Abstract
BACKGROUND
Currently available semiautomated insulin-delivery systems require individualized insulin regimens for the initialization of therapy and meal doses based on carbohydrate counting for routine operation. In contrast, the bionic pancreas is initialized only on the basis of body weight, makes all dose decisions and delivers insulin autonomously, and uses meal announcements without carbohydrate counting.
METHODS
In this 13-week, multicenter, randomized trial, we randomly assigned in a 2:1 ratio persons at least 6 years of age with type 1 diabetes either to receive bionic pancreas treatment with insulin aspart or insulin lispro or to receive standard care (defined as any insulin-delivery method with unblinded, real-time continuous glucose monitoring). The primary outcome was the glycated hemoglobin level at 13 weeks. The key secondary outcome was the percentage of time that the glucose level as assessed by continuous glucose monitoring was below 54 mg per deciliter; the prespecified noninferiority limit for this outcome was 1 percentage point. Safety was also assessed.
RESULTS
A total of 219 participants 6 to 79 years of age were assigned to the bionic-pancreas group, and 107 to the standard-care group. The glycated hemoglobin level decreased from 7.9% to 7.3% in the bionic-pancreas group and did not change (was at 7.7% at both time points) in the standard-care group (mean adjusted difference at 13 weeks, −0.5 percentage points; 95% confidence interval [CI], −0.6 to −0.3; P<0.001). The percentage of time that the glucose level as assessed by continuous glucose monitoring was below 54 mg per deciliter did not differ significantly between the two groups (13-week adjusted difference, 0.0 percentage points; 95% CI, −0.1 to 0.04; P<0.001 for noninferiority). The rate of severe hypoglycemia was 17.7 events per 100 participant-years in the bionic-pancreas group and 10.8 events per 100 participant-years in the standard-care group (P = 0.39). No episodes of diabetic ketoacidosis occurred in either group.
CONCLUSIONS
In this 13-week, randomized trial involving adults and children with type 1 diabetes, use of a bionic pancreas was associated with a greater reduction than standard care in the glycated hemoglobin level. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; ClinicalTrials.gov number, NCT04200313.)
The current glycated hemoglobin goal of less than 7.0% is met in only approximately 20% of patients with type 1 diabetes in the United States.1,2 Automated and semiautomated insulin-delivery systems have the potential to increase the number of persons with diabetes in whom this goal would be met.3 Commercially available hybrid closed-loop systems, which partially automate insulin delivery, require the inputting of basal rates, insulin-sensitivity factors, carbohydrate-to-insulin ratios, the total daily dose of insulin, or a subset of these metrics on initialization. Insulin doses at mealtime are determined by having the user enter the number of grams of carbohydrate in the meal, and effective therapy may be dependent on user-initiated correction doses for hyperglycemia. A warm-up period, during which the system gathers information about insulin doses that are controlled by the user, may be required before automation can begin.
In contrast, the iLet bionic pancreas (Beta Bionics) does not use information about the patient’s previous insulin regimen (e.g., basal and bolus dose settings), is initialized only on the basis of body weight, and automates the determination and delivery of all insulin doses immediately after body-weight data have been entered, with no warm-up period. Meal announcements consist of a qualitative estimate of carbohydrate content (“usual for me,” “more,” or “less”) as compared with a typical meal of that type (“breakfast,” “lunch,” or “dinner”). The algorithms that determine the insulin dose by the bionic pancreas were designed to continually adapt to the user’s insulin needs. Because all the therapeutic insulin doses are determined by the bionic pancreas (including basal, correction, and meal-announcement doses), it is not possible for the user to determine or modify insulin doses.
The bionic pancreas that we used in this trial was developed as both an insulin-only system and a bihormonal system that administers both insulin and glucagon. Here, we report the results of a multicenter, randomized trial evaluating the efficacy and safety of the insulin-only configuration of the bionic pancreas in adults and children 6 years of age or older with type 1 diabetes. We compared the bionic pancreas with standard care, which was defined as any method of insulin delivery combined with unblinded, real-time continuous glucose monitoring.
METHODS
TRIAL CONDUCT AND OVERSIGHT
We conducted this parallel-group, unblinded trial at 16 centers in the United States. The trial protocol, which is available with the full text of this article at NEJM.org, was approved by a central institutional review board. Written informed consent was obtained from all adult participants (≥18 years of age), with parental consent and participant assent obtained for children. An investigational device exemption was approved by the Food and Drug Administration. An independent data and safety monitoring board provided trial oversight.
Trial funding was provided by the National Institute of Diabetes and Digestive and Kidney Diseases and others. The Jaeb Center for Health Research was the trial coordinating center and was responsible for the randomization scheme, the database, data validation, analyses, and trial coordination. The steering committee was responsible for the design of the trial and for the decision to submit the manuscript for publication. The first three authors wrote the first draft of the manuscript and vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol.
Beta Bionics provided the experimental bionic-pancreas devices that were used in the trial. Fast-acting insulin aspart and insulin aspart were provided by Novo Nordisk, and insulin lispro was provided by Eli Lilly. Blood-glucose meters and test strips (Contour Next One Blood Glucose Monitoring System) were provided by Ascensia Diabetes Care. Continuous-glucose-monitor sensors and transmitters were purchased from Dexcom at a discounted price.
Two authors were involved in the design of the trial before the founding of Beta Bionics and were involved in the oversight of the trial as Beta Bionics employees and shareholders. Otherwise, no external funder had a role in the design or conduct of the trial, the collection or analysis of the data, or the preparation of the manuscript. There were no agreements concerning the confidentiality of the data with respect to publication rights between the funders and the authors or their institutions.
TRIAL DESIGN AND PARTICIPANTS
Eligible participants were at least 6 years of age, had received a diagnosis of type 1 diabetes, and had used insulin for at least 1 year. The complete criteria are listed in Table S1 in the Supplementary Appendix, available at NEJM.org. Participants who were not already using the Dexcom G6 continuous glucose monitor used a blinded Dexcom G6 monitor in order to obtain 2 weeks of baseline glucose data just before randomization.
Participants 18 years of age or older were randomly assigned in a 2:2:1 ratio to use the bionic pancreas with insulin aspart or insulin lispro (bionic-pancreas group), the bionic pancreas with fast-acting insulin aspart, or standard-care insulin delivery plus use of the unblinded Dexcom G6 continuous glucose monitor (standard-care group). Analyses of the fast-acting insulin-aspart group were prespecified as secondary analyses and are not reported here.4 Participants 6 to 17 years of age were randomly assigned in a 2:1 ratio to the bionic-pancreas group or the standard-care group. Randomization was performed separately for adults and children with the use of a computer-generated sequence, with a permuted block design and with stratification according to site (see the Supplementary Appendix).
Participants who were assigned to the bionic-pancreas group were trained on the use of the system, which included the iLet device with embedded bionic-pancreas insulin-dose algorithms, the Dexcom G6 continuous glucose monitor, and the Inset I infusion set (Unomedical), which is an insulin-infusion set with a Teflon cannula inserted at a 90° angle to the skin surface. The algorithms were initialized by entering the participant’s body weight; there was no run-in or warm-up period before automated insulin delivery commenced. The default glucose target was “usual” (120 mg per deciliter [6.7 mmol per liter]) and could be shifted by 10 mg per deciliter (0.6 mmol per liter) down to “lower” or up to “higher”; a different target could be set for part of the day. In response to qualitative meal announcements to the system by the user, the system delivered approximately 75% of the autonomously estimated insulin immediately, a dose that could not be modified by the user (see the Supplementary Appendix). All glucose-correction boluses were fully automated; there was no mechanism for manual administration of insulin through the bionic pancreas.
When data from continuous glucose monitoring were not available, the bionic pancreas continued to administer insulin on the basis of a basal profile that had been autonomously determined by the bionic pancreas when such data were available. The bionic pancreas administered meal doses as usual in response to meal announcements and delivered glucose-correction doses on the basis of entered blood-glucose values. Information about adaptation and changes in body weight is provided in the Supplementary Appendix. Participants in the bionic-pancreas group were provided with a blood glucose meter (Contour Next One, Ascensia Diabetes Care), a ketone meter (Precision Xtra, Abbott Diabetes Care), and guidelines for identifying and managing infusion-set failure (Fig. S1).
Participants who were assigned to the standard-care group continued to use the insulin-delivery method they were using at the time of enrollment (which could include hybrid closed-loop systems) and used a real-time unblinded Dexcom G6 continuous glucose monitor that was provided by the trial. They were not provided with blood-glucose or ketone meters, nor were they given guidelines regarding infusion-set failure. Participants were instructed to contact their own health care provider for guidance on diabetes management.
Scheduled visits and contacts were the same in each group. Participants were contacted by telephone on day 1 or 2 and had follow-up visits, which could be completed by means of video conference, at 2, 6, 10, and 13 weeks. Glycated hemoglobin was measured at a central laboratory at randomization and at the completion of 6 weeks and 13 weeks.
OUTCOMES
The primary outcome was the glycated hemoglobin level at 13 weeks. The key secondary outcome, which was second in the hierarchical analysis, was the percentage of time that the glucose level as measured by the continuous glucose monitor was below 54 mg per deciliter (3.0 mmol per liter); the testing for noninferiority of this outcome was a margin of 1 percentage point. Other secondary outcomes that were included in the hierarchy were ordered as follows: the mean glucose level; the percentage of time with the glucose level in the range of 70 to 180 mg per deciliter (3.9 to 10.0 mmol per liter); the percentage of time with the glucose level above 180 mg per deciliter; the percentage of time with the glucose level above 250 mg per deciliter (13.9 mmol per liter); the glucose-level standard deviation; the percentage of time with the glucose level below 70 mg per deciliter; the percentage of time with the glucose level below 54 mg per deciliter, to be tested for superiority; and the glucose coefficient of variation. Additional secondary and exploratory outcomes are listed in the Supplementary Appendix. Safety outcomes included the incidence of severe hypoglycemia (defined as hypoglycemia with cognitive impairment requiring the assistance of a third party for treatment), diabetic ketoacidosis, and other serious adverse events.
STATISTICAL ANALYSIS
An overall sample size of 440 was selected for regulatory purposes; 110 participants were to be enrolled in the fast-acting insulin-aspart group, the results for which are not reported here.4 We calculated that if 200 participants in the bionic-pancreas group using insulin aspart or insulin lispro and 100 participants in the standard-care group completed the trial, the trial would have more than 99% power for the primary analysis, assuming a difference in the mean glycated hemoglobin level of 0.4 percentage points between the bionic-pancreas group and the standard-care group, a standard deviation of the 13-week glycated hemoglobin level of 0.8 percentage points in each group, and a correlation between the glycated hemoglobin levels at baseline and 13 weeks of 0.40 with a two-sided type I error of 5%.
Statistical analyses, except for the per-protocol efficacy analysis (see the Supplementary Appendix), were performed on an intention-to-treat basis and included all the participants who had undergone randomization. In the primary and key secondary analyses, we compared the bionic pancreas and standard care using a linear mixed-effects regression model with adjustment for the baseline value, age, and site (random factor); 95% confidence intervals are reported. For the key secondary outcome, noninferiority was assessed by comparing the upper boundary of the confidence interval to a noninferiority margin of 1 percentage point. Secondary outcomes were tested in a hierarchical fashion, as specified in the protocol, to maintain a type I error rate of 5%; an outcome was tested only if the previous outcome met significance criteria. Per-protocol analyses, sensitivity analyses, and subgroup analyses were performed for the primary outcome, and on-treatment analyses were performed for all hierarchical outcomes as described in the Supplementary Appendix. Other additional statistical methods are described in the Supplementary Appendix.
RESULTS
PARTICIPANTS AND FOLLOW-UP
Between January 4, 2021, and July 7, 2021, a total of 326 adults and children were randomly assigned to the bionic-pancreas group (219 participants) or the standard-care group (107 participants) (Fig. S2). The age of the participants ranged from 6 to 79 years, and the baseline glycated hemoglobin level ranged from 5.5 to 13.1% (Tables 1 and S2). A total of 74% of the participants identified as being non-Hispanic White, 10% as non-Hispanic Black, 10% as Hispanic, and 6% as another or more than one race or ethnic group. The relevance and representativeness of the trial population is discussed in Table S3.
Table 1.
Characteristics of the Participants at Baseline.*
| Characteristic | Bionic Pancreas (N = 219) |
Standard Care (N = 107) |
|---|---|---|
| Age — yr | ||
| Mean | 28±19 | 28±20 |
| Range | 6–73 | 6–79 |
| Glycated hemoglobin — %† | ||
| Mean | 7.9±1.2 | 7.7±1.1 |
| Range | 5.5–13.1 | 5.5–11.3 |
| Female sex — no. (%) | 107 (49) | 41 (38) |
| Race or ethnic group — no. (%)‡ | ||
| White, non-Hispanic | 157 (72) | 83 (78) |
| Black, non-Hispanic | 27 (12) | 5 (5) |
| Hispanic | 23 (11) | 11 (10) |
| Asian | 2 (1) | 3 (3) |
| American Indian or Alaskan Native | 1 (<1) | 1 (1) |
| Multiple | 7 (3) | 4 (4) |
| Unknown or not reported | 2 (1) | 0 |
| Annual household income — no. (%) | ||
| <$50,000 | 24 (11) | 12 (11) |
| $50,000 to <$100,000 | 53 (24) | 25 (23) |
| ≥$100,000 | 124 (57) | 52 (49) |
| Unknown or not reported | 18 (8) | 18 (17) |
| Education level — no. (%) | ||
| <Bachelor’s degree | 72 (33) | 37 (35) |
| Bachelor’s degree | 76 (35) | 39 (36) |
| >Bachelor’s degree§ | 68 (31) | 28 (26) |
| Unknown or not reported | 3 (1) | 3 (3) |
| Insulin-delivery method — no. (%) | ||
| Multiple daily injections | 71 (32) | 39 (36) |
| Pump without automation | 71 (32) | 31 (29) |
| Pump with predictive low-glucose suspension | 9 (4) | 5 (5) |
| Hybrid closed-loop system¶ | 68 (31) | 32 (30) |
| Use of continuous glucose-monitoring system — no. (%) | 194 (89) | 97 (91) |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding.
Data on the glycated hemoglobin level were missing for one participant in the standard-care group.
Race and ethnic group were reported by the participants or their parents or guardians.
Participants (or the parents or guardians of participating children) reported holding a master’s, professional, or doctorate degree.
In the bionic-pancreas group, 17 participants were using the MiniMed 670G or 770G system (Medtronic) and 51 were using the Control-IQ system (Tandem Diabetes Care). In the standard-care group, 12 participants were using the MiniMed 670G or 770G system and 20 were using the Control-IQ system
At screening, 100 participants (31%) were using a hybrid closed-loop system, 14 (4%) a system with predictive low-glucose suspension, 102 (31%) an insulin pump without automation, and 110 (34%) multiple daily injections of insulin. In the standard-care group, 30% of the participants were using a hybrid closed-loop system (with 19% using a t:slim X2 insulin pump with the Control-IQ system [Tandem Diabetes Care] and 11% using a MiniMed 670G or 770G system [Medtronic]).
Of the enrolled participants, 323 (99%) completed the trial. A total of 19 participants (9%) in the bionic-pancreas group stopped using the bionic pancreas before the completion of the trial; 16 of these participants completed the trial (Table S4). Insulin was administered autonomously by the bionic pancreas for a median of 96% of the possible time during the 13-week trial (97% of the time when the bionic pancreas was in use), with input from the continuous glucose monitor available for dose decisions for 89% of the possible time during 13 weeks (90% of the time when the bionic pancreas was in use) (Table S5). Glucose-level targets that were used during the trial are shown in Table S6. The mean (±SD) number of meal announcements per day was 3.0±1.2, and the proportions of qualitative meal sizes that were used for announcements are reported according to meal type in Table S7. In the standard-care group, continuous-glucose-monitoring data were available for 96% of the possible time during 13 weeks. There were 64 unscheduled visits by 53 participants in the bionic-pancreas group and 12 unscheduled visits by 9 participants in the standard-care group (Tables S8 and S9).
EFFICACY OUTCOMES
The mean glycated hemoglobin level at 13 weeks (primary outcome) decreased from 7.9% at baseline to 7.3% in the bionic-pancreas group at week 13 and did not change (was 7.7% at both time points) in the standard-care group (Table 2 and Figs. 1A and S3 through S5). The mean adjusted between-group difference in the glycated hemoglobin level at 13 weeks was −0.5 percentage points (95% confidence interval [CI], −0.6 to −0.3; P<0.001).
Table 2.
Primary and Secondary Hierarchical Efficacy Outcomes.*
| Outcome | Baseline | Follow-up over 13 Wk or at 13 Wk |
Adjusted Difference (95% CI)† |
P Value | ||
|---|---|---|---|---|---|---|
| Bionic Pancreas (N = 219) |
Standard Care (N = 107) |
Bionic Pancreas (N = 219) |
Standard Care (N = 107) |
|||
| Primary outcome | ||||||
| Glycated hemoglobin — % | 7.9±1.2 | 7.7±1.1 | 7.3±0.7 | 7.7±1.0 | −0.5 (−0.6 to −0.3) | <0.001 |
| Key secondary outcome | ||||||
| Median percentage of time with glucose level <54 mg/dl (IQR) — % | 0.2 (0.02 to 0.6) | 0.2 (0.0 to 0.4) | 0.3 (0.2 to 0.6) | 0.2 (0.1 to 0.6) | 0.0 (−0.1 to 0.04) | <0.001‡ |
| Other secondary hierarchical outcomes in prespecified order | ||||||
| Mean glucose level — mg/dl§ | 187±40 | 190±42 | 164±15 | 181±32 | −16 (−19 to −12) | <0.001 |
| Percentage of time with glucose level in range 70–180 mg/dl — % | 51±19 | 51±20 | 65±9 | 54±17 | 11 (9 to 13) | <0.001 |
| Percentage of time with glucose level >180 mg/dl — % | 46±20 | 47±21 | 33±9 | 44±18 | −10 (−12 to −8) | <0.001 |
| Median percentage of time with glucose level >250 mg/dl (IQR) — % | 16.0 (7.0 to 27.3) | 17.8 (6.0 to 33.5) | 8.5 (5.3 to 13.2) | 14.9 (6.3 to 25.3) | −5.0 (−6.6 to −3.6) | <0.001 |
| Glucose SD — mg/dl¶ | 67±16 | 68±18 | 60±11 | 67±16 | −7 (−8 to −5) | <0.001 |
| Median percentage of time with glucose level <70 mg/dl (IQR) — % | 1.5 (0.5 to 2.8) | 1.4 (0.4 to 2.9) | 1.8 (1.1 to 2.9) | 1.8 (0.8 to 3.1) | −0.1 (−0.3 to 0.2) | 0.51 |
| Median percentage of time with glucose level <54 mg/dl (IQR) — %∥ | 0.2 (0.02 to 0.6) | 0.2 (0.0 to 0.4) | 0.3 (0.2 to 0.6) | 0.2 (0.1 to 0.6) | 0.0 (−0.1 to 0.04) | — |
| Glucose coefficient of variation — %¶ | 36±6 | 36±6 | 36±5 | 37±5 | −0.8 (−1.6 to 0.0) | — |
Plus–minus values are means ±SD. Data on the glycated hemoglobin level at baseline were missing for one participant in the standard-care group; data on the glycated hemoglobin level at week 13 were missing for seven participants in the bionic-pancreas group and for three in the standard-care group. Follow-up continuous glucose-monitoring data were missing for one participant in the bionic-pancreas group. All the participants who had undergone randomization were included in the models. To control the type I error, a hierarchical approach was used in which hypothesis testing was performed sequentially in the order listed. When a P value of 0.05 or higher was observed, the subsequent outcomes on the list were not formally tested. All P values reflect testing for superiority, except for the P value for the key secondary outcome, which was tested for noninferiority. To convert values for glucose to millimoles per liter, multiply by 0.05551. CI denotes confidence interval, and IQR interquartile range.
The adjusted difference was computed from a mixed-effect model with adjustment for baseline value of the metric, age at randomization, and site (random effect). Differences in percentages are shown in percentage points. Missing data were handled with the use of direct-likelihood analyses. Owing to a skewed distribution, the percentages of time with the glucose level above 250 mg per deciliter (13.9 mmol per liter), below 70 mg per deciliter (3.9 mmol per liter), and below 54 mg per deciliter (3.0 mmol per liter) were transformed with the use of a rank normal transformation.
The P value for the key secondary outcome was from testing for noninferiority with a margin of 1.0 percentage point.
Shown are the means of the individual participants’ mean glucose levels.
The glucose SD and coefficient of variation values indicate within-participant variability of sensor glucose measurements.
The outcome of the percentage of time with the glucose level below 54 mg per deciliter was to be tested for superiority at this level in the hierarchical analysis. This and the subsequent analysis were not conducted since the result for the percentage of time with the glucose level below 70 mg per deciliter was not significant.
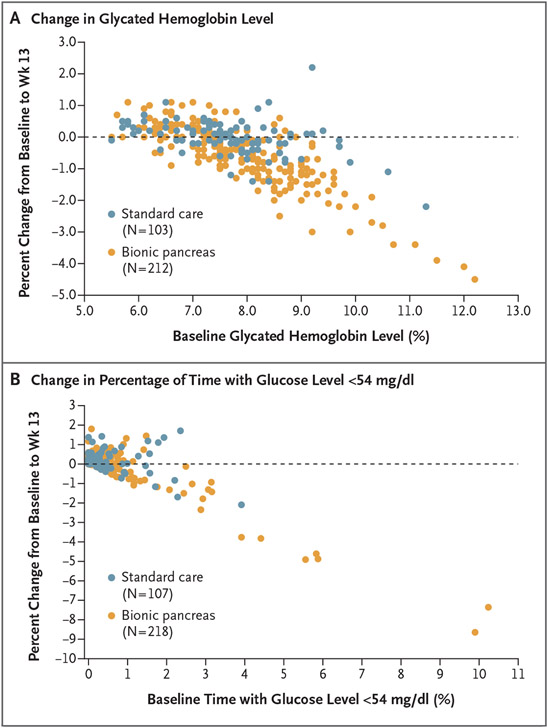
Figure 1. Changes in the Glycated Hemoglobin Level and the Percentage of Time with the Glucose Level below 54 mg per Deciliter from Baseline to Week 13, as Compared with Baseline.
Panel A shows a scatterplot of the change in the glycated hemoglobin level from baseline (randomization) to 13 weeks, as compared with the glycated hemoglobin level at baseline. Panel B shows a scatterplot of the change in the percentage of time with the glucose level below 54 mg per deciliter (3.0 mmol per liter), as assessed by continuous glucose monitoring, from baseline to week 13 as compared with the percentage of time with the glucose level below 54 mg per deciliter at baseline. In both panels, each point represents an individual participant, and participants with data plotted on the dashed horizontal line had no difference in the value at 13 weeks as compared with the baseline value.
In the key secondary analysis, the percentage of time that the glucose level as assessed by continuous glucose monitoring was below 54 mg per deciliter was noninferior in the bionic-pancreas group as compared with the standard-care group. The median values at baseline and over the 13-week period were 0.2% and 0.3%, respectively, in the bionic-pancreas group and 0.2% and 0.2%, respectively, in the standard-care group; the 13-week adjusted between-group difference was 0.0 percentage points (95% CI, −0.1 to 0.0; P<0.001 for noninferiority) (Table 2 and Figs. 1B, S6, and S7)
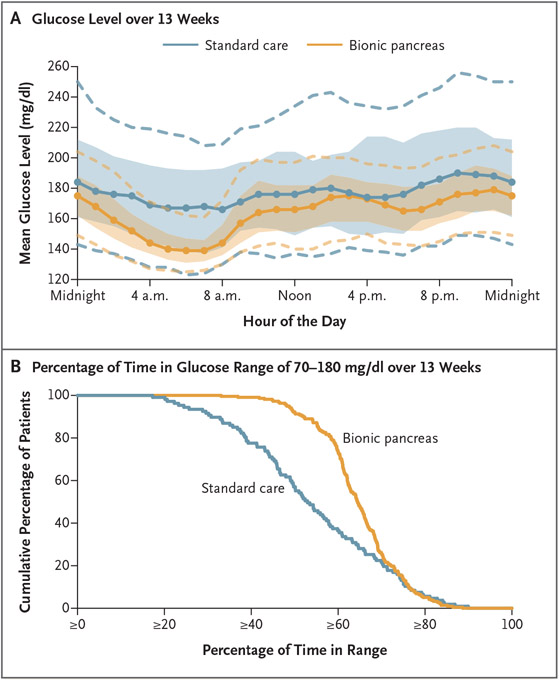
The mean adjusted difference between the bionic-pancreas group and the standard-care group in the mean glucose level at 13 weeks as assessed by continuous glucose monitoring was −16 mg per deciliter (−0.9 mmol per liter; 95% CI, −19 to −12 mg per deciliter [−1.1 to −0.7 mmol per liter]; P<0.001) (Table 2 and Figs. 2A and S8). The difference in the percentage of time that the glucose level was in the target range of 70 to 180 mg per deciliter was 11 percentage points (95% CI, 9 to 13; P<0.001) (Table 2 and Figs. 2B and S9), which equated to an increase of 2.6 hours per day in the bionic-pancreas group. The percentages of time that the glucose level as assessed by continuous glucose monitoring was above 180 mg per deciliter and above 250 mg per deciliter and the standard deviation of the glucose level were all lower in the bionic-pancreas group than in the standard-care group (P<0.001 for all comparisons) (Table 2 and Figs. S10 through S12). The percentage of time that the glucose level was below 70 mg per deciliter did not differ significantly between the two groups (P = 0.51) (Table 2 and Fig. S13), so significance was not tested for the remaining items in the hierarchy.
Figure 2. Mean Glucose Levels According to Time of Day and the Cumulative Distribution of Time in the Target Glucose Range.
Panel A shows an envelope plot of the glucose level as measured by continuous glucose monitoring over the 13-week trial, according to time of day. Solid circles denote the hourly median values of the participants’ mean glucose levels, and shaded regions indicate the interquartile range; dashed curves indicate the 10th and 90th percentiles. Panel B shows the cumulative distribution plot of the cumulative percentage of participants as compared with the percentage of time that the glucose level was within the range of 70 to 180 mg per deciliter (3.9 to 10.0 mmol per liter) as assessed by continuous glucose monitoring over the 13-week trial. To convert values for glucose to millimoles per liter, multiply by 0.05551.
Secondary outcomes regarding the glycated hemoglobin and glucose levels that were reflective of hyperglycemia were consistent with the primary and secondary hierarchical analyses, with all favoring the bionic-pancreas group overall and during daytime and nighttime. In addition, the incidence of prolonged hyperglycemia (defined as a glucose level >300 mg per deciliter [16.6 mmol per liter] for ≥90 minutes during a 120-minute period) also favored the bionic-pancreas group. The hypoglycemia and coefficient of variation outcomes as assessed by continuous glucose monitoring were similar in the two groups (Tables S10 through S13 and Figs. S14 through S16). Differences in the outcomes that were assessed by continuous glucose monitoring were apparent in the first 4 weeks and were stable throughout the trial period (Table S14 and Figs. S17 and S18). Analyses that excluded participants who had been using a hybrid closed-loop system before the trial and analyses that were restricted to participants with a baseline glycated hemoglobin level of more than 7.0% showed a larger treatment effect, with mean adjusted between-group differences in the glycated hemoglobin level of −0.6 percentage points (95% CI, −0.7 to −0.4) and −0.7 percentage points (95% CI, −0.9 to −0.5), respectively (Tables S15 and S16).
The mean adjusted difference in the glycated hemoglobin level at 13 weeks was similar in the adult cohort (participants ≥18 years of age: between-group difference, −0.5 percentage points; 95% CI, −0.6 to −0.3) and in the pediatric cohort (participants 6 to <18 years of age: between-group difference, −0.5 percentage points; 95% CI, −0.7 to −0.2). The 13-week adjusted difference between the treatment groups in the percentage of time with the glucose level below 54 mg per deciliter was 0.02 percentage points (95% CI, −0.04 to 0.08) in the adult cohort and −0.04 percentage points (95% CI, −0.13 to 0.03) in the pediatric cohort. The treatment effect of the bionic pancreas on the glycated hemoglobin level was greater among participants with a higher baseline glycated hemoglobin level and was greater among participants with a lower baseline time in the range of 70 to 180 mg per deciliter (Table S17). The benefit of the bionic pancreas over standard care was evident in the subgroups that were defined according to education status (higher and lower) and in the subgroups involving users of multiple daily injections and users of insulin pumps without automation (Table S17). The per-protocol, on-treatment, and sensitivity analyses produced results very similar to those of the primary intention-to-treat analysis (Tables S18 through S20). Treatment effects according to site for the glycated hemoglobin level and for the time with the glucose level below 54 mg per deciliter are shown in Tables S21 and S22. Data regarding the total daily insulin dose, body weight, and bodymass index are provided in Tables S23 and S24.
ADVERSE EVENTS
A total of 244 adverse events were reported in 126 participants in the bionic-pancreas group, and 10 adverse events were reported in 8 participants in the standard-care group (Table 3). There were 214 episodes of hyperglycemia with or without ketosis in the bionic-pancreas group and 2 episodes in the standard-care group; nearly all the events in the bionic-pancreas group were adjudicated by the medical monitor as being due to infusion-set failure. There were no episodes of diabetic ketoacidosis. The incidence of presumed infusion-set failure associated with prolonged hyperglycemia is shown in Table S25. There were 10 episodes of severe hypoglycemia in 10 participants in the bionic-pancreas group and 3 episodes in 2 participants in the standard-care group (incidence rate, 17.7 events and 10.8 events per 100 participant-years, respectively; P = 0.39). Two children received prescriptions to use insulin glargine with the bionic pancreas owing to prolonged periods of hyperglycemia despite the bionic pancreas administering the maximum amount of insulin allowed by its algorithms. A summary of the device issues that occurred in the bionic-pancreas group is provided in Table S26.
Table 3.
Safety Outcomes during the 13-Week Trial Period.*
| Event | Bionic Pancreas (N = 219) |
Standard Care (N = 107) |
P Value† |
|---|---|---|---|
| Any adverse event‡ | |||
| No. of participants with event (%) | 126 (58) | 8 (7) | — |
| No. of events | 244 | 10 | |
| Severe hypoglycemia§ | |||
| No. of participants with event (%) | 10 (5) | 2 (2) | 0.39 |
| No. of events | 10 | 3 | |
| Incidence rate per 100 participant-yr | 17.7 | 10.8 | |
| Diabetic ketoacidosis — no. of events¶ | 0 | 0 | ND |
| Other serious adverse event∥ | |||
| No. of participants (%) | 3 (1) | 2 (2) | 0.77 |
| No. of events | 3 | 2 | |
| Incidence rate per 100 participant-yr | 5.3 | 7.2 | |
| Increase in the glycated hemoglobin level by ≥0.5 percentage points — no. of participants (%) | 17 (8) | 8 (7) | 0.74 |
| Hyperglycemia with or without ketosis related to trial device** | |||
| No. of participants with event (%) | 95 (43) | NA | — |
| No. of events | 160 | NA | |
| Hyperglycemia with or without ketosis not related to trial device | |||
| No. of participants with event (%) | 44 (20) | 1 (1) | — |
| No. of events | 54 | 2 | |
| Nonsevere hypoglycemia | |||
| No. of participants with event (%) | 2 (1) | 0 | — |
| No. of events | 2 | 0 | |
| Other reportable adverse events | |||
| No. of participants with event (%) | 14 (6) | 3 (3) | — |
| No. of events | 15 | 3 |
NA denotes not applicable, and ND not done (according to the statistical analysis plan).
P values were calculated only for the outcomes that were prespecified in the statistical analysis plan. The P value for the number of severe hypoglycemic events per participant was produced from a Poisson regression model with adjustment for age at randomization, the glycated hemoglobin level as assessed by the central laboratory at randomization, the occurrence of at least one event of severe hypoglycemia before randomization, and site (random effect). A P value for the number of other serious adverse events per participant was produced from a Poisson regression model with adjustment for age at randomization, the glycated hemoglobin level as assessed by the central laboratory at randomization, and site (random effect). The P value for the percentage of participants with a glycated hemoglobin level that increased by at least 0.5 percentage points was produced from a marginal logistic-regression model with adjustment for age at randomization and the glycated hemoglobin level as assessed by the central laboratory at randomization, with a compound symmetry covariance structure to handle the correlated outcomes within site.
Reportable adverse events as defined in the protocol included the following: serious adverse event; adverse device effect (i.e., an adverse event related to use of a trial device); adverse event occurring in association with a trial procedure; adverse event not related to a device issue that led to temporary or permanent discontinuation of a trial device; adverse event for which a visit was made to the emergency department; severe hypoglycemia with cognitive impairment requiring the assistance of a third party for treatment; diabetic ketoacidosis; and hyperglycemic or ketosis event for which a visit occurred, a health care provider was contacted, or the blood ketone level was at least 1.0 mmol per liter.
Among participants 18 years of age or older, seven events of severe hypoglycemia occurred in seven participants (6.5%) in the bionic-pancreas group and two events in one participant (1.9%) in the standard-care group (incidence rate per 100 participant-years, 25.5 vs. 14.2; P = 0.40). Among participants younger than 18 years of age, three events of severe hypoglycemia occurred in three participants (2.7%) in the bionic-pancreas group and one event in one participant (1.9%) in the standard-care group (incidence rate per 100 participant-years, 10.4 vs. 7.3).
In the group of 114 participants using the bionic pancreas with fast-acting insulin aspart (for which data are not otherwise reported in this article), diabetic ketoacidosis occurred in 2 participants (2%) (on the basis of device evaluation, both events were considered by the medical monitor to be caused by an infusion-set failure) and severe hypoglycemia occurred in 3 (3%; incidence rate per 100 participant-years, 10.2).4
In the bionic-pancreas group, attempted suicide occurred in two participants and hypoglycemia in one. In the standard-care group, spontaneous pneumothorax and epiglottitis occurred in one participant each. The hypoglycemic event did not meet criteria for severe hypoglycemia related to cognitive impairment but was considered to be a serious adverse event (clinically significant medical event) as judged by the investigator.
The trial device included the insulin pump, infusion set, and continuous glucose monitor. Most adverse events of hyperglycemia that were considered to be related to the trial device were due to infusion-set failures.
DISCUSSION
In this multicenter, randomized trial involving adults and children with type 1 diabetes, the use of the insulin-only configuration of a bionic pancreas was associated with a lower glycated hemoglobin level, a lower mean glucose level, an increase of 2.6 hours per day in the target glucose range, and less time in a hyperglycemic state without an increase in the incidence of hypoglycemia as assessed by continuous glucose monitoring. The glycated hemoglobin level was 0.5 percentage points lower in the bionic-pancreas group than in the standard-care group, overall and in both the pediatric and adult subgroups. Further data regarding the age cohorts have been reported separately.5,6
Inappropriate insulin regimens and errors in estimation and calculation can lead to suboptimal glycemic control.7 Since all insulin doses, including for meals, were autonomously determined by the bionic pancreas, the results of this trial suggest that good glycemic control can be achieved by the bionic pancreas with only qualitative meal announcements and without a prespecified insulin regimen, carbohydrate counting, user-initiated correction doses, or any adjustment of the insulin dose by the user or health care provider.
Randomized trials involving adolescents and adults and children 6 to 13 years of age showed a mean difference of 11 percentage points in the percentage of time in the target range of 70 to 180 mg per deciliter with the Control-IQ system as compared with a pump plus continuous glucose monitoring; the mean adjusted difference in the glycated hemoglobin level was −0.3 percentage points among adults and adolescents, and there was a nonsignificant difference in the glycated hemoglobin level of −0.4 percentage points among children.8,9 The use of the bionic pancreas in our trial was associated with a greater effect on the glycated hemoglobin level than in these previous trials and with a similar increase in the time in the range of 70 to 180 mg per deciliter while requiring only body weight for initialization and no quantitative input or carbohydrate counting from the user, even though 31% of the cohort had been using a hybrid closed-loop system, such as the Control-IQ system, at baseline.
The most frequently reported adverse event in the bionic-pancreas group was hyperglycemia, which was often attributed to infusion-set failure and which occurred at an incidence that was similar to that reported for Teflon infusion sets having a 90° angle of insertion in another automated insulin-delivery system.10 According to the protocol, infusion-set failures were reportable adverse events only in the bionic-pancreas group. In addition, only participants in the bionic-pancreas group were provided with blood glucose and ketone meters, as well as guidelines on managing ketosis that required notification of the trial staff. Participants in the standard-care group followed their usual practices and were instructed to contact their diabetes health care provider for guidance on diabetes management. Therefore, infusion-set failures were not reported as adverse events in the standard-care group.
There were fewer episodes of prolonged hyperglycemia and less time with a glucose level of more than 180 mg per deciliter and more than 250 mg per deciliter with the bionic pancreas than with standard care. These findings indicate that the infusion-set failures did not adversely affect glycemia in the bionic-pancreas group.
The rates of severe hypoglycemia events did not differ significantly between the bionic-pancreas group and the standard-care group and were lower in both groups than the rate of 24.1 events per 100 participant-years that has been reported in the general type 1 diabetes population by the T1D Exchange registry,1 despite the use of a more-demanding definition of severe hypoglycemia requiring unconsciousness or seizure in that study. In addition, the percentage of time with the glucose level below 54 mg per deciliter did not differ significantly between the groups.
Strengths of this trial included a randomized, controlled design and the use of hybrid closed-loop systems by 30% of the participants in the standard-care group. This trial also had a larger population that was more diverse with respect to race and ethnic group and educational and economic status than previous trials of hybrid closed-loop systems.8,9
Our trial has certain limitations. First, the low frequency of baseline hypoglycemia precluded determination of whether the insulin-only bionic pancreas could reduce the risk and severity of hypoglycemia as assessed with continuous glucose monitoring, although the bionic pancreas did not increase the risk of such hypoglycemia. Second, the trial used different approaches for the management and reporting of hyperglycemia and ketosis in the two groups. Third, a single type of infusion set was used by the participants in the bionic-pancreas group, which, despite its being a commonly used set, may have contributed to the frequency of infusion-set failures. Fourth, the number of unscheduled contacts was greater in the bionic-pancreas group than the standard-care group; this situation was inherent to the trial design, in which participants in the standard-care group followed their usual care guidelines and contacted their own health care provider with questions.
In this 13-week, multicenter, randomized trial, adults and children with type 1 diabetes who had been randomly assigned to automated glycemic control with a bionic pancreas had lower glycated hemoglobin levels than participants in the standard-care group, who were using insulin therapy augmented by continuous glucose monitoring.
Supplementary Material
Acknowledgments
Supported by a grant (1UC4DK108612-01) from the National Institute of Diabetes and Digestive and Kidney Diseases, by an investigator-initiated study award from Novo Nordisk, and by Beta Bionics, which also provided the experimental bionic pancreas devices used in the trial.
APPENDIX
The authors’ full names and academic degrees are as follows: Steven J. Russell, M.D., Ph.D., Roy W. Beck, M.D., Ph.D., Edward R. Damiano, Ph.D., Firas H. El-Khatib, Ph.D., Katrina J. Ruedy, M.S.P.H., Courtney A. Balliro, R.N., C.D.C.E.S., C.R.N.-B.C., Zoey Li, M.S., Peter Calhoun, Ph.D., R. Paul Wadwa, M.D., Bruce Buckingham, M.D., Keren Zhou, M.D., Mark Daniels, M.D., Philip Raskin, M.D., Perrin C. White, M.D., Jane Lynch, M.D., Jeremy Pettus, M.D., Irl B. Hirsch, M.D., Robin Goland, M.D., John B. Buse, M.D., Ph.D., Davida Kruger, M.S.N., A.P.N.-B.C., B.C.-A.D.M., Nelly Mauras, M.D., Andrew Muir, M.D., Janet B. McGill, M.D., Fran Cogen, M.D., C.D.C.E.S., Jill Weissberg-Benchell, Ph.D., C.D.C.E.S., Jordan S. Sherwood, M.D., Luz E. Castellanos, M.D., Mallory A. Hillard, M.S.N., N.P., A.G.P.C.N.P.-B.C., Marwa Tuffaha, M.D., Melissa S. Putman, M.D., Mollie Y. Sands, M.D., Gregory Forlenza, M.D., Robert Slover, M.D., Laurel H. Messer, Ph.D., R.N., C.D.C.E.S., Erin Cobry, M.D., Viral N. Shah, M.D., Sarit Polsky, M.D., M.P.H., Rayhan Lal, M.D., Laya Ekhlaspour, M.D., Michael S. Hughes, M.D., Marina Basina, M.D., Betul Hatipoglu, M.D., Leann Olansky, M.D., Amrit Bhangoo, M.D., Nikta Forghani, M.D., Himala Kashmiri, M.D., Francoise Sutton, P.N.P., M.S.N., Abha Choudhary, M.D., Jimmy Penn, M.S.N., A.P.R.N., F.N.P.-C., C.D.C.E.S., Rabab Jafri, M.D., Maria Rayas, M.D., Elia Escaname, M.D., Catherine Kerr, M.D., Ruby Favela-Prezas, M.S.N., A.P.R.N., F.N.P.-B.C., Schafer Boeder, M.D., Subbulaxmi Trikudanathan, M.D., Kristen M. Williams, M.D., Natasha Leibel, M.D., M. Sue Kirkman, M.D., Kate Bergamo, F.N.P.-C., Klara R. Klein, M.D., Ph.D., Jean M. Dostou, M.D., Sriram Machineni, M.D., Laura A. Young, M.D., Ph.D., Jamie C. Diner, M.S.N., F.N.P.-C., R.N., C.D.E., Arti Bhan, M.D., J. Kimberly Jones, A.P.N.-B.C., B.C.-A.D.M., Matthew Benson, M.D., Keisha Bird, D.N.P., A.P.R.N., B.C.-A.D.M., Kimberly Englert, R.N., C.C.R.C., Joe Permuy, M.S.N., A.P.R.N., Kristina Cossen, M.D., Eric Felner, M.D., Maamoun Salam, M.D., Julie M. Silverstein, M.D., Samantha Adamson, M.D., Ph.D., Andrea Cedeno, M.D., Seema Meighan, C.P.N.P., and Andrew Dauber, M.D.
The authors’ affiliations are as follows: the Diabetes Research Center, Massachusetts General Hospital (S.J.R., C.A.B., J.S.S., L.E.C., M.A.H., M.T., M.S.P., M.Y.S.), and Boston University (E.R.D.), Boston, and Beta Bionics, Concord (E.R.D., F.H.E.-K.) — all in Massachusetts; the Jaeb Center for Health Research, Tampa (R.W.B., K.J.R., Z.L., P.C.), and Nemours Children’s Health Jacksonville, Jacksonville (N.M., M. Benson, K. Bird, K.E., J. Permuy) — both in Florida; the Barbara Davis Center for Diabetes, University of Colorado, Aurora (R.P.W., G.F., R.S., L.H.M., E.C., V.N.S., S.P.); Stanford University School of Medicine, Palo Alto (B.B., R.L., L.E., M.S.H., M. Basina), Children’s Hospital of Orange County, Orange (M.D., A. Bhangoo, N.F., H.K., F.S.), and the University of California, San Diego, La Jolla (J. Pettus, S.B.) — all in California; Cleveland Clinic, Cleveland (K.Z., B.H., L.O.); University of Texas Southwestern Medical Center, Dallas (P.R., P.C.W., A. Choudhary, J. Penn), and University of Texas Health Science Center, San Antonio (J.L., R.J., M.R., E.E., C.K., R.F.-P.); the University of Washington, Seattle (I.B.H., S.T.); the Naomi Berrie Diabetes Center, Columbia University, New York (R.G., K.M.W., N.L.); the University of North Carolina, Chapel Hill (J.B.B., M.S.K., K. Bergamo, K.R.K., J.M.D., S. Machineni, L.A.Y., J.C.D.); the Henry Ford Health System, Detroit (D.K., A. Bhan, J.K.J.); Emory University, Atlanta (A.M., K.C., E.F.); Washington University in St. Louis, St. Louis (J.B.M., M.S., J.M.S., S.A., A. Cedeno); Children’s National Hospital, Washington, DC (F.C., S. Meighan, A.D.); and the Pritzker Department of Psychiatry and Behavioral Health, Ann and Robert Lurie Children’s Hospital, Chicago (J.W.-B.).
Footnotes
REFERENCES
- 1.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pettus JH, Zhou FL, Shepherd L, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care 2019;42:2220–7. [DOI] [PubMed] [Google Scholar]
- 3.Forlenza GP, Lal RA. Current status and emerging options for automated insulin delivery systems. Diabetes Technol Ther 2022;24:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck RW, Russell SJ, Damiano ER, et al. A multicenter randomized trial evaluating fast-acting insulin aspart in the bionic pancreas in adults with type 1 diabetes. Diabetes Technol Ther 2022;24:681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruger D, Kass A, Pettus J, et al. A multicenter randomized trial evaluating the insulin-only configuration of the bionic pancreas in adults with type 1 diabetes. Diabetes Technol Ther 2022;24:697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messer LH, Buckingham BA, Cogen F, et al. Positive impact of the bionic pancreas on diabetes control in youth 6-17 years old with type 1 diabetes: a multicenter randomized trial. Diabetes Technol Ther 2022;24:712–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meade LT, Rushton WE. Accuracy of carbohydrate counting in adults. Clin Diabetes 2016;34:142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breton MD, Kanapka LG, Beck RW, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020;383:836–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanapka LG, Lum JW, Beck RW. Insulin pump infusion set failures associated with prolonged hyperglycemia: frequency and relationship to age and type of infusion set during 22,741 infusion set wears. Diabetes Technol Ther 2022;24:396–402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.