substantial overlap of clinical findings between corticobasal syndrome–AD and corticobasal syndrome–corticobasal degeneration (CBD), molecular imaging or CSF biomarkers could prove critical to establishing AD as the causative etiology antemortem.
Within atypical AD, overlapping symptoms can make distinguishing between phenotypes challenging. Differentiation can be assisted by identifying the most prominent symptoms and the timeline of onset.
The pharmacologic management of early-onset or atypical AD is similar to that of late-onset AD.
The atypical AD variants pose even greater safety and day-to-day caregiving challenges than typical AD.
The syndromic diversity in early-onset AD and especially the atypical variants provides a rich platform to improve our understanding of brain region vulnerability and the associated factors driving these atypical presentations.
ACKNOWLEDGMENT
Funding/Support: This article was supported by the National Institutes of Health (U01 AG057195).
Footnotes
RELATIONSHIP DISCLOSURE:
Dr Polsinelli receives research/grant support from the Alzheimer’s Association, the Kessler Foundation, and the National Institute on Aging (U01 AG057195). Dr Apostolova has received personal compensation in the range of $500 to $4999 for serving as a consultant for the Florida Department of Health, GE Healthcare, Lilly, the National Institutes of Health, and the National Institutes of Health NeuroBioBank and for serving on a scientific advisory or data safety monitoring board for Eisai Co, Ltd; IQVIA, Inc; and TwoLabs, LLC. Dr Apostolova has received personal compensation in the range of $5000 to $9999 for serving as a consultant and on a scientific advisory or data safety monitoring board for Biogen. Dr Apostolova has received personal compensation in the range of $10,000 to $49,999 for serving as an editor, associate editor, or editorial advisory board member for the Alzheimer’s Association. An immediate family member of Dr Apostolova has stock in Cassava Sciences, Inc; Golden Seeds; and Semiring. The institution of Dr Apostolova has received research support from the Alzheimer’s Association, Avid Radiopharmaceuticals, F. Hoffman-La Roche Ltd, Life Molecular Imaging, and the National Institute on Aging (U01 AG057195).
UNLABELED USE OF PRODUCTS/INVESTIGATIONAL USE DISCLOSURE:
Drs Polsinelli and Apostolova discuss the unlabeled/investigational use of psychotropic medications for the management of behavioral symptoms in atypical Alzheimer disease.
USEFUL WEBSITES
ALZHEIMER’S ASSOCIATION
The Alzheimer’s Association website offers information about dementia, research programs, and support and local resources for patients and caregivers.
LEADS LONGITUDINAL EARLY-ONSET ALZHEIMER’S DISEASE STUDY
This website offers information about the LEADS study for potential participants, referring clinicians, and researchers.
REFERENCES
- 1.Association A. Alzheimer’s disease facts and figures. Alzheimers Dement 2019;2019;15(3):321–387. doi: 10.1016/j.jalz.2019.01.010 [DOI] [Google Scholar]
- 2.Harvey R, Skelton-Robinson M, Rossor M. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry 2003;74(9):1206–1209. doi: 10.1136/jnnp.74.9.1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercy L, Hodges JR, Dawson K, et al. Incidence of early-onset dementias in Cambridgeshire. United Kingdom Neurology 2008;71(19):1496–1499. doi: 10.1212/01.wnl.0000334277.16896.fa [DOI] [PubMed] [Google Scholar]
- 4.Newens A, Forster DP, Kay DW, et al. Clinically diagnosed presenile dementia of the Alzheimer type in the Northern Health Region: ascertainment, prevalence, incidence and survival. Psychol Med 1993;23(3):631–644. doi: 10.1017/s0033291700025411 [DOI] [PubMed] [Google Scholar]
- 5.Jayadev S Genetics of Alzheimer disease. Continuum (Minneap Minn) 28(3, Dementia): 852–871. [DOI] [PubMed] [Google Scholar]
- 6.Heyman A, Wilkinson WE, Hurwitz BJ, et al. Early-onset Alzheimer’s disease: clinical predictors of institutionalization and death. Neurology 1987;37(6):980–984. doi: 10.1212/wnl.37.6.980 [DOI] [PubMed] [Google Scholar]
- 7.Koss E, Edland S, Fillenbaum G, et al. Clinical and neuropsychological differences between patients with earlier and later onset of Alzheimer’s disease: a CERAD analysis, Part XII. Neurology 1996;46(1):136–141. doi: 10.1212/wnl.46.1.136 [DOI] [PubMed] [Google Scholar]
- 8.Seltzer B, Sherwin I. A comparison of clinical features in early- and late-onset primary degenerative dementia. One entity or two? Arch Neurol 1983;40(3):143–146. doi: 10.1001/archneur.1983.04050030037006 [DOI] [PubMed] [Google Scholar]
- 9.Jacobs D, Sano M, Marder K, et al. Age at onset of Alzheimer’s disease: relation to pattern of cognitive dysfunction and rate of decline. Neurology 1994;44(7):1215–1220. doi: 10.1212/wnl.44.7.1215 [DOI] [PubMed] [Google Scholar]
- 10.Loring DW, Largen JW. Neuropsychological patterns of presenile and senile dementia of the Alzheimer type. Neuropsychologia 1985;23(3):351–357. doi: 10.1016/0028-3932(85)90021-1 [DOI] [PubMed] [Google Scholar]
- 11.Filley CM, Kelly J, Heaton RK. Neuropsychologic features of early- and late-onset Alzheimer’s disease. Arch Neurol 1986;43(6):574–576. doi: 10.1001/archneur.1986.00520060038014 [DOI] [PubMed] [Google Scholar]
- 12.Imamura T, Takatsuki Y, Fujimori M, et al. Age at onset and language disturbances in Alzheimer’s disease. Neuropsychologia 1998;36(9):945–949. doi: 10.1016/s0028-3932(98)00010-4 [DOI] [PubMed] [Google Scholar]
- 13.Fujimori M, Imamura T, Yamashita H, et al. Age at onset and visuocognitive disturbances in Alzheimer disease. Alzheimer Dis Assoc Disord 1998;12(3):163–166. doi: 10.1097/00002093-199809000-00007 [DOI] [PubMed] [Google Scholar]
- 14.Binetti G, Magni E, Padovani A, et al. Neuropsychological heterogeneity in mild Alzheimer’s disease. Dementia 1993;4(6):321–326. doi: 10.1159/000107340 [DOI] [PubMed] [Google Scholar]
- 15.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 2nd Ed. Oxford University Press; 1998:xvi, 736. [Google Scholar]
- 16.Wechsler D Wechsler Memory Scale IV (WMS-IV). Psychological Corporation; 2009. [Google Scholar]
- 17.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea & Febiger; 1983. [Google Scholar]
- 18.Lezak K. Neuropsychological Assessment. Oxford University Press; 1995. [Google Scholar]
- 19.Wechsler D Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV). Pearson Assessment; 2008. [Google Scholar]
- 20.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry 1983;40(7). doi: 10.1001/archpsyc.1983.01790060110016 [DOI] [PubMed] [Google Scholar]
- 21.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chui H Dementia attributable to subcortical ischemic vascular disease. Neurologist 2001;7:208–219. [Google Scholar]
- 24.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56(9):1143–1153. doi: 10.1212/wnl.56.9.1143 [DOI] [PubMed] [Google Scholar]
- 25.Whitwell JL, Dickson DW, Murray ME, et al. Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control study. Lancet Neurol 2012;11(10):868–877. doi: 10.1016/S1474-4422(12)70200-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavedo E, Pievani M, Boccardi M, et al. Medial temporal atrophy in early and late-onset Alzheimer’s disease. Neurobiol Aging 2014;35(9):2004–2012. doi: 10.1016/j.neurobiolaging.2014.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frisoni GB, Pievani M, Testa C, et al. The topography of grey matter involvement in early and late onset Alzheimer’s disease. Brain 2007;130(pt 3):720–730. doi: 10.1093/brain/awl377 [DOI] [PubMed] [Google Scholar]
- 28.Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement 2013;9(1). doi: 10.1016/j.jalz.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement 2013;9(4). doi: 10.1016/j.jalz.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 30.Stage EC Jr, Svaldi D, Phillips M, et al. Neurodegenerative changes in early- and late-onset cognitive impairment with and without brain amyloidosis. Alzheimers Res Ther 2020;12(1). doi: 10.1186/s13195-020-00647-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabinovici GD, Furst AJ, Alkalay A, et al. Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain 2010;133(pt 2):512–528. doi: 10.1093/brain/awp326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall GA, Fairbanks LA, Tekin S, et al. Early-onset Alzheimer’s disease is associated with greater pathologic burden. J Geriatr Psychiatry Neurol 2007;20(1):29–33. doi: 10.1177/0891988706297086 [DOI] [PubMed] [Google Scholar]
- 33.Bigio EH, Hynan LS, Sontag E, et al. Synapse loss is greater in presenile than senile onset Alzheimer disease: implications for the cognitive reserve hypothesis. Neuropathol Appl Neurobiol 2002;28(3):218–227. doi: 10.1046/j.1365-2990.2002.00385.x [DOI] [PubMed] [Google Scholar]
- 34.Chen MK, Mecca AP, Naganawa M, et al. Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol 2018;75(10):1215–1224. doi: 10.1001/jamaneurol.2018.1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmqvist S, Janelidze S, Stomrud E, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related β-amyloid status. JAMA Neurol 2019;76(9):1060–1069. doi: 10.1001/jamaneurol.2019.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janelidze S, Stomrud E, Palmqvist S, et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci Rep 2016;6(26801). doi: 10.1038/srep26801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattsson N, Zetterberg H, Janelidze S, et al. Plasma tau in Alzheimer disease. Neurology 2016;87(17):1827–1835. doi: 10.1212/WNL.0000000000003246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schindler SE. Fluid Biomarkers in Dementia Diagnosis. Continuum (Minneap Minn) 28(3, Dementia):822–833. [DOI] [PubMed] [Google Scholar]
- 39.Crutch SJ, Schott JM, Rabinovici GD, et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement 2017;13(8):870–884. doi: 10.1016/j.jalz.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crutch SJ, Lehmann M, Schott JM, et al. Posterior cortical atrophy. Lancet Neurol 2012;11(2):170–178. doi: 10.1016/S1474-4422(11)70289-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galton CJ, Patterson K, Xuereb JH, Hodges J. Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 2000;3:484–498. doi: 10.1093/brain/123.3.484 [DOI] [PubMed] [Google Scholar]
- 42.Snowden JS, Stopford CL, Julien CL, et al. Cognitive phenotypes in Alzheimer’s disease and genetic risk. Cortex 2007;43(7):835–845. doi: 10.1016/s0010-9452(08)70683-x [DOI] [PubMed] [Google Scholar]
- 43.Magnin E, Sylvestre G, Lenoir F, et al. Logopenic syndrome in posterior cortical atrophy. J Neurol 2013;260(2):528–533. doi: 10.1007/s00415-012-6671-7 [DOI] [PubMed] [Google Scholar]
- 44.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA 2015;313(19):1939–1949. doi: 10.1001/jama.2015.4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergeron D, Gorno-Tempini ML, Rabinovici GD, et al. Prevalence of amyloid-β pathology in distinct variants of primary progressive aphasia. Ann Neurol 2018;84(5):729–740. doi: 10.1002/ana.25333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howard D, Patterson K. The Pyramids and Palm Trees Test: a test for semantic access from words and pictures. Thames Valley Test Company; 1992. [Google Scholar]
- 48.Owens TE, Machulda MM, Duffy J, et al. Patterns of neuropsychological dysfunction and cortical volume changes in logopenic aphasia. J Alzheimers Dis 2018;66(3):1015–1025. doi: 10.3233/JAD-171175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramanan S, Roquet D, Goldberg ZL, et al. Establishing two principal dimensions of cognitive variation in logopenic progressive aphasia. Brain Commun 2020;2(2). doi: 10.1093/braincomms/fcaa125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machulda MM, Whitwell JL, Duffy J, et al. Identification of an atypical variant of logopenic progressive aphasia. Brain Lang 2013;127(2):139–144. doi: 10.1016/j.bandl.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knopman DS, Boeve BF, Parisi JE, et al. Antemortem diagnosis of frontotemporal lobar degeneration. Ann Neurol 2005;57(4):480–488. doi: 10.1002/ana.20425 [DOI] [PubMed] [Google Scholar]
- 52.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain 2007;130(pt 10):2636–2645. doi: 10.1093/brain/awm213 [DOI] [PubMed] [Google Scholar]
- 53.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol 2006;59(6):952–962. doi: 10.1002/ana.20873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendez MF, Joshi A, Tassniyom K, et al. Clinicopathologic differences among patients with behavioral variant frontotemporal dementia. Neurology 2013;80(6):561–568. doi: 10.1212/WNL.0b013e3182815547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perry DC, Brown JA, Possin KL, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain 2017;140(12):3329–3345. doi: 10.1093/brain/awx254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Townley RA, Graff-Radford J, Mantyh WG, et al. Progressive dysexecutive syndrome due to Alzheimer’s disease: a description of 55 cases and comparison to other phenotypes. Brain Commun 2020;2(1). doi: 10.1093/braincomms/fcaa068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 58.Li CH, Fan SP, Chen TF, et al. Frontal variant of Alzheimer’s disease with asymmetric presentation mimicking frontotemporal dementia: case report and literature review. Brain Behav 2020;10(3). doi: 10.1002/brb3.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ossenkoppele R, Pijnenburg YAL, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain 2015;138(pt 9):2732–2749. doi: 10.1093/brain/awv191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bergeron D, Sellami L, Poulin S, et al. The behavioral/dysexecutive variant of Alzheimer’s disease: a case series with clinical, neuropsychological, and FDG-PET characterization. Dement Geriatr Cogn Disord 2020;49(5):518–525. doi: 10.1159/000511210 [DOI] [PubMed] [Google Scholar]
- 61.Lehingue E, Gueniat J, Jourdaa S, et al. Improving the diagnosis of the frontal variant of Alzheimer’s disease with the DAPHNE Scale. J Alzheimers Dis 2021;79(4):1735–1745. doi: 10.3233/JAD-201088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singleton EH, Pijnenburg YAL, Sudre CH, et al. Investigating the clinico-anatomical dissociation in the behavioral variant of Alzheimer disease. Alzheimers Res Ther 2020;12(1). doi: 10.1186/s13195-020-00717-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paquin V, Therriault J, Pascoal TA, et al. Frontal variant of Alzheimer disease differentiated from frontotemporal dementia using in vivo amyloid and tau imaging. Cogn Behav Neurol 2020;33(4):288–293. doi: 10.1097/WNN.0000000000000251 [DOI] [PubMed] [Google Scholar]
- 64.Boeve BF, Maraganore DM, Parisi JE, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 1999;53(4):795–800. doi: 10.1212/wnl.53.4.795 [DOI] [PubMed] [Google Scholar]
- 65.Grimes D, Lang A, Bergeron C. Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology 1999;53(9):1969–1974. doi: 10.1212/wnl.53.9.1969 [DOI] [PubMed] [Google Scholar]
- 66.Lee SE, Rabinovici GD, Mayo MC, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol 2011;70(2):327–340. doi: 10.1002/ana.22424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mathew R, Bak TH, Hodges J. Diagnostic criteria for corticobasal syndrome: a comparative study. J Neurol Neurosurg Psychiatry 2012;83(4):405–410. doi: 10.1136/jnnp-2011-300875 [DOI] [PubMed] [Google Scholar]
- 68.De Stefano F, Kas A, Habert MO, et al. The phenotypical core of Alzheimer’s disease-related and nonrelated variants of the corticobasal syndrome: a systematic clinical, neuropsychological, imaging, and biomarker study. Alzheimers Dement 2016;12(7):786–795. doi: 10.1016/j.jalz.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 69.Hassan A, Whitwell JL, Josephs KA. The corticobasal syndrome–Alzheimer’s disease conundrum. Expert Rev Neurother 2011;11(11):1569–1578. doi: 10.1586/ern.11.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pardini M, Huey ED, Spina S, et al. FDG-PET patterns associated with underlying pathology in corticobasal syndrome. Neurology 2019;92(10). doi: 10.1212/WNL.0000000000007038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134(9):2456–2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sakae N, Josephs KA, Litvan I, et al. Clinicopathologic subtype of Alzheimer’s disease presenting as corticobasal syndrome. Alzheimers Dement 2019;15(9):1218–1228. doi: 10.1016/j.jalz.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sha SJ, Ghosh PM, Lee SE, et al. Predicting amyloid status in corticobasal syndrome using modified clinical criteria, magnetic resonance imaging and fluorodeoxyglucose positron emission tomography. Alzheimers Res Ther 2015;7(1). doi: 10.1186/s13195-014-0093-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cummings J, Aisen P, Apostolova LG, et al. Aducanumab: appropriate use recommendations. J Prev Alzheimers Dis 2021;8(4):398–410. doi: 10.14283/jpad.2021.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Centers for Medicare & Medicaid Services. Monoclonal antibodies directed against amyloid for the treatment of Alzheimer’s disease. Accessed May 4, 2022. cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=305&fromTracking=Y&
- 76.Cummings J, Rabinovici GD, Atri A, et al. Aducanumab: appropriate use recommendations update. J Prev Alzheimers Dis 2022;9:221–230. doi: 10.14283/jpad.2022.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ 2007;176(5):627–632. doi: 10.1503/cmaj.061250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Apostolova LG, Aisen P, Eloyan A, et al. The Longitudinal Early-onset Alzheimer’s Disease Study (LEADS): framework and methodology. Alzheimers Dement 2021;17(12):2043–2055. doi: 10.1002/alz.12350 [DOI] [PMC free article] [PubMed] [Google Scholar]