Abstract
Background:
People living with chronic kidney disease (CKD) have been disproportionately affected by the coronavirus disease 2019 (COVID-19) pandemic, including higher rates of infection, hospitalization, and death. Data on responsiveness to COVID-19 vaccination strategies and immunogenicity are limited, yet required to inform vaccination strategies in this at-risk population.
Objective:
The objective of this study is to characterize the longitudinal serologic response to COVID-19 vaccination.
Design:
This is a prospective observational cohort study.
Setting:
Participating outpatient kidney programs within Ontario and British Columbia.
Patients:
Up to 2500 participants with CKD G3b-5D receiving COVID-19 vaccination, including participants receiving dialysis and kidney transplant recipients (CKD G1T-5T).
Measurements:
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG antibodies (anti-spike, anti-receptor binding domain, anti-nucleocapsid) will be detected by ELISA (enzyme-linked immunosorbent assay) from serum or dried blood spot testing. In a subset of participants, neutralizing antibodies against novel variants of concern will be evaluated. Peripheral blood mononuclear cells will be collected for exploratory immune profiling of SARS-CoV-2 specific cellular immunity.
Methods:
Participants will be recruited prior to or following any COVID-19 vaccine dose and have blood sampled for serological testing at multiple timepoints: 1, 3, 6, 9, and 12 months post vaccination. When possible, samples will be collected prior to a dose or booster. Participants will remain in the study for at least 1 year following their last COVID-19 vaccine dose.
Strengths and limitations:
The adaptive design of this study allows for planned modification based on emerging evidence or rapid changes in public health policy surrounding vaccination. Limitations include incomplete earlier timepoints for blood collection due to rapid vaccination of the population.
Conclusions:
This large multicenter serologic study of participants living with kidney disease will generate data on the kinetics of SARS-CoV-2 immune response to vaccination across the spectrum of CKD, providing insights into the amplitude and duration of immunity conferred by COVID-19 vaccination and allowing for characterization of factors associated with immune response. The results of this study may be used to inform immunization guidelines and public health recommendations for the 4 million Canadians living with CKD.
Keywords: COVID-19, serology, vaccine, chronic kidney disease, dialysis
Abrégé
Contexte:
Les personnes atteintes d’insuffisance rénale chronique (IRC) ont été touchées de façon disproportionnée par la pandémie de COVID-19 ayant notamment présenté des taux plus élevés d’infection, d’hospitalisation et de décès. Les données sur la réactivité aux stratégies de vaccination de la COVID-19 et à l’immunogénicité sont limitées, mais elles sont nécessaires pour développer des stratégies de vaccination dans cette population à risque.
Objectif:
Caractériser la réponse sérologique longitudinale à la vaccination contre la COVID-19.
Conception:
Étude de cohorte observationnelle prospective.
Cadre:
Les programmes ambulatoires de santé rénale participants en Ontario et en Colombie-Britannique.
Sujets:
Jusqu’à 2 500 personnes atteintes d’IRC G3B-5D recevant un vaccin contre la COVID-19, y compris des patients suivant des traitements de dialyse et des receveurs d’une greffe rénale (IRC G1T-5T).
Mesures:
Les anticorps IgG anti-SARS-CoV-2 (anti-spike, anti-domaine de liaison au récepteur, anti-nucléocapside) seront détectés par ELISA à partir du sérum ou de taches de sang séché. Un sous-groupe de sujets participera également à l’évaluation d’anticorps neutralisants dirigés contre les nouveaux variants préoccupants. Des cellules mononuclées de sang périphérique seront prélevées pour établir un profil immunitaire exploratoire de l’immunité cellulaire spécifique au SARS-CoV-2.
Méthodologie:
Les sujets seront recrutés avant ou après toute dose du vaccin contre la COVID-19 et se soumettront à des prélèvements sanguins pour les tests sérologiques à 1, 3, 6, 9 et 12 mois post-vaccination. Lorsque possible, des échantillons seront prélevés avant l’administration d’une dose ou d’un rappel. Les sujets demeureront dans l’étude pendant au moins un an après leur dernière dose de vaccin contre la COVID-19.
Points forts et limites:
La conception adaptative de l’étude permet d’apporter des modifications planifiées fondées sur de nouvelles données ou des changements rapides dans les politiques de santé publique entourant la vaccination. Les résultats sont limités par l’absence de certains prélèvements sanguins antérieurs (point temporels) en raison de la vaccination rapide de la population.
Conclusion:
Cette vaste étude sérologique multicentrique menée auprès de personnes atteintes de néphropathie fournira des données sur la cinétique de la réponse immunitaire à la vaccination contre le SARS-CoV-2 dans l’ensemble du spectre de l’IRC. Elle fournira des informations sur l’amplitude et la durée de l’immunité conférée par la vaccination contre la COVID-19 et permettra de caractériser les facteurs associés à la réponse immunitaire. Ces résultats serviront à orienter les recommandations de santé publique et les lignes directrices en matière d’immunisation pour les quatre millions de Canadiens et Canadiennes qui vivent avec l’IRC.
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has resulted in a devastating global pandemic. Chronic kidney disease (CKD), which affects more than 10% of Canadians,1 is a major risk factor for severe disease and mortality in patients infected with COVID-19.2,3 Furthermore, individuals who receive in-center hemodialysis are at increased risk for exposure to SARS-CoV-2.4
Fortunately, vaccines against SARS-CoV-2 have been developed at an unprecedented speed and are now being further tailored given the emergence of novel variants of concern which have led to high rates of breakthrough infections, including the development of bivalent vaccines targeting the B.1.1.529 (Omicron) subvariants.5 -7 However, in the CKD population, multiple factors such as uremia, chronic inflammation, malnutrition, endothelial cell dysfunction, and immunosuppressive medication contribute to humoral and cellular dysfunction. Thus, after immunization, individuals with CKD typically have lower rates of seroconversion, lower antibody titers, and accelerated waning of immunity in comparison with the general population.8
To evaluate the immune response related to COVID-19 vaccination, we are conducting a longitudinal multicenter study focused on individuals with CKD across the spectrum of kidney function and modalities, including recipients of kidney transplants and those undergoing dialysis therapies in Ontario and British Columbia. This large cohort will be essential to strengthen our understanding of the immune response to COVID-19 vaccination in this population. The results of this study will provide critical information regarding longitudinal immune response following COVID-19 vaccination in the CKD population and explore factors associated with immunogenicity. As such, this work will inform the current and future care of people with CKD in Canada and may inform policy changes at the provincial and/or national level.
Methods
General Design
This is a prospective observational cohort study involving individuals with CKD G3b-5D and kidney transplant recipients (CKD G1T-5T) who have received or plan to receive a COVID-19 vaccine. This study aims to recruit up to 2500 participants with a glomerular filtration rate (GFR) of less than 45 mL/min/1.73m2, including those receiving dialysis and kidney transplant recipients. Participants will remain in the study for 1 year following their last COVID-19 vaccine dose and will have repeated serologic measurements. The study protocol will be revised based on the emergence of variants of concern and availability of additional vaccine doses. Demographics, comorbidities, medications, laboratory values, and prior hospital admissions will be obtained from the Ontario Renal Reporting System (ORRS) and the PROMIS database in British Columbia, and supplemented with electronic patient records. Serious adverse events related to the COVID-19 vaccination will be tracked by the Ontario Renal Network and British Columbia Renal Agency. COVID-19 infections will be tracked based on prior confirmed infections in provincial tracking systems, self-reported rapid antigen testing, or anti-nucleocapsid antibody seroconversion.
Study Objectives
The primary objectives of this study are to determine the longitudinal serologic response to vaccination in the CKD population through quantitative measurement of SARS-CoV-2 anti-spike, receptor binding domain (anti-RBD) and anti-nucleocapsid IgG antibodies and to monitor for adverse events related to vaccination. The secondary objective of this study is to perform exploratory immune profiling of humoral and cellular immunity in an enriched subset of the study population, including poor serologic responders, those with rapidly waning immunity, and those who contract COVID-19.
Participant Selection
Participants will be recruited from participating kidney programs within British Columbia and Ontario, consented when they are offered the COVID-19 vaccine, at routine visits, or through mailouts and phone calls.
Inclusion Criteria
Each participant must meet all of the following inclusion criteria to participate in this study: age ≥18 at study enrolment, G3b-5D CKD defined as GFR of less than 45 mL/min/1.73m2, or be a kidney transplant recipient, or on immunosuppressive treatment, and receiving the BNT162b2 COVID-19 vaccine (Pfizer-BioNTech), mRNA-1273 COVID-19 vaccine (Moderna), or ChAdOx1 nCoV-19 vaccine (Oxford-AstraZeneca). Of note, no participants received the Ad26.COV2.S vaccine (Johnson & Johnson).
Exclusion Criteria
The CKD participants declining COVID-19 vaccination or unable to provide informed consent due to cognitive impairment or a language barrier if a translator is unavailable were excluded.
Study Endpoints
The primary endpoints of this study are the SARS CoV-2 full-length spike protein (anti-spike), its anti-RBD, and anti-nucleocapsid IgG binding antibodies measured serially for up to 12 months following the most recent vaccine dose.
Secondary endpoints include viral neutralization against SARS-CoV-2 variants of concern and SARS-CoV-2-specific cellular immunity measured through a panel of cytokines and cytotoxic molecules.
Study Schedule and Procedures
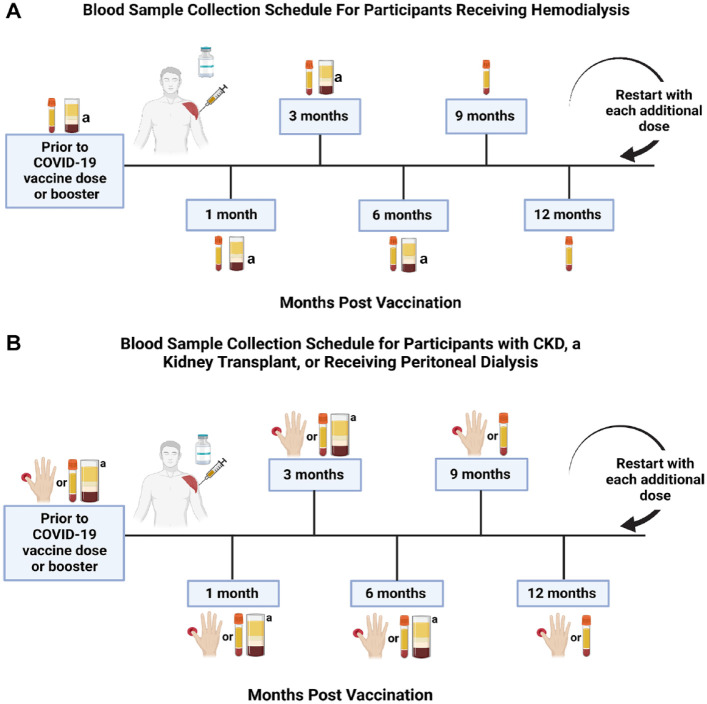
Consented participants will have samples drawn for serologic studies prior to each vaccine dose, and 1, 3, 6, 9, and 12 months following the most recent vaccine dose (Figure 1). Booster doses may include the bivalent mRNA-1273 original and Omicron B.1.1.529 BA.1 COVID-19 vaccine (Moderna) and bivalent BNT162b2 original and Omicron BA.4/BA.5 (Pfizer-BioNTech). All participants will remain in the study until at least 12 months after their last vaccine dose/booster. Details regarding COVID-19 infections will be recorded throughout the study.
Figure 1.
Sample collection schedule for study participants in relation to time from COVID-19 vaccination.
Note. When participants receive an additional vaccine dose or booster, the collection schedule will reset and begin again at the 1-month post dose/booster timepoint. The thin tube represents serum collection, the thick tube represents peripheral blood mononuclear cell collection, and the hand represents blood collection through dried blood spot kits. Samples will be analyzed for quantitative SARS-CoV-2 anti-spike, anti-receptor binding domain, and anti-nucleocapsid antibodies. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; CKD = chronic kidney disease.
aCollection for a subset of participants.
For participants receiving hemodialysis, serum samples will be drawn pre-dialysis. Those receiving home hemodialysis will either collect their own serum samples or have their samples collected by a dialysis nurse at the home dialysis unit. Dried blood spot (DBS) sampling allows participants to participate in the study without in-person visits. For feasibility, participants receiving peritoneal dialysis, kidney transplant recipients, and participants with non-dialysis CKD may have samples collected through DBS testing and/or serum sampling at the discretion of the Site Investigator. A 14-day window (14 days prior to or 14 days after a timepoint) will be allowed for sample collection; otherwise, collection will occur at the next scheduled timepoint.
A subset of the study participants will be selected for exploratory cellular immune profiling based on their humoral response to enrich for individuals with varying levels of SARS-CoV-2 anti-spike or anti-RBD antibodies, including those who fail to seroconvert following vaccination and those who contract COVID-19. For these participants, in addition to serum or DBS, peripheral blood samples will be collected for peripheral blood mononuclear cells (PBMCs) processing prior to a vaccine dose, then at 1, 3, and 6 months post vaccination. When participants in this subset receive an additional dose, both the serum and PBMC blood sample collection schedule will be reset to the first timepoint.
SARS-CoV-2 Binding Antibody Testing
Anti-SARS-CoV-2 IgG against anti-spike and anti-RBD are binding antibodies measured to determine the humoral response to COVID-19 vaccination. The RBD allows for SARS-CoV-2 to enter human cells by binding to the angiotensin-converting enzyme 2 (ACE2) receptor. Spike protein and RBD antibodies prevent SARS-CoV-2 from interacting with the ACE2 receptor resulting in viral neutralization. Conversely, the nucleocapsid protein is not targeted by available vaccines and therefore measuring anti-nucleocapsid antibodies can identify prior COVID-19 infection.
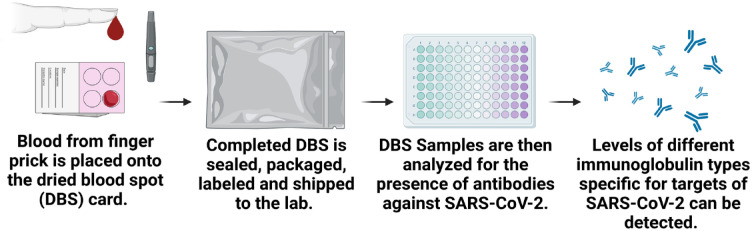
Serum and DBS sampling collection and storage
For serum, 5-mL samples will be collected in a serum separator tube and allowed to clot for 30 minutes and centrifuged for 10 minutes at 3000 × g at room temperature. Aliquots are made in 4 small −80°C stable microtubes each with a volume of 300 µL. DBS will be collected by placing a few drops of blood on Whatman 903 cards using a blade lancet for fingerpick puncture (Figure 2). Participants will be provided with an instruction sheet on how to complete DBS, which includes a link to an instructional video. Once the DBS cards are dry, they will be placed individually in sealable bags, along with a desiccant pouch. The cards can be stored and transported at ambient temperature to centralized laboratories through regular mail services. On reception in the laboratory, the cards will be placed at 4°C for short-term storage or frozen (at −80°C) for long-term storage. Cards will be brought to room temperature before the packages are opened, and a portion of the spot (2 × 3 mm) will be placed directly into a 96-well plate with a semi-automated hole puncher. Antibodies will be eluted from the punches by the addition of PBS-Tween with 1% Triton-X100, and incubated at room temperature with shaking for ≥ 4 hours.9
Figure 2.
Analysis of DBS samples.
Note. DBS may be used as an alternative to serum sampling for convenience. Using a spring-loaded lancet provided in the test kit, drops of blood from a finger prick are placed onto the DBS filter paper card. The DBS is then sealed, packaged, labeled, and shipped to an analytical lab, where it is processed, eluted, and analyzed by ELISA for the presence of anti-SARS-CoV-2 IgG antibodies. DBS = dried blood spot; ELISA = enzyme-linked immunosorbent assay; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Sample testing
SARS-CoV-2 enzyme-linked immunosorbent assay (ELISA) testing will be performed on DBS and serum samples on a robotic platform to quantitatively measure the anti-nucleocapsid, anti-spike, and anti-RBD total IgG antibodies present in the samples.9 Levels of antibodies to each of the antigens (produced by the National Research Council of Canada) will be normalized to reference standards included on each plate and expressed as relative ratios or World Health Organization International Standard units (BAU/mL).
In a subset of participants, a spiked-pseudotyped lentiviral neutralization assay will be performed to detect neutralization against the wild-type (D614G) SARS-CoV-2 and novel variants of concern.10 Cellular immune responses, including T-cell immunity, also play a key role in viral clearance and will be evaluated through PBMC collection at pre-specified time points using the LEGENDplex multiplex bead assay (BioLegend) and flow cytometry from selected participants.11,12 Cytokines and cytotoxic molecules will be measured including IL-2, 4, 10, 17, IFN-γ, TNF, Granzyme A, B, Perforin, and sFASL.13,14 Further details regarding the spiked-pseudotyped lentiviral neutralization assay and cellular immunity testing are available in the Supplementary Material.
Sample Size Considerations
Given that SARS-CoV-2 antibody levels and their variance in the study population were unknown at the beginning of the study, sample size calculation was not performed based on the study outcomes. Based on feasibility of recruitment and the aim of examining risk factors associated with immune response, the target sample size is 2500 participants, including a pre-specification of aiming for greater than 1000 participants receiving in-center hemodialysis and 500 participants with non-dialysis CKD. This sample size was determined based on the rule of 10 observations per parameter estimate in a multivariable model, allowing evaluation of up to 25 potential risk factors were determined a priori. The final number of covariates will be adjusted based on achieved sample size, with backward selection based on the effect sizes.
Statistical Analysis Plan
Antibody levels will be described using parametric or non-parametric summary measures as appropriate. Longitudinal antibody levels will be modeled using mixed effect models to account for repeated measures. Analyses will be performed using the R Statistical Program (version 4.1.0). A priori risk groups to be explored are age, sex, ethnicity, residence, kidney function, dialysis modality, and use of immunosuppression. The results of these subgroups will be reported separately, and interactions between these groups and antibody response will be investigated. Continuous adverse events will be summarized using means (standard deviations) or medians (25th, 75th percentiles) as appropriate. Categorical events will be summarized as percentages. Figures were created using Biorender.com.
Discussion
This protocol outlines an ambitious observational study to assess the immune response to COVID-19 vaccination in a diverse group of CKD populations, in 2 large Canadian provinces. Recruitment to this study has been completed with 2236 participants consenting to participate. Among consenting participants, 1022 are receiving in-center hemodialysis, 64 are receiving home hemodialysis, 143 are receiving peritoneal dialysis, 245 are kidney transplant recipients, 647 have G3b-5ND CKD, and 115 are healthy controls.
Knowledge Translation Plan
A knowledge translation (KT) strategy will be facilitated by the Can-SOLVE CKD Network (www.cansolveckd.ca) to inform research, generate awareness, and influence policy change based on the aims and results of this study.15 The short-term goal of the KT plan is to increase understanding of both serology testing and antibody response to COVID-19 vaccines in people with CKD. The long-term goal is to inform best practices and policies for maximal COVID-19 vaccine effectiveness in individuals living with CKD based on the initial vaccine types (heterologous vs homologous COVID-19 boosting), active treatments (ie, dialysis vs immunosuppressive drugs), and comorbidities. Working closely with patient partners, Can-SOLVE has created FAQ sheets, diagrams, and patient facing handouts on serology and antibody testing. These materials highlight how serology testing differs from PCR tests, how to understand serology test results, and how antibody levels may correlate with protection against SARS-CoV-2 (Supplementary Material). Study participants will receive their individual results as they become available. The research project teams will hold webinars and present at conferences about the aims of the study, preliminary findings, progress updates, and the final results of this study. A survey to assess patient understanding and knowledge of vaccination will be sent to participants enrolled in the study.
Strengths and Limitations
This open observational cohort has several strengths. First, this collaborative multicenter study with a large sample size will be able to describe the heterogeneity in serologic response in the CKD population and allow for assessment of important factors associated with the immune response against COVID-19. Second, the assays used in this study are quantitative, standardized, and have been deployed in more than 40 other clinical studies, which allows for comparison of results to other cohorts being studied by the Canadian COVID-19 Immunity Task Force. Third, a subset will undergo evaluation for cellular immunity which will fill a key gap in current studies, as research has primarily focused on humoral response. Finally, due to rapidly changing evidence, emergence of novel SARS-CoV-2 variants of concern, and public health recommendations, the protocol for this study is continuously being amended including the inclusion criteria, intervention, and methods (Table 1). Standard operating procedures on recruitment, labeling of blood samples, and data management will change dynamically as required.
Table 1.
Protocol Modifications to the Study From Conception.
| Original protocol (March 18, 2021) | Current protocol (September 30, 2022) | |
|---|---|---|
| Participants (inclusion criteria) | Individuals with chronic kidney disease G3b, 4, and 5, including those receiving dialysis, who intend to or have already received at least 1 dose of a COVID-19 vaccine | Individuals with chronic kidney disease G3b, 4, and 5, including those receiving dialysis or kidney transplant recipients who intend to or have already received at least 1 dose or booster of a COVID-19 vaccine |
| Intervention | Two-dose primary vaccine series | Extended 3-dose primary vaccine series and booster(s), including bivalent vaccines |
| Methods (study schedule) | Blood sample collection timepoints are pre-dose, between doses 1 and 2, then 1, 3, 6, and 12-months post dose 2 (with additional collections at 2, 4, and 5 months for participants receiving hemodialysis) | Blood sample collection timepoints are prior to a dose or booster, then 1, 3, 6, 9, and 12 months post dose or booster. With each new dose or booster, sample collection resets to the 1-month post timepoint. Additional neutralizing antibody testing against novel variants of concern, including Beta, Delta, Omicron (BA.1, BA.5, BQ.1.1, XBB) was added |
A limitation of this study is that not all participants will have serology collected at earlier or all timepoints given the rapid pace of vaccination. The adaptive nature of the study can be challenging to navigate in a multicenter study across 2 provinces that have variations in public health recommendations, COVID-19 PCR testing intensity, lockdowns, outbreaks, and COVID-19 vaccine schedules and strategies. However, this will not detract from the primary objective of understanding immunity in this population over at least a 1-year period. Second, for feasibility, a portion of participants will have DBS testing rather than serum samples. DBS assays have demonstrated strong correlation with serum measurements and have been deployed in other large cohort studies in COVID-19, but do not allow for assessment of viral neutralization or cellular immunity. Third, given the labor-intensive nature of evaluating cellular immunity, this will only be performed in a small subset of the participants, but attempts will be made to enrich the population selected for this testing for groups of interest including poor serologic responders.
Given the evolving nature of the COVID-19 pandemic with respect to access to services, non-pharmaceutical interventions, emergence of variants of concern, and vaccination policies and practices, there has been a need to adapt and modify the study protocols, while maintaining the longitudinal nature originally intended.
Study Progress
This observational study has already discovered practice-changing findings, including low seroconversion following a single dose of BNT162b216 and higher immunogenicity with the mRNA-1273 vaccine in comparison to BNT162b2 among individuals receiving maintenance hemodialysiss.17 During the course of this study, the Canadian National Advisory Committee on Immunization recommended a third dose of a COVID-19 vaccine as part of an extended primary series for individuals who are moderately to severely immunocompromised due to concerns regarding waning immunity and evolving variants of concern. Building on our findings of differences in immunogenicity by vaccine type, we have undertaken a randomized controlled trial in participants with CKD evaluating the immunogenicity of mRNA-1273 vs BNT162b2 third-dose mRNA COVID-19 vaccination (NCT05022329). In addition, there is concern that despite multiple vaccine doses, kidney transplant recipients remain vulnerable to severe COVID-19. In a cohort of kidney transplant recipients undergoing neutralizing antibody testing, we found an improved immune response to the third mRNA vaccine dose; however, more than 50% of kidney transplant recipients lacked an Omicron (BA.1)-specific response even after receiving the third dose.10
Ongoing Challenges and Future Directions
Despite the progress to date, much work lies ahead as the continuous mutation of SARS-CoV-2 and our response to the pandemic leave important questions unanswered. While much of the focus in individuals with kidney disease has been on the in-center hemodialysis population, less work has examined patients receiving peritoneal dialysis and the non-dialysis CKD population. Differing levels of natural immunity from prior infections and use of different vaccine combinations have resulted in a heterogeneous humoral response in individuals with CKD, and there are insufficient data available on long-term antibody kinetics following COVID-19 vaccination in people with advanced kidney disease.
An ongoing challenge for individuals with CKD (and indeed the broader population) is the correlation between measurements of antibodies and clinical outcomes. While neutralizing antibody levels are known to correlate with COVID-19 disease severity,18,19 this relationship has not been as robustly evaluated during the period in which Omicron has been the dominant variant of concern. In addition, the safety and effectiveness of newer bivalent vaccines against the Omicron sublineages (BA.1, BA.2, BA.5, BQ.1, XBB) have yet to be studied in people with CKD. Furthermore, limited studies have correlated the humoral response with the cellular response, and work with PBMCs will be essential in addressing this knowledge gap. Given the likelihood of ongoing COVID-19 vaccine doses in the future in people with CKD, serologic studies will be essential in informing the need, timing, and effectiveness of additional booster doses.
Conclusion
In summary, we describe a prospective observational serologic study of participants in Ontario and British Columbia with CKD G3b to 5D who have received COVID-19 vaccines at variable intervals, with homologous and heterologous vaccine types, which will provide longitudinal data on the SARS-CoV-2 antibody response. A subset of the enrolled cohort will undergo detailed neutralizing antibody and cellular immune profiling. This study will provide insight into the immune response to COVID-19 vaccination and systematically assess risk factors associated with differential amplitude or longevity of response, complementing ongoing epidemiologic studies. Ultimately, we aim to guide public health policy regarding vaccination strategies during the ongoing COVID-19 pandemic to inform care of people with kidney disease in Canada.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581231160511 for Determining the Longitudinal Serologic Response to COVID-19 Vaccination in the Chronic Kidney Disease Population: A Clinical Research Protocol by Kevin Yau, Omosomi Enilama, Adeera Levin, Marc G. Romney, Joel Singer, Peter Blake, Jeffrey Perl, Jerome A. Leis, Robert Kozak, Hubert Tsui, Shelly Bolotin, Vanessa Tran, Christopher T. Chan, Paul Tam, Miten Dhruve, Christopher Kandel, Jose Estrada-Codecido, Tyler Brown, Aswani Siwakoti, Kento T. Abe, Queenie Hu, Karen Colwill, Anne-Claude Gingras, Matthew J. Oliver and Michelle A. Hladunewich in Canadian Journal of Kidney Health and Disease
Acknowledgments
We would like to thank Mandana Rahimi and Gail Klein for valuable assistance in coordinating this study.
Footnotes
Ethics Approval and Consent to Participate: REB approval of both the protocol (including amendments) and the consent form were obtained at Sunnybrook Health Sciences Centre, Unity Health Toronto, University Health Network (CTO #3604), Michael Garron Hospital (REB # 856-2201-Inf-066), Scarborough Health Network (COV-21-021), and British Columbia (REB #H21-00848).
Consent for Publication: All authors read and approved the final version of this manuscript.
Availability of Data and Materials: All or portions of the de-identified data and serum samples are available upon reasonable request to the corresponding author, Michelle Hladunewich after approval by the local ethics committee.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kevin Yau has received speaker fees from AstraZeneca. Adeera Levin reports being a scientific advisor to, or member of, AstraZeneca, Bayer, Boehringer-Ingelheim, Canadian Journal of Kidney Health and Disease, Canadian Institutes of Health Research, Certa, Chinook Therapeutics, Johnson and Johnson, Kidney Foundation of Canada, National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Otsuka, Reata, Retrophin, and The George Institute; receiving research funding from AstraZeneca, Boehringer-Ingelheim, Canadian Institute of Health Research, Janssen, Johnson and Johnson, Kidney Foundation of Canada, Merck, NIDDK, NIH, Ortho Biotech, Otsuka, and Oxford Clinical Trials; and having consultancy agreements with Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Johnson and Johnson/Jansen, Reata, and Retrophin. Marc Romney has received research support from Public Health Agency of Canada and the COVID-19 Immunity Task Force. Jeffrey Perl reports receiving speaking honoraria and consultancy fees from Baxter Healthcare; grants from Agency for Healthcare Research and Quality grant support; speaking honoraria from Fresenius Medical Care, AstraZeneca, Davita Healthcare, and US Renal Care; and consultancy fees from LiberDi Dialysis outside the submitted work. Shelly Bolotin reports funding from the Canadian Institutes of Health Research, the Canadian Immunization Research Network, the COVID-19 Immunity Task Force, and the Public Health Agency of Canada, outside the submitted work. She is a member of the Canadian Immunization Research Network Management Committee, COVID-19 Immunity Task Force Leadership Group. Vanessa Tran reports that Public Health Ontario received funding from the Public Health Agency of Canada and test kits from the Canadian Immunity Task Force for COVID-19 serosurveillance studies. Public Health Ontario is also involved in a COVID-19 mix-and-match vaccine clinical trial. Shelly Bolotin and Vanessa Tran are employees of Public Health Ontario. Anne-Claude Gingras has received research funds from a research contract with Providence Therapeutics Holdings, Inc, for other projects, participates in the COVID-19 Immunity Task Force (CITF) Immune Science and Testing working party, chairs the CIHR Institute of Genetics Advisory Board, and is a member of the SAB of the National Research Council of Canada Human Health Therapeutics Board. Matthew Oliver and Michelle Hladunewich are contracted Medical Leads at Ontario Renal Network, Ontario Health. Matthew Oliver is owner of Oliver Medical Management Inc., which licenses Dialysis Management Analysis and Reporting System software. He has received honoraria for speaking from Baxter Healthcare. Michelle Hladunewich reports receiving grants from Pfizer for a study in focal segmental glomerulosclerosis; Ionis, Calliditas, and Chinook for studies in Immunoglobulin A nephropathy; and Roche for a preeclampsia study. No other competing interests were declared.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by a grant from the COVID-19 Immunity Task Force (CITF), which is funded by the Government of Canada (grant no. 2122-HQ-000071).
ORCID iDs: Kevin Yau  https://orcid.org/0000-0001-8653-6778
https://orcid.org/0000-0001-8653-6778
Michelle A. Hladunewich  https://orcid.org/0000-0001-9227-4292
https://orcid.org/0000-0001-9227-4292
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Arora P, Vasa P, Brenner D, et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ. 2013;185:E417-E423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gok M, Cetinkaya H, Kandemir T, et al. Chronic kidney disease predicts poor outcomes of COVID-19 patients. Int Urol Nephrol. 2021;53(9):1891-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Council E-E, Group EW. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021;36(1):87-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yau K, Muller MP, Lin M, et al. COVID-19 outbreak in an urban hemodialysis unit. Am J Kidney Dis. 2020;76(5):690-695.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rössler A, Riepler L, Bante D, et al. SARS-CoV-2 omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022;386:698-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chalkias S, Harper C, Vrbicky K, et al. A bivalent omicron-containing booster vaccine against covid-19. N Engl J Med. 2022;387:1279-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown PE, Fu SH, Bansal A, et al.Omicron BA.1/1.1 SARS-CoV-2 infection among vaccinated Canadian adults. N Engl J Med. 2022;386:2337-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DaRoza G, Loewen A, Djurdjev O, et al. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis. 2003;42:1184-1192. [DOI] [PubMed] [Google Scholar]
- 9. Colwill K, Galipeau Y, Stuible M, et al. A scalable serology solution for profiling humoral immune responses to SARS-CoV-2 infection and vaccination. Clin Transl Immunology. 2022;11(3):e1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McEvoy CM, Hu Q, Abe KT, et al. Humoral responses in the omicron era following three-dose SARS-CoV-2 vaccine series in kidney transplant recipients. Transplant Direct. 2022;9:e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt ME, Varga SM. The CD8 T cell response to respiratory virus infections. Front Immunol. 2018;9:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Windpessl M, Bruchfeld A, Anders HJ, et al. COVID-19 vaccines and kidney disease. Nat Rev Nephrol. 2021;17:291-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Law JC, Koh WH, Budylowski P, et al. Systematic examination of antigen-specific recall T cell responses to SARS-CoV-2 versus influenza virus reveals a distinct inflammatory profile. J Immunol. 2021;206:37-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Law JC, Girard M, Chao GYC, et al. Persistence of T cell and antibody responses to SARS-CoV-2 up to 9 months after symptom onset. J Immunol. 2022;208:429-443. [DOI] [PubMed] [Google Scholar]
- 15. Levin A, Adams E, Barrett BJ, et al. Canadians seeking solutions and innovations to overcome chronic kidney disease (Can-SOLVE CKD): form and function. Can J Kidney Health Dis. 2018;5:2054358117749530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yau K, Abe KT, Naimark D, et al. Evaluation of the SARS-CoV-2 antibody response to the BNT162b2 vaccine in patients undergoing hemodialysis. JAMA Netw Open. 2021;4:e2123622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yau K, Chan CT, Abe KT, et al. Differences in mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 vaccine immunogenicity among patients undergoing dialysis. CMAJ. 2022;194:E297-E305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205-1211. [DOI] [PubMed] [Google Scholar]
- 19. Garcia-Beltran WF, Lam EC, Astudillo MG, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476-488.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581231160511 for Determining the Longitudinal Serologic Response to COVID-19 Vaccination in the Chronic Kidney Disease Population: A Clinical Research Protocol by Kevin Yau, Omosomi Enilama, Adeera Levin, Marc G. Romney, Joel Singer, Peter Blake, Jeffrey Perl, Jerome A. Leis, Robert Kozak, Hubert Tsui, Shelly Bolotin, Vanessa Tran, Christopher T. Chan, Paul Tam, Miten Dhruve, Christopher Kandel, Jose Estrada-Codecido, Tyler Brown, Aswani Siwakoti, Kento T. Abe, Queenie Hu, Karen Colwill, Anne-Claude Gingras, Matthew J. Oliver and Michelle A. Hladunewich in Canadian Journal of Kidney Health and Disease