Abstract
Objective
To determine if there is an association between the timing of testosterone discontinuation and assisted reproductive technology (ART) outcomes.
Design
Retrospectivse cohort study.
Setting
Single academic center.
Patient(s)
We included consecutive transgender patients seeking fertility preservation between October 2019 and April 2021. Patients who identified as transgender on androgens for >1 month on presentation were included.
Intervention(s)
None.
Main outcome measure(s)
A linear regression model was used to evaluate the effect of testosterone discontinuation duration on the number of mature oocytes retrieved.
Result(s)
Eighteen patients (mean age 27.7 [SD 5.2] years, mean body mass index 27.3 [SD 4.6] kg/m2, mean antimüllerian hormone 27.2 [SD 11.8], median antral follicle count 20 [interquartile range (IQR) 14–32]) were included in the analysis. No patient underwent transition-related surgery (eg, oophorectomy, hysterectomy). None of the patients were previously pregnant. Mean time o,n testosterone was 44 (SD 29.6) months. The median time off testosterone until the start of ovarian stimulation was 7.7 weeks (IQR 4.3–20.7). All patients underwent oocyte cryopreservation except one who had embryo cryopreservation. The median total number of oocytes was 11 (IQR 7–14). The median number of mature oocytes was 7.5 (IQR 5–12) oocytes. The univariate regression model evaluating the duration of time off testosterone before ART demonstrated no significant association with the outcome of mature oocytes (regression coefficient, 0.19; 95% confidence interval, –0.13 to 0.50).
Conclusion
In a retrospective analysis of transgender patients recently on testosterone undergoing ART, no association was detected between the timing of testosterone cessation and the number of mature oocytes.
Key Words: ART, IVF, transgender
The transgender population has been marginalized and understudied in medicine (1, 2, 3). Nearly half of the transgender patients reported a desire to have children. Of the transgender patients with ovaries, 37% would consider freezing oocyte if that option were available (4). However, a considerable proportion of transgender patients decline to proceed with fertility preservation because of the need for testosterone discontinuation (5).
There are variable reported durations in the literature on when to discontinue testosterone before starting the fertility preservation cycle. Presently, no guidelines specify the duration or necessity of testosterone discontinuation. Therefore, counseling transgender patients who have already started testosterone therapy is challenging. In a series of 16 transgender men reporting a mean testosterone discontinuation time of 4.5 months, most patients resumed menses before starting assisted reproductive technology (ART). A matched retrospective cohort study reported that transgender patients with a history of testosterone therapy were able to have cycle outcomes similar to that of cisgender women (6). Several observational studies have described oocyte cryopreservation among transgender men before initiation or after discontinuation of androgen therapy (6, 7, 8, 9, 10, 11).
The literature includes reports that transgender men demonstrate similar oocyte retrieval outcomes to cisgender women (6); however, there are no reports examining whether the timing of testosterone cessation impacts fertility preservation outcomes. Therefore, we aimed to evaluate whether the timing of testosterone cessation was associated with ART outcomes in transgender men presenting to a high-volume fertility center.
Materials and methods
This study is a retrospective cohort study of consecutive transgender men seeking fertility preservation at a single, high-volume academic fertility clinic between October 2019 and April 2021. The institutional research ethics board approval was obtained. Transgender male patients taking androgens for at least one month were included in the study. Transgender patients who did not start hormone replacement therapy or stopped hormone replacement therapy for more than one year were excluded. Patients were followed until the completion of their ART cycle. The primary analysis evaluated the association existing between the duration of testosterone cessation and the number of mature oocytes retrieved in transgender men.
All patients who had initiated testosterone therapy stopped before their fertility preservation cycle started. The standard recommendation at this clinic was the discontinuation of testosterone at least four weeks before ovarian stimulation; however, some patients voluntarily stopped testosterone earlier. Resumption of menses was not required before starting the cycle. Gonadotropin dosing was determined by the treating physician based on patient factors such as age, weight, and ovarian reserve. Transvaginal ultrasound and bloodwork monitoring were initiated to monitor the ovarian response. A gonadotropin releasing hormone (GnRH) antagonist was started between day 7 and 9 of the stimulation cycle once the patient met one of the following two criteria: serum estradiol (E2) concentration greater than 2,000 pmol/L or a follicle measuring ≥14 mm. A daily subcutaneous injection of a GnRH antagonist (0.25 mg) was administered by the patient subcutaneously to prevent an endogenous surge in luteinizing hormone (LH) concentration. Monitoring of the ovarian response continued until three or more follicles were visualized by transvaginal ultrasound with a mean diameter of ≥17 mm. Ovulation was subsequently triggered with a subcutaneous injection of human chorionic gonadotropin or GnRH agonist, depending on the managing in vitro fertility physician. If a GnRH agonist trigger was chosen 12 hours after their trigger medication, the patient returned to the clinic for blood work, including LH and progesterone levels. This was a routine blood test to confirm that an endogenous LH surge had occurred. Transvaginal ultrasound-guided oocyte retrieval was performed approximately 36 hours after the administration of the initial trigger.
Statistical Analysis
Continuous variables were examined for normality using normality probability plots and the Shapiro-Wilk test (12). Normally distributed variables were reported as mean with standard deviation. Non-normally distributed variables were reported as median with interquartile range (IQR).
The primary outcome of this study was the association between testosterone discontinuation duration (weeks) and the number of mature oocytes. Other covariates evaluated as secondary outcomes included: patient age, antimüllerian hormone (AMH), antral follicle count (AFC), and duration on testosterone (weeks). All covariates were continuous variables. These models were also run for the outcome of the total number of oocytes. The total dataset was >99% complete; no missing data for covariates or outcomes were used in the regression analyses. All patients completed the follow-up necessary to obtain outcome data for their respective ART cycles. Statistical analysis was performed using R statistical software (version 4.1.2) (13).
Results
A total of 18 transgender men were included. The mean age at cycle start was 27.7 (SD, 5.2; IQR, 24–30) years. None of the patients were previously pregnant. None of the patients who pursued treatment had undergone transition-related surgery (eg, oophorectomy or hysterectomy). The mean AMH was 27.2 (SD, 11.8) pmol/L. One patient had an AMH level of 7.1 pmol/L. The median AFC was 20 (IQR, 14–32). All patients had previously been on testosterone treatment and had stopped before their fertility preservation cycle. Mean time on testosterone therapy was 44 (SD, 29.6) months. The median time off testosterone until the start of ovarian stimulation was 7.7 (IQR, 4.3–20.7) weeks. There were no complications secondary to the fertility preservation cycle, including no thromboembolic events. Table 1 details the remaining demographics of included patients.
Table 1.
Characteristics of patients
| Characteristic | Value∗ |
|---|---|
| Age (y) | 27.7 (5.2) |
| BMI (kg/m2) (n = 16) | 27.3 (4.6) |
| Smoking, n (%) | |
| Never | 13 (72.2) |
| Present | 4 (22.2) |
| Past | 1 (5.6) |
| AMH (pmol/L) | 27.2 (11.8) |
| AFC | 20 (14 to 32) |
| Partner sex (n = 17) | |
| Female, n (%) | 11 (64.7) |
| Male, n (%) | 1 (5.9) |
| No partner, n (%) | 5 (29.4) |
| Time on testosterone therapy (w) | 191 (129) |
| Time off testosterone therapy before IVF (w) | 7.7 (4.3 to 20.7) |
| Resumed bleeding before stimulation, n (%) | 10 (55.6) |
| Estradiol at trigger (pmol/L) | 4695 (3135 to 6430) |
| Progesterone at trigger (nmol/L) | 2.7 (1.5) |
| Luteal phase priming | |
| None, n (%) | 15 (83.3) |
| Estrace, n (%) | 1 (5.6) |
| OCP, n (%) | 2 (11.1) |
| Recombinant FSH dose (IU) | 210.4 (52.6) |
| Total gonadotropin dose per patient (IU) | 2284 (858) |
| FSH only protocol, n (%) | 13 (72.2) |
| FSH + LH protocol, n (%) | 5 (27.8) |
| Days of stimulation, n (%) | 10.6 (1.5) |
| Trigger | |
| hCG, n (%) | 14 (77.8) |
| GnRH Agonist, n (%) | 4 (22.2) |
| Endometrial lining at time of trigger (mm) | 9.1 (3.3) |
All variables have complete data unless otherwise specified.
AFC = antral follicle count; AMH = antimüllerian hormone; BMI = body mass index; FSH = follicle-stimulating hormone; hCG = human chorionic gonadotropin; IVF = in- vitro fertilization; LH = luteinizing home; OCP = oral contraceptive pill; PCOS = polycystic ovarian syndrome.
Continuous variables reported as mean (SD) or median (interquartile range).
The median number of mature oocytes was 7.5 (IQR, 5–12; Table 2). The median number of total oocytes was 11 (IQR, 7–14). One patient cryopreserved seven embryos. For this patient, 14 oocytes were retrieved: 12 were fertilized through conventional in vitro fertility using donor sperm. Of these, seven progressed to the embryo stage. A total of seven embryos were cryopreserved: five were cryopreserved on day five, and two were cryopreserved on day 6. The remainder of the patients underwent oocyte cryopreservation. The median total number of cryopreserved oocytes was 7.5 (IQR, 5–12).
Table 2.
In vitro fertilization outcomes
| Outcome | Value∗ |
|---|---|
| Follicles over 11 mm at time of trigger | 15.8 (6.2) |
| Follicles 16–22 mm at time of trigger | 8 (6 to 10) |
| Total oocytes | 11 (7 to 14) |
| Mature oocytes | 7.5 (5 to 12) |
Continuous variables reported as mean (SD) or median (interquartile range).
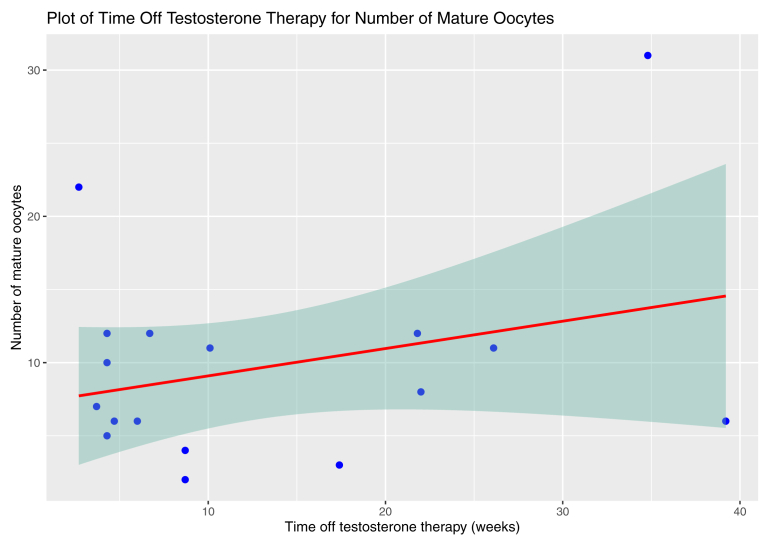
There was no significant association between testosterone discontinuation duration and number of mature oocytes (regression coefficient 0.19, 95% confidence interval (CI), 0.13 to 0.50, P=.226). AMH (P=.039) and AFC (P=.001) were associated with the number of mature oocytes. There was no statistical significance demonstrated between age and the number of mature oocytes (P=.059) and between age and the total number of oocytes (P=.058). There was no association detected between the duration on testosterone and number of mature oocytes (P=.339). The remaining regression outcomes are reported in Table 3. Figure 1 plots the testosterone discontinuation duration and mature oocyte count with 95% CI bands.
TABLE 3.
Simple unadjusted regression analyses for mature oocytes and total oocytes
| Outcome | Covariates | Regression coefficient (95% CI) | P value |
|---|---|---|---|
| Mature oocytes | Age | –0.62 (–1.26 to –0.03) | .059 |
| AMH | 0.29 (0.02 to 0.57) | .039 | |
| AFC | 0.43 (0.20 to 0.67) | .001 | |
| Duration on testosterone | –0.01 (–0.04 to 0.02) | .339 | |
| Testosterone discontinuation duration | 0.19 (–0.13 to 0.50) | .226 | |
| Total oocytes | Age | –0.74 (–1.51 to –0.03) | .058 |
| AMH | 0.34 (0.003 to 0.68) | .048 | |
| AFC | 0.58 (0.33 to 0.83) | .0002 | |
| Duration on testosterone | –0.01 (–0.05 to 0.02) | .434 | |
| Testosterone discontinuation duration | 0.24 (–0.13 to 0.62) | .188 |
AFC = antral follicle count; AMH = antimüllerian; CI = confidence interval.
Figure 1.
Plot of testosterone discontinuation duration vs. mature oocytes with 95% confidence interval band, demonstrating no association
Discussion
Our study is among the largest cohort of transgender patients previously on testosterone therapy undergoing fertility preservation. Our analyses did not detect an association between testosterone discontinuation time and the number of oocytes obtained, as well as the number of mature oocytes. These results have important implications for transgender men who wish to undergo ART but are concerned about the potential undesirable effects of stopping testosterone therapy (14) or are concerned about the effects of testosterone on fertility outcomes (15).
Studies have reported that approximately 0.6% of the population in the United States (16) and 0.24% of the population in Canada (17) identifies as transgender. Approximately 47% of transgender persons wish to have a child that is genetically related to them (18). Notably, 37% of transgender men with ovaries reported considering freezing oocytes if that option were available (4). Unfortunately, in addition to fertility preservation being a costly, time-consuming, and invasive process (19, 20, 21), the effects of testosterone cessation can be psychologically stressful (14). A study found that even although 76% of transgender men and women had considered fertility preservation before transition, only 3% of transgender men had completed this process (22). The resumption of pelvic bleeding is one of the causes of distress for this patient population (14). A study of 41 transgender men examined the timing of resumption of pelvic bleeding after testosterone cessation: 8% of patients had resumption at <1 month after testosterone cessation; 24% had resumption at 1 month after cessation; 28% after 2 months; 16% after three months; 4% after 4–6 months (23). Twenty percent had no pelvic bleeding before pregnancy (23). The patients in our study were on testosterone for varying durations ranging from one month to eight years. Univariate analyses showed no association between the duration of testosterone before the cycle started and oocyte yield. Because patients who discontinued testosterone for only one month did not have inferior results to those who stopped for more than one month, discontinuing testosterone for one month may be a reasonable compromise to reduce the discomfort associated with the return of menses without compromising good oocyte yield. Future studies are needed to evaluate even shorter periods of testosterone discontinuation. A recent case report by Gale and colleagues reported a 20-year-old transgender man on testosterone treatment for 18 months who chose to continue treatment throughout the fertility preservation cycle (18). The patient had a robust outcome with 22 mature oocytes.
In our study, most patients were on testosterone hormone therapy for more than 12 months, with no negative result on the baseline ovarian reserve given a mean AMH of 27.2 pmol/L. Most patients had normal baseline E2 and follicle-stimulating hormone levels, with average to above-average ovarian reserve results for a given age. Several case reports and case series have reported similar normal ovarian reserve parameters and ovarian responses (6, 7, 8, 9, 10, 11, 24). In the present study, the average total dose of gonadotropins used was 2284 IU, and the median number of total oocytes was 11 (mature oocytes 7.5). Leung and colleagues matched cisgender and transgender patients across several variables, including age and AMH, with transgender patients receiving significantly higher doses of gonadotropins (4155 vs. 2707, P=.002) (6). The mean number of oocytes retrieved per patient was 18.6, of which 77% were mature oocytes (6). Notably, the mean number of gonadotropins used in cisgender patients (2707 IU) was similar to the amount of gonadotropins used in our study in transgender patients (2284 IU). Leung and colleagues explained that the higher doses used are because of the mindset that this is a "one-shot deal" because of the intent of maximizing oocyte yield given the transition-related and financial costs of performing a cycle. Accordingly, higher gonadotropin doses may be needed in transgender patients to obtain a similar number of oocytes as in cisgender patients. Another larger study showed a similar number of retrieved oocytes compared with our results (25). Law and colleagues performed a population-based cohort study of 221,221 treatment cycles in cisgender patients (25): in the 18–29-year-old group, 36.1% had 4–9 oocytes retrieved, 28.5% had 10–14 oocytes retrieved, and 15.7% had 15–19 oocytes retrieved. This right-skewed distribution, with the majority (64.6%) of patients having between 4–14 oocytes retrieved, is similar to the right-skewed distribution in our study of transgender patients, whereby 72.2% had between 4–14 oocytes retrieved. Future studies evaluating ART outcomes between transgender and cisgender patients may consider differences in populations, gonadotropin dosage, or other cycle factors should differences in oocyte yield be demonstrated. Should future studies support higher gonadotropin dosages explaining this discrepancy, higher doses of gonadotropins may be considered to maximize the success of a single cycle.
Part of the lack of consensus on the timing of testosterone cessation may be secondary to an incomplete understanding of the effects of androgen therapy on the ovarian environment. A study by Caanen and colleagues reported a reduction in AMH levels (median 3.5 to 0.3 μg/L, P<.0001) in 22 female-to-male transgender patients with a mean age of 22.4 years (24). The investigators conclude that androgens are important in regulating AMH (26). Leung and colleagues performed a matched cohort study and found that after being matched on age, BMI, and AMH, transgender men showed a similar number of oocytes compared with cisgender women (6). These patients discontinued testosterone for a mean of 4 months (6). The transgender patients had a mean AMH of 24.3 (SD 13.6) pmol/L and a mean age of 28.3 (SD 6.7) (6). In our study, the mean AMH was 27.3 pmol/L, but the median total oocytes count was 11, and the median mature oocytes count was 7.5. As the transgender population remains to be investigated in large studies, several hypotheses can explain these results: transgender patients may inherently have lower oocyte yield, the effect of using testosterone before the cycle may reduce the number of retrieved oocytes, ART cycles may require optimization (eg, higher gonadotropin dosage), or there is no true difference, and the observed difference is secondary to selection bias.
Our study had several strengths. Our center is one that aims to reduce the period of testosterone discontinuation and the start of stimulation to 4 weeks. This period is shorter than other discontinuation periods (27), and accordingly permits evaluating patients with both short and long discontinuation periods. In addition, our primary analysis attempted to evaluate whether a relationship exists between testosterone discontinuation duration and oocyte yield. This analysis is the first step toward ultimately identifying a minimum evidence-based threshold for when to discontinue testosterone in this population. Nevertheless, our study contains limitations. First, our sample size is low, so the lack of association in our primary outcome may be based on an unpowered sample. Unfortunately, there always remains a probability of an outcome being negative because of insufficient statistical power. With limited knowledge of the true effect size in transgender patients, determining the statistical power of analysis is difficult. We aimed to minimize the effect of our low sample size by only evaluating a single covariate in the model. Future studies, including patients from several centers, must validate our findings with higher statistical power. Second, our study was retrospective, which increases the risk of selection bias. Third, there was clinical heterogeneity, such as differences in trigger (human chorionic gonadotropin vs. lupron), which may have differences in effect on oocyte maturity. Fourth, pregnancy success rates were not assessed in this study, so the pragmatic question of the effect of testosterone discontinuation on oocyte quality remains to be completely evaluated. Finally, the transgender population is a diverse population, likely requiring larger studies to capture the diversity of patients interested in pursuing ART. Larger studies would increase the clinical generalizability of our results and allow for subgroup analyses that may predict differences in ART outcomes.
Conclusions
In a sample of 18 transgender patients undergoing fertility preservation, there was no association between testosterone discontinuation duration and ART, including the number of mature oocytes. Transmale patients who have already started hormone therapy and who are interested in minimizing their time off of testosterone may, therefore, still consider fertility preservation. Larger studies are needed to confirm these findings.
Footnotes
M.A. has nothing to disclose. A.K. has nothing to disclose. E.M. has nothing to disclose. C.C. has nothing to disclose. K.L. has nothing to disclose.
References
- 1.Giblon R., Bauer G.R. Health care availability, quality, and unmet need: a comparison of transgender and cisgender residents of Ontario, Canada. BMC Health Serv Res. 2017;17:283. doi: 10.1186/s12913-017-2226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer G.R., Scheim A.I., Deutsch M.B., Massarella C. Reported emergency department avoidance, use, and experiences of transgender persons in Ontario, Canada: results from a respondent-driven sampling survey. Ann Emerg Med. 2014;63:713–720. doi: 10.1016/j.annemergmed.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 3.James-Abra S., Tarasoff L.A., Green D., Epsein R., Andeson S., Mavel S., et al. Trans people's experiences with assisted reproduction services: a qualitative study. Hum Reprod. 2015;30:1365–1374. doi: 10.1093/humrep/dev087. [DOI] [PubMed] [Google Scholar]
- 4.Wierckx K., Van Caenegem E., Pennings G., Elaut E., Dedecker D., Van de Peer F., et al. Reproductive wish in transsexual men. Hum Reprod. 2012;27:483–487. doi: 10.1093/humrep/der406. [DOI] [PubMed] [Google Scholar]
- 5.Jones C.A., Reiter L., Greenblatt E. Fertility preservation in transgender patients. Int J Transgend. 2016;17:76–82. [Google Scholar]
- 6.Leung A., Sakkas D., Pang S., Thornton K., Resetkova N. Assisted reproductive technology outcomes in female-to-male transgender patients compared with cisgender patients: a new frontier in reproductive medicine. Fertil Steril. 2019;112:858–865. doi: 10.1016/j.fertnstert.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Cho K., Harjee R., Roberts J., Dunne C. Fertility preservation in a transgender man without prolonged discontinuation of testosterone: a case report and literature review. F S Rep. 2020;1:43–47. doi: 10.1016/j.xfre.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adeleye A.J., Cedars M.I., Smith J., Mok-Lin E. Ovarian stimulation for fertility preservation or family building in a cohort of transgender men. J Assist Reprod Genet. 2019;36:2155–2161. doi: 10.1007/s10815-019-01558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gidoni Y.S., Raziel A., Strassburger D., Kasterstein E., Ben-Ami I., Ron-El R. Can we preserve fertility in a female-to-male trangender after a long term testosterone treatment-case report. Fertil Steril. 2013;100:S169–S170. [Google Scholar]
- 10.Broughton D., Omurtag K. Care of the transgender or gender-nonconforming patient undergoing in vitro fertilization. Int J Transgend. 2017;18:372–375. [Google Scholar]
- 11.Rothenberg S.S., Witchel S.F., Menke M.N. Oocyte cryopreservation in a transgender male adolescent. N Engl J Med. 2019;380:886–887. doi: 10.1056/NEJMc1813275. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro S.S., Wilk M.B. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 13.R Core Team R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria; 2016. https://www.R-project.org/ Available at:
- 14.Armuand G., Dhejne C., Olofsson J.I., Rodriguez-Wallberg K.A. Transgender men's experiences of fertility preservation: a qualitative study. Hum Reprod. 2017;32:383–390. doi: 10.1093/humrep/dew323. [DOI] [PubMed] [Google Scholar]
- 15.Tasker F., Gato J. Gender identity and future thinking about parenthood: a qualitative analysis of focus group data with transgender and non-binary people in the United Kingdom. Front Psychol. 2020;11:865. doi: 10.3389/fpsyg.2020.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman J.L., Flores A.R., Brown T.N., Wilson B.D., Conron K.J. University of California; 2017. Age of individuals who identify as transgender in the United States. eScholarship.https://escholarship.org/uc/item/45f0k759 Available at: [Google Scholar]
- 17.Jaffray B. Juristat: Canadian Centre for Justice Statistics; 2020. Experiences of violent victimization and unwanted sexual behaviours among gay, lesbian, bisexual and other sexual minority people, and the transgender population, in Canada, 2018; pp. 1–27.https://www150.statcan.gc.ca/n1/pub/85-002-x/2020001/article/00009-eng.htm Available at: [Google Scholar]
- 18.Tornello S.L., Bos H. Parenting intentions among transgender individuals. LGBT Health. 2017;4:115–120. doi: 10.1089/lgbt.2016.0153. [DOI] [PubMed] [Google Scholar]
- 19.Moravek M.B., Kinnear H.M., George J., Batchelor J., Shikanov A., Radmanabhan V., et al. Impact of exogenous testosterone on reproduction in transgender men. Endocrinology. 2020;161:bqaa014. doi: 10.1210/endocr/bqaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartholomaeus C., Riggs D.W. Transgender and non-binary Australians’ experiences with healthcare professionals in relation to fertility preservation. Cult Health Sex. 2020;22:129–145. doi: 10.1080/13691058.2019.1580388. [DOI] [PubMed] [Google Scholar]
- 21.Baram S., Myers S.A., Yee S., Librach C.L. Fertility preservation for transgender adolescents and young adults: a systematic review. Hum Reprod Update. 2019;25:694–716. doi: 10.1093/humupd/dmz026. [DOI] [PubMed] [Google Scholar]
- 22.Auer M.K., Fuss J., Nieder T.O., Briken P., Biedermann S.V., Stalla G.K., et al. Desire to have children among transgender people in Germany: A cross-sectional multi-center study. J Sex Med. 2018;15:757–767. doi: 10.1016/j.jsxm.2018.03.083. [DOI] [PubMed] [Google Scholar]
- 23.Light A.D., Obedin-Maliver J., Sevelius J.M., Kerns J.L. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014;124:1120–1127. doi: 10.1097/AOG.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 24.Amir H., Yaish I., Samara N., Hasson J., Groutz A., Azem F. Ovarian stimulation outcomes among transgender men compared with fertile cisgender women. J Assist Reprod Genet. 2020;37:2463–2472. doi: 10.1007/s10815-020-01902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law Y.J., Zhang N., Venetis C.A., Chambers G.M., Harris K. The number of oocytes associated with maximum cumulative live birth rates per aspiration depends on female age: a population study of 221 221 treatment cycles. Hum Reprod. 2019;34:1778–1787. doi: 10.1093/humrep/dez100. [DOI] [PubMed] [Google Scholar]
- 26.Caanen M.R., Soleman R.S., Kuijper E.A., Kreukels B.P.C., Roo C.D., Tillemen K., et al. Antimüllerian hormone levels decrease in female-to-male transsexuals using testosterone as cross-sex therapy. Fertil Steril. 2015;103:1340–1345. doi: 10.1016/j.fertnstert.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Sterling J., Garcia M.M. Fertility preservation options for transgender individuals. Trans Andol Urol. 2020;9:S215–S226. doi: 10.21037/tau.2019.09.28. [DOI] [PMC free article] [PubMed] [Google Scholar]