Abstract
Prostate cancer (PCa) is one of the most prominent causes of cancer-related mortality in the male population. A highly impactful prognostic factor for patients diagnosed with PCa is the presence or absence of bone metastases. The formation of secondary tumours at the bone is the most commonly observed site for the establishment of PCa metastases and is associated with reduced survival of patients in addition to a cohort of life-debilitating symptoms, including mobility issues and chronic pain. Despite the prevalence of this disease presentation and the high medical relevance of bone metastases, the mechanisms underlying the formation of metastases to the bone and the understanding of what drives the osteotropism exhibited by prostate tumours remain to be fully elucidated. This lack of in-depth understanding manifests in limited effective treatment options for patients with advanced metastatic PCa and culminates in the low rate of survival observed for this sub-set of patients. The present review aims to summarise the most recent promising advances in the understanding of how and why prostate tumours metastasise to the bone, with the ultimate aim of highlighting novel treatment and prognostic targets, which may provide the opportunity to improve the diagnosis and treatment of patients with PCa with bone metastases.
Keywords: prostate cancer, bone metastases, osteoclasts, osteoblasts, treatment, biomarker
1. Introduction
Prostate cancer (PCa) is one of the most significant causes of morbidity and mortality in the global male population, presenting as the second most commonly diagnosed malignancy and the fifth most predominant cause of mortality in men worldwide (1). A number of risk factors relating to PCa incidence have been identified, with the most prominent being age; 75% of all patients diagnosed with PCa are over the age of 65. Additionally to this, other risk factors which predispose an individual to developing PCa have been identified, including being of African-American heritage, living in a more economically-developed country, and certain genetic factors (1–3).
Prostate cancer diagnosis and treatment
Patients with suspected PCa are primarily diagnosed through the use of an initial digital rectal examination (DRE) and the current gold-standard prostate-specific antigen (PSA) blood test, followed by confirmation of diagnosis through magnetic resonance imaging (MRI) or transrectal ultrasound (TRUS) guided biopsy (4,5). The growth and development of PCa is largely driven through androgen receptor (AR) signalling. Therefore, following diagnosis, the most widely utilised approach to treating patients is androgen deprivation therapy (ADT). However, despite the initial disease regression commonly observed with ADT, 2–3 years after commencing treatment a significant majority of patients will go on to develop ADT-resistant tumours, which are termed castrate-resistant PCa (CRPC) (6). The treatment options available for treating CRPC patients has significantly improved in recent years, most predominantly having been revolutionised through the development of androgen receptor-axis targeted (ARAT) agents, such as abiraterone and enzalutamide (7,8). Despite these advances, CRPC remains to be a predominantly fatal disease, with the median survival for these patients being relatively dire at 9–36 months (9,10).
Prostate cancer bone metastases
Bone metastases is a commonly observed clinical occurrence for PCa patients, with secondary skeletal tumours being apparent in around 10% of patients diagnosed with primary disease and up to 80% of patients with later-stage PCa (11,12). The presence or absence of bone metastases in PCa patients has significant prognostic implications. This can be highlighted by the findings of one study in which it was reported that the 3- and 5-year survival rate for PCa patients with bone metastases was 47.70 and 32.42%, respectively, in comparison to 98.43 and 97.28% in patients without bone metastases (12). In addition to the prognostic impact of bone metastases, it is also associated with significant debilitating co-morbidities, including severe bone pain and fractures (13).
One of the most extensively described features of PCa cells which possess the capability to form secondary tumours at the bone is the ability to interact with and modulate the activity of the resident bone cells, osteoblasts and osteoclasts, in order to generate a preferential microenvironment to support tumour establishment. Osteoblasts and osteoclasts are involved in the bone remodelling process, which is a naturally occurring process in which bone tissue is progressively broken down and built back up again in order to maintain healthy skeletal structure and function, as well as playing an integral role in calcium homeostasis (14,15). However, a number of studies have documented that cancer cells which colonise the bone have the ability to interact with these resident bone cells in order to disrupt the carefully regulated balance of bone resorption in order to promote the formation of secondary tumours (16–18).
Despite PCa having a well-known predilection for forming secondary tumours at the bone, the molecular mechanisms which underpin this tropism remain to be fully elucidated. Gaining a better understanding of these mechanisms may provide the opportunity to identify more clinically relevant biomarkers to enable the earlier detection of metastatic disease to the bone, in addition to potentially providing novel therapeutic targets.
2. Bone metastases formation in prostate cancer
In order for a secondary skeletal tumour to be established, PCa cells must first undergo a step-wise process in which successive molecular expression changes must occur to support detachment from the primary tumour site, survival in the blood stream and settlement at the distal site (19). According to the ‘seed and soil’ hypothesis first stipulated by Paget in 1889, in order for a circulating tumour cell to colonise a secondary site the microenvironment of the distal loci must be hospitable to support the settlement and establishment of a secondary tumour (20). A key feature underpinning the capacity of a circulating PCa cell in establishing a secondary tumour at the site of the bone is the ability to interact with the resident osteoblasts and osteoclasts in order to generate a microenvironment that can support the growth of a tumour (18).
Prostate cancer cell interactions with osteoblasts and osteoclasts in bone metastases formation
Bone metastases can be defined as either osteoblastic, osteolytic or mixed, depending on the mechanistic involvement of osteoblasts and osteoclasts in the formation of secondary tumours (21). In the instance of PCa, the significant majority of cases present with osteoblastic metastases (22–24).
Osteoblastic (also called sclerotic) bone lesions are characterised by an imbalance in resorption activity in which osteoblasts are preferentially activated whilst the activity of osteoclasts are downregulated (25). Excessive osteoblast activity results in a net gain in bone tissue that presents in patients as irregular woven bone that is highly under-mineralised. This results in the generation of highly frail bone tissue that is liable to fracture (26). Areas of osteoblastic bone metastases formation are commonly associated with parallel areas of heightened osteoclast activity, which gives rise to both osteopenic and osteodense areas arising within the same lesion (27,28).
In comparison, osteolytic bone lesions arise as a result of hyper-activity of osteoclasts; the cells responsible for bone resorption (29). Osteolytic bone metastases are characterised by excessive bone degradation due to a heightened bone resorption activity of osteoclasts. The formation of osteolytic bone metastases has been described as a ‘vicious cycle’ in which PCa cells secrete factors which enhance the differentiation and activity of osteoclasts, and in-turn, osteoclasts secrete factors which further promote the survival and growth of localised PCa cells (18).
Despite the marked difference in the activity of osteoblasts and osteoclasts in sclerotic and osteolytic bone lesions, the subtype of bone lesion is not currently utilised to inform treatment approach in the clinic. This may be a result of the fact that the subtype of bone lesion has been shown to result in no difference in patient survival (30). However, personalised treatment approaches and patient stratification based on bone metastases presentation may provide the opportunity to improve the current survival statistics and quality of life for patients that have PCa which has metastasised to the bone.
Osteoblastic bone metastases
The vast majority of PCa patients that present with bone metastases have osteoblastic lesions. For example, one study of 55 PCa patients found that 70.9% had osteoblastic metastases, compared to 16.4% of patients having osteolytic metastases and the remaining 12.7% having mixed osteolytic and osteoblastic lesions (30). Despite the high occurrence of osteoblastic bone lesions in PCa and a number of other cancer types the mechanisms through which cancer cells may promote the formation of osteoblastic lesions are not well established. However, studies have demonstrated an irrefutable relationship between PCa cells and osteoblasts in the formation of sclerotic lesions, and some of the potential signalling pathways involved have been highlighted in the literature. For example, one study reported that culturing the CRPC cell line, C4-2B, in osteoblast conditioned media, resulted in increased PCa cell proliferation through an androgen-independent signalling mechanism (31). Furthermore, it has been demonstrated that PCa cells secrete a number of factors which promote the differentiation and activity of osteoblasts. One example of this is prostatic acid phosphatase (PAP) which has been shown to be secreted by PCa cells and plays an important role in osteoblastogenesis. The importance of PAP in sclerotic bone metastases can be highlighted by the findings of Kirschenbaum et al (32) who reported that 100% of PCa bone metastases tissue samples had high expression of PAP. Furthermore, it was found that co-culture of mouse pre-osteoblast cells with the PAP-secretory human PCa cell line, VCaP, induced osteoblast maturation. The secretion of PAP has been shown to play a role in modulating the key nuclear factor-κB (RANK)/RANK-L/osteoprotegerin (OPG) signalling axis involved in controlling osteoclast and osteoblast differentiation. For example, it has been demonstrated that PAP overexpression in a metastatic bone-derived PCa cell line results in a concurrent increase in OPG and decrease in RANK/RANK-L expression. This change in expression profile was demonstrated to result in elevated osteoblast maturation and the generation of osteoblastic lesions in an in vivo model (33).
Another factor which may be utilised by PCa cells in order to promote the formation of osteoblastic bone lesions is OPG (34). OPG is a soluble decoy receptor which acts to prevent the interaction between RANK and RANK-L, and thereby inhibits osteoclast maturation (35,36). It has been previously shown that OPG expression is significantly higher in PCa cell lines compared to normal cell lines (34,37,38). Furthermore, in one study it was reported that 73% of patient samples taken from metastatic PCa (mPCa) were positive for OPG expression, in comparison to 19% of primary carcinoma samples, with a marked increase in OPG expression being most apparent for bone lesions (34). Additionally to this, C4-2 PCa cells which were stably transfected to overexpress OPG were found to inhibit osteoclast maturation and resulted in heightened bone mineral density and bone volume in vivo (36). In addition to PAP and OPG, a number of additional PCa-derived secretory factors, such as fibroblast growth factors (FGFs), endothelin-1 (ET-1), insulin-like growth factor-1 (IGF-1) and urokinase-type plasminogen activator (uPA) have all been demonstrated to induce dysfunctional osteoblastic activity giving rise to sclerotic lesions (39–43).
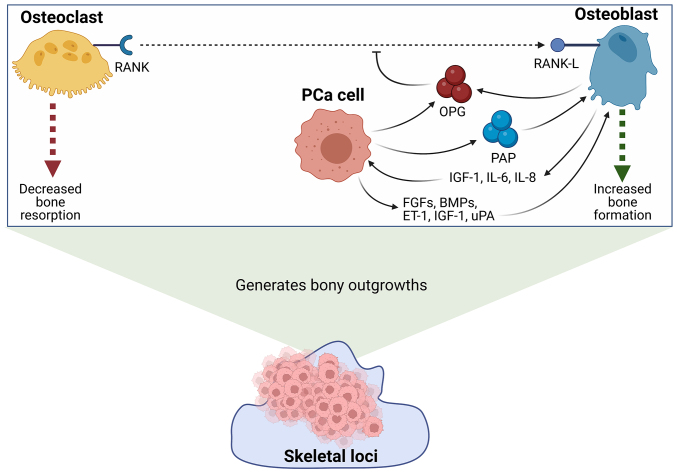
The enhancement of osteoblast differentiation and activity then in turn further promotes the proliferation of localised PCa cells through the release of growth factors, such as IGF-1, and cytokines, such as interleukin-6 and −8 (IL-6 and −8) (40,43). A summary of the mechanisms through which osteoblasts and PCa cells may interact in order to facilitate the formation of sclerotic lesions are summarised below in Fig. 1.
Figure 1.
A summary of the key molecular interactions between metastatic PCa cells and osteoblasts in the formation of osteoblastic bone metastases. PCa cells and osteoblasts secrete OPG, which acts as a soluble decoy receptor to inhibit the interaction between RANK and its ligand, RANK-L. The inhibition of this interaction prevents the maturation of osteoclasts, and promotes the activation of osteoblasts, thereby facilitating bone reformation. Furthermore, PCa cells have also been shown to secrete PAP. PAP has been demonstrated to stimulate osteoblast maturation through modulating the RANK/RANK-L/OPG axis, and promotes collagen synthesis and alkaline phosphatase production to facilitate further bone formation. In addition to this, PCa cells secrete a number of additional factors which stimulate the maturation and activity of osteoblasts, such as FGFs, BMPs, ET-1, IGF-1 and uPA. Mature osteoblasts have similarly been shown to secrete a number of factors which further support the survival and growth of localised PCa cells, such as IGF-1, IL-6 and IL-8. The net increase in osteoblast activity results in the formation of bony outgrowths which are colonised by sclerotic bone metastatic PCa cells (32–43,132). Created with BioRender.com. BMPs, bone morphogenic proteins; ET-1, endothelin-1; FGFs, fibroblast growth factors; IGF-1, insulin-like growth factor 1; IL, interleukin; OPG, osteoprotegerin; PAP, prostatic acid phosphatase; PCa, prostate cancer; RANK, receptor activator of nuclear factor κB; RANK-L, RANK-ligand; uPA, urokinase-type plasminogen activator.
Osteolytic bone metastases
Osteolytic bone metastases are not a common occurrence in PCa and when they do occur, they primarily present as a solitary metastasis (44,45). However, there are some limited documented cases of diffuse osteolytic lesions occurring in PCa patients (45–53).
The generation of osteolytic bone metastases arises as a result of specific signalling factors expressed by localised PCa cells that promote the maturation and activity of osteoclasts. For example, it has been demonstrated that PCa cells with the ability to form osteolytic bone lesions express elevated levels of parathyroid hormone-related peptide (PTHrP), which binds to receptors on osteoblasts and facilitates a downregulation in expression of OPG and an upregulation in receptor activator of RANK-L expression (18,54–56). The expression of RANK-L on the surface of osteoblasts facilitates binding to the RANK receptor which is expressed on the surface of pre-osteoclasts, resulting in enhanced osteoclast maturation and activity (57,58). In addition to these factors, PCa cells may also upregulate osteoclast activity through the release of a number of pro-inflammatory factors, such as tumour necrosis factor α (TNF-α), and cytokines, such as interleukin-1 (IL-1) and IL-6, via downstream signalling cascades (59).
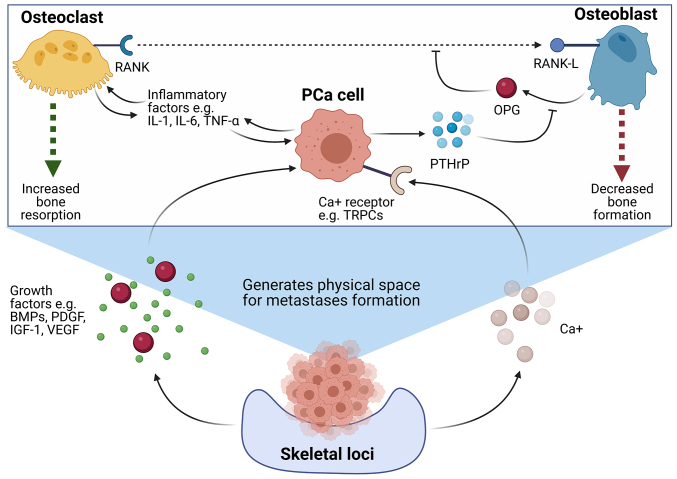
Ultimately, the excessive resorptive activity of osteoclasts and inhibition of osteoblasts results in a bone resorption imbalance that favours uncontrolled bone degradation. This generates a ‘vicious cycle’, as the degradation of bone tissue leads to the release of a number of growth factors, including IGF-1, vascular endothelial growth factor (VEGF), bone morphogenic proteins (BMPs), platelet-derived growth factor (PDGF) and excessive calcium, which then further promotes the growth and proliferation of PCa cells (18,60–62). Not only do these changes in the microenvironment help to further aid and support the growth of PCa cells, but it also leads to the formation of the physical space to enable the formation of a metastatic tumour (63). The interplay between PCa cells and osteoclasts in order to promote the establishment of secondary tumours is a highly complex and dynamic process which remains to be fully investigated. However, a summary of some of the key interactions outlined in the literature to date can be summarised in Fig. 2.
Figure 2.
Molecular interactions between osteoclasts and PCa cells leading to the formation of lytic bone metastases. PCa cells with the ability to give rise to lytic bone metastases have been shown to secrete a number of factors which promote osteoclast maturation and activity, and concurrently, downregulate osteoblast maturation and activity, in order to generate a supportive niche for the growth of a bone lytic tumour. PCa cells secrete PTHrP, which inhibits the release of osteoblast-derived OPG, an inhibitor of the interaction between RANK and its ligand, RANK-L. This facilitates the binding of RANK-L to RANK on the surface of pre-osteoclasts, which initiates an intracellular signalling cascade resulting in osteoclast maturation. In addition to this, it has been demonstrated that PCa cells secrete a number of inflammatory factors, including IL-1, IL-6 and TNFα, which act to further stimulate osteoclast maturation and activity. Mature osteoclasts have been demonstrated to further promote the growth of PCa cells through the secretion of a similar subset of pro-inflammatory factors. The heightened levels of bone resorption results in a net loss in bone tissue, and generates a ‘vicious cycle’ in which the degradation of bone leads to the release of a number of growth factors, including BMPs, PDGFs, IGF-1 and VEGF, which act to further promote the survival and development of localised PCa cells. Furthermore, the excessive breakdown of bone leads to elevated levels of calcium in the tumour microenvironment, which has been demonstrated to interact with calcium ion receptors expressed on the surface of PCa cells, in order to further facilitate cancer cell survival and growth (18,54–63,180). Created with BioRender.com. BMPs, bone morphogenic proteins; IGF-1, insulin-like growth factor 1; IL, interleukin; OPG, osteoprotegerin; PCa, prostate cancer; PDGFs, platelet-derived growth factors; PTHrP, parathyroid hormone-related peptide; RANK, receptor activator of nuclear factor κB; RANK-L, RANK-ligand; TRPC, transient receptor potential canonical; VEGF, vascular endothelial growth factor.
Molecular changes involved in bone metastases formation
In addition to interacting with the resident bone cells in order to promote secondary bone metastases formation, PCa cells with the ability to metastasise must undergo sequential changes to protein expression which will facilitate dissociation from the primary tumour site, survival in the circulatory system, extravasation at a distal site, and establishment of a secondary tumour. Due to the highly complex, multi-step nature of this process vast numbers of molecular changes must occur to enable a metastatic event to take place. Although the principle molecular changes which underpin the metastatic process are already well-documented in literature, novel aberrations to both protein expression and post-translational modifications specific to bone cancer metastases are becoming apparent in the literature (19).
Role of integrins and cadherins in prostate cancer bone metastases
One example of an important molecular change which underpins the ability of a circulating cancer cell to establish secondary tumours at the site of the bone is alternative integrin expression. Integrins are a family of transmembrane receptors that mediate cellular adhesion to the extracellular matrix (ECM) and to other neighbouring cells (64,65). A number of changes to integrin expression have been reported to play a role in cancer metastases, including integrin αvβ3. Abnormal expression of αvβ3 plays a role in facilitating bone metastases in PCa through a multitude of mechanisms. For example, it has been shown that αvβ3 on PCa cells may enable tumour cell adhesion to the localised bone ECM through interacting with the RDG motif on fibronectin. This facilitates a downstream signalling cascade that ultimately results in cytoskeletal protein rearrangement, leading to protein fibre contraction and enabling invasion of the PCa cell into the bone ECM (66,67). In addition to the direct interactions of αvβ3 in facilitating ECM invasion, it has also been shown that αvβ3 modulates the activity of matrix metalloproteinases via aberrations to the phosphoinositide 3-kinase (PI3K) signalling pathway in order to promote the degradation of localised ECM (68,69). Similarly, integrin αvβ3 interactions promotes vascularisation via PI3K/Akt pathway activation leading to VEGF expression, further supporting the establishment of a secondary tumour (70–72). Furthermore, αvβ3 has been shown to recruit and activate additional downstream proteins which help to prevent immune-recognition, such as TGFβ, which further promotes tumour cell survival in the circulation (73).
The therapeutic potential of targeting integrins to prevent bone metastases in PCa patients has been investigated. An example of this is in one phase II study which investigated the use of a pan-αv integrin inhibitor, abituzumab, in a cohort of patients with asymptomatic or mildly symptomatic mCRPC. Despite finding no significant difference in progression-free survival (PFS) between the abituzumab-treatment group and the placebo group, significantly fewer patients in the abituzumab-treatment group experienced bone lesion progression relative to the patients on placebo (74,75).
In addition to integrins, a number of other protein expression changes have been shown to play a role in modulating the ability of PCa cells to colonise the bone. For example, alterations to the expression profile of cadherins are a well-characterised aberration which play a key role in cancer metastases through promoting epithelial-to-mesenchymal transition (EMT). EMT is generally characterised by a loss of E-cadherin expression and an increase in N-cadherin expression, which promotes cellular dissociation from the primary site and enables migration and invasion of cancer cells at distal sites (65,76). For example, in one study looking at paired prostate bone metastases and primary tumour samples, it was found that both E-cadherin and β-catenin were uniformly downregulated in expression in all of the metastatic tissues assessed compared to the matched primary samples (77). Furthermore, a number of studies have reported an upregulation of N-cadherin in patients and in vivo studies in the instance of metastases (78–80). The clinical utility of targeting N-cadherin using antibodies have been shown to block metastases and result in disease regression in in vivo studies (81).
Role of glycosylation in bone metastases formation
In addition to the interactions between PCa cells and bone cells in the establishment of secondary bone tumours, a number of other biological processes have been implicated as playing a key role in bone metastases formation. One example of this is glycosylation. Glycosylation is a ubiquitous feature of cellular proteins in which sugar groups are enzymatically added post-translationally to proteins and lipids in order to generate glycan chains (82). Alterations to glycosylation in cancer has been identified as a potential driver of metastasis, and moreover, may play a role in premeditating the site-preference exhibited by cancer cells when forming secondary metastatic tumours (83,84). Although not widely investigated, a cohort of studies have published findings indicating that changes to glycans and their associated glycosylation enzymes may play a role in predisposing PCa cells to establishing secondary tumours at the bone.
One example of how abnormal glycosylation may impact metastasis and osteotropism in PCa is the glycosphingolipid, GM1. The bone-mPCa cell line, C4-2B, has been shown to express an abnormally glycosylated form of GM1, which has the capacity to interact the integrin, α2β1, to facilitate invasive cell behaviour. The role of the α2β1 integrin in bone metastasis has been previously outlined in literature, with PCa patients that have bone metastases being found to have significantly greater expression of α2β1 relative to patients with soft tissue metastases (85). In addition to this, elevated levels of α2,3-sialylation and expression of the responsible sialyltransferase enzyme, ST3GAL3, were found to be more highly expressed in the bone-invasive PCa cell lines relative to non-invasive counterparts (86). This may provide a potential insight into one of the mechanisms through which PCa exhibits a preference for settlement at the bone, through altering the cellular glycosylation profile to promote attachment and invasiveness at secondary skeletal sites.
Furthermore, one of the most extensively documented interactions involved in bone metastasis is the interaction between E-selectin and its preferential ligand, sialyl-LewisX (sLeX) (87). It has been previously demonstrated that extravasation of PCa cells at the site of the bone is mediated by interactions between E-selectin ligands, such as sLeX, and E-selectin, which is expressed on endothelial bone cells. Moreover, it has been demonstrated that PCa cell lines with the capacity to invade bone tissue express the fucosyltransferase enzymes, 3 and 6 (FUT3 and FUT6), at a 31- and 10-fold higher expression, respectively, in comparison to normal, non-invasive prostate tissue. These FUT3 and FUT6 enzymes play an integral role in the catalytic creation of sLeX ligand (88).
Another example which highlights a potential role for aberrant glycosylation in PCa metastasis is cleaved galectin-3 (Gal-3). Cleaved Gal-3 expression is positively associated with metastasis in PCa and is closely related to PSA levels (89). Furthermore, secreted Gal-3 has been found to be elevated specifically in PCa patients with bone metastases. The secreted, cleaved version of Gal-3 interacts with myosin-2A which attenuates osteoclast differentiation, and thereby generates a bone niche which is supportive of sclerotic bone metastases formation (90).
Although glycosylation appears to play a role in determining the ability of circulating PCa cells to colonise secondary skeletal sites, the metastatic process is highly complex and involves a multitude of interactions and alterations to cellular expression profiles to facilitate each stage of metastasis (91). Extensive and dynamic sequential changes to the expression of a great number of proteins, including cadherins, integrins and GTP-binding proteins underpins the ability of PCa to establish a secondary tumour at the bone. However, as alterations to glycosylation poses as a plausible contributing factor to the ability of PCa to metastasise to the bone it may provide a potentially efficacious therapeutic target for the discovery of novel treatments and biomarkers for patient disease identification and treatment stratification.
In vitro models of prostate cancer bone metastasis
Due to the clinical relevance of bone metastases in cancer, the ability to study this process in vitro is of high importance. However, due to the extensive complexity of the molecular pathways involved in the metastatic cascade, in addition to difficulties in recapitulating patient physiology, models are often limited and lack translational efficacy (92).
However, significant recent advances have led the development of promising models for studying metastases both in vitro and in vivo. For example, improved knowledge of the molecular pathways which underpin a metastatic event have led to studies which have demonstrated that knocking down CD44 and CD147 in PCa cells results in reduced invasiveness. Furthermore, xenografts of these knock-down cell lines in in vivo models have demonstrated reduced tumour growth and metastatic capacity (93). This knowledge can then be applied to generate models in which key drivers of metastases, such as CD44 and CD147, can be modulated to somewhat recapitulate the molecular profile of metastatic tumour cells (94,95).
Despite advances in the understanding of the molecular pathways which underpin metastasis, human cell line-based models remain limited in their translational efficacy. This is due to a lack of clinical relevance in recapitulating the complex interplay between the multiple cell types that are involved in tumour formation. For example, increasing evidence has indicated that the stromal microenvironment plays an integral role in the metastatic cascade (96,97). Therefore, 2-dimensional (2D) models which aim to allow the investigation of tumour cell interactions with the surrounding ECM have been generated, including scratch or wound healing assays and transwell cell migration assays (92). Although these assays provide improved translational efficacy in comparison to human cell line-based models, they fail to fully recapitulate the architectural and cellular complexity of metastatic tumour formation in vivo. Therefore, an alternative approach to modelling metastasis experimentally is through the use of 3-D models. One example of a 3-D model of metastasis in vitro is the use of perfusion bioreactors which aim to experimentally recreate the mechanical tension on the ECM caused by constant tissue perfusion. Studies have indicated that the inclusion of perfusion bioreactors in modelling metastasis enables a better representation of the cellular functionality of human mesenchymal stem cells (hMSCs) and initiates the expression of EMT markers in bone-metastasised PCa models (98). In addition to this, the capacity to model metastasis in vitro has further been improved through the ability to grow 3-D models of prostate tumours. For example, establishing human prostate organoids from induced pluripotent stem cells (iPSCs) and the growth of patient-derived xenograft models. These models then have the capacity to be co-cultured alongside other cell types commonly observed in the tumour microenvironment to better model tumour-stromal interactions (99,100).
Furthermore, the translational capacity of these models has been significantly improved due to recent advances in the development of novel nanocomposite materials and scaffolds. These materials can provide biophysical and biochemical signals to cells to promote tissue formation, whilst also enabling nutrient supply and waste removal to occur due the porous nature of the structures. Therefore, these materials enable better recreation of the metastatic microenvironment in which a secondary tumour forms in vivo (101,102). These novel biomaterials can be utilised in models which allow the study of tumour cells which metastasise to the bone. For example, the use of calcium-phosphate-based biomaterials, such as hydroxyapatite (HAP), have been used to model bone metastases due to their similarity with the mineral composition observed in human bone. These HAP nanoparticles in combination with collagen have been shown to support osteoblast colonisation and promote bone-ingrowths. Additionally to this, it has been shown that PCa cell lines undergo morphogenic changes and have the ability to colonise these scaffolds (102,103).
These recent developments in the generation of novel, efficacious models of metastasis will enable more in-depth study of the molecular mechanisms which underpin the formation of secondary tumours, and hopefully inform the development of future targets for treating and preventing bone metastasis occurrence.
3. Treatments for patients with prostate cancer with bone metastases
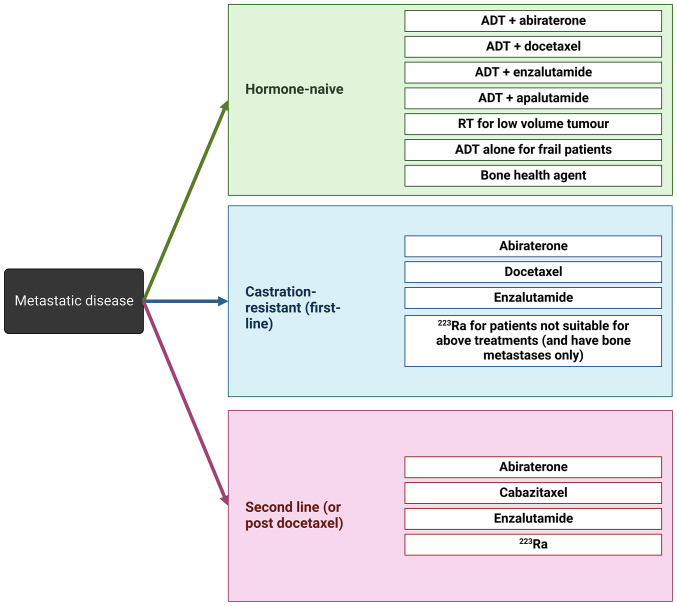
The treatment options for patients diagnosed with metastatic disease has improved significantly over recent years, however the survival statistics for this subset of patients still remains relatively poor, with the medium overall survival for patients with mPCa being around 21 months (10). For patients diagnosed with mPCa, the treatment options available are dependent on the hormone-status of the disease and prior treatment regimes. Patients diagnosed with hormone-naïve metastatic disease may be treated with ADT in combination with abiraterone, docetaxel, enzalutamide or apalutamide, or for patients that are deemed to not have the capacity to tolerate combination therapy may receive ADT alone. Patients who have a low tumour volume may alternatively be treated with radiotherapy. In addition to this, patients may be prescribed bone health agents, such as zoledronic acid, which are utilised to help manage bone pain, initiate disease regression and prevent further skeletal related events (SREs) (104,105). For patients diagnosed with castrate-resistant metastatic disease, the first-line treatment approaches are abiraterone, docetaxel or enzalutamide monotherapeutically. For those patients who are found to be not suitable for these treatment approaches and have the presence of bone metastases, patients may be prescribed the bone-targeting agent, radium 223 dichloride (radium-223). Patients needing treatment as a second-line approach or following disease progression that is refractory to docetaxel are treated with abiraterone, cabazitaxel, enzalutamide, or radium-223 (104). The currently utilised approach to clinical management of PCa patients with metastatic disease is summarised in Fig. 3.
Figure 3.
Clinical management options for patients diagnosed with metastatic prostate cancer, depending on the clinical presentation of disease being hormone-naïve or castration-resistant (first-line), or as a second line (/post-docetaxel treatment) therapeutic intervention according to European Society for Medical Oncology clinical practice guidelines (104). Created with BioRender.com. ADT, androgen deprivation therapy; RT, radiotherapy.
Current treatments for prostate cancer bone metastases
The high prevalence of PCa bone metastases and resultant life-limiting nature of the associated symptoms necessitates the ability to successfully prevent and treat bone metastases formation and progression. A number of treatment approaches with the aim of limiting the progression of bone metastases and managing the associated symptoms have been developed, including bisphosphonates and other bone-targeting therapies, corticosteroids, radiation therapies and surgical intervention.
Despite the fact that the vast majority of metastatic events in PCa are osteoblastic, osteoclast-inhibiting therapeutics have, until recently, been the only treatment approaches approved for managing PCa bone metastases. Osteolytic-based interventions are thought to demonstrate a degree of success as previous studies have indicated that a significant proportion of patients with bone metastases have mixed osteolytic and osteoblastic lesions. Furthermore, it is a widely accepted dogma that simultaneous activation of osteoclasts may be concurrent with osteoblastic lesions. This is thought to be due to the natural feedback system designed to maintain the homeostasis of bone resorption, in which activated osteoblasts may promote the maturation of pre-osteoclasts in order to achieve an equilibrated rate of bone turnover (27,28).
Bisphosphonates
Bisphosphonates act to inhibit osteoclast maturation and ultimately result in osteoclast apoptosis (106,107). Bisphosphonates were originally developed with the view to treat osteolytic bone lesions. However, due to the concurrent upregulation of osteolytic activity in areas of sclerotic metastases, these drugs have been applied to the clinical management of mPCa disease, with variable success (108–110). For example, pamidronate disodium has been investigated for its utility in treating skeletal metastases progression and the associated bone pain experienced by patients. However, results have been largely inconclusive and show no notable significant improvement to disease or symptom management (111,112). This may be a result of pamidronate disodium not being as potent as some of the more recently developed bisphosphate treatments available in the clinic, such as zoledronic acid (113). Zoledronic acid is the most widely utilised bisphosphonate for the management of bone metastasis in PCa patients due to its reported ability to delay the time to SRE and a reduction in bone pain experienced by patients (114). Despite reported efficacy for disease management with zoledronic acid, results are variable and some studies report inconclusive findings. For example, in one study assessing the clinical utility of zoledronic acid in 645 patients with early stage PCa, it was found there was no significant improvement to the time to first SRE (31.9 months with zoledronic acid vs. 29.8 months with placebo) or overall survival (111). However, a number of studies have reported findings in support of a clinical benefit for the use of zoledronic in treating mPCa disease. For example, Kamba et al (115) reported finding a significant improvement to the time to the first SRE of 18.8 months in patients treated with zoledronic acid and ADT compared to patients that received ADT alone. As a result of the findings of this study and others, NICE guidelines currently recommend the use of bisphosphonates for pain management in PCa patients with bone metastases, and specifically zoledronic acid for preventing or reducing the occurrence of SREs (116,117).
A number of studies have reported the results of treating bone mPCa patients with bisphosphonates, as summarised below Table I.
Table I.
A summary of the key clinical trials investigating the clinical benefit and improvement in quality of life in patients diagnosed with prostate cancer following treatment with the different classes of bisphosphonates (181).
| First author/s, year | Bisphosphonate | Pain experienced | Skeletal related events | Overall survival | (Refs.) |
|---|---|---|---|---|---|
| Elomaa et al, 1992 | Clodronate | Benefit | N/A | No benefit | (182) |
| Vorreuther et al, 1992 | Benefit | No benefit | N/A | (183) | |
| Adami et al, 1989 | Benefit | N/A | N/A | (184) | |
| Strang et al, 1997 | No benefit | N/A | N/A | (185) | |
| Kylmälä et al, 1997 | Benefit | N/A | N/A | (186) | |
| Ernst et al, 2003 | No benefit | N/A | No benefit | (187) | |
| Mason et al, 2007 | N/A | No benefit | No benefit | (188) | |
| Dearnaley et al, 2003 | N/A | N/A | Benefit | (189,190) | |
| Small et al, 2003 | Pamidronate | No benefit | No benefit | N/A | (111) |
| Lipton et al, 1994 | Benefit | N/A | N/A | (191) | |
| Figg et al, 2005 | Alendronate | N/A | N/A | No benefit | (192) |
| Sweeney et al, 2010 | Risedronate | N/A | No benefit | N/A | (193) |
| Meulenbeld et al, 2012 | No benefit | N/A | No benefit | (194) | |
| Hahn et al, 2014 | N/A | Benefit | N/A | (195) | |
| Hoskin et al, 2015 | Ibandronate | No benefit | N/A | No benefit | (196) |
| Saad et al, 2002 | Zoledronate | Benefit | Benefit | No benefit | (197) |
| Abetz et al, 2006 | Benefit | N/A | N/A | (198) | |
| Weinfurt et al, 2006 | Benefit | N/A | No benefit | (116) | |
| Leto et al, 2006 | Benefit | N/A | N/A | (199) | |
| Cózar Olmo et al, 2008 | Benefit | Benefit | N/A | (200) | |
| Saad et al, 2004 | N/A | Benefit | N/A | (201) | |
| Fulfaro et al, 2005 | Benefit | N/A | N/A | (202) | |
| Saad et al, 2005; | Benefit | Benefit | N/A | (203–205) | |
| Saad et al, 2007; | |||||
| Saad et al, 2010 | |||||
| Paparella et al, 2011 | Benefit | N/A | N/A | (206) | |
| Uemura et al, 2013 | N/A | N/A | Benefit | (207) | |
| Wang et al, 2013 | Benefit | Benefit | No benefit | (208) | |
| Ueno et al, 2013 | Benefit | Benefit | Benefit | (209) | |
| Chiang et al, 2013 | Benefit | Benefit | N/A | (210) | |
| Pan et al, 2014 | N/A | N/A | Benefit | (211) | |
| Smith et al, 2014 | No benefit | No benefit | No benefit | (212) | |
| Wirth et al, 2015 | N/A | No benefit | N/A | (213) | |
| James et al, 2016 | No benefit | Benefit | No benefit | (214) | |
| Kamba et al, 2017 | N/A | Benefit | No benefit | (115) | |
| Denham et al, 2012; | N/A | N/A | No benefit | (215–217) | |
| Denham et al, 2014; | |||||
| Denham et al, 2019 |
N/A, not assessed.
Denosumab
As an alternative to bisphosphonates, a human monoclonal antibody which targets RANK-L, called denosumab, has been developed. In one phase 3 study, 1,904 patients with CRPC with no previous exposure to bisphosphonates were allocated to either denosumab, or the current gold-standard therapy, zoledronic acid. It was found that there was a significant improvement to the median duration to the first detected SRE in the denosumab group (20.7 months), compared to zoledronic acid (17.1 months). Both drugs were found to be similarly well-tolerated between the treatment groups, however a greater occurrence of adverse events such as hypocalcaemia (13% vs. 6%) and osteonecrosis of the jaw (2% vs. 1%) was observed with the denosumab treatment group (118). Despite the relatively improved efficacy of treating mPCa patients with denosumab relative to bisphosphonate intervention, the cost associated with denosumab treatment is considerably higher. For example, one cost-benefit study carried out indicated that 1 year treatment of a PCa patient with denosumab would cost an estimated $35,341 in comparison to $27,528 for zoledronic acid treatment, and this increase in cost could not be justified by the predicted improvement to the occurrence of SREs (119).
Despite denosumab being approved for the treatment of other solid tumours which have metastasised to the bone, such as breast cancers, denosumab is not currently recommended for PCa bone metastases treatment or management according to NICE guidelines (120).
Radiopharmaceuticals
An alternative, more recently introduced approach to managing bone metastases in patients with PCa is the use of radiopharmaceuticals. A number of radiopharmaceutical agents have received approval by the FDA to manage skeletal secondary tumours in patients with advanced PCa [Phosphorus-32 (32P), Strontium-89 (89Sr), Samarium-153 (153Sm) in combination with ethylenediaminetetramethylenephosphonic acid (Sm-EDTMP or 153Sm lexidronam)]. Despite improvements to patient-reported pain, these interventions have been shown to provide no survival benefit (121–126). However, a promising alternative is radium-223. Radium-223 is an FDA-approved alpha-emitting radionucleotide that is selectively taken up in areas of high bone turnover (due it being a calcium-mimetic) and subsequently initiates apoptosis in resident PCa cells, thereby facilitating tumour regression (127). Despite other bone-specific radiopharmaceuticals not having demonstrated a significant impact on patient survival to date, a phase 3 trial reported finding treatment with radium-223 achieved a significant improvement to patient quality of life, and an improvement to overall survival of 3.6 months (128,129). As a result of this trial, radium-223 is now approved for the treatment of patients with mCRPC with symptomatic bone metastases and no known visceral metastases (104,127).
Despite the recent improvements to the currently available therapeutic interventions for managing bone metastases in patients with PCa, there remains only a marginal improvement to patient survival and pain management. The current limitations to the efficacy of therapeutic interventions necessitates the development of novel treatment targets for the clinical management of patients with mPCa to the bone.
Future potential treatment targets for prostate cancer bone metastases
The mainstay of intervention for PCa patients with bone metastases is currently predominantly limited to osteoclast-targeting therapies despite the significant majority of PCa patients having sclerotic (osteoblastic) bone lesions (30). Therefore, alternative therapeutic targets based on inhibiting the aberrant activity of osteoblasts may be a more efficacious approach to treating PCa bone metastases. One target that may have future clinical utility for preventing osteoblast-mediated metastatic tumour formation is PAP. PAP is a secretory glycoprotein produced by PCa cells that was the first described human tumour marker (130). Circulatory levels of PAP have been found to be higher in PCa patients with more advanced disease, particularly in those with the presence of bone metastases (131,132). PAP has been shown to promote osteoblast maturation and activity through modulating the RANK/RANK-L/OPG system to promote OPG levels, leading to a concurrent inhibition of osteoclasts and promotion of osteoblast activity (32,33). The clinical efficacy of inhibiting PAP for the treatment of PCa patients has already been demonstrated through the development of the first FDA-approved anti-tumour vaccine, sipuleucel-T, which targets PAP for generating a targeted host immune response (133,134). Sipuleucel-T had been shown to reduce the risk of death by 33% in patients with advanced PCa in two phase III randomised, double-blind, placebo-controlled trials, leading to its FDA approval (135). Furthermore, early phase trials are currently being conducted to investigate the potential clinical utility of combining sipuleucel-T treatment with radium-223 and have shown improvements to overall survival in patients with mCRPC (136). However, no treatments directly targeting PAP enzymatic activity for the treatment of sclerotic bone metastasis in PCa are currently in clinical trials.
Another alternative proposed target for the development of therapies for treating PCa bone metastases is ET-1. ET-1 is secreted by PCa cells and promotes osteoblastogenesis (41,137,138). This knowledge has led to the development of an inhibitor targeting the ET-1 receptor, ETA, called atrasentan (ABT627). Despite pre-clinical indications yielding positive results, the reported findings of clinical trials to date have been variable and inconclusive for treatment efficacy in PCa patients with skeletal secondary tumours (139–141). Atrasentan has also been investigated for clinical efficacy in combination with docetaxel for the treatment of patients with mCRPC in a phase III trial, however the trial was terminated prematurely due to no indication of patient benefit (142). The potential to develop novel, more potent inhibitors of ET-1 may have the capacity to provide clinical benefit for PCa patients with sclerotic bone metastases.
A number of alternative therapeutic approaches to treating patients with mPCa are currently being investigated, however, further future elucidation of the molecular interplay between metastasising PCa cells and resident bone cells will hopefully enable the identification of novel, more efficacious therapeutic targets for the clinical management of PCa bone metastasis (125).
4. Biomarkers for the diagnosis and treatment stratification of patients with prostate cancer with bone metastases
The presence of bone metastases for PCa patients has significant prognostic implications, and the treatments available to manage patients at this stage of disease remain limited and have little effect on prolonging patient survival (12,143). Current diagnostic approaches utilised for patients which present with suspected bone metastases in the clinic encompass a blood test for a variety of factors (serum calcium, phosphorous, 25-hydroxyvitamin D, alkaline phosphatase, creatine, parathyroid hormone, thyroid-stimulating hormone and specific globulins detected by serum protein electrophoresis) in combination with imaging approaches (144). Although this method of diagnosis has a relatively high success rate for identifying bone metastases in patients, secondary tumours are already well-established at the point of being detectable through imaging and blood-based assessments. Therefore, it would be beneficial to have the capacity to identify patients with a high risk of developing secondary bone metastases prior to the metastatic event taking place, or in lieu of this, at the earliest stage of development possible. This would enable better stratification of the clinical management and treatment of PCa patients. Currently, there is no singular reliable biomarker widely utilised in the clinic to identify or stratify PCa patients in regards to the presence or absence of bone metastases, however a number of approaches have been suggested in the literature to date.
Biomarkers of bone remodelling
Identifying specific biomarkers with the ability to distinguish between osteolytic and osteoblastic metastatic lesions would be desirable to achieve better patient stratification and enable personalised treatment approaches. However, areas of osteolytic bone metastases are commonly associated with a concurrent, parallel upregulation of osteoblastic activity, which is thought to be as a result of a localised bone-formation response in an attempt to repair an osteolytic lesion (145). This phenomenon gives rise to a difficulty in establishing biomarkers that are specific to the individual sub-class of bone cell involvement in metastases formation.
One of the most widely utilised biomarkers in identifying bone metastases currently in the clinic is serum alkaline phosphatase. Alkaline phosphatase is a marker of osteoblast differentiation and activity, and studies have indicated a link between the progression of bone metastases and serum phosphatase levels, although the clinical utility of serum phosphatase as a biomarker of PCa disease progression remains inconclusive (21,146). Despite this, alkaline phosphatase is currently implicated as part of a panel of factors assessed to monitor disease progression and treatment response in PCa (144). Previous studies have indicated that serum alkaline phosphatase may prove to be an efficacious marker for the identification of patients with bone metastases. For example, Karhade et al (147) found that following investigation of serum alkaline phosphatase levels in 732 patients with metastatic disease that levels were significantly higher in patients with both bone and visceral metastases. Furthermore, it was found that elevated serum alkaline phosphatase levels acted as a prognostic factor for the survival of patients with bone metastases (147). However, it was reported that elevated serum alkaline phosphatase was associated with both skeletal and non-skeletal metastases, and therefore this marker may not be exclusive to the presence of bone metastases.
Another potential marker of the presence of bone metastases is serum levels of calcium. Hypercalcaemia has been demonstrated to be associated with a higher incidence of osteolytic bone lesions, greater bone pain and a significantly poorer prognosis in PCa patients (148). In addition to this, elevated calcium levels may act as a predictor for the risk of patients developing fatal PCa. One study of 2,814 men found that those with the highest serum calcium levels were three times more likely to die from PCa in later life (149). In addition to the potential prognostic use of calcium for the identification of lytic bone lesions, it may similarly be utilised in identifying sclerotic bone lesions. Heightened osteoblast activity and subsequent bone formation could result in a reduction in circulatory calcium levels as calcium is utilised in the bone formation process. One study investigating the clinical utility of urine-based calcium for predicting the success of treating osteoblastic bone lesions found that PCa patients with sclerotic metastasis that demonstrated regression after treatment had increased urine calcium levels following chemotherapy, and a concurrent decrease to urine calcium levels in those patients which had disease progression in spite of chemotherapy (150). This indicates that monitoring serum calcium levels may enable the identification of sclerotic bone lesions in PCa patients, in addition to having the potential utility to monitor treatment success following chemotherapeutic intervention.
Relying on the discovery of a singular biomarker for accurate identification of bone metastases in PCa patients may be limiting due to the highly dynamic nature of the bone resorption process, individual patient variability and the impact of biological variance on the expression of these factors. Therefore, a more efficacious approach may be to identify a panel of biomarkers of bone turnover to identify aberrations to this process in metastases formation. In one study, the diagnostic accuracy of a panel of serum biomarkers for bone formation and bone osteoclastogenesis markers were assessed in 117 PCa patients in comparison to 35 patients with benign prostatic hyperplasia (BPH) and a further 35 normal samples. It was found that the bone formation markers, total and bone-specific alkaline phosphatase (tALP and bALP) and amino terminal procollagen pro-peptides of type 1 collagen (P1NP), and the bone resorption markers, bone sialoprotein (BSP), tartrate-resistant acid phosphatase (TRAP), cross-linked N-terminal (NTX) telopeptide of type 1 collagen and OPG, were all found at significantly greater levels in the serum of patients with metastases compared to those without metastases. Furthermore, it was found that elevated levels of these factors was associated with significantly reduced survival compared to patients with lower detected serum levels (151). Despite the efficacy of these biomarkers collaboratively in identifying the presence of bone metastases, the serum levels of these factors individually do not reliably distinguish between lytic and sclerotic bone metastases.
Attempts to identify efficacious biomarkers that are specific to the predominant bone cell activity in metastases formation have been carried out. For example, markers of osteoblast activity, such as osteocalcin, hydroxyproline, pyridinolene and markers of collagen degradation have all been investigated for their efficacy as biomarkers for sclerotic bone metastases formation, with variable success (152–155).
Despite some biomarkers, such as serum alkaline phosphatase and calcium, already being utilised as part of a panel of factors in some clinics to identify and diagnose bone metastases, these factors remain to have limited specificity and sensitivity in their diagnostic capacity. Therefore, future identification of novel biomarkers specific for osteolytic or sclerotic bone tumours may provide earlier and more efficacious diagnostic potential for the identification and treatment of patients with bone metastases.
Circulating tumour cells as blood-based biomarkers
Circulating tumour cells (CTCs) are cancer cells that have originated from a primary or metastatic tumour that can be isolated from patient blood. CTCs have been investigated in a number of cancer subtypes for their prognostic efficacy with variable success reported to date (156). It has been demonstrated that the use of CTCs in order to identify PCa patients with metastatic disease may have some clinical utility. For example, in one study in which 76 mCRPC patient blood samples were compared to 180 patient samples with localised disease and 19 healthy samples, the expression levels of KLK3, KLK2 and PSCA in isolated CTCs was assessed to determine variability in expression between metastatic and non-metastatic disease. It was reported that patients with metastatic disease were found to be significantly more likely to test positive for KLK2 and KLK3 expression in comparison to patients with localised disease (49% vs. 8%), and normal samples were negative for the expression of both genes. Furthermore, the expression of KLK2 and KLK3 was found to be correlated with survival and acted as a better prognostic indicator in this cohort in comparison to serum PSA (157). Furthermore, circulating tumour cells have been shown to recapitulate the expression profile of skeletal metastasis, which could be utilised to identify the presence of secondary bone metastases and also inform the implementation of individualised treatment approaches for patients with skeletal disease (158).
Micro RNAs as biomarkers of bone metastases
Another potential avenue for the discovery of an efficacious biomarker for the diagnosis of bone metastatic disease in PCa patients is the use of microRNAs (miRNAs). miRNAs are short, non-coding RNAs which are typically 19–26 nucleotides in length. They are thought to function by suppressing gene expression post-transcriptionally by binding to complementary messenger RNA (159). miRNAs can be found free in the serum or contained in extracellular vesicles in the serum, urine and semen. Once isolated, they can then be reliably profiled through easily accessible techniques such as quantitative polymerase chain reaction (qPCR) (160–163). A number of miRNAs have been identified as potentially playing a role in PCa development and progression (160,162,164–166). One miRNA which has gained particular interest in the respect of being a potential biomarker for the identification of bone metastases in PCa patients is miR-141. For example, in one study comparing the serum miR-141 levels in 6 patients with BPH, 20 with localised PCa and 30 with bone-mPCa it was found that miR-141 levels were significantly higher in those patients with bone metastases and were also correlated with the number of bone metastatic lesions. Interestingly, no correlation was found between serum miR-141 and PSA (the currently utilised gold-standard biomarker for the diagnosis of PCa), indicating an additive diagnostic potential to what is currently available in the clinic (167).
Another potential miRNA that could be used to identify bone metastases in PCa patients is miR-205. miR-205 is predominantly expressed by basal cells in the prostate tissue and has been shown to inhibit tumour proliferation, apoptosis and cancer cell aggressiveness (165,168). Despite miR-205 expression levels being lower in the serum of individuals with PCa relative to healthy controls, it has been shown that miR-205 expression is significantly elevated in patients with bone metastases compared to PCa patients without bone metastases (164). Furthermore, a number of additional miRNAs have been identified as showing promising indications for the ability to distinguish patients with bone metastases, including miR-96, miR-135b, miR-15, miR-16, miR-21 and miR505-3p (169–173).
In addition to the potential application of miRNAs as clinical biomarkers, they have also been investigated for their utility as potential therapeutic interventions (174,175).
Despite promising emerging evidence supporting the potential of using miRNAs as biomarkers in PCa, inconsistencies in the findings of different studies are a common occurrence making it difficult to draw definitive conclusions on the efficacy of individual miRNA-based biomarkers. This may largely be a result of variability in experimental design in the way that miRNAs are isolated from PCa patients and subsequently processed. To circumvent these issues, specific guidelines outlining recommendations for sample acquisition, handling and analytical processing have been suggested in the literature in order to improve reliability and reproducibility in this field of research (176). An example of one method which may enable more accurate quantification of miRNAs in patient samples is the use of spike-ins in order to allow for sample normalisation (176,177). Another approach to improving the certainty in identifying which miRNAs may act as the most efficacious biomarkers for diagnosing and monitoring PCa is through the use of meta-analysis approaches (178,179). For example, Pashaei et al (179) carried out a meta-analysis on six miRNA datasets looking at PCa recurrence following radical prostatectomy and identified a common upregulation in 15 miRNAs, and a common downregulation in 22 miRNA genes across the datasets analysed.
As a result of the seemingly high efficacy in identifying bone metastases, coupled with being easily and non-invasively isolated from patients and quantitatively assessed through widely accessible laboratory techniques, miRNAs may pose an attractive alternative diagnostic tool for the identification of bone-metastatic disease in PCa patients in the future.
5. Conclusions
Despite recent advances to the treatment options available for PCa patients with bone metastases, significant limitations still remain which is reflected in the insubstantial options for patient symptom management and poor overall survival for this subset of patients. Improvements to our knowledge of the molecular signalling changes which occur during the formation of bone metastases, and the molecular interplay between metastasising PCa cells and localised osteoclasts and osteoblasts, will in the future potentially enable the development of improved novel targeted therapeutics and biomarkers for disease diagnosis and management. An example of how this knowledge can be utilised to improve the morbidity and mortality of patients with mPCa is the implementation of Radium-223 in the clinical pathway, manifesting in significant improvements to both patient survival and pain experienced as a result of bone metastases. An alternative future directive for diagnosing and treating PCa patients with metastatic disease may come from further elucidation of the roles of post-translational modification processes, such as glycosylation, which have been shown to play an integral role in bone metastases formation and development.
However, despite promising advances in the field of developing novel targeted therapeutics and identification of bone metastases specific biomarkers, significant further work remains to be carried out in order to improve the treatment landscape for patients with incurable mPCa.
Acknowledgements
Not applicable.
Funding Statement
This work was supported by the MRC Discovery Medicine North Doctoral Training Partnership and Prostate Cancer Research (grant reference 6961).
Availability of data and materials
Not applicable.
Authors' contributions
EAG carried out a literature search, wrote and formatted the review, and produced the figures. JM and NW contributed to the review content and reviewed the original draft. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
References
- 1.Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pernar CH, Ebot EM, Wilson KM, Mucci LA. The epidemiology of prostate cancer. Cold Spring Harb Perspect Med. 2018;8:a030361. doi: 10.1101/cshperspect.a030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vietri MT, D'Elia G, Caliendo G, Resse M, Casamassimi A, Passariello L, Albanese L, Cioffi M, Molinari AM. Hereditary prostate cancer: Genes related, target therapy and prevention. Int J Mol Sci. 2021;22:3753. doi: 10.3390/ijms22073753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet. 2017;389:815–822. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 5.Descotes JL. Diagnosis of prostate cancer. Asian J Urol. 2019;6:129–136. doi: 10.1016/j.ajur.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadosky KM, Koochekpour S. Molecular mechanisms underlying resistance to androgen deprivation therapy in prostate cancer. Oncotarget. 2016;7:64447–64470. doi: 10.18632/oncotarget.10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi K, Hotte SJ, Joshua AM, North S, Wyatt AW, Collins LL, Saad F. Treatment of mCRPC in the AR-axis-targeted therapy-resistant state. Ann Oncol. 2015;26:2044–2056. doi: 10.1093/annonc/mdv267. [DOI] [PubMed] [Google Scholar]
- 8.Shah H, Vaishampayan U. Therapy of advanced prostate cancer: Targeting the androgen receptor axis in earlier lines of treatment. Target Oncol. 2018;13:679–689. doi: 10.1007/s11523-018-0611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Antonov P, Raycheva G, Popov V. Unexpected long-term survival in an adult patient with metastatic prostate cancer. Urol Case Rep. 2021;37:101634. doi: 10.1016/j.eucr.2021.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 12.Liu D, Kuai Y, Zhu R, Zhou C, Tao Y, Han W, Chen Q. Prognosis of prostate cancer and bone metastasis pattern of patients: A SEER-based study and a local hospital based study from China. Sci Rep. 2020;10:9104. doi: 10.1038/s41598-020-64073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieder C, Haukland E, Pawinski A, Dalhaug A. Pathologic fracture and metastatic spinal cord compression in patients with prostate cancer and bone metastases. BMC Urol. 2010;10:23. doi: 10.1186/1471-2490-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, Christakos S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016;4:16041. doi: 10.1038/boneres.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018;59:99–107. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: Mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208–218. doi: 10.1038/nrendo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordstrand A, Bovinder Ylitalo E, Thysell E, Jernberg E, Crnalic S, Widmark A, Bergh A, Lerner UH, Wikström P. Bone cell activity in clinical prostate cancer bone metastasis and its inverse relation to tumor cell androgen receptor activity. Int J Mol Sci. 2018;19:1223. doi: 10.3390/ijms19041223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong SK, Mohamad NV, Giaze TR, Chin KY, Mohamed N, Ima-Nirwana S. Prostate cancer and bone metastases: The underlying mechanisms. Int J Mol Sci. 2019;20:2587. doi: 10.3390/ijms20102587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. doi: 10.1016/S0140-6736(00)49915-0. [DOI] [PubMed] [Google Scholar]
- 21.Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, Goncalves F. Bone metastases: An overview. Oncol Rev. 2017;11:321. doi: 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 23.Conti G, La Torre G, Cicalese V, Micheletti G, Ludovico MG, Vestita GD, Cottonaro G, Introini C, Cecchi M. Prostate cancer metastases to bone: Observational study for the evaluation of clinical presentation, course and treatment patterns. Presentation of the METAURO protocol and of patient baseline features. Arch Ital Urol Androl. 2008;80:59–64. [PubMed] [Google Scholar]
- 24.Kitajima K, Yamamoto S, Kawanaka Y, Komoto H, Shimatani K, Hanasaki T, Taguchi M, Nagasawa S, Yamada Y, Kanematsu A, Yamakado K. Assessment of the viability and treatment response of bone metastases in patients with metastatic castration-resistant prostate cancer using choline PET/CT. Medicine (Baltimore) 2021;100:e26206. doi: 10.1097/MD.0000000000026206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin SC, Yu-Lee LY, Lin SH. Osteoblastic factors in prostate cancer bone metastasis. Curr Osteoporos Rep. 2018;16:642–647. doi: 10.1007/s11914-018-0480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roudier MP, Morrissey C, True LD, Higano CS, Vessella RL, Ott SM. Histopathological assessment of prostate cancer bone osteoblastic metastases. J Urol. 2008;180:1154–1160. doi: 10.1016/j.juro.2008.04.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garnero P, Buchs N, Zekri J, Rizzoli R, Coleman RE, Delmas PD. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br J Cancer. 2000;82:858–864. doi: 10.1054/bjoc.1999.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan MA, Partin AW. Bisphosphonates in metastatic prostate cancer. Rev Urol. 2003;5:204–206. [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast-osteoclast communication and bone homeostasis. Cells. 2020;9:2073. doi: 10.3390/cells9092073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheville JC, Tindall D, Boelter C, Jenkins R, Lohse CM, Pankratz VS, Sebo TJ, Davis B, Blute ML. Metastatic prostate carcinoma to bone: Clinical and pathologic features associated with cancer-specific survival. Cancer. 2002;95:1028–1036. doi: 10.1002/cncr.10788. [DOI] [PubMed] [Google Scholar]
- 31.Ribelli G, Simonetti S, Iuliani M, Rossi E, Vincenzi B, Tonini G, Pantano F, Santini D. Osteoblasts promote prostate cancer cell proliferation through androgen receptor independent mechanisms. Front Oncol. 2021;11:789885. doi: 10.3389/fonc.2021.789885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirschenbaum A, Liu XH, Yao S, Leiter A, Levine AC. Prostatic acid phosphatase is expressed in human prostate cancer bone metastases and promotes osteoblast differentiation. Ann N Y Acad Sci. 2011;1237:64–70. doi: 10.1111/j.1749-6632.2011.06198.x. [DOI] [PubMed] [Google Scholar]
- 33.Kirschenbaum A, Izadmehr S, Yao S, O'Connor-Chapman KL, Huang A, Gregoriades EM, Yakar S, Levine AC. Prostatic acid phosphatase alters the RANKL/OPG system and induces osteoblastic prostate cancer bone metastases. Endocrinology. 2016;157:4526–4533. doi: 10.1210/en.2016-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen G, Sircar K, Aprikian A, Potti A, Goltzman D, Rabbani SA. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer. 2006;107:289–298. doi: 10.1002/cncr.21978. [DOI] [PubMed] [Google Scholar]
- 35.Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K, Suda T. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology. 2000;141:3478–3484. doi: 10.1210/endo.141.9.7634. [DOI] [PubMed] [Google Scholar]
- 36.Corey E, Brown LG, Kiefer JA, Quinn JE, Pitts TE, Blair JM, Vessella RL. Osteoprotegerin in prostate cancer bone metastasis. Cancer Res. 2005;65:1710–1718. doi: 10.1158/0008-5472.CAN-04-2033. [DOI] [PubMed] [Google Scholar]
- 37.Brown JM, Corey E, Lee ZD, True LD, Yun TJ, Tondravi M, Vessella RL. Osteoprotegerin and rank ligand expression in prostate cancer. Urology. 2001;57:611–616. doi: 10.1016/S0090-4295(00)01122-5. [DOI] [PubMed] [Google Scholar]
- 38.Holen I, Croucher PI, Hamdy FC, Eaton CL. Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res. 2002;62:1619–1623. [PubMed] [Google Scholar]
- 39.Chiao JW, Moonga BS, Yang YM, Kancherla R, Mittelman A, Wu-Wong JR, Ahmed T. Endothelin-1 from prostate cancer cells is enhanced by bone contact which blocks osteoclastic bone resorption. Br J Cancer. 2000;83:360–365. doi: 10.1054/bjoc.2000.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fizazi K, Yang J, Peleg S, Sikes CR, Kreimann EL, Daliani D, Olive M, Raymond KA, Janus TJ, Logothetis CJ, et al. Prostate cancer cells-osteoblast interaction shifts expression of growth/survival-related genes in prostate cancer and reduces expression of osteoprotegerin in osteoblasts. Clin Cancer Res. 2003;9:2587–2597. [PubMed] [Google Scholar]
- 41.Yin JJ, Mohammad KS, Käkönen SM, Harris S, Wu-Wong JR, Wessale JL, Padley RJ, Garrett IR, Chirgwin JM, Guise TA. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci USA. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valta MP, Tuomela J, Bjartell A, Valve E, Väänänen HK, Härkönen P. FGF-8 is involved in bone metastasis of prostate cancer. Int J Cancer. 2008;123:22–31. doi: 10.1002/ijc.23422. [DOI] [PubMed] [Google Scholar]
- 43.Quiroz-Munoz M, Izadmehr S, Arumugam D, Wong B, Kirschenbaum A, Levine AC. Mechanisms of osteoblastic bone metastasis in prostate cancer: Role of prostatic acid phosphatase. J Endocr Soc. 2019;3:655–664. doi: 10.1210/js.2018-00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikuerowo SO, Omisanjo OA, Bioku MJ, Ajala MO, Mordi VP, Esho JO. Prevalence and characteristics of prostate cancer among participants of a community-based screening in Nigeria using serum prostate specific antigen and digital rectal examination. Pan Afr Med J. 2013;15:129. doi: 10.11604/pamj.2013.15.129.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Idowu BM. Prostate carcinoma presenting with diffuse osteolytic metastases and supraclavicular lymphadenopathy mimicking multiple myeloma. Clin Case Rep. 2017;6:253–257. doi: 10.1002/ccr3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maharaj B, Kalideen JM, Leary WP, Pudifin DJ. Carcinoma of the prostate with multiple osteolytic metastases simulating multiple myeloma. A case report. S Afr Med J. 1986;70:227–228. [PubMed] [Google Scholar]
- 47.Fukuoka H, Ishibashi Y, Masuda M, Gotoh A, Murai T, Kitamura H. A case of prostatic carcinoma with osteolytic bone metastases. Hinyokika Kiyo. 1988;34:1805–1809. (In Japanese) [PubMed] [Google Scholar]
- 48.Migita T, Maeda K, Ogata N. A case of prostate cancer associated with osteolytic bone metastases. Hinyokika Kiyo. 1999;45:371–374. (In Japanese) [PubMed] [Google Scholar]
- 49.Rajendiran G, Green L, Chhabra G. A rare presentation of prostate cancer with diffuse osteolytic metastases and PSA of 7242 ng/ml. Int J Case Rep Image. 2011;2:16–20. doi: 10.5348/ijcri-2011-09-55-CR-5. [DOI] [Google Scholar]
- 50.Segamwenge IL, Mgori NK, Abdallahyussuf S, Mukulu CN, Nakangombe P, Ngalyuka PK, Kidaaga F. Cancer of the prostate presenting with diffuse osteolytic metastatic bone lesions: A case report. J Med Case Rep. 2012;6:425. doi: 10.1186/1752-1947-6-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma P, Karunanithi S, Singh Dhull V, Jain S, Bal C, Kumar R. Prostate cancer with lytic bone metastases: 18F-fluorodeoxyglucose positron emission tomography-computed tomography for diagnosis and monitoring response to medical castration therapy. Indian J Nucl Med. 2013;28:178–179. doi: 10.4103/0972-3919.119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bird VY, Domino PM, Sutkowski R, Stillings SA, Trejo-Lopez JA. Prostate cancer with metastatic lytic bone lesions: Positive bone scan post docetaxel chemotherapy in the setting of clinically successful treatment. Urol Case Rep. 2016;6:12–14. doi: 10.1016/j.eucr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rummel K, Benson J, Roller L. Prostate adenocarcinoma with osteolytic metastases: Case report and review of the literature. Radiol Case Rep. 2021;16:3565–3568. doi: 10.1016/j.radcr.2021.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bryden AAG, Hoyland JA, Freemont AJ, Clarke NW, George NJR. Parathyroid hormone related peptide and receptor expression in paired primary prostate cancer and bone metastases. Br J Cancer. 2002;86:322–325. doi: 10.1038/sj.bjc.6600115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang JC, Sakata T, Pfleger LL, Bencsik M, Halloran BP, Bikle DD, Nissenson RA. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res. 2004;19:235–244. doi: 10.1359/JBMR.0301226. [DOI] [PubMed] [Google Scholar]
- 56.Ongkeko WM, Burton D, Kiang A, Abhold E, Kuo SZ, Rahimy E, Yang M, Hoffman RM, Wang-Rodriguez J, Deftos LJ. Parathyroid hormone related-protein promotes epithelial-to-mesenchymal transition in prostate cancer. PLoS One. 2014;9:e85803. doi: 10.1371/journal.pone.0085803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X, Zhi X, Wang J, Su J. RANKL signaling in bone marrow mesenchymal stem cells negatively regulates osteoblastic bone formation. Bone Res. 2018;6:34. doi: 10.1038/s41413-018-0035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, Kariya Y, Kato G, Tabata Y, Penninger JM, et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561:195–200. doi: 10.1038/s41586-018-0482-7. [DOI] [PubMed] [Google Scholar]
- 59.Wang M, Xia F, Wei Y, Wei X. Molecular mechanisms and clinical management of cancer bone metastasis. Bone Res. 2020;8:30. doi: 10.1038/s41413-020-00105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng J, Xu X, Li B, Brown E, Farris AB, Sun SY, Yang JJ. Prostate cancer metastatic to bone has higher expression of the calcium-sensing receptor (CaSR) than primary prostate cancer. Receptors Clin Investig. 2014;1:e270. doi: 10.14800/rci.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuchimaru T, Hoshino T, Aikawa T, Yasuda H, Kobayashi T, Kadonosono T, Kizaka-Kondoh S. Bone resorption facilitates osteoblastic bone metastatic colonization by cooperation of insulin-like growth factor and hypoxia. Cancer Sci. 2014;105:553–559. doi: 10.1111/cas.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russo S, Scotto di Carlo F, Gianfrancesco F. The osteoclast traces the route to bone tumors and metastases. Front Cell Dev Biol. 2022;10:886305. doi: 10.3389/fcell.2022.886305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–396. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 64.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: Emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 65.Schneider JG, Amend SR, Weilbaecher KN. Integrins and bone metastasis: Integrating tumor cell and stromal cell interactions. Bone. 2011;48:54–65. doi: 10.1016/j.bone.2010.10.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh R, Kapur N, Mir H, Singh N, Lillard JW, Jr, Singh S. CXCR6-CXCL16 axis promotes prostate cancer by mediating cytoskeleton rearrangement via Ezrin activation and αvβ3 integrin clustering. Oncotarget. 2016;7:7343–7353. doi: 10.18632/oncotarget.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu J, Doyle AD, Shinsato Y, Wang S, Bodendorfer MA, Zheng M, Yamada KM. Basement membrane regulates fibronectin organization using sliding focal adhesions driven by a contractile winch. Dev Cell. 2020;52:631–646.e4. doi: 10.1016/j.devcel.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He Y, Liu XD, Chen ZY, Zhu J, Xiong Y, Li K, Dong JH, Li X. Interaction between cancer cells and stromal fibroblasts is required for activation of the uPAR-uPA-MMP-2 cascade in pancreatic cancer metastasis. Clin Cancer Res. 2007;13:3115–3124. doi: 10.1158/1078-0432.CCR-06-2088. [DOI] [PubMed] [Google Scholar]
- 69.Sun LC, Luo J, Mackey LV, Fuselier JA, Coy DH. A conjugate of camptothecin and a somatostatin analog against prostate cancer cell invasion via a possible signaling pathway involving PI3K/Akt, alphaVbeta3/alphaVbeta5 and MMP-2/-9. Cancer Lett. 2007;246:157–166. doi: 10.1016/j.canlet.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 70.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12:177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong Y, Xie X, Wang Z, Hu C, Zheng Q, Wang Y, Chen R, Xue T, Chen J, Gao D, et al. Increasing matrix stiffness upregulates vascular endothelial growth factor expression in hepatocellular carcinoma cells mediated by integrin β1. Biochem Biophys Res Commun. 2014;444:427–432. doi: 10.1016/j.bbrc.2014.01.079. [DOI] [PubMed] [Google Scholar]
- 72.Tang L, Xu M, Zhang L, Qu L, Liu X. Role of αVβ3 in prostate cancer: Metastasis initiator and important therapeutic target. Onco Targets Ther. 2020;13:7411–7422. doi: 10.2147/OTT.S258252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown NF, Marshall JF. Integrin-mediated TGFβ activation modulates the tumour microenvironment. Cancers (Basel) 2019;11:1221. doi: 10.3390/cancers11091221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 75.Hussain M, Le Moulec S, Gimmi C, Bruns R, Straub J, Miller K, PERSEUS Study Group Differential effect on bone lesions of targeting integrins: Randomized phase II trial of abituzumab in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2016;22:3192–3200. doi: 10.1158/1078-0432.CCR-15-2512. [DOI] [PubMed] [Google Scholar]
- 76.Gheldof A, Berx G. Cadherins and epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci. 2013;116:317–336. doi: 10.1016/B978-0-12-394311-8.00014-5. [DOI] [PubMed] [Google Scholar]
- 77.Bryden AAG, Hoyland JA, Freemont AJ, Clarke NW, Schembri Wismayer D, George NJR. E-cadherin and beta-catenin are down-regulated in prostatic bone metastases. BJU Int. 2002;89:400–403. doi: 10.1046/j.1464-4096.2001.01712.x. [DOI] [PubMed] [Google Scholar]
- 78.Jennbacken K, Tesan T, Wang W, Gustavsson H, Damber JE, Welén K. N-cadherin increases after androgen deprivation and is associated with metastasis in prostate cancer. Endocr Relat Cancer. 2010;17:469–479. doi: 10.1677/ERC-10-0015. [DOI] [PubMed] [Google Scholar]
- 79.Cui Y, Yamada S. N-cadherin dependent collective cell invasion of prostate cancer cells is regulated by the N-terminus of α-catenin. PLoS One. 2013;8:e55069. doi: 10.1371/journal.pone.0055069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun Y, Jing J, Xu H, Xu L, Hu H, Tang C, Liu S, Wei Q, Duan R, Guo J, Yang L. N-cadherin inhibitor creates a microenvironment that protect TILs from immune checkpoints and Treg cells. J Immunother Cancer. 2021;9:e002138. doi: 10.1136/jitc-2020-002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanaka H, Kono E, Tran CP, Miyazaki H, Yamashiro J, Shimomura T, Fazli L, Wada R, Huang J, Vessella RL, et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med. 2010;16:1414–1420. doi: 10.1038/nm.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15:346–366. doi: 10.1038/s41581-019-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garnham R, Scott E, Livermore KE, Munkley J. ST6GAL1: A key player in cancer. Oncol Lett. 2019;18:983–989. doi: 10.3892/ol.2019.10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bindeman WE, Fingleton B. Glycosylation as a regulator of site-specific metastasis. Cancer Metastasis Rev. 2022;41:107–129. doi: 10.1007/s10555-021-10015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sottnik JL, Daignault-Newton S, Zhang X, Morrissey C, Hussain MH, Keller ET, Hall CL. Integrin alpha2beta 1 (α2β1) promotes prostate cancer skeletal metastasis. Clin Exp Metastasis. 2013;30:569–578. doi: 10.1007/s10585-012-9561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Slambrouck S, Groux-Degroote S, Krzewinski-Recchi MA, Cazet A, Delannoy P, Steelant WF. Carbohydrate-to-carbohydrate interactions between α2,3-linked sialic acids on α2 integrin subunits and asialo-GM1 underlie the bone metastatic behaviour of LNCAP-derivative C4-2B prostate cancer cells. Biosci Rep. 2014;34:e00138. doi: 10.1042/BSR20140096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Julien S, Ivetic A, Grigoriadis A, QiZe D, Burford B, Sproviero D, Picco G, Gillett C, Papp SL, Schaffer L, et al. Selectin ligand sialyl-Lewis × antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 2011;71:7683–7693. doi: 10.1158/0008-5472.CAN-11-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barthel SR, Gavino JD, Wiese GK, Jaynes JM, Siddiqui J, Dimitroff CJ. Analysis of glycosyltransferase expression in metastatic prostate cancer cells capable of rolling activity on microvascular endothelial (E)-selectin. Glycobiology. 2008;18:806–817. doi: 10.1093/glycob/cwn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao J, Li T, Mo Z, Hu Y, Yi Q, He R, Zhu X, Zhou X, She S, Chen Y. Overexpression of the galectin-3 during tumor progression in prostate cancer and its clinical implications. Int J Clin Exp Pathol. 2018;11:839–846. [PMC free article] [PubMed] [Google Scholar]
- 90.Nakajima K, Kho DH, Yanagawa T, Harazono Y, Hogan V, Chen W, Ali-Fehmi R, Mehra R, Raz A. Galectin-3 cleavage alters bone remodeling: different outcomes in breast and prostate cancer skeletal metastasis. Cancer Res. 2016;76:1391–1402. doi: 10.1158/0008-5472.CAN-15-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat Res. 2011;728:23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pouliot N, Pearson HB, Burrows A. Madame Curie Bioscience Database [Internet] Austin (TX): Landes Bioscience; 2013. Investigating metastasis using in vitro platforms. [Google Scholar]
- 93.Hao J, Madigan MC, Khatri A, Power CA, Hung TT, Beretov J, Chang L, Xiao W, Cozzi PJ, Graham PH, et al. In vitro and in vivo prostate cancer metastasis and chemoresistance can be modulated by expression of either CD44 or CD147. PLoS One. 2012;7:e40716. doi: 10.1371/journal.pone.0040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li W, Qian L, Lin J, Huang G, Hao N, Wei X, Wang W, Liang J. CD44 regulates prostate cancer proliferation, invasion and migration via PDK1 and PFKFB4. Oncotarget. 2017;8:65143–65151. doi: 10.18632/oncotarget.17821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fang F, Li Q, Wu M, Nie C, Xu H, Wang L. CD147 promotes epithelial-mesenchymal transition of prostate cancer cells via the Wnt/β-catenin pathway. Exp Ther Med. 2020;20:3154–3160. doi: 10.3892/etm.2020.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo S, Deng CX. Effect of stromal cells in tumor microenvironment on metastasis initiation. Int J Biol Sci. 2018;14:2083–2093. doi: 10.7150/ijbs.25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jasuja H, Kar S, Katti DR, Katti KS. Perfusion bioreactor enabled fluid-derived shear stress conditions for novel bone metastatic prostate cancer testbed. Biofabrication. 2021;13 doi: 10.1088/1758-5090/abd9d6. [DOI] [PubMed] [Google Scholar]
- 99.Fong ELS, Wan X, Yang J, Morgado M, Mikos AG, Harrington DA, Navone NM, Farach-Carson MC. A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials. 2016;77:164–172. doi: 10.1016/j.biomaterials.2015.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hepburn AC, Curry EL, Moad M, Steele RE, Franco OE, Wilson L, Singh P, Buskin A, Crawford SE, Gaughan L, et al. Propagation of human prostate tissue from induced pluripotent stem cells. Stem Cells Transl Med. 2020;9:734–745. doi: 10.1002/sctm.19-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fitzgerald KA, Guo J, Tierney EG, Curtin CM, Malhotra M, Darcy R, O'Brien FJ, O'Driscoll CM. The use of collagen-based scaffolds to simulate prostate cancer bone metastases with potential for evaluating delivery of nanoparticulate gene therapeutics. Biomaterials. 2015;66:53–66. doi: 10.1016/j.biomaterials.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 102.Katti KS, Jasuja H, Kar S, Katti DR. Nanostructured biomaterials for in vitro models of bone metastasis cancer. Curr Opin Biomed Eng. 2021;17:100254. doi: 10.1016/j.cobme.2020.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cruz-Neves S, Ribeiro N, Graça I, Jerónimo C, Sousa SR, Monteiro FJ. Behavior of prostate cancer cells in a nanohydroxyapatite/collagen bone scaffold. J Biomed Mater Res A. 2017;105:2035–2046. doi: 10.1002/jbm.a.36070. [DOI] [PubMed] [Google Scholar]
- 104.Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, Tombal B, Gillessen S, ESMO Guidelines Committee. Electronic address clinicalguidelines@esmo.org: Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1119–1134. doi: 10.1016/j.annonc.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 105.Kuppen MCP, Westgeest HM, van den Eertwegh AJM, van Moorselaar RJA, van Oort IM, Tascilar M, Mehra N, Lavalaye J, Somford DM, Aben KKH, et al. Symptomatic skeletal events and the use of bone health agents in a real-world treated metastatic castration resistant prostate cancer population: Results from the CAPRI-study in the netherlands. Clin Genitourin Cancer. 2022;20:43–52. doi: 10.1016/j.clgc.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 106.Benford HL, McGowan NW, Helfrich MH, Nuttall ME, Rogers MJ. Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone. 2001;28:465–473. doi: 10.1016/S8756-3282(01)00412-4. [DOI] [PubMed] [Google Scholar]
- 107.Gralow J, Tripathy D. Managing metastatic bone pain: The role of bisphosphonates. J Pain Symptom Manage. 2007;33:462–472. doi: 10.1016/j.jpainsymman.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 108.Percival RC, Urwin GH, Harris S, Yates AJ, Williams JL, Beneton M, Kanis JA. Biochemical and histological evidence that carcinoma of the prostate is associated with increased bone resorption. Eur J Surg Oncol. 1987;13:41–49. [PubMed] [Google Scholar]
- 109.Clarke NW, McClure J, George NJ. Morphometric evidence for bone resorption and replacement in prostate cancer. Br J Urol. 1991;68:74–80. doi: 10.1111/j.1464-410X.1991.tb15260.x. [DOI] [PubMed] [Google Scholar]
- 110.Berruti A, Dogliotti L, Tucci M, Tarabuzzi R, Fontana D, Angeli A. Metabolic bone disease induced by prostate cancer: Rationale for the use of bisphosphonates. J Urol. 2001;166:2023–2031. doi: 10.1016/S0022-5347(05)65498-5. [DOI] [PubMed] [Google Scholar]
- 111.Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21:4277–4284. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 112.Liu J, Zhao C, Liu B, Liu H, Wang L. Analgesia and curative effect of pamidronate disodium combined with chemotherapy on elderly patients with advanced metastatic bone cancer. Oncol Lett. 2019;18:771–775. doi: 10.3892/ol.2019.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Widler L, Jaeggi KA, Glatt M, Müller K, Bachmann R, Bisping M, Born AR, Cortesi R, Guiglia G, Jeker H, et al. Highly potent geminal bisphosphonates. From pamidronate disodium (Aredia) to zoledronic acid (Zometa) J Med Chem. 2002;45:3721–3738. doi: 10.1021/jm020819i. [DOI] [PubMed] [Google Scholar]
- 114.Finianos A, Aragon-Ching JB. Zoledronic acid for the treatment of prostate cancer. Expert Opin Pharmacother. 2019;20:657–666. doi: 10.1080/14656566.2019.1574754. [DOI] [PubMed] [Google Scholar]
- 115.Kamba T, Kamoto T, Maruo S, Kikuchi T, Shimizu Y, Namiki S, Fujimoto K, Kawanishi H, Sato F, Narita S, et al. A phase III multicenter, randomized, controlled study of combined androgen blockade with versus without zoledronic acid in prostate cancer patients with metastatic bone disease: Results of the ZAPCA trial. Int J Clin Oncol. 2017;22:166–173. doi: 10.1007/s10147-016-1037-2. [DOI] [PubMed] [Google Scholar]
- 116.Weinfurt KP, Anstrom KJ, Castel LD, Schulman KA, Saad F. Effect of zoledronic acid on pain associated with bone metastasis in patients with prostate cancer. Ann Oncol. 2006;17:986–989. doi: 10.1093/annonc/mdl041. [DOI] [PubMed] [Google Scholar]
- 117.NICE. Prostate cancer, corp-author. Diagnosis and management. https://www.nice.org.uk/guidance/ng131/chapter/Recommendations 2019 15/12/2021 [cited 2022 21/11/2022]; Available from: [Google Scholar]
- 118.Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie J, Namjoshi M, Wu EQ, Parikh K, Diener M, Yu AP, Guo A, Culver KW. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J Manag Care Pharm. 2011;17:621–643. doi: 10.18553/jmcp.2011.17.8.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.NICE, corp-author. Denosumab is not recommended for preventing skeletal-related events in adults with bone metastases from prostate cancer. https://www.nice.org.uk/donotdo/denosumab-is-not-recommended-for-preventing-skeletalrelated-events-inadults-with-bone-metastases-from-prostate-cancer 2012 Available from. [Google Scholar]
- 121.Smart JG. The use of P32 in the treatment of severe pain from bone metastases of carcinoma of the prostate1. Br J Urol. 1965;37:139–147. doi: 10.1111/j.1464-410X.1965.tb09584.x. [DOI] [PubMed] [Google Scholar]
- 122.Porter AT, McEwan AJ, Powe JE, Reid R, McGowan DG, Lukka H, Sathyanarayana JR, Yakemchuk VN, Thomas GM, Erlich LE, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 1993;25:805–813. doi: 10.1016/0360-3016(93)90309-J. [DOI] [PubMed] [Google Scholar]
- 123.Serafini AN, Houston SJ, Resche I, Quick DP, Grund FM, Ell PJ, Bertrand A, Ahmann FR, Orihuela E, Reid RH, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: A double-blind placebo-controlled clinical trial. J Clin Oncol. 1998;16:1574–1581. doi: 10.1200/JCO.1998.16.4.1574. [DOI] [PubMed] [Google Scholar]
- 124.Sartor O, Reid RH, Hoskin PJ, Quick DP, Ell PJ, Coleman RE, Kotler JA, Freeman LM, Olivier P, Quadramet 424Sm10/11 Study Group Samarium-153-lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63:940–945. doi: 10.1016/j.urology.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 125.Powers E, Karachaliou GS, Kao C, Harrison MR, Hoimes CJ, George DJ, Armstrong AJ, Zhang T. Novel therapies are changing treatment paradigms in metastatic prostate cancer. J Hematol Oncol. 2020;13:144. doi: 10.1186/s13045-020-00978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Terrisse S, Karamouza E, Parker CC, Sartor AO, James ND, Pirrie S, Collette L, Tombal BF, Chahoud J, Smeland S, et al. Overall survival in men with bone metastases from castration-resistant prostate cancer treated with bone-targeting radioisotopes: A meta-analysis of individual patient data from randomized clinical trials. JAMA Oncol. 2020;6:206–216. doi: 10.1001/jamaoncol.2019.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Den RB, George D, Pieczonka C, McNamara M. Ra-223 treatment for bone metastases in castrate-resistant prostate cancer: Practical management issues for patient selection. Am J Clin Oncol. 2019;42:399–406. doi: 10.1097/COC.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 129.Nilsson S, Cislo P, Sartor O, Vogelzang NJ, Coleman RE, O'Sullivan JM, Reuning-Scherer J, Shan M, Zhan L, Parker C. Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann Oncol. 2016;27:868–874. doi: 10.1093/annonc/mdw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gutman EB, Sproul EE, Gutman AB. Significance of increased phosphatase activity of bone at the site of osteoplastic metastases secondary to carcinoma of the prostate gland. Am J Cancer. 1936;28:485–495. doi: 10.1158/ajc.1936.485a. [DOI] [Google Scholar]
- 131.Ozu C, Nakashima J, Horiguchi Y, Oya M, Ohigashi T, Murai M. Prediction of bone metastases by combination of tartrate-resistant acid phosphatase, alkaline phosphatase and prostate specific antigen in patients with prostate cancer. Int J Urol. 2008;15:419–422. doi: 10.1111/j.1442-2042.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- 132.Larson SR, Chin J, Zhang X, Brown LG, Coleman IM, Lakely B, Tenniswood M, Corey E, Nelson PS, Vessella RL, Morrissey C. Prostate cancer derived prostatic acid phosphatase promotes an osteoblastic response in the bone microenvironment. Clin Exp Metastasis. 2014;31:247–256. doi: 10.1007/s10585-013-9625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 134.Wargowski E, Johnson LE, Eickhoff JC, Delmastro L, Staab MJ, Liu G, McNeel DG. Prime-boost vaccination targeting prostatic acid phosphatase (PAP) in patients with metastatic castration-resistant prostate cancer (mCRPC) using sipuleucel-T and a DNA vaccine. J Immunother Cancer. 2018;6:21. doi: 10.1186/s40425-018-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 136.Marshall CH, Fu W, Wang H, Park JC, DeWeese TL, Tran PT, Song DY, King S, Afful M, Hurrelbrink J, et al. Randomized phase II trial of sipuleucel-T with or without radium-223 in men with bone-metastatic castration-resistant prostate cancer. Clin Cancer Res. 2021;27:1623–1630. doi: 10.1158/1078-0432.CCR-20-4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kasperk CH, Börcsök I, Schairer HU, Schneider U, Nawroth PP, Niethard FU, Ziegler R. Endothelin-1 is a potent regulator of human bone cell metabolism in vitro. Calcif Tissue Int. 1997;60:368–374. doi: 10.1007/s002239900245. [DOI] [PubMed] [Google Scholar]
- 138.Montironi R, Mazzucchelli R, Barbisan F, Stramazzotti D, Santinelli A, Lòpez Beltran A, Cheng L, Montorsi F, Scarpelli M. Immunohistochemical expression of endothelin-1 and endothelin-A and endothelin-B receptors in high-grade prostatic intraepithelial neoplasia and prostate cancer. Eur Urol. 2007;52:1682–1690. doi: 10.1016/j.eururo.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 139.Vogelzang NJ, Nelson JB, Schulman CC, Dearnaley DP, Saad F, Sleep DJ, Isaacson D, Carducci MA. Meta-analysis of clinical trials of atrasentan 10 mg in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2005;23((Suppl 16)):S4563. doi: 10.1200/jco.2005.23.16_suppl.4563. [DOI] [Google Scholar]
- 140.Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, Sleep DJ, Isaacson JD, Nelson JB, Atrasentan Phase III Study Group Institutions A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–1966. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 141.Drake JM, Danke JR, Henry MD. Bone-specific growth inhibition of prostate cancer metastasis by atrasentan. Cancer Biol Ther. 2010;9:607–614. doi: 10.4161/cbt.9.8.11112. [DOI] [PubMed] [Google Scholar]
- 142.Quinn DI, Tangen CM, Hussain M, Lara PN, Jr, Goldkorn A, Moinpour CM, Garzotto MG, Mack PC, Carducci MA, Monk JP, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): A randomised phase 3 trial. Lancet Oncol. 2013;14:893–900. doi: 10.1016/S1470-2045(13)70294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479–499. doi: 10.1146/annurev-med-051517-011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: A review. Crit Rev Oncol Hematol. 2005;56:365–378. doi: 10.1016/j.critrevonc.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 145.Keller ET, Brown J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J Cell Biochem. 2004;91:718–729. doi: 10.1002/jcb.10662. [DOI] [PubMed] [Google Scholar]
- 146.Li D, Lv H, Hao X, Hu B, Song Y. Prognostic value of serum alkaline phosphatase in the survival of prostate cancer: Evidence from a meta-analysis. Cancer Manag Res. 2018;10:3125–3139. doi: 10.2147/CMAR.S174237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Karhade AV, Thio QCBS, Kuverji M, Ogink PT, Ferrone ML, Schwab JH. Prognostic value of serum alkaline phosphatase in spinal metastatic disease. Br J Cancer. 2019;120:640–646. doi: 10.1038/s41416-019-0407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tucci M, Mosca A, Lamanna G, Porpiglia F, Terzolo M, Vana F, Cracco C, Russo L, Gorzegno G, Tampellini M, et al. Prognostic significance of disordered calcium metabolism in hormone-refractory prostate cancer patients with metastatic bone disease. Prostate Cancer Prostatic Dis. 2009;12:94–99. doi: 10.1038/pcan.2008.10. [DOI] [PubMed] [Google Scholar]
- 149.Skinner HG, Schwartz GG. Serum calcium and incident and fatal prostate cancer in the national health and nutrition examination survey. Cancer Epidemiol Biomarkers Prev. 2008;17:2302–3205. doi: 10.1158/1055-9965.EPI-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Francini G, Petrioli R, Gonnelli S, Correale P, Pozzessere D, Marsili S, Montagnani A, Lucani B, Rossi S, Monaco R, et al. Urinary calcium excretion in the monitoring of bone metastases from prostatic carcinoma. Cancer. 2001;92:1468–1474. doi: 10.1002/1097-0142(20010915)92:6<1468::AID-CNCR1471>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 151.Jung K, Lein M, Stephan C, Von Hösslin K, Semjonow A, Sinha P, Loening SA, Schnorr D. Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: Diagnostic and prognostic implications. Int J Cancer. 2004;111:783–791. doi: 10.1002/ijc.20314. [DOI] [PubMed] [Google Scholar]
- 152.Cooper EH, Whelan P, Purves D. Bone alkaline phosphatase and prostate-specific antigen in the monitoring of prostate cancer. Prostate. 1994;25:236–242. doi: 10.1002/pros.2990250503. [DOI] [PubMed] [Google Scholar]
- 153.Kylmälä T, Tammela TL, Risteli L, Risteli J, Kontturi M, Elomaa I. Type I collagen degradation product (ICTP) gives information about the nature of bone metastases and has prognostic value in prostate cancer. Br J Cancer. 1995;71:1061–1064. doi: 10.1038/bjc.1995.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maeda H, Koizumi M, Yoshimura K, Yamauchi T, Kawai T, Ogata E. Correlation between bone metabolic markers and bone scan in prostatic cancer. J Urol. 1997;157:539–543. doi: 10.1097/00005392-199702000-00034. [DOI] [PubMed] [Google Scholar]
- 155.Klepzig M, Jonas D, Oremek GM. Procollagen type 1 amino-terminal propeptide: A marker for bone metastases in prostate carcinoma. Anticancer Res. 2009;29:671–673. [PubMed] [Google Scholar]
- 156.Sundling KE, Lowe AC. Circulating tumor cells: Overview and opportunities in cytology. Adv Anat Pathol. 2019;26:56–63. doi: 10.1097/PAP.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 157.Helo P, Cronin AM, Danila DC, Wenske S, Gonzalez-Espinoza R, Anand A, Koscuiszka M, Väänänen RM, Pettersson K, Chun FK, et al. Circulating prostate tumor cells detected by reverse transcription-PCR in men with localized or castration-refractory prostate cancer: Concordance with CellSearch assay and association with bone metastases and with survival. Clin Chem. 2009;55:765–773. doi: 10.1373/clinchem.2008.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Josefsson A, Larsson K, Månsson M, Björkman J, Rohlova E, Åhs D, Brisby H, Damber JE, Welén K. Circulating tumor cells mirror bone metastatic phenotype in prostate cancer. Oncotarget. 2018;9:29403–29413. doi: 10.18632/oncotarget.25634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Saxby H, Mikropoulos C, Boussios S. An update on the prognostic and predictive serum biomarkers in metastatic prostate cancer. Diagnostics (Basel) 2020;10:549. doi: 10.3390/diagnostics10080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang HL, Yang LF, Zhu Y, Yao XD, Zhang SL, Dai B, Zhu YP, Shen YJ, Shi GH, Ye DW. Serum miRNA-21: Elevated levels in patients with metastatic hormone-refractory prostate cancer and potential predictive factor for the efficacy of docetaxel-based chemotherapy. Prostate. 2011;71:326–331. doi: 10.1002/pros.21246. [DOI] [PubMed] [Google Scholar]
- 161.Bhagirath D, Yang TL, Bucay N, Sekhon K, Majid S, Shahryari V, Dahiya R, Tanaka Y, Saini S. microRNA-1246 is an exosomal biomarker for aggressive prostate cancer. Cancer Res. 2018;78:1833–1844. doi: 10.1158/0008-5472.CAN-17-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tinay I, Tan M, Gui B, Werner L, Kibel AS, Jia L. Functional roles and potential clinical application of miRNA-345-5p in prostate cancer. Prostate. 2018;78:927–937. doi: 10.1002/pros.23650. [DOI] [PubMed] [Google Scholar]
- 163.Roest HP, IJzermans JNM, van der Laan LJW. Evaluation of RNA isolation methods for microRNA quantification in a range of clinical biofluids. BMC Biotechnol. 2021;21:48. doi: 10.1186/s12896-021-00706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guo X, Han T, Hu P, Guo X, Zhu C, Wang Y, Chang S. Five microRNAs in serum as potential biomarkers for prostate cancer risk assessment and therapeutic intervention. Int Urol Nephrol. 2018;50:2193–2200. doi: 10.1007/s11255-018-2009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yamada Y, Nishikawa R, Kato M, Okato A, Arai T, Kojima S, Yamazaki K, Naya Y, Ichikawa T, Seki N. Regulation of HMGB3 by antitumor miR-205-5p inhibits cancer cell aggressiveness and is involved in prostate cancer pathogenesis. J Hum Genet. 2018;63:195–205. doi: 10.1038/s10038-017-0371-1. [DOI] [PubMed] [Google Scholar]
- 166.Casanova-Salas I, Rubio-Briones J, Fernández-Serra A, López-Guerrero JA. miRNAs as biomarkers in prostate cancer. Clin Transl Oncol. 2012;14:803–811. doi: 10.1007/s12094-012-0877-0. [DOI] [PubMed] [Google Scholar]
- 167.Zhang HL, Qin XJ, Cao DL, Zhu Y, Yao XD, Zhang SL, Dai B, Ye DW. An elevated serum miR-141 level in patients with bone-metastatic prostate cancer is correlated with more bone lesions. Asian J Androl. 2013;15:231–235. doi: 10.1038/aja.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nordby Y, Richardsen E, Ness N, Donnem T, Patel HRH, Busund LT, Bremnes RM, Andersen S. High miR-205 expression in normal epithelium is associated with biochemical failure-an argument for epithelial crosstalk in prostate cancer? Sci Rep. 2017;7:16308. doi: 10.1038/s41598-017-16556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Haflidadóttir BS, Larne O, Martin M, Persson M, Edsjö A, Bjartell A, Ceder Y. Upregulation of miR-96 enhances cellular proliferation of prostate cancer cells through FOXO1. PLoS One. 2013;8:e72400. doi: 10.1371/journal.pone.0072400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ma Y, Yang HZ, Dong BJ, Zou HB, Zhou Y, Kong XM, Huang YR. Biphasic regulation of autophagy by miR-96 in prostate cancer cells under hypoxia. Oncotarget. 2014;5:9169–9182. doi: 10.18632/oncotarget.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bonci D, Coppola V, Patrizii M, Addario A, Cannistraci A, Francescangeli F, Pecci R, Muto G, Collura D, Bedini R, et al. A microRNA code for prostate cancer metastasis. Oncogene. 2016;35:1180–1192. doi: 10.1038/onc.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tang Y, Wu B, Huang S, Peng X, Li X, Huang X, Zhou W, Xie P, He P. Downregulation of miR-505-3p predicts poor bone metastasis-free survival in prostate cancer. Oncol Rep. 2019;41:57–66. doi: 10.3892/or.2018.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Olivan M, Garcia M, Suárez L, Guiu M, Gros L, Méndez O, Rigau M, Reventós J, Segura MF, de Torres I, et al. Loss of microRNA-135b enhances bone metastasis in prostate cancer and predicts aggressiveness in human prostate samples. Cancers (Basel) 2021;13:6202. doi: 10.3390/cancers13246202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aigner A, Fischer D. Nanoparticle-mediated delivery of small RNA molecules in tumor therapy. Pharmazie. 2016;71:27–34. [PubMed] [Google Scholar]
- 175.Oh-Hohenhorst SJ, Lange T. Role of metastasis-related microRNAs in prostate cancer progression and treatment. Cancers (Basel) 2021;13:4492. doi: 10.3390/cancers13174492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Abramovic I, Ulamec M, Katusic Bojanac A, Bulic-Jakus F, Jezek D, Sincic N. miRNA in prostate cancer: Challenges toward translation. Epigenomics. 2020;12:543–558. doi: 10.2217/epi-2019-0275. [DOI] [PubMed] [Google Scholar]
- 177.Locati MD, Terpstra I, de Leeuw WC, Kuzak M, Rauwerda H, Ensink WA, van Leeuwen S, Nehrdich U, Spaink HP, Jonker MJ, et al. Improving small RNA-seq by using a synthetic spike-in set for size-range quality control together with a set for data normalization. Nucleic Acids Res. 2015;43:e89. doi: 10.1093/nar/gkv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang W, Zang J, Jing X, Sun Z, Yan W, Yang D, Shen B, Guo F. Identification of candidate miRNA biomarkers from miRNA regulatory network with application to prostate cancer. J Transl Med. 2014;12:66. doi: 10.1186/1479-5876-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pashaei E, Pashaei E, Ahmady M, Ozen M, Aydin N. Meta-analysis of miRNA expression profiles for prostate cancer recurrence following radical prostatectomy. PLoS One. 2017;12:e0179543. doi: 10.1371/journal.pone.0179543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sottnik JL, Keller ET. Understanding and targeting osteoclastic activity in prostate cancer bone metastases. Curr Mol Med. 2013;13:626–639. doi: 10.2174/1566524011313040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Macherey S, Monsef I, Jahn F, Jordan K, Yuen KK, Heidenreich A, Skoetz N. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev. 2017;12:Cd006250. doi: 10.1002/14651858.CD006250.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Elomaa I, Kylmälä T, Tammela T, Viitanen J, Ottelin J, Ruutu M, Jauhiainen K, Ala-Opas M, Roos L, Seppänen J, et al. Effect of oral clodronate on bone pain. A controlled study in patients with metastic prostatic cancer. Int Urol Nephrol. 1992;24:159–166. doi: 10.1007/BF02549644. [DOI] [PubMed] [Google Scholar]
- 183.Vorreuther R, Klotz T, Engelking R. Clodronate in the palliative therapy of bone-metastasized prostatic carcinoma. Urologe A. 1992;31:63–66. (In German) [PubMed] [Google Scholar]
- 184.Adami S, Mian M. Clodronate therapy of metastatic bone disease in patients with prostatic carcinoma. Recent Results Cancer Res. 1989;116:67–72. doi: 10.1007/978-3-642-83668-8_6. [DOI] [PubMed] [Google Scholar]
- 185.Strang P, Nilsson S, Brändstedt S, Sehlin J, Borghede G, Varenhorst E, Bandman U, Borck L, Englund G, Selin L. The analgesic efficacy of clodronate compared with placebo in patients with painful bone metastases from prostatic cancer. Anticancer Res. 1997;17:4717–4721. [PubMed] [Google Scholar]
- 186.Kylmälä T, Taube T, Tammela TL, Risteli L, Risteli J, Elomaa I. Concomitant i.v. and oral clodronate in the relief of bone pain-a double-blind placebo-controlled study in patients with prostate cancer. Br J Cancer. 1997;76:939–942. doi: 10.1038/bjc.1997.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ernst DS, Tannock IF, Winquist EW, Venner PM, Reyno L, Moore MJ, Chi K, Ding K, Elliott C, Parulekar W. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21:3335–3342. doi: 10.1200/JCO.2003.03.042. [DOI] [PubMed] [Google Scholar]
- 188.Mason MD, Sydes MR, Glaholm J, Langley RE, Huddart RA, Sokal M, Stott M, Robinson AC, James ND, Parmar MK, et al. Oral sodium clodronate for nonmetastatic prostate cancer-results of a randomized double-blind placebo-controlled trial: Medical research council PR04 (ISRCTN61384873) J Natl Cancer Inst. 2007;99:765–776. doi: 10.1093/jnci/djk178. [DOI] [PubMed] [Google Scholar]
- 189.Dearnaley DP, Sydes MR, Mason MD, Stott M, Powell CS, Robinson AC, Thompson PM, Moffat LE, Naylor SL, Parmar MK, Mrc Pr05 Collaborators A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 trial) J Natl Cancer Inst. 2003;95:1300–1311. doi: 10.1093/jnci/djg038. [DOI] [PubMed] [Google Scholar]
- 190.Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: Long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10:872–876. doi: 10.1016/S1470-2045(09)70201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lipton A, Glover D, Harvey H, Grabelsky S, Zelenakas K, Macerata R, Seaman J. Pamidronate in the treatment of bone metastases: Results of 2 dose-ranging trials in patients with breast or prostate cancer. Ann Oncol. 1994;5((Suppl 7)):S31–S35. [PubMed] [Google Scholar]
- 192.Figg WD, Liu Y, Arlen P, Gulley J, Steinberg SM, Liewehr DJ, Cox MC, Zhai S, Cremers S, Parr A, et al. A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. J Urol. 2005;173:790–796. doi: 10.1097/01.ju.0000147013.09157.8e. [DOI] [PubMed] [Google Scholar]
- 193.Sweeney C, Dugan WM, II, Dreicer R, Chu F, Parks G, Baker K, Reed D, Jansz K, Zadra J, Yiannoutsos CT. A randomized placebo-controlled trial of daily high-dose oral risedronate in men with metastatic prostate cancer commencing androgen deprivation therapy (ADT) J Clin Oncol. 2010;28((15 Suppl)):e15000. doi: 10.1200/jco.2010.28.15_suppl.e15000. [DOI] [Google Scholar]
- 194.Meulenbeld HJ, van Werkhoven ED, Coenen JL, Creemers GJ, Loosveld OJ, de Jong PC, Ten Tije AJ, Fosså SD, Polee M, Gerritsen W, et al. Randomised phase II/III study of docetaxel with or without risedronate in patients with metastatic castration resistant prostate cancer (CRPC), the Netherlands prostate study (NePro) Eur J Cancer. 2012;48:2993–3000. doi: 10.1016/j.ejca.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 195.Hahn NM, Yiannoutsos CT, Kirkpatrick K, Sharma J, Sweeney CJ. Failure to suppress markers of bone turnover on first-line hormone therapy for metastatic prostate cancer is associated with shorter time to skeletal-related event. Clin Genitourin Cancer. 2014;12:33–40.e4. doi: 10.1016/j.clgc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 196.Hoskin P, Sundar S, Reczko K, Forsyth S, Mithal N, Sizer B, Bloomfield D, Upadhyay S, Wilson P, Kirkwood A, et al. A multicenter randomized trial of ibandronate compared with single-dose radiotherapy for localized metastatic bone pain in prostate cancer. J Natl Cancer Inst. 2015;107:djv197. doi: 10.1093/jnci/djv197. [DOI] [PubMed] [Google Scholar]
- 197.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B, Zoledronic Acid Prostate Cancer Study Group A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 198.Abetz L, Barghout V, Arbuckle R, Bosch V, Shirina N, Saad F. Impact of zoledronic acid (Z) on pain in prostate cancer patients with bone metastases in a randomised placebo-control trial. J Clin Oncol. 2006;24((Suppl 18)):S4638. doi: 10.1200/jco.2006.24.18_suppl.4638. [DOI] [Google Scholar]
- 199.Leto G, Badalamenti G, Arcara C, Crescimanno M, Flandina C, Tumminello FM, Incorvaia L, Gebbia N, Fulfaro F. Effects of zoledronic acid on proteinase plasma levels in patients with bone metastases. Anticancer Res. 2006;26:23–26. [PubMed] [Google Scholar]
- 200.Cózar Olmo JM, Carballido Rodriguez J, Luque Galvez P, Tabernero Gómez AG, Barreiro Mouro A, Sánchez Sánchez E, González Enguita C, Alcover García J, Garcia-Galisteo E, Abascal García JM, et al. Effectiveness and tolerability of zoledronic acid in the treatment of metastatic prostate cancer. Actas Urol Esp. 2008;32:492–501. doi: 10.1016/S0210-4806(08)73873-7. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 201.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Zheng M, Zoledronic Acid Prostate Cancer Study Group Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh295. [DOI] [PubMed] [Google Scholar]
- 202.Fulfaro F, Leto G, Badalamenti G, Arcara C, Cicero G, Valerio MR, Di Fede G, Russo A, Vitale A, Rini GB, et al. The use of zoledronic acid in patients with bone metastases from prostate carcinoma: Effect on analgesic response and bone metabolism biomarkers. J Chemother. 2005;17:555–559. doi: 10.1179/joc.2005.17.5.555. [DOI] [PubMed] [Google Scholar]
- 203.Saad F. Clinical benefit of zoledronic acid for the prevention of skeletal complications in advanced prostate cancer. Clin Prostate Cancer. 2005;4:31–37. doi: 10.3816/CGC.2005.n.009. [DOI] [PubMed] [Google Scholar]
- 204.Saad F, Chen YM, Gleason DM, Chin J. Continuing benefit of zoledronic acid in preventing skeletal complications in patients with bone metastases. Clin Genitourin Cancer. 2007;5:390–396. doi: 10.3816/CGC.2007.n.022. [DOI] [PubMed] [Google Scholar]
- 205.Saad F, Eastham J. Zoledronic acid improves clinical outcomes when administered before onset of bone pain in patients with prostate cancer. Urology. 2010;76:1175–1181. doi: 10.1016/j.urology.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 206.Paparella S, Finkelberg E, Varisco D, Tondelli E, Rocco F. Use of zoledronic acid in patients with prostate cancer bone metastases: Control of pain and musculoskeletal complications. Urologia. 2011;78:300–304. doi: 10.5301/RU.2011.8877. (In Italian) [DOI] [PubMed] [Google Scholar]
- 207.Uemura H, Yanagisawa M, Ikeda I, Fujinami K, Iwasaki A, Noguchi S, Noguchi K, Kubota Y, Yokohama Bone Metastasis Study Group Possible anti-tumor activity of initial treatment with zoledronic acid with hormonal therapy for bone-metastatic prostate cancer in multicenter clinical trial. Int J Clin Oncol. 2013;18:472–477. doi: 10.1007/s10147-012-0406-8. [DOI] [PubMed] [Google Scholar]
- 208.Wang F, Chen W, Chen H, Mo L, Jin H, Yu Z, Li C, Liu Q, Duan F, Weng Z. Comparison between zoledronic acid and clodronate in the treatment of prostate cancer patients with bone metastases. Med Oncol. 2013;30:657. doi: 10.1007/s12032-013-0657-x. [DOI] [PubMed] [Google Scholar]
- 209.Ueno S, Mizokami A, Fukagai T, Fujimoto N, Oh-Oka H, Kondo Y, Arai G, Ide H, Horie S, Ueki O, et al. Efficacy of combined androgen blockade with zoledronic acid treatment in prostate cancer with bone metastasis: The ZABTON-PC (zoledronic acid/androgen blockade trial on prostate cancer) study. Anticancer Res. 2013;33:3837–3844. [PubMed] [Google Scholar]
- 210.Chiang PH, Wang HC, Lai YL, Chen SC, Yen-Hwa W, Kok CK, Ou YC, Huang JS, Huang TC, Chao TY. Zoledronic acid treatment for cancerous bone metastases: A phase IV study in Taiwan. J Cancer Res Ther. 2013;9:653–659. doi: 10.4103/0973-1482.126471. [DOI] [PubMed] [Google Scholar]
- 211.Pan Y, Jin H, Chen W, Yu Z, Ye T, Zheng Y, Weng Z, Wang F. Docetaxel with or without zoledronic acid for castration-resistant prostate cancer. Int Urol Nephrol. 2014;46:2319–2326. doi: 10.1007/s11255-014-0824-9. [DOI] [PubMed] [Google Scholar]
- 212.Smith MR, Halabi S, Ryan CJ, Hussain A, Vogelzang N, Stadler W, Hauke RJ, Monk JP, Saylor P, Bhoopalam N, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: Results of CALGB 90202 (alliance) J Clin Oncol. 2014;32:1143–1150. doi: 10.1200/JCO.2013.51.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wirth M, Tammela T, Cicalese V, Gomez Veiga F, Delaere K, Miller K, Tubaro A, Schulze M, Debruyne F, Huland H, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: Efficacy and safety results of the Zometa European Study (ZEUS) Eur Urol. 2015;67:482–491. doi: 10.1016/j.eururo.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 214.James ND, Pirrie SJ, Pope AM, Barton D, Andronis L, Goranitis I, Collins S, Daunton A, McLaren D, O'Sullivan J, et al. Clinical outcomes and survival following treatment of metastatic castrate-refractory prostate cancer with docetaxel alone or with strontium-89, zoledronic acid, or both: The TRAPEZE randomized clinical trial. JAMA Oncol. 2016;2:493–499. doi: 10.1001/jamaoncol.2015.5570. [DOI] [PubMed] [Google Scholar]
- 215.Denham JW, Wilcox C, Joseph D, Spry NA, Lamb DS, Tai KH, Matthews J, Atkinson C, Turner S, Christie D, et al. Quality of life in men with locally advanced prostate cancer treated with leuprorelin and radiotherapy with or without zoledronic acid (TROG 03.04 RADAR): Secondary endpoints from a randomised phase 3 factorial trial. Lancet Oncol. 2012;13:1260–1270. doi: 10.1016/S1470-2045(12)70423-0. [DOI] [PubMed] [Google Scholar]
- 216.Denham JW, Joseph D, Lamb DS, Spry NA, Duchesne G, Matthews J, Atkinson C, Tai KH, Christie D, Kenny L, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): An open-label, randomised, phase 3 factorial trial. Lancet Oncol. 2014;15:1076–1089. doi: 10.1016/S1470-2045(14)70328-6. [DOI] [PubMed] [Google Scholar]
- 217.Denham JW, Joseph D, Lamb DS, Spry NA, Duchesne G, Matthews J, Atkinson C, Tai KH, Christie D, Kenny L, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-Year results from a randomised, phase 3, factorial trial. Lancet Oncol. 2019;20:267–281. doi: 10.1016/S1470-2045(18)30757-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.