Abstract
Background:
The purpose of this study was to estimate the cost of syphilis in the United States, in terms of the average lifetime direct medical cost per infection.
Methods:
We used a decision tree model of the natural history of syphilis. The model allowed for numerous possible outcomes of infection, including treatment for syphilis at various stages, inadvertent treatment, and late syphilis outcomes in those who are alive and still infected 30 years after acquisition. Future costs were discounted at 3% annually. Model inputs such as the cost and probability of each outcome were based on published sources. The probabilities we applied yielded outcomes consistent with reported cases of syphilis by stage from national surveillance data and number of deaths due to late syphilis from national mortality data.
Results:
The estimated, discounted lifetime cost per infection was $1,190 under base case assumptions (2019 dollars). Treatment costs associated with late syphilis outcomes such as cardiovascular syphilis accounted for only $26 of the average lifetime cost per infection. Results were most sensitive to assumptions regarding the treatment cost per case of unknown duration or late syphilis. In the probabilistic sensitivity analyses, the 2.5th and 97.5th percentiles of the 10,000 simulations of the lifetime cost per infection were $729 and $1,884, respectively.
Conclusions:
Our estimate of the lifetime cost per infection is about 50% higher than in a previous study, a difference due in large part to our higher cost assumptions for benzathine penicillin G.
Brief summary:
We estimated the lifetime cost of syphilis to be $1,190, in terms of the average present value of the direct medical treatment and care costs per infection.
Introduction
Estimates of the average lifetime cost per infection can help to quantify the economic burden of syphilis in the United States and can inform cost-effectiveness analyses of interventions to prevent syphilis and related sequelae. The direct medical cost of syphilis in 2008 in the United States was estimated in a 2013 study at $39.3 million, calculated as the product of two estimates: 55,400 incident infections and an expected lifetime cost per infection of $709 in 2010 US dollars.1 These estimates correspond to $807 per infection and $44.7 million overall when adjusted for inflation to 2019 US dollars.
The purpose of this study was to update the 2013 study’s estimate of the expected lifetime cost of syphilis per infection in the United States. This update addresses a notable gap in the literature on costs of STDs in the United States, not only in providing an updated cost estimate for syphilis but also in providing a detailed accounting of the methodology employed. To our knowledge, no stand-alone study of the lifetime cost per infection has ever been published for syphilis. Of four available estimates of the lifetime cost of syphilis per infection in the United States, three were reported as part of assessments of the overall cost of STDs in the United States1–3 and one was reported as a conference poster presentation.4 In this study, we use a modified version of the decision tree model applied in these previous analyses, in which we incorporate recent cost information and include updated, evidence-based probabilities of the possible outcomes of infection.
Methods
We used a decision tree model of the natural history of syphilis (Figure 1) to estimate the average lifetime cost per infection. This decision tree was based on an earlier version developed by Schmid and Zaidi5 which has been adapted by others for the purpose of estimating the lifetime cost per infection1–4 and the cost-effectiveness of syphilis screening strategies.6 Adaptations of the Schmid and Zaidi model are necessary when estimating the lifetime cost per incident infection, as their model was developed to estimate the costs and benefits of screening for prevalent infection.
Figure 1.
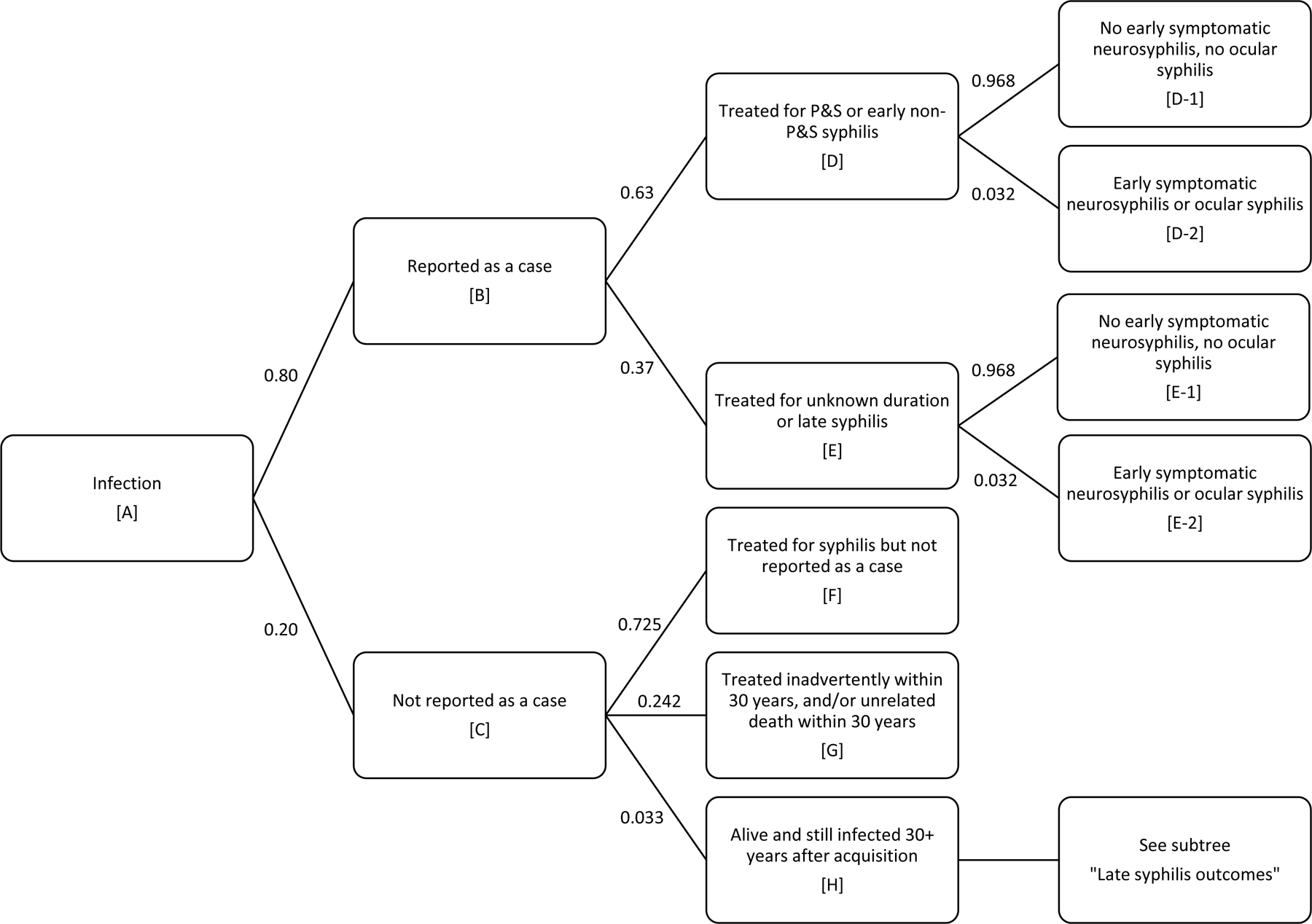
Decision tree of natural history of syphilis used to estimate the average lifetime cost per infection
Events in the decision tree are labeled by letters and numbers in brackets. We estimated the expected lifetime cost of syphilis, per infection, discounted to the time of infection (Event A). Of those reported as a syphilis case, the probabilities of treatment by stage (Event D and Event E) were consistent with the distribution of reported syphilis cases in the United States. Of those never reported as a case, we assumed three possible outcomes: treated for syphilis but not reported as a case (Event F), treated inadvertently through receipt of antibiotics for purposes other than syphilis treatment and/or death within 30 years from a cause other than syphilis (Event G),or alive and still infected 30+ years after infection (Event H). For those in Event H, we assumed one of six outcomes would occur (Events H-1 through H-6 in Panel B). The probability of Event H given Event C was estimated so that the decision tree would yield estimates consistent with current data regarding deaths due to syphilis in the United States.
We developed estimates of the probabilities and costs needed for the decision tree in order to generate estimates of the average discounted, lifetime direct medical cost per infection. In addition to establishing base case values for each parameter, we also assigned lower and upper bound values for use in one-way sensitivity analyses and assigned a distribution for use in the probabilistic sensitivity analyses. These sensitivity analyses are described in more detail later.
We limited our analysis to lifetime medical costs incurred for treatment of syphilis and related sequelae arising from a single infection with Treponema pallidum in an adolescent or adult. We used a health care sector perspective and included all direct medical costs regardless of who incurred the costs (e.g., costs paid by an insurer or other third-party, costs paid out-of-pocket by the patient). We did not include medical costs associated with congenital syphilis. In our analysis, the accrual of costs ended when the infection was treated, except for the small percentage of infections that resulted in long-term sequelae (late benign syphilis, cardiovascular syphilis, tabes dorsalis, meningovascular syphilis, general paresis) which were assumed to impose additional costs even after the infection was treated. We did not include costs associated with reinfection or with transmission of infection to another person, as these were considered to be new infections and not part of the index infection. We also did not include costs of syphilis prevention, such as syphilis screening and partner services. However, we did assume that all persons treated for syphilis incurred diagnostic testing costs. We did not include non-medical costs, such as patient time and travel costs for seeking treatment. All analyses were conducted using Microsoft Excel for Office 365.
Probabilities
The probabilities we assigned for the decision tree (Table 1) were estimated as follows. We assumed that 80% of infections would be diagnosed and reported as a syphilis case, denoted as Event B in the decision tree.7–9 This 80% assumption, applied in a 1999 incidence study9 and cited in subsequent updates,7,8 likely reflected a best guess based on the expertise of the authors of the 1999 study, as no data or references were provided in support of this assumption.9 Given the lack of data to support this 80% assumption, we applied a wide range of plausible values (0.65 to 0.95) in sensitivity analyses. Among reported syphilis cases, the probability of being treated for primary and secondary (P&S) syphilis or early non-P&S syphilis (Event D) was calculated as 0.63, based on the average annual distribution of reported cases over the 40-year period from 1979 through 2018.10 This multi-year average value of 0.63 is consistent with the single-year value of 0.65 reported in 2018 (35,063 P&S; 38,539 early non-P&S; and 40,137 unknown duration or late; the value 0.65 was calculated as [35,063 + 38,539] divided by [35,063 + 38,539 + 40,137]). The range (0.46 to 0.81) reflects the minimum and maximum values observed from 1979 through 2018. We assumed that 3.2% (range: 0.4% to 7.8%)11–13 of those with reported syphilis would also be treated for early symptomatic neurosyphilis or ocular syphilis) (Events D-2 and E-2).
Table 1.
Probabilities applied in decision tree: Base case value, lower bound, upper bound, and distribution assumptions
| Outcome | Base case value | Lower bound value | Upper bound value | Distribution used in probabilistic sensitivity analysis |
|---|---|---|---|---|
| Probability that infection is reported as a case | 0.8007–9 | 0.650 | 0.950 | Beta (21.05, 5.26) |
| Probability of treatment for P&S or early non-P&S syphilis, among those with reported syphilis | 0.63010 | 0.460 | 0.810 | Beta (17.79, 10.45) |
| Probability of early symptomatic neurosyphilis or ocular syphilis, among those with reported syphilis* | 0.03211,23 | 0.00412 | 0.07812 | Beta (2.75, 83.17) |
| Probability of remaining alive and still infected 30 years after acquisition, among those not reported as cases† | 0.033 | 0.016 | 0.091 | Beta (2.84, 83.33) |
| Among those not reported as a case and who do not remain alive and still infected 30 years after acquisition, the proportion treated for syphilis‡ | 0.750 | 0.250 | 1 | Beta (3.09, 1.03) |
| Probability of latent syphilis in those still infected after 30 years | 0.6805,17,24 | 0.480 | 0.880 | Beta (13.53, 6.37) |
| Probability of late benign syphilis in those still infected after 30 years§ | 0.1605,17,24 | NA | NA | NA |
| Probability of cardiovascular syphilis in those still infected after 30 years§ | 0.0905,17,24 | NA | NA | NA |
| Probability of tabes dorsalis in those still infected after 30 years§ | 0.0205,17,24 | NA | NA | NA |
| Probability of meningovascular syphilis in those still infected after 30 years§ | 0.0305,17,24 | NA | NA | NA |
| Probability of general paresis in those still infected after 30 years§ | 0.0205,17,24 | NA | NA | NA |
NA: Not applicable.
The base case value was calculated as the sum of two probabilities, 0.017 for neurosyphilis23 and 0.015 for ocular syphilis.11 The probability of ocular syphilis ranged from 0.002 to 0.039 across eight U.S. jurisdictions from 2014 through 2015;12 we assumed a similar range of plausible values for neurosyphilis and calculated the lower and upper bounds for the combined outcomes of ocular syphilis and early symptomatic neurosyphilis as the sum of the probabilities of each outcome (i.e., 2 × 0.002 = 0.004 and 2 × 0.039 = 0.780).
The calculation of the base case value and range for this parameter is described briefly in the manuscript text and in detail in Appendix Table 1.
The proportion 0.750 was used to calculate the probabilities for Event F and Event G as follows, such that the probabilities of Events F, G, and H would sum to 1 (given event C). The probability of 0.725 for Event F was calculated as 0.750 x (1 – 0.033), and the probability of 0.242 for Event G was calculated as (1 – 0.750) x (1 – 0.033), where 0.033 is the probability that those not reported as a case will remain alive and still infected 30 years after acquisition. The value of 0.750 for this proportion was selected so that the resulting probability for Event G (0.242) would be consistent with the estimated probability of death within 30 years of 0.103 plus the 0.134 estimated probability of receiving inadvertent treatment for conditions other than syphilis.1 The 0.103 probability of death within 30 years of infection was calculated using mortality statistics25 available online from: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/NVSR/67_07/Table01.xlsx and assuming a median age of 31 years at infection.1
The probabilities of late benign syphilis, cardiovascular syphilis, tabes dorsalis, meningovascular syphilis, and general paresis were not varied directly in the sensitivity analyses but instead were varied indirectly when the probability of latent syphilis (Platent = 0.68 in the base case) was varied. In all sensitivity analyses, the probabilities of late benign syphilis, cardiovascular syphilis, tabes dorsalis, meningovascular syphilis, and general paresis calculated so that they (1) summed to 1 - Platent and (2) maintained their same relative proportion to one another as in the base case (e.g., the probability of late benign syphilis was always 8 times the probability of tabes dorsalis). For the assumptions attributed to the Schmid and Zaidi model5 in this manuscript and the appendix, details of their model were obtained from an unpublished manuscript “Screening for syphilis: Preliminary results of a decision tree and economic analysis” acquired from George Schmid circa 1997.
Among those infected but not reported as a case, we assumed one of the following three outcomes occurred: treated for syphilis, but not reported as a case (Event F); inadvertently cured through receipt of antibiotics for a condition other than syphilis, or an unrelated death within 30 years of infection, or both (Event G); and still alive and infected 30 years after acquiring the infection (Event H). We assumed long-term sequelae costs were possible for those still alive and infected after 30 years (Event H), but not for Events F and G.
We calculated the probability that a person who acquired syphilis today would be alive and still infected in 30 years (i.e., the probability of Event H given Event C) such that the annual incidence of long-term sequelae implied by our decision tree model would be consistent with data on syphilis mortality in the United States.14 This calculation, described in detail in the Appendix, was based on reported syphilis cases from 1969 through 1987, syphilis mortality (obtained from CDC Wonder at https://wonder.cdc.gov/) from 1999 through 2017 (thereby assuming 30 years from infection to mortality), and was adjusted for other factors such as the probability of death attributable to syphilis among those with serious consequences of syphilis (0.73),15 and the probability that a death attributable to late syphilis would be listed as such on the death certificate (0.50).16
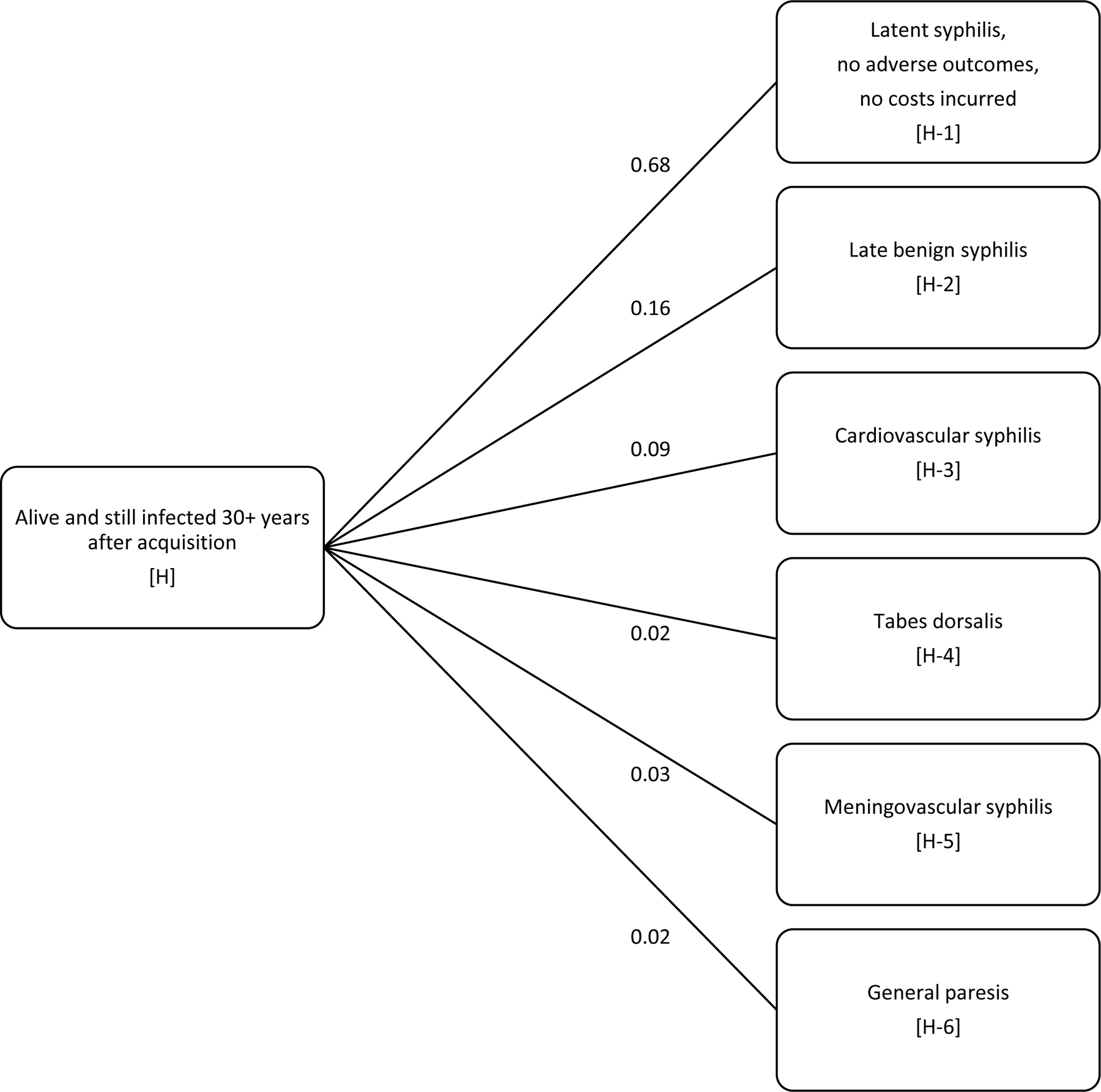
Among those still alive and still infected 30 years after acquisition, we assumed one of the following six outcomes occurred: latent syphilis, with no adverse outcomes or costs incurred (68%, Event H-1); late benign syphilis (16%, Event H-2), cardiovascular syphilis (9%, Event H-3); tabes dorsalis (2%, Event H-4); meningovascular syphilis (3%, Event H-5); and general paresis (2%, Event H-6). The probabilities of events H-1 through H-6 were obtained from the Schmid and Zaidi model,5 which incorporated data from the Oslo study as interpreted by Gjestland (1955).17
Costs
The costs we assigned for the decision tree (Table 2) were calculated using the unit costs listed in Appendix Table A-2. All cost estimates have been updated to 2019 dollars using the personal consumption expenditures price index for health care (https://www.bea.gov/). The resources required for each outcome in the decision tree (Appendix Tables A-3 through A-10) were based on the Schmid and Zaidi model.5
Table 2.
Cost per case estimates applied in decision tree: Base case value, lower bound, upper bound, and distribution assumptions
| Cost per case | Base case value | Lower bound value | Upper bound value | Distribution used in probabilistic sensitivity analysis |
|---|---|---|---|---|
| P&S syphilis or early non-P&S syphilis | $680 | $340 | $1,130 | lognormal (6.48, 0.29) |
| Unknown duration or late syphilis | $1,660 | $900 | $2,940 | lognormal (7.37, 0.30) |
| Early neurosyphilis and ocular syphilis | $7,900 | $2,200 | $21,000 | lognormal (8.81, 0.56) |
| Late benign syphilis | $3,000 | $1,800 | $4,600 | lognormal (7.98, 0.24) |
| Cardiovascular syphilis | $35,100 | $6,300 | $109,400 | lognormal (10.24, 0.67) |
| Tabes dorsalis | $40,700 | $11,800 | $97,200 | lognormal (10.49, 0.50) |
| Meningovascular syphilis | $78,400 | $17,000 | $399,200 | lognormal (10.80, 0.97) |
| General paresis | $131,500 | $33,000 | $290,100 | lognormal (11.68, 0.47) |
The lower and upper bound values were applied in the one-way sensitivity analyses in which we varied one parameter value at a time while holding other parameters at their base case value. The distributions were used in the probabilistic sensitivity analyses in which all parameters in the model (costs, probability, and outcome timing assumptions) were varied simultaneously.
These costs as shown are discounted to the time of initiation of treatment. In the analysis, these costs are further discounted to the time of infection as needed. Treatment costs for cardiovascular syphilis, tabes dorsalis, meningovascular syphilis, and general paresis include costs of follow-up care and long-term care.
Costs are in 2019 U.S. dollars.
For example, as noted in Appendix Table A-3, we assumed that treatment for P&S and early non-P&S syphilis would include 1.5 office visits on average (1 new patient visit and 0.5 established patient visits), 1 single dose of benzathine penicillin G 2.4 million units, and 1 injection. We also assumed that 37.3% (range: 7% to 80.9%) of patients would be followed-up as recommended by STD treatment guidelines18 and would require an additional 2 office visits (established patient), 2 blood draws, and 2 rapid plasma reagin (RPR) tests. The base case value of $680 (Table 1) was calculated using the base case values of the unit costs (Appendix Table A-1) and the base case value of the probability of follow-up of 37.3% (Appendix Table A-11). The lower bound value of $340 was calculated using the lower bound values of the unit costs and the lower bound probability of follow-up. The upper bound value of $1,130 was calculated using the upper bound values of the unit costs and the upper bound probability of follow-up.
Of note, the base case value we applied for the cost of treatment of P&S and early non-P&S syphilis ($680) was notably higher than in the previous cost study ($141 when updated to 2019 dollars), primarily due to an increase in cost of benzathine penicillin G. We applied a base case cost per dose of $326 for benzathine penicillin G based on the Federal Supply Schedule,19 which reflects the approximate cost of drugs from the healthcare sector and societal perspectives according to recent health economics guidelines.20
Outcome timing assumptions
To express the lifetime cost per infection in present value terms, future costs were discounted to the time of infection at a rate of 3% annually. We assumed that treatment for P&S syphilis and early non-P&S syphilis would occur within 1 year of infection, and thus did not discount these costs. Costs of treatment for syphilis of unknown duration or late syphilis and costs of early neurosyphilis and ocular syphilis were discounted for 1 year (range: 0 to 2 years). Costs associated with late benign syphilis (Event H-2), cardiovascular syphilis (Event H-3), tabes dorsalis (Event H-4), meningovascular syphilis (Event H-5), and general paresis (Event H-6) were discounted 30 years (range: 10 to 50 years).
Estimation of lifetime cost per infection
In estimating the discounted, average lifetime cost per infection, we applied the probabilities and costs listed in Tables 1 and 2 to the decision tree described in Figure 1 and applied the outcome timing assumptions described above in order to discount the future costs. For treatment of syphilis among those not reported as cases (Event F), we assumed the same distribution of treatment by syphilis stage as for reported cases (i.e., a 63% probability of treatment in the P&S or early non-P&S stage). Inadvertent treatment for syphilis or death prior to the onset of sequelae costs (Event G), and latent syphilis with no adverse outcomes (Event H-1) were assumed not to impose costs.
The average lifetime cost per infection was calculated by summing the cost of every possible outcome of that infection times the probability of that outcome occurring. Specifically, the lifetime cost per infection was calculated as the sum of the expected costs of each final outcome (Events D-1, D-2, E-1, E-2, F, G, H-1, H-2, H-3, H-4, H-5, and H-6) in the decision tree, where the expected cost due to each of these outcomes was calculated as the discounted cost of the outcome multiplied by the probability of the outcome (e.g., the expected cost due to Event D-1 was calculated as $680 multiplied by 0.8 × 0.63 × 0.968).
Sensitivity analyses
We estimated the lifetime cost per infection using the base case values listed in Tables 1 and 2. We then conducted sensitivity analyses to see how the lifetime cost estimate would change when we varied our model assumptions. We first conducted one-way sensitivity analyses in which we varied each probability parameter (Table 1), each cost parameter (Table 2), and each outcome timing assumption one at a time from its lower bound to upper bound range, while holding all other parameter values at their base case values. We then conducted probabilistic sensitivity analyses using Monte Carlo simulations in which we calculated the lifetime cost per infection 10,000 times. In each of the 10,000 simulations, we drew a random value for each probability and cost parameter (according to the distributions listed in Tables 1 and 2) and for the outcome timing assumption (assuming a uniform distribution). The distributions we used (e.g., beta distributions for probabilities and lognormal distributions for costs) are common in health economic analyses.20 For each model input listed in Tables 1 and 2, we estimated the beta distribution parameters and the lognormal distribution parameters by assuming the base case value reflected the mean value and that the standard deviation could be approximated based on the difference between the upper bound and lower bound values, following methods described elsewhere.21
Results
The estimated, discounted lifetime cost per infection was $1,190 under base case assumptions (Table 3). The most common outcome of infection was to be treated and reported as a case, which occurred in 80% of infections. Most of the remaining 20% of infections resulted in treatment for syphilis without being reported as a case, inadvertent treatment, or an unrelated death prior to treatment or long-term sequelae. Symptomatic late syphilis outcomes (Outcomes H-2 through H-6 in the decision tree) occurred in only 0.21% of infections.
Table 3.
Summary of results of decision tree analysis of syphilis costs: Components of lifetime cost per infection
| Outcome | Percentage of infections in which outcome occurs | Discounted cost of outcome if outcome occurs | Contribution of outcome to average cost per infection |
|---|---|---|---|
| Outcomes among infections that are reported as cases | |||
| Treated for P&S or early non-P&S syphilis, no early neurosyphilis/ocular syphilis | 48.79% | $680 | $331.75 |
| Treated for P&S or early non-P&S syphilis, and early neurosyphilis/ocular syphilis | 1.61% | $8,350 | $134.67 |
| Treated for late syphilis,* no early neurosyphilis/ocular syphilis | 28.65% | $1,612 | $461.78 |
| Treated for late syphilis,* and early neurosyphilis/ocular syphilis | 0.95% | $9,282 | $87.91 |
| Outcomes among infections that are not reported as cases ** | |||
| Treated for syphilis but not reported as a case | 14.50% | $1,025 | $148.58 |
| Treated inadvertently, or unrelated death within 30 years, or both | 4.84% | $0 | $0.00 |
| Latent syphilis, no adverse outcomes, no treatment costs | 0.45% | $0 | $0.00 |
| Treated for late benign syphilis | 0.11% | $1,236 | $1.31 |
| Treated for cardiovascular syphilis | 0.06% | $14,461 | $8.59 |
| Treated for tabes dorsalis | 0.01% | $16,768 | $2.21 |
| Treated for meningovascular syphilis | 0.02% | $32,300 | $6.40 |
| Treated for general paresis | 0.01% | $54,176 | $7.15 |
| Total (all outcomes) | 100% | Not applicable | $1,190.36 |
The “treated for late syphilis” outcome also includes those treated for syphilis of unknown duration. See manuscript text and Figure 1 for a more detailed description of the outcomes.
“Infections that are not reported as cases” refers to those who are not reported as cases within 30 years as shown in the decision tree in Figure 1.
For those treated for syphilis but not reported as a case, we assumed that the proportion treated for P&S or early non-P&S syphilis would be the same as for the reported cases (e.g., the $1,025 value shown here was calculated as (0.630 x $680) + ((1 – 0.630) x $1,612), where 0.630 is the base case probability of treatment for P&S or early non-P&S syphilis among reported cases. We assumed no early symptomatic neurosyphilis and no ocular syphilis among those treated for syphilis but not reported as a case.
The discounted costs in this table have been discounted to the time of infection.
Costs are in 2019 U.S. dollars.
Of the $1,190 average cost per infection, treatment for syphilis of unknown duration or late syphilis accounted for $462 and $88 (without and with early neurosyphilis or ocular syphilis, respectively), treatment for P&S syphilis or early non-P&S syphilis accounted for $332 and $135 (without and with early neurosyphilis or ocular syphilis, respectively), and treatment for syphilis without being reported as a case accounted for $149 (Table 3). All of the remaining outcomes combined to account for $26 of the average cost per infection.
In one-way sensitivity analyses, the results were most sensitive to changes in assumptions regarding the treatment cost per case of unknown duration or late syphilis; the treatment cost per case of P&S syphilis or early non-P&S syphilis; the treatment cost per case of early neurosyphilis and ocular syphilis; the probability of early neurosyphilis and ocular syphilis; and the probability of being treated for P&S or early non-P&S syphilis, among reported cases (Figure 2). When varying any of the other parameters individually, the estimated lifetime cost per infection remained within $100 of the base case estimate. In the probabilistic sensitivity analyses, the 2.5th and 97.5th percentiles of the 10,000 Monte Carlo simulations were $729 and $1,884, respectively, and the 25th and 75th percentiles were $985 and $1,352, respectively.
Figure 2.
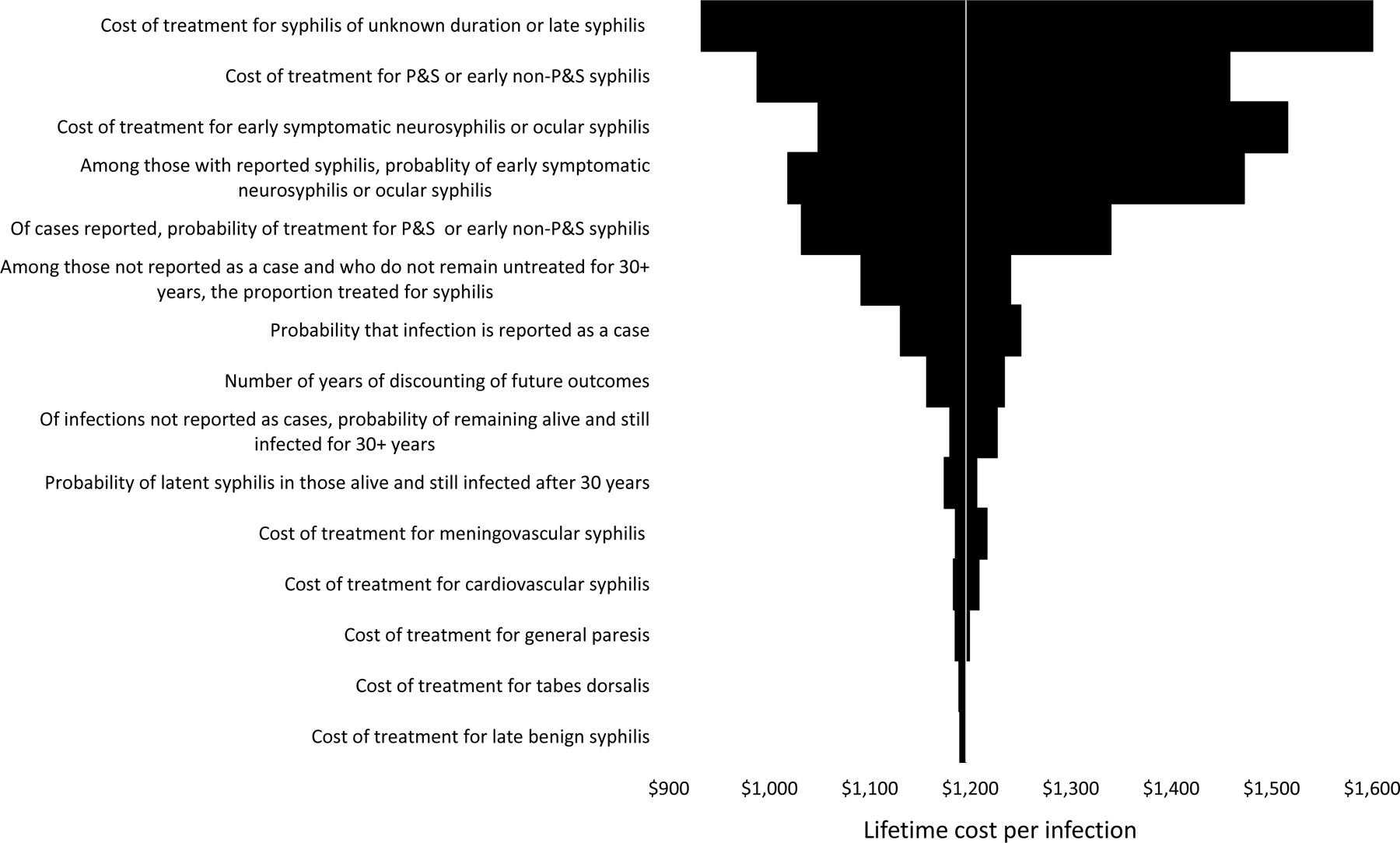
Results of one-way sensitivity analysis of cost of syphilis: Estimated lifetime cost per infection when varying one parameter value at a time
This diagram shows the estimated lifetime cost per infection when a single parameter value was changed from its base case value to its lower or upper bound. For example, when we varied the treatment cost per case of unknown duration or late syphilis while holding all other parameters at their base case values, the lifetime cost per infection was $932 when applying the lower bound and $1,625 when applying the upper bound (top entry of diagram). For ease of illustration, the parameter descriptions have been shortened; see Table 1 and Table 2 and manuscript text for more precise descriptions.
Discussion
Our estimate of the expected lifetime cost of syphilis of $1,190 per infection is about 50% higher than the most recent previous estimate of $807 when adjusted to 2019 dollars.1 Our intermediate results differed substantially from those of the previous study: treatment for early syphilis accounted for $466 of the average lifetime cost in our model vs. approximately $86 in the previous model; treatment for unknown duration or late syphilis accounted for $550 in our model vs. approximately $204 in the previous model; and treatment for late syphilis outcomes accounted for $26 in our model vs. approximately $516 in the previous model.
There are three main reasons why our intermediate outcomes differed notably from those of the previous study. The first main difference is that we applied higher costs for treatment of syphilis than the previous study, which assumed that two-thirds of those treated for P&S syphilis would be treated in a public clinic setting at a cost of $90.1 In contrast, our higher cost estimate allowed for a higher cost of the physician visit, a higher cost of the penicillin regimen, and the possibility of costs associated with follow-up. The cost of the penicillin regimen we assumed ($360 per dose) accounted for most of the $680 cost we applied for the treatment of P&S and early non-P&S syphilis. The second main difference is that we explicitly accounted for the possibility of treatment for early neurosyphilis and ocular syphilis among those with reported syphilis. The third main difference is that we applied much lower probabilities for the late syphilis outcomes. For example, the proportion of infections incurring costs of long-term serious consequences of syphilis was 0.0021 in our model, which is roughly one-eighth the corresponding value of 0.016 in the previous model. Our use of lower probabilities for late syphilis outcomes yielded model results more consistent with current data on mortality due to late syphilis.
As illustrated by the differences in our study and the previous study, many parameter values needed to estimate the lifetime cost of syphilis are informed by limited data and are subject to considerable uncertainty. We addressed this issue in two main ways. First, we ensured that the probabilities applied in our model yielded outcomes that were consistent with two key sources of data: reported cases of syphilis by stage from national surveillance data and number of deaths due to late syphilis from national mortality data. The incorporation of the latter data source was perhaps the biggest improvement in our model over previous versions. Second, we conducted extensive sensitivity analyses to illustrate how the results change when model inputs were varied and to identify the most influential assumptions. For example, the percentage of infections that are ever reported as syphilis cases is one of the most difficult parameter values to estimate from empirical data, and our base case value of 80% was based solely on expert opinion. Fortunately, our sensitivity analysis indicated that this assumption was not particularly influential, and thus the lack of data to inform this particular assumption is not of particular concern.
Unfortunately, however, several of the most influential parameter values in our model were among the most uncertain. For example, the treatment cost per case of early neurosyphilis and ocular syphilis was one of the most influential parameter values observed in our one-way sensitivity analysis, and limited data exist to inform this cost estimate. One key factor in this estimate is whether the patient receives the recommended regimen of intravenous (IV) aqueous crystalline penicillin G or the alternative regimen of intramuscular (IM) procaine penicillin G plus oral probenecid.18 We applied a higher cost for the recommended regimen than the alternative regimen, primarily because we assumed hospitalization would be required for IV therapy. However, the value we applied for the percent of patients with neurosyphilis or ocular syphilis that receive IV therapy (21.3%, range: 14.0% to 29.3%) was likely conservative, as it was based on a study where participants were recruited primarily from a public STD clinic where the standard neurosyphilis treatment was the IM regimen.13 We note that a study of patients with ocular syphilis in North Carolina11 found that a much higher percentage of patients (72%) received IV therapy.
Our estimate of the average lifetime cost per infection can be interpreted as the present value of the direct medical treatment and care costs that could be saved by preventing one instance of syphilis acquisition in an adolescent or adult, in the context of existing STD prevention efforts. We did not include congenital syphilis costs. Further, the lifetime medical cost per infection does not include a wide range of other costs imposed by syphilis, such as the costs of syphilis prevention activities like screening and partner services, costs of presumptive treatment of those not actually infected, and syphilis testing of biologics such as blood donations. Syphilis also imposes productivity costs and intangible costs (e.g., pain and suffering). Although these costs were beyond the scope of our study, a study is currently underway to estimate the health burden of syphilis in the United States in terms of quality-adjusted life years (QALYs) lost.22 Such studies can help to quantify the intangible costs associated with syphilis.
In summary, there are numerous challenges and limitations associated with estimating the lifetime cost of syphilis per infection. We have attempted to address these issues by applying conservative assumptions (i.e., assumptions that result in a lower cost estimate) where warranted and by ensuring the probabilities we used for the potential outcomes of infection were consistent with available national-level data. Our estimate of the average lifetime cost per infection ($1,190) is about $400 higher than that of the most recent previous study ($807),1 a difference mainly due to our higher cost assumptions for benzathine penicillin G. Differences across these two studies are even more notable when itemized results are compared. In updating the estimated lifetime cost per infection, we have provided extensive documentation of our methods and assumptions. We hope this documentation will facilitate future studies of the cost of syphilis as better data become available and improved methodologies are developed.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Owusu-Edusei K Jr., Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013;40(3):197–201. [DOI] [PubMed] [Google Scholar]
- 2.Chesson HW, Blandford JM, Gift TL, Tao G, Irwin KL. The estimated direct medical cost of sexually transmitted diseases among American youth, 2000. Perspect Sex Reprod Health 2004;36(1):11–19. [DOI] [PubMed] [Google Scholar]
- 3.American Social Health Association. Sexually transmitted diseases in America: How many cases and at what cost? Menlo Park, CA: Kaiser Family Foundation;1998. [Google Scholar]
- 4.Chesson HW, Rein D, Kassler WJ, et al. Direct medical costs of syphilis in the United States: The potential for a cost-saving national elimination program Poster presentation. 1998 National STD Prevention Conference; Dallas, December 6–9, 1998. [Google Scholar]
- 5.Schmid GP, Zaidi A. Serologic screening for syphilis: a decision model Eleventh Meeting of the International Society for STD Research; New Orleans, August 27–30, 1995. [Google Scholar]
- 6.Silberstein GS, Coles FB, Greenberg A, Singer L, Voigt R. Effectiveness and cost-benefit of enhancements to a syphilis screening and treatment program at a county jail. Sex Transm Dis 2000;27(9):508–517. [DOI] [PubMed] [Google Scholar]
- 7.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013;40(3):187–193. [DOI] [PubMed] [Google Scholar]
- 8.Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases in American youth: incidence and prevalence estimates. Perspect Sex Reprod Health 2004;36(1):6–10. [DOI] [PubMed] [Google Scholar]
- 9.Cates W Jr. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex Transm Dis 1999;26(4 Suppl):S2–S7. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2018 Atlanta: U.S. Department of Health and Human Services; 2019. [Google Scholar]
- 11.Oliver SE, Cope AB, Rinsky JL, et al. Increases in ocular syphilis-North Carolina, 2014–2015. Clin Infect Dis 2017;65(10):1676–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver SE, Aubin M, Atwell L, et al. Ocular syphilis - eight jurisdictions, United States, 2014–2015. MMWR Morb Mortal Wkly Rep 2016;65(43):1185–1188. [DOI] [PubMed] [Google Scholar]
- 13.Dunaway SB, Maxwell CL, Tantalo LC, Sahi SK, Marra CM. Neurosyphilis treatment outcomes after intravenous penicillin G versus intramuscular procaine penicillin plus oral probenecid. Clin Infect Dis 2019:ciz795. [DOI] [PMC free article] [PubMed]
- 14.Peterman TA, Kidd SE. Trends in deaths due to syphilis, United States, 1968–2015. Sex Transm Dis 2019;46(1):37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosahn PD. Autopsy Studies in Syphilis: A Monograph Washington, DC: US Department of Health, Education, and Welfare, Public Health Service; 1955. [Google Scholar]
- 16.McGivern L, Shulman L, Carney JK, Shapiro S, Bundock E. Death certification errors and the effect on mortality statistics. Public Health Rep 2017;132(6):669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gjestland T The Oslo study of untreated syphilis; an epidemiologic investigation of the natural course of the syphilitic infection based upon a re-study of the Boeck-Bruusgaard material. Acta Derm Venereol Suppl (Stockh) 1955;35(Suppl 34):3-LVI. [DOI] [PubMed] [Google Scholar]
- 18.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and reports: 2015;64(Rr-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 19.United States Department of Veterans Affairs. Federal supply schedule - pharmaceutical prices https://www.va.gov/opal/nac/fss/pharmPrices.asp. Accessed 10/20/2020.
- 20.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine: Second Edition. New York, NY: Oxford University Press; 2016. [Google Scholar]
- 21.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine 2010;28(42):6858–6867. [DOI] [PubMed] [Google Scholar]
- 22.Lee K Estimated burden of STIs in terms of quality-adjusted life years Presented at NCHHSTP Epidemiologic and Economic Modeling Agreement (NEEMA) Meeting; October 15, 2020, 2020; Atlanta (virtual conference). [Google Scholar]
- 23.Centers for Disease Control and Prevention. Symptomatic early neurosyphilis among HIV-positive men who have sex with men--four cities, United States, January 2002-June 2004. MMWR Morb Mortal Wkly Rep 2007;56(25):625–628. [PMC free article] [PubMed] [Google Scholar]
- 24.Clark EG, Danbolt N. The Oslo study of the natural history of untreated syphilis; an epidemiologic investigation based on a restudy of the Boeck-Bruusgaard material; a review and appraisal. J Chronic Dis 1955;2(3):311–344. [DOI] [PubMed] [Google Scholar]
- 25.Arias E, Xu J. United States Life Tables, 2015. Natl Vital Stat Rep 2018;67(7):1–64. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


